Abstract
Many patients with early stage cancers will go on to develop metastases. Blood based tests for circulating tumor markers can provide an invaluable minimally invasive method for assessing tumor and monitoring patients. Several markers are purported to be available for this purpose. These include some newer biomarkers such as tissue polypeptide antigens and serum autoantibodies against tumor associated antigens. In this chapter, we critically evaluate the available markers and describe their advantages and more importantly their limitations. A thorough review of the data available for these biomarkers leads us to conclude that sufficient evidence exists for the use of CEA, CA15-3, CA27.29 in metastatic breast cancers. However, none of the biomarkers are suitable for routine use in patients with early stage breast cancer. Novel blood based-biomarkers are urgently required to monitor patients with early stage breast cancer and predict the long-term outcomes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
14.1 Introduction
Breast cancer is the most common solid tumor in women, with a lifetime risk of 1 in 8, if a woman lives to age 80. While in general, breast cancer is a very treatable disease, with 75–80 % cure rates, it accounted for an estimated 40,356 deaths in 2014 in the United States alone and approximately 400,000 deaths worldwide (Porter 2008; Kamangar et al. 2006). Improvement in survival rates have been seen over the last few decades and these are largely attributed to earlier detection and improvements in treatment of micro-metastases that have spread to distant organs. These achievements are the result of better screening technologies and a better understanding of the underlying molecular makeup of the disease.
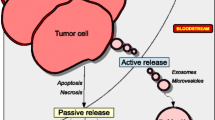
Improvement in treatment of cancer is best achieved when the disease is well understood from a biological perspective. This approach has been successful in breast cancer where tumor tissue ‘biomarkers’ are used to classify the disease. The ‘molecular classification’ allows the identification of relevant targets that are most likely to eradicate micro metastatic disease in early stages of breast cancer. In addition to tissue biomarkers, the disease can be assessed by circulating markers that are typically proteins, nucleic acids and cellular fragments that are shed by the cells and represent the underlying biology of the tumor. The detection of these circulating biomarkers is a significant challenge as they are much less abundant in blood and require special techniques to measure them. While, they represent ‘surrogate markers’ of the tumor tissue, there are additional challenges in distinguishing these markers from those of the host, as many of them are also seen in other conditions such as inflammatory diseases and benign causes of organ dysfunction. Typically, circulating markers from cancer are more abundant than those of normal processes, although the lines become blurry when small amounts of tumor are present in the body. The applications of these markers range from early detection, to diagnosis, to treatment and the following review will focus on these areas, in the context of each marker discussed. The markers included in this chapter are serum or plasma-based markers and nucleic acid and cellular components are discussed elsewhere.
14.2 Traditional Tumor Markers
Several proteins have been classically associated with breast tumors and also detected in the circulation. Traditionally, used tumor markers include carcinoembryonic antigen (CEA ), soluble mucin1 protein or MUC 1 protein (CA27.29 or CA15.3), and the ectodomain (ECD) of the human epidermal growth factor receptor (serum HER2). In addition, autoantibodies that are tumor-specific are detectable in plasma and serum and are thought to be part of the host reaction to the presence of tumor tissue. These glycoproteins are thought to be secreted by tumor cells or the cells in the tumor microenvironment and can be detected in the peripheral blood with immunoassays.
14.2.1 Carcinoembryonic Antigen (CEA )
CEA is a glycoprotein, attached to the membrane by a GPI (glycosyl phosphatidyl inositol) anchor and is involved in adhesion to the extracellular matrix and plays an important role in cancer growth, invasion and metastasis (Blumenthal et al. 2005). It is a normal constituent of mucus that is secreted into the lumen by the glandular epithelial cells. With disruption of the normal tissue architecture, CEA is released into the vascular and lymphatic system. It is thought that the release of CEA into the extracellular matrix is due to GPI anchor cleavage catalysis by GPI specific phospholipase D type enzyme in in vitro experiments, but its mechanism of release is still under study.
CEA has been evaluated as a diagnostic/screening test, a prognostic marker and to monitor breast cancer during therapy. CEA has not been found to be useful for screening at the population level, as it is not sensitive or specific enough to differentiate between benign breast disease and breast cancer (Rimsten et al. 1979).
CEA is more promising in the setting of prognosis, as it is clearly associated with important outcomes and has been found to be reflective of disease burden. In a multivariate analysis, breast cancer patients undergoing surgery with elevated pre-operative levels of CEA had worse prognosis and higher risk of relapse after therapy (Gaglia et al. 1988). In early or localized breast cancer, CEA levels were noted to be similar before and after mastectomy, however, increases in levels after mastectomy are associated with increased recurrence rate (Wang et al. 1975). In patients with metastatic disease, approximately 50–60 % of the patients have elevated CEA levels (Hogan-Ryan et al. 1980; Gray 1984; Tormey and Waalkes 1978; Veronesi et al. 1982). Furthermore, increases in CEA levels have been noted in cancer with metastasis to lymph nodes and distant organs (Laessig et al. 2007). While there is clearly a relationship between the detection and level of CEA and prognosis in early stage patients, there is no evidence that adding or changing therapy alters this prognosis. This concept, ‘clinical utility’, needs to be established in order for the biomarker to be recommended for clinical use. As a result, ASCO 2007 guidelines do not recommend CEA for determining prognosis among breast cancer patients, since clinical utility has not been established (Harris et al. 2007).
