Abstract
High-dose rate (HDR) brachytherapy can play an important role in the management of patients with gastrointestinal tumors, in particular those with rectal and anal cancers. Endorectal/endoanal brachytherapy is a safe, effective, and well-tolerated option for treatment of patients with medically inoperable primary and recurrent rectal and anal tumors. It also may be a nonoperative alternative for patients with low-lying rectal cancer who refuse surgery that will leave them with a colostomy (abdominoperineal resection). There are also ongoing studies evaluating the use of preoperative endorectal brachytherapy as an alternative to standard pelvic radiotherapy for locally advanced rectal cancer. An overview of the technical aspects of the procedure as well as issues regarding treatment planning, delivery, and follow-up after endorectal/endoanal brachytherapy are discussed in this chapter.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Endorectal
- Endoanal
- High-dose rate (HDR)
- Brachytherapy
- Medically inoperable
- Recurrent
- Cancer
- Gastrointestinal • Rectal
- Anal
Introduction
Rationale for Brachytherapy in Rectal Cancer
-
According to the American Cancer Society , approximately 39,220 cases of rectal cancer are diagnosed annually in the United States [1]
-
Standard management of rectal cancer
-
Stage I: Total mesorectal excision (TME) alone
-
Stage II–III: Neoadjuvant chemoradiation therapy (CRT) → TME → adjuvant chemotherapy
-
-
Local recurrence rates after standard management are <10 % [2]
-
TME provides excellent disease control in the management of primary rectal cancer; however, it may have an unfavorable impact on patients’ functioning
-
Approximately 20 % of patients have a pathologic complete response to preoperative chemoradiation, thus more recently, there has been interest in nonoperative management for patients who have a clinical complete response to CRT
-
However, 80 % of patients may still harbor residual tumor cells and will benefit from surgical resection of the tumor
-
While patients who have had a complete tumor response to CRT may experience excellent outcomes in the absence of TME , patients with biopsy-proven residual or recurrent disease after external beam radiation therapy ± chemotherapy are at high risk of local progression if surgery is omitted from their management
-
Nonetheless, TME may not be performed either due to prohibitive surgical risk or patient refusal
-
Elderly patients and patients with multiple comorbidities may not be good candidates for radical rectal surgery
-
Some patients refuse any treatment that will leave them with a stoma, including standard-of-care TME
-
-
Endorectal brachytherapy can be delivered to address local persistence or recurrence of disease in the rectal wall to improve local control for patients who are unable to undergo a radical rectal surgery or have refused a stoma. The goal is to deliver a high focal dose to the tumor cells and achieve a pathologic complete response
Rationale for Brachytherapy in Anal Cancer
-
It is estimated that approximately 8080 people in the United States will be diagnosed with anal cancer in 2016 [1]
-
Unlike rectal cancer, primary anal cancer is managed non operatively with chemoradiation alone
-
The standard treatment, introduced by Nigro, involves concurrent external beam radiation therapy (EBRT), 5-Fluorouracil (5-FU), and mitomycin-C (MMC)
-
Local failure after completion of this regimen ranges from approximately 10–25 %, with higher rates in patients unable to receive any component of the Nigro regimen. Some patients also have persistent disease after definitive chemoradiation
-
Salvage of local recurrence or persistent disease is typically performed with an abdominoperineal resection (APR)
-
If patients with persistent or locally recurrent anal cancer decline or are unfit for APR, endorectal/endoanal brachytherapy may represent an alternative option to provide local disease control
HDR Brachytherapy in Rectal and Anal Cancer
HDR Brachytherapy in the Management of Rectal Cancer
-
The use of brachytherapy in the treatment of rectal cancer dates back to the early 1900s
-
One of the initial approaches involved application of a radium source internally, within the rectum [9, 10]
-
Contact X-ray therapy , using a low-energy X-ray endorectal tube to deliver a high dose of radiation to the tumor, has demonstrated efficacy in controlling small, early-stage rectal tumors without external beam radiation. For many decades, contact X-ray therapy (CXRT) alone was used to effectively treat T1N0 rectal cancer [11, 12]. While this type of superficial radiotherapy does not utilize a radioactive source, the technique of applying high-dose radiotherapy directly to the tumor works in a way very similar to brachytherapy. CXRT is performed with a 50 kVp endorectal tube that delivers high doses of radiation to the tumor, while rapid dose falloff ensures low doses to deeper tissues. Because this approach provides only superficial dose, it is insufficient to control ≥T2 or node-positive disease. In this setting, a combination of CXRT and/or 192Ir high-dose rate (HDR) brachytherapy with EBRT may be used to deliver adequate dose
-
-
Many groups, especially in Europe, have reported on combination external beam radiotherapy followed by a “boost” to the tumor using brachytherapy in patients with locally advanced rectal cancer
-
