Abstract
Breast cancer is the most common malignancy among women. Most of breast cancer patients are diagnosed in early stages and will be treated with curative intent. Despite this, some patients will relapse. The identification of patients at high risk remains an important challenge. CTCs can be useful to identify this patients, to assess tumor dynamics and to monitoring therapy. There is definitive evidence on the prognostic role of CTCs in early breast cancer (eBC) but its clinical utility in daily practice is still lacking. We have to take into consideration that the studies published to date mainly evaluated the presence of CTC based on the expression of epithelial surface markers. Future studies need to overcome this limitation and important advances in technical methods can assess CTCs and capture the heterogeneity of the tumor landscape. It is also tempting to speculate that CTCs may also provide complementary information on the interplay of tumor cells with the immune system. The combination of different methods to detect tumoral disease by liquid biopsy may provide new ways to personalize in an unprecedented manner the management of patients with eBC.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Breast cancer is the most common malignancy among women, accounting for 2,088,849 of new cancer diagnoses (11.6% of total cancer burden) worldwide [1]. Thanks to important advances in screening and prevention strategies, most of breast cancer patients are diagnosed early and can thus be offered treatment with curative intent [2]. Despite this, some patients will relapse with metastatic spread of the disease months, years or decades after the treatment of the primary tumor [3]. The identification of patients at high risk of relapse remains an important challenge in the field. Here, we review the rationale for studying Circulating Tumor Cells (CTCs) in patients with early breast cancer (eBC) and discuss their potential clinical applications.
Potential clinical applications of CTCs in eBC include: (1) identifying patients at risk of relapse; (2) assessing tumor dynamics to characterize the tumor evolution; (3) monitoring therapy efficacy; (4) identifying potential biomarkers for personalized therapy development [4].
9.2 CTCs Biology and Molecular Characterization in Early Breast Cancer
At the time of initial diagnosis, disseminated tumor cells (DTC) can be detected in the bone marrow in 30% of operable breast cancer patients that lack any clinical or histopathological signs of metastasis. Nevertheless DTCs require invasive methods for their detection and its clinical potential is therefore very limited. According to several studies the concordance between CTC and DTC ranged 66–94% [5].
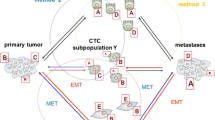
CTCs are tumor cells that depart, actively or passively, from the primary tumor or from a metastatic site. Even during early stages of cancer, tumor cells can disseminate into the circulation at an estimated rate of 106 cells per gram of primary tumor per day [6]. In blood circulation, CTCs can travel as single cells, cell clusters or apoptotic bodies [7], and have the ability to disseminate to distant localizations, where they can adapt and survive [8] (Fig. 9.1).
CTCs depart from primary tumor and enter in blood circulation. CTCs may undergo phenotypic changes to acquire a survival advantage. In the bloodstream, CTCs can travel as single cells or as cell clusters together with platelets, neutrophils, and/or other immune cells increasing their metastatic potential
CTCs are a heterogeneous cell population, constituted mainly by differentiated tumor cells but also harboring sub-populations of cells with resistance, self-renewal and/or tumor-initiating capabilities, otherwise known as cancer stem-like cells (CSCs), which may present important phenotypic differences with regard to the main CTC population [9,10,11].
Furthermore, the functional capabilities of CTCs may vary depending on the disease context. In eBC, for example, mitotic CTCs are very rare, while most of the CTCs of metastatic breast cancer patients actively divide and can be identified at all stages of mitosis [12,13,14].
It is estimated that only 0.1% of single CTCs survive more than 24 h in the bloodstream (their half time ranging from 1 to 3 h), and that less than 0.01% of these cells have the ability to produce metastases [4, 15, 16]. These cells must acquire phenotypic changes (e.g. epithelial mesenchymal transition or EMT) that provide a survival advantage in the bloodstream as well as in foreign tissues [9, 17, 18].
Another important biological aspect to consider with regard to the biological behavior of CTCs is that in the bloodstream, they can be present as single cells or form clusters with other blood cells or endothelial cells, forming aggregates with each other and with blood cells through cytoskeletal protrusions supported by α-tubulin (TUB), vimentin (VIM) and Detyrosinated α-tubulin (GLU) [19]. Recently it has been described the importance of Plakoglobin [20] as adhesion molecule to maintain this aggregation of cells that allow them to be protected from the action of immune system, keeping the aggregation of cells and conferring important advantage to survive in bloodstream and to arrive to the metastatic niche [7]. Clustered cells have 23–50-fold increased metastatic potential compared with single cells. The study of the cluster circulating tumor cells, is an important point of research. In this scenario, an elegant study recently published by Gkountela et al. reveal in preclinical models a different pattern of methylation between CTCs and Clusters, identifying specifics hypomethylated binding sites for OCT4, SOX2, NANOG, and SIN3A that promote stemness and metastatic dissemination [21, 22].
EMT allows CTCs to survive in blood circulation inducing the loss of both cell junctions and cell polarity, enabling cell motility and assisting CTCs during intravasation into the bloodstream [23]. This process is extremely complex and involves different molecular pathways, ultimately yielding a survival advantage.