Perhaps the most useful setting to date for the use of CEA is in monitoring metastatic disease. Studies have shown that, among advanced breast cancer patients receiving hormonal therapy and chemotherapy, a drop in CEA levels correlates with response to therapy. Tormey et al., found that CEA levels >5 ng/ml pre therapy were associated with poor response or early failure of chemotherapy (Tormey and Waalkes 1978). However, monitoring CEA requirement for clinical utility as it does not alter the prognosis when used for monitoring. Having said that, CEA and other similar markers may aid in therapy decision-making, in conjunction with other features of the clinical scenario. As a result, they are sanctioned by the ASCO Tumor Marker Guidelines Panel 2015 as reasonable adjuncts to physical examination and radiographic tests in the metastatic setting (Van Poznak et al. 2015).
14.2.2 Mucin 1 or MUC1 (CA15.3/CA27.29 )
MUC1, a transmembrane glycoprotein (Fig. 14.1), is involved in oncogenesis by promotion of tyrosine kinase signaling, loss of epithelial cell polarity and constitutive activation of growth and survival pathways (Ren et al. 2006; Rajabi et al. 2014). In breast adenocarcinoma, MUC1 is overexpressed and under glycosylated resulting in loss of architectural demarcation between the apical and basolateral membrane in the cell. The most widely studied biomarkers are the soluble form of MUC1 (CA15.3), and mucin like associated antigen (MCA or CA27.29 ).
MUC1 Molecule. To the right of the figure we have MUC1 molecule, the variant and nonvariant tandem repeat that form the major part of MUC1-N, and its glycosylation in normal and tumor associated mucin. To the left of the figure immunohistochemistry showing the expression of underglycosylated MUC1 in (a) apocrine metaplasia of the breast (b) ductal carcinoma in situ of the breast (c) invasive ductal adenocarcinoma of the breast (d) capillary with tumor embolus from an invasive ductal adenocarcinoma of the breast
Similar to CEA , CA27.29 and CA15.3 have not been found to be adequately sensitive to be used for diagnosis. However, CA15.3 is found in the circulation of 10–15 % of stage I, 20–25 % and 30–45 % in stage II and stage III breast cancers, respectively (Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer. Adopted on May 17, 1996 by the American Society of Clinical Oncology 1996). Its usefulness is limited as marker in early disease but can be used in advanced stages for disease monitoring.
In early stage disease, elevated levels of CA15.3 are associated with worse outcome (McLaughlin et al. 2000; Gion et al. 2002). In addition, the prognostic impact of CA15.3 is independent of the tumor size and lymph node status (Tampellini et al. 2006; Gray 1984). However, as with CEA , there is no evidence that measuring these markers at diagnosis would not influence treatment decisions in a way that affects patient outcomes.
CA15.3 has been used in follow-up of breast cancer patients after the diagnosis and treatment of early stage disease. While measurement of the marker can provide a lead-time of 5–6 months for the detection of recurrent/metastatic disease in some women (Ren et al. 2006; Rajabi et al. 2014), there is no evidence that early intervention based on this lead-time improves outcomes or quality of life (Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer. Adopted on May 17, 1996 by the American Society of Clinical Oncology 1996). This is likely due to the fact that macrometastases that are detected at the time of recurrence are not curable with current treatment strategies and therefore finding these recurrences a few months earlier does not influence overall survival. This underlies the issue of sensitivity of many markers that makes them inadequate to detect micrometastases at a curable stage. As with CEA , the MUC-1, CA15.3 and CA27.29 markers are not recommended for follow-up of early stage patients (Harris et al. 2007).
There have been a number of studies of CA15.3 in the metastatic setting. In an anthracycline based-phase II and III trial, median survival and clinical progression correlated with CA15.3 levels (McLaughlin et al. 2000). However, concordance with disease response was inconsistent. It has also been suggested that CA15.3 is useful for monitoring unevaluable disease such as pleural effusions, ascites, lytic and sclerotic bone disease, which are present in around one third of metastatic patients.
Studies utilizing CA15.3 and CA27.29 for monitoring patients have shown mixed results. (D’Alessandro et al. 2001; De La Lande et al. 2002; Guadagni et al. 2001; Kokko et al. 2002; Nicolini et al. 2006), and there are no randomized prospective clinical trials that determine the clinical utility of monitoring patients with metastatic disease. As a result, ASCO 2015 guidelines state that CA15.3 and CA27.29 should only be used in conjunction with other modalities like history, examination, and imaging to monitor treatment response in patients with metastatic disease (Harris et al. 2007; Van Poznak et al. 2015).
14.2.3 HER2/Neu Oncogene
The HER2 gene is located in chromosome 17q11-12 which encodes for transmembrane receptor protein with tyrosine kinase activity. The overexpression of HER2 protein is detected in 15–30 % of breast cancer patients and has traditionally correlated with poorer outcomes. The extracellular domain (ECD) of HER2 is detectable in the serum and has been proposed as a surrogate for tissue levels of HER2 to predict early relapse or response to therapy. Like the other circulating markers, HER2 also lacks sensitivity and specificity for early detection and is not recommended for use in that setting.
