Abstract
Drug interactions are a commonplace occurrence, and with the majority of pharmaceuticals, such interactions are well characterized. These interactions may be beneficial, with augmentation of the effects of one of the agents used. However, interactions with adverse outcomes are also common. Harmful interactions can result in therapeutic failure of a drug or in a toxic outcome. Just as two drugs can interact, so can complementary products and drugs; such interactions are referred to as “herb-drug interactions” and, like all drug interactions, can be potentially harmful. Herb-drug interactions present their unique additional complications due to the complex and variable nature of the products and their somewhat random use.
Investigations into herb-drug interactions are challenging due to the very nature of the product; many components that may be active or inactive when isolated may behave very differently when in a mixture. Additionally, there is great product variability and currently, regulations requiring standardization or demonstration of product safety, are carried out mainly in developed parts of the world. Also, the methodology used to study herb-drug interactions can have a significant impact on the results obtained, and an understanding of these methods and the extrapolation of the data to human impact is required.
In this chapter we discuss herb-drug interactions of potential or reported clinical significance.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
Drug interactions can significantly alter the effect of a drug. This may manifest as increased or decreased effectiveness of the drug, or an atypical effect. Drug-drug and food-drug interactions are often well documented, and many resources, including Web-based tools, exist to help practitioners and consumers to be aware of, and to avoid, common interactions. For the well-documented and extensively studied interactions, the mechanisms are often identified or suspected, and it is possible to predict the probable outcomes with reasonable certainty.
This is not necessarily the situation with herb-drug interactions, which are becoming an increasingly important area of interest, as their market share and use continue to grow. In the USA, their reported use has been estimated to be 18 % of the adult population (Barnes et al. 2008). Similar figures have been reported in Australia, with 25 % reportedly using complementary products. In the UK, the use of herbal products has been reported to be as high as 22 % (Thomas et al. 2001).
The World Health Organization (WHO) reports that the use of traditional medicine accounts for 80–95 % of primary health care in Africa and Asia, although this figure includes other forms of traditional medicine, such as acupuncture and homeopathy (World Health Organization 2008). The global market for these products was estimated at US $83 billion annually in 2008 (Robinson and Zhang 2011). Demand for herbal products worldwide has increased at an annual rate of 8 % between 1994 and 2001, and according to WHO forecasts, the global herbal market will be worth $5 trillion by the year 2050.
Irrespective of the outcome of the interaction (not all interactions are harmful), the unpredictable and often unknown nature of these interactions is cause for concern. Ideally these products should undergo the standard safety testing that is required during pre-clinical investigations into new therapeutic agents and continues throughout the market life of a product. Currently, pre-clinical testing and regulatory investigations that are carried out on pharmaceutical agents are not required (although in some countries testing is required if a therapeutic claim is made), and post-market surveillance is limited and inconsistent. Complicating the issue further, each country and the various regulators have their own requirements.
Nevertheless, in many parts of the world, herbal products continue to be generally unregulated and untested for quality, efficacy, and more importantly, safety. In that way, most of the products on the global market are untested and used by the general public without medical supervision. Post-marketing surveillance and adverse drug-interaction reporting are hampered by this global market that involves diverse and conflicting proprietary trade names, Internet sales, and unlicensed practitioners.
Under European medicines legislation (Directive 2004/24/EC), medicinal products containing herbal substances/preparations must fall within one of the following three categories in order to reach the market:
-
A product can be classified under traditional medicinal use provisions (“traditional use”), accepted on the basis of sufficient safety data and plausible efficacy: the product is granted a traditional use registration.
-
A product can be classified under well-established medicinal use provisions (“well-established use”). This is demonstrated with sufficient safety and efficacy data. As a result, the product is granted a marketing authorization.
-
A product can be authorized after the evaluation of a marketing authorization application consisting of only product-specific safety and efficacy data (“full dossier”). As a result, the product is granted marketing authorization.
Since September 2015, the European Medical Agency (EMA) has been responsible for monitoring a number of substances and selected medical literature to identify suspected adverse reactions with medicines authorized in the European Union, and for entering the relevant information into the EudraVigilance database. The list of substances being monitored includes many herbal products, including valerian, gingko and angelica.
In the United States, the National Center for Complementary and Alternative Medicine (NCCAM), a center within the National Institutes of Health (NIH), advises the U.S. Food and Drug Administration (FDA) on issues relating to complementary products. The regulation of these products is under the control of the FDA, and many of them fall under the regulation of the Dietary Supplement Health and Education Act (DSHEA) of 1994. This act regulates products that are intended to supplement the diet and includes vitamins, minerals, herbs, or other botanicals, amino acids, and substances such as enzymes, organ tissues, glandular, and metabolites. Under this act, the manufacturer is responsible for ensuring that the ingredient is safe before it is marketed, although products do not need to be registered with the FDA or gain approval prior to being marketed. Manufacturers must make sure that product label information is truthful and not misleading, and that products are manufactured under Good Manufacturing Practices (GMP). The FDA is responsible for taking action against any unsafe dietary supplement product after it reaches the market.
The frequency of side effects due to herbal products is not known, as the current systems in place are inadequate; the U.S. Department of Health and Human Services has estimated that only 1 % of events are detected (General 2001). It is proposed that consumers are less likely to consider an adverse event linked to a herbal product that is generally perceived as “natural and safe” (Eisenberg et al. 1998).
