Abstract
Sepsis-related circulatory dysfunction is usually manifested as an early hypovolemic state that can be reversed with initial fluid resuscitation. If not reversed early, this can progress into a persistent circulatory dysfunction. In contrast to a quite predictable course during the initial phase, persistent circulatory dysfunction is a complex and heterogeneous syndrome. All individual perfusion parameters have extensive limitations to adequately reflect tissue perfusion status in this phase. However, a multimodal approach integrating observation, macrohemodynamics, metabolic parameters, and peripheral and eventually microcirculatory perfusion parameters can overcome their individual limitations. This proposal may provide a holistic understanding of the predominant underlying mechanisms of hypoperfusion and lead to individualized and physiologically oriented therapeutic strategies.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Shock was recently defined, by a taskforce of the European Society of Intensive Care, as a life-threatening, generalized form of acute circulatory failure associated with inadequate oxygen utilization by the cells [1]. In this state, the circulation is unable to deliver sufficient oxygen to meet the demands of the tissues, resulting in cellular dysfunction. The result is cellular dysoxia, i.e., the loss of the physiological independence between oxygen delivery and oxygen consumption, associated with increased lactate levels [1]. Septic shock would thus represent this syndrome in the presence of an acute infection.
In older definitions, much more significance was given to the frequently present clinical symptoms in order to facilitate recognition. In the 1992 consensus definition by an American College of Chest Physicians and Society of Critical Care Medicine consensus conference, both included both volume-refractory hypotension and perfusion abnormalities as obligatory components of a septic shock definition [2]. Over the last decade, an even simpler definition has been used, relying mainly on vasopressor requirements [3]. In this definition, perfusion abnormalities were not required for the diagnosis of septic shock. More recently, the Sepsis-3 conference defined septic shock as the combination of hypotension and hyperlactatemia in a patient with infection [4] while disregarding other markers of circulatory dysfunction such as peripheral perfusion abnormalities that were incorporated in the definition of shock by the European Society Task Force [1]. In the Sepsis-3 definition, increased lactate levels in the absence of hypotension do not classify as septic shock.
The purpose of this chapter is to provide a holistic integrative view of perfusion monitoring and treatment based on the pathophysiological definition that includes macrohemodynamic and microcirculatory symptoms and their relation to tissue dysoxia in septic shock [1].
2 Holistic View
In the diagnosis of the condition of a critically ill patient, physical exam still has an important place [5] even though some argue that correction of vital signs prevails detailed physical examination [6] and others even think it could be abandoned [7]. A simple assessment of pelvic instability in trauma patients [8], subjective assessment of the peripheral temperature of an ICU patient’s skin [9], or even simple assessment of the extent of skin discoloration in septic shock patients [10] reveal important prognostic information. In addition, simple physical exam can even accurately distinguish different categories of shock [11]. On an even more holistic view, an uneasy feeling about the condition of a patient may already contribute the ultimate morbidity and mortality in trauma patients [12].
In the old days, clinical observation was even more important and treatment limited. In traditional Chinese medicine, stasis/stagnation, deficiency, and collapse are important characteristics of the important concepts of energy (Qi), blood, and Yin and Yang. Although the assessment of these concepts doesn’t easily translate to modern intensive care medicine, the principles are frequently observed in critically ill patients.
A Qi deficiency may be characterized by lethargy, weakness, and sweating, where a Qi stagnation would be characterized by emotional distress and pain.
Blood deficiency may relate to anemia in traditional Chinese medicine although it may also refer to local blood deficiency as in abnormally perfused areas. Even more interesting is the translation of the Yin and Yang concept. This could be translated into the balance between the branches of the autonomic nervous system. In this context, the Yin would be the parasympathetic restorative branch where the sympathetic system would be the emergency response branch. In the immediate response to critical illness, the sympathetic nervous system plays an important role, and also in the treatment, we frequently use drugs to stimulate this system in order to improve hemodynamics or block this system with beta-blockers. Even using these old concepts, the presence of lethargy, sweating, and abnormal peripheral perfusion (so a Qi and blood-deficient patient) has been shown to characterize a patient population with high chances of mortality [13].
