Abstract
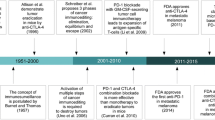
Breaking tolerance represents a major paradigm shift that marks the beginning of a new era in immunotherapy. The impact of the first checkpoint inhibitors, i.e., anti-CTLA-4 (cytotoxic T lymphocyte antigen-4) and anti-PD1/anti-PDL1 (programmed death-1 receptor and its ligand PD-L1) is unprecedented. In advanced melanoma, response rates are about 12 % for anti-CTLA-4 and about 40 % for anti-PD1. Most importantly, these responses are remarkably durable and have a clear impact on survival. In melanoma anti-CTLA4 (ipilimumab) was approved in 2011 and the anti-PD1 molecules pembrolimumab and nivolumab in 2014. The combination of ipilimumab and nivolumab has further significantly improved response rates and impact of progression-free survival (PFS) in stage IV disease. Various combinations with checkpoint inhibitors with agonists, cytokines, and vaccines will be explored to improve results in the coming years. Ipilimumab has already been evaluated in the adjuvant setting (EORTC 18071) and was shown to significantly improve recurrence-free survival in stage III patients at high risk of relapse. An adjuvant trial to evaluate pembrolizumab in this population (EORTC 1325) is scheduled to start in the first quarter of 2015 as is the case for nivolumab in patients with resected stage IIIB/C-IV disease.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
17.1 The New Paradigm: Breaking Tolerance Is the Prerequisite
Advances in melanoma therapies are at present mainly in the field of immunotherapy and mutation-driven drug development (Eggermont et al. 2014). Breaking tolerance represents a major paradigm shift and the impact of the first checkpoint inhibitors, i.e. anti-CTLA-4 (cytotoxic T lymphocyte antigen-4) and anti-PD1/anti-PDL1 (programmed death-1 receptor and its ligand PD-L1) is unprecedented (Pardoll 2012). In only 5 years, advanced melanoma has been transformed from an incurable disease into a curable disease (Eggermont et al. 2013; Robert et al. 2013). Breaking tolerance has a transversal impact throughout solid tumor oncology.
17.2 Anti-CTLA4
17.2.1 Ipilimumab in the Therapeutic Setting of Advanced Melanoma
Monoclonal antibody blocking of cytotoxic T lymphocyte antigen 4 (CTLA-4) leads to breaking immune tolerance and can induce tumor regressions. In 2011, the fully humanized monoclonal anti-CTLA4 antibody ipilimumab was approved in the USA in first- and second- line for patients with advanced melanoma and in second line in Europe at a dose of 3 mg/kg. The approval was based on randomized controlled trial (RCT) results that showed that ipilimumab alone or combined with a peptide vaccination provided a significant survival benefit of about 33 % compared to vaccination alone (Hodi et al. 2010). In another RCT, but in first-line, ipilimumab at 10 mg/kg combined with dacarbazine provided only a small, albeit statistically significant, benefit over treatment with dacarbazine alone, but there seems no reason to advocate the use of this combination (Robert et al. 2011). Mature data in thousands of patients indicate that about 20 % of patients treated with ipilimumab have the potential to survive for at least 3 years and up to 10 years from treatment initiation (Schadendorf et al. 2015). Also the efficacy in patients with brain metastases has been established and reported (Margolin et al. 2012). Ipilimumab responses can occur after the initial tumor progression or the appearance of new lesions. For this reason, immune-related response criteria (irRC) have been developed to avoid premature treatment cessation (Wolchok et al. 2009; Hoos et al. 2010).
Adverse events (AE) occur in about 40 % of patients and are mostly immune-related (irAE), such as skin rashes, colitis, hepatitis, and hypophysitis. Grades 3–4 adverse events occur in about 20 % of patients and can, in rare cases, be fatal. Usually, they resolve spontaneously or after steroid therapy. Endocrinopathies behave differently and pituitary–adrenal axis failure usually requires permanent hormonal substitution. High-dose steroids are indicated for severe irAEs, but other immunosuppressive agents, like anti-TNF-alpha antibodies may also be needed, especially in the context of severe colitis (Weber et al. 2012).