Investigations on Herb-Drug Interactions
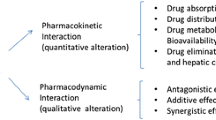
To carry out investigations into complementary products involves additional challenges to what is already a complex area of study. Like all drug interactions, herb-drug interactions may result from a pharmacokinetic or a pharmacodynamic interaction. The complexity is exacerbated when you consider the variable nature of these herbal and complementary products. The qualitative and quantitative composition of important components can vary greatly. This variability was recognized well over a century ago with the cardiac glycosides containing foxglove, Digitalis purpurea L. (Breckenridge 2006). The growth conditions, seasonal and geographic variability, the processing, and the storage of the product can alter the composition and concentration of the constituents (Braun and Cohen 2010). The eventual extraction and final compounding is also variable as the products are used in creams, teas, tablets, and capsules, all of which may lead to the patient's exposure to various compounds and varying concentrations from the same starting material. An additional difficulty with complementary products is that multiple constituents often exhibit biological activity.
The manufacturers’ claim about the main ingredient may also be misleading, as it may not be the constituent responsible for an interaction. Furthermore, the claimed concentrations are often inaccurate. In a review of 25 ginseng products marketed in the USA, all were found to be correctly labeled with regards to the genus of the plant used (Panax or Eleutherococcus), but with regards to the claimed concentration of the marker compounds, a 15- to 36-fold variability of ginsenosides and 43- to 200-fold variability of eleutherosides was reported (Harkey et al. 2001).
In a study of 880 individual preparations marketed in the USA, only 43 % were consistent with labeling: another 20 % were consistent with respect to ingredients, but not dosage. A total of 37 % were either not consistent with respect to ingredients, or the label information was so vague that determination of ingredients was impossible (Garrard et al. 2003). This highlights the misleading nature of the product labeling provided by the manufacturers, which results in confusion among customers. While the products may have multiple active components, an additional complication in safety evaluation or testing is that a pharmacologically inactive component may be responsible for any herb-drug interaction.
A simple search for the term “herb-drug interaction” in the NCBI database yields over 1,700 hits (database accessed January 2016). Many of these investigations focus on isolated components tested using in vitro systems, and while these studies are crucial for understanding the underlying mechanism, they may not always translate into clinically significant interactions and are generally not supported by evidence-based clinical trials. Many of the claimed herb-drug interactions reported are based on theoretical suspicion or anecdotal case reports. On the other hand, in some cases, despite clear clinical evidence of a herb-drug interaction, the underlying mechanism of action is poorly understood.
It is important to remember that, as with drug-drug interactions, the risk of an interaction occurring and being clinically relevant must always be considered. This is particularly so when the interaction involves a drug with a narrow therapeutic window, such as warfarin, or a drug for which therapeutic failure would be life-threatening. In these situations, the potential for an interaction must be weighed against any potential benefit of using a herbal product.
Methods for Investigating Herb-Drug Interactions
A rational approach to interaction studies can be based on understanding the mechanisms of interaction or a relative risk assessment. The mechanistic studies can be broadly categorized into pharmacodynamic and pharmacokinetic interactions. The risk assessment approach, on the other hand, relates to the likelihood of two compounds being consumed concurrently, and clinical significance of relevant interaction.
The concurrent use of complementary products with prescription medications is an issue for prescribing practitioners as consumers conceal the use of complementary products both unintentionally and intentionally. The unintentional failure to reveal their use is a consequence of not considering these products relevant. They may intentionally conceal their use for fear of being judged by medical practitioners. Medical professionals must assure their patients that they are asking them specifically about their use of herbal products, and encourage them to discuss these products as part of their treatment.
Criteria for Prioritization (Ranking Based on Risk)
With the large number of products available to the general public and the variability in their composition and formulation, undertaking interaction studies in each circumstance may not be practical. Therefore, a system of prioritizing products for study should be considered. In order to best identify the significant interactions, several factors can be considered. In each of the following circumstances, an interaction is likely to produce a significant risk to the patient.
-
Likelihood of concurrent use with therapeutic agents based on therapeutic claims:
-
Many complementary products make broad therapeutic claims or claims of certain health benefits. If these claims indicate that a product is likely to be concurrently used with a therapeutic agent − for example a product to treat nausea or boost the immune system − something that patients receiving chemotherapy may likely want, then there is a high risk of concurrent use and the potential for any interaction should be investigated.
-
-
Likelihood of a complementary product being used concurrently with drugs with a narrow therapeutic index:
-
In cases where a therapeutic agent known to have a narrow therapeutic index, such as warfarin, is administered, then products likely to be concurrently used should be tested for interaction potential.
-
-
Circumstances for which therapeutic failure would be significant or life-threatening:
-
In cases where the patient requires therapeutic treatment for a life- threatening disease, such as cancer or HIV, then products that are likely to be taken concurrently should be investigated for their interaction potential.
-
A product that definitely meets these criteria is St. John’s wort (Hypericum perforatum L.), which is one of the most-studied herbal products and is responsible for both pharmacokinetic and pharmacodynamic interactions (Henderson et al. 2002; Zhou et al. 2003, 2004a, b; Gurley et al. 2005a). Many of the interactions are clinically significant, causing therapeutic failure with life-threatening outcomes; several key interactions are summarized in Table 5.1. The therapeutic claims of St. John’s wort − including treatment for depression and anxiety − indicates that it could be concurrently used with antidepressant medications.
Methodology for Assessment
Considerations When Studying Complex Mixtures
Many supporters of these products suggest that they cannot be studied with the same protocols as therapeutic agents, as this does not allow for the complexity of the product to be considered. It is claimed that data obtained by isolating and standardizing the components are not reflective of the true holistic and complex dosages required for herbal products. The claim is that if these products are studied as isolated components, then it is not reflective of what the consumer is exposed to (Benzie and Wachtel-Galor 2011). Thus, one of the biggest challenges in studying complementary products for their interaction potential is in the preparation of the extract(s) to be tested.