Circulating HER2 levels have been studied in both the early and advanced stage settings of breast cancer and are consistently associated with a worse prognosis (Lee et al. 2016; Leitzel et al. 1995; Yamauchi et al. 1997; Volas et al. 1996). Serum HER2 levels are positively correlated with tumor size, tumor grade, and worse disease free survival in early stage disease (Burstein et al. 2003). In addition, studies have shown that ECD levels are associated with response to trastuzumab and hormonal therapy. In a study done by Ali et al. (2008), data was collected from 307 metastatic breast cancer patients from seven different institutions receiving trastuzumab based therapy. The serum samples were collected at baseline and at 30–120 days after initiation of trastuzumab. Sixty two percent patients had significant decline (>20 %) in serum HER2/neu and thirty eighty percent did not. The response rate was 57 % in patients with decline in serum HER2/neu compared to 28 % who did not. Patients with decline in HER2/neu levels had significantly longer time to disease progression (320 days vs. 180 days; p < 0.001), longer duration of response (369 days vs. 230 days; p < 0.0001) and longer overall survival (898 days vs. 593 days; p < 0.018). Based on this study data, patients with significant decrease in the HER2/neu levels >20 % were known to have decreased benefit from trastuzumab therapy (Ali et al. 2008). Given the complexity of calculating percentage declines and the variability around this number, HER2-ECD is not felt to be a practical measure for clinical use.
HER2-ECD has also been evaluated in metastatic patients in the context of trastuzumab with hormonal therapy. In a randomized controlled trial, patients with elevated HER2-ECD had lower response to letrozole versus tamoxifen. Serial measurement of HER2-ECD levels in these two groups of patients showed that patients with elevated HER2-ECD had overall lower response rates and had no advantage of letrozole over tamoxifen (Lipton et al. 2002). This suggests that this marker might be used to determine which patients are unlikely to respond to the combination of trastuzumab and any hormonal therapy and would be better served by a chemotherapy-based HER therapy combination.
Unfortunately, associations of HER2-ECD with therapy response are confounded by the fact that HER2-ECD levels are associated with increased tumor burden and a decrease in the half-life of trastuzumab antibody due to increase in the binding sites and accelerated clearance of immune complexes. These complex interactions make the use of HER2-ECD impractical and therefore it is not recommended in either the early or advanced disease setting (Harris et al. 2007).
14.3 Tumor Markers in Development: Protein Markers
Although, no biomarker is currently approved for early detection in clinical practice, emerging research on novel biomarkers for diagnosis, prognosis and response to treatment is underway and many promising markers are under development.
14.3.1 Tissue Polypeptide Antigens (TPA)
TPA is a complex polypeptide filament made up of cytokeratin 8, 18 and 19 produced mainly during the late S and G2 phase of the cell cycle. TPA can be elevated in benign conditions like renal failure, liver failure, pregnancy, diabetes mellitus (Tramonti et al. 2000), as well as a number of cancers, limiting its utility as a biomarker for early detection or diagnosis.
In the advanced stage setting, serum TPA levels were shown to be elevated in advanced cancers (stage III and IV) patients, compared to localized breast cancer patients (Al-Youzbaki et al. 2014; Sliwowska et al. 2006). It was also shown that TPA levels are lower in breast cancer patients who received chemotherapy compared to patients who did not suggesting that it is associated with a worse prognosis as the patients who receive chemotherapy tend to have a higher disease burden and worse clinical features (Al-Youzbaki et al. 2014).
Tissue polypeptide–specific antigen (TPS) is a peptide epitope of cytokeratin 18 that can be detected in the serum (Bonfrer et al. 1994; Rydlander et al. 1996; D'Alessandro et al. 2001). As such it is thought to be a more specific serum marker than TPA and has been evaluated in several disease contexts. TPS has been found to be associated with higher tumor grade, and early stage patients with elevated tumor TPS levels have a higher risk of recurrence (O'Hanlon et al. 1996). A number of studies have suggested the utility of TPS as a prognostic marker. There are conflicting results in the literature regarding value of TPS marker in breast cancer (Given et al. 2000). On the contrary, TPS levels are known to be elevated in loco regional recurrence and significantly elevated to greater extent in metastatic diseases predicting different stages of the disease (O'Hanlon et al. 1996). Patients with elevated levels during follow up were likely to experience disease progression on further follow up. When compared to CEA or CA 15-3 , TPS indicates proliferative activity, which is one of the most important phenotypic characteristics of tumor aggressiveness and is thus more beneficial as prognostic marker than serum markers as mentioned earlier (Bodenmuller et al. 1994; Weber et al. 1984; Hwa et al. 2008). Some studies have found that elevated pre-operative levels associated with poor disease free survival (p < 0.001) and low pre-treatment levels correlated with increased survival in advanced breast cancer patients (Ahn et al. 2013).
Several studies have suggested that TPS, particularly when combined with CA15.3, may be more specific and sensitive at predicting the likelihood of recurrence among breast cancer patients. However, larger scale studies and those aimed at clinical utility are needed to confirm these findings and support the recommendation of this marker in early stage disease. Thus, there are no recommendations as per ASCO Tumor Marker Guidelines for use of TPA or TPS in breast cancer (Harris et al. 2007).
TPS is known to be elevated in other inflammatory conditions like liver cirrhosis (van Dalen 1992) and in post-menopausal versus premenopausal women (Given et al. 2000), and is thus not specific enough to be recommended for screening or early detection.