In Chinese medicine, the concept of balance is extremely important. Optimizing health would imply the restoration of all deficiencies/stagnations. This is an interesting concept when we come to the topic of monitoring. If optimal restorative capabilities should be used to make the patient survive his critical illness, then monitoring cannot be limited to only a few macro-circulatory variables. Additionally, treatment should be targeted on all systems that we can possibly monitor. In the following, we will thus unfold a holistic monitoring plan based on our current knowledge of the (patho)physiology of critical illness.
3 Physiology-Based Perfusion Monitoring
A fundamental challenge in septic shock resuscitation, independent of the diagnostic criteria employed, is to evaluate tissue perfusion. During the past decades, several parameters such as gastric tonometry [14]; lactate [15, 16], mixed (SvO2) [17], or central venous oxygen saturations (ScvO2) [16, 18]; peripheral perfusion [9, 19]; oxygen tissue saturation (StO2) [20, 21]; and central venous-arterial pCO2 gradient (P(cv-a)CO2) [22] or mixed venous to arterial pCO2 gradient [23] have been used to monitor perfusion status or as potential resuscitation goals in septic shock. More recently, the pathophysiological relevance of septic-related microvascular dysfunction has been highlighted [24,25,26], and trials testing microcirculatory-oriented therapeutic strategies start to appear in the literature [27]. However, given that sepsis is a pan system disease affecting all aspects of the circulation (myocardium, pulmonary vasculature, systemic vasculature, and microcirculation), none of these markers have earned universal acceptance as the unique parameter to be considered as the hallmark to guide septic shock resuscitation. Moreover, they have been tested in rather mutually exclusive protocols [16]. As a result, the lack of an integrative comprehensive approach is evident, with notable exceptions [15]. This trend contrasts with our holistic approach. It also contrasts with suggestions to use all available techniques to monitor brain perfusion/function in neurocritical care patients and to not rely on only one or two [28]. However, as with many organ-specific protocols, they lack significant detailing on the other systems [29].
The case of central venous oxygen saturation (ScvO2), a complex physiological parameter, is paradigmatic. It was widely used as the resuscitation goal in critically ill patients since the landmark study of Rivers et al. [18] until some recent major trials couldn’t confirm these findings [30]. However, using a fixed end point of ScvO2 without including the complicated interpretation of its changes [31,32,33] or many other parameters that affect ScvO2 precludes a straightforward abandoning of its clinical use. The presence of low ScvO2 clearly indicates an imbalance in the DO2/oxygen consumption (VO2) relationship. This finding should prompt an aggressive DO2/VO2 optimization strategy as was demonstrated by Rivers et al. [18]. This could already be in part realized by just decreasing oxygen demand [31]. In contrast, the presence of normal ScvO2 values, as frequently observed in ICU patients, should not be interpreted as evidence of normal global tissue perfusion as ScvO2 is in strict terms a superior vena cava territory regional monitor. Thus, its correction does not assure the correction of global tissue hypoxia [31,32,33]. In addition, severe microcirculatory derangements could theoretically impair tissue oxygen extraction capabilities resulting in normal or even supranormal ScvO2 values despite the presence of tissue hypoxia [33].
The preceding example demonstrates that the idea of a single perfusion-related parameter representing the adequacy of the whole cardiovascular system in its essential role to provide oxygenation to tissues according to local demands appears as oversimplistic and anti-physiological under a critical view [33].
In effect, there are several conceptual problems with the single representative parameter paradigm:
-
1.
The relative or comparative hierarchy is relatively unknown at least in terms of prognosis. Persistent hyperlactatemia appears as the strongest prognostic factor when analyzing literature [34], although its involved pathogenic mechanisms are complex and time dependent [35, 36] that eventually may represent an unbalanced state rather than a simple manifestation of hypoxia and thus questionable as a target of treatment [37,38,39]. In contrast to patients with abnormal lactate levels, patients able to maintain normal lactate levels under severe circulatory stress are probably optimal physiological responders and exhibit an extremely low mortality [40]. Thus, besides its prognostic significance, development of hyperlactatemia is a powerful systemic biological signal. However, some guidelines recommend the indistinct use of lactate or ScvO2 as resuscitation goals [41], a too simplistic approach that neglects other important aspects of the circulation.
-
2.
