Abstract
Astrocytes perform various homeostatic functions in the nervous system beyond that of a supportive or metabolic role for neurons. A growing body of evidence indicates that astrocytes are crucial for central respiratory chemoreception. This review presents a classical overview of respiratory central chemoreception and the new evidence for astrocytes as brainstem sensors in the respiratory response to hypercapnia. We review properties of astrocytes for chemosensory function and for modulation of the respiratory network. We propose that astrocytes not only mediate between CO2/H+ levels and motor responses, but they also allow for two emergent functions: (1) Amplifying the responses of intrinsic chemosensitive neurons through feedforward signaling via gliotransmitters and; (2) Recruiting non-intrinsically chemosensitive cells thanks to volume spreading of signals (calcium waves and gliotransmitters) to regions distant from the CO2/H+ sensitive domains. Thus, astrocytes may both increase the intensity of the neuron responses at the chemosensitive sites and recruit of a greater number of respiratory neurons to participate in the response to hypercapnia.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Respiratory rhythm
- Central chemoreception
- Raphe nuclei
- Locus coeruleus nuclei
- Retrotrapezoid nuclei
- Brainstem
- Glia
- Gliotransmitters
- Astrocytes
The Respiratory Network
The neural network responsible for generating the respiratory rhythm , the respiratory pattern generator (RPG), is composed of neurons preferentially discharging during inspiration or expiration and distributed along the ventral (VRC) and the dorsal (DRC) respiratory columns (Fig. 1) (Feldman et al. 2003; von Euler 1986). The RPG projects into respiratory motoneurons located at different cranial nerve nuclei (V, VII, IX, X, XII), which innervate muscles controlling airway flow and resistance. In addition, the RPG sends projections and synapses on various spinal cord motoneurons, particularly the phrenic motoneurons (C3–C5), which innervate the diaphragm muscle, and intercostal motoneurons (T1–T10), which innervate intercostal muscles. The RPG imposes on these motoneurons a synchronic and rhythmic activity responsible for generating a sequence of inspiratory, post-inspiratory, and expiratory phases observable in recordings from phrenic, abductor laryngeal, and internal intercostal nerves, respectively (Richter and Spyer 2001). The coordinated activation of these motoneurons results in a sequence of air pressure gradients commanding the inspiratory and expiratory phases of ventilation.
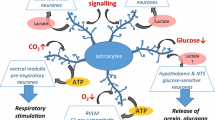
Schema of the respiratory neural network and central respiratory chemoreceptors. The dorsal respiratory column (DRC) is represented by the nucleus tractus solitarius (NTS), while the ventral respiratory column (VRC), by the retrotrapezoid /parafacial respiratory group (pFRG), the Bötzinger nucleus, the pre-Bötzinger complex (preBötC), and the rostral and caudal ventral respiratory group (rVRG and cVRG). Input and output to the central respiratory network are indicated with white and black arrows, respectively; note that the output was represented by respiratory motoneurons localized in cranial and phrenic nuclei. The phrenic nerve (Ph n.) controls the diaphragm muscle, main responsible for generating air pressure gradients during breathing. The main peripheral input is provided by vagal mechanoafferents and peripheral arterial chemoreceptors. Central chemosensitive sites (NTS, preBötc, LC, RTN, raphe ) containing cells that sense changes in pH or PCO2 in the interstitial or the cerebrospinal fluid of the brainstem are indicated in red. Note that the raphe and LC provide inputs into the respiratory network, while NTS, preBötc, and RTN belong to the respiratory network. KF, pontine Kölliker-Fuse nucleus; LC, locus coeruleus ; LPBR, lateral parabrachial nucleus; VII, facial nucleus; XII, hypoglossal nucleus
At the RPG, within the VRC, at least two oscillators can be recognized: at the rostral area of the VRC, the pre-inspiratory retrotrapezoid /parafacial respiratory group (RTN/pFRG), arising from Phox2b expressing progenitors (Guyenet and Mulkey 2010; Onimaru and Homma 2006; Onimaru et al. 2006, 2009; Stornetta et al. 2006; Wang et al. 2013; Takakura et al. 2014; Dubreuil et al. 2009b; Abbott et al. 2011), and at the caudal portions of the VRC, the inspiratory preBötzinger Complex (preBötC), which is derived from Dbx1 progenitors and considered essential for generating the inspiratory activity (Fig. 1) (Smith et al. 1991; Feldman et al. 2003; Gray et al. 2010).
The RPG receives input from several central nervous system (CNS) structures, including cortex, cerebellum, hypothalamus, and brainstem nuclei, and from the peripheral nervous system (PNS), including vagal mechanosensory afferents and peripheral arterial chemoreceptors (Feldman 1986; von Euler 1986). Peripheral arterial chemoreceptors (carotid and aortic bodies) sense changes in PaO2, PaCO2, pH, osmolarity, temperature, and flow of blood circulating through great arteries (Eyzaguirre et al. 1983). In contrast, central chemoreceptors (Fig. 1 indicated in red) are activated by changes in pH or PCO2 in the interstitial or the cerebrospinal fluid of the brainstem (Nattie 1999). In fact, CO2- and H+-sensitive neurons are a major source of the tonic input that drives the mammalian respiratory pattern generator (Nattie 1999).
Central Chemoreception
Central chemoreception can be defined as: “the detection of CO2/pH at sites within the central nervous system and the resultant effects on ventilation” (Nattie and Li 2010), or in more systemic terms as “the feedback process whereby changes in the brain CO2 (or pH) bring about adaptive (homeostatic) changes in breathing to maintain arterial CO2 (or pH) near steady-state levels” (Funk 2010). Central chemoreception is crucial for matching breathing to physiological demands relative to H+ or CO2 elimination. In addition, it appears essential for generating and maintaining the respiratory rhythm (Eugenin 1995), allowing brainstem respiratory neurons to be coordinated and excitable in an optimal manner (Nattie and Li 2012). In fact, CO2- and H+-sensitivities are a major source of the tonic drive that sustains the activity of the RPG (Nattie 1999; Nattie and Li 2012). For example, in en bloc preparations from newborn opossum and mice, alkaline superfusion of the brainstem arrests the respiratory rhythm (Eugenin and Nicholls 1997; Infante et al. 2003; Eugenin et al. 2006).
Localization of Central Chemoreceptors
Various strategies have been used to localize chemosensitive sites in the brain. Detection of c-Fos protein as a marker of neuronal activity revealed that those nuclei in which the number of c-Fos positive neurons increase after exposure to hypercapnia also contain neurons with electrophysiological responses to hypercapnic acidosis (Belegu et al. 1999; Mulkey et al. 2004; Ritucci et al. 2005; Wang and Richerson 1999; Wickstrom et al. 2002; Teppema et al. 1997). In these nuclei we can find neurons that fire in association with or in correlation with the respiratory response to hypercapnia. On the other hand, destruction (Akilesh et al. 1997; Biancardi et al. 2008; da Silva et al. 2011; Dias et al. 2007), genetic ablation (Hodges et al. 2011; Dubreuil et al. 2009b; Ramanantsoa et al. 2011), inactivation, or synaptic inhibition (Nattie and Li 2000; Curran et al. 2001) of specific nuclei reduces the ventilatory response to hypercapnia. More direct evidence of the existence of chemosensitive sites was obtained by focal acidification. Either local application of acetazolamide (Coates et al. 1993), an inhibitor of the enzyme carbonic anhydrase, or reverse microdialysis of artificial cerebrospinal fluid equilibrated with high CO2 within specific CNS areas, increased ventilation (Li et al. 1999; Nattie and Li 2001, 2002a; Li and Nattie 2002; Dias et al. 2008; da Silva et al. 2010; Krause et al. 2009; Kuwaki et al. 2010; Coates et al. 1993). Notably, as illustrated in Fig. 1 (nuclei in red), central respiratory chemosensitivity was localized to multiple sites, including such nuclei belonging to the RPG as the pFRG/RTN, preBötC, and nucleus tractus solitarius (NTS), and such nuclei or identified regions outside the RPG but projecting into as the medullary raphe (RN) , locus coeruleus (LC, A6), ventrolateral medullary surface, hypothalamus, and fastigial nucleus (Ballantyne and Scheid 2001; Coates et al. 1993; Mitchell et al. 1963; Nattie 2001; Oyamada et al. 1998; Wang and Richerson 1999; Li et al. 2006, 2013; Guyenet et al. 2005; Nattie and Li 2006, 2009, 2010; Xu et al. 2001; Xu and Frazier 1995; Martino et al. 2007; Krause et al. 2009). The contribution of specific groups of cells within chemosensitive nuclei were evident from the effects of lesions of neurokinin-1 receptor expressing cells in the RTN, or serotonergic cells in the RN, or catecholaminergic cells in the LC. In all these specific lesions, the CO2 response decreased by 15–30 % during both sleep and wakefulness (Nattie and Li 2008).
Roles of the RTN and Raphe RN Neurons in Central Chemoreception
RTN Neurons
RTN neurons are glutamatergic, chemosensitive, express the transcription factor Phox2b, provide excitatory projections to other sites in the central respiratory network, and when stimulated activate breathing (Mulkey et al. 2004; Onimaru et al. 2008; Stornetta et al. 2006; Wang et al. 2013; Guyenet and Mulkey 2010; Goridis et al. 2010; Dubreuil et al. 2009b).
Inhibition of RTN neurons by muscimol dialysis or their chemical (kainic acid injection) or electrical destruction reduces basal ventilation and the ventilatory responses to hypercapnia in anesthetized rats (Nattie and Li 1994). More selective lesions restricted to RTN neurons expressing the neurokinin 1 receptor (NK1R), obtained with a saporin–substance P conjugate (SSP-SAP), impairs ventilatory response to hypercapnia in rats (Nattie and Li 2002b). In anesthetized rats, elimination of at least 70 % of Phox2b+ tyrosine hydroxylase negative (TH−) RTN neurons is required for a significant increase of the apnea threshold, but does not affect the sensitivity of the subsequent responses to hypercapnia (Takakura et al. 2008). Allatostatin inhibition of RTN Phox2b-expressing neurons transformed with a lentiviral construct to express the G-protein-coupled Drosophila allatostatin receptor did not affect the basal respiratory activity in unanesthetized, conscious rats (Marina et al. 2010). Nevertheless, allatostatin reduced the amplitude of the phrenic nerve discharge and the CO2-evoked ventilatory responses in anesthetized rats, in in situ preparations, and in conscious rats with denervated or intact peripheral chemoreceptors (by 28 and 60 %, respectively) (Marina et al. 2010; Ramanantsoa et al. 2011).
In contrast, photostimulation of RTN neurons expressing channel rhodopsin-2 under the control of the Phox2-responsive promoter PRSx8, increases ventilation in both anesthetized and conscious animals (Abbott et al. 2009, 2011; Kanbar et al. 2010; Burke et al. 2015).