French physician Jean Papillon (University of Lyon) was one of the first clinicians to combine endocavitary irradiation and EBRT. He treated 71 elderly patients with T2–T3 adenocarcinoma with cobalt arc therapy (30 Gy in 10 fractions over 13 days) followed 2 months later by CXRT (25 Gy) and an 192Ir implant (20–30 Gy). The tumors were staged without imaging. Papillon reported that “the tolerance of this treatment was generally good. Benign and superficial radionecrosis, which healed spontaneously, was observed in five cases.” At a minimum follow-up of 3 years, 46 patients (64.7 %) were alive and well, and 44 of these patients reported normal bowel function. The rate of cancer-specific death was 16 % at 5 years [13]
-
Maingon et al. reported on 151 rectal cancer patients who received radiotherapy alone with curative intent. By clinical examination, 76 (50 %) had T1 lesions, 62 (41 %) T2, and 13 (9 %) T3. Of the 26 patients evaluated by endorectal ultrasound (ERUS) , 6 (23 %) had pelvic lymphadenopathy. CXRT was given to 129 patients (69 %), brachytherapy to 45 (30 %), and EBRT to 34 (22.5 %). No acute grade ≥3 toxicity was observed. Ten patients (7 %) experienced late grade ≥3 toxicity, 3 of whom required a colostomy (1 for rectal stenosis, 1 for rectal bleeding, and 1 for fecal incontinence). A clinical complete response was achieved in 93 % of patients at 3 months after treatment. Ultimately, local failure was observed in 50 cases (28 %). The risk of local recurrence increased with tumor size and tethering and with omission of EBRT. After salvage surgery, the local control rate for the entire cohort was 82 %. The 5-year disease-specific survival was 66 %. Of the 124 patients available for long-term follow-up, sphincter preservation was obtained in 104 (84 %), 102 (98 %) of whom had normal sphincter function [14]
-
Gerard et al. published a pilot study of 63 patients with T2-3N0-1M0, mid-to distal rectal cancer who were treated with radiotherapy alone. In this cohort, 41 patients (65 %) had T2 disease, 22 (35 %) T3, 45 (71 %) N0, and 18 (29 %) N1. Most patients (53/63 = 84 %) were staged by ERUS. All patients received CXRT (median 80 Gy in 3 fractions over 21 days), followed by EBRT (39 Gy in 13 fractions over 17 days) with a concomitant boost (4 Gy in 4 fractions). After a 4–6-week interval, all but 7 patients received a low-dose rate 192Ir implant (20 Gy over 22 h). There was no instance of grade ≥3 toxicity. Although most patients experienced acute proctitis, no treatment was interrupted because of intolerance or severe toxicity. Intermittent late rectal bleeding occurred in 24 patients (38 %), one of whom required occasional blood transfusions. Among the 39 living patients at the time of analysis, bowel function was scored as excellent in 19, good in 17, and fair in 3, based on the Memorial Sloan Kettering Cancer Center (MSKCC) scale . At a median follow-up time of 54 months, the rate of local tumor control was 63 %. Five patients required an abdominoperineal resection (APR) , because they had residual disease at 2 months after treatment. Local recurrence was observed in 18 patients (28.5 %) who initially had a clinical complete response; 5 were salvaged by APR and 1 by a second course of CXRT. After primary or salvage treatment, the ultimate rate of pelvic control was 73 %. The 5-year overall survival rate was 64.4 % for the entire cohort and 78 % for the subset of 42 patients aged <80 years (84 % for T2 and 53 % for T3 lesions) [15]
-
Aumock et al. reported the experience at Washington University treating 199 patients with endocavitary RT ± EBRT. The majority of tumors were freely mobile to palpation (n = 128), ≤3 cm in greatest dimension (n = 136), without clinical evidence of nodal metastases (n = 177), and well to moderately differentiated (n = 190). ERUS was used to evaluate 77 patients (39 %). Early during the study period, some patients received CXRT alone; however, an interim analysis revealed that tumor control improved with EBRT. Therefore, all patients treated after 1987 received EBRT (mean 45 Gy in 25 fractions) followed 6 weeks later by CXRT (mean 60 Gy divided in 2 fractions over 2 weeks). The primary toxicity was proctitis (n = 19) that typically resolved within 10 months. Two patients required transfusions for rectal bleeding and 2 required dilation of a rectal stricture. Local recurrence was observed in 58 patients (29 %), 20 of whom were effectively salvaged surgically. Thus, after primary or salvage treatment, the ultimate pelvic control rate was 81 % [16]
-
Hoskin et al. reported on 50 patients who received endorectal HDR brachytherapy at a single institution. The majority of patients were elderly and frail (median age 82 years) and were therefore poor surgical candidates. Patients who had received prior CRT were treated to 12 Gy in 2 fractions (n = 18); those who had not received prior EBRT were treated with a single 10 Gy fraction for palliation (n = 22) or with 6 Gy fractions up to 36 Gy (n = 8). Among the 25 patients with follow-up information, 14 achieved complete clinical regression, 7 partial (>50 %) regression, and 4 minimal (<50 %) regression. The authors reported significant palliation of mucous discharge, bleeding, pain, and diarrhea. In 2 patients who were treated palliatively with a single fraction of 10 Gy, the treatment was repeated 10 months later for recurrent symptoms and again provided good relief with no additional toxicity [17]
-
-
Taken together, these groups have shown that a combination of endorectal brachytherapy and EBRT is feasible and safe in the nonoperative management of rectal cancer
-
The most common side effect is acute and late proctitis Patients typically maintain good bowel function
-
Furthermore, these studies have demonstrated good rates of tumor control [13–17]
-
The feasibility and efficacy of endorectal brachytherapy have been evaluated in the preoperative setting.