Good biomarkers for response to ipilimumab therapy still remain to be established. Immune-related adverse events, an increase in lymphocyte counts, an increase in eosinophil counts, the presence of NY-ESO-1 antigen, and the resistance in vitro to T-regulatory cell functions seem to be associated with higher response rates (Attia et al. 2005; Ku et al. 2010; Delyon et al. 2013; Ménard et al. 2008). Recently, the high levels of soluble CD25 in the serum, especially in combination with high levels of LDH, were demonstrated to be a very strong prognostic factor for poor outcome (Hannani et al. 2015).
Even the optimal dose and schedule for ipilimumab remain to be established. A randomized phase II trial comparing 0.3 mg/kg, 3 mg/kg, and 10 mg/kg suggested 10 mg/kg to be the more effective dose, but associated it with more toxicity (Wolchok et al. 2010). The results of the RCT comparing 3 mg/kg versus 10 mg/kg are not yet mature. The value of four thrice-weekly administrations (induction) compared to induction followed by further administrations (maintenance) has not been established.
17.2.2 Ipilimumab in the Adjuvant Setting of Resected Stage III Melanoma
The results of a double-blind placebo-controlled adjuvant trial EORTC18071 in stage III patients at high risk for relapse were recently published (Eggermont et al. 2015). In 951 patients with high-risk stage III disease (palpable nodal disease or sentinel node positive disease with metastases >1 mm in diameter according to the Rotterdam Criteria (van Akkooi et al. 2008; van der Ploeg et al. 2011, 2014)), ipilimumab was dosed at 10 mg/kg and administered every 3 weeks over the first 12 weeks (induction) and thereafter every 12 weeks for up to 3 years or relapse. A significant impact on RFS (HR 0.75, p = 0.0013) for the ITT population was reported. Most patients came off treatment after four to five administrations of ipilimumab. The potential value of maintenance therapy will therefore remain unanswered. irAEs were consistent with what has been observed in advanced melanoma trials, but at a higher frequency, especially regarding endocrinopathies. Post hoc analyses demonstrated a significant impact both in patients with sentinel node-positive disease and palpable node-positive disease. Similar to EORTC adjuvant trials 18952 and 18991 with IFN and pegylated-IFN, patients with sentinel-positive disease derived a greater benefit (Eggermont et al. 2005, 2008, 2012a). Patients with an ulcerated primary derived the greatest benefit like in the meta-analysis of the IFN trials 18952 and 18991, indicating that ulcerated melanoma is a separate biologic entity (Eggermont et al. 2012b, c). In contrast, however, to the experience in the adjuvant IFN trials EORTC 18952 and 18991, patients with non-ulcerated melanomas also derived a benefit in the adjuvant ipilimumab setting (van Akkooi et al. 2008). This is in contrast to the total lack of benefit in IFN trials, which has also recently been confirmed in the individual patient data (IPD) meta-analysis of all adjuvant IFN versus observation trials (Suciu et al. 2014).
17.2.3 Combination Therapies with Ipilimumab
Various combinations of ipilimumab with other immune-modulating, anti-angiogenic or chemotherapeutic, or targeted agents have been reported or are ongoing. Guiding principles for combination treatment designs could be to use drugs that lead to immunogenic cell death (Kroemer et al. 2013; Vacchelli et al. 2014a; Galluzzi et al. 2012; Zitvogel et al. 2013). Since radiotherapy can also induce immunogenic cell death, the reported observation of abscopal antitumor effects after radiotherapy and ipilimumab has led to a number of clinical studies to further investigate this phenomenon (Postow et al. 2012).
17.2.3.1 Chemotherapy
Three studies regarding the combination of chemotherapy with ipilimumab in melanoma patients have been published thus far.
-
1.