The human disease called central congenital hypoventilation syndrome (CCHS) shows a spectrum of defects comparable with the ontogenic defects of the autonomic nervous system in Phox2b mutant mice (Brunet and Pattyn 2002; Pattyn et al. 1999). CCHS is a life threatening human disease characterized by hypoventilation periods or apnea during sleep and a variable reduction of ventilatory response to hypercapnia, from moderate to severe. CCHS was attributable to a mutation consisting of a polyalanine expansion in the Phox2b transcription factor (Amiel et al. 2003, 2009). Moreover, genetic generation of a knock-in mouse having the most frequent of the CCHS-mutations, the Phox2b27Ala allele, resulted in the selective ablation of glutamatergic neurons in the RTN and a CCHS-like phenotype. These mice showed gasping behavior, cyanosis, disruption of the respiratory chemo reflex at birth and, in contrast to human CCHS patients, they died during the first hours of postnatal life from respiratory failure (Dubreuil et al. 2008, 2009a, b; Goridis et al. 2010; Ramanantsoa et al. 2011).
Raphe Nucleus Neurons
In brainstem slices, CO2/H+ responsive neurons can be found in the midline Raphe nucleus (RN) (Richerson 1995; Wang et al. 1998). As mentioned above, ventilation increases with focal acidification of the midline RN by microinjection of acetazolamide in anesthetized rats or by reverse microdialysis of acidified cerebrospinal fluid (CSF) in conscious rats or goats (Nattie and Li 2001; Hodges et al. 2004a, b). Inhibition of RN neurons by microdialysis of muscimol (Taylor et al. 2006), by administration of 5-hydroxytryptamine (5-HT)1A autoreceptor agonist (8-OH-DPAT), which inhibits serotonergic neurons, or by microinjections of lidocaine or ibotenic acid significantly decreased the response to hypercapnia in piglets (Messier et al. 2002, 2004; Dreshaj et al. 1998). In the unanesthetized juvenile rat brainstem preparation perfused in situ, 5-HT2 receptor antagonism with ketanserin or 5-HT1A autoreceptor activation with 8-OH-DPAT blunted the respiratory response (Corcoran et al. 2013). In rats, injections of a monoclonal antibody against the serotonin transporter (SERT) conjugated to saporin into the RN specifically killed serotonergic neurons, and as result decreased the average CO2 response (Nattie et al. 2004). In addition, hypercapnic ventilatory response decreased by 50 % in adult knock out (KO) mice (Lmx1bf/f/p and Pet-1 knockout mice) with near complete absence of central 5-HT neurons (Hodges et al. 2008, 2011) or with absence of the 5HT transporter (Li and Nattie 2008). Egr2-null mice have, among others defects, altered serotonergic progeny, low respiratory rate, and severe apneas, dying perinatally due to respiratory insufficiency.
Selective hyperpolarization of Egr2 expressing neurons or 5HT neurons was achieved by clozapine-N-oxide (CNO) activation of the synthetic Gi/o protein–coupled receptor Di expressed selectively on 5-HT neurons using conditional intersectional genetics. Hyperpolarization of Egr2 neurons reduced the ventilatory response by 63 % (Ray et al. 2013). Hyperpolarization restricted to serotonergic neurons reduced the ventilatory chemoreflex in vivo by almost 50 % and reduced the CO2-induced firing rate increase of 5HT neurons in culture (Ray et al. 2011). When Di expression was targeted to a specific subtype of 5HT neuron, the Egr2-Pet1 serotonergic subgroup was found to contribute most to the ventilatory response to hypercapnia and acidosis. Egr2-Pet1 neurons project to other chemosensory areas and show intrinsic chemosensitivity firing in response to a hypercapnic stimulus (Brust et al. 2014).
Relative Contribution of Chemosensitive Sites to the Overall Response
Determination of the relative contribution of each chemosensory site to the full expression of chemosensitivity has been elusive. Pronounced effects after unilateral chemical or electrolytic lesion of the RTN, NTS, or RN led to the notion that each nucleus provides an essential, indispensable, and singular contribution to the full expression of central chemosensitivity (Berger and Cooney 1982; Nattie and Li 1994). However, these deleterious effects caused by lesion of chemosensitive nucleus were strongly influenced by anesthesia (Nattie and Li 2012; Nattie 2011). In fact, lesion-related impairment of the responses to systemic hypercapnia largely disappeared with recovery of consciousness (Berger and Cooney 1982). Thus, under anesthesia, destruction of the rat RTN reduced the integrated baseline activity of the phrenic nerve and the respiratory response to hypercapnia (Nattie and Li 1994). In contrast, in conscious, unanesthetized rats, similar unilateral lesions of RTN produced minor effects on baseline ventilation and the respiratory response to hypercapnia (Akilesh et al. 1997). In agreement with these results, the magnitude of the ventilatory effects evoked by acidification of chemosensitive areas using reverse microdialysis in conscious, unanesthetized animals was lower than that observed in anesthetized animals (Nattie and Li 2012; Nattie 2011). The reduction in ventilatory effects observed in conscious animals may be explained in part by an enhanced clearance of focal stimulus as a result of an increased cerebral blood flow in unanesthetized mammals.
The relative contributions of chemosensory nuclei in the conscious animal has been studied using either focal inhibition of chemosensitive sites or focal acidification by reverse microdialysis of artificial cerebrospinal fluid (aCSF) equilibrated with high CO2 (Nattie and Li 2009). Assuming that the contributions of chemosensitive sites are independent, the overall respiratory response does not appear to be the result of simple additive interactions of individual contributions (Nattie and Li 2010). Clear synergisms could be inferred, as for example, observing the ventilatory depression when RTN and caudal RN were simultaneously inhibited (Li et al. 2006). More direct evidence of this synergism was obtained with simultaneous focal acidification of the RTN and caudal RN (Dias et al. 2008). However, unrealistically complex experiments with multiple probes stimulating each chemosensory area individually or several simultaneously during wakefulness and sleep seem to be necessary to fully address this question.
Interestingly, the full expression of central chemoreception also depends on the peripheral chemoreceptor input. In fact, in unanesthetized awake dogs the ventilatory responsiveness to four progressively increasing levels of central hypercapnia depended on the degree of carotid body inhibition or stimulation with respect to basal eupneic conditions (normoxic, normocapnic carotid body perfusion). The increase in carotid body activity via carotid body perfusion with a hypoxic, normocapnic perfusate increased the ventilatory response to hypercarbia by 223 % respect basal conditions. By contrast, silencing of carotid bodies activity with hyperoxic, hypocapnic perfusate reduced the ventilatory response to hypercarbia by 81 %. This interdependence between peripheral and central chemoreception suggests that the whole system of central and peripheral chemosensory structures are functionally interrelated and integrated.
Central Chemoreception Dependency on Functional State of the Respiratory Network
Special attention should be focused to the fact that contribution of each chemosensitive site to the overall response to hypercapnia depends on the functional state of the respiratory network. Such dependency not only may give account of the differences between conscious and anesthetized animals that are mentioned above, but also of differences in ventilation and ventilatory responses between wakefulness and sleep (Newton et al. 2014). Studies in rats with focal acidosis by reverse microdialysis along the sleep–wake cycle have shown that acidification of the RTN or the perifornical-lateral hypothalamic area (PF-LHA), where orexin neurons are found, or the caudal ventrolateral medulla (cVLM) increased ventilation predominantly in wakefulness (Li and Nattie 2002; Li et al. 2013; da Silva et al. 2010). By contrast, acidification of rostral RN increased ventilation predominantly in sleep (Nattie and Li 2001) while focal acidification of the NTS increased ventilation in both wakefulness and sleep (Nattie and Li 2002a).
Orexin neurons are good candidates to be the link between arousal state and chemoreceptive properties at the brainstem (Nattie and Li 2010, 2012). Orexin neurons are critical for generating wakefulness (Ohno and Sakurai 2008; Sakurai 2014; Alexandre et al. 2013) and controlling breathing (Nakamura et al. 2007; Li et al. 2013; Li and Nattie 2010; Dias et al. 2010; Terada et al. 2008; Dutschmann et al. 2007; Deng et al. 2007; Young et al. 2005b; Toyama et al. 2009). They are sensitive to H+/CO2 (Williams et al. 2007; Li et al. 2013; Sunanaga et al. 2009) and their firing rate is maximal during wakefulness (Lee et al. 2005) and minimal during sleep.
As mentioned above, focal acidification of the hypothalamic area containing orexin neurons increased ventilation up to 15 % only in wakefulness but not in sleep (Li et al. 2013). In prepro-orexin knockout mice (ORX-KO) basal ventilation is not affected along the sleep–wake cycle. Neither their ventilatory responses to hypercarbia during sleep period nor their ventilatory responses to hypoxia during wake–sleep cycle when compared with those in wild type mice. However, ORX-KO mice have a ventilatory response to hypercapnia reduced to the half of that in wild type mice during quiet wakefulness. The ventilatory response to hypercapnia was partially restored in ORX-KO mice administered intracerebroventricular with orexin-A or orexin–B, the two orexin subtypes derived from prepro-orexin (Deng et al. 2007).
Such results are compatible with those obtained by dialyzing the rat RTN with SB-334867, orexin receptor-1 antagonist that reduced the hyperventilation caused by hypercapnia by 30 % during wakefulness and 9 % during sleep. A much smaller effect (16 % reduction of hypercapnia-induced hyperventilation) was observed when microdialysis of SB-334867 was performed into rostral RN during wakefulness in dark period and null effect in the ventilatory chemo reflex when administered during sleep (Dias et al. 2010). In addition, almorexant, antagonist of both orexin receptor-1 and orexin receptor-2, administered orally reduced the ventilatory response to hypercapnia by 26 % only in wakefulness during the dark, active period of the diurnal cycle (Li and Nattie 2010). Then, we can conclude that projections of orexin-containing neurons to the RTN and rostral RN contribute, via orexin receptor-1, to the hypercapnic chemoreflex control during wakefulness and to a lesser extent during sleep (Dias et al. 2009). However, a possible role for orexin neurons as a “wakefulness” driver of chemosensitive properties is still uncertain.
Astrocytes
Astrocytes are not mere intermingled cells of the CNS that outnumber neurons. As already described in Chapter “Glial Cells and Integrity of the Nervous System”, they serve multiple functions: structure of the nervous tissue, trophism, metabolic support as for example the lactate shuttle, energy storage in the form of glycogen, ionic and water homeostasis, homeostasis of the synaptic environment buffering the concentration of extracellular potassium and the excess of extracellular neurotransmitters and release of gliotransmitters and neurotransmitters (most of them influencing synaptic strength, Table 1), formation and remodeling of synapses, defense against oxidative stress, scar formation, and tissue repair. Even more, astrocytes are involved in complex processes like neural network plasticity, inflammation, and neurodegeneration (Belanger et al. 2011; Grass et al. 2004; Rodriguez-Arellano et al. 2015).
There is a remarkable heterogeneity among astrocytes, being their phenotype largely a function of both local anatomy and regional functional demands (Oberheim et al. 2012). They are in intimate contact with most of the structures of the nervous system being largely responsible of its compartmentalization. Astrocytes send end-feet processes that enwrap blood vessels and interact with endothelial cells determining the formation of the blood brain barrier. Astrocytic end-feet processes express, among others, glucose transporters and aquaporin 4. They are involved in the cerebral neurovascular coupling regulating the microvascular flow for matching this to synaptic activity (Iadecola and Nedergaard 2007).