CTCs in blood circulation, can interact with all the elements of immune system and platelets. Interestingly, platelets had a main role in metastatic spread in breast cancer [24]. In preclinical models with cell lines, researchers have shown the adhesion of platelets to CTCs surface. This interaction prevents the recognition of CTCs by the immune system. Other important interaction between CTCs and platelets is related with transforming growth factor beta (TGFβ) pathway. This signaling pathway, promoted by platelets, assists a process of epithelial to mesenchymal transition [25]. This cell to cell communication confers a survival advantage and promotes the metastatic spread.
The prevalence of detected CTCs in the bloodstream, as expected, is higher in metastatic than in localized breast cancer [26, 27]. An important limitation to use CTCs in clinical practice is, at least as yet, the difficulty to detect them. In eBC, CTC prevalence increases with disease stage, ranging between 10% and 30% in different studies across all stages [28] (Table 9.1).
There are some methods to isolate CTCs by size or by identification of cell surface markers. The FDA-approved CellSearch® system [36,37,38,39] is a platform commonly used for the isolation and enrichment of CTCs in breast cancer to identify the presence of CTCs in bloodstream. This platform is based on the positive selection of CTCs by expression of the epithelial cell adhesion molecule (EpCAM) surface maker. However, as mentioned previously a mechanism that could (at least partially) explain the metastatic process and CTC dissemination is the phenotypic change of epithelial to mesenchymal and the loss of epithelial surface proteins. Therefore, we have to be aware that CellSearch® method excludes the CTCs that lack EpCAM, resulting in the underestimation of mesenchymal-like CTCs that have lost their epithelial features. Recently, a work in primary breast cancer published by Mego et al. revealed a different behavior of CTCs accordingly to the expression of epithelial or mesenchymal surface proteins [40]. This fact strongly suggests the necessity to incorporate new methods to identify the various subpopulation of CTCs from clinical samples. To overcome this limitation, some researchers describe new methods to detect CTCs independently of epithelial biomarkers, as using nucleases as CTCs biomarkers. Previous studies provided information about the elevated amplification of these enzymes in cancer patients regardless its mesenchymal or epithelial phenotype. Kruspe et al. described this novel method to detect these more aggressive cells, concluding that this approach was promising to examine CTC levels in early diagnosis [12].
One of the characteristics of CTCs as mentioned above, is their important heterogeneity, like the differences between epithelial versus mesenchymal phenotypes; but we also have to take into account the heterogeneity of the various breast cancer subtypes (based on hormone receptors and HER2 [41]). CTCs can be isolated and molecularly profiled to evaluate important clinical biomarkers to monitor disease and help guide therapy. In this setting, Riethdorf et al. assessed CTCs by CellSearch® in patients with non-metastatic breast cancer enrolled into the GeparQuattro phase III neoadjuvant trial [42]. Two hundred and thirteen patients were included in the analysis, and 21% had CTCs before neoadjuvant treatment and 10.6% after neoadjuvant treatment. HER2-overexpressing CTCs were observed in 24.1% of CTCs positive patients and was restricted to ductal carcinoma and associated with high tumor stage.
In the same line, Ignatiadis et al. conducted a study with the aim to identify CTCs assessed by CellSeach® method, and HER2-positive CTCs in breast cancer patients [43]. According to experiments performed in cell lines, HER2-positive CTCs were defined by a population of CTCs with HER2 immunofluorescence intensity that was at least 2.5 times higher than the background. The study showed that 4.1% of patients with ductal/lobular carcinoma in situ had at least 1 HER2–positive CTC, 7.3% in eBC and 39.5% in metastatic breast cancer. No CTCs HER2 positive were detected in 42 women without breast cancer. In this line of research, Ligthart et al. in a prospective study evaluating HER2 CTCs in adjuvant and metastatic patients, defined CTCs HER2 positivity as overexpression in 3.5 times higher than the CD45 immunofluorescence intensity in 75% of CTCs in patients with ≥5 CTCs. Using this cut-off, 9% of M1 patients that were HER2 negative had HER2-positive CTC status and conversely 29% with HER2 positive primary had negative HER2 CTCs [44, 45], showing the heterogeneity of tumor cells presents in blood circulation.
Krishnamurthy et al. evaluated HER2-positive CTCs by FISH from 88 patients with breast cancer stages I–IV [46]. Cells with a ratio of HER2:CEP17 > 2 in any CK+/CD45 or CK-/CD45 cell was regarded as positive for HER2 gene amplification. CTCs were detected in 27.3% of patients and HER2-positive CTCs in 11.1%. Among patients with a HER2-negative primary tumor, 6.3% had CTCs-HER2. The overall rate of discordance in HER2 status was 15% between primary tumor and CTCs.
ER expression in CTCs has been less extensively studied, however in eBC, only approximated 25% of CTCs are ER positive, despite most primary tumors being ER positive. However, the lack of a validated assay for determining ER-positivity in CTCs and a lack of larger studies examining ER CTC expression limits the clinical utility of this finding [47].
Taken together, these findings suggest potential clinical implications for evaluating molecular markers in CTCs in breast cancer patients.
9.3 Prognostic Studies
The main body of evidence published related to CTCs is related to its capacity to provide prognostic information. Here we review the most relevant studies related to prognostic information in eBC according to the use of systemic chemotherapy or not. However, CTCs are not yet routinely used in clinical practice as a prognostic marker due to the lack of definitive studies showing clinical utility in terms of helping to safely select those patients who will benefit from adjuvant therapy.