Alcohol- and water-based extractions will isolate different components that are present in the product, which may produce very different results when testing for activity and interactions. Also, low-polarity compounds would be absent from such extracts, whereas they could be solubilized in preparations for traditional use and so be bioavailable to the consumers.
In vitro studies into Dong Quai (Angelica sinensis (Oliv.) Diels) with both aqueous and methanolic extracts, showed the variability in results that are seen with various extraction methods. Using these extracts to study the inhibition of cytochrome P450 enzymes, aqueous extracts resulted in an IC50 <100 μg/ml for the nine CYP enzymes tested for inhibition (Sevior et al. 2010; Sevior 2012). The methanolic extracts caused significant inhibition of three isoenzymes: CYP2A6, CYP2B6 and CYP2C19 with IC50’s of 94.7, 11.4 and 14.0 μg/ml, respectively. Inhibition by Dong Quai may be significant, since well-known CYP2C19 inhibitors, including isoniazid (K i = 25.4 μM), are likely to cause adverse clinical interactions when administered concurrently with CYP2C19 substrates such as diazepam or phenytoin (Kay et al. 1985; Ochs et al. 1981).
Advanced separation and isolation using techniques, such as supercritical fluid extraction, microwave and ultrasonication-assisted extraction, are now used and have been shown to influence the composition of the extracts (Zhang et al. 2015; Anubala et al. 2014). The reality is that these techniques are also used in the preparation of products offered to the consumers, thus changing their composition.
The inactivation of active components when in mixture, compared to isolated compound studies, can be suspected whenever the in vitro studies are not supported by clinical findings. In vitro investigation of valerian (Valeriana officinalis L.) reported that (1) commercial extracts caused inhibition of CYP3A4 (Lefebvre and Foster 2004; Hellum and Nilsen 2008), and (2) methanolic extracts inhibited additional Cytochrome P450 enzymes, including CYP1A2 and CYP2C19 (Sevior et al. 2010); but in vivo investigations did not correlate with these findings (Gurley et al. 2005b).
Reporting of Adverse Drug Reactions
Case reports are one way to detect potential interactions, but post-marketing surveillance and patient reporting is not required under current regulations for products marketed as nutrients or food supplements. A comprehensive database to collate suspected herb-drug interactions may provide researchers with guidance helping to focus studies, but until both practitioners and consumers understand the risk of these interactions, case studies will continue to be limited in their use. They nevertheless constitute important warning signals to detect harmful combinations (Shaw et al. 2012; Zhang et al. 2012).
At present, suspected interactions can be found using various online tools (Table 5.2). Although these resources are limited and provide varying levels in the quality of the information, they may act as a guide to practitioners and researchers.
In Vitro
In vitro studies are often relatively inexpensive and rapid and can be upscaled as a way of screening many compounds in a relatively short time. Such studies are often used during the pre-clinical phase of drug development to assess the potential for a compound to bind to therapeutic targets and pharmacokinetic profiles. Pharmacokinetic profiling of test compounds includes elucidation of metabolites and interference by other drugs. This same information can be obtained for herbal products by in vitro studies; similarly these studies can identify potential herb-drug interactions and assist in the identification of the component(s) responsible.
In vitro studies may be used as the basis for extrapolation to in vivo studies, but this can be complex and relies on assumptions regarding the behavior of the compound in vivo, and is more difficult when working with complex herbal products. Without the information regarding the metabolic clearance, metabolite formation, and stability, in vitro-in vivo extrapolation is practically impossible (Wilk-Zasadna et al. 2015).
In Silico
With the development of in silico technology, it may eventually be possible to identify the components present in complementary products that are likely to be involved in interactions. If the composition of a product is known, the compounds can be subjected to quantitative structure-activity relationship (QSAR) studies in order to predict behavior with therapeutic targets and metabolizing enzymes, such as cytochrome P450.
The ‘omics-based technologies, which include NMR and LC-MS-based metabolomics, may provide significant advances in the studies of herbal products. The promise of these technologies not only involves the identification of herb-drug interactions with the identification of biomarkers, but also the identification and tracking of the active constituents and establishing chemical variability (Yang et al. 2012; Sheridan et al. 2012). These advanced analytical and highly sensitive techniques can track biomarkers that could reveal both adverse effects and interactions in vivo.
Evidence for using gene based ‘omics technologies with interesting outcomes has involved products such as kava kava (Piper methysticum) (Guo et al. 2009, 2010b). In these studies, kava kava was administered to rats; subsequent genome-wide gene expression revealed differences in the expression of drug metabolizing enzymes in the exposed group, and alterations in the mitochondrial function and oxidative stress response pathways.
Microarrays have also been used to identify gene expression changes. Ginkgo biloba L. has been shown to alter a number of genes in a dose-dependent manner (Guo et al. 2010a). Of the 31,802 genes investigated, 2,011 were altered. This included an increase in the expression of alcohol dehydrogenase and cytochrome P450 family 1 and a decrease in the expression of aldehyde dehydrogenase and cytochrome P450 family 2.
These “omics” and computational technologies are rapidly expanding and may act as a complement to the standard in vitro and in vivo tests. These studies do, however, require strict quality control and reliable and repeatable methods to analyze the large amounts of data that are generated.
Pharmacokinetic Studies
Isolating individual components of herbal preparations is one approach that can be used to assist in the determination of the pharmacokinetic profiles of these products, although this is time-consuming, and in the end may not provide the answers required as these products are not taken as isolated components, but as complex mixtures.
Practitioners of herbal medicine do not consider the multitude of components of complementary products in isolation. Instead, the multiple components present are often considered integral to the action, with “a concerted pharmacological intervention of multiple compounds interacting with multiple targets and possessing mutually interdependent activities that are required for an optimal effect” (Chan 1995). For this reason, proponents of herbal products often discredit studies involving isolated components; however, these studies still provide valuable information and insights into the specific components possibly responsible for an interaction.