Dacarbazine: A phase III trial comparing DTIC versus DTIC plus ipilimumab at 10 mg/kg in first-line in patients with advanced melanoma showed a survival benefit for patients treated with the combination (Robert et al. 2011). The median benefit of only 2.1 months was, however, disappointing and the combination is not believed to bring a benefit over ipilimumab alone.
-
2.
Fotemustine: In an open-label, single-arm phase II trial, 86 patients with advanced melanoma, 20 of them with asymptomatic brain metastases, received induction treatment of 10 mg/kg intravenous ipilimumab every 3 weeks for a total of four doses, and 100 mg/m2 intravenous fotemustine weekly for 3 weeks and then every 3 weeks from week 9 to week 24 (Di Giacomo et al. 2012). Patients with a confirmed clinical response were eligible for maintenance treatment from week 24, with ipilimumab every 12 weeks and fotemustine every 3 weeks. Forty patients (46.5 %) in the study population achieved disease control, as did 10 patients with brain metastases (50 %). Toxicity was considerable with 47 patients (55 %) having grade 3 or 4 treatment-related adverse events.
-
3.
Carboplatin/taxol: Very preliminary results of a randomized phase II trial comparing concurrent carboplatin plus paclitaxel and ipilimumab (four doses at 3 mg/kg) with sequential treatment of these agents were reported recently (Jamal et al. 2014). In 31 patients, response rates (RR) and disease control rates (DCR) for 14 evaluable patients at 24 weeks were 21.4 % and 42.9 % by mWHO, and 35.7 % and 64.3 % by irRC, respectively. Grades 3 to 4 AEs were observed in 63 % of patients.
17.2.3.2 Antiangiogenic Agents
Bevacizumab
Four dosing cohorts of ipilimumab (3 or 10 mg/kg) with four doses at 3-week intervals and then every 12 weeks, and bevacizumab (7.5 or 15 mg/kg) every 3 weeks, were studied in 46 patients with metastatic melanoma (Hodi et al. 2014a). There were 8 PRs and 22 SDs, and a disease control rate of 67.4 %. Median survival was 25.1 months. Extensive CD8(+) and macrophage cell infiltration were observed in on-treatment tumor biopsies. From this initial experience, it appears that the combination of bevacizumab and ipilimumab can be safely administered. VEGF-A blockade influences inflammation, lymphocyte trafficking, and immune regulation that should be studied further.
17.2.3.3 Cytokines
-
1.
Interleukin-2 (IL-2): The most mature data on the combination of IL-2 and ipilimumab regard 36 patients treated at the NCI Surgery Branch (Prieto et al. 2012). There were six complete responders (17 %), which was higher than the 6 % CR rate in 56 patients treated with ipilimumab alone and the 7 % CR rate among 85 patients who received ipilimumab with gp100 peptide vaccination. All CRs except one were ongoing at 54+ to 99+ months at the time of the report. The combination with IL-2 did not seem to increase toxicity. The combination with IL-2 should be explored further.
-
2.
Interferon-alpha (IFN): The first phase II trial report on the combination of IFN was a study with the anti-CTLA4 drug tremelimumab (Tarhini et al. 2012). In this study, 37 stage IV melanoma patients received tremelimumab 15 mg/kg/course (three cycles [one cycle = 4 weeks]) intravenously every 12 weeks. High-dose interferon alfa-2b (HDI) was administered concurrently, at 20 MU/m2/day i.v. for 5 days/week for 4 weeks followed by 10 MU/m2/day s.c. three times a week for 8 weeks per course. In 35 evaluable patients, overall response rate was 24 % (four CRs and five PRs), 38 % SD, with a median progression-free survival of 6.4 months and a median overall survival of 21 months. These results seemed to indicate additive antitumor activity.
-
3.
Pegylated-IFN: In 31 patients, ipilimumab (3 mg/kg for four doses) was administered in combination with peg-interferon alfa-2b at 3 mcg/kg weekly for up to 156 weeks (Kudchadkar et al. 2014). Among 26 evaluable patients, there were two CRs, nine PRs, three SDs, and twelve PDs. Peg-interferon alfa-2b added to ipilimumab resulted in a response rate of 42.3 % and was well tolerated except for a high grade 3 rash rate of 20 %. The combination warrants further exploration.