9.3.1 Prognostic Studies of CTCs in Patients Who Did not Receive Adjuvant Chemotherapy
CTCs have been reported as an independent poor prognostic factor in eBC. European groups firstly showed the prognostic impact of disseminated tumor cells (DTC) in the bone marrow of breast cancer patients [48]; Molloy et al. evaluated CTCs and DTCs at primary surgery in 733 stage I or II breast cancer patients. CTCs were detected in 7.9% of patients, while DTCs were found in 11.7%. Both CTC and DTC positivity independently predicted poor outcomes: metastasis-free survival (MFS) and breast cancer-specific survival (BCSS) [5].
In 2012 Lucci et al. conducted a clinical trial with the aim of identifying CTCs by CellSearch® system and their association with prognosis in eBC. They prospectively collected blood samples in patients chemo-naive, with eBC. They found ≥1 CTCs in 24% of patients. The detection of one or more CTCs identified a subset of patients with worse prognostic, both decreased progression-free survival and overall survival [32]. As it will be mentioned below, the prognostic significance of CTCs not only is qualitative but quantitative, so that patients with rising numbers of CTCs had poor outcomes.
9.3.2 Prognostic Studies of CTCs in Patients Who Received Chemotherapy for Early Breast Cancer
The study conducted by Rack et al. assayed CTCs by CellSearch® system in 2026 patients with eBC before adjuvant chemotherapy and in 1496 patients after adjuvant chemotherapy [33]. The rates of detection of CTCs were similar in patients receiving adjuvant or neoadjuvant chemotherapy (in order to 21.5–22%). The presence of CTCs was an independent poor prognostic factor and was associated with poor disease-free survival, poor distant disease-free survival (DFS), breast cancer-specific survival (BCSS), and overall survival (OS). The group of patients with at least five CTCs had significantly worse outcomes (DFS: HR = 4.51, 95% CI = 2.59 to 7.86; OS: HR = 3.60, 95% CI = 1.56 to 8.45). In this trial, the authors found that the patients with persisting CTCs before and after chemotherapy treatment had worse outcomes compared with the other subgroups in terms of DFS, and an important negative prognostic effect in the presence of CTCs previously systemic treatment [49].
In the neoadjuvant setting, Pierga et al. investigated the presence of CTC in pre and post neoadjuvant blood samples in 118 non-metastatic breast cancer patients [30, 50]. They found a significantly decreased DFS and OS in patients with ≥1CTC. Similar findings in the neoadjuvant setting were found by Riethdorf et al. in patients enrolled in GeparQuattro trial [42]. The Beverly study, included 137 patients with inflammatory breast cancer (IBC) candidates to neoadjuvant treatment [51, 52]. The study analyzed the possible benefit of incorporated bevacizumab to standard chemotherapy and trastuzumab in the neoadjuvant scenario. Prior to neoadjuvant chemotherapy, 39% of patients had detectable CTCs. The detection of CTCs after four cycles of chemotherapy decreased from 39% to 9%. The authors found that the presence of CTCs at baseline was associated with shorter 3-year DFS (39% versus 70%, P < 0.01, HR 2.80) and shorter 3-year OS (P < 0.01) compared with the patients with undetected CTCs [52]. The pooled analysis including Beverly 1 and Beverly 2, suggests that the combination of pathological complete response (pCR) and CTCs detection could be a potential tool to identify a subgroup with better outcomes after neoadjuvant treatment: the subgroup of patients that achieved a pCR and undetected CTCs had an excellent OS (94% 3-year OS) [50]. The authors suggested that the prognosis of IBC relies on the achievement of pCR and highlighted the role of early hematogenous tumor dissemination as assessed by CTCs. Combining these two prognostic factors they reported a subgroup of IBC with excellent survival when treated with bevacizumab and trastuzumab-containing regimens.
9.3.3 Pooled Analysis of the Prognostic Value of CTCs in Early Breast Cancer
Janni et al. published a pooled analysis including 3173 patients with stage I–III breast cancer [34]. A total of 58% of patients included had nodal involvement and 42 had high-grade tumor. In this series, only 8.2% patients received neoadjuvant treatment and 79.9% received adjuvant treatment, including hormonal therapy and radiotherapy according to guidelines. The presence of CTCs was assessed by CellSearch® at time of primary diagnosis. The prevalence of CTCs was 20% and the presence of ≥1 CTCs was an independent negative prognostic factor for DFS [HR, 1.82; 95% confidence interval (CI), 1.47–2.26], distant DFS (HR, 1.89; 95% CI, 1.49–2.40), BCSS (HR, 2.04; 95% CI, 1.52–2.75), and overall survival. The presence of CTCs was correlated with large size, high histological grade and nodal involvement. In a subgroup analysis, CTCs were not able to provide prognostic information in very low risk patients (T1 N0) and in hormone receptor negative, HER2 positive breast cancer subtype, probably by the small sample size in the last subgroup.
A Meta-analysis was published in 2018 by Bidard et al. Data from 2185 patients from EEUU, Japan and European countries were included. Blood samples from patients were collected before neoadjuvant treatment (n = 1574) and before surgery (n = 1200) and presence of CTCs was assessed by CellSearch® system. One or more CTC were detected in 25.2% of patients before neoadjuvant chemotherapy. The presence of CTCs was associated with tumor size. In concordance with previous studies mentioned above, the number of CTCs detected had a detrimental impact in OS, DFS and locorregional relapse-free interval, although not correlated with pCR [35]. The higher number of CTCs detected before neoadjuvant chemotherapy was associated with the HR of death.