If the hallmark of shock is tissue hypoperfusion or hypoxia, then abnormalities in the proposed parameters should be related to the presence of hypoperfusion. However, this is not the case for several parameters. Hyperlactatemia or a prolonged capillary refill time may be simply related to adrenergic-induced aerobic lactate production or vasoconstriction [33]. Oliguria is frequently multifactorial. Thus, some relevant parameters may be influenced by non-hypoxic conditions and therefore are nonspecific and occasionally unreliable as unique perfusion markers.
-
3.
Currently recommended septic shock treatment strategies are based on the assumption that perfusion-related variables will improve after increasing oxygen delivery (mainly by increasing cardiac output), a concept that can be defined as flow responsiveness [35, 42]. However, parameters traditionally considered as representing tissue perfusion can also be mechanistically determined by non-flow-dependent or mixed mechanisms. Thus, to propose DO2 increasing maneuvers to normalize any single abnormal parameter without considering specific involved pathogenic mechanisms appears as nonrational and may eventually lead to severe adverse events such as fluid overload and arrhythmias [43, 44], stressing the fact that overstimulation of one system might have significant side effects for the whole. Furthermore, to focus resuscitation efforts on a wrong target can lead to dangerous unbalanced therapies: e.g., using fluid unresponsiveness as a target might induce fluid overload without any benefit if hypoperfusion has already been corrected [45].
-
4.
The dynamics of recovery for individual parameters has not been well addressed in experimental or clinical studies. A predominant hypoxic versus a non-hypoxic pathogenic mechanism may result in a wide variability in the recovery time courses of individual parameters after DO2 optimization [19, 35]. This fact should be taken into account when selecting a resuscitation strategy in order to determine the most appropriate target at different time points, to avoid over- or under-resuscitation.
-
5.
The relationship of macrohemodynamics with metabolic, peripheral, regional, or microcirculatory perfusion parameters is controversial and may change throughout the resuscitation process [19, 35, 42].
-
6.
The normalization of one parameter does not necessarily assure the normalization of others. Even more, in case of ScvO2, a normalization trend to supranormal values may occasionally reflect a worsening microvascular dysfunction rather than a systemic flow improvement [32].
-
7.
Normal/adequate values for some parameters are unknown, e.g., microcirculatory perfused vessel density or thenar muscle tissue saturation, among others.
When analyzing potentially useful perfusion-related parameters under the above described considerations, it is clear that all individual parameters have extensive limitations to adequately reflect tissue perfusion during persistent sepsis-related circulatory dysfunction. Therefore, the only rational approach to perfusion monitoring is a multimodal one, integrating macrohemodynamic, metabolic, peripheral, regional, and microcirculatory perfusion parameters to overcome those limitations. This approach may also provide a thorough understanding on the predominant driving forces of hypoperfusion and lead to physiologically oriented interventions. As an example, it is far more easy to understand the underlying mechanism of an increasing lactate level, if a low-flow state is first ruled out by simultaneous assessment of systemic hemodynamics, Scvo2, P(cv-a)CO2, and peripheral perfusion [33, 46].
4 Initial Circulatory Dysfunction
Sepsis-related circulatory dysfunction is usually manifested as an early hypovolemic state that can be completely reversed with initial fluid resuscitation or eventually progresses into a persistent circulatory dysfunction. In contrast to a quite predictable course during the initial phase where all perfusion parameters tend to improve in parallel, persistent circulatory dysfunction can be expressed in complex and heterogeneous patterns. Although many mechanisms are involved in the pathogenesis of sepsis-related circulatory dysfunction, hypovolemia is clearly the predominant factor in pre-resuscitated patients early following hospital admission [1, 33]. Depending on the severity and time course of hypovolemia, patients may exhibit an impaired peripheral perfusion, hyperlactatemia, low ScvO2, and altered microcirculatory flow, whether or not they are hypotensive.