-
Vuong et al. reported on 100 patients with T2 to early T4, operable rectal cancer treated with high-dose endorectal brachytherapy using 3-dimensional treatment planning (26 Gy in 4 consecutive daily fractions) followed in 6–8 weeks by definitive surgery. From 1998 to 2005, those with pathologically positive nodes received postoperative EBRT (45 Gy in 25 fractions) with concurrent 5-FU. By ERUS and MRI, the clinical staging of the tumors were T2 (n = 3), T3 (n = 93), T4 (n = 4), N0 (n = 58), and N1–N2 (n = 42). Ninety-six patients underwent surgery; 2 refused the operation based on a normal ERUS after treatment; 2 died before surgery, one from a stroke and the other from a myocardial infarction. Acute toxicity related to brachytherapy was limited to grade 2 proctitis in 99 patients, with 1 patient receiving grade 3 proctitis requiring transfusion. One patient who refused surgery developed mild rectal stenosis but did not require dilation. Of the group that underwent surgery, 29 % were ypT0N0-2, 34 % demonstrated residual tumor, and 37 % had micro-foci of residual disease. Only one patient had microscopic positive margins; this patient had no evidence of disease at five year follow-up. Postoperative adjuvant external beam therapy and chemotherapy were given in 27 of the 31 patients with positive nodes. The median follow-up time was 60 months. At 5 years, the actual local recurrence rate was 5 %, disease-free survival was 65 %, and the overall survival rate was 70 % [18]
-
This cohort was updated in 2015; a total of 483 patients received neoadjuvant endorectal HDR brachytherapy alone; 43 received postoperative external beam radiation therapy. The complete sterilization rate was 27 % and the rate of positive nodes was 30.7 %. Median follow-up time was 63 months. Actuarial local recurrence rate was 4.8 %. Disease-free survival was 65.5 %. Overall survival rate was 72.8 % [19]
-
The authors concluded that HDR endorectal brachytherapy is an effective neoadjuvant treatment for patients with resectable rectal cancer that offers excellent local control with a favorable toxicity profile [18, 19]
-
Ongoing Clinical Trials
-
Phase I, Dose Escalation Trial of Endoluminal High-Dose Rate Brachytherapy with Concurrent Chemotherapy for Rectal or Anal Cancer in Patients with Recurrent Disease or Undergoing Nonoperative Management
-
Ongoing single-institution (MSKCC) trial involving anorectal cancer patients to determine the maximum tolerated dose and assess rates of acute and late toxicity after endorectal brachytherapy with concurrent capecitabine or 5-fluorouracil
-
Patients who previously received pelvic EBRT ± chemotherapy and will not undergo surgery
-
Dose escalation 1200 cGy in a systematic fashion
-
Three dose tiers
-
1500 cGy (500 cGy per fraction)
-
1800 cGy (600 cGy per fraction)
-
2100 cGy (700 cGy per fraction)
-
-
-
Brachytherapy will be administered in 3 fractions, 1 fraction/week
-
Dose will be prescribed to the minimum peripheral dose, or the dose-line encompassing the tumor as contoured on the CT or MRI. In addition to brachytherapy, patients will receive concurrent capecitabine or 5-FU
-
The primary objective of this trial is to establish the maximum tolerated dose (MTD) and to determine toxicity rates of endorectal brachytherapy with concurrent 5-FU-based chemotherapy
-
-
Phase II, Study of High-Dose Rate Endorectal Brachytherapy in the Treatment of Locally Advanced Low Rectal Cancer (Sidney Kimmel Cancer Institute—Johns Hopkins)
-
Ongoing Phase II study (but no longer recruiting participants) of locally advanced resectable rectal cancer patients (T2N1 or T3N0-1) examining pathologic response of neoadjuvant high-dose endorectal brachytherapy in comparison to standard-of-care neoadjuvant chemoradiation
-
Daily dose of 6.5 Gy over 4 consecutive days
-
Primary Endpoint: Pathologic complete response. Secondary endpoints include biologic and radiographic predictors of response to therapy, adverse events (gastrointestinal toxicity), quality of life as measured by the QLQ-C30, and tumor regression/response
-
Goal was to enroll 30 patients; will report primary outcome in 2020
-
-
Phase II: CORRECT (Chemoradiation OR Brachytherapy for RECTal Cancer) (PI: Sidney Kimmel Cancer Institute—Johns Hopkins—Multiple institutions)
-
Ongoing Phase II study (currently recruiting participants)
-
Neoadjuvant IMRT with concurrent capecitabine vs. neoadjuvant endorectal HDR brachytherapy (6.5 Gy daily over 4 consecutive days)
-
To be followed by FOLFOX6 x 12 cycles followed by surgical resection
-
Primary outcome: pathologic complete response rate
-
Secondary outcomes: biologic and radiographic predictors of response, acute and long-term toxicity, quality of life, sphincter preservation rates, locoregional control, distant metastases, and overall survivalGoal enrollment 138 patients; estimated completion 2018
-
HDR Brachytherapy in the Management of Anal Cancer
-
No reported series focusing specifically on the use of brachytherapy to treat recurrent anal cancer; however, several groups have reported their experience using EBRT with an HDR brachytherapy boost in the upfront management of primary anal cancer
-