Absorption/Distribution
Interactions of herbal products with the absorption and distribution of clinical drugs have the potential to alter the concentration of the drug in the systemic circulation.
Interaction with Transporters
The extensive family of transporters is responsible for the flux of a wide range of compounds in and out of cells. Consequently, they are important in the absorption, redistribution, and elimination of drugs. Any change in the relative abundance or activity of transporters can have consequences. These transporters can be induced or inhibited by a wide variety of compounds. The pharmacokinetic behavior of drugs that are substrates for transporters can be influenced by concurrently administered compounds including conventional medicines, foods, and complementary products that function as inhibitors or inducers of transporter function.
To date, several complementary and alternative products have been identified as substrates, inhibitors, and/or inducers of P-glycoprotein (P-gp), which is a major efflux transporter in many organs, such as the intestine, liver, kidney, and brain; P-gp notably opposes the intestinal absorption of xenobiotics. One of the most clinically significant examples is St. John’s wort (Hypericum perforatum L.), which is commonly used to treat mild depression (Nathan 1999).
In vitro and in vivo studies have indicated that St. John’s wort induces intestinal P-gp (Hennessy et al. 2002; Perloff et al. 2001). These findings have been consistent in healthy volunteers who consumed the product for 14 days, with a subsequent 1.4--to 1.5-fold increase in their expression of P-gp (Dürr et al. 2000). This induction of P-gp can be expected to enhance the clearance of therapeutic agents from the circulation of patients. In the case of patients undergoing treatment with the chemotherapeutic drugs etoposide and doxorubicin, there is a risk of a decrease in the therapeutic effect and therapeutic failure (Hennessy and Spiers 2007).
Milk thistle is suspected to inhibit P-gp, and studies have shown that silymarin, a mixture of flavolignanes, major components of milk thistle, inhibits the transport of P-gp substrates such as calcein into the cell in in vitro experiments, although in vivo studies with healthy human volunteers determined no statistically significant effect on digoxin pharmacokinetics with milk thistle (900 mg a day) (Gurley et al. 2006). Studies such as this with milk thistle highlight the problems that can occur when isolated components are investigated for drug-interactions and the need for additional clinical data.
Interaction with Protein Binding
Binding to plasma proteins can influence the distribution, metabolism, and excretion of many endogenous and exogenous compounds. In vivo, drug molecules are either bound to plasma and tissue proteins and plasma lipids, or are free. It is generally accepted that the free molecules represent the fraction of a drug that is able to interact with the therapeutic target to produce an effect.
The fraction of a drug or ligand that is bound to protein can change significantly due to co-administered drugs/ligands. Thus the ability to bind to protein and to displace a previously bound compound from protein is an important consideration in drug development and in the prediction of drug interactions. Several human plasma proteins, such as albumin and alpha-1-acid glycoprotein, play a key role in binding drugs.
One significant example of herbal constituents binding to a plasma protein and causing a potential herb-drug interaction is seen with albumin and Danshen (a traditional Chinese medicine prepared from the root of Salvia miltiorrhiza Bunge). Studies claim that Danshen components “are 50-70 % bound to albumin, and can displace salicylate from its binding site” (Gupta et al. 2002); this study appears to be alarming due to the potential for concurrent use of Danshen (recommended for the treatment of “stagnation of blood flow”) with a drug such as warfarin, which has a narrow therapeutic index. While the methods used in this study are valid, the interpretation by these authors is highly misleading and highlights the need for caution when assessing the literature for potential interactions; the authors should specify that “at the Danshen level used, 50-70 % of salicylate binding sites are occupied by as yet unidentified components of Danshen.” Limitations of this study include using non-physiological concentrations of albumin and very high concentrations of Danshen, measuring the binding and subsequent displacement of salicylate instead of digoxin, and assuming the Danshen would displace the digoxin from the albumin, rather than a direct measurement of binding.
Drug interactions due to protein-binding and displacement highlight the importance of considering the in vitro and in vivo results in the clinical setting. Let us assume that two compounds (A and B) are administered concurrently (or when an herb and a drug are co-administered). If compound A is displaced by compound B, an increase in the free concentration of compound A results, but the increase in free compound A may not correlate with an increase in compound A at the receptor site because A will also be available for redistribution to the rest of the body. Any increase in free A following redistribution will also be available for elimination. Even for low clearance drugs, where intrinsic clearance of free compounds is the only determinant of mean steady-state free drug concentration, free concentration for A will return to the pre-B level. Thus any increase in the pharmacological effect of A will generally be transient and cannot be sustained; this means that the interaction may become significant if the transient increase in the free compound is for a drug with a very narrow therapeutic index.
Predicting clinically significant interactions from in vitro data is further complicated by the possibility for drug metabolites to bind to plasma proteins and potentially displace previously bound compounds. As the metabolic stability of a molecule and the rate of the biotransformation can affect its toxic potential, disposition in the body and eventual excretion (Wilk-Zasadna et al. 2015; Coecke et al. 2013), in vitro studies should consider the possible metabolites that may also be biologically active in vivo.
Metabolism
Drug interactions involving metabolism are very important as they are often the cause of adverse outcomes.
The cytochrome P450 (CYP) system is comprised of a family of heme-containing (hemoprotein) enzymes with closely related isoforms classified as “mixed function oxidases.” This enzymatic system is found in almost all organisms, including mammals, bacteria, and plants,being crucial for the oxidative, per-oxidative, and reductive metabolism of exogenous and endogenous compounds. More than >11,000 CYPs have been identified, and new CYPS are continuously being added (Nelson 2009).
