Abstract
Prostate cancer is a complex, heterogeneous disease. Factors related to detection, particularly PSA screening, further increase heterogeneity in the manifestation of the disease. It is thus not possible to provide a simple summary of the relationship between obesity and prostate cancer. Findings on obesity, often defined using body mass index (BMI), and total prostate cancer risk have been mixed; however, obesity is relatively consistently associated with a higher risk of aggressive prostate cancer, with aggressiveness defined in various ways (e.g., advanced stage, fatal, poorer prognosis in men with prostate cancer). Many methodologic issues (e.g., influence of PSA screening, detection bias and treatment) need to be thoroughly considered in both existing and future etiologic and prognostic research. Biological mechanisms supporting the link are under investigation, but may involve insulin and IGF axis, sex steroid hormones and alterations in metabolism. Some promising data suggest that molecular sub-types of prostate cancer may offer insights into etiology, but further study is required. A full evaluation of body fatness and weight change over the life course would not only provide insights to the underlying mechanisms but also allow more effective interventions.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Burden of Prostate Cancer
An estimated 1.1 million men worldwide were diagnosed with prostate cancer in 2012, accounting for 15 % of the cancers diagnosed in men, with almost 70 % of the cases occurring in more developed regions. Age-standardized prostate cancer incidence rates vary more than 25-fold worldwide [1]. In the USA, prostate cancer is the most frequently diagnosed cancer in men, with 60 % higher incidence rates in blacks than in non-Hispanic whites [2]. Although differences in prostate-specific antigen (PSA) screening may account largely for the global variation in incidence, geographic differences were apparent already in the era prior to PSA screening, highlighting a potential role of lifestyle factors, including obesity, to account for the variation in rates.
Prostate cancer is the fifth leading cause of death from cancer globally in men with an estimated 307,000 deaths in 2012 [1]. There is less variation in mortality rates worldwide (~10-fold) than is observed for incidence. Rates of mortality are highest in countries in the Caribbean and among African-American men in the USA. During the past decades, prostate cancer mortality rates have shown declines in some countries, most notably in the USA since 1990s, which may be attributable in part to earlier detection through PSA screening and subsequent earlier treatment [3]. Notwithstanding the considerable mortality associated with this disease, most men with prostate cancer die with and not from their cancer. Accumulating evidence suggests that overweight and obese men may have poor outcome compared to men with normal weight, and it is crucial to review evidence on obesity and mortality and recurrence of prostate cancer, as proper management of this modifiable lifestyle factor may help improve prostate cancer outcomes.
In considering the epidemiology of prostate cancer, it is critical to take into account certain features of the natural history and heterogeneity of the disease. In a recent synthesis of 19 available autopsy studies, among men aged 70–79, prostate cancer was found in 36 % of Caucasians and 51 % of African-Americans [4]. PSA screening detects many indolent cancers that otherwise would not have been diagnosed. Thus, it is important to consider, in addition to total prostate cancer, those cancers with lethal potential for several reasons. First, some evidence suggests that risk factor patterns may differ for potentially lethal and indolent disease. This suggests either that there may be separate etiologies for indolent and aggressive prostate cancer, or that some risk factors may affect progression rather than incidence [5]. Secondly, some forms of detection biases may, for example, lead to an underdetection of prostate cancer in obese men, yet despite the apparent lower incidence, these men could still be at greater risk of fatal cancer. Potential useful indicators are advanced stage disease at diagnosis, high-grade cancer and fatal or metastatic cancer.
2 Epidemiological Evidence
2.1 Adulthood Obesity
2.1.1 Overall Obesity
Findings on obesity, often defined using body mass index (BMI), and total prostate cancer risk have yielded inconsistent findings. Nonetheless, cumulative data support a positive association between obesity and advanced/fatal prostate cancer, while the association with total or non-advanced prostate cancer has been mostly null or even inverse [6–9]. A recent meta-analysis suggests that for localized prostate cancer, there was an inverse linear relationship with BMI (relative risk (RR) 0.94; 95 % confidence interval (CI) 0.91–0.97 for every 5 kg/m2 higher BMI). In contrast, obesity was positively associated with advanced stage prostate cancer (RR 1.09; 95 % CI 1.02–1.16 for every 5 kg/m2 higher BMI) [7]. A meta-analysis that included studies up to 2010 suggests that among healthy population, a 5 kg/m2 increase in BMI was associated with a 15 % (RR 1.15; 95 % CI 1.06–1.25) higher risk of dying of prostate cancer. Obese men also have higher rates of cancer-specific mortality after diagnosis. A 5 kg/m2 higher BMI was associated with a 20 % (RR 1.20; 95 % CI 0.99–1.46) increased risk of prostate cancer-specific mortality [6]. However, in contrast, a recent analysis of 18 prospective cohort studies across 6 countries in southern and eastern Asia found that obesity was not associated with prostate cancer mortality [10]. The fact that Asian populations may be more susceptible to abdominal and visceral fat accumulation rather than overall adiposity as measured by BMI, as well as differences in detection and potentially distinct nature of disease among Asians [11] compared to Western populations may contribute to the null association. More research in other race/ethnicities is warranted.
Evidence on obesity and prostate cancer recurrence was limited. Meta-analysis for studies up to 2010 suggests that a 5 kg/m2 higher BMI was significantly associated with a 21 % increased risk of biochemical recurrence (RR 1.21; 95 % CI 1.11–1.31), with a slightly stronger association for patients who had radical prostatectomy (RR 1.25; 95 % CI 1.12–1.40) compared to patients treated with radiation therapy (RR 1.15; 95 % CI 1.03–1.28) [6]. Some [12, 13] but not all the subsequent studies [14] supported the positive association. Thus, the evidence for a role of obesity on recurrence appears suggestive but not definitive at this point.
2.1.2 Abdominal Obesity
The association between abdominal obesity and risk of total prostate cancer has been inconsistent. A recent updated analysis from the Health Professionals Follow-up Study (HPFS) suggests that waist circumference was not associated with total prostate cancer risk [15], consistent with previous report with shorter follow-up [14], and two partly overlapping meta-analyses [8, 16]. However, more recent meta-analysis showed a 56 % (P = 0.007) increased risk of total prostate cancer for waist circumference >102 cm (40.2 in) [17]. Some evidence suggests that abdominal obesity is associated with more advanced disease. In the European Prospective Investigation into Cancer and Nutrition (EPIC), waist circumference (RR per 5 cm 1.06; 95 % CI 1.01–1.10) and waist-to-hip ratio (RR per 0.1 unit 1.21; 95 % CI 1.04–1.39) were positively associated with diagnosis of more advanced prostate cancer [18]. Waist circumference was significantly associated with more aggressive disease in the Melbourne Collaborative Cohort Study [19], but it was not associated with advanced stage or high-grade disease in the HPFS [15].