As one example, Oehler-Janne et al. treated 81 anal cancer patients with EBRT (45 Gy in 25 fractions) followed either immediately by an EBRT boost (14.4 Gy in 8 fractions) or 3 weeks later by an interstitial 192Ir interstitial brachytherapy boost (14 Gy in 7 fractions over 3 days). There was a lower rate of acute dermatitis and hematologic toxicity in the brachytherapy group. There was no difference in other acute toxicities, late toxicity, or quality of life between the two cohorts. Chronic toxicity in the brachytherapy group consisted of grade ≥2 proctitis in 19 % of patients and grade 1–2 incontinence in 18 %. At 5 years, the local failure rate was 10.3 % in the brachytherapy group and 15.4 % in the EBRT group (P = 0.5) [20]
-
Kapp et al. treated 39 patients with T1-2N0-2M0 anal cancer with split-course EBRT (50–50.4 Gy) ± chemotherapy (5-FU and MMC) and an integrated 192Ir brachytherapy boost (6 Gy) during the 1–2 week split. Patients who achieved a partial tumor response (n = 7) received a second 192Ir brachytherapy boost (6 Gy) after a 6–8 week delay. Brachytherapy was provided with interstitial needles and/or an endorectal cylinder. Acute toxicity among brachytherapy patients was similar to that of patients receiving EBRT ± chemotherapy alone. Late complications included proctitis (n = 2), occasional sphincter dysfunction (n = 1), and circumscribed ulcers at the site of the primary tumor (n = 7). The rate of 5-year local control was 76 % and colostomy-free survival was 73 %. For the patients in whom the anus was conserved, full continence was recorded in 28/30 (93 %) [21]
-
These groups have demonstrated the feasibility and safety of using HDR brachytherapy to treat cancers of the anal canal
Timing for Endorectal Brachytherapy
-
Definitive treatment for small rectal (T1, T2) tumor
-
Neoadjuvant therapy for patients with newly diagnosed rectal cancer with previous pelvic radiation
-
After EBRT ± chemotherapy, concurrent with capecitabine or 5-FU
-
Medically inoperable rectal tumor
-
Patients requiring an APR and refuse
-
Palliative treatment for patients with Stage IV disease
-
Salvage for small recurrent, anal cancers
Goal for Endorectal Brachytherapy of Anorectum
-
Local control: If patients with locally persistent or recurrent disease after chemoradiation decline to undergo an abdominoperineal resection (APR) or are unfit for radical surgery, endorectal brachytherapy may represent an option for providing local disease control. Brachytherapy enables delivery of high-dose radiation directly to a rectal tumor, with relative sparing of the surrounding normal tissues. In these patients, brachytherapy can be employed with the goal of improving complete response and avoiding radical resection. Given the conformal dose distribution, treatment may be provided in several large fractions, providing a radiobiological advantage over conventionally fractionated RT
-
Improved quality of life: Endorectal brachytherapy can be applied to improve outcomes and individualize treatment of patients with rectal cancer. Given the morbidity of radical surgery, and its long-term impact on quality of life, selective nonoperative treatment may be an alternative in some cases. With appropriate use of endorectal brachytherapy, rectal surgery may be selectively omitted from the management of some rectal cancer patients. These include individuals for whom surgery poses a prohibitive risk, or who refuse to have an APR, or whose cancers respond dramatically to chemoradiation
-
Palliative: Endorectal brachytherapy also serves a palliative purpose in the management of patients with metastatic disease who may benefit from local therapy, but for whom radical resection is inappropriate. With advances in treatment options for rectal cancer and continued collaboration with our colleagues across disciplines, endorectal brachytherapy provides an additional option to better tailor therapies based on patient risk factoring and tumor characteristics, taking into account the impact of treatment on each patient’s quality of life
Selection Criteria for Implantation
-
Palpable or MRI-visible low-lying tumor
-
Tumor may be concentric or eccentric
-
Maximum tumor length of 7 cm at time of brachytherapy start to allow accessibility by 10 cm applicator
-
Rectal: preferably no invasion of anal canal (increased risk of anal necrosis). Invasion of anal canal permitted for recurrent anal primary
Patients Who Are Not Candidates for Endorectal Brachytherapy
-
Patients with contraindications to general anesthesia
-
Proximal rectal tumors (>10 cm from the anal verge)
-
ECOG performance status 3
Medical Operability
-
Comprehensive evaluation including
-
Within 45 days of treatment start:
-
History and Physical examination
-
Performance status
-
Weight
-
Review of current medications
-
Assessment of baseline comorbidities, including baseline pain assessment
-
Imaging:
-
MRI of the pelvis with DCE and DWI series (unless contraindicated)
-
CT of the chest/abdomen/pelvis as a baseline to evaluate for systemic disease
-
-
-
Within 30 days of treatment start:
-
Quality of life assessments: EORTC QLQ-C30, EuroQol 5D-5L, and BFI assessments
-
Standard preoperative evaluation including determination of normal cardiac function
-
No active coronary artery disease
-
No New York Heart Association class II, III, or IV disease
-
No arrhythmia requiring treatment
-
-
Proctoscopy, with photograph of the tumor if possible
-
Anesthesia consent
-
-
Within 14 days of treatment start