9.4 Other Prognostic Studies
9.4.1 CTCs and Late Recurrences in Early Breast Cancer
Recently, a trial published by Sparano et al. provided evidence of an association of CTCs and late recurrence in hormone receptor positive HER2 negative breast cancer [53]. Analysis of CTCs were assessed by CellSearch® system in 547 patients without clinical evidence of recurrence between 4.5 and 7.5 years after primary treatment of stage II–III breast cancer. Only 5% of patients had detectable CTCs in blood circulation. They found a 12.5 risk-fold increased risk of recurrence in patients with CTCs compared to patients with undetected CTCs detected. An interesting finding was that the patients with CTCs were still receiving hormonal therapy and 4.4% had clinical recurrence, and these were predominantly in HR+ breast cancer. The detection of CTCs was observed 2.8 years prior to clinical recurrence. This provided for the first time evidence on the potential value of CTC detection during patient follow up and late clinical recurrence.
9.4.2 CTCs with EMT Phenotype and Prognosis
As mentioned above, circulating tumors cells are a heterogeneous population of cells including CTCs with partial or complete EMT phenotype. The prognostic value of CTCs has been demonstrated for epithelial CTCs. However, a subset of primary breast cancer patients shows EMT and stem cell characteristics [54]. EMT phenotype in breast cancer have been shown to be prognostically unfavorable, but the prognostic value of CTCs with EMT is poorly known in eBC and the currently used detection methods for CTC are not efficient to identify a subtype of CTC which underwent EMT. An interesting work published recently by Mego et al. identify a subset of CTCs with more aggressive behavior and patients with an inferior outcome in this setting [40]. In this work the authors evaluated the expression of EMT transcription factors (TWIST1, SANAIL1, SLUG and ZEB1) by PCR in real time. The patients with EMT-CTCs had inferior outcomes compared with patients without detectable CTC EMT. In this work the presence of CTC EMT was associated with p53 status and after a median of follow-up of 55 months, patients with CTC EMT in the peripheral blood had significantly poor DFS. This prognostic value was demonstrated in all subgroups and was most pronounced in the hormone receptor positive, HER2 negative subgroup independently of the adjuvant treatment administrated. Despite of the small sample size of the study, it provides for the first time evidence fo the prognostic value of CTCs with an EMT phenotype in eBC. The poor prognostic associated to EMT features of CTCs is in line with observations reported in primary breast cancer tissue. Along this line, Creighton et al. reported that the residual breast tumor tissue cell populations surviving after letrozole or docetaxel treatment were enriched for subpopulations of cells with both tumor-initiating and mesenchymal features, which may explain resistance to antihormonal and conventional chemotherapeutic drugs [55].
9.4.3 Dynamic Evolution of CTC During (Neo)adjuvant Treatment
Muller et al. analyzed patients with primary breast cancer at stage M0. They found that 8.3% of patients had CTCs after surgery and before initiation of adjuvant chemotherapy. During the course of adjuvant chemotherapy, repeated analysis of 20 M(0) patients revealed the occurrence of CTCs in 7 of 16 patients that were initially negative [29].
Pachmann et al. analyzed the presence of CTCs to monitor residual disease during adjuvant treatment with the aim to detect patients early who are at risk of relapse [56]. They analyzed serially the presence of CTC by epithelial surface markers in 91 non-metastatic primary breast cancer patients by an EpCAM-based laser scanning cytometric approach. Patients with initial reduction in CTC number followed by a significant increase (>10 fold compared with the nadir (lowest value) were the subgroup with the highest risk of subsequent relapse. Kwan et al. have developed a novel breast cancer CTC-specific assay, selecting 17 transcripts strongly expressed in breast derived tissue but absent in blood cells [57]. They tested its clinical utility monitoring response in high-risk breast cancer patients receiving neoadjuvant therapy. In 52 patients with localized breast cancer, the increase in a CTC-score after three cycles of neoadjuvant therapy was associated with residual disease at surgery. This study suggests a novel CTC assay to monitor response to neoadjuvant chemotherapy. Further research is needed to further study its potential clinical implications.
9.5 Clinical Trials Based on CTCs
The presence of CTCs in eBC has been the bases for a few clinical trials (Table 9.2). Georgoulias et al. conducted a randomized phase II trial in patients with non-metastatic breast cancer with detectable CTCs before and after adjuvant chemotherapy based on an anthracycline regimen [58]. CK19 mRNA-positive CTCs were detected by RT-PCR and double stained CK(+)/HER2(+) cells by immunofluorescence. A total of 378 patients (310 HER2-negative and 68 with HER2-positive eBC) were treated with adjuvant chemotherapy and 148 (39%) patients had detectable CK19 mRNA-positive CTCs before any adjuvant systemic treatment. The patients with persistence of CTCs (26%) at the end of adjuvant chemotherapy were randomized to receive trastuzumab or observation. Fifty-one (89%) of the 57 analyzed patients had HER2-expressing CTCs. In HER2-negative breast cancer patients, after trastuzumab administration, 27 of 36 (75%) women became CK19 mRNA-negative compared to seven of 39 (17.9%) in the observation arm. In that study, the median DFS was significantly higher for the trastuzumab-treated patients. This result suggested that the administration of trastuzumab may eliminate chemotherapy-resistant CK19 mRNA positive CTCs and improve patient’s outcome.