The role of abdominal obesity in prostate cancer recurrence and survival has been less investigated. A recent study measured visceral adipose tissue using computed tomography among prostate cancer patients who underwent radical prostatectomy and found that BMI, waist circumference or VAT was not associated with biochemical recurrence [20].
2.1.3 Weight Change
Research on weight change and prostate cancer risk, recurrence and survival is limited. In a recent meta-analysis of four studies, adult weight gain was not associated with risk of total prostate cancer (RR per 5 kg increase 0.98; 95 % CI 0.94–1.02), and the summary RR per 5 kg adult weight gain was 0.96 (95 % CI 0.92–1.00) for localized prostate cancer and 1.04 (95 % CI 0.99–1.09) for advanced prostate cancer [21]. In a retrospective cohort study of 1337 men with clinically localized prostate cancer who underwent prostatectomy, compared with men who had stable weight (from 5 years before surgery to 1 year after surgery), those whose weight increased by more than 2.2 kg had almost twice the recurrence risk (RR 1.94; 95 % CI 1.14–3.32) [12]. Analysis among 4376 men diagnosed with clinically localized prostate cancer suggests that a weight gain > 5 % after diagnosis was associated with an almost doubled increased rate of prostate cancer-specific mortality (RR 1.93; 95 % CI 1.18–3.16) [22]. These findings merit further research.
2.2 Early-Life Obesity
Overweight and obesity in childhood and adolescence may influence or reflect sex hormone levels during periods of growth and development and thus may be important for later prostate cancer risk [23]. For body size in early adulthood (≤30 years), the findings were inconsistent [24–28]. Obesity in childhood has been inversely associated with risk of total, advanced or aggressive prostate cancer [27, 29, 30], whereas other studies have shown no association [31, 32]. In a recent updated analysis of the HPFS, high BMI at age 21 was inversely associated with total prostate cancer (RR 0.89; 95 % CI 0.80–0.98 for BMI ≥ 26 versus 20–21.9 kg/m2, P trend = 0.01) and with fatal and advanced disease [15]. In addition, higher cumulative average BMI was associated with reduced risk of total, non-advanced and less aggressive disease in men ≤65 years at diagnosis. However, no clear association was observed between childhood body size and prostate cancer. The authors observed greater attenuation overall when adjusting for BMI at age 21 in analyses of cumulative average BMI or waist circumference than the reverse, supporting the possibility that body size in early adulthood is more strongly related to prostate cancer development than body size later in life.
3 Methodology Issues
3.1 PSA Screening
Before PSA screening was introduced, potentially lethal cases could be identified as those with advanced stage (T3b or higher) at diagnosis; thus, pre-PSA cases were enriched with those of lethal potential, as compared to the distribution in screened populations, among which over 90 % of cases are well-differentiated tumors with low metastatic potential [33]. Therefore, epidemiologic studies of overall prostate cancer in the pre-PSA era tended to observe relative risk estimates closer to those found for lethal disease in contemporary studies.
Additionally, PSA screening may also be a potential confounder in epidemiological studies. Men who take part in regular screening practice, including PSA screening, tend also to take part in other health-related behaviors [34]. Thus, studies in the PSA era should account for PSA screening practices in their study design or data analysis.
3.2 Detection Bias
It has also been suggested that obesity makes the early detection of prostate cancer more difficult due to less PSA screening, lower accuracy of digital rectal examination in obese men and high missing rates due to large prostate [35]. In addition, obese men have lower PSA values due to increased blood volume and PSA hemodilution [36]. Among men with prostate cancer, PSA values are 7 % lower in overweight patients (BMI 25–30 kg/m2), 14 % lower in obese patients (BMI 30–35 kg/m2) and 18 % lower in severely obese patients (BMI > 35 kg/m2), compared to men with normal weight patients (BMI < 25 kg/m2) [36], with similar reductions in PSA levels reported for overweight and obese cancer-free men [37]. As such, obese men have lower PSA-driven biopsy rates. In the USA, where prostate biopsies are largely driven by PSA screening, obese men have a reduced chance of undergoing biopsy compared to normal weight men, leading to the detection of fewer cancers in obese individuals and biasing the association between obesity and prostate cancer toward the null. In countries with lower PSA screening rates, such as Europe and Australia, this detection bias is reduced and meta-analysis of studies from these regions demonstrates a positive association between obesity and prostate cancer risk [9].
Although the existence of detection bias could not be fully ruled out, studies suggest that elevated BMI was significantly associated with a higher risk of prostate cancer-specific mortality in those without PSA screening [38] and in both pre- and PSA screening eras [39], suggesting that biological mechanisms play a role.
3.3 Treatment
Alternatively, difficulties in treatment, such as increased risk of positive surgical margins [40–42], and the greater day-to-day variation in prostate location that leads to lower dose and less effective radiation [43] could also contribute to the poorer outcome observed in diagnosed patients. However, the association with recurrence was still significant after adjusting for margin status in many studies [6]. A study among only patients with organ-confined disease and negative surgical margins also suggests that obesity remains associated with biochemical recurrence following radical prostatectomy [44].
4 Biological Mechanisms
A recent large case–control analysis of 22 studies in the PRACTICAL consortium found that genetic variants previously associated with higher BMI were minimally inversely associated with prostate cancer risk (RR per standard deviation higher BMI genetic score 0.98; 95 % CI 0.96–1.00; P = 0.07) [45], supporting that other factors play major roles in the link between obesity and prostate cancer. However, higher BMI genetic score appeared to be associated suggestively with higher risk of prostate cancer-specific mortality, especially among men with low-grade disease. These findings require confirmation. The following mechanisms haven been proposed so far.
4.1 Insulin and IGF Axis
Obesity induces insulin resistance [46], a condition whereby some organs become resistant to insulin’s ability to shuttle glucose into cells, especially after eating a meal high in carbohydrates. To compensate for this resistance to insulin, the pancreas produces more insulin, which leads to an increase in blood insulin levels. Insulin could directly signal growth, or it could do this by increasing the levels of other growth factors (insulin-like growth factors [IGF]), or it could make cells more sensitive to other growth factors and therefore may exert a cancer-promoting influence.
Direct epidemiologic evidence linking circulating insulin and prostate cancer risk is limited and mixed: A case–control [47] and a recent case–cohort study [48] observed positive associations, whereas three other prospective studies observed no association [49–51]. In addition, findings are inconclusive between C-peptide, a marker for insulin secretion, and risk of prostate cancer [52–54], including a recent analysis in HPFS (highest vs lowest quartile RR 1.05; 95 % CI 0.82–1.34, P trend = 0.95) [52].