On the other hand, astrocytes send processes that ensheath most synapses. These perisynaptic processes express receptors for cytokines and growth factors. In addition, they express different kind of neurotransmitter receptors, transporters, and ion channels as expected of an active participant in the homeostasis of the synapse. Thus, at the synaptic compartment, astrocytes can sense the synaptic activity by means of neurotransmitter receptors activation (Araque et al. 2014), regulate the levels of neurotransmitters at the synaptic cleft influencing their recapture and release (Hamilton and Attwell 2010), modulate the synaptic transmission through gliotransmitters release, and modulate the neuron excitability by extracellular potassium buffering (Perea et al. 2014).
In hippocampus and cortex from rodent and humans, astrocytes are organized in discrete spatial domains (Oberheim et al. 2012). Each astrocyte extends its processes on a defined territory without important overlap between adjacent astrocytes. On other terms, all cellular structures in a territory (blood vessels, perikarya and synapses) interact with processes from a single astrocyte only (Oberheim et al. 2009). It is estimated that a single spatial domain for a protoplasmic astrocyte in rodent contains 20,000–120,000 synapses, while that in humans contains the extraordinary amount of 270 thousand to 2 million synapses (Oberheim et al. 2009).
A particular feature of astrocytes is that each one of them is coupled to others, through gap junction channels forming an extensive functional syncytium. In hippocampus, each astrocyte forms gap junctions with 11 others astrocytes, in average (Xu et al. 2010). This syncytium offers a route of low electrical resistance for propagation of electronic signaling and ionic currents and for cell-to-cell propagation of second messengers. This syncytium represents a huge sink for buffering the changes in potassium composition of the extracellular space. In addition, this syncytium allows the spreading of calcium waves, which, in humans reach the speed of 37–43 μm/s (Cornell-Bell et al. 1990; Oberheim et al. 2009), into neighboring astrocytes. Thus, astrocytes can be sequentially activated and recruited for performing a common task. Since each astrocytic domain represents an elementary glio-neuronal unit for monitoring the changes in activity of contiguous synapses, the existence of a functional syncytium implies the capability of influencing other astrocytic domains and the spreading of a potential astrocytic response to domains placed far away from an immediate neighborhood. This organization of highly organized and interconnected anatomical domains will allow the recruitment of distant domains, which in turn will influence a larger number of synapses within a neural network. As a consequence, a more intense, and may be, a more synchronized response will arise.
Calcium management of one astrocyte can affect many thousands of excitatory synapses nearby as shown by clamping intracellular Ca2+ experiments. In these, clamping of calcium in individual hippocampal astrocytes is made through a whole-cell pipette while an extracellular field excitatory postsynaptic potential (EPSP) recording is done with an extracellular electrode placed either in the immediate vicinity of the clamped astrocyte or in a more distanced CA1 pyramidal cells group. Astrocytic Ca2+ clamping blocked long term potentiation (LTP) induced by tetanic stimulation of Schaeffer collaterals, at nearby, but not far away positions (Henneberger et al. 2010).
Astrocytes are ideally located to sense synapse activity with the perisynaptic processes and metabolic supply from blood vessels with the end feet processes. In fact, they mediate the response consisting in the modification of the local blood flow as function of synaptic or neuronal activity. It has been shown that astrocytes respond to increased neuronal activity by consuming more glucose and producing more lactate, this latter transferred into neighbor neurons as fuel during hyperactivity. As previously mentioned in Chapter “Glial Cells and Integrity of the Nervous System”, this is known as the “astrocyte-neuron lactate shuttle” hypothesis (Pellerin et al. 2007).
Astrocytes in the PreBötzinger Complex (preBötC)
The preBötC is the main generator of the inspiratory activity and a chemosensitive nucleus (Solomon 2003; Solomon et al. 2000). Fluctuations of the extracellular potassium concentrations are induced by the occurrence of rhythmic bursts of action potentials (Richter et al. 1978), which in turn are associated to fluctuation in the neurotransmitter release. Since astrocytes express K+ channels (Kir4.1; KCNJ10), fluctuations in potassium concentrations generates fluctuations in the resting membrane potential, which can induce fluctuations in intracellular calcium concentration in astrocytes. Using whole-cell recordings from astrocytes and two-photon calcium imaging from rhythmic slices, none coupling between respiratory neuronal activity and astrocytic calcium signals was observed. The absence of correlation between respiratory neuronal activity and astrocytic calcium fluctuation (Schnell et al. 2011) indicates that astrocytic release of gliotransmitters is not commanding the respiratory like activity in neurons. Likely, one role of astrocytes in the preBötC is the control of extracellular levels of neurotransmitters and ions, both largely influencing the excitability of respiratory neurons.
Astrocytes in Central Chemoreception
Over the last two decades, multiple pieces of evidence revealed that astrocytes can contribute to central chemoreception. Such contribution may be accomplished by astrocytes directly playing a role as H+/CO2 sensors or as part of the mechanisms underlying the cholinergic and glutamatergic hypothesis. Reduction in chemosensitivity of astrocytes may be involved in the pathogenesis of Rett syndrome and may explain the deficit in ventilatory responses to hypercapnia in these patients (Turovsky et al. 2015). Also, it has been proposed that astrocytes can play a modulatory role of the network in charge of the respiratory pattern generation by controlling the extracellular ion and transmitter concentrations (Neusch et al. 2006; Szoke et al. 2006; Ballanyi et al. 2010; Erlichman and Leiter 2010). Likely, astrocytes in different chemosensitive regions also differ in their contributions to central chemoreception and the mechanisms underlying such contribution.
Astrocyte Chemosensitivity
As illustrated in Fig. 2, several molecular mechanisms by which astrocytes detect H+/CO2 have been proposed
-
(1)
Inwardly rectifying potassium (Kir) heteromeric channels Kir4.1–Kirk5.1. These channels contribute to the extracellular potassium regulation and are expressed in brainstem nuclei, including, among others, the LC, the ventrolateral medullary (VLM) area, the RTN, and the NTS (Wu et al. 2004). Kir4.1, and Kir5.1 channel subunits are observed in astrocytic processes contacting the pia mater, blood vessels, and synapses associated to PDZ domains containing syntrophins (Hibino et al. 2004). Depolarization of astrocytes by CO2 would involve inhibition of heteromeric Kir4.1–Kir5.1 channels and contribution of Na+-HCO3 − cotransporter (Wenker et al. 2010).
-
(2)
Carbonic anhydrase enzyme and, in addition to the Na+-HCO3 − cotransporter, several other transporters that contribute to pH regulation like the Na+/H+ exchanger and the Na+-dependent or Na+-independent Cl−/HCO3 − antiporters (Brookes 1997; Baird et al. 1999; Makara et al. 2001; Schmitt et al. 2000; Deitmer and Rose 1996).
-
(3)
Connexins presenting a carbamylation motif (Cx26, Cx30, and Cx32), a site for binding CO2 to induce the opening of connexin hemichannels (Meigh et al. 2013) (see Chapter “Physiological Functions of Glial Cell Hemichannels” for further information on hemichannels). In particular, connexin 26 is abundantly expressed at the ventral medullary surface and its CO2 sensitivity is within physiological range having a steep change in conductance centered around 40 mmHg PCO2 (Huckstepp et al. 2010a, b). It is known that heterologous expression of Cx26 endows HeLa cells with CO2-sensitivity and the capacity for releasing adenosine triphosphate (ATP) as a function of PCO2 at constant extracellular pH (Huckstepp et al. 2010a). Accordingly, connexin hemichannel blockers reduce both the ATP release and the ventilatory response induced by hypercapnia in vivo and the ATP release induced by hypercarbia in vitro (Huckstepp et al. 2010b).
-
(4)
Transient receptor potential (TRP) channels endows to astrocytes with the ability for responding to hypercapnic acidosis. This was assayed in enriched glia cells cultures using intracellular calcium- and pH-imaging in addition to perforated patch-clamp methods (Hirata and Oku 2010).
Astrocytes may sense acidosis or hypercapnia through different molecular sensors. Inwardly rectifying potassium (Kir) heteromeric channels Kir4.1–Kirk5.1 are inhibited by CO2 resulting in depolarization of astrocytes (Wenker et al. 2010); carbonic anhydrase (CA) enzyme, the Na+-HCO3 − cotransporter, Na+/H+ exchanger and the Na+-dependent or Na+-independent Cl−/HCO3 − antiporters contribute to pH regulation (Brookes 1997; Baird et al. 1999; Makara et al. 2001; Schmitt et al. 2000; Deitmer and Rose 1996); connexins with a carbamylation motif (Cx26, Cx30, and Cx32), a site for binding CO2 to induce the opening of connexin hemichannels (Meigh et al. 2013) endows cells with CO2-sensitivity and the capacity for releasing ATP as a function of PCO2 at constant extracellular pH (Huckstepp et al. 2010a); TRP channels endows to astrocytes with the ability for responding to hypercapnic but not isocapnic acidosis (Hirata and Oku 2010). It is possible that TRP activation could be given by extracellular acidification (Cui et al. 2011)
Astrocyte Involvement in Respiratory Rhythm Modulation
Specific glial metabolic inhibitors have been used to evaluate astrocyte contribution to the ventilatory process. Fluorocitrate or fluoroacetate at low doses, are incorporated selectively by astrocytes and block the tricarboxylic acid (Krebs) cycle by inhibiting the enzyme aconitase. Administration of fluorocitrate into the RTN in either anesthetized mechanically ventilated or conscious adult rats increased the respiratory output (Erlichman et al. 1998; Holleran et al. 2001). This response can be explained on basis of the fluorocitrate-induced ATP and tissue pH decrease. Inhibition of Krebs cycle reduces ATP levels, which in turn, reduces Na+-K+ATPase activity. Pump inactivation increases the extracellular potassium concentration and, subsequently, depolarizes, among others, chemosensitive neurons. Since chemosensitive neurons also respond to the acidification of the medium, and at the end, as overall result, the respiratory output is increased (Erlichman and Leiter 2010).
In contrast to in vivo experiments, fluoroacetate as well as methionine sulfoximine (MS), an inhibitor of glutamine synthetase, an enzyme present only in astrocytes that catalyzes the synthesis of glutamine from glutamate (see Chapter “Pharmacological Tools to Study the Role of Astrocytes in Neural Network Functions”), reduced the amplitude and frequency of the integrated inspiratory burst recorded from rhythmically active brainstem slices. At a first glance, these results suggest that astrocyte metabolic support or astrocyte functions depend on Krebs cycle and are necessary for the maintenance of the respiratory rhythm (Hulsmann 2000). In brainstem slices, evoked depolarization of the hypoglossal neurons by electrical stimulation of the ventral respiratory column (measured by optical imaging using voltage-sensitive dye) was reduced and delayed after fluoroacetate administration which is compatible with metabolic inhibition of fast synaptic transmission (Hulsmann et al. 2003). Accordingly, after fluoroacetate or MS treatment of brainstem slices, addition of glutamine restored the respiratory rhythm indicating that likely, the respiratory effects of both inhibitors were related, essentially, to impairment of the glutamate neurotransmission. In fact, fluoroacetate also impairs the astrocytic uptake of glutamate and the formation of glutamine (Swanson and Graham 1994)
In vivo administration of MS reduces basal ventilation and the ventilatory response to hypercapnia in conscious neonatal rats (Young et al. 2005a). By contrast, fluorocitrate administered into the RTN in vivo did not affect the respiratory response to acidosis or hypercapnia (Erlichman et al. 1998). Likely, the effects of fluorocitrate-induced reduction in ATP tissue pH oppose and predominate to the impairment in glutamate neurotransmission.