However, these results were not confirmed by a phase II trial conducted by Ignatiadis et al. that included 95 HER2 negative eBC patients with CTCs detected by CellSearch® after completing neoadjuvant chemotherapy and surgery [59]. These patients were randomized to receive trastuzumab or no treatment. The aim of the study was the eradication of CTCs at week 18 in the experimental arm. In 23.8% of the patients there was at least one HER2-positive CTC (6 patients in the trastuzumab arm and 9 in the observational arm). Fifty-eight patients were assessable for the primary end point, 29 in each arm. In 9 of the 58 patients, CTC(s) were still detected at week 18; 5 in the trastuzumab and 4 in the observation arm. The study was stopped by Independent Data Monitoring Committee recommendation for futility for the primary end point, concluding that the use of trastuzumab according to detection of CTC was of no benefit in terms of DFS or OS. Further research is needed in this area.
Related to radiotherapy, Goodman et al. recently published a potential relation between CTC and benefit of radiotherapy in patients with eBC that undergo conserving surgery [60]. The study included patients enrolled in SUCCESS trial, and patients from NCDB. CTCs detection ranged from 19% to 24% and DFS and OS were related with the presence of CTCs. The patients that received radiotherapy after conservative surgery had longer local recurrence-free survival (LRFS), DFS and OS if at least 1 CTC was detected compared to patients that did not receive radiation therapy. In contrast, in patients with undetected CTCs, the addition of radiation therapy did not significantly improve patient outcomes. This clinical trial was the first study that incorporated CTCs as a predictive biomarker of radiation therapy in non-metastatic breast cancer and these results suggest CTCs as a potential tool to identify patients that potentially benefit of radiation. Based on these promising results, additional studies appear warranted.
There are additional registered ongoing studies of CTCs in eBC that will shed light on their potential utility in the upcoming years. A selection of these studies is presented in Table 9.2. Furthermore, CTCs may become a target per se in eBC, particularly CTC clusters, albeit current technology still does not allow their reliable detection in a sufficient proportion of eBC patients. This view is based on an interesting preclinical study suggesting the use of drugs that could segregate and prevent clusters formation in blood in metastatic setting [21].
9.6 Conclusions and Future Directions
There is definitive evidence on the prognostic role of CTCs in eBC but its clinical utility in daily practice is still lacking. However, the challenge is to develop biomarkers for prediction rather than prognosis. Clinical trials based on CTCs have provide promising, but not robust, responses to the potential use of CTC-guided therapy in eBC. Therefore further efforts should be directed at deepening the molecular characterization of CTCs and, importantly, to the design of clinical trials that could exploit the unique information that CTCs may reveal. It is also tempting to speculate that CTCs may also provide complementary information on the interplay of tumor cells with the immune system.
We have to take into consideration that the studies published to date mainly evaluated the presence of CTC based on the expression of epithelial surface markers. Future studies need to overcome this limitation and, hence, be based on methods that can detect CTCs with mesenchymal phenotype or detect CTCs based on their physical properties. There are also new methods to assess CTCs that can capture the heterogeneity of the tumor landscape and this may prove valuable in the future. In addition, the information of CTC enumeration and biological features should be combined with the emerging data provided by ctDNA. It is entirely reasonable to believe that the combination of CTCs and ctDNA testing may provide new ways to personalize in an unprecedented manner the management of patients with eBC.
References
Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. https://doi.org/10.3322/caac.21492.
Moschetti I, Cinquini M, Lambertini M, Levaggi A, Liberati A. Follow-up strategies for women treated for early breast cancer. Cochrane Database Syst Rev. 2016;5:CD001768. https://doi.org/10.1002/14651858.CD001768.pub3.
Voduc KD, Cheang MC, Tyldesley S, Gelmon K, Nielsen TO, Kennecke H. Breast cancer subtypes and the risk of local and regional relapse. J Clin Oncol. 2010;28(10):1684–91. https://doi.org/10.1200/JCO.2009.24.9284.
Braun M, Markiewicz A, Kordek R, Sadej R, Romanska H. Profiling of invasive breast carcinoma circulating tumour cells-are we ready for the ‘liquid’ revolution? Cancers (Basel). 2019;11(2) https://doi.org/10.3390/cancers11020143.
Molloy TJ, Bosma AJ, Baumbusch LO, Synnestvedt M, Borgen E, Russnes HG, et al. The prognostic significance of tumour cell detection in the peripheral blood versus the bone marrow in 733 early-stage breast cancer patients. Breast Cancer Res. 2011;13(3):R61. https://doi.org/10.1186/bcr2898.
Castle J, Shaker H, Morris K, Tugwood JD, Kirwan CC. The significance of circulating tumour cells in breast cancer: a review. Breast. 2014;23(5):552–60. https://doi.org/10.1016/j.breast.2014.07.002.
Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, et al. Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell. 2014;158(5):1110–22. https://doi.org/10.1016/j.cell.2014.07.013.
Bednarz-Knoll N, Alix-Panabieres C, Pantel K. Clinical relevance and biology of circulating tumor cells. Breast Cancer Res. 2011;13(6):228. https://doi.org/10.1186/bcr2940.