-
Labs: CBC, PT and PTT, CMP, CEA (for rectal cancer patients only), and pregnancy test drawn within 14 days prior to procedure start
-
ANC ≥ 1.5 cells/mm3 and PLT ≥100,000/mm3
-
Adequate Renal function : Creatinine <1.5× the upper limit of normal (ULN) or calculated creatinine clearance of ≥50 cc/min
-
Adequate Hepatic functions : Bilirubin less than 1.5 mg/dL; (except in patients with Gilbert’s Syndrome, who must have a total bilirubin less than 3.0 mg/dL); AST or ALT <3× ULN, or <5× ULN if known liver metastases
-
-
-
Applicator
-
There are several options for anorectal applicators including Varian’s Capri™ rectal and vaginal applicator (Varian, Palo Alto, CA, USA), the Nucletron® Intracavitary Mold Applicator Set (Elekta AB, Stockholm, Sweden), and the Anorectal (AR) applicator (Ancer Medical, Hialeah, FL, USA)
The Capri™ Applicator (Fig. 11.1)
-
Allows for highly asymmetric dose distributions
-
One central channel and 12 peripheral channels
-
Barium sulfate to allow simple catheter detection
-
Inflatable with air or saline
-
Internal markers allow for identification of catheters
-
Soft silicone exterior
-
Single use
-
CT Compatible
The Intracavitary Mold Applicator Set (IMAS) from Nucletron®
-
Made of a flexible material with several channels for the radioactive source
-
One central catheter and eight peripheral catheters, enabling delivery of a variety of dose patterns, limiting normal tissue toxicity, and improving dose delivery to the tumor
-
Markers on the handle, the base, and one or more catheters of the device assist with positioning
-
High torsional stiffness prevents twisting during placement
-
Tip: A saline-inflatable balloon may help to push the applicator flush against the rectal mucosa
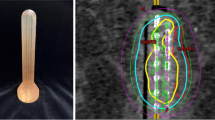
The Anorectal (AR) Applicator [22, 23] (Fig. 11.2)
Anorectal (AR) applicator (left) and phantom study to simulate a protruding tumor structure (right). (Left: Used with permission of Ancer Medical, Hialeah, FL, USA; Right: Used with permission from Cohen, Gilad N. et al. Evaluation of a New MRI Compatible Brachytherapy Ano-rectal Applicator. Brachytherapy 2014; 13: S48)
-
Multichannel applicator with two concentric balloons
-
The inner balloon supports eight radially symmetric source lumens; the compliant outer balloon expands to separate the normal rectal mucosal wall and the source lumens yet deform around a firm, exophytic rectal mass
-
The effective treatment zone of the applicator, in which the source lumens maintain the cylindrical geometry, is 10 cm long and is delineated by two central markers for positioning and treatment verification
-
Single use
-
Reduces the dose to the contralateral rectal wall to less than 50 % of prescription [22]
-
CT and MRI compatible (Fig. 11.3)
Guidelines for Implantation
-
At least 4 weeks from prior major surgery or radiotherapy (recommend waiting 4 weeks after EBRT prior to implant)
-
On an outpatient basis
-
MSKCC approach: In the operating room under general anesthesia
-
Canadian approach: In operating or procedure room without general anesthesia
-
Can be delivered once weekly for 3 consecutive weeks or a daily dose of 6.5 Gy over 4 consecutive days
Pre-procedure Advice
-
Golytely prep prior to each procedure with water enema the morning of the procedure
-
Semi-sterile procedure
-
Antibiotics at the time of the procedure
-
Exam under anesthesia prior to each procedure. Patient in dorsal lithotomy position with legs up in whole leg stirrups
-
Colorectal surgeon present at first fraction to place gold fiducial markers above and below the tumor
Procedure Tips
-
Identify and mark extent of tumor
-
Insert and secure endorectal applicator
-
In the event of circumferential narrowing that prevents insertion of the endorectal applicator, a single-channel Bougie applicator (similar to a Savary Dilator with a single internal lumen for placement of the HDR catheter) has been used
Treatment Planning
-
CT and MRI will be obtained for treatment planning to develop a conformal radiation dose specific to the rectal tumor, thus minimizing the dose to the bladder and small bowel [23] (Fig. 11.4)
-
Prescription dose and fractionation:
-
MSKCC approach: 3 fractions, each spaced apart by 7 days (±1 day)
-
Clinical trial for dose escalation currently ongoing to evaluate maximum tolerated dose: 12–21 Gy in 4–7 Gy per fraction
-
-
Canadian approach: Daily dose of 6.5 Gy over 4 consecutive days
-
-
Structures to be contoured
-
Gross tumor volume (GTV)
-
Normal rectum
-
Applicator
-
Bladder
-
Urethra
-
Bowel
-
-
Dose constraints for organs at risk
-
Rectal surface Dmax < 15 Gy/fraction
-
Bladder Dmax < 5 Gy/fraction
-
Urethra Dmax < 4 Gy/fraction
-
Bowel <4 Gy/fraction
-
-
Treatment planning will be performed with computerized dosimetry. The dose will be prescribed to the isodose line that best covers the target volume, while minimizing dose to adjacent normal tissue
-
A dose volume histogram (DVH) is generated and reviewed [23] (Fig. 11.5)
Fig. 11.5. DVH for the plan in Fig. 11.4 shows GTV (yellow), CTV (magenta, an expansion of the GTV overlapping some healthy rectal and anal muscle), anus (blue), healthy rectum (cyan), urethra (dark green), bladder (light green), and superior and inferior gold fiducial markers (red). (Courtesy of Gil’ad Cohen, PhD, Memorial Sloan Kettering Cancer Center, NY, NY)