To date, there have been 107 genes identified as encoding for CYPs in humans, with 18 families and 45 subfamilies. Of the genes identified, 57 have been isolated, identified, and classified, and most appear to be expressed primarily in the endoplasmic reticulum. Of the CYPs with known catalytic activities, 14 are involved in steroidogenesis, 4 in the metabolism of vitamins, 5 in eicosanoid metabolism, 4 have fatty acids as their substrates, and 15 catalyze transformation of xenobiotic chemicals (Guengerich 2005).
The investigation of interactions involving the drug-metabolizing enzymes, and in particular the CYP group, is a key area of study as it has been shown that ≈75 % of all drugs can be metabolized by three CYPs (CYP3A4, CYP2D6, and CYP2C9) (Rendic 2002), and a set of six to seven CYPs (CYP1A1, CYP1A2, CYP1B1, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 CYP2E1 and CYP3A4) account for 90–95 % of all drug metabolism (Evans and Relling 1999). CYPs are involved in the metabolism of 78 % of the 200 top-selling prescription medications in the U.S. (Zanger et al. 2008).
Complementary products are usually complex mixtures containing many potentially pharmacologically active components, notably essential oils, tannins, flavonoids, anthraquinones, alkaloids or polyphenols (Liu et al. 2006; Zhou et al.. 2004a, b, 2005; Izzo and Ernst 2009). Several complementary products, such as St. John’s wort (Hypericum perforatum L.), garlic (Allium sativum L.), liquorice (Glycyrrhiza glabra L.), and ginseng (Panax ginseng C.A.Mey.) have been proven to interact with CYPs. They act either as inhibitors or inducers of more or less specific CYPs, with St. John’s wort known to induce CYP3A4 (Henderson et al. 2002).
Furanocoumarins found in several herbal medicines, including Chinese angelica (Angelica sinensis) and also in grapefruit (Citrus × paradisi Macfad.), are known to be potent inhibitors of CYP1A2 and CYP3A4, and therefore the cause of drug interactions (Tassaneeyakul et al. 2000). Products suspected of containing furanocoumarins − and therefore likely to cause a drug interaction − can be screened using standard HPLC analysis, allowing the rapid identification of potential drug interactions without the need for more complex experiments.
Furanocoumarins isolated from grapefruit juice are mechanism-based inhibitors of CYP3A4 (Tassaneeyakul et al. 2000), and resveratrol from Vitis vinifera L., have been shown to be mechanism-based inhibitors of CYP1A2 and CYP3A4 (Chan and Delucchi 2000). The analysis of 30 herbal plants from Indonesia showed their ability to inhibit CYP2D6 and CYP3A4 (Subehan et al. 2006). With an increase in the incubation time, five plants showed more than a 30 % increase in mechanism-based inhibition of CYP3A4, and three showed a 30 % increase in mechanism-based inhibition of CYP2D6.
CYP2E1 inhibitors from natural sources include compounds originating from garlic (diallyl sulfide, diallyl sulfoxide, and diallyl sulfone) that were shown to competitively inhibit CYP2E1 (Gurley et al. 2005a; Brady et al. 1991). This was confirmed by two clinical trials, as revealed by the decreased 6-hydroxychlorzoxazone/ chlorzoxazone serum ratios (Gurley et al. 2005a).
In vivo studies in humans initially found that black cohosh (Actaea racemosa L.) may weakly inhibit CYP2D6 (Gurley et al. 2005b), but this observation was negated by later studies conducted by the same group (Gurley et al. 2008). In both these studies, the method for determining inhibition is the same but the product investigated was different. The 2005 study used a black cohosh that was standardized to 0.2 % triterpene glycosides per tablet, while the 2008 study used a product that was standardized to 2.5 % triterpene glycosides per tablet. This finding suggests that while the triterpene glycosides are often reported as the principal component of these preparations, they may not be the components that cause CYP inhibition; as such other components are not yet identified and may be present or absent; depending on the extraction conditions, this complicates safety assessments.
The induction of CYPs can have a major impact on drug metabolism, pharmacokinetic behavior, drug-drug interactions, on the toxicity of foreign chemicals, and on the activity and disposition of endogenous hormones (Conney 1982). It is now known that a wide range of chemicals are capable of causing induction; these include therapeutic agents, pesticides, food additives, industrial chemicals, dietary constituents, natural products, and environmental pollutants (Pelkonen et al. 2008).
Pharmacodynamic Interactions
Pharmacodynamic interactions with herbal products are not as well studied or identified. Early reports suggested that garlic and ginkgo interacted with anticoagulants and caused increased bleeding in consumers. For gingko, adverse events were particularly severe for aspirin (spontaneous hyphema), warfarin (intracerebral hemorrhage) and ibuprofen (comatose state with an intracerebral mass bleeding from which the patient died). However, recent trials did not confirm these effects (Izzo and Ernst 2009).
Ginger (Zingiber officinale) was also suspected to interact with warfarin but, as with ginkgo and garlic, in vitro studies indicated that it could inhibit platelet aggregation, and in vivo studies with healthy human volunteers showed that co-administration of warfarin with ginseng did not alter the pharmacokinetics or the pharmacodynamics of warfarin (Jiang et al. 2005).
Warfarin is a complex target of interacting substances. Apart from pharmacokinetic interactions, warfarin therapy is subject to any dietary product containing vitamin K; these include many vegetables and green tea.
Clinically significant interactions have shown that when green tea is consumed along with warfarin, the antagonism may result in dramatic reductions of a patient's INR value (Taylor and Wilt 1999).