Yu M, Bardia A, Wittner BS, Stott SL, Smas ME, Ting DT, et al. Circulating breast tumor cells exhibit dynamic changes in epithelial and mesenchymal composition. Science. 2013;339(6119):580–4. https://doi.org/10.1126/science.1228522.
Micalizzi DS, Maheswaran S, Haber DA. A conduit to metastasis: circulating tumor cell biology. Genes Dev. 2017;31(18):1827–40. https://doi.org/10.1101/gad.305805.117.
Celia-Terrassa T, Kang Y. Distinctive properties of metastasis-initiating cells. Genes Dev. 2016;30(8):892–908. https://doi.org/10.1101/gad.277681.116.
Kruspe S, Dickey DD, Urak KT, Blanco GN, Miller MJ, Clark KC, et al. Rapid and sensitive detection of breast cancer cells in patient blood with nuclease-activated probe technology. Mol Ther Nucleic Acids. 2017;8:542–57. https://doi.org/10.1016/j.omtn.2017.08.004.
Adams DL, Adams DK, Stefansson S, Haudenschild C, Martin SS, Charpentier M, et al. Mitosis in circulating tumor cells stratifies highly aggressive breast carcinomas. Breast Cancer Res. 2016;18(1):44. https://doi.org/10.1186/s13058-016-0706-4.
Serrano MJ, Nadal R, Lorente JA, Salido M, Rodriguez R, Rodriguez M, et al. Circulating cancer cells in division in an early breast cancer patient. Ann Oncol. 2011;9:2150–1.
Hedley BD, Chambers AF. Tumor dormancy and metastasis. Adv Cancer Res. 2009;102:67–101. https://doi.org/10.1016/S0065-230X(09)02003-X.
Gomis RR, Gawrzak S. Tumor cell dormancy. Mol Oncol. 2017;11(1):62–78. https://doi.org/10.1016/j.molonc.2016.09.009.
Battula VL, Evans KW, Hollier BG, Shi Y, Marini FC, Ayyanan A, et al. Epithelial-mesenchymal transition-derived cells exhibit multilineage differentiation potential similar to mesenchymal stem cells. Stem Cells. 2010;28(8):1435–45. https://doi.org/10.1002/stem.467.
Pantel K, Speicher MR. The biology of circulating tumor cells. Oncogene. 2016;35(10):1216–24. https://doi.org/10.1038/onc.2015.192.
Kallergi G, Aggouraki D, Zacharopoulou N, Stournaras C, Georgoulias V, Martin SS. Evaluation of alpha-tubulin, detyrosinated alpha-tubulin, and vimentin in CTCs: identification of the interaction between CTCs and blood cells through cytoskeletal elements. Breast Cancer Res. 2018;20(1):67. https://doi.org/10.1186/s13058-018-0993-z.
Holen I, Whitworth J, Nutter F, Evans A, Brown HK, Lefley DV, et al. Loss of plakoglobin promotes decreased cell-cell contact, increased invasion, and breast cancer cell dissemination in vivo. Breast Cancer Res. 2012;14(3):R86. https://doi.org/10.1186/bcr3201.
Gkountela S, Castro-Giner F, Szczerba BM, Vetter M, Landin J, Scherrer R, et al. Circulating tumor cell clustering shapes DNA methylation to enable metastasis seeding. Cell. 2019;176(1–2):98–112e14. https://doi.org/10.1016/j.cell.2018.11.046.
Maltoni R, Gallerani G, Fici P, Rocca A, Fabbri F. CTCs in early breast cancer: a path worth taking. Cancer Lett. 2016;376(2):205–10. https://doi.org/10.1016/j.canlet.2016.03.051.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell. 2008;133(4):704–15. https://doi.org/10.1016/j.cell.2008.03.027.
Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells – mechanisms of immune surveillance and escape. Nat Rev Clin Oncol. 2017;14(3):155–67. https://doi.org/10.1038/nrclinonc.2016.144.
Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–90. https://doi.org/10.1016/j.ccr.2011.09.009.
Allard WJ, Matera J, Miller MC, Repollet M, Connelly MC, Rao C, et al. Tumor cells circulate in the peripheral blood of all major carcinomas but not in healthy subjects or patients with nonmalignant diseases. Clin Cancer Res. 2004;10(20):6897–904. https://doi.org/10.1158/1078-0432.CCR-04-0378.
Meng S, Tripathy D, Frenkel EP, Shete S, Naftalis EZ, Huth JF, et al. Circulating tumor cells in patients with breast cancer dormancy. Clin Cancer Res. 2004;10(24):8152–62. https://doi.org/10.1158/1078-0432.CCR-04-1110.
Cristofanilli M, Budd GT, Ellis MJ, Stopeck A, Matera J, Miller MC, et al. Circulating tumor cells, disease progression, and survival in metastatic breast cancer. N Engl J Med. 2004;351(8):781–91. https://doi.org/10.1056/NEJMoa040766.
Muller V, Stahmann N, Riethdorf S, Rau T, Zabel T, Goetz A, et al. Circulating tumor cells in breast cancer: correlation to bone marrow micrometastases, heterogeneous response to systemic therapy and low proliferative activity. Clin Cancer Res. 2005;11(10):3678–85. https://doi.org/10.1158/1078-0432.CCR-04-2469.