Treatment Delivery
-
Prior to each treatment , fluoroscopic imaging is used to verify catheter positioning and position/rotation of the applicator and confirm the treatment geometry using rigid registration of the CBCT and planning MRI. After registration of the applicator images, positioning was evaluated based on the match of the pre-implanted gold fiducial markers [22, 23] (Figs. 11.6 and 11.7)
Fig. 11.6. Fig. 11.7. Pretreatment verification. Planning MRI is registered to pretreatment CBCT. Built-in applicator fiducials (green) are matched. The quality of registration is assessed based on the match of fiducials placed in the patient (red). (Courtesy of Gil’ad Cohen, PhD, Memorial Sloan Kettering Cancer Center, NY, NY)
-
Despite nonrigid nature of the applicators and use of new applicator at each treatment session, treatment geometry should be reproducible within 2.5 mm [23]
-
HDR brachytherapy will be administered using an HDR afterloader with an 192Ir source in a shielded room
-
Following treatment planning, the HDR afterloader will be attached to the applicator
-
After completion of treatment, the applicator will be removed, anesthesia will be reversed, and the patient will be extubated or monitored anesthesia care (MAC) with local anesthesia together with sedation and analgesia is completed. Epidural or local anesthesia may be appropriate in some patients
-
After adequate observation (≥1 h) to ensure safety, the patient will be discharged home if appropriate
-
If discharge is not deemed appropriate on the day of the procedure, the patient may be admitted overnight for further observation and management
Image Guidance Utilization
Use of Image Guidance Pre-procedure
-
MRI of the pelvis with T1, T2, DCE and DWI series
-
CT pelvis
Types of Image Guidance to Potentially Use and Pros/Cons
-
Tumor is better seen on MRI
-
Gold fiducials are better seen on CT; gold appears black on MRI
Use of Image Guidance During Procedure
-
If MR simulator available in department, this can be utilized to evaluate placement of applicator and can be fused with CT images for contouring
-
An O-arm can be used in the OR to obtain a CT scan to confirm placement of the applicator prior to delivery of treatment
Evaluation and Distribution of Implantation
-
A dose volume histogram (DVH) is generated and reviewed (Fig. 11.5)
-
Isodose lines are reviewed to confirm GTV coverage, identify potential hot spots, cold spots, and doses to the normal tissues [24] (Fig. 11.8)
Fig. 11.8. Distribution of implant showing sparing of the contralateral rectal wall in a patient. (King, M, Cohen G, Wu, A et al. Prospective Evaluation of Endoluminal High Dose Rate Brachytherapy with Concurrent Chemotherapy for Rectal or Anal Cancer Patients: Initial Clinical Results. Brachytherapy May–June, 2016; 15,Supplement 1:S142)
Follow-up and Assessment of Response
-
Clinical Assessment of Response: More frequent follow-up is recommended to survey for potential recurrence
-
Follow-up by Radiation Oncology and Surgery departments every 3 months for the first 6 months, then every 6 months until 2 years after completion of brachytherapy
-
Evaluated by proctoscopy at each of these time points to assess response
-
During follow-up, any nodularity, mass, ulcer, or radiographic abnormality should prompt consideration of a biopsy
-
-
Radiographic assessment of Tumor Response after Brachytherapy
-
Prior to treatment and at 3, 6, and 12 months (±3 weeks) after the completion of brachytherapy, Pelvic MRI with DCE and DWI series
-
At baseline and at 6, 12, and 24 months (±3 weeks) after brachytherapy, CT of the chest/abdomen/pelvis to evaluate for distant disease progression
-
-
Re-staging of rectal cancer patients may be difficult after RT, due to inflammation and fibrosis. Many researchers have evaluated how to accurately identify a complete tumor response to chemoradiation therapy (CRT)
-
Digital rectal examination (DRE) is insufficient
-
In a prospective trial of 94 patients with T3, T4, or N1 rectal cancer who were evaluated by the same surgeon at diagnosis and preoperatively, only 21 % (3 of 14) who had a pCR were correctly identified by preoperative DRE. Furthermore, the extent of pathological downstaging was underestimated in almost 80 % of patients [25]
-
-
Endoscopy may be used to evaluate the rectum; however, biopsies obtained after CRT must be interpreted with caution
-
One group found the negative predictive value of a benign biopsy after CRT to be only 21 % [26]. Furthermore, regional nodal disease may persist despite a complete response of the primary tumor. In one study, 12 % of patients with a pCR in the rectal wall had nodal metastases [27]. Therefore, additional evaluation is necessary
-
Radiographic response parallels tumor regression and predicts patient outcomes [28]
-
-
ERUS and MRI may be difficult to interpret after CRT. Accuracy for T-stage has been reported as 48–72 % by ERUS and 47–52 % by standard MRI, and for N-stage as 77–80 % and 64–68 %, respectively [29–32]
-
Use of PET to determine response to CRT remains investigational [33, 34]. Several studies correlate the extent of metabolic tumor response on positron emission tomography (PET) with pathological response; however, the results have not been consistent
-