-
4.
GM-CSF: In a randomized phase II trial, conducted by ECOG in 245 patients with unresectable stage III/IV melanoma, ipilimumab plus GM-CSF (sargramostim) treatment was compared with ipilimumab alone (Hodi et al. 2014b). Patients received ipilimumab at 10 mg/kg, intravenously on day 1 plus sargramostim, 250 μg subcutaneously, on days 1–14 of a 21-day cycle versus ipilimumab alone. Ipilimumab treatment included induction for four cycles followed by maintenance every fourth cycle. At a rather short median follow-up of 13.3 months, overall survival was superior for the combination treatment (17.5 months versus 12.7 months), the 1-year survival rates were 68.9 % versus 52.9 %. Surprisingly, no differences for PFS were observed (median PFS of 3.1 months for both treatment arms). The combination treatment was associated with less toxicity. Further studies are needed to elucidate these observations, which is true for all combinations with cytokines (Vacchelli et al. 2014b).
17.2.3.4 Vaccines
-
1.
gp100 vaccines: Theoretically, a combination of a vaccine with anti-CTLA4 is very attractive. Yet the results from the RCT comparing ipilimumab versus ipilimumab plus gp100 vaccine versus gp100 vaccination alone did not show a benefit for the combination of ipilimumab plus the vaccine compared to ipilimumab alone (Hodi et al. 2010) and similar observations were made with the mature results of the NCI Surgery Branch experience (Prieto et al. 2012).
-
2.
Laherparepvec (T-VEC): The first combination study of ipilimumab with the vaccine laherparepvec (T-VEC) was reported at the 2014 ASCO annual meeting (Puzanov et al. 2014a). In 17 patients, the response rate was 41 % (24 % CR, 18 % PR); and 35 % had SD. Median time to response was 2.9 months. No DLTs were reported. Grade 3/4 AEs occurred in 32 %, with only two patients having irAEs at grades 3/4. These very preliminary results seem promising, but more mature data are awaited.
17.2.4 BRAF and MEK Inhibitors
Combinations of BRAF inhibitors and MEK inhibitors with immune checkpoint inhibitors such as anti-CTLA are theoretically attractive, but have, in practice, proven to be not so simple to develop.
-
1.
Vemurafenib: A phase I trial combining vemurafenib and ipilimumab was stopped early, after only 11 patients, because of several cases of grades 3–4 hepatitis (Ribas et al. 2013).
-
2.
Dabrafenib + Trametenib: A phase I trial with dabrafenib + ipilimumab did not evoke a high rate of hepatitis, and an expansion cohort is ongoing (Puzanov et al. 2014b). However, the combination of dabrafenib + trametenib + ipilimumab phase I study was stopped because of life-threatening colitis in three of the first seven patients (Puzanov et al. 2014b).