Pierga JY, Bidard FC, Mathiot C, Brain E, Delaloge S, Giachetti S, et al. Circulating tumor cell detection predicts early metastatic relapse after neoadjuvant chemotherapy in large operable and locally advanced breast cancer in a phase II randomized trial. Clin Cancer Res. 2008;14(21):7004–10. https://doi.org/10.1158/1078-0432.CCR-08-0030.
Bidard FC, Mathiot C, Delaloge S, Brain E, Giachetti S, de Cremoux P, et al. Single circulating tumor cell detection and overall survival in nonmetastatic breast cancer. Ann Oncol. 2010;21(4):729–33. https://doi.org/10.1093/annonc/mdp391.
Lucci A, Hall CS, Lodhi AK, Bhattacharyya A, Anderson AE, Xiao L, et al. Circulating tumour cells in non-metastatic breast cancer: a prospective study. Lancet Oncol. 2012;13(7):688–95. https://doi.org/10.1016/S1470-2045(12)70209-7.
Rack B, Schindlbeck C, Juckstock J, Andergassen U, Hepp P, Zwingers T, et al. Circulating tumor cells predict survival in early average-to-high risk breast cancer patients. J Natl Cancer Inst. 2014;106(5) https://doi.org/10.1093/jnci/dju066.
Janni WJ, Rack B, Terstappen LW, Pierga JY, Taran FA, Fehm T, et al. Pooled analysis of the prognostic relevance of circulating tumor cells in primary breast cancer. Clin Cancer Res. 2016;22(10):2583–93. https://doi.org/10.1158/1078-0432.CCR-15-1603.
Bidard FC, Michiels S, Riethdorf S, Mueller V, Esserman LJ, Lucci A, et al. Circulating tumor cells in breast cancer patients treated by neoadjuvant chemotherapy: a meta-analysis. J Natl Cancer Inst. 2018;110(6):560–7. https://doi.org/10.1093/jnci/djy018.
Riethdorf S, Fritsche H, Muller V, Rau T, Schindlbeck C, Rack B, et al. Detection of circulating tumor cells in peripheral blood of patients with metastatic breast cancer: a validation study of the CellSearch system. Clin Cancer Res. 2007;13(3):920–8. https://doi.org/10.1158/1078-0432.CCR-06-1695.
Kraan J, Sleijfer S, Strijbos MH, Ignatiadis M, Peeters D, Pierga JY, et al. External quality assurance of circulating tumor cell enumeration using the CellSearch((R)) system: a feasibility study. Cytometry B Clin Cytom. 2011;80(2):112–8. https://doi.org/10.1002/cyto.b.20573.
Went PT, Lugli A, Meier S, Bundi M, Mirlacher M, Sauter G, et al. Frequent EpCam protein expression in human carcinomas. Hum Pathol. 2004;35(1):122–8. https://doi.org/10.1016/j.humpath.2003.08.026.
Peeters DJ, De Laere B, Van den Eynden GG, Van Laere SJ, Rothe F, Ignatiadis M, et al. Semiautomated isolation and molecular characterisation of single or highly purified tumour cells from CellSearch enriched blood samples using dielectrophoretic cell sorting. Br J Cancer. 2013;108(6):1358–67. https://doi.org/10.1038/bjc.2013.92.
Mego M, Karaba M, Minarik G, Benca J, Silvia J, Sedlackova T, et al. Circulating tumor cells with epithelial-to-mesenchymal transition phenotypes associated with inferior outcomes in primary breast cancer. Anticancer Res. 2019;39(4):1829–37. https://doi.org/10.21873/anticanres.13290.
Jordan NV, Bardia A, Wittner BS, Benes C, Ligorio M, Zheng Y, et al. HER2 expression identifies dynamic functional states within circulating breast cancer cells. Nature. 2016;537(7618):102–6. https://doi.org/10.1038/nature19328.
Riethdorf S, Muller V, Loibl S, Nekljudova V, Weber K, Huober J, et al. Prognostic impact of circulating tumor cells for breast cancer patients treated in the neoadjuvant “Geparquattro” trial. Clin Cancer Res. 2017;23(18):5384–93. https://doi.org/10.1158/1078-0432.CCR-17-0255.
Ignatiadis M, Rothe F, Chaboteaux C, Durbecq V, Rouas G, Criscitiello C, et al. HER2-positive circulating tumor cells in breast cancer. PLoS One. 2011;6(1):e15624. https://doi.org/10.1371/journal.pone.0015624.
Chen W, Zhang J, Huang L, Chen L, Zhou Y, Tang D, et al. Detection of HER2-positive circulating tumor cells using the LiquidBiopsy system in breast cancer. Clin Breast Cancer. 2019;19(1):e239–e46. https://doi.org/10.1016/j.clbc.2018.10.009.
Ligthart ST, Bidard FC, Decraene C, Bachelot T, Delaloge S, Brain E, et al. Unbiased quantitative assessment of Her-2 expression of circulating tumor cells in patients with metastatic and non-metastatic breast cancer. Ann Oncol. 2013;24(5):1231–8. https://doi.org/10.1093/annonc/mds625.
Krishnamurthy S, Bischoff F, Ann Mayer J, Wong K, Pham T, Kuerer H, et al. Discordance in HER2 gene amplification in circulating and disseminated tumor cells in patients with operable breast cancer. Cancer Med. 2013;2(2):226–33. https://doi.org/10.1002/cam4.70.