Circulating IGF-1 concentrations are regulated by growth hormone, present in systemic circulation and expressed in body tissues. Approximately 0.5–1 % of IGF-1 is free, with the majority being bound in the serum by acid-labile subunit and IGFBPs, including IGFBP-3, the most ubiquitous of the binding proteins. Whether obesity is associated with increased IGF levels is controversial, with most studies showing no association between total IGF-1 and obesity [55–57]. Some studies suggest that free IGF-1 levels may be more relevant. However, free IGF-1 measured by immunoassays has not been consistently associated with obesity. Because immunoassays are not able to take into account the modifying effects of IGFBPs on interactions between IGF-1 and its receptor, other measures, such as kinase receptor activation assay, have been proposed. A recent study using such method found that 24-h mean bioactive IGF-1 levels were not reduced in obese women and did not correlate with BMI [58].
Higher circulating IGF-1 levels are consistently associated with an increased risk of prostate cancer in epidemiologic studies [59]. A stronger association with low-grade prostate cancer was suggested in a pooled analysis of 12 prospective studies [60] and recent reports from EPIC [61] and the HPFS [62]. The growth of poorly differentiated cancers may be more autonomous because the PI3k-Akt pathway is constitutively active due to molecular defects (e.g., loss of PTEN, which is associated with higher-grade tumors). Thus, high-grade prostate cancers may be less sensitive to the action of IGF-1 than low-grade cancers [63]. Findings between IGFBP-3 and risk of prostate cancer have been inconsistent [60].
It is unclear whether the insulin and IGF axes interact to affect prostate cancer carcinogenesis. Recently, the role of IGFBP-1, a marker for insulin activity, which also binds IGF-1 and inhibits its action, has emerged [51, 62, 64]. High insulin levels inhibit production/release of IGFBP-1 and are associated with lower IGFBP-1 concentrations. In HPFS, higher pre-diagnostic fasting IGFBP-1 levels were associated with a lower risk of prostate cancer (highest vs. lowest quartile RR 0.67; 95 % CI 0.52–0.86, P trend = 0.003), which remained similar after adjusting for IGF-1, and was primarily driven by lower-grade and non-advanced prostate cancer. Men in the bottom IGFBP-1 and top IGF-1 tertiles had a 78 % increased risk of prostate cancer compared with men in the top IGFBP-1 and bottom IGF-1 tertiles [62].
4.2 Sex Steroid Hormones
Androgens are required for the normal growth and development of the prostate gland. Most prostate tumors respond to androgen deprivation therapy until they establish an androgen-independent growth mechanism. Inhibition of the conversion of testosterone to the more potent dihydrotestosterone (DHT) by finasteride, a 5α-reductase inhibitor, reduced the occurrence of prostate cancer by approximately 25 % (95 % CI 19–31 %) during a 7-year follow-up [65]. The decrease was entirely observed in low-grade cancers, so the ultimate impact on fatal prostate cancer may be minimal [66]. Some evidence suggests that lower levels of testosterone in obese men might be linked to poorly differentiated and hormone-insensitive tumors [67, 68]. However, the Endogenous Hormones and Prostate Cancer Collaborative Group pooled 18 prospective studies that included 3886 men with incident prostate cancer and 6438 control subjects and observed no associations between the risk of prostate cancer and serum concentrations of testosterone, calculated free testosterone, DHT, dehydroepiandrosterone sulfate, androstenedione, androstanediol glucuronide, estradiol or calculated free estradiol, with similar findings for both localized and advanced diseases, suggesting that endogenous estrogen concentrations are not related to prostate cancer risk [69].
Interestingly, in the same pooled analysis by the Endogenous Hormones and Prostate Cancer Collaborative Group, serum concentration of sex hormone-binding globulin (SHBG) was modestly inversely associated with prostate cancer risk (highest vs. lowest quintile RR 0.86, 95 % CI 0.75–0.98; P trend = 0.01) [69]. SHBG is a glycoprotein with high binding affinity for testosterone and DHT and lower affinity for estradiol. SHBG is negatively associated with obesity [69, 70] and IGF-1 [69]. Although the possibility that the inverse association observed was due to the negative relationship between concentrations of SHBG and IGF-1 could not be ruled out, the role of SHBG in prostate cancer carcinogenesis requires further research.
Early studies among prostate cancer patients showed that low pretreatment testosterone levels were associated with higher Gleason score [71], advanced stage [72, 73], positive surgical margins [73] and worse overall survival for men with metastatic prostate cancer [74, 75]. However, reverse causality is likely as low pretreatment testosterone levels could be a consequence of prostate cancer itself rather than that aggressive prostate is a result of the hormonal milieu in which it develops. To address these concerns, a recent analysis of 963 prostate cancer cases from the Physicians’ Health Study (PHS) and the HPFS suggests that pre-diagnostic circulating sex hormones, including total testosterone, SHBG, SHBG-adjusted testosterone, free testosterone, DHT, androstanediol glucuronide or estradiol, were not associated with lethal prostate cancer or total mortality [76].
4.3 Metabolic Syndrome and Diabetes Mellitus
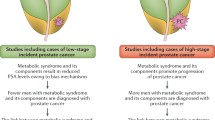
The role of obesity in cardiovascular disease and other diseases including some cancers is mediated in part or largely through alterations in metabolism. Although the basis for clustering of these factors is controversial, empirical definitions such as the metabolic syndrome may yield some etiologic insights. A recent meta-analysis summarized the result of 14 studies that evaluated the association between metabolic syndrome, its components and risk of prostate cancer [17]. A nonsignificant increased risk was observed, though no clear association was observed in cohort studies. Interestingly, a significant association was observed in the 8 European studies (RR = 1.30, P = 0.03), but not in the 4 US or 2 Asiatic studies. Also of note, the associations were driven by waist circumference and high blood pressure, rather than BMI, dysglycemia or dyslipidemia (including high triglycerides or low HDL cholesterol).
A diagnosis of type 2 diabetes mellitus has been associated with a decreased risk of total prostate cancer [77]. This association has been stronger for studies conducted in the PSA era, suggesting that the inverse association is stronger for more indolent cancers. Detection bias does not appear to entirely account for the association [77]. In contrast to incidence, diabetes may be associated with worse prognosis in prostate cancer. A recent meta-analysis included 11 cohort studies that examined mortality in men with prostate cancer [78]. In the meta-analysis, diabetes mellitus was associated with an increased incidence of all-cause mortality among men with prostate cancer (RR 1.50; 95 % CI 1.25–1.79), prostate cancer-specific mortality (RR 1.26; 95 % CI 1.20–1.33) and non-prostate cancer mortality (RR 1.83; 95 % CI 1.33–2.52).
4.4 Tissue-Based Factors
Molecular factors in prostate cancer tissue linking obesity and poor prostate cancer outcome is an emerging area of research. Such studies, by incorporating molecular features that underlie tumor heterogeneity, may yield insights. Such studies in the future may help unravel some of the inconsistent findings regarding obesity and prostate cancer, but research to date has been limited to a few examples.