17.2.5 Anti-PD1 and Anti-PDL1
PD1 protein is another immune checkpoint expressed in many tumor-infiltrating lymphocytes in response to inflammation. It has two ligands, PD-L1 (B7-H1) and PD-L2 (B7-DC). The engagement of PD1 on the lymphocyte surface by PD-L1 on melanoma cells delivers inhibitory signals down-regulating T-cell function (Topalian et al. 2012a). Remarkable results of phase I trials evaluating two anti-PD1 antibodies (nivolumab and pembrolizumab) reported response rates of 30 % (Topalian et al. 2012b; Robert et al. 2014). Anti-PD-L1 antibody also gave an encouraging long-term response rate of 17.3 % in melanoma patients in a phase I study (Brahmer et al. 2012). Importantly, the safety profile is very favorable compared to ipilimumab, with much lower rates of irAEs, in particular the troublesome colitis and hypophysitis. Both pembrolizumab and nivolumab have been reported to induce response rates around 30 % in advanced melanoma patients, even in patients that previously failed ipilimumab (Hamid et al. 2013; Topalian and Sznol 2014). Responses tend to be very durable, up to 2 years. Moreover, PDL-1 expression in the tumor is a good biomarker for response for monotherapy with either agent. Nivolumab proved to be vastly superior dacarbazine in first-line in a RCT in 418 patients with advanced non-BRAF-mutant melanoma (Robert et al. 2015a). Pembrolizumab proved to be superior to therapy of choice in ipilimumab failures (Ribas et al. 2015). Moreover, in a cohort of 655 patients treated with pemborlizumab it was demonstrated that response rates in BRAF wild-type patients and in BRAF-mutant patients are similar (45 % and 50 %, respectively) (Daud et al. 2015). Moreover, pembrolizumab has been shown to be superior to ipilimumab in a phase III trial (Robert et al. 2015b). Overall, it leads to the conclusion that anti-PD1 can be considered to be proposed to all patients with advanced melanoma in first-line, irrespective of mutational status, perhaps with the only exception of patients with bulky rapidly progressive BRAF-mutant melanoma. However, the incredible impact of anti-PD1 and anti-PDL1 monoclonal antibodies lies in its broad transversal impact in oncology, now with activity demonstrated against a wide panel of neoplasms other than melanoma, including lung cancer, renal cell cancer, bladder cancer, stomach cancer, head and neck cancer, ovarian cancer, and colorectal cancer with microsatellite instability and Hodgkin lymphoma (Lorenzo Galluzzi et al. 2014).
17.2.6 Anti-PD1 Plus Anti-CTLA4
Very impressive data have been reported on the efficacy of the combination of ipilimumab and nivolumab in the last 2 years (Wolchok et al. 2013; Sznol et al. 2014). The rationale to combine these two checkpoint inhibitors is that they have different mechanisms of action, with anti-CTLA4 mainly acting at the central level in the lymph node compartment, perpetuating and/or restoring the induction and proliferation of activated T-cells, and with anti-PD1 mainly acting at the peripheral level at the tumor site, preventing the neutralization of cytotoxic T cells by PDL1 expressing tumor cells and PDL2 expressing plasmoid dendritic cells in the tumor infiltrate. Very deep and long-lasting responses are observed, and in the update on the current experience presented by Sznol et al. at the 2014 ASCO annual meeting, with impressive survival rates of >90 % at 1 year and >80 % at 2 years in advanced melanoma patients (Sznol et al. 2014). In 2015, the RCT comparing nivolumab + ipilimumab versus nivolumab versus ipilimumab in advanced melanoma patients was published and demonstrated that the combination is superior to either monotherapy and that nivolumab alone is superior to ipilimumab regarding PFS (Larkin et al. 2015). The trial is not mature regarding OS data. Importantly, patients with PDL1-positive tumors seemed to benefit equally from nivolimumab monotherapy compared with combination therapy. PDL-1-negative patients had the best results with the combination therapy. It will be very interesting to have the mature results of this trial in 1–1.5 years’ time. Clearly, all these results are unprecedented in the melanoma world and demonstrate the power of the current concepts of breaking tolerance.
Immunotherapy combinations in general are expected to be perhaps the most dynamic drug development field for years to come. Once breaking tolerance is achieved, or even further improved with candidate molecules such as anti-LAG3 and others, the door is open to combine with agonists such as OX40, CD137, and others. Deepening breaking tolerance and combining with various agonistic approaches is a complex scenario to work out, but obviously, smart immune combos are the future (Eggermont and Robert 2014).