14.3.2 Serum Autoantibodies Against Tumor Associated Antigens (TAA)
The ‘Holy Grail’ of serum tumor markers is to be able to use them for early detection, as this would reduce the need for non-specific radiographic screening of the entire population of women at risk for breast cancer, which currently is thought to be any woman over the age of 40 years. For many years, mammography has been the gold standard for screening that has been proven to have reduced mortality (Brooks 2009), but its sensitivity is reduced in patients with dense breasts (Brooks 2009). In addition, non-specific mammographic screening is thought to lead to over diagnosis and unnecessary treatments (Brooks 2009). Recently, there have been many serum tumor markers introduced like CEA , CA 15.3, estrogen receptor (ER) and progesterone receptor (PR), Circulating tumor cells (CTCs) which have been studied but unfortunately none of which have been approved for screening or early diagnosis of breast cancer due to lack of sensitivity and specificity of these circulating proteins. This prompted the evaluation of other serum markers that could improve these endpoints and led to intensive research on serum autoantibodies against tumor-associated antigens (Fig. 14.2).
ELISA method using antibody microarray to capture tumor associated antigens in the circulating blood. This figure depicts the use of autoantibodies purified from serum, are tagged with fluorescent dye. Native antigens in the circulating blood are bound to antibody on incubated array and result in fluorescent color from antigen antibody reaction
Autoantibodies against p53 (Crawford et al. 1982), HER2 (Disis et al. 1997), MUC1 (von Mensdorff-Pouilly et al. 1996) and NY-ESO-1 (Stockert et al. 1998) were the first to be discovered in breast cancer patients. Studies have showed that serum collected prior to diagnosis, at diagnosis, and during treatment showed that HER2 and p53 autoantibodies were significantly increased in samples from breast cancer patients. Elevated levels of HER2 and p53 autoantibodies can be detected in sera more than 150 days prior to diagnosis in breast cancer patients compared to controls (Lu et al. 2012). Recently, new autoantibodies such as SOX2 were found to be significantly elevated in patients with breast cancer (18.4 %) compared to healthy women (2.6 %) and (6.4 %) in patients with benign breast conditions. SOX2 antibodies were also associated with high tumor grade and positive nodal status. Other autoantibodies including p90/CIP2A show promising results (Sun et al. 2012) and are under investigation.
In patients with breast cancer, only 10–30 % of patients had a humoral response against a specific TAA, thought to be due to heterogeneous nature of the underlying biology (Tan et al. 2009). Looi et al. (2006) showed that p16 antibodies were relatively higher in nasopharyngeal cancer than in breast cancer. To confirm the specificity of p16 antibodies and to increase the frequency of antibody detection, a combination of TAA (p16, p53, and c-myc) was used. Antibodies to this antigen panel were found to be increased in frequency at p < 0.01. The combination of TAAs together increased the positive antibody detection rate to a sensitivity of 44 %. Multiple studies have looked into TAA panels and the focus later has shifted to developing TAA panels to increase the sensitivity and specificity of the test (Fernandez Madrid 2005; Lu et al. 2008). This led to developing of different TAA panels with application of SEREX (autoantibodies like XI-A, p80, S6, RPA32 (Tomkiel et al. 2002) and NY-BR-I (Fernandez-Madrid et al. 2004; Brooks 2009; Levenson 2007; Jager et al. 2001) or SERPA (autoantibodies against RNA-binding protein regulatory subunit (RS), DJ-1 oncogene, glucose 6 phosphate dehydrogenase, heat shock 70-kDa protein-1 (HS71) and dihydrolipoamide dehydrogenase (Le Naour et al. 2001; Fernandez Madrid 2005). The TAA panels also increase the sensitivity and specificity for primary breast cancer and for ductal carcinoma in situ (Chapman et al. 2007) which can help in early diagnosis and can aid along with mammography for screening breast cancer.
Mammography has been shown to decrease the breast cancer mortality rates. The relative risk reduction is only 23 % and has recall rate for additional testing is 5–10 % in whom cancer would be detected. In women undergoing screening mammography, approximately 4 to 9 % have false positive test. There is clinical need for additional tests to aid in diagnosis of breast cancer, particularly in young patients under the age of 50 years in whom mammography is less sensitive (Levenson 2007). Chapman et al. investigated the use of autoantibodies to p53, c-myc, and HER2, NY-ESO-1, BRCA2 and MUC1 antigens by using the enzyme linked immunosorbent assay (Chapman et al. 2007). It was shown that autoantibodies were elevated repeatedly for one of six antigens in 64 % of primary breast cancer patients and 45 % of patients with ductal carcinoma in situ with 85 % specificity. Individual assay specificity for each antigen varied from 91 to 98 %. Hence these autoantibody assay against panel of antigens could be used with mammography for early detection of primary breast cancer especially in young women at risk. Due to heterogeneity of breast cancer and our limited understanding about autoantibodies against TAA, we need more definitive studies before tthey can be used in clinical practice.
14.4 Challenges in Utility of Circulating Tumor Markers
Multiple studies have shown the potential for utilization of circulating biomarkers for the clinical care of breast cancer from screening and diagnosis, to prognosis and treatment monitoring. However, only a few of these markers have successfully transitioned to routine clinical use. This section addresses some of the issues surrounding these challenges.