Clinical Considerations
Predicting adverse herb-drug interactions in humans is complicated by the influence of genes, disease state, and age on the expression of many proteins and enzymes that are involved in interactions. As the liver contains the greatest concentration of CYPs, diseases affecting the liver, including cirrhosis, alcoholic liver disease, hepatitis, and hepatocarcinoma, all alter the level and activities of the CYPs and therefore the drug metabolism. CYP activity may also be decreased due to altered hepatic blood flow and hypoalbuminemia.
While the changes in the drug metabolizing enzymes that occur with age and in disease states are well documented, there are also changes in the serum protein levels. Alpha-1-acid glycoprotein (AGP) is a protein that is known to bind mainly to basic drugs in the serum. A critically important characteristic of AGP is the change in plasma levels that occurs with conditions such as inflammation (e.g., arthritis) and chronic disease (e.g,, cancer). In these patients, expression is increased two- to fivefold (Fournier et al. 2000). An increase in AGP also occurs following myocardial infarction (Johansson et al. 1972) and surgery (Voulgari et al. 1982), while lower levels are seen during pregnancy (Perucca and Crema 1982), in thyroid disease (Feely et al. 1981), and in patients with liver cirrhosis (Serbouce-Hougel et al. 1981). Such wild fluctuations must be considered when the potential for drug interactions are investigated with regards to AGP.
Conclusion
The inescapable reality is that most patients resorting to the use of herbal products are likely to be treated with prescription or over-the-counter drugs (MacLennan et al. 2002; Eisenberg et al. 1998). Additionally, many patients see multiple therapists along with medical practitioners.
Safety studies and interaction studies of herbal products are lacking and are urgently required to ensure consumer safety. Several steps should be mandatory regarding the standardization and quality of these products.
-
Worldwide databases need to be established with full product monographs to ensure correct product identification;
-
the composition of products should be determined and compounds known to cause drug interactions flagged.
To monitor the safety of these products, the cooperation between traditional practitioners, pharmacists, medical professionals, and consumers needs to improve to ensure that accurate reports of adverse events are identified. Due to the complex nature of these products and the specific challenges associated with them, pharmacovigilance needs to be expanded to incorporate all medications and herbal products, whatever their marketing status, and consumers need to be educated to consider complementary products in this light. Mandatory labelling requirements can be set so that consumers are warned of potential interactions.
Reported suspected interactions with complementary products should include additional information, such as the origin of the product, the presence of any contaminant and adulterants, and the name attributed to the product (traditional, botanical, common). If this information were available to regulators worldwide, then consistent risk assessments could be carried out and the risk to patients would be decreased.
Studying herbal products as pure compounds is relatively uncomplicated; however, complementary products present a number of difficult problems that must be solved in order to conduct any meaningful studies with them. These problems include:
-
Product selection
-
Extraction method
-
Metabolic profiling and constituent concentration
Finally, the study of drug interactions is complicated by the variation in response by individual subjects; causes of this variability include ethnicity, age, genetic factors, disease state (in particular, kidney and liver failure), and pregnancy.
References
Anubala S, Sekar R, Nagaiah K (2014) Development and validation of an analytical method for the separation and determination of major bioactive curcuminoids in curcuma longa rhizomes and herbal products using non-aqueous capillary electrophoresis. Talanta 123:10–17
Barnes P, Bloom B, Nahin R (2008) Complementary and alternative medicine use among adults and children: United States, 2007. National Health Statistics Report. U.S. Department of Health and Human Services
Benzie IFF, Wachtel-Galor S (2011) Herbal medicine: bimolecular and clinical aspects. CRC Press/Taylor & Francis, Florida
Brady JF, Xiao F, Wang MH, Li Y, Ning SM, Gapac JM, Yang CS (1991) Effects of disulfiram on hepatic P450IIE1, other microsomal enzymes, and hepatotoxicity in rats. Toxicol Appl Pharmacol 108:366–373
Braun L, Cohen M (2010) Herbs & natural supplements − an evidence-based guide. Elsevier Australia (Sydney)
Breckenridge A (2006) William Withering’s legacy − for the good of the patient. Clin Med 6:393–397
Chan K (1995) Progress in traditional Chinese medicine. Trends Pharmacol 16:182–187
Chan WK, Delucchi AB (2000) Resveratrol, a red wine constituent, is a mechanism-based inactivator of cytochrome P450 3A4. Life Sci 67:3103–3112
Coecke S, Pelkonen O, Leite S, Bernauer U, Bessems JGM, Bois FY, Gundert-Remy U, Loizou G, Testai E, Zaldivar JM (2013) Toxicokientics as a key to the integrated toxicity risk assessment based primarily on non-animal approaches. Toxicol In Vitro 27:1570–1577