References
Attia P, Phan GQ, Maker AV et al (2005) Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol 23:6043–6053
Brahmer JR, Tykodi SS, Chow LQM et al (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366:2455–2465
Daud A, Ribas A, Robert C, et al (2015) Long-term efficacy of pembrolizumab (pembro; MK-3475) in a pooled analysis of 655 patients (pts) with advanced melanoma (MEL) enrolled in KEYNOTE-001. J Clin Oncol 33, (suppl; abstr 9005)
Delyon J, Mateus C, Lefevres D et al (2013) Experience in daily practice with ipilimumab for the treatment of patients with metastatic melanoma: an early increase in lymphocytes and eosinophils is associated with improved survival. Ann Oncol 24:1697–1703
Di Giacomo AM, Ascierto PA, Pilla L et al (2012) Ipilimumab and fotemustine in patients with advanced melanoma (NIBIT-M1): an open-label, single-arm phase 2 trial. Lancet Oncol 13(9):879–886
Eggermont AM, Robert C (2014) Melanoma: smart therapeutic strategies in immuno-oncology. Nat Rev Clin Oncol 11:181–182
Eggermont AMM, Suciu S, MacKie R et al (2005) Post-surgery adjuvant therapy with intermediate doses of interferon alfa 2b versus observation in patients with stage IIb/III melanoma (EORTC 18952): randomised controlled trial. Lancet 366:1189–1196
Eggermont AMM, Suciu S, Santinami M et al (2008) Adjuvant therapy with pegylated interferon alfa-2b versus observation alone in resected stage III melanoma: final results of EORTC 18991, a randomised phase III trial. Lancet 372:117–126
Eggermont AM, Suciu S, Testori A et al (2012a) Long term results of the randomized phase III trial EORTC 18991 of adjuvant therapy with pegylated interferon alfa-2b versus observation in resected stage III melanoma. J Clin Oncol 30:3810–3818
Eggermont AMM, Suciu S, Testori A et al (2012b) Ulceration and stage are predictive of interferon efficacy in melanoma: Results of the phase III adjuvant trials EORTC 18952 and EORTC 18991. Eur J Cancer 48:218–225
Eggermont AM, Spatz A, Lazar V, Robert C (2012c) Is ulceration in cutaneous melanoma just a prognostic and predictive factor or is ulcerated melanoma a distinct biologic entity? Curr Opin Oncol 24:137–140
Eggermont AM, Kroemer G, Zitvogel L (2013) Immunotherapy and the concept of a clinical cure. Eur J Cancer 49(14):2965–2967
Eggermont AM, Spatz A, Robert C (2014) Cutaneous melanoma. Lancet 383(9919):816–827. doi:10.1016/S0140-6736(13)60802-8
Eggermont AM, Chiarion-Sileni V, Grob JJ et al (2015) Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol 16(5):522–530
Galluzzi L, Senovilla L, Zitvogel L, Kroemer G (2012) The secret ally: immunostimulation by anticancer drugs. Nat Rev Drug Discov 11:215–233
Hamid O, Robert C, Daud A et al (2013) Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med 369(2):134–144
Hannani D, Vétizou M, Enot D et al (2015) Anticancer immunotherapy by CTLA-4 blockade: obligatory contribution of IL-2 receptors and negative prognostic impact of soluble CD25. Cell Res 25(2):208–224. doi:10.1038/cr.2015.3, Epub 2015 Jan 13. Erratum in: Cell Res. 2015 Mar;25(3):399–400. Zörnig, Inka [added]; Hassel, Jessica [added]
Hodi FS, O’Day SJ, McDermott DF et al (2010) Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 363:711–723
Hodi FS, Lawrence D, Lezcano C et al (2014a) Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer Immunol Res 2:632–642
Hodi FS, Lee S, McDermott DF et al (2014b) Ipilimumab plus sargramostim vs ipilimumab alone for treatment of metastatic melanoma: a randomized clinical trial. JAMA 312:1744–1753
Hoos A, Eggermont AMM, Janetzki S et al (2010) Improved endpoints for cancer immunotherapy trials. J Natl Cancer Inst 102:1388–1397