TMPRSS2:ERG, a hormonally regulated gene fusion, presents in about half of prostate tumors [79]. In a recent analysis in the PHS and HPFS, the detrimental effects of obesity on prostate cancer outcomes are limited primarily to men with tumors harboring the TMPRSS2:ERG gene fusion. Among men with ERG-positive tumors, the RR for lethal prostate cancer was 1.48 (95 % CI 0.98–2.23) per 5-unit higher BMI before diagnosis, 2.51 (95 % CI 1.26–4.99) per 8-inch higher waist circumference before diagnosis and 2.22 (95 % CI 1.35–3.63) per 5-unit higher BMI at baseline. In contrast, the corresponding RR among men with ERG-negative tumors was 1.10 (95 % CI 0.76–1.59; Pinteraction = 0.24), 1.14 (95 % CI 0.62–2.10; Pinteraction = 0.09) and 0.78 (95 % CI 0.52–1.19; Pinteraction = 0.001) [80]. If confirmed, this finding could potentially inform prostate cancer therapy development and secondary prevention strategies.
Fatty acid synthase (FASN) is an enzyme critical in the synthesis of endogenous fatty acids, which can be modified and packaged into structural lipids required for cell division. Elevated FASN enzyme, mRNA and enzymatic activity have been seen in human breast cancer cell lines [81], ovarian tumors [82] and prostate tumors [83], and polymorphisms in FASN were associated with BMI [84]. In HPFS, SNP rs1127678 was significantly related to higher BMI and interacted with BMI for both prostate cancer risk (Pinteraction = 0.004) and prostate cancer mortality (Pinteraction = 0.056). Among overweight men (BMI ≥ 25 kg/m2), but not leaner men, the homozygous variant allele carried a relative risk of advanced prostate cancer of 2.49 (95 % CI 1.00–6.23) compared with lean men with the wild type. Overweight patients carrying the variant allele had a 2.04 (95 % CI 1.31–3.17) times higher risk of prostate cancer mortality. Similarly, overweight patients with elevated tumor FASN expression had a 2.73 (95 % CI 1.05–7.08) times higher risk of lethal prostate cancer. In contrast, among men who had normal body weight, FASN expression level was not significantly associated with lethal prostate cancer (Pinteraction = 0.02). Significant interactions of BMI with FASN polymorphisms and FASN tumor expression suggest that FASN may be a potential link between obesity and poor prostate outcome and raise the possibility that FASN inhibition could reduce prostate cancer-specific mortality, particularly in overweight men [83].
5 Conclusions
Prostate cancer is a complex, heterogeneous disease. Factors related to detection, particularly PSA screening, further increase heterogeneity in the manifestation of the disease. It is thus not possible to provide a simple summary of the relationship between obesity and prostate cancer. At a first level, it is useful to separate manifestations of relatively indolent disease (e.g., organ-confined, low-grade, non-lethal, total cancer in the PSA era) and aggressive disease (advanced stage, lethal, poor prognosis). Overall, although there are some exceptions, obesity does not appear to increase risk of indolent prostate cancer. Some evidence even suggests a potential protective association, though it is unclear whether this is biologically related (e.g., low testosterone levels) or related to lower detection (e.g., obesity may lower PSA levels).
In contrast, obesity is relatively consistently, though with exceptions, associated with a higher risk of aggressive prostate cancer, with aggressiveness defined in various ways (e.g., advanced stage, fatal, poorer prognosis in men with prostate cancer). A number of mechanisms, not mutually exclusive, may account for this association: (1) later detection in obese men, (2) poorer response to treatment and (3) direct biological mechanisms (e.g., hyperinsulinemia). In part, if less aggressive cancers are detected in obese men, for any reason (see above), obese men will be selected to have more aggressive cancer if diagnosed by the exclusion of diagnosed indolent cancers. Whatever the explanation(s), under most circumstances it is reasonable to assume from a clinical perspective that obese men diagnosed with prostate cancer are likely to have a worse prognosis independent of other known clinical predictors. Some promising data suggest that molecular sub-types of prostate cancer may offer insights into etiology, but further study is required.
Together, these data provide encouraging evidence for using weight management to prevent aggressive prostate cancer, prostate cancer disease progression and reduce prostate cancer-specific mortality. However, many methodologic issues (e.g., influence of PSA screening, detection bias and treatment) need to be addressed in etiologic and prognostic research. More data are needed to understand the timing of body fatness/weight gain relative to the diagnosis/treatment of prostate cancer. A full evaluation of body fatness and weight gain over the life course would not only provide insights into the underlying mechanisms but also allow more effective interventions. Such interventions may include increasing self-awareness and more early detection efforts among overweight or obese healthy individuals, and more counseling on healthy lifestyle (e.g., physical activity) after diagnosis, and appropriate personalized treatment for overweight and obese patients.
References
Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray, F (2013) GLOBOCAN 2012 v1.0, cancer incidence and mortality worldwide. IARC CancerBase No. 11. IARC Press, Lyon
Cancer Facts & Figures 2015 (2015). American Cancer Society, Atlanta
Chu KC, Tarone RE, Freeman HP (2003) Trends in prostate cancer mortality among black men and white men in the United States. Cancer 97(6):1507–1516. doi:10.1002/cncr.11212