The hypothesis that astrocytes contribute to H+/CO2 sensitivity concatenates several steps: first, a subset of glial cells is depolarized in response to acidification (Fukuda et al. 1978; Fukuda and Honda 1975; Ritucci et al. 2005). Second, and derived from glial cell depolarization, intracellular Ca2+ increases, which is required also for the inter-cellular propagation of calcium waves in glia (Guthrie et al. 1999); third, as a consequence of the intracellular Ca2+ increase, ATP is released from astrocytes, likely through connexin hemichannels (Huckstepp et al. 2010b). In fact, electrochemical sensors placed at the ventral medullary surface can detect high levels of ATP (3.8 ± 0.9 µM) during hypercapnia in anesthetized rats (Spyer et al. 2004; Gourine et al. 2005). Activation of glial purinoceptors by ATP can initiate self-propagating calcium waves that are proposed to influence local network excitability (Fiacco and McCarthy 2006). Finally, ATP, or other neuroactive molecules, will activate central chemoreceptor neurons such as those found in RTN/pFG (Spyer et al. 2004; Gourine et al. 2005).
ATP can act by binding to 7 subtypes of ionotropic P2X receptors (P2X1–7Rs) and eight subtypes of metabotropic P2YRs (P2Y1,2,4,6,11–14) (North 2002; Abbracchio et al. 2009).
According to this sequence of events, purinoceptor antagonists should impair the respiratory effects evoked by CO2 stimulation. In fact, reduction and even abolition of ATP induced respiratory responses have been observed in vivo and in vitro (Thomas and Spyer 2000; Gourine et al. 2005; Zwicker et al. 2011; Gourine and Kasparov 2011; Gourine et al. 2010). Hypercapnia induces the release of ATP from the ventral surface of the medulla (Gourine et al. 2005). Further, application of ATP into the most rostral ATP-releasing site, corresponding likely to the retrotrapezoid nucleus , stimulated respiratory output, whereas application of ATP receptor antagonists like PPADS (pyridoxal-phosphate-6-azophenyl-2′,4′-disulfonate) to this area reduced CO2 respiratory responses (Gourine et al. 2005). Hypercapnia-induced ATP-mediated excitation of the respiratory rhythm in rats is likely to involve a potent P2Y1R- activation of the preBötC (Lorier et al. 2007; Gourine et al. 2010). Such P2Y receptor-dependency has also found in vitro RTN neurons (Mulkey et al. 2006). Since pH sensitivity of RTN neurons in bicarbonate-free HEPES medium is not affected after purinergic receptor blockade with PPADS, ATP would play a role of modulator of the activity of pH-sensitive neurons, amplifying their responses to hypercapnia (Mulkey et al. 2006, 2004).
New insights of astrocyte contribution in modulating the function of respiratory neuronal circuits arise from application of molecular and electrophysiological methodology in conjunction with genetically engineered optical stimulation and Ca2+ imaging tools. In an elegant work, Gourine et al. (2010) tested the hypothesis that rat astrocytes residing in RTN/pFRG behave as pH sensors, and trigger the respiratory response through the release of ATP. Astrocytes were genetically encoded with a Ca2+ indicator associated to the promoter for glial fibrillary acidic protein (GFAP). They could confirm that these astrocytes, but not those from cerebral cortex, responded to physiological decreases in pH with elevations in intracellular Ca2+ and ATP release. Accordingly, studies of vesicular fusion using total internal reflection fluorescence (TIRF) microscopy show that 35 % of astrocytes from rat brainstem in dissociated cultures respond to acidification with exocytosis of ATP-containing vesicles. Vesicles were visualized with fluorescent dyes quinacrine, an acridine derivative with very high affinity for ATP, and MANT-ATP, an ATP analogue esterified by the fluorescent methylisatoic acid. Vesicular exocytosis requires intracellular Ca2+ signaling and was independent of autocrine ATP actions (Kasymov et al. 2013). By contrast, ATP was necessary to propagate astrocytic Ca2+ excitation, since elimination of ATP by the ATP-hydrolyzing enzyme, apyrase, reduced importantly the CO2-evoked astrocytic calcium responses (Gourine et al. 2010). In addition, ATP activating P2Y1Rs excited chemoreceptor neurons leading to the increase in the respiratory rhythm frequency (Gourine et al. 2010). Optogenetic stimulation of astrocytes expressing channelrhodopsin-2 associated under the command of GFAP promoter, resulted in a robust increase in breathing, associated to the increase in intracellular Ca2+ in astrocytes. This optogenetic stimulation mimicked the hypercapnia and acidosis induced activation of chemoreceptor neurons via an ATP-dependent mechanism (Gourine et al. 2010). In agreement with these results, disruption of purinergic signaling decreases CO2 sensitivity of RTN neurons by 25 % (Wenker et al. 2010) as well as gap junction blockers, which decrease CO2-evoked ATP release in the RTN, reduced the whole-animal ventilatory response to CO2 also by 25 % (Huckstepp et al. 2010b). In addition, fluorocitrate-induced depolarization of astrocytes evoked a reversible increase in firing rate of RTN neurons. This increase in neuronal firing rate was abolished by the presence of P2 receptor antagonists (PPADS or suramin) (Wenker et al. 2012) suggesting that a purinergic signaling was a mediator. Purinergic blockade also blunted the hypercapnic ventilatory response in vivo and the firing rate response of RTN neurons to hypercapnic stimulus of slices (10–15 % CO2) (Wenker et al. 2012).
Regional Differences in Contribution of Astrocytes to Central Chemoreception
As mentioned above, neurons responding to CO2 with increased firing rate can be found, among other sites, at the RTN (Nattie et al. 1993a), RN (Iceman et al. 2013), and the caudal portion of the NTS (Dean et al. 1990; Nichols et al. 2009). Furthermore, focal acidification either by injection of acetazolamide within these three regions in anesthetized cats (Coates et al. 1993) or by microdialysis within these nuclei in unanesthetized awake or sleeping rats (Li et al. 1999; Nattie and Li 2001, 2002a) increases ventilation. Since RTN and RN neurons in culture have intrinsic CO2-pH-sensitivities (Wang et al. 1998; Wang and Richerson 1999; Wang et al. 2013), glia would play a coadjuvant, synergic role in these chemoreceptive nuclei. There is not any study detailing the cytoarchitecture and properties of astrocytes at the different areas of the brainstem . Hitherto, the degree of cell-to-cell interconnections, the extension of astrocyte domains, and the differential expression of receptors, gliotransmitters , are mostly unknown. Since in other regions of the CNS, the population of astrocytes is heterogeneous in shapes and functions (Oberheim et al. 2012), it would not be strange that astrocytes belonging to different chemosensory nuclei at the brainstem differ in their structure and properties. Therefore, it is possible that the mechanisms through which astrocytes interact with the respiratory network at different nuclei could also be different.
As expressed before, data obtained at the RTN suggest the existence of a cascade of events triggered by hypercapnia or acidosis: depolarization of astrocytes, cytoplasmic calcium increase, ATP release, and ATP activation of respiratory neurons. It is worth to remember that this constitute the glial pathway for RTN neurons activation since RTN neurons are chemosensitive themselves. At that respect, the glial pathway appears as intensifier of the RTN neurons response to hypercapnia.
At the NTS and the RN, there is some controversial evidence pointing to the role of ATP as mediator of the response to hypercapnia. P2 receptors are expressed in the NTS and with less intensity, at the RN (Yao et al. 2000).
The administration of ATP or its analogues into the NTS, in awake rats produced cardiorespiratory responses (Antunes et al. 2005; De Paula et al. 2004). On the other hand, the injection of P2 receptor antagonists into NTS reduces the sympatho-excitatory response to peripheral chemoreflex activation (Braga et al. 2007; Boscan et al. 2002). Microinjection of ATP into the raphe magnus reduces the respiratory activity while that into the raphe pallidus increase it in anesthetized and artificially ventilated rats (Cao and Song 2007). The injection of the P2X broad-spectrum antagonist, PPADS, into the rostral medullary raphe blunted the ventilatory response to hypercapnia in conscious rats (da Silva et al. 2012), while this unaffected ventilation when injection was placed into the caudal RN of conscious rats (da Silva et al. 2012) or when it was done into raphe magnus or pallidus in anesthetized rats (Cao and Song 2007).
To test whether an astrocytic ATP-dependent mechanism was involved in central chemoreception at the RN and NTS, ATP antagonists were applied into these nuclei while chemoreflexes were evaluated in vivo as in vitro (Sobrinho et al. 2014). ATP injections into the caudal NTS (cNTS) increased cardiorespiratory activity in anesthetized rats (Sobrinho et al. 2014) confirming results obtained with the rat working heart-brainstem preparation (Antunes et al. 2005). By contrast, the injection of broad range purinergic receptor antagonists like PPADS or suramin into the cNTS did not affect basal ventilation or the ventilatory responses to changes in CO2/H+ as it does at the RTN (Sobrinho et al. 2014). In the case of RN the results were more negative, because both the injections of ATP or PPADS in anesthetized rats did not affect neither the basal ventilation nor the responsiveness to H+/CO2 (Sobrinho et al. 2014). Cell-attached NTS neurons recorded from brainstem slices increased their firing rate in response to ATP, while P2 receptors antagonists (PPDAS or suramin) did not modified NTS neurons response to hypercarbia. Likewise, the firing rate of RN neurons were not modified by ATP and their responses to changes in PCO2/pH were unaffected by ATP-receptor blockade (Sobrinho et al. 2014).
Sobrinho et al. (2014) results are unexpected from previous reports indicating the existence of P2 receptors, and the respiratory-related effects of ATP agonist and antagonist injected into the NTS or RN. In fact, it is known that ATP in NTS plays a role in modulating the glutamatergic excitatory transmission as evidenced by the reduction in the amplitude of tractus solitaries-evoked excitatory postsynaptic currents (TS-eEPSCs) by purinergic antagonist (iso-PPADS). The glial cells are the source of ATP released by tractus solitarius electrical stimulation is suggested by the reduction in this TS-eEPSCs induced by the glia toxin, fluoroacetate (Accorsi-Mendonca et al. 2013). Likely, the inconsistency in results may be partly due to methodological differences, for example the use of anesthesia or the use of broad-spectrum antagonists which are weakly effective for blocking specific subset of P2 receptors. However, it remains possible that astrocytes contribute to the CO2/H+ responsiveness of cNTS and RN neurons, perhaps by an ATP-independent mechanism.