14.4.1 Cost Effectiveness
Health care costs are continually rising and becoming an increasing concern, particularly in the United States. Adding more tests to the treatment of a patient may increase costs and offers only limited benefits. A SEER-Medicare database analysis from 2001 to 2007 of the early breast cancer survivor patients evaluated the tumor marker tests for CEA , CA 15-3 , CA 27.29 and health care claims through the billing codes and found that 42 % had received these tumor marker test within 2 years of diagnosis and the utilization increased over time from 38 % in 2001 to 46 % in 2007 (Ramsey et al. 2015). They found that the total cost of care for those patients with one test performed was 29 % higher than those not tested, often due to higher rates of advanced imaging (Ramsey et al. 2015). Given the financial constraints of current medical system, it is important to consider the benefit of a test before recommending routine use.
14.4.2 Poor Specificity
Certain tumor markers are also known to be de-regulated in other benign conditions. For example, like CA15.3 is elevated in chronic hepatitis, liver cirrhosis, hypothyroidism, and sarcoidosis. Therefore, the utility of these biomarkers for early detection of breast cancer is low.
Further, paradoxically there can be increase in tumor markers concentration after commencement of chemotherapy possibly secondary to tumor cell necrosis. For example, Hayes et al. reported that there could be a spike in the CEA or CA 15-3 in 7 of 16 patients undergoing chemotherapy (Tondini et al. 1988). Therefore, many biomarkers used for monitoring ›treatment response need to be carefully defined as to when and how they are useful.
14.4.3 Lack of Reproducibility
Unfortunately, many promising new biomarkers that are reported in the literature fail to replicate in subsequent studies. For example, although circulating miRNAs were thought to hold great potential for breast cancer early detection, a review showed that the positive findings from these studies overlapped less than would be expected by chance (Tondini et al. 1988). Many studies of circulating biomarkers are not done in a rigorous manner and are done ad hoc with samples that are readily available. For example, using samples from a case-control design study to analyze a biomarker for early detection. Then, when the biomarker is tested in a sample of screening-eligible women in a prospective manner, the test does not replicate, as it is unclear how levels of these biomarkers change post-biopsy. Research networks, such as the Early Detection Research Network (EDRN) have been developed to help facilitate access to more appropriate samples.
Further, studies are often inconsistent in their protocols for collection and quantification of the biomarker. Some biomarkers, particularly many of these emerging cell-free markers, may be sensitive to time, temperature or processing. It is important for researchers from groups to collaborate and design strong biomarker studies with a number of independent replication sets.
14.5 Conclusion
Future direction towards identifying new tumor markers or new use of old tumor markers are essential. Many early stage breast cancer patients that are being treated surgically for cure, are prone to develop metastatic disease. We have insufficient data to recommend tumor markers like CEA , CA 15-3 and CA27.29 for diagnosis or monitoring of early stage disease but they can be used as adjunctive for monitoring the response to treatment in the metastatic setting. It is important that the clinicians are aware of sensitivities, specificities and limitation of each tumor marker before its use. In the recent past, investigators have focused on identifying new autoantibodies against tumor specific antigens and their role in breast cancer management. Many of these markers are still under study and have shown some promising results. It is crucial that we identify more of these tumor markers and explore their clinical applications. When new markers are identified, it is essential that we address the reliability and clinical utility of each marker. Only in this way, can we make progress in the management of breast cancer and improve outcomes for our patients.
REFERENCES:
Ahn SK, Moon HG, Ko E, Kim HS, Shin HC, Kim J, You JM, Han W, Noh DY (2013) Preoperative serum tissue polypeptide-specific antigen is a valuable prognostic marker in breast cancer. Int J Cancer 132(4):875–881. doi:10.1002/ijc.27727
Al-Youzbaki WB, Al-Youzbaki NB, Telfah MM (2014) Tissue polypeptide antigen & interleukin-6: Are their serum levels a predictor for response to chemotherapy in breast cancer? Pak J Med Sci 30(5):1108–1112. doi:10.12669/pjms.305.5199