Jamal R, Belanger K, Friedmann JE et al (2014) A randomized phase II study of ipilimumab (IPI) with carboplatin and paclitaxel (CP) in patients with unresectable stage III or IV metastatic melanoma (MM). J Clin Oncol 32(5s): abstract 9066
Kroemer G, Galluzzi L, Kepp O, Zitvogel L (2013) Immunogenic cell death in cancer therapy. Ann Rev Immunol 31:51–72
Ku GY, Yuan J, Page DB et al (2010) Single-institution experience with ipilimumab in advanced melanoma patients in the compassionate use setting: lymphocyte count after 2 doses correlates with survival. Cancer 116:1767–1775
Kudchadkar RR, Gibney GT, Dorman D, et al (2014) A phase IB study of ipilimumab with peginterferon alfa-2b for patients with unresectable stages IIIB/C/IV melanoma. J Clin Oncol 32(5s): abstract 9098
Larkin J, Chiarion-Sileni V, Gonzalez R et al (2015) Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med 373(1):23–34
Lorenzo Galluzzi L, Kroemer G, Eggermont A (2014) Novel immune checkpoint blocker approved for the treatment of advanced melanoma. Oncoimmunology 3:e29030
Margolin K, Ernstoff MS, Hamid O et al (2012) Ipilimumab in patients with melanoma and brain metastases: an open-label, phase 2 trial. Lancet Oncol 13:459–465
Ménard C, Ghiringhelli F, Roux S et al (2008) Ctla-4 blockade confers lymphocyte resistance to regulatory T-cells in advanced melanoma: surrogate marker of efficacy of tremelimumab? Clin Cancer Res 14:5242–5249
Pardoll D (2012) The blockade of immune checkpoints in immunotherapy. Nat Rev Cancer 12(4):252–264
Postow MA, Callahan MK, Barker CA et al (2012) Immunologic correlates of the abscopal effect in a patient with melanoma. N Engl J Med 366:925–931
Prieto PA, Yang JC, Sherry RM et al (2012) CTLA-4 blockade with ipilimumab: long-term follow-up of 177 patients with metastatic melanoma. Clin Cancer Res 18:2039–2047
Puzanov I, Milhem MM, Andtbacka RH, et al (2014) Primary analysis of a phase 1b multicenter trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage IIIB-IV melanoma. J Clin Oncol 32 suppl: Abstract 9092
Puzanov I, Callahan ML, Linette GP, et al (2014) Phase 1 study of the BRAF inhibitor dabrafenib (D) with or without the MEK inhibitor trametinib (T) in combination with ipilimumab (Ipi) for V600E/K mutation–positive unresectable or metastatic melanoma (MM). J Clin Oncol;32 suppl: abstract 2511
Ribas A, Hodi FS, Callahan M et al (2013) Hepatotoxicity with combination of vemurafenib and ipilimumab. N Engl J Med 368:1365–1366
Ribas A, Puzanov I, Dummer R et al (2015) Pembrolizumab versus investigator-choice chemotherapy for Ipilimumab-refractory melanoma (KEYNOTE-002): a randomised, controlled, phase 2 trial. Lancet Oncol 16:908–918
Robert C, Thomas L, Bondarenko I et al (2011) Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med 364:2517–2526
Robert C, Soria JC, Eggermont AM (2013) Drug of the year: programmed death-1 receptor/programmed death-1 ligand-1 receptor monoclonal antibodies. Eur J Cancer 49(14):2968–2971
Robert C, Ribas A, Wolchok JD et al (2014) Anti-programmed-death-receptor-1 treatment with pembrolizumab in ipilimumab-refractory advanced melanoma: a randomised dose-comparison cohort of a phase 1 trial. Lancet 384:1109–1117
Robert C, Long GV, Brady B et al (2015a) Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372(4):320–330
Robert C, Schachter J, Long GV et al (2015b) KEYNOTE-006 investigators. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N Engl J Med 372(26):2521–2532
Schadendorf D, Hodi FS, Robert C, Weber JS, Margolin K, Hamid O, Patt D, Chen TT, Berman DM, Wolchok JD (2015) Pooled Analysis of Long-Term Survival Data From Phase II and Phase III Trials of ipilimumab in Unresectable or Metastatic Melanoma. J Clin Oncol 33(17):1889–1894