Jahn JL, Giovannucci EL, Stampfer MJ (2015) The high prevalence of undiagnosed prostate cancer at autopsy: implications for epidemiology and treatment of prostate cancer in the Prostate-specific Antigen-era. Int J Cancer J Int Cancer 137(12):2795–2802. doi:10.1002/ijc.29408
Giovannucci E, Liu Y, Platz EA, Stampfer MJ, Willett WC (2007) Risk factors for prostate cancer incidence and progression in the health professionals follow-up study. Int J Cancer J Int Cancer 121(7):1571–1578. doi:10.1002/ijc.22788
Cao Y, Ma J (2011) Body mass index, prostate cancer-specific mortality, and biochemical recurrence: a systematic review and meta-analysis. Cancer Prev Res (Phila) 4(4):486–501. doi:10.1158/1940-6207.CAPR-10-0229
Discacciati A, Orsini N, Wolk A (2012) Body mass index and incidence of localized and advanced prostate cancer—a dose-response meta-analysis of prospective studies. Ann Oncol (Official Journal of the European Society for Medical Oncology/ESMO) 23(7):1665–1671. doi:10.1093/annonc/mdr603
MacInnis RJ, English DR (2006) Body size and composition and prostate cancer risk: systematic review and meta-regression analysis. Cancer Causes Control (CCC) 17(8):989–1003. doi:10.1007/s10552-006-0049-z
Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M (2008) Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet 371(9612):569–578. doi:10.1016/S0140-6736(08)60269-X
Fowke JH, McLerran DF, Gupta PC, He J, Shu XO, Ramadas K, Tsugane S, Inoue M, Tamakoshi A, Koh WP, Nishino Y, Tsuji I, Ozasa K, Yuan JM, Tanaka H, Ahn YO, Chen CJ, Sugawara Y, Yoo KY, Ahsan H, Pan WH, Pednekar M, Gu D, Xiang YB, Sauvaget C, Sawada N, Wang R, Kakizaki M, Tomata Y, Ohishi W, Butler LM, Oze I, Kim DH, You SL, Park SK, Parvez F, Chuang SY, Chen Y, Lee JE, Grant E, Rolland B, Thornquist M, Feng Z, Zheng W, Boffetta P, Sinha R, Kang D, Potter JD (2015) Associations of body mass index, smoking, and alcohol consumption with prostate cancer mortality in the Asia Cohort Consortium. Am J Epidemiol 182(5):381–389. doi:10.1093/aje/kwv089
Chia SJ, Tang WY, Elnatan J, Yap WM, Goh HS, Smith DR (2000) Prostate tumours from an Asian population: examination of bax, bcl-2, p53 and ras and identification of bax as a prognostic marker. Br J Cancer 83(6):761–768. doi:10.1054/bjoc.2000.1355
Joshu CE, Mondul AM, Menke A, Meinhold C, Han M, Humphreys EB, Freedland SJ, Walsh PC, Platz EA (2011) Weight gain is associated with an increased risk of prostate cancer recurrence after prostatectomy in the PSA era. Cancer Prev Res (Phila) 4(4):544–551. doi:10.1158/1940-6207.CAPR-10-0257
Asmar R, Beebe-Dimmer JL, Korgavkar K, Keele GR, Cooney KA (2013) Hypertension, obesity and prostate cancer biochemical recurrence after radical prostatectomy. Prostate Cancer Prostatic Dis 16(1):62–66. doi:10.1038/pcan.2012.32
Tomaszewski JJ, Richman EL, Sadetsky N, O’Keefe DS, Carroll PR, Davies BJ, Chan JM (2014) Impact of folate intake on prostate cancer recurrence following definitive therapy: data from CaPSURE. J Urol 191(4):971–976. doi:10.1016/j.juro.2013.09.065
Moller E, Wilson KM, Batista JL, Mucci LA, Balter K, Giovannucci E (2015) Body size across the life course and prostate cancer in the health professionals follow-up study. Int J Cancer J Int Cancer. doi:10.1002/ijc.29842
Hsing AW, Sakoda LC, Chua S Jr (2007) Obesity, metabolic syndrome, and prostate cancer. Am J Clin Nutr 86(3):s843–s857
Esposito K, Chiodini P, Capuano A, Bellastella G, Maiorino MI, Parretta E, Lenzi A, Giugliano D (2013) Effect of metabolic syndrome and its components on prostate cancer risk: meta-analysis. J Endocrinol Invest 36(2):132–139. doi:10.1007/BF03346748
Pischon T, Boeing H, Weikert S, Allen N, Key T, Johnsen NF, Tjonneland A, Severinsen MT, Overvad K, Rohrmann S, Kaaks R, Trichopoulou A, Zoi G, Trichopoulos D, Pala V, Palli D, Tumino R, Sacerdote C, Bueno-De-Mesquita HB, May A, Manjer J, Wallstrom P, Stattin P, Hallmans G, Buckland G, Larranaga N, Chirlaque MD, Martinez C, Cornejo MLR, Ardanaz E, Bingham S, Khaw KT, Rinaldi S, Slimani N, Jenab M, Riboli E (2008) Body size and risk of prostate cancer in the European prospective investigation into cancer and nutrition. Cancer Epidem Biomar 17(11):3252–3261. doi:10.1158/1055-9965.Epi-08-0609
MacInnis RJ, English DR, Gertig DM, Hopper JL, Giles GG (2003) Body size and composition and prostate cancer risk. Cancer Epidem Biomar 12(12):1417–1421
Ohwaki K, Endo F, Hattori K (2015) Abdominal obesity, hypertension, antihypertensive medication use and biochemical recurrence of prostate cancer after radical prostatectomy. Eur J Cancer 51(5):604–609. doi:10.1016/j.ejca.2015.01.003
Keum N, Greenwood DC, Lee DH, Kim R, Aune D, Ju W, Hu FB, Giovannucci EL (2015) Adult weight gain and adiposity-related cancers: a dose-response meta-analysis of prospective observational studies. J Natl Cancer Inst 107(2). doi:10.1093/jnci/djv088
Bonn SE, Wiklund F, Sjolander A, Szulkin R, Stattin P, Holmberg E, Gronberg H, Balter K (2014) Body mass index and weight change in men with prostate cancer: progression and mortality. Cancer Causes Control (CCC) 25(8):933–943. doi:10.1007/s10552-014-0393-3
Sutcliffe S, Colditz GA (2013) Prostate cancer: is it time to expand the research focus to early-life exposures? Nat Rev Cancer 13(3):208–518. doi:10.1038/nrc3434
Littman AJ, White E, Kristal AR (2007) Anthropometrics and prostate cancer risk. Am J Epidemiol 165(11):1271–1279. doi:10.1093/aje/kwm013
Discacciati A, Orsini N, Andersson SO, Andren O, Johansson JE, Wolk A (2011) Body mass index in early and middle-late adulthood and risk of localised, advanced and fatal prostate cancer: a population-based prospective study. Br J Cancer 105(7):1061–1068. doi:10.1038/bjc.2011.319
Giovannucci E, Rimm EB, Liu Y, Leitzmann M, Wu K, Stampfer MJ, Willett WC (2003) Body mass index and risk of prostate cancer in U.S. health professionals. J Natl Cancer Inst 95(16):1240–1244
Robinson WR, Stevens J, Gammon MD, John EM (2005) Obesity before age 30 years and risk of advanced prostate cancer. Am J Epidemiol 161(12):1107–1114. doi:10.1093/aje/kwi150