Ali SM, Carney WP, Esteva FJ, Fornier M, Harris L, Kostler WJ, Lotz JP, Luftner D, Pichon MF, Lipton A, Serum HERnSG (2008) Serum HER-2/neu and relative resistance to trastuzumab-based therapy in patients with metastatic breast cancer. Cancer 113 (6):1294-1301. doi:10.1002/cncr.23689
Blumenthal RD, Hansen HJ, Goldenberg DM (2005) Inhibition of adhesion, invasion, and metastasis by antibodies targeting CEACAM6 (NCA-90) and CEACAM5 (Carcinoembryonic Antigen). Cancer Res 65(19):8809–8817. doi:10.1158/0008-5472.CAN-05-0420
Bodenmuller H, Ofenloch-Hahnle B, Lane EB, Dessauer A, Bottger V, Donie F (1994) Lung cancer-associated keratin 19 fragments: development and biochemical characterisation of the new serum assay Enzymun-Test CYFRA 21-1. Int J Biol Markers 9(2):75–81
Bonfrer JM, Groeneveld EM, Korse CM, van Dalen A, Oomen LC, Ivanyi D (1994) Monoclonal antibody M3 used in tissue polypeptide-specific antigen assay for the quantification of tissue polypeptide antigen recognizes keratin 18. Tumour Biol 15(4):210–222
Brooks M (2009) Breast cancer screening and biomarkers. Methods Mol Biol 472:307–321. doi:10.1007/978-1-60327-492-0_13
Burstein HJ, Harris LN, Gelman R, Lester SC, Nunes RA, Kaelin CM, Parker LM, Ellisen LW, Kuter I, Gadd MA, Christian RL, Kennedy PR, Borges VF, Bunnell CA, Younger J, Smith BL, Winer EP (2003) Preoperative therapy with trastuzumab and paclitaxel followed by sequential adjuvant doxorubicin/cyclophosphamide for HER2 overexpressing stage II or III breast cancer: a pilot study. J Clin Oncol 21(1):46–53
Chapman C, Murray A, Chakrabarti J, Thorpe A, Woolston C, Sahin U, Barnes A, Robertson J (2007) Autoantibodies in breast cancer: their use as an aid to early diagnosis. Ann Oncol 18(5):868–873. doi:10.1093/annonc/mdm007
Clinical practice guidelines for the use of tumor markers in breast and colorectal cancer. Adopted on May 17, 1996 by the American Society of Clinical Oncology (1996). J Clin Oncol 14 (10):2843-2877
Crawford LV, Pim DC, Bulbrook RD (1982) Detection of antibodies against the cellular protein p53 in sera from patients with breast cancer. Int J Cancer 30(4):403–408
D’Alessandro R, Roselli M, Ferroni P, Mariotti S, Spila A, Aloe S, Carone MD, Abbolito MR, Carlini S, Perri P, Ricciotti A, Botti C, Conti F, Vici P, Chiappetta NR, Cognetti F, Buonomo O, Guadagni F (2001) Serum tissue polypeptide specific antigen (TPS): a complementary tumor marker to CA 15-3 in the management of breast cancer. Breast Cancer Res Treat 68(1):9–19
De La Lande B, Hacene K, Floiras JL, Alatrakchi N, Pichon MF (2002) Prognostic value of CA 15.3 kinetics for metastatic breast cancer. Int J Biol Markers 17(4):231–238
Disis ML, Pupa SM, Gralow JR, Dittadi R, Menard S, Cheever MA (1997) High-titer HER-2/neu protein-specific antibody can be detected in patients with early-stage breast cancer. J Clin Oncol 15(11):3363–3367
Fernandez-Madrid F, Tang N, Alansari H, Granda JL, Tait L, Amirikia KC, Moroianu M, Wang X, Karvonen RL (2004) Autoantibodies to Annexin XI-A and Other Autoantigens in the Diagnosis of Breast Cancer. Cancer Res 64(15):5089–5096. doi:10.1158/0008-5472.CAN-03-0932
Fernandez Madrid F (2005) Autoantibodies in breast cancer sera: candidate biomarkers and reporters of tumorigenesis. Cancer Lett 230(2):187–198. doi:10.1016/j.canlet.2004.12.017
Gaglia P, Caldarola B, Bussone R, Potente F, Lauro D, Jayme A, Caldarola L (1988) Prognostic value of CEA and ferritin assay in breast cancer: a multivariate analysis. Eur J Cancer Clin Oncol 24(7):1151–1155
Gion M, Boracchi P, Dittadi R, Biganzoli E, Peloso L, Mione R, Gatti C, Paccagnella A, Marubini E (2002) Prognostic role of serum CA15.3 in 362 node-negative breast cancers. An old player for a new game. Eur J Cancer 38(9):1181–1188
Given M, Scott M, Mc Grath JP, Given HF (2000) The predictive of tumour markers CA 15-3, TPS and CEA in breast cancer recurrence. Breast 9(5):277–280. doi:10.1054/brst.1999.0154
Gray BN (1984) Value of CEA in breast cancer. Aust N Z J Surg 54(1):1–2
Guadagni F, Ferroni P, Carlini S, Mariotti S, Spila A, Aloe S, D’Alessandro R, Carone MD, Cicchetti A, Ricciotti A, Venturo I, Perri P, Di Filippo F, Cognetti F, Botti C, Roselli M (2001) A re-evaluation of carcinoembryonic antigen (CEA) as a serum marker for breast cancer: a prospective longitudinal study. Clin Cancer Res 7(8):2357–2362
Harris L, Fritsche H, Mennel R, Norton L, Ravdin P, Taube S, Somerfield MR, Hayes DF, Bast RC, Jr., American Society of Clinical O (2007) American Society of Clinical Oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol 25(33):5287–5312. doi:10.1200/JCO.2007.14.2364
Hogan-Ryan A, Fennelly JJ, Jones M, Cantwell B, Duffy MJ (1980) Serum sialic acid and CEA concentrations in human breast cancer. Br J Cancer 41(4):587–592