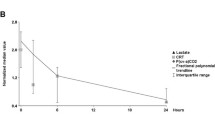
A couple of studies have explored the relationship between hemodynamic and perfusion parameters in this pre-resuscitative phase. Trzeciak et al. [47] found an early significant correlation between macrohemodynamic parameters, lactate, and microcirculatory flow alterations. Payen et al. [48] confirmed these findings in 43 septic shock patients undergoing initial resuscitation. The cornerstone of initial resuscitation is fluid loading. A series of dynamic studies evaluated the effects of a fluid challenge in this setting. Pottecher et al. [49] observed an improvement in sublingual microcirculatory perfusion after fluid administration in septic shock patients. Interestingly, improvement in microcirculatory flow correlated significantly with changes in global hemodynamics. However, in the presence of an already normal microcirculation, increasing cardiac output or blood pressure by fluids doesn’t offer any advantages [45]. In another septic shock study, early fluid loading improved mean arterial pressure (MAP), cardiac index, SvO2 or ScvO2 values, lactate levels, pulse pressure variation, and microcirculatory flow in parallel [50]. Another study evaluated changes in metabolic and peripheral perfusion parameters at different time points during initial resuscitation. In 41 patients with septic shock, Hernandez et al. [19] found that capillary refill time, lactate, and heart rate improved in parallel during 6 h of fluid-based resuscitation.
These data taken together suggest an intricate relationship between macrohemodynamics, perfusion parameters, and microcirculatory flow indices. All these elements are affected by hypovolemia and tend to improve in parallel in fluid-responsive patients. The clinical expression of these effects is variable according to several preexisting factors such as preload responsiveness, the magnitude of adrenergic-induced redistributive vasoconstriction, or local microvascular dysfunction. The fundamental challenge in this phase is rapid and complete reversal of the low-flow state secondary to hypovolemia. Simple, readily available and validated monitoring tools such as subjective peripheral perfusion and lactate can be used to guide this process. Normalization of these parameters indicates a successful reversal of initial circulatory dysfunction [51].
5 Persistent Circulatory Dysfunction
In contrast to the pre-resuscitative phase, more complex mechanisms may lead the pathogenesis of persistent circulatory dysfunction. Vascular dysfunction induces vasoplegia, capillary leak, and distributive abnormalities. Myocardial depression is frequently manifested by a decreased left ventricle ejection fraction [1]. The role of microcirculatory derangements has been highlighted in recent years, and these abnormalities may hasten the development of tissue hypoxia and/or multiple organ dysfunction [26]. It is likely that evolution into different expressions of persistent sepsis-related circulatory dysfunction is influenced by the relative preponderance of any of these mechanisms at the individual level. Several recent publications support the heterogeneity of hemodynamic and perfusion profiles in persistent sepsis-related circulatory dysfunction. Therefore, in contrast to the pre-resuscitative phase where all perfusion markers tend to improve in parallel, during persistent circulatory dysfunction individual perfusion markers may change in unpredictable or even opposite directions. Consequently, the assessment of perfusion status based solely on one marker can lead to incomplete, inaccurate, or misleading conclusions. This highlights the necessity of a multimodal holistic approach for this phase.
References
Cecconi M, De Backer D, Antonelli M, Beale R, Bakker J, Hofer C, Jaeschke R, Mebazaa A, Pinsky MR, Teboul JL, Vincent JL, Rhodes A. Consensus on circulatory shock and hemodynamic monitoring. Task force of the European Society of Intensive Care Medicine. Intensive Care Med. 2014;40:1795–815.
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55.
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G, International Sepsis Definitions Conference. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med. 2003;29:530–8.
Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, Ferrer R, Kumar A, Sevransky JE, Sprung CL, Nunnally ME, Rochwerg B, Rubenfeld GD, Angus DC, Annane D, Beale RJ, Bellinghan GJ, Bernard GR, Chiche JD, Coopersmith C, De Backer DP, French CJ, Fujishima S, Gerlach H, Hidalgo JL, Hollenberg SM, Jones AE, Karnad DR, Kleinpell RM, Koh Y, Lisboa TC, Machado FR, Marini JJ, Marshall JC, Mazuski JE, McIntyre LA, McLean AS, Mehta S, Moreno RP, Myburgh J, Navalesi P, Nishida O, Osborn TM, Perner A, Plunkett CM, Ranieri M, Schorr CA, Seckel MA, Seymour CW, Shieh L, Shukri KA, Simpson SQ, Singer M, Thompson BT, Townsend SR, Van der Poll T, Vincent JL, Wiersinga WJ, Zimmerman JL, Dellinger RP. Surviving Sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intensive Care Med. 2017;43(3):304–77.