Schuurman AG, Goldbohm RA, Dorant E, van den Brandt PA (2000) Anthropometry in relation to prostate cancer risk in the Netherlands Cohort Study. Am J Epidemiol 151(6):541–549
Barba M, Terrenato I, Schunemann HJ, Fuhrman B, Sperati F, Teter B, Gallucci M, D’Amato A, Muti P (2008) Indicators of sexual and somatic development and adolescent body size in relation to prostate cancer risk: results from a case-control study. Urology 72(1):183–187. doi:10.1016/j.urology.2007.09.065
Giovannucci E, Rimm EB, Stampfer MJ, Colditz GA, Willett WC (1997) Height, body weight, and risk of prostate cancer. Cancer Epidemiol Biomark Prev (A Publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology) 6(8):557–563
Aarestrup J, Gamborg M, Cook MB, Sorensen TI, Baker JL (2014) Childhood body mass index and the risk of prostate cancer in adult men. Br J Cancer 111(1):207–212. doi:10.1038/bjc.2014.266
Hsing AW, Deng J, Sesterhenn IA, Mostofi FK, Stanczyk FZ, Benichou J, Xie T, Gao YT (2000) Body size and prostate cancer: a population-based case-control study in China. Cancer Epidemiol Biomark Prev (A Publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology) 9(12):1335–1341
Li J, Djenaba JA, Soman A, Rim SH, Master VA (2012) Recent trends in prostate cancer incidence by age, cancer stage, and grade, the United States, 2001–2007. Prostate Cancer 2012:691380. doi:10.1155/2012/691380
Satia JA, Galanko JA (2007) Demographic, behavioral, psychosocial, and dietary correlates of cancer screening in African Americans. J Health Care Poor Underserved 18(4 Suppl):146–164
Freedland SJ, Platz EA, Presti JC Jr, Aronson WJ, Amling CL, Kane CJ, Terris MK (2006) Obesity, serum prostate specific antigen and prostate size: implications for prostate cancer detection. J Urol 175(2):500–504; discussion 504. doi:10.1016/S0022-5347(05)00162-X
Banez LL, Hamilton RJ, Partin AW, Vollmer RT, Sun L, Rodriguez C, Wang Y, Terris MK, Aronson WJ, Presti JC Jr, Kane CJ, Amling CL, Moul JW, Freedland SJ (2007) Obesity-related plasma hemodilution and PSA concentration among men with prostate cancer. JAMA 298(19):2275–2280. doi:10.1001/jama.298.19.2275
Grubb RL 3rd, Black A, Izmirlian G, Hickey TP, Pinsky PF, Mabie JE, Riley TL, Ragard LR, Prorok PC, Berg CD, Crawford ED, Church TR, Andriole GL Jr, Team PP (2009) Serum prostate-specific antigen hemodilution among obese men undergoing screening in the prostate, lung, colorectal, and ovarian cancer screening trial. Cancer Epidemiol Biomark Prev (A Publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology) 18(3):748–751. doi:10.1158/1055-9965.EPI-08-0938
Wright ME, Chang SC, Schatzkin A, Albanes D, Kipnis V, Mouw T, Hurwitz P, Hollenbeck A, Leitzmann MF (2007) Prospective study of adiposity and weight change in relation to prostate cancer incidence and mortality. Cancer 109(4):675–684. doi:10.1002/cncr.22443
Ma J, Li H, Giovannucci E, Mucci L, Qiu W, Nguyen PL, Gaziano JM, Pollak M, Stampfer MJ (2008) Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol 9(11):1039–1047. doi:10.1016/S1470-2045(08)70235-3
Freedland SJ, Aronson WJ, Kane CJ, Presti JC Jr, Amling CL, Elashoff D, Terris MK (2004) Impact of obesity on biochemical control after radical prostatectomy for clinically localized prostate cancer: a report by the shared equal access regional cancer hospital database study group. J Clin Oncol (Official Journal of the American Society of Clinical Oncology) 22(3):446–453. doi:10.1200/JCO.2004.04.181
Amling CL, Riffenburgh RH, Sun L, Moul JW, Lance RS, Kusuda L, Sexton WJ, Soderdahl DW, Donahue TF, Foley JP, Chung AK, McLeod DG (2004) Pathologic variables and recurrence rates as related to obesity and race in men with prostate cancer undergoing radical prostatectomy. J Clin Oncol 22(3):439–445
Ho T, Gerber L, Aronson WJ, Terris MK, Presti JC, Kane CJ, Amling CL, Freedland SJ (2012) Obesity, prostate-specific antigen nadir, and biochemical recurrence after radical prostatectomy: biology or technique? Results from the SEARCH database. Eur Urol 62(5):910–916. doi:10.1016/j.eururo.2012.08.015
Millender LE, Aubin M, Pouliot J, Shinohara K, Roach M 3rd (2004) Daily electronic portal imaging for morbidly obese men undergoing radiotherapy for localized prostate cancer. Int J Radiat Oncol Biol Phys 59(1):6–10. doi:10.1016/j.ijrobp.2003.12.027
Freedland SJ, Terris MK, Presti JC Jr, Amling CL, Kane CJ, Trock B, Aronson WJ, Search Database Study G (2004) Obesity and biochemical outcome following radical prostatectomy for organ confined disease with negative surgical margins. J Urol 172(2):520–524. doi:10.1097/01.ju.0000135302.58378.ae
Davies NM, Gaunt TR, Lewis SJ, Holly J, Donovan JL, Hamdy FC, Kemp JP, Eeles R, Easton D, Kote-Jarai Z, Al Olama AA, Benlloch S, Muir K, Giles GG, Wiklund F, Gronberg H, Haiman CA, Schleutker J, Nordestgaard BG, Travis RC, Neal D, Pashayan N, Khaw KT, Stanford JL, Blot WJ, Thibodeau S, Maier C, Kibel AS, Cybulski C, Cannon-Albright L, Brenner H, Park J, Kaneva R, Batra J, Teixeira MR, Pandha H, consortium P, Lathrop M, Smith GD, Martin RM (2015) The effects of height and BMI on prostate cancer incidence and mortality: a Mendelian randomization study in 20,848 cases and 20,214 controls from the PRACTICAL consortium. Cancer Causes Control (CCC) 26(11):1603–1616. doi:10.1007/s10552-015-0654-9
Kahn BB, Flier JS (2000) Obesity and insulin resistance. J Clin Investig 106(4):473–481. doi:10.1172/JCI10842
Hsing AW, Chua S Jr, Gao YT, Gentzschein E, Chang L, Deng J, Stanczyk FZ (2001) Prostate cancer risk and serum levels of insulin and leptin: a population-based study. J Natl Cancer Inst 93(10):783–789
Albanes D, Weinstein SJ, Wright ME, Mannisto S, Limburg PJ, Snyder K, Virtamo J (2009) Serum insulin, glucose, indices of insulin resistance, and risk of prostate cancer. J Natl Cancer Inst 101(18):1272–1279. doi:10.1093/jnci/djp260