Other Gliotransmitters
It is possible that other gliotransmitter, different to ATP, could serve as mediator in NTS or RN. A good candidate is D-serine. D-serine is a D-amino acid synthesized from L-serine by a pyridoxal 5′-phosphate-dependent serine racemase (SR) enzyme, which is present in neurons and astrocytes (Rosenberg et al. 2010; Wolosker 2011). D-serine binds with high affinity to the co-agonist (glycine) site of the N-methyl-D-aspartate (NMDA) glutamate receptor. D-serine effects have not been evaluated in the respiratory network, despite of NMDAR activation increases the respiratory frequency in vivo (Connelly et al. 1992) and in vitro (Greer et al. 1991). Preliminary data from our laboratory indicates that in en bloc preparations from neonatal mice, D-serine applied into the superfusion bath increases the respiratory rhythm of neonatal mice (Fig. 3).
Increase of respiratory frequency induced by D-serine. a integrated inspiratory burst recorded from C4 ventral root in en bloc preparation obtained from CF1 mouse neonate at the third postnatal day before (basal), during, and after (recovery) the superfusion with aCSF containing D-serine 10 μM. b Instantaneous respiratory frequency measured cycle-to-cycle before, during (indicated by horizontal bar), and after the superfusion with aCSF containing D-serine 10 μM in the preparation from (a)
Astrocytes and Cholinergic-Glutamatergic Hypothesis of Central Chemoreception
Historically, two neurotransmitters have been involved in central chemoreception , acetylcholine and glutamate, what is known as “the cholinergic and glutamate hypothesis of central chemoreception”.
Cholinergic (ACh) hypothesis: Cholinergic neurons form part of input and output of the respiratory network. They are found at the NTS (Ruggiero et al. 1990; Armstrong et al. 1988; Gotts et al. 2015), the hypoglossal nuclei, facial nuclei, ambiguous nuclei (Kang et al. 2007), within the RN (Tatehata et al. 1987; Ruggiero et al. 1990), the nucleus reticularis rostroventrolateralis (RVL), and the ventral medullary surface (VMS); although cholinergic neurons are also detected in other localizations of the brainstem , like those in the medial portion of the rostral ventrolateral medulla (mRVLM), these would not be involved in cardiorespiratory events (Stornetta et al. 2013). The most important cholinergic inputs to the brainstem are originated from the pedunculopontine tegmental (PPT) and laterodorsal tegmental (LDT) nuclei. These inputs, as well as those provided by the serotonergic RN and the noradrenergic LC may be a clue for understanding pathogenia of respiratory dysfunctions associated to sleep–wake cycle, like sudden infant death syndrome (SIDS).
Muscarine or nicotine applied on the ventral surface of rostral and caudal medulla increase ventilation in anesthetized cats (Dev and Loeschcke 1979a, b). An endogenous cholinergic drive of the respiratory rhythm is revealed with acetylcholinesterase inhibitors (physostigmine, eserine) within rostral and caudal medulla (Dev and Loeschcke 1979a). In part, the respiratory cholinergic drive is exerted on the preBötC where activation of M3 and α4β2 nicotinic receptors increases the frequency of the respiratory rhythm in neonatal rats and mice slices (Shao and Feldman 2005; Shao et al. 2008; Shao and Feldman 2009). A tonic cholinergic respiratory drive in the mouse en bloc preparation is revealed by application of atropine, a muscarinic receptor antagonist, which reduces the amplitude and frequency of the respiratory rhythm (Coddou et al. 2009).
That a cholinergic relay may be involved in central chemoreception at the surface of ventral medulla is derived from the fact that acetylcholine-sensitive areas and H+- or CO2-sensitive areas overlapped. In addition, application of cholinergic agonists on these sensitive areas elicits similar patterns of respiratory responses than those evoked by acidic stimulation (Loeschcke 1982; Eugenin and Nicholls 1997). Furthermore, central chemoreception and muscarinic cholinergic neurotransmission are strongly linked (Loeschcke 1982) as indicated by the brainstem distribution of muscarinic receptors (Nattie and Li 1990; Nattie et al. 1994; Mallios et al. 1995). Application of atropine to the rostral and caudal medulla decreases ventilation and, at the same time, reduces importantly the ventilatory response to CO2 (Dev and Loeschcke 1979a; Nattie et al. 1989). Muscarinic blockade also reduces and, sometimes, abolishes the respiratory responses induced by H+ or CO2 in in vitro preparations from neonatal rats (Monteau et al. 1990), newborn opossum (Eugenin and Nicholls 1997), and neonatal mouse (Coddou et al. 2009). Microinjection of muscarinic M3 antagonist on the rostral ventrolateral medulla (RVLM) has a great efficacy for inhibiting respiratory CO2-evoked response (Nattie and Li 1990). Interestingly, the arcuate nucleus, which is the human homologue of the RVLM, shows decreased muscarinic binding in SIDS infants (Kinney et al. 1995). Such probable reduction of the muscarinic binding in SIDS is compatible with the reduction of the muscarinic contribution to the chemosensory responses in en bloc and slices preparations from P0-P3 nicotine-exposed neonates by the prenatal-perinatal nicotine exposure (Coddou et al. 2009; Eugenin et al. 2008).
Unexpected results were obtained when muscarinic receptor knockout (KO) mice were challenged with hypercapnia (3 and 5 % CO2). M1 single KO mice showed normal, while M3 single KO mice showed reduced VT response slope to hypercapnia (Boudinot et al. 2004). Surprisingly, M1/3R or M2/4R double-KO mice showed unaltered chemosensory ventilatory responses (Boudinot et al. 2008). These results are puzzling and will require future research with conditional KO mice to evaluate muscarinic contribution to chemo reflexes in adults in absence of possible compensatory mechanisms exerted during development.
Glutamate (Glu) hypothesis: Excitatory glutamate neurotransmission predominates within the mammalian RPG, and the ventral surface of medulla is not an exception. Injection of glutamate into the RVLM increases ventilation in anesthetized cats (Li and Nattie 1995; Nattie and Li 1995). By contrast, microinjection of kynurenic acid, a nonselective glutamate receptor antagonist, or AP5, an NMDA receptor antagonist, or CNQX, a non-NMDA receptor antagonist, into the RVLM region decreased both the amplitude of the integrated phrenic nerve activity and the CO2 sensitivity in a dose-dependent manner in anaesthetized cats (Nattie et al. 1993b). In contrast to in vivo experiments (Connelly et al. 1992), blockade of NMDARs in brainstem slices had a negligible effect on respiratory rhythm (Morgado-Valle and Feldman 2007; Greer et al. 1991), while the blockade of AMPARs completely abolished the rhythm. Similarly, NMDA receptor R1 subunit (NMDAR1) mutant mice were completely unresponsive to NMDA applications and showed a respiratory rhythm almost identical to that of controls. These results indicate that NMDA receptors are not relevant for generating the rhythm and for the development of circuits in charge of it (Funk et al. 1997). As for muscarinic receptors, the effects of glutamate antagonists have not been demonstrated to be specific for chemoreception.
Till now, acetylcholine (ACh) or glutamate (Glu) actions on chemosensitive areas are attributed to direct effects on neurons and a probable contribution of astrocytes in such responses has not been evaluated. Numerous studies demonstrate that astrocytes in different CNS regions express functional neurotransmitter receptors, which allow them to be sensitive to neurotransmitters like ACh and Glu (Perea and Araque 2010; Halassa and Haydon 2010; Ben Achour and Pascual 2010; Paixao and Klein 2010; Attwell et al. 2010; Sidoryk-Wegrzynowicz et al. 2011; Stipursky et al. 2011; Haydon and Carmignoto 2006; Erlichman et al. 2010). It is worth noting that astrocytes in the ventral respiratory group (VRG) express receptors for 5-HT, substance P (SP), and thyrotropin releasing hormone (TRH). So, projections from chemosensitive RN neurons may modify the activation of astrocytes within the respiratory network (Hartel et al. 2009).
Astrocytes in the respiratory network respond to prevailing neuromodulators with an increase of intracellular calcium concentration (Huxtable et al. 2010; Gourine et al. 2010; Hartel et al. 2009). Besides, astrocytes are also capable of synthesizing and releasing neuro- and glio-transmitters such as ACh, Glu, ATP/adenosine, and D-serine (Haydon and Carmignoto 2006; Hamilton and Attwell 2010; Carmignoto et al. 1998; Araque et al. 2002; Hosli and Hosli 1994b; Hosli et al. 1988). So, theoretically, astrocytes may be involved in mediating or amplifying the ventilatory response to cholinergic and glutamatergic inputs by releasing gliotransmitters able of modifying the activity of the respiratory network. In addition, astrocytes can remove neurotransmitters from the synaptic cleft so they may participate in the control of the synaptic neurotransmitter concentration (Carmignoto et al. 1998; Araque et al. 2002; Hosli and Hosli 1994b; Hosli et al. 1988; Haydon and Carmignoto 2006). For example at the NTS, acidification can depolarize astrocytes by inhibition of both K+ channel current and voltage-sensitive glutamate transporters (Huda et al. 2013). Therefore, as consequence of acidification at the NTS, the inhibition of this glutamate transporter, increases the levels of glutamate at the synaptic cleft affecting the excitatory synaptic transmission (Huda et al. 2013).
In the human infant, about 95 % of the arcuate nucleus neurons (corresponding to the chemosensitive RVLM in cats and rats) are glutamatergic. A large number of astrocytes in the ventral medullary surface express the vesicular glutamate transporter 2 and low levels of 5-HT1A and kainate (GluR5) receptors. So, it is reasonable to propose that astrocytes, which can also express muscarinic and nicotinic receptors (Gahring et al. 2004; Hosli et al. 1994; Hosli and Hosli 1994a, b), may store and release glutamate, possibly in response to stimulation by 5-HT, by ACh, or by glutamate itself (Paterson et al. 2006) affecting, in addition to the inhibition of glutamate uptake, the levels of glutamate at the synaptic cleft.
In addition, astrocytes play an essential role in glutamatergic synapses. Glutamate in the synaptic space is uptaken by astrocytes, converted by them into glutamine, and then transferred as glutamine to the presynaptic terminals for renewal of the glutamate presynaptic pool (Haydon and Carmignoto 2006). In the RPG, most of the excitatory synapses are glutamatergic; interestingly, 5 mM fluoroacetate or 0.1 mM methionine sulfoximine, both glial metabolic toxins, reduce the increase in respiratory frequency induced by ATP in brainstem slices, but they do not affect substance P evoked increase, suggesting that astrocytes contribute to the purinergic drive of the inspiratory rhythm generating network (Huxtable et al. 2010).