Conney AH (1982) Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G.H.A. Clowes memorial lecture. Cancer Res 42:4875–4917
Dürr D, Stieger B, Kullak-Ublick GA, Rentsch KM, Steinert HC, Meier PJ, Fattinger K (2000) St John’s wort induces intestinal P-glycoprtoein/MDR1 and intestinal and hepatic CYP3A4. Clin Pharmacol Ther 68:598–604
Eisenberg DM, Davis RB, Ettner SL, Appel S, Wilkey S, Van Rompay MI, Kessler RC (1998) Trends in alternative medicine use in the United States, 1990–1997. J Am Med Assoc 280:1569–1575
Evans WE, Relling MV (1999) Pharmacogenomics: translating functional genomics into rational therapeutics. Science 286:487–491
Feely J, Stevenson I, Crooks J (1981) Alteres plasma protein binding of drugs in thyroid disease. Clin Pharmacokinet 6:298–305
Fournier T, Medjoubi-N N, Porquet D (2000) Alpha-1- acid glycoprotein. Biochim Biophys Acta 1482:157–171
Garrard J, Harms S, Eberly LE, Matiak A (2003) Variations in product choices of frequently purchased herbs. Arch Intern Med 163:2290–2295
Office of Inspector General (2001) Adverse event reporting for dietary supplements. An inadequate safety valve. Department of Health and Human Services - USA. Boston Regional Office, OEI-01-00-00180. Available at: http://www.dhhs.gov/oig/oei
Guengerich FP (2005) Human cytochrome P450 enzymes. In: Ortiz De Montellano PR (ed) Cytochrome 450. Structure, mechanism and biochemistry, 3rd edn. Kluwer Academic/Plenum Publishers, New York
Guo L, Li QZ, Xia Q, Dial S, Chan PC, Fu P (2009) Analysis of gene expression changes of drug metabolizing enzymes in the livers of F344 rats following oral treatment with kava extract. Food Chem Toxicol 47:433–442
Guo L, Mei N, Lioa W, Chan PC, Fu PP (2010a) Ginkgo biloba extract induces gene expression changes in xenobiotics metabolism and the Myc-centered network. OMICS 14:75–90
Guo L, Shi Q, Dial S, Xia Q, Mei N, Li QZ, Chan PC, Fu P (2010b) Gene expression profiling in male B6C3F1 mouse livers exposed to kava identifies changes in drug metabolizing genes and potential mechanisms linked to kava toxicity. Food Chem Toxicol 48:686–696
Gupta D, Jalali M, Wells A, Dasgupta A (2002) Drug-herb interactions: unexpected suppression of free danshen concentrations by salicylate. J Clin Lab Anal 16:293–294
Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Cui Y, Ang CY (2005a) Clinical assessment of effects of botanical supplementation on cytochrome P450 phenotypes in the elderly: St. John’s wort, garlic oil, Panax ginseng and Ginkgo biloba. Drugs Aging 22:525–539
Gurley BJ, Gardner SF, Hubbard MA, Williams DK, Gentry WB, Khan IA, Shah A (2005b) In vivo effects of goldenseal, kava kava, black cohosh and valerian on human cytochrome P450 1A2, 2D6, 2E1 and 3A4 phenotypes. Clin Pharmacol Ther 77:415–426
Gurley BJ, Barone GW, Williams DK, Carrier J, Breen P, Yates CR, Song PF, Hubbard MA, Tong Y, Cheboyina S (2006) Effect of milk thistle (Silybum marianum) and black cohosh (Cimicifugaq racemosa) supplementation on digoxin pharmacokinetics in humans. Drug Metab Dispos 34:69–74
Gurley BJ, Swain A, Hubbard MA, Williams DK, Barone GW, Hartsfield F, Tong Y, Carrier DJ, Cheboyina S, Battu SK (2008) Clinical assessment of CYP2D6-mediated herb-drug interactions in humans: effects of milk thistle, black cohosh, goldenseal, kava kava, St. John’s wort and echinacea. Mol Nutr Food Res 52:1–9
Harkey MR, Henderson GL, Gershwin ME, Stern JS, Hackman RM (2001) Variability in commercial ginseng products: an analysis of 25. Clin Nutr 73:1101–1106
Hellum BH, Nilsen OG (2008) In vitro inhibition of CYP3A4 metabolism and P-glycoprotein mediated transport by trade herbal products. Basic Clin Pharmacol Toxicol 102:466–475
Henderson L, Yue QY, Bergquist C, Gerden B, Arlett P (2002) St. John’s wort (hypericum perforatum): drug interactions and clinical outcomes. Br J Clin Pharmacol 54:349–356
Hennessy M, Spiers JP (2007) A primer on the mechanics of P-glycportein the multidrug transporter. Pharmacol Res 55:1–15
Hennessy M, Kelleher D, Spiers JP, Barry M, Kavanagh P, Back D, Mulcahy F, Feely J (2002) St. Johns wort increases expression of P-glycoprotein: implications for drug interactions. Br J Pharmacol 53:75–82
Izzo AA, Ernst E (2009) Interactions between herbal medicines and prescribed drugs. An updated systematic review. Drugs 69:1777–1798
Jiang X, Williams KM, Liauw WS, Ammit AJ, Roufogalis BO, Duke CC, Day RO, Mclachlan AJ (2005) Effect of ginkgo and ginger on the pharmacokientics and pharmacodynamics of warfarin in healthy subjects. Br J Clin Pharmacol 59:425–432
Johansson B, Kindmark C, Trell E, Wollheim F (1972) Sequential changes of plasma proteins after myocardial infarction. Scand J Clin Lab Investig 124:117–126
Kay L, Kampmann JP, Svendsen TL, Vergman B, Hansen JEM, Skovsted L, Kristensen M (1985) Influence of rifampicin and isoniazid on the kinetics of phenytoin. Br J Clin Pharmacol 20:323–326
Lefebvre T, Foster BC (2004) In vitro activity of commercial valerian root extracts against human cytochrome P450 3A4. J Pharmacol Pharm Sci 7:265–273
Liu KH, Kim MJ, Jeon BH, Shon JH, Cha IJ, Cho KH, Lee SS, Shin JG (2006) Inhibition of human cytochrome P450 isoforms and NADPH-CYP reductase in vitro by 15 herbal medicines, including Epimedii herba. J Clin Pharm Ther 31:83–91