Suciu S, Ives N, Eggermont AM, et al (2014) Predictive importance of ulceration on the efficacy of adjuvant interferon-a (IFN): An individual patient data (IPD) meta-analysis of 15 randomized trials in more than 7,500 melanoma patients (pts). J Clin Oncol 32(5s): abstract 9067
Sznol M, Kluger HM, Callahan MK et al (2014) Survival, response duration, and activity by BRAF mutation (MT) status of nivolumab (NIVO, anti-PD-1, BMS-936558, ONO-4538) and ipilimumab (IPI) concurrent therapy in advanced melanoma (MEL). J Clin Oncol 32(18_suppl):LAB9003
Tarhini AA, Cherian J, Moschos SJ et al (2012) Safety and efficacy of combination immunotherapy with interferon alfa-2b and tremelimumab in patients with stage IV melanoma. J Clin Oncol 30(3):322–328
Topalian SL, Sznol M (2014) McDermott DF, et al Survival, durable tumor remission, and long-term safety in patients with advanced melanoma receiving nivolumab. J Clin Oncol 32:1020–1030
Topalian SL, Drake CG, Pardoll DM (2012a) Targeting the PD-1/B7-H1(PD-L1) pathway to activate anti-tumor immunity. Curr Opin Immunol 24:207–212
Topalian SL, Hodi FS, Brahmer JR et al (2012b) Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366:2443–2454
Vacchelli E, Aranda F, Eggermont A, Galon J, Sautes-Fridman C, Cremer I et al (2014a) Trial Watch: Chemotherapy with immunogenic cell death inducers. Oncoimmunology 3:e27878
Vacchelli E, Aranda F, Obrist F et al (2014b) Trial watch: Immunostimulatory cytokines in cancer therapy. Oncoimmunology 3:e29030
van Akkooi AC, Nowecki ZI, Voit C et al (2008) Sentinel node tumor burden according to the Rotterdam criteria is the most important prognostic factor for survival in melanoma patients: a multicenter study in 388 patients with positive sentinel nodes. Ann Surg 248(6):949–955
van der Ploeg AP, van Akkooi AC, Rutkowski P et al (2011) Prognosis in patients with sentinel node-positive melanoma is accurately defined by the combined Rotterdam tumor load and Dewar topography criteria. J Clin Oncol 29(16):2206–2214
van der Ploeg AP, van Akkooi AC et al (2014) The prognostic significance of sentinel node tumour burden in melanoma patients: an international, multicenter study of 1539 sentinel node-positive melanoma patients. Eur J Cancer 50(1):111–120
Weber JS, Kaehler KC, Hauschild A (2012) Management of immune-related adverse events and kinetics of response with ipilimumab. J Clin Oncol 30:2691–2697
Wolchok JD, Hoos A, O’Day S et al (2009) Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin Cancer Res 15:7412–7420
Wolchok JD, Neyns B, Linette G et al (2010) Ipilimumab monotherapy in patients with pretreated advanced melanoma: a randomised, double-blind, multicentre, phase 2, dose-ranging study. Lancet Oncol 11:155–164
Wolchok JD, Kluger H, Callahan MK et al (2013) Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med 369:122–133
Zitvogel L, Galluzzi L, Smyth MJ, Kroemer G (2013) Mechanism of action of conventional and targeted anticancer therapies: reinstating immunosurveillance. Immunity 39:74–88
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Eggermont, A.M.M., Schadendorf, D., Robert, C. (2017). Immune Checkpoint Inhibitors in Melanoma Define a New Era in Immunotherapy Aiming for Cure. In: Bosserhoff, A. (eds) Melanoma Development. Springer, Cham. https://doi.org/10.1007/978-3-319-41319-8_17
Download citation
DOI: https://doi.org/10.1007/978-3-319-41319-8_17
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-41317-4
Online ISBN: 978-3-319-41319-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)