Concluding Remarks
Astrocytes have diverse roles in modulation of the respiratory rhythm . These involve controlling neural network excitability through potassium buffering, regulation of synaptic transmitter concentrations via their synthesis, reuptake and release; in particular, at glutamatergic synapses, astrocyte is the source of glutamine, essential for replenish synaptic vesicles of glutamatergic neurons. Respect to respiratory central chemoreception , astrocytes have the ability of monitoring PCO2 and pH and release gliotransmitters like ATP in the RTN, in response to changes in CO2 and H+. In addition, they contribute to the regulation of the extracellular pH either by generating acidic substances derived from metabolic coupling (lactate shuttle) leading to amplification of hypercapnic stimulus or through proton buffering (transporters and channels).
On basis to the discussed properties of astrocytes (calcium waves, coupling of astrocytic domains through gap junctions, regulation of neurotransmitters and release of gliotransmitters) we propose that astrocytes may play two emergent roles in central respiratory chemoreception. A first role, as amplifiers of the responses of intrinsic chemosensitive neurons through feedforward signaling via gliotransmitters and a second role as recruiter of non-intrinsic chemosensitive cells thanks to volume spreading of signals (calcium waves and gliotransmitters) to regions far away the CO2/H+ sensitive domains (Fig. 4).
Schema of astrocyte contribution to the chemosensory response. Astrocytes enwrapping blood vessels or exposed to the CNS environment are continuously monitoring pH and PCO2. As consequence of their activation (astrocyte depolarization) by acidosis or hypercapnia, there is an increase in intracellular calcium concentration, which may trigger the release, among others, of ACh, Glu, ATP, or D-serine and calcium waves that travel from astrocyte-to-astrocyte influencing the behavior of astrocyte according other astrocyte domains. Thus, the action of gliotransmitters at the local chemosensitive site may enhance the response of chemosensitive cells in the immediate environment. In addition, by volume diffusion of gliotransmitters and by activation of faraway astrocytes influenced by calcium waves more neurons of the respiratory network may be recruited
Abbreviations
- 5-HT:
-
5-hydroxytryptamine (Serotonin)
- ACh:
-
Acetylcholine
- aCSF:
-
Artificial cerebrospinal fluid
- AMPAR:
-
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor
- ANP:
-
Atrial natriuretic peptide
- ATP:
-
Adenosine triphosphate
- CA:
-
Carbonic anhydrase enzyme
- CCHS:
-
Central congenital hypoventilation syndrome
- CNS:
-
Central nervous system
- CNO:
-
Clozapine-N-oxide
- CO2 :
-
Carbon dioxide
- CNQX:
-
6-cyano-7-nitroquinoxaline-2,3-dione—competitive AMPA/kainate receptor antagonist
- cNTS:
-
Caudal nucleus tractus solitarius
- CSF:
-
Cerebrospinal fluid
- cVLM:
-
Caudal ventrolateral medulla
- cVRG:
-
Caudal ventral respiratory group
- Cx:
-
Connexins
- DRC:
-
Dorsal respiratory columns
- EPSP:
-
Excitatory postsynaptic potentials
- GABA:
-
γ-aminobutyric acid
- GFAP:
-
Glial fibrillary acidic protein
- KF:
-
Pontine Kölliker-Fuse nucleus
- KO:
-
Knock out
- LC:
-
Locus coeruleus
- LDT:
-
Laterodorsal tegmental nucleus
- LPBR:
-
Lateral parabrachial nucleus
- LTP:
-
Long-term potentiation
- mRVLM:
-
Medial portion of the rostral ventrolateral medulla
- MS:
-
Methionine sulfoximine
- NK1R:
-
Neurokinin 1 receptor
- NMDA:
-
N-methyl-D-aspartate
- NMDAR:
-
N-methyl-D-aspartate receptor
- NO:
-
Nitric oxide
- NTS:
-
Nucleus tractus solitarius
- PaCO2 :
-
Partial arterial pressure of carbon dioxide
- PCO2 :
-
Partial pressure of carbon dioxide
- PaO2 :
-
Partial arterial pressure of oxygen
- PF-LHA:
-
Perifornical-lateral hypothalamic area
- PNS:
-
Peripheral nervous system
- PPADS:
-
Pyridoxal-phosphate-6-azophenyl-2=,4=-disulfonate
- PPT:
-
Pedunculopontine tegmental nucleus
- preBötC:
-
PreBötzinger Complex
- ORX:
-
Orexin
- ORX-KO:
-
Prepro-orexin knockout mice
- RN:
-
Medullary raphe nucleus
- RPG:
-
Respiratory pattern generator
- RTN/pFRG:
-
Retrotrapezoid/parafacial respiratory group
- RVL:
-
Nucleus reticularis rostroventrolateralis
- RVLM:
-
Rostral ventrolateral medulla
- rVRG:
-
Rostral ventral respiratory group
- SERT:
-
Serotonin transporter
- SIDS:
-
Sudden infant death syndrome
- SP:
-
Substance P
- SSP-SAP:
-
Saporin–substance P conjugate
- TH:
-
Tyrosine hydroxylase
- TIRF:
-
Total internal reflection fluorescence
- TRH:
-
Thyrotropin releasing hormone
- TRP:
-
Channels Transient receptor potential channels
- TS-eEPSCs:
-
Tractus solitaries-evoked excitatory postsynaptic currents
- VLM:
-
Ventrolateral medullary surface
- VMS:
-
Ventral medullary surface
- VRC:
-
Ventral respiratory columns
- VRG:
-
Ventral respiratory group
References
Abbott SB, Stornetta RL, Fortuna MG, Depuy SD, West GH, Harris TE, Guyenet PG (2009) Photostimulation of retrotrapezoid nucleus phox2b-expressing neurons in vivo produces long-lasting activation of breathing in rats. J Neurosci 29(18):5806–5819. doi:10.1523/JNEUROSCI.1106-09.2009
Abbott SB, Stornetta RL, Coates MB, Guyenet PG (2011) Phox2b-expressing neurons of the parafacial region regulate breathing rate, inspiration, and expiration in conscious rats. J Neurosci 31(45):16410–16422. doi:10.1523/JNEUROSCI.3280-11.2011
Abbracchio MP, Burnstock G, Verkhratsky A, Zimmermann H (2009) Purinergic signalling in the nervous system: an overview. Trends Neurosci 32(1):19–29. doi:10.1016/j.tins.2008.10.001
Accorsi-Mendonca D, Zoccal DB, Bonagamba LG, Machado BH (2013) Glial cells modulate the synaptic transmission of NTS neurons sending projections to ventral medulla of Wistar rats. Physiol Rep 1(4):e00080. doi:10.1002/phy2.80
Akilesh MR, Kamper M, Li A, Nattie EE (1997) Effects of unilateral lesions of retrotrapezoid nucleus on breathing in awake rats. J Appl Physiol 82(2):469–479
Albrecht J, Simmons M, Dutton GR, Norenberg MD (1991) Aluminum chloride stimulates the release of endogenous glutamate, taurine and adenosine from cultured rat cortical astrocytes. Neurosci Lett 127(1):105–107
Alexandre C, Andermann ML, Scammell TE (2013) Control of arousal by the orexin neurons. Curr Opin Neurobiol 23(5):752–759. doi:10.1016/j.conb.2013.04.008
Amiel J, Laudier B, Attie-Bitach T, Trang H, de Pontual L, Gener B, Trochet D, Etchevers H, Ray P, Simonneau M, Vekemans M, Munnich A, Gaultier C, Lyonnet S (2003) Polyalanine expansion and frameshift mutations of the paired-like homeobox gene PHOX2B in congenital central hypoventilation syndrome. Nat Genet 33(4):459–461. doi:10.1038/ng1130
Amiel J, Dubreuil V, Ramanantsoa N, Fortin G, Gallego J, Brunet JF, Goridis C (2009) PHOX2B in respiratory control: lessons from congenital central hypoventilation syndrome and its mouse models. Respir Physiol Neurobiol 168(1–2):125–132. doi:10.1016/j.resp.2009.03.005
Antunes VR, Braga VA, Machado BH (2005) Autonomic and respiratory responses to microinjection of ATP into the intermediate or caudal nucleus tractus solitarius in the working heart-brainstem preparation of the rat. Clin Exp Pharmacol Physiol 32:467–472
Araque A, Martin ED, Perea G, Arellano JI, Buno W (2002) Synaptically released acetylcholine evokes Ca2+ elevations in astrocytes in hippocampal slices. J Neurosci 22(7):2443–2450
Araque A, Carmignoto G, Haydon PG, Oliet SH, Robitaille R, Volterra A (2014) Gliotransmitters travel in time and space. Neuron 81(4):728–739. doi:10.1016/j.neuron.2014.02.007
Armstrong DM, Rotler A, Hersh LB, Pickel VM (1988) Localization of choline acetyltransferase in perikarya and dendrites within the nuclei of the solitary tracts. J Neurosci Res 20(3):279–290. doi:10.1002/jnr.490200302
Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA (2010) Glial and neuronal control of brain blood flow. Nature 468(7321):232–243. doi:10.1038/nature09613
Baird NR, Orlowski J, Szabó EZ, Zaun HC, Schultheis PJ, Menon AG, Shull GE (1999) Molecular cloning, genomic organization, and functional expression of Na+/H+ exchanger isoform 5 (NHE5) from human brain. J Biol Chem 274(7):4377–4382
Ballantyne D, Scheid P (2001) Central respiratory chemosensitivity: cellular and network mechanisms. Adv Exp Med Biol 499:17–26
Ballanyi K, Panaitescu B, Ruangkittisakul A (2010) Control of breathing by “nerve glue”. Sci Signal 3(147):e41. doi:10.1126/scisignal.3147pe41
Belanger M, Allaman I, Magistretti PJ (2011) Brain energy metabolism: focus on astrocyte-neuron metabolic cooperation. Cell Metab 14(6):724–738. doi:10.1016/j.cmet.2011.08.016
Belegu R, Hadziefendic S, Dreshaj IA, Haxhiu MA, Martin RJ (1999) CO2-induced c-fos expression in medullary neurons during early development. Respir Physiol 117(1):13–28
Ben Achour S, Pascual O (2010) Glia: the many ways to modulate synaptic plasticity. Neurochem Int 57(4):440–445. doi:10.1016/j.neuint.2010.02.013
Berger AJ, Cooney KA (1982) Ventilatory effects of kainic acid injection of the ventrolateral solitary nucleus. J Appl Physiol 52:131–140
Biancardi V, Bicego KC, Almeida MC, Gargaglioni LH (2008) Locus coeruleus noradrenergic neurons and CO2 drive to breathing. Pflugers Arch 455(6):1119–1128. doi:10.1007/s00424-007-0338-8
Boscan P, Pickering AE, Paton JFR (2002) The nucleus of the solitary tract: an integrating station for nociceptive and cardiorespiratory afferents. Exp Physiol 87(2):259–266
Boudinot E, Yamada M, Wess J, Champagnat J, Foutz AS (2004) Ventilatory pattern and chemosensitivity in M1 and M3 muscarinic receptor knockout mice. Respir Physiol Neurobiol 139(3):237–245. doi:10.1016/j.resp.2003.10.006