Maclennan AH, Wilson DH, Taylor AW (2002) The escalating cost and prevalence of alternative medicine. Preventative Med 35:166–173
Nathan P (1999) The experimental and clinical pharmacology of St. John’s Wort (hypericum perforatum L.). Mol Psychiatry 4:333–338
Nelson DR (2009) The cytochrome P450 homepage. Hum Genomics 4(1):59–65
Ochs HR, Greenblatt DJ, Roberts GM, Dengler HJ (1981) Diazepam interaction with antituberculosis drugs. Clin Pharmacol Ther 29:671–678
Pelkonen OP, Turpeinen M, Hakkola J, Honkakoski P, Hukkanen J, Raunio H (2008) Inhibition and induction of human cytochrome P450 enzymes: current status. Arch Toxicol 82:667–715
Perloff MD, Von Moltke LL, Störmer E, Shader RI, Greenblatt DJ (2001) St. John’s wort: an in vitro analysis of P-glycoprotein induction due to extended exposure. Br J Pharmacol 134:1601–1608
Perucca E, Crema A (1982) Plasma protein binding of drugs during pregnancy. Clin Pharmacokinet 7:336–352
Rendic S (2002) Summary of information on human CYP enzymes: human P450 metabolism data. Drug Metab Rev 34:83–448
Robinson MM, Zhang X (2011) Traditional medicines: global situation, issues and challenges. In: WHO/EMP/MIE (ed) The world medicines situation 2011. World Health Organization, Geneva
Serbouce-Hougel S, Durand G, Corbii M, Agneray J, Feger J (1981) Alterations in relative proportions of microheterogenous forms of human alpha 1-acid glycoprotein in liver disease. J Hepatol 2:245–252
Sevior DK (2012) Complementary products and drug interactions – screening for the potential to cause pharmacokinetic interactions. Doctor of Philosophy, RMIT University
Sevior D, Hokkanen J, Tolonen A, Abass K, Tursas L, Pelkonen O, Ahokas JT (2010) Rapid screening of commercially available herbal products for the inhibition of major human hepatic cytochrome P450 enzymes using the N-in-One cocktail. Xenobiotica 40:245–254
Shaw D, Graeme L, Pierre D, Elizabeth W, Kelvin C (2012) Pharmacovigilance of herbal medicine. J Ethnopharmacol 140:513–518
Sheridan H, Krenn L, Jiang R, Sutherland I, Ignatova S, Marmann A, Liang X, Sendker J (2012) The potential of metabolic fingerprinting as a tool for the modernization of TCM preparations. J Ethnopharmacol 140:482–491
Subehan A, Usia T, Iwata H, Kadota S, Tezuka Y (2006) Mechanism-based inhibition of CYP3A4 and CYP2D6 by Indonesian medicinal plants. J Ethnopharmacol 105:449–455
Tassaneeyakul W, Guo LQ, Fukuda K, Ohta T, Yamazoe Y (2000) Inhibition selectivity of grapefruit juice components on human cytochromes P450. Arch Biochem Biophys 378:356–363
Taylor JR, Wilt VM (1999) Probable antagonism of warfarin by green tea. Ann Pharmacother 33:426–428
Thomas KJ, Nicholl JP, Coleman P (2001) Use and expenditure on complementary medicine in England: a population based survey. Complement Ther Med 9:2–11
Voulgari F, Cummins P, Gardecki T, Beeching N, Stone P, Stuart J (1982) Serum levels of acute phase and cardiac proteins after myocardial infarction, surgery, and infection. Br Heart J 1982:4
Wilk-Zasadna I, Bernasconi C, Pelkonen O, Coecke S (2015) Biotransformation in vitro: an essential consideration in the quantitative in vitro-to-iv-vivo extrapolation (QIVIVE) of toxicity data. Toxicology 332:8–19
World Health Organization (2008) Traditional medicine. WHO. http://www.who.int/mediacentre/factsheets/2003/fs134/en/
Yang SO, Shin YS, Hyun SH, Cho S, Bang KH, Lee D, Choi SP, Choi HK (2012) NMR-based metabolic profiling and differentiation of ginseng roots according to cultivation ages. J Pharm Biomed Anal 58:19–26
Zanger UM, Turpeinen M, Klein K, Schwab M (2008) Functional pharmacogenetics/genomics of human cytochromes P450 involved in drug biotransformation. Anal Bioanal Chem 392:1093–1108
Zhang L, Yan J, Liu X, Ye Z, Yang X, Meyboom R, Chan K, Shaw D, Duez P (2012) Pharmacovigilance practice and risk control of traditional Chinese medicine drugs in China: current status and future perspective. J Ethnopharmacol 140:519–525
Zhang Y, Liu C, Qi Y, Li Y, Wang L, Ren J, Tang Y (2015) Efficient combination of circulating ultrasound-assisted extraction partition chromatography for extraction and on-line separation of chemical constituents from Stellera chamaejasme L. Phytochem Anal 26:301–309
Zhou S, Gao Y, Jiang W, Huang M, Xu A, Paxton JW (2003) Interactions of herbs with cytochrome P450. Drug Metab Rev 35:35–98
Zhou S, Lim L, Chowbray B (2004a) Herbal modulation of P-glycoprotein. Drug Metab Rev 36:57–104
Zhou SF, Koh HL, Gao Y, Gong ZY, Lee EJD (2004b) Herbal bioactivation: the good, the bad and the ugly. Life Sci 74:935–968
Zhou S, Huang M, Xu A, Yang H, Duan W, Paxton JW (2005) Prediction of herb-drug metabolic interactions: a simulation study. Phytother Res 19:464–471
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Sevior, D., Ahokas, J. (2017). Interactions Between Conventional and Herbal Medicinal Products. In: Pelkonen, O., Duez, P., Vuorela, P., Vuorela, H. (eds) Toxicology of Herbal Products. Springer, Cham. https://doi.org/10.1007/978-3-319-43806-1_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-43806-1_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43804-7
Online ISBN: 978-3-319-43806-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)