Hwa HL, Kuo WH, Chang LY, Wang MY, Tung TH, Chang KJ, Hsieh FJ (2008) Prediction of breast cancer and lymph node metastatic status with tumour markers using logistic regression models. J Eval Clin Pract 14(2):275–280. doi:10.1111/j.1365-2753.2007.00849.x
Jager D, Stockert E, Gure AO, Scanlan MJ, Karbach J, Jager E, Knuth A, Old LJ, Chen YT (2001) Identification of a tissue-specific putative transcription factor in breast tissue by serological screening of a breast cancer library. Cancer Res 61(5):2055–2061
Kamangar F, Dores GM, Anderson WF (2006) Patterns of cancer incidence, mortality, and prevalence across five continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 24(14):2137–2150. doi:10.1200/JCO.2005.05.2308
Kokko R, Holli K, Hakama M (2002) Ca 15-3 in the follow-up of localised breast cancer: a prospective study. Eur J Cancer 38(9):1189–1193
Laessig D, Nagel D, Heinemann V, Untch M, Kahlert S, Bauerfeind I, Stieber P (2007) Importance of CEA and CA 15-3 during disease progression in metastatic breast cancer patients. Anticancer Res 27(4A):1963–1968
Le Naour F, Misek DE, Krause MC, Deneux L, Giordano TJ, Scholl S, Hanash SM (2001) Proteomics-based identification of RS/DJ-1 as a novel circulating tumor antigen in breast cancer. Clin Cancer Res 7(11):3328–3335
Lee MH, Jung SY, Kang SH, Song EJ, Park IH, Kong SY, Kwon YM, Lee KS, Kang HS, Lee ES (2016) The Significance of Serum HER2 Levels at Diagnosis on Intrinsic Subtype-Specific Outcome of Operable Breast Cancer Patients. PLoS One 11(10):e0163370. doi:10.1371/journal.pone.0163370
Leitzel K, Teramoto Y, Konrad K, Chinchilli VM, Volas G, Grossberg H, Harvey H, Demers L, Lipton A (1995) Elevated serum c-erbB-2 antigen levels and decreased response to hormone therapy of breast cancer. J Clin Oncol 13(5):1129–1135
Levenson VV (2007) Biomarkers for early detection of breast cancer: what, when, and where? Biochim Biophys Acta 1770(6):847–856. doi:10.1016/j.bbagen.2007.01.017
Lipton A, Ali SM, Leitzel K, Demers L, Chinchilli V, Engle L, Harvey HA, Brady C, Nalin CM, Dugan M, Carney W, Allard J (2002) Elevated serum Her-2/neu level predicts decreased response to hormone therapy in metastatic breast cancer. J Clin Oncol 20(6):1467–1472
Looi K, Megliorino R, Shi FD, Peng XX, Chen Y, Zhang JY (2006) Humoral immune response to p16, a cyclin-dependent kinase inhibitor in human malignancies. Oncol Rep 16(5):1105–1110
Lu H, Goodell V, Disis ML (2008) Humoral immunity directed against tumor-associated antigens as potential biomarkers for the early diagnosis of cancer. J Proteome Res 7(4):1388–1394. doi:10.1021/pr700818f
Lu H, Ladd J, Feng Z, Wu M, Goodell V, Pitteri SJ, Li CI, Prentice R, Hanash SM, Disis ML (2012) Evaluation of known oncoantibodies, HER2, p53, and cyclin B1, in prediagnostic breast cancer sera. Cancer Prev Res (Phila) 5(8):1036–1043. doi:10.1158/1940-6207.CAPR-11-0558
McLaughlin R, McGrath J, Grimes H, Given HF (2000) The prognostic value of the tumor marker CA 15-3 at initial diagnosis of patients with breast cancer. Int J Biol Markers 15(4):340–342
Nicolini A, Tartarelli G, Carpi A, Metelli MR, Ferrari P, Anselmi L, Conte M, Berti P, Miccoli P (2006) Intensive post-operative follow-up of breast cancer patients with tumour markers: CEA, TPA or CA15.3 vs MCA and MCA-CA15.3 vs CEA-TPA-CA15.3 panel in the early detection of distant metastases. BMC Cancer 6:269. doi:10.1186/1471-2407-6-269
O’Hanlon DM, Kerin MJ, O’Boyle C, Grimes H, Given HF (1996) Tissue polypeptide specific antigen (TPS) in breast cancer–an initial evaluation. Eur J Surg Oncol 22(1):38–41
Porter P (2008) “Westernizing” women’s risks? Breast cancer in lower-income countries. N Engl J Med 358(3):213–216. doi:10.1056/NEJMp0708307
Rajabi H, Alam M, Takahashi H, Kharbanda A, Guha M, Ahmad R, Kufe D (2014) MUC1-C oncoprotein activates the ZEB1/miR-200c regulatory loop and epithelial-mesenchymal transition. Oncogene 33(13):1680–1689. doi:10.1038/onc.2013.114
Ramsey SD, Henry NL, Gralow JR, Mirick DK, Barlow W, Etzioni R, Mummy D, Thariani R, Veenstra DL (2015) Tumor marker usage and medical care costs among older early-stage breast cancer survivors. J Clin Oncol 33(2):149–155. doi:10.1200/JCO.2014.55.5409
Ren J, Bharti A, Raina D, Chen W, Ahmad R, Kufe D (2006) MUC1 oncoprotein is targeted to mitochondria by heregulin-induced activation of c-Src and the molecular chaperone HSP90. Oncogene 25(1):20–31. doi:10.1038/sj.onc.1209012
Rimsten A, Adami HO, Wahren B, Nordin B (1979) Carcinoembryonic antigen in serum of unselected breast-cancer patients and of non-hospitalized controls. Br J Cancer 39(2):109–115