Hubbard JS, Rohrmann S, Landis PK, Metter EJ, Muller DC, Andres R, Carter HB, Platz EA (2004) Association of prostate cancer risk with insulin, glucose, and anthropometry in the Baltimore longitudinal study of aging. Urology 63(2):253–258. doi:10.1016/j.urology.2003.09.060
Chen C, Lewis SK, Voigt L, Fitzpatrick A, Plymate SR, Weiss NS (2005) Prostate carcinoma incidence in relation to prediagnostic circulating levels of insulin-like growth factor I, insulin-like growth factor binding protein 3, and insulin. Cancer 103(1):76–84. doi:10.1002/cncr.20727
Stattin P, Bylund A, Rinaldi S, Biessy C, Dechaud H, Stenman UH, Egevad L, Riboli E, Hallmans G, Kaaks R (2000) Plasma insulin-like growth factor-I, insulin-like growth factor-binding proteins, and prostate cancer risk: a prospective study. J Natl Cancer Inst 92(23):1910–1917
Lai GY, Giovannucci EL, Pollak MN, Peskoe SB, Stampfer MJ, Willett WC, Platz EA (2014) Association of C-peptide and leptin with prostate cancer incidence in the health professionals follow-up study. Cancer Causes Control (CCC) 25(5):625–632. doi:10.1007/s10552-014-0369-3
Stocks T, Lukanova A, Rinaldi S, Biessy C, Dossus L, Lindahl B, Hallmans G, Kaaks R, Stattin P (2007) Insulin resistance is inversely related to prostate cancer: a prospective study in Northern Sweden. Int J Cancer J Int Cancer 120(12):2678–2686. doi:10.1002/ijc.22587
Borugian MJ, Spinelli JJ, Sun Z, Kolonel LN, Oakley-Girvan I, Pollak MD, Whittemore AS, Wu AH, Gallagher RP (2007) Prediagnostic C-peptide and risk of prostate cancer. Cancer Epidemiol Biomark Prev (A Publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology) 16(10):2164–2165. doi:10.1158/1055-9965.EPI-07-0495
Gram IT, Norat T, Rinaldi S, Dossus L, Lukanova A, Tehard B, Clavel-Chapelon F, van Gils CH, van Noord PA, Peeters PH, Bueno-de-Mesquita HB, Nagel G, Linseisen J, Lahmann PH, Boeing H, Palli D, Sacerdote C, Panico S, Tumino R, Sieri S, Dorronsoro M, Quiros JR, Navarro CA, Barricarte A, Tormo MJ, Gonzalez CA, Overvad K, Paaske Johnsen S, Olsen A, Tjonneland A, Travis R, Allen N, Bingham S, Khaw KT, Stattin P, Trichopoulou A, Kalapothaki V, Psaltopoulou T, Casagrande C, Riboli E, Kaaks R (2006) Body mass index, waist circumference and waist-hip ratio and serum levels of IGF-I and IGFBP-3 in European women. Int J Obes 30(11):1623–1631. doi:10.1038/sj.ijo.0803324
Nam SY, Lee EJ, Kim KR, Cha BS, Song YD, Lim SK, Lee HC, Huh KB (1997) Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord (Journal of the International Association for the Study of Obesity) 21(5):355–359
Lukanova A, Lundin E, Zeleniuch-Jacquotte A, Muti P, Mure A, Rinaldi S, Dossus L, Micheli A, Arslan A, Lenner P, Shore RE, Krogh V, Koenig KL, Riboli E, Berrino F, Hallmans G, Stattin P, Toniolo P, Kaaks R (2004) Body mass index, circulating levels of sex-steroid hormones, IGF-I and IGF-binding protein-3: a cross-sectional study in healthy women. Eur J Endocrinol/Eur Fed Endocr Soc 150(2):161–171
Frystyk J, Brick DJ, Gerweck AV, Utz AL, Miller KK (2009) Bioactive insulin-like growth factor-I in obesity. J Clin Endocrinol Metab 94(8):3093–3097. doi:10.1210/jc.2009-0614
Rowlands MA, Gunnell D, Harris R, Vatten LJ, Holly JM, Martin RM (2009) Circulating insulin-like growth factor peptides and prostate cancer risk: a systematic review and meta-analysis. Int J Cancer J Int Cancer 124(10):2416–2429. doi:10.1002/ijc.24202
Roddam AW, Allen NE, Appleby P, Key TJ, Ferrucci L, Carter HB, Metter EJ, Chen C, Weiss NS, Fitzpatrick A, Hsing AW, Lacey JV Jr, Helzlsouer K, Rinaldi S, Riboli E, Kaaks R, Janssen JA, Wildhagen MF, Schroder FH, Platz EA, Pollak M, Giovannucci E, Schaefer C, Quesenberry CP Jr, Vogelman JH, Severi G, English DR, Giles GG, Stattin P, Hallmans G, Johansson M, Chan JM, Gann P, Oliver SE, Holly JM, Donovan J, Meyer F, Bairati I, Galan P (2008) Insulin-like growth factors, their binding proteins, and prostate cancer risk: analysis of individual patient data from 12 prospective studies. Ann Intern Med 149(7):461–471, W483–W468
Price AJ, Allen NE, Appleby PN, Crowe FL, Travis RC, Tipper SJ, Overvad K, Gronbaek H, Tjonneland A, Johnsen NF, Rinaldi S, Kaaks R, Lukanova A, Boeing H, Aleksandrova K, Trichopoulou A, Trichopoulos D, Andarakis G, Palli D, Krogh V, Tumino R, Sacerdote C, Bueno-de-Mesquita HB, Arguelles MV, Sanchez MJ, Chirlaque MD, Barricarte A, Larranaga N, Gonzalez CA, Stattin P, Johansson M, Khaw KT, Wareham N, Gunter M, Riboli E, Key T (2012) Insulin-like growth factor-I concentration and risk of prostate cancer: results from the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomark Prev (A Publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology) 21(9):1531–1541. doi:10.1158/1055-9965.EPI-12-0481-T
Cao Y, Nimptsch K, Shui IM, Platz EA, Wu K, Pollak MN, Kenfield SA, Stampfer MJ, Giovannucci EL (2015) Prediagnostic plasma IGFBP-1, IGF-1 and risk of prostate cancer. Int J Cancer J Int Cancer 136(10):2418–2426. doi:10.1002/ijc.29295
Nimptsch K, Platz EA, Pollak MN, Kenfield SA, Stampfer MJ, Willett WC, Giovannucci E (2011) Plasma insulin-like growth factor 1 is positively associated with low-grade prostate cancer in the health professionals follow-up study 1993–2004. Int J Cancer J Int Cancer 128(3):660–667. doi:10.1002/ijc.25381
Chokkalingam AP, Pollak M, Fillmore CM, Gao YT, Stanczyk FZ, Deng J, Sesterhenn IA, Mostofi FK, Fears TR, Madigan MP, Ziegler RG, Fraumeni JF Jr, Hsing AW (2001) Insulin-like growth factors and prostate cancer: a population-based case-control study in China. Cancer Epidemiol Biomark Prev (A Publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology) 10(5):421–427
Thompson IM, Goodman PJ, Tangen CM, Lucia MS, Miller GJ, Ford LG, Lieber MM, Cespedes RD, Atkins JN, Lippman SM, Carlin SM, Ryan A, Szczepanek CM, Crowley JJ, Coltman CA Jr (2003) The influence of finasteride on the development of prostate cancer. N Engl J Med 349(3):215–224. doi:10.1056/NEJMoa030660