Metkus TS, Kim BS. Bedside diagnosis in the intensive care unit. Is looking overlooked? Ann Am Thorac Soc. 2015;12:1447–50.
John BV, Thomas SM. Physical examination. Lancet. 2003;362:2023; author reply 2024.
Ioannidis JP. Physical examination. Lancet. 2003;362:2023; author reply 2024.
Pehle B, Nast-Kolb D, Oberbeck R, Waydhas C, Ruchholtz S. [Significance of physical examination and radiography of the pelvis during treatment in the shock emergency room]. Unfallchirurg. 2003;106:642–8.
Lima A, Jansen TC, Van Boommel J, Ince C, Bakker J. The prognostic value of the subjective assessment of peripheral perfusion in critically ill patients. Crit Care Med. 2009;37:934–8.
Ait-Oufella H, Lemoinne S, Boelle PY, Galbois A, Baudel JL, Lemant J, Joffre J, Margetis D, Guidet B, Maury E, Offenstadt G. Mottling score predicts survival in septic shock. Intensive Care Med. 2011;37:801–7.
Vazquez R, Gheorghe C, Kaufman D, Manthous CA. Accuracy of bedside physical examination in distinguishing categories of shock: a pilot study. J Hosp Med. 2010;5:471–4.
Smith T, Den Hartog D, Moerman T, Patka P, Van Lieshout EM, Schep NW. Accuracy of an expanded early warning score for patients in general and trauma surgery wards. Br J Surg. 2012;99:192–7.
Vincent JL, Ince C, Bakker J. Clinical review: circulatory shock—an update: a tribute to Professor Max Harry Weil. Crit Care. 2012;16:239.
Palizas F, Dubin A, Regueira T, Bruhn A, Knobel E, Lazzeri S, Baredes N, Hernandez G. Gastric tonometry versus cardiac index as resuscitation goals in septic shock: a multicenter, randomized, controlled trial. Crit Care. 2009;13:R44.
Jansen TC, van Bommel J, Schoonderbeek FJ, Sleeswijk Visser SJ, van der Klooster JM, Lima AP, Willemsen SP, Bakker J. Early lactate-guided therapy in intensive care unit patients: a multicenter, open-label, randomized controlled trial. Am J Respir Crit Care Med. 2010;182:752–61.
Jones AE, Shapiro NI, Trzeciak S, Arnold RC, Claremont HA, Kline JA. Lactate clearance vs central venous oxygen saturation as goals of early sepsis therapy: a randomized clinical trial. JAMA. 2010;303:739–46.
Gattinoni L, Brazzi L, Pelosi P, Latini R, Tognoni G, Pesenti A, Fumagalli R. A trial of goal-oriented hemodynamic therapy in critically ill patients. SvO2 Collaborative Group. N Engl J Med. 1995;333:1025–32.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, Peterson E, Tomlanovich M. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77.
Hernandez G, Pedreros C, Veas E, Bruhn A, Romero C, Rovegno M, Neira R, Bravo S, Castro R, Kattan E, Ince C. Evolution of peripheral vs metabolic perfusion parameters during septic shock resuscitation. A clinical-physiologic study. J Crit Care. 2012;27:283–8.
Creteur J, Carollo T, Soldati G, Buchele G, De Backer D, Vincent JL. The prognostic value of muscle StO2 in septic patients. Intensive Care Med. 2007;33:1549–56.
Lima A, van Bommel J, Jansen TC, Ince C, Bakker J. Low tissue oxygen saturation at the end of early goal-directed therapy is associated with worse outcome in critically ill patients. Crit Care. 2009;13 Suppl 5:S13.
Ospina-Tascon GA, Bautista-Rincon DF, Umana M, Tafur JD, Gutierrez A, Garcia AF, Bermudez W, Granados M, Arango-Davila C, Hernandez G. Persistently high venous-to-arterial carbon dioxide differences during early resuscitation are associated with poor outcomes in septic shock. Crit Care. 2013;17:R294.
Bakker J, Vincent JL, Gris P, Leon M, Coffernils M, Kahn RJ. Veno-arterial carbon dioxide gradient in human septic shock. Chest. 1992;101:509–15.