Rydlander L, Ziegler E, Bergman T, Schoberl E, Steiner G, Bergman AC, Zetterberg A, Marberger M, Bjorklund P, Skern T, Einarsson R, Jornvall H (1996) Molecular characterization of a tissue-polypeptide-specific-antigen epitope and its relationship to human cytokeratin 18. Eur J Biochem 241(2):309–314
Sliwowska I, Kopczynski Z, Grodecka-Gazdecka S (2006) Diagnostic value of measuring serum CA 15-3, TPA, and TPS in women with breast cancer. Postepy Hig Med Dosw (Online) 60:295–299
Stockert E, Jager E, Chen YT, Scanlan MJ, Gout I, Karbach J, Arand M, Knuth A, Old LJ (1998) A survey of the humoral immune response of cancer patients to a panel of human tumor antigens. J Exp Med 187(8):1349–1354
Sun Y, Zhang R, Wang M, Zhang Y, Qi J, Li J (2012) SOX2 autoantibodies as noninvasive serum biomarker for breast carcinoma. Cancer Epidemiol Biomarkers Prev 21(11):2043–2047. doi:10.1158/1055-9965.EPI-12-0498
Tampellini M, Berruti A, Bitossi R, Gorzegno G, Alabiso I, Bottini A, Farris A, Donadio M, Sarobba MG, Manzin E, Durando A, Defabiani E, De Matteis A, Ardine M, Castiglione F, Danese S, Bertone E, Alabiso O, Massobrio M, Dogliotti L (2006) Prognostic significance of changes in CA 15-3 serum levels during chemotherapy in metastatic breast cancer patients. Breast Cancer Res Treat 98(3):241–248. doi:10.1007/s10549-005-9155-y
Tan HT, Low J, Lim SG, Chung MC (2009) Serum autoantibodies as biomarkers for early cancer detection. FEBS J 276(23):6880–6904. doi:10.1111/j.1742-4658.2009.07396.x
Tomkiel JE, Alansari H, Tang N, Virgin JB, Yang X, VandeVord P, Karvonen RL, Granda JL, Kraut MJ, Ensley JF, Fernandez-Madrid F (2002) Autoimmunity to the M(r) 32,000 subunit of replication protein A in breast cancer. Clin Cancer Res 8(3):752–758
Tondini C, Hayes DF, Gelman R, Henderson IC, Kufe DW (1988) Comparison of CA15-3 and carcinoembryonic antigen in monitoring the clinical course of patients with metastatic breast cancer. Cancer Res 48(14):4107–4112
Tormey DC, Waalkes TP (1978) Clinical correlation between CEA and breast cancer. Cancer 42(3 Suppl):1507–1511
Tramonti G, Ferdeghini M, Donadio C, Norpoth M, Annichiarico C, Bianchi R, Bianchi C (2000) Renal function and serum concentration of five tumor markers (TATI, SCC, CYFRA 21-1, TPA, and TPS) in patients without evidence of neoplasia. Cancer Detect Prev 24(1):86–90
van Dalen A (1992) TPS in breast cancer–a comparative study with carcinoembryonic antigen and CA 15–3. Tumour Biol 13(1–2):10–17
Van Poznak C, Somerfield MR, Bast RC, Cristofanilli M, Goetz MP, Gonzalez-Angulo AM, Hicks DG, Hill EG, Liu MC, Lucas W, Mayer IA, Mennel RG, Symmans WF, Hayes DF, Harris LN (2015) Use of Biomarkers to Guide Decisions on Systemic Therapy for Women With Metastatic Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 33(24):2695–2704. doi:10.1200/JCO.2015.61.1459
Veronesi A, Talamini R, Longhi S, Crivellari D, Galligioni E, Tirelli U, Trovo MG, Magri MD, Frustaci S, Figoli F, Zagonel V, Tumolo S, Grigoletto E (1982) Carcinoembryonic antigen (CEA) in the follow-up of disease-free breast cancer patients. Tumori 68(6):477–480
Volas GH, Leitzel K, Teramoto Y, Grossberg H, Demers L, Lipton A (1996) Serial serum c-erbB-2 levels in patients with breast carcinoma. Cancer 78(2):267–272. doi:10.1002/(SICI)1097-0142(19960715)78:2<267:AID-CNCR12>3.0.CO;2-U
von Mensdorff-Pouilly S, Gourevitch MM, Kenemans P, Verstraeten AA, Litvinov SV, van Kamp GJ, Meijer S, Vermorken J, Hilgers J (1996) Humoral immune response to polymorphic epithelial mucin (MUC-1) in patients with benign and malignant breast tumours. Eur J Cancer 32A(8):1325–1331
Wang DY, Bulbrook RA, Hayward JL, Hendrick JC, Franchimont P (1975) Relationship between plasma carcinoembryonic antigen and prognosis in women with breast cancer. Eur J Cancer 11(9):615–618
Weber K, Osborn M, Moll R, Wiklund B, Luning B (1984) Tissue polypeptide antigen (TPA) is related to the non-epidermal keratins 8, 18 and 19 typical of simple and non-squamous epithelia: re-evaluation of a human tumor marker. EMBO J 3(11):2707–2714
Yamauchi H, O’Neill A, Gelman R, Carney W, Tenney DY, Hosch S, Hayes DF (1997) Prediction of response to antiestrogen therapy in advanced breast cancer patients by pretreatment circulating levels of extracellular domain of the HER-2/c-neu protein. J Clin Oncol 15(7):2518–2525
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Prabhakar, D., Harris, L. (2016). Circulating Tumor Markers for Breast Cancer Management. In: Badve, S., Gökmen-Polar, Y. (eds) Molecular Pathology of Breast Cancer. Springer, Cham. https://doi.org/10.1007/978-3-319-41761-5_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-41761-5_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-41759-2
Online ISBN: 978-3-319-41761-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)