Boudinot E, Champagnat J, Foutz AS (2008) M(1)/M(3) and M(2)/M(4) muscarinic receptor double-knockout mice present distinct respiratory phenotypes. Respir Physiol Neurobiol 161(1):54–61. doi:10.1016/j.resp.2007.12.001
Bowery NG, Brown DA, Collins GG, Galvan M, Marsh S, Yamini G (1976) Indirect effects of amino-acids on sympathetic ganglion cells mediated through the release of gamma-aminobutyric acid from glial cells. Br J Pharmacol 57(1):73–91
Braga VA, Soriano RN, Braccialli AL, de Paula PM, Bonagamba LG, Paton JF, Machado BH (2007) Involvement of L-glutamate and ATP in the neurotransmission of the sympathoexcitatory component of the chemoreflex in the commissural nucleus tractus solitarii of awake rats and in the working heart-brainstem preparation. J Physiol 581(Pt 3):1129–1145. doi:10.1113/jphysiol.2007.129031
Brookes N (1997) Intracellullar pH as a regulatory signal in astrocyte metabolism. Glia 21:64–73
Brunet JF, Pattyn A (2002) Phox2 genes—from patterning to connectivity. Curr Opin Genet Dev 12(4):435–440
Brust RD, Corcoran AE, Richerson GB, Nattie E, Dymecki SM (2014) Functional and developmental identification of a molecular subtype of brain serotonergic neuron specialized to regulate breathing dynamics. Cell Rep 9(6):2152–2165. doi:10.1016/j.celrep.2014.11.027
Burke PG, Kanbar R, Viar KE, Stornetta RL, Guyenet PG (2015) Selective optogenetic stimulation of the retrotrapezoid nucleus in sleeping rats activates breathing without changing blood pressure or causing arousal or sighs. J Appl Physiol 118(12):1491–1501. doi:10.1152/japplphysiol.00164.2015 (1985)
Cao Y, Song G (2007) Purinergic modulation of respiration via medullary raphe nuclei in rats. Respir Physiol Neurobiol 155(2):114–120. doi:10.1016/j.resp.2006.04.013
Caravagna C, Soliz J, Seaborn T (2013) Brain-derived neurotrophic factor interacts with astrocytes and neurons to control respiration. Eur J Neurosci 38(9):3261–3269. doi:10.1111/ejn.12320
Carmignoto G, Pasti L, Pozzan T (1998) On the role of voltage-dependent calcium channels in calcium signaling of astrocytes in situ. J Neurosci 18(12):4637–4645
Chen X, Wang L, Zhou Y, Zheng LH, Zhou Z (2005) “Kiss-and-run” glutamate secretion in cultured and freshly isolated rat hippocampal astrocytes. J Neurosci 25(40):9236–9243. doi:10.1523/JNEUROSCI.1640-05.2005
Coates EL, Li A, Nattie EE (1993) Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol 75(1):5–14
Coddou C, Bravo E, Eugenin J (2009) Alterations in cholinergic sensitivity of respiratory neurons induced by pre-natal nicotine: a mechanism for respiratory dysfunction in neonatal mice. Philos Trans R Soc Lond 364(1529):2527–2535
Connelly CA, Otto-Smith MR, Feldman JL (1992) Blockade of NMDA receptor-channels by MK-801 alters breathing in adult rats. Brain Res 596(1–2):99–110
Constam DB, Philipp J, Malipiero UV, ten Dijke P, Schachner M, Fontana A (1992) Differential expression of transforming growth factor-beta 1,—beta 2, and—beta 3 by glioblastoma cells, astrocytes, and microglia. J Immunol 148(5):1404–1410
Corcoran AE, Richerson GB, Harris MB (2013) Serotonergic mechanisms are necessary for central respiratory chemoresponsiveness in situ. Respir Physiol Neurobiol 186(2):214–220. doi:10.1016/j.resp.2013.02.015
Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ (1990) Glutamate induces calcium waves in cultured astrocytes: long-range glial signaling. Science 247(4941):470–473
Corsini E, Dufour A, Ciusani E, Gelati M, Frigerio S, Gritti A, Cajola L, Mancardi GL, Massa G, Salmaggi A (1996) Human brain endothelial cells and astrocytes produce IL-1 beta but not IL-10. Scand J Immunol 44(5):506–511
Cui N, Zhang X, Tapedalli JS, Yu L, Gai H, Petit J, Pamulapati RT, Jin X, Jiang C (2011) Involvement of TRP channels in the CO2 chemosensitivity of locus coeruleus neurons. J Neurophysiol 105:2791–2801. doi:10.1152/jn.00759.2010.-Catecholaminergic
Curran AK, Darnall RA, Filiano JJ, Li A, Nattie EE (2001) Muscimol dialysis in the rostral ventral medulla reduced the CO(2) response in awake and sleeping piglets. J Appl Physiol 90(3):971–980
da Silva GS, Li A, Nattie E (2010) High CO2/H+ dialysis in the caudal ventrolateral medulla (Loeschcke’s area) increases ventilation in wakefulness. Respir Physiol Neurobiol 171(1):46–53. doi:10.1016/j.resp.2010.01.014
da Silva GS, Giusti H, Benedetti M, Dias MB, Gargaglioni LH, Branco LG, Glass ML (2011) Serotonergic neurons in the nucleus raphe obscurus contribute to interaction between central and peripheral ventilatory responses to hypercapnia. Pflugers Arch 462(3):407–418. doi:10.1007/s00424-011-0990-x
da Silva GS, Moraes DJ, Giusti H, Dias MB, Glass ML (2012) Purinergic transmission in the rostral but not caudal medullary raphe contributes to the hypercapnia-induced ventilatory response in unanesthetized rats. Respir Physiol Neurobiol 184(1):41–47. doi:10.1016/j.resp.2012.07.015
De Paula PM, Antunes VR, Bonagamba LG, Machado BH (2004) Cardiovascular responses to microinjection of ATP into the nucleus tractus solitarii of awake rats. Am J Physiol 287:R1164–R1171. doi:10.1152/ajpregu.00722.2003
Dean JB, Bayliss DA, Erickson JT, Lawing WL, Millhorn DE (1990) Depolarization and stimulation of neurons in nucleus tractus solitarii by carbon dioxide does not require chemical synaptic input. Neuroscience 36(1):207–216
Deitmer JW, Rose CR (1996) pH regulation and proton signalling by glial cells. Prog Neurobiol 48(2):73–103
Deng BS, Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T (2007) Contribution of orexin in hypercapnic chemoreflex: evidence from genetic and pharmacological disruption and supplementation studies in mice. J Appl Physiol 103(5):1772–1779. doi:10.1152/japplphysiol.00075.2007 (1985)
Dev NB, Loeschcke HH (1979a) A cholinergic mechanism involved in the respiratory chemosensitivity. Pflügers Arch 379:29–36
Dev NB, Loeschcke HH (1979b) Topography of the respiratory and circulatory responses to acetylcholine and nicotine on the ventral surface of the medulla oblongata. Pflügers Arch 379:19–27
Dias MB, Nucci TB, Margatho LO, Antunes-Rodrigues J, Gargaglioni LH, Branco LG (2007) Raphe magnus nucleus is involved in ventilatory but not hypothermic response to CO2. J Appl Physiol 103(5):1780–1788
Dias MB, Li A, Nattie E (2008) Focal CO2 dialysis in raphe obscurus does not stimulate ventilation but enhances the response to focal CO2 dialysis in the retrotrapezoid nucleus. J Appl Physiol 105(1):83–90
Dias MB, Li A, Nattie EE (2009) Antagonism of orexin receptor-1 in the retrotrapezoid nucleus inhibits the ventilatory response to hypercapnia predominantly in wakefulness. J Physiol 587(Pt 9):2059–2067. doi:10.1113/jphysiol.2008.168260
Dias MB, Li A, Nattie E (2010) The orexin receptor 1 (OX1R) in the rostral medullary raphe contributes to the hypercapnic chemoreflex in wakefulness, during the active period of the diurnal cycle. Respir Physiol Neurobiol 170(1):96–102. doi:10.1016/j.resp.2009.12.002
Dreshaj IA, Haxhiu MA, Martin RJ (1998) Role of the medullary raphe nuclei in the respiratory response to CO2. Respir Physiol 111(1):15–23
Dubreuil V, Ramanantsoa N, Trochet D, Vaubourg V, Amiel J, Gallego J, Brunet JF, Goridis C (2008) A human mutation in phox2b causes lack of CO2 chemosensitivity, fatal central apnea, and specific loss of parafacial neurons. Proc Natl Acad Sci USA 105(3):1069–1072. doi:10.1073/pnas.0709115105
Dubreuil V, Barhanin J, Goridis C, Brunet JF (2009a) Breathing with Phox2b. Philos Trans R Soc Lond 364:2477–2483. doi:10.1098/rstb.2009.0085
Dubreuil V, Thoby-Brisson M, Rallu M, Persson K, Pattyn A, Birchmeier C, Brunet JF, Fortin G, Goridis C (2009b) Defective respiratory rhythmogenesis and loss of central chemosensitivity in Phox2b mutants targeting retrotrapezoid nucleus neurons. J Neurosci 29(47):14836–14846. doi:10.1523/JNEUROSCI.2623-09.2009
Dutschmann M, Kron M, Morschel M, Gestreau C (2007) Activation of Orexin B receptors in the pontine Kolliker-Fuse nucleus modulates pre-inspiratory hypoglossal motor activity in rat. Respir Physiol Neurobiol 159(2):232–235
Erlichman JS, Leiter JC (2010) Glia modulation of the extracellular milieu as a factor in central CO2 chemosensitivity and respiratory control. J Appl Physiol 108:1803–1811. doi:10.1152/japplphysiol.01321.2009.-We
Erlichman JS, Li A, Nattie EE (1998) Ventilatory effects of glial dysfunction in a rat brain stem chemoreceptor region. J Appl Physiol 85(5):1599–1604
Erlichman JS, Leiter JC, Gourine AV (2010) ATP, glia and central respiratory control. Respir Physiol Neurobiol 173(3):305–311. doi:10.1016/j.resp.2010.06.009
Eugenin J (1995) Generation of the respiratory rhythm: modelling the inspiratory off switch as a neural integrator. J Theor Biol 172(2):107–120
Eugenin J, Nicholls JG (1997) Chemosensory and cholinergic stimulation of fictive respiration in isolated CNS of neonatal opossum. J Physiol (London) 501(Pt 2):425–437
Eugenin J, von Bernhardi R, Muller KJ, Llona I (2006) Development and pH sensitivity of the respiratory rhythm of fetal mice in vitro. Neuroscience 141(1):223–231
Eugenin J, Otarola M, Bravo E, Coddou C, Cerpa V, Reyes-Parada M, Llona I, von Bernhardi R (2008) Prenatal to early postnatal nicotine exposure impairs central chemoreception and modifies breathing pattern in mouse neonates: a probable link to sudden infant death syndrome. J Neurosci 28(51):13907–13917