Sakr Y, Dubois MJ, De Backer D, Creteur J, Vincent JL. Persistent microcirculatory alterations are associated with organ failure and death in patients with septic shock. Crit Care Med. 2004;32:1825–31.
De Backer D, Donadello K, Sakr Y, Ospina-Tascon G, Salgado D, Scolletta S, Vincent JL. Microcirculatory alterations in patients with severe sepsis: impact of time of assessment and relationship with outcome. Crit Care Med. 2013;41:791–9.
Ince C. The microcirculation is the motor of sepsis. Crit Care. 2005;9(Suppl 4):S13–9.
Boerma EC, Koopmans M, Konijn A, Kaiferova K, Bakker AJ, van Roon EN, Buter H, Bruins N, Egbers PH, Gerritsen RT, Koetsier PM, Kingma WP, Kuiper MA, Ince C. Effects of nitroglycerin on sublingual microcirculatory blood flow in patients with severe sepsis/septic shock after a strict resuscitation protocol: a double-blind randomized placebo controlled trial. Crit Care Med. 2010;38:93–100.
Tisdall MM, Smith M. Multimodal monitoring in traumatic brain injury: current status and future directions. Br J Anaesth. 2007;99:61–7.
Werdan K, Russ M, Buerke M, Delle-Karth G, Geppert A, Schondube FA, German Cardiac Society, German Society of Intensive Care and Emergency Medicine, German Society for Thoracic and Cardiovascular Surgery, German Interdisciplinary Association of Intensive Care and Emergency Medicine, Austrian Society of Cardiology, German Society of Anaesthesiology and Intensive Care Medicine, German Society of Preventive Medicine and Rehabilitation. Cardiogenic shock due to myocardial infarction: diagnosis, monitoring and treatment: a German-Austrian S3 Guideline. Dtsch Arztebl Int. 2012;109:343–51.
Angus DC, Barnato AE, Bell D, Bellomo R, Chong CR, Coats TJ, Davies A, Delaney A, Harrison DA, Holdgate A, Howe B, Huang DT, Iwashyna T, Kellum JA, Peake SL, Pike F, Reade MC, Rowan KM, Singer M, Webb SA, Weissfeld LA, Yealy DM, Young JD. A systematic review and meta-analysis of early goal-directed therapy for septic shock: the ARISE, ProCESS and ProMISe Investigators. Intensive Care Med. 2015;41:1549–60.
Hernandez G, Pena H, Cornejo R, Rovegno M, Retamal J, Navarro JL, Aranguiz I, Castro R, Bruhn A. Impact of emergency intubation on central venous oxygen saturation in critically ill patients: a multicenter observational study. Crit Care. 2009;13:R63.
Textoris J, Fouche L, Wiramus S, Antonini F, Tho S, Martin C, Leone M. High central venous oxygen saturation in the latter stages of septic shock is associated with increased mortality. Crit Care. 2011;15:R176.
Hernandez G, Bruhn A, Castro R, Regueira T. The holistic view on perfusion monitoring in septic shock. Curr Opin Crit Care. 2012;18:280–6.
Vincent JL, Quintairos ESA, Couto L Jr, Taccone FS. The value of blood lactate kinetics in critically ill patients: a systematic review. Crit Care. 2016;20:257.
Hernandez G, Luengo C, Bruhn A, Kattan E, Friedman G, Ospina-Tascon GA, Fuentealba A, Castro R, Regueira T, Romero C, Ince C, Bakker J. When to stop septic shock resuscitation: clues from a dynamic perfusion monitoring. Ann Intensive Care. 2014;4:30.
Jansen TC, van Bommel J, Bakker J. Blood lactate monitoring in critically ill patients: a systematic health technology assessment. Crit Care Med. 2009;37:2827–39.
Monnet X, Delaney A, Barnato A. Lactate-guided resuscitation saves lives: no. Intensive Care Med. 2016;42:470–1.
Bloos F, Zhang Z, Boulain T. Lactate-guided resuscitation saves lives: yes. Intensive Care Med. 2016;42:466–9.
Bakker J, de Backer D, Hernandez G. Lactate-guided resuscitation saves lives: we are not sure. Intensive Care Med. 2016;42:472–4.