DCE-MRI : Several groups have shown that the addition of dynamic contrast enhancement (DCE) to standard MRI series increases the sensitivity and specificity for detection of tumor response. DCE-MRI quantitates the movement of injected contrast between the intracellular, extracellular/interstitial, and vascular compartments. The vascularity and cell density of tissue determine the pharmacokinetics of contrast enhancement [35, 36]
-
DWI-MRI : DWI-MRI reflects the microscopic motion of water molecules in tissues and thus reveals information about the tissue architecture. DWI-MRI provides a quantity, the apparent diffusion coefficient (ADC), as an indicator of water motion restriction or tissue cellularity. As a tumor becomes necrotic and cell membranes more leaky, the ADC increases. Multiple groups have shown that changes in ADC provide a more accurate indication of tumor response than conventional MRI alone [37–40]
-
Toxicity
-
Anticipated Toxicities of Anorectal Brachytherapy
-
Likely
-
Proctitis, resulting in rectal bleeding, mucous discharge, tenesmus, and/or discomfort
-
Urinary urgency, dysuria
-
Perianal skin erythema for low-lying rectal or anal tumors
-
Fatigue
-
-
Less Likely
-
Rectal ulceration
-
Abdominal pain, cramping
-
Diarrhea
-
Decreased production of red blood cells, possibly requiring transfusion
-
Decreased production of white blood cells, possibly predisposing to infection
-
Decreased number of platelets, possibly resulting in bleeding
-
-
Rare, but Serious
-
Severe rectal bleeding
-
Rectal fistulization
-
Large bowel obstruction
-
Urinary obstruction
-
Reaction to general anesthesia
-
Death
-
-
-
Late Effects of Radiation Therapy May Include
-
Proctitis
-
Altered sphincter functioning
-
Infertility
-
Early menopause in premenopausal women who have not undergone an ovarian transposition
-
Vaginal dryness and narrowing
-
-
Acute toxicity is defined as occurring from 0 to 90 days after brachytherapy, and late toxicity is defined as occurring from 91 days to 2 years after brachytherapy
-
Management of Brachytherapy Toxicity
-
Antidiarrheals: For symptoms of diarrhea and/or abdominal cramping, patients can take loperamide. Additional antidiarrheal measures can be used at the discretion of the treating physician. Patients should be instructed to increase fluid intake to help maintain fluid and electrolyte balance during episodes of diarrhea
-
References
American Cancer Society. Cancer facts & figures 2016. Atlanta: American Cancer Society; 2016.
Sauer R, Becker H, Hohenberger W, et al. Preoperative versus postoperative chemoradiotherapy for rectal cancer. N Engl J Med. 2004;351(17):1731–40.
Bosset JF, Collette L, Calais G, et al. Chemotherapy with preoperative radiotherapy in rectal cancer. N Engl J Med. 2006;355(11):1114–23.
Valenti V, Hernandez-Lizoain JL, Baixauli J, et al. Analysis of early postoperative morbidity among patients with rectal cancer treated with and without neoadjuvant chemoradiotherapy. Ann Surg Oncol. 2007;14(5):1744–51.
Pucciarelli S, Toppan P, Friso ML, et al. Preoperative combined radiotherapy and chemotherapy for rectal cancer does not affect early postoperative morbidity and mortality in low anterior resection. Dis Colon Rectum. 1999;42(10):1276–83, discussion 1283–4.
Peeters KC, van de Velde CJ, Leer JW, et al. Late side effects of short-course preoperative radiotherapy combined with total mesorectal excision for rectal cancer: increased bowel dysfunction in irradiated patients—a Dutch colorectal cancer group study. J Clin Oncol. 2005;23(25):6199–206.
Hendren SK, O’Connor BI, Liu M, et al. Prevalence of male and female sexual dysfunction is high following surgery for rectal cancer. Ann Surg. 2005;242(2):212–23.
Lange MM, den Dulk M, Bossema ER, et al. Risk factors for faecal incontinence after rectal cancer treatment. Br J Surg. 2007;94(10):1278–84.
Binkley GE. Radiation in the treatment of rectal cancer. Ann Surg. 1929;90(6):1000–14.
Binkley GE. Gold radon seeds in rectal cancer. Ann Surg. 1935;102(1):72–7.
Sischy B, Hinson EJ, Wilkinson DR. Definitive radiation therapy for selected cancers of the rectum. Br J Surg. 1988;75(9):901–3.
Gerard JP, Ayzac L, Coquard R, et al. Endocavitary irradiation for early rectal carcinomas T1 (T2). A series of 101 patients treated with the Papillon’s technique. Int J Radiat Oncol Biol Phys. 1996;34(4):775–83.
Papillon J. Present status of radiation therapy in the conservative management of rectal cancer. Radiother Oncol. 1990;17(4):275–83.
Maingon P, Guerif S, Darsouni R, et al. Conservative management of rectal adenocarcinoma by radiotherapy. Int J Radiat Oncol Biol Phys. 1998;40(5):1077–85.
Gerard JP, Chapet O, Ramaioli A, et al. Long-term control of T2-T3 rectal adenocarcinoma with radiotherapy alone. Int J Radiat Oncol Biol Phys. 2002;54(1):142–9.
Aumock A, Birnbaum EH, Fleshman JW, et al. Treatment of rectal adenocarcinoma with endocavitary and external beam radiotherapy: results for 199 patients with localized tumors. Int J Radiat Oncol Biol Phys. 2001;51(2):363–70.
Hoskin PJ, de Canha SM, Bownes P, et al. High dose rate afterloading intraluminal brachytherapy for advanced inoperable rectal carcinoma. Radiother Oncol. 2004;73(2):195–8.
Vuong T, Devic S, Podgorsak E. High dose rate endorectal brachytherapy as a neoadjuvant treatment for patients with resectable rectal cancer. Clin Oncol. 2007;19(9):701–5.