Aktas B, Kasimir-Bauer S, Muller V, Janni W, Fehm T, Wallwiener D, et al. Comparison of the HER2, estrogen and progesterone receptor expression profile of primary tumor, metastases and circulating tumor cells in metastatic breast cancer patients. BMC Cancer. 2016;16:522. https://doi.org/10.1186/s12885-016-2587-4.
Alix-Panabieres C. EPISPOT assay: detection of viable DTCs/CTCs in solid tumor patients. Recent Results Cancer Res. 2012;195:69–76. https://doi.org/10.1007/978-3-642-28160-0_6.
Trapp E, Janni W, Schindlbeck C, Juckstock J, Andergassen U, de Gregorio A, et al. Presence of circulating tumor cells in high-risk early breast cancer during follow-up and prognosis. J Natl Cancer Inst. 2019;111(4):380–7. https://doi.org/10.1093/jnci/djy152.
Pierga JY, Bidard FC, Autret A, Petit T, Andre F, Dalenc F, et al. Circulating tumour cells and pathological complete response: independent prognostic factors in inflammatory breast cancer in a pooled analysis of two multicentre phase II trials (BEVERLY-1 and -2) of neoadjuvant chemotherapy combined with bevacizumab. Ann Oncol. 2017;28(1):103–9. https://doi.org/10.1093/annonc/mdw535.
Bertucci F, Fekih M, Autret A, Petit T, Dalenc F, Levy C, et al. Bevacizumab plus neoadjuvant chemotherapy in patients with HER2-negative inflammatory breast cancer (BEVERLY-1): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2016;17(5):600–11. https://doi.org/10.1016/S1470-2045(16)00011-5.
Pierga JY, Petit T, Levy C, Ferrero JM, Campone M, Gligorov J, et al. Pathological response and circulating tumor cell count identifies treated HER2+ inflammatory breast cancer patients with excellent prognosis: BEVERLY-2 survival data. Clin Cancer Res. 2015;21(6):1298–304. https://doi.org/10.1158/1078-0432.CCR-14-1705.
Sparano J, O’Neill A, Alpaugh K, Wolff AC, Northfelt DW, Dang CT, et al. Association of circulating tumor cells with late recurrence of estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol. 2018;4(12):1700–6. https://doi.org/10.1001/jamaoncol.2018.2574.
Kasimir-Bauer S, Hoffmann O, Wallwiener D, Kimmig R, Fehm T. Expression of stem cell and epithelial-mesenchymal transition markers in primary breast cancer patients with circulating tumor cells. Breast Cancer Res. 2012;14(1):R15. https://doi.org/10.1186/bcr3099.
Creighton CJ, Li X, Landis M, Dixon JM, Neumeister VM, Sjolund A, et al. Residual breast cancers after conventional therapy display mesenchymal as well as tumor-initiating features. Proc Natl Acad Sci USA. 2009;106(33):13820–5. https://doi.org/10.1073/pnas.0905718106.
Pachmann K, Camara O, Kavallaris A, Krauspe S, Malarski N, Gajda M, et al. Monitoring the response of circulating epithelial tumor cells to adjuvant chemotherapy in breast cancer allows detection of patients at risk of early relapse. J Clin Oncol. 2008;26(8):1208–15. https://doi.org/10.1200/JCO.2007.13.6523.
Kwan TT, Bardia A, Spring LM, Giobbie-Hurder A, Kalinich M, Dubash T, et al. A digital RNA signature of circulating tumor cells predicting early therapeutic response in localized and metastatic breast cancer. Cancer Discov. 2018;8(10):1286–99. https://doi.org/10.1158/2159-8290.CD-18-0432.
Georgoulias V, Bozionelou V, Agelaki S, Perraki M, Apostolaki S, Kallergi G, et al. Trastuzumab decreases the incidence of clinical relapses in patients with early breast cancer presenting chemotherapy-resistant CK-19mRNA-positive circulating tumor cells: results of a randomized phase II study. Ann Oncol. 2012;23(7):1744–50. https://doi.org/10.1093/annonc/mds020.
Ignatiadis M, Litiere S, Rothe F, Riethdorf S, Proudhon C, Fehm T, et al. Trastuzumab versus observation for HER2 nonamplified early breast cancer with circulating tumor cells (EORTC 90091-10093, BIG 1-12, Treat CTC): a randomized phase II trial. Ann Oncol. 2018;29(8):1777–83. https://doi.org/10.1093/annonc/mdy211.
Goodman CR, Seagle BL, Friedl TWP, Rack B, Lato K, Fink V, et al. Association of circulating tumor cell status with benefit of radiotherapy and survival in early-stage breast cancer. JAMA Oncol. 2018;4(8):e180163. https://doi.org/10.1001/jamaoncol.2018.0163.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this chapter
Cite this chapter
Martos, T., Casadevall, D., Albanell, J. (2020). Circulating Tumor Cells: Applications for Early Breast Cancer. In: Piñeiro, R. (eds) Circulating Tumor Cells in Breast Cancer Metastatic Disease. Advances in Experimental Medicine and Biology, vol 1220. Springer, Cham. https://doi.org/10.1007/978-3-030-35805-1_9
Download citation
DOI: https://doi.org/10.1007/978-3-030-35805-1_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-35804-4
Online ISBN: 978-3-030-35805-1
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)