Hernandez G, Castro R, Romero C, de la Hoz C, Angulo D, Aranguiz I, Larrondo J, Bujes A, Bruhn A. Persistent sepsis-induced hypotension without hyperlactatemia: Is it really septic shock? J Crit Care. 2011;26:435 e439–14.
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM, Sevransky JE, Sprung CL, Douglas IS, Jaeschke R, Osborn TM, Nunnally ME, Townsend SR, Reinhart K, Kleinpell RM, Angus DC, Deutschman CS, Machado FR, Rubenfeld GD, Webb S, Beale RJ, Vincent JL, Moreno R, Surviving Sepsis Campaign Guidelines Committee including The Pediatric Subgroup. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock. Intensive Care Med. 2012;39:165–228.
Bakker J. Lactate levels and hemodynamic coherence in acute circulatory failure. Best Pract Res Clin Anaesthesiol. 2016;30:523–30.
Dunser MW, Ruokonen E, Pettila V, Ulmer H, Torgersen C, Schmittinger CA, Jakob S, Takala J. Association of arterial blood pressure and vasopressor load with septic shock mortality: a post hoc analysis of a multicenter trial. Crit Care. 2009;13:R181.
Sakr Y, Rubatto Birri PN, Kotfis K, Nanchal R, Shah B, Kluge S, Schroeder ME, Marshall JC, Vincent JL, Intensive Care Over Nations Investigators. Higher fluid balance increases the risk of death from sepsis: results from a large international audit. Crit Care Med. 2017;45:386–94.
Klijn E, van Velzen MHN, Lima AP, Bakker J, van Bommel J, Groeneveld ABJ. Tissue perfusion and oxygenation to monitor fluid responsiveness in critically ill, septic patients after initial resuscitation: a prospective observational study. J Clin Monit Comput. 2015;29:707–12.
Ospina-Tascon GA, Umana M, Bermudez W, Bautista-Rincon DF, Hernandez G, Bruhn A, Granados M, Salazar B, Arango-Davila C, De Backer D. Combination of arterial lactate levels and venous-arterial CO2 to arterial-venous O2 content difference ratio as markers of resuscitation in patients with septic shock. Intensive Care Med. 2015;41:796–805.
Trzeciak S, Dellinger RP, Parrillo JE, Guglielmi M, Bajaj J, Abate NL, Arnold RC, Colilla S, Zanotti S, Hollenberg SM, Microcirculatory Alterations in Resuscitation and Shock Investigators. Early microcirculatory perfusion derangements in patients with severe sepsis and septic shock: relationship to hemodynamics, oxygen transport, and survival. Ann Emerg Med. 2007;49:88–98, 98 e81–82
Payen D, Luengo C, Heyer L, Resche-Rigon M, Kerever S, Damoisel C, Losser MR. Is thenar tissue hemoglobin oxygen saturation in septic shock related to macrohemodynamic variables and outcome? Crit Care. 2009;13 Suppl 5:S6.
Pottecher J, Deruddre S, Teboul JL, Georger JF, Laplace C, Benhamou D, Vicaut E, Duranteau J. Both passive leg raising and intravascular volume expansion improve sublingual microcirculatory perfusion in severe sepsis and septic shock patients. Intensive Care Med. 2010;36:1867–74.
Ospina-Tascon G, Neves AP, Occhipinti G, Donadello K, Buchele G, Simion D, Chierego ML, Silva TO, Fonseca A, Vincent JL, De Backer D. Effects of fluids on microvascular perfusion in patients with severe sepsis. Intensive Care Med. 2010;36:949–55.
Dunser MW, Takala J, Brunauer A, Bakker J. Re-thinking resuscitation: leaving blood pressure cosmetics behind and moving forward to permissive hypotension and a tissue perfusion-based approach. Crit Care. 2013;17:326.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this chapter
Cite this chapter
Hernández, G., Rosenthal, L., Bakker, J. (2018). Holistic Monitoring and Treatment in Septic Shock. In: Pinto Lima, A., Silva, E. (eds) Monitoring Tissue Perfusion in Shock. Springer, Cham. https://doi.org/10.1007/978-3-319-43130-7_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-43130-7_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-43128-4
Online ISBN: 978-3-319-43130-7
eBook Packages: MedicineMedicine (R0)