Vuong T, Devic S. High-dose-rate pre-operative endorectal brachytherapy for patients with rectal cancer. J Contemp Brachytherapy. 2015;7(2):183–8.
Oehler-Janne C, Seifert B, Lutolf UM, et al. Clinical outcome after treatment with a brachytherapy boost versus external beam boost for anal carcinoma. Brachytherapy. 2007;6(3):218–26.
Kapp KS, Geyer E, Gebhart FH, et al. Experience with split-course external beam irradiation +/- chemotherapy and integrated Ir-192 high-dose-rate brachytherapy in the treatment of primary carcinomas of the anal canal. Int J Radiat Oncol Biol Phys. 2001;49(4):997–1005.
Cohen GN, et al. Evaluation of a new MRI compatible brachytherapy ano-rectal applicator. Brachytherapy. 2014;13:S48.
Cohen G, Goodman K. TU-AB-201-07: image guided endorectal HDR brachytherapy using a compliant balloon applicator. Med Phys. 2015;42:3595.
King, M, Cohen G, Wu, A, et al. Prospective evaluation of endoluminal high dose rate brachytherapy with concurrent chemotherapy for rectal or anal cancer patients: Initial clinical results. Submitted to American Brachytherapy Society Annual Meeting; 2016.
Guillem JG, Chessin DB, Shia J, et al. Clinical examination following preoperative chemoradiation for rectal cancer is not a reliable surrogate end point. J Clin Oncol. 2005;23(15):3475–9.
Perez RO, Habr-Gama A, Pereira GV, et al. Role of biopsies in patients with residual rectal cancer following neoadjuvant chemoradiation after downsizing: can they rule out persisting cancer? Colorectal Dis. 2012;14(6):714–20.
Zmora O, Dasilva GM, Gurland B, et al. Does rectal wall tumor eradication with preoperative chemoradiation permit a change in the operative strategy? Dis Colon Rectum. 2004;47(10):1607–12.
Patel UB, Taylor F, Blomqvist L, et al. Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience. J Clin Oncol. 2011;29(28):3753–60.
Maor Y, Nadler M, Barshack I, et al. Endoscopic ultrasound staging of rectal cancer: diagnostic value before and following chemoradiation. J Gastroenterol Hepatol. 2006;21(2):454–8.
Vanagunas A, Lin DE, Stryker SJ. Accuracy of endoscopic ultrasound for restaging rectal cancer following neoadjuvant chemoradiation therapy. Am J Gastroenterol. 2004;99(1):109–12.
Kuo LJ, Chern MC, Tsou MH, et al. Interpretation of magnetic resonance imaging for locally advanced rectal carcinoma after preoperative chemoradiation therapy. Dis Colon Rectum. 2005;48(1):23–8.
Chen CC, Lee RC, Lin JK, et al. How accurate is magnetic resonance imaging in restaging rectal cancer in patients receiving preoperative combined chemoradiotherapy? Dis Colon Rectum. 2005;48(4):722–8.
Capirci C, Rubello D, Chierichetti F, et al. Long-term prognostic value of 18F-FDG PET in patients with locally advanced rectal cancer previously treated with neoadjuvant radiochemotherapy. AJR Am J Roentgenol. 2006;187(2):W202–8.
Cascini GL, Avallone A, Delrio P, et al. 18F-FDG PET is an early predictor of pathologic tumor response to preoperative radiochemotherapy in locally advanced rectal cancer. J Nucl Med. 2006;47(8):1241–8.
Dinter DJ, Horisberger K, Zechmann C, et al. Can dynamic MR imaging predict response in patients with rectal cancer undergoing cetuximab-based neoadjuvant chemoradiation? Onkologie. 2009;32(3):86–93.
Gollub MJ, Gultekin DH, Akin O, et al. Dynamic contrast enhanced-MRI for the detection of pathological complete response to neoadjuvant chemotherapy for locally advanced rectal cancer. Eur Radiol. 2012;22(4):821–31.
Kim SH, Lee JM, Hong SH, et al. Locally advanced rectal cancer: added value of diffusion-weighted MR imaging in the evaluation of tumor response to neoadjuvant chemo- and radiation therapy. Radiology. 2009;253(1):116–25.
Curvo-Semedo L, Lambregts DM, Maas M, et al. Rectal cancer: assessment of complete response to preoperative combined radiation therapy with chemotherapy—conventional MR volumetry versus diffusion-weighted MR imaging. Radiology. 2011;260(3):734–43.
Jung SH, Heo SH, Kim JW, et al. Predicting response to neoadjuvant chemoradiation therapy in locally advanced rectal cancer: diffusion-weighted 3 Tesla MR imaging. J Magn Reson Imaging. 2012;35(1):110–6.
Patterson DM, Padhani AR, Collins DJ. Technology insight: water diffusion MRI—a potential new biomarker of response to cancer therapy. Nat Clin Pract Oncol. 2008;5(4):220–33.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Jain, S.K., Goodman, K.A. (2017). Gastrointestinal Brachytherapy: Anal and Rectal Cancer. In: Mayadev, J., Benedict, S., Kamrava, M. (eds) Handbook of Image-Guided Brachytherapy. Springer, Cham. https://doi.org/10.1007/978-3-319-44827-5_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-44827-5_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-44825-1
Online ISBN: 978-3-319-44827-5
eBook Packages: MedicineMedicine (R0)