Preston MA, Wilson KM, Markt SC, Ge R, Morash C, Stampfer MJ, Loda M, Giovannucci E, Mucci LA, Olumi AF (2014) 5alpha-Reductase inhibitors and risk of high-grade or lethal prostate cancer. JAMA Intern Med 174(8):1301–1307. doi:10.1001/jamainternmed.2014.1600
Severi G, Morris HA, MacInnis RJ, English DR, Tilley W, Hopper JL, Boyle P, Giles GG (2006) Circulating steroid hormones and the risk of prostate cancer. Cancer Epidemiol Biomark Prev (A Publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology) 15(1):86–91. doi:10.1158/1055-9965.EPI-05-0633
Platz EA, Leitzmann MF, Rifai N, Kantoff PW, Chen YC, Stampfer MJ, Willett WC, Giovannucci E (2005) Sex steroid hormones and the androgen receptor gene CAG repeat and subsequent risk of prostate cancer in the prostate-specific antigen era. Cancer Epidemiol Biomark Prev (A Publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology) 14(5):1262–1269. doi:10.1158/1055-9965.EPI-04-0371
Endogenous H, Prostate Cancer Collaborative G, Roddam AW, Allen NE, Appleby P, Key TJ (2008) Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst 100(3):170–183. doi:10.1093/jnci/djm323
Hautanen A (2000) Synthesis and regulation of sex hormone-binding globulin in obesity. Int J Obes Relat Metabol Disord (Journal of the International Association for the Study of Obesity) 24(Suppl 2):S64–S70
Schatzl G, Madersbacher S, Thurridl T, Waldmuller J, Kramer G, Haitel A, Marberger M (2001) High-grade prostate cancer is associated with low serum testosterone levels. Prostate 47(1):52–58. doi:10.1002/pros.1046
Isom-Batz G, Bianco FJ Jr, Kattan MW, Mulhall JP, Lilja H, Eastham JA (2005) Testosterone as a predictor of pathological stage in clinically localized prostate cancer. J Urol 173(6):1935–1937. doi:10.1097/01.ju.0000158040.33531.e7
Massengill JC, Sun L, Moul JW, Wu H, McLeod DG, Amling C, Lance R, Foley J, Sexton W, Kusuda L, Chung A, Soderdahl D, Donahue T (2003) Pretreatment total testosterone level predicts pathological stage in patients with localized prostate cancer treated with radical prostatectomy. J Urol 169(5):1670–1675. doi:10.1097/01.ju.0000062674.43964.d0
Chodak GW, Vogelzang NJ, Caplan RJ, Soloway M, Smith JA (1991) Independent prognostic factors in patients with metastatic (stage D2) prostate cancer. The Zoladex Study Group. JAMA 265(5):618–621
Ribeiro M, Ruff P, Falkson G (1997) Low serum testosterone and a younger age predict for a poor outcome in metastatic prostate cancer. Am J Clin Oncol 20(6):605–608
Gershman B, Shui IM, Stampfer M, Platz EA, Gann PH, Sesso HL, DuPre N, Giovannucci E, Mucci LA (2014) Prediagnostic circulating sex hormones are not associated with mortality for men with prostate cancer. Eur Urol 65(4):683–689. doi:10.1016/j.eururo.2013.01.003
Kasper JS, Giovannucci E (2006) A meta-analysis of diabetes mellitus and the risk of prostate cancer. Cancer Epidemiol Biomark Prev (A Publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology) 15(11):2056–2062. doi:10.1158/1055-9965.EPI-06-0410
Cai H, Xu Z, Xu T, Yu B, Zou Q (2015) Diabetes mellitus is associated with elevated risk of mortality amongst patients with prostate cancer: a meta-analysis of 11 cohort studies. Diabetes/Metab Res Rev 31(4):336–343. doi:10.1002/dmrr.2582
Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM (2005) Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310(5748):644–648. doi:10.1126/science.1117679
Pettersson A, Lis RT, Meisner A, Flavin R, Stack EC, Fiorentino M, Finn S, Graff RE, Penney KL, Rider JR, Nuttall EJ, Martin NE, Sesso HD, Pollak M, Stampfer MJ, Kantoff PW, Giovannucci EL, Loda M, Mucci LA (2013) Modification of the association between obesity and lethal prostate cancer by TMPRSS2:ERG. J Natl Cancer Inst 105(24):1881–1890. doi:10.1093/jnci/djt332
Hunt DA, Lane HM, Zygmont ME, Dervan PA, Hennigar RA (2007) MRNA stability and overexpression of fatty acid synthase in human breast cancer cell lines. Anticancer Res 27(1A):27–34
Gansler TS, Hardman W 3rd, Hunt DA, Schaffel S, Hennigar RA (1997) Increased expression of fatty acid synthase (OA-519) in ovarian neoplasms predicts shorter survival. Hum Pathol 28(6):686–692
Nguyen PL, Ma J, Chavarro JE, Freedman ML, Lis R, Fedele G, Fiore C, Qiu W, Fiorentino M, Finn S, Penney KL, Eisenstein A, Schumacher FR, Mucci LA, Stampfer MJ, Giovannucci E, Loda M (2010) Fatty acid synthase polymorphisms, tumor expression, body mass index, prostate cancer risk, and survival. J Clin Oncol (Official Journal of the American Society of Clinical Oncology) 28(25):3958–3964. doi:10.1200/JCO.2009.27.0793
Campa D, McKay J, Sinilnikova O, Husing A, Vogel U, Hansen RD, Overvad K, Witt PM, Clavel-Chapelon F, Boutron-Ruault MC, Chajes V, Rohrmann S, Chang-Claude J, Boeing H, Fisher E, Trichopoulou A, Trichopoulos D, Palli D, Villarini A, Sacerdote C, Mattiello A, Tumino R, Peeters PH, van Gils CH, Bas Bueno-de-Mesquita H, Lund E, Chirlaque MD, Sala N, Suarez LR, Barricarte A, Dorronsoro M, Sanchez MJ, Lenner P, Hallmans G, Tsilidis K, Bingham S, Khaw KT, Gallo V, Norat T, Riboli E, Rinaldi S, Lenoir G, Tavtigian SV, Canzian F, Kaaks R (2009) Genetic variation in genes of the fatty acid synthesis pathway and breast cancer risk. Breast Cancer Res Treat 118(3):565–574. doi:10.1007/s10549-009-0347-8
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Cao, Y., Giovannucci, E. (2016). Obesity and Prostate Cancer. In: Pischon, T., Nimptsch, K. (eds) Obesity and Cancer. Recent Results in Cancer Research, vol 208. Springer, Cham. https://doi.org/10.1007/978-3-319-42542-9_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-42542-9_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-42540-5
Online ISBN: 978-3-319-42542-9
eBook Packages: MedicineMedicine (R0)