Eyzaguirre C, Fitzgerald RS, Lahiri S, Zapata P (1983) Arterial chemoreceptors. In: Shepherd JT, Abboud FM (eds) American physiological society: handbook of physiology, vol 3., The Cardiovascular SystemWilliams & Wilkins Co., Baltimore, Maryland, pp 557–621
Feldman JL (1986) Neurophysiology of breathing in mammals. In: Bloom FE (ed) Handbook of physiology, vol IV. Williams & Wilkins Co., Bethesda, Maryland, pp 463–524
Feldman JL, Mitchell GS, Nattie EE (2003) Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26:239–266. doi:10.1146/annurev.neuro.26.041002.131103
Fiacco TA, McCarthy KD (2006) Astrocyte calcium elevations: properties, propagation, and effects on brain signaling. Glia 54(7):676–690. doi:10.1002/glia.20396
Fukuda Y, Honda Y (1975) pH sensitive cells at ventrolateral surface of the rat medulla oblongata. Nat New Biol 256:317–318
Fukuda Y, Honda Y, Schlaefke ME, Loeschcke HH (1978) Effect of H+ on the membrane potential of silent cells in the ventral and dorsal surface layer of the rat medulla in vitro. Pflügers Arch 376:229–235
Funk GD (2010) The ‘connexin’ between astrocytes, ATP and central respiratory chemoreception. J Physiol 588(Pt 22):4335–4337. doi:10.1113/jphysiol.2010.200196
Funk GD, Johnson SM, Smith JC, Dong X-W, Lai J, Feldman JL (1997) Functional respiratory rhythm generating networks in neonatal mice lacking NMDAR1 gene. J Neurophysiol 78:1414–1420
Furukawa S, Furukawa Y, Satoyoshi E, Hayashi K (1986) Synthesis and secretion of nerve growth factor by mouse astroglial cells in culture. Biochem Biophys Res Commun 136(1):57–63
Gahring LC, Persiyanov K, Rogers SW (2004) Neuronal and astrocyte expression of nicotinic receptor subunit beta4 in the adult mouse brain. J Comp Neurol 468(3):322–333. doi:10.1002/cne.10942
Gebicke-Haerter PJ, Seregi A, Schobert A, Hertting G (1988) Involvement of protein kinase C in prostaglandin D(2) synthesis by cultured astrocytes. Neurochem Int 13(4):475–480
Goridis C, Dubreuil V, Thoby-Brisson M, Fortin G, Brunet JF (2010) Phox2b, congenital central hypoventilation syndrome and the control of respiration. Sem Cell Dev Biol 21(8):814–822. doi:10.1016/j.semcdb.2010.07.006
Gotts J, Atkinson L, Edwards IJ, Yanagawa Y, Deuchars SA, Deuchars J (2015) Co-expression of GAD67 and choline acetyltransferase reveals a novel neuronal phenotype in the mouse medulla oblongata. Auton Neurosci. doi:10.1016/j.autneu.2015.05.003
Gourine AV, Kasparov S (2011) Astrocytes as brain interoceptors. Exp Physiol 96(4):411–416. doi:10.1113/expphysiol.2010.053165
Gourine AV, Llaudet E, Dale N, Spyer KM (2005) ATP is a mediator of chemosensory transduction in the central nervous system. Nature 436(7047):108–111. doi:10.1038/nature03690
Gourine AV, Kasymov V, Marina N, Tang F, Figueiredo MF, Lane S, Teschemacher AG, Spyer KM, Deisseroth K, Kasparov S (2010) Astrocytes control breathing through pH-dependent release of ATP. Science 329(5991):571–575. doi:10.1126/science.1190721
Grass D, Pawlowski PG, Hirrlinger J, Papadopoulos N, Richter DW, Kirchhoff F, Hulsmann S (2004) Diversity of functional astroglial properties in the respiratory network. J Neurosci 24(6):1358–1365. doi:10.1523/JNEUROSCI.4022-03.2004
Gray PA, Hayes JA, Ling GY, Llona I, Tupal S, Picardo MC, Ross SE, Hirata T, Corbin JG, Eugenin J, Del Negro CA (2010) Developmental origin of preBotzinger complex respiratory neurons. J Neurosci 30(44):14883–14895. doi:10.1523/JNEUROSCI.4031-10.2010
Greer JJ, Smith JC, Feldman JL (1991) Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J Physiol 437:727–749
Guček A, Vardjan N, Zorec R (2012) Exocytosis in astrocytes: transmitter release and membrane signal regulation. Neurochem Res 37(11):2351–2363. doi:10.1007/s11064-012-0773-6
Guthrie PB, Knappenberger J, Segal M, Bennett MV, Charles AC, Kater SB (1999) ATP released from astrocytes mediates glial calcium waves. J Neurosci 19(2):520–528
Guyenet PG, Mulkey DK (2010) Retrotrapezoid nucleus and parafacial respiratory group. Respir Physiol Neurobiol 173(3):244–255. doi:10.1016/j.resp.2010.02.005
Guyenet PG, Mulkey DK, Stornetta RL, Bayliss DA (2005) Regulation of ventral surface chemoreceptors by the central respiratory pattern generator. J Neurosci 25(39):8938–8947
Halassa MM, Haydon PG (2010) Integrated brain circuits: astrocytic networks modulate neuronal activity and behavior. Annu Rev Physiol 72:335–355. doi:10.1146/annurev-physiol-021909-135843
Hamilton NB, Attwell D (2010) Do astrocytes really exocytose neurotransmitters? Nat Rev 11(4):227–238. doi:10.1038/nrn2803
Hartel K, Schnell C, Hulsmann S (2009) Astrocytic calcium signals induced by neuromodulators via functional metabotropic receptors in the ventral respiratory group of neonatal mice. Glia 57(8):815–827. doi:10.1002/glia.20808
Hartung HP, Toyka KV (1987) Phorbol diester TPA elicits prostaglandin E release from cultured rat astrocytes. Brain Res 417(2):347–349
Hartung HP, Heininger K, Schafer B, Toyka KV (1988) Substance P stimulates release of arachidonic acid cyclooxygenation products from primary culture rat astrocytes. Ann N Y Acad Sci 540:427–429
Haydon PG, Carmignoto G (2006) Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev 86(3):1009–1031. doi:10.1152/physrev.00049.2005
Henneberger C, Papouin T, Oliet SH, Rusakov DA (2010) Long-term potentiation depends on release of D-serine from astrocytes. Nature 463(7278):232–236. doi:10.1038/nature08673
Hibino H, Fujita A, Iwai K, Yamada M, Kurachi Y (2004) Differential assembly of inwardly rectifying K+ channel subunits, Kir4.1 and Kir5.1, in brain astrocytes. J Biol Chem 279(42):44065–44073. doi:10.1074/jbc.M405985200
Hirata Y, Oku Y (2010) TRP channels are involved in mediating hypercapnic Ca2+ responses in rat glia-rich medullary cultures independent of extracellular pH. Cell Calcium 48(2–3):124–132. doi:10.1016/j.ceca.2010.07.006
Hodges MR, Klum L, Leekley T, Brozoski DT, Bastasic J, Davis S, Wenninger JM, Feroah TR, Pan LG, Forster HV (2004a) Effects on breathing in awake and sleeping goats of focal acidosis in the medullary raphe. J Appl Physiol 96(5):1815–1824. doi:10.1152/japplphysiol.00992.2003 (1985)
Hodges MR, Opansky C, Qian B, Davis S, Bonis J, Bastasic J, Leekley T, Pan LG, Forster HV (2004b) Transient attenuation of CO2 sensitivity after neurotoxic lesions in the medullary raphe area of awake goats. J Appl Physiol 97(6):2236–2247
Hodges MR, Tattersall GJ, Harris MB, McEvoy SD, Richerson DN, Deneris ES, Johnson RL, Chen ZF, Richerson GB (2008) Defects in breathing and thermoregulation in mice with near-complete absence of central serotonin neurons. J Neurosci 28(10):2495–2505
Hodges MR, Best S, Richerson GB (2011) Altered ventilatory and thermoregulatory control in male and female adult Pet-1 null mice. Respir Physiol Neurobiol 177(2):133–140. doi:10.1016/j.resp.2011.03.020
Holleran J, Babbie M, Erlichman JS (2001) Ventilatory effects of impaired glial function in a brainstem chemoreceptor region in the conscious rat. J Appl Physiol 90:1539–1547
Hosli E, Hosli L (1994a) Binding of cholecystokinin, bombesin and muscarine to neurons and astrocytes in explant cultures of rat central nervous system: autoradiographic and immunohistochemical studies. Neuroscience 61(1):63–72
Hosli E, Hosli L (1994b) Colocalization of binding sites for somatostatin, muscarine and nicotine on cultured neurones of rat neocortex, cerebellum, brain stem and spinal cord: combined autoradiographic and immunohistochemical studies. Neurosci Lett 173(1–2):71–74
Hosli L, Hosli E, Della-Briotta G, Quadri L, Heuss L (1988) Action of acetylcholine, muscarine, nicotine and antagonists on the membrane potential of astrocytes in cultured rat brainstem and spinal cord. Neurosci Lett 92(2):165–170
Hosli L, Hosli E, Winter T, Stauffer S (1994) Coexistence of cholinergic and somatostatin receptors on astrocytes of rat CNS. NeuroReport 5(12):1469–1472
Huckstepp RT, Eason R, Sachdev A, Dale N (2010a) CO2-dependent opening of connexin 26 and related beta connexins. J Physiol 588(Pt 20):3921–3931. doi:10.1113/jphysiol.2010.192096
Huckstepp RT, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N (2010b) Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. J Physiol 588(Pt 20):3901–3920. doi:10.1113/jphysiol.2010.192088
Huda R, McCrimmon DR, Martina M (2013) pH modulation of glial glutamate transporters regulates synaptic transmission in the nucleus of the solitary tract. J Neurophysiol 110:368–377. doi:10.1152/jn.01074.2012
Hulsmann S (2000) Metabolic coupling between glia and neurons is necessary for maintaining respiratory activity in transverse medulary slices of neonatal mouse. Eur J Neurosci 12:7
Hulsmann S, Straub H, Richter DW, Speckmann EJ (2003) Blockade of astrocyte metabolism causes delayed excitation as revealed by voltage-sensitive dyes in mouse brainstem slices. Exp Brain Res 150(1):117–121. doi:10.1007/s00221-003-1410-z
Huxtable AG, Zwicker JD, Alvares TS, Ruangkittisakul A, Fang X, Hahn LB, Posse de Chaves E, Baker GB, Ballanyi K, Funk GD (2010) Glia contribute to the purinergic modulation of inspiratory rhythm-generating networks. J Neurosci 30(11):3947–3958. doi:10.1523/JNEUROSCI.6027-09.2010
Iadecola C, Nedergaard M (2007) Glial regulation of the cerebral microvasculature. Nat Neurosci 10(11):1369–1376. doi:10.1038/nn2003
Iceman KE, Richerson GB, Harris MB (2013) Medullary serotonin neurons are CO2 sensitive in situ. J Neurophysiol 110(11):2536–2544. doi:10.1152/jn.00288.2013
Infante CD, von Bernhardi R, Rovegno M, Llona I, Eugenin JL (2003) Respiratory responses to pH in the absence of pontine and dorsal medullary areas in the newborn mouse in vitro. Brain Res 984(1–2):198–205
Kadle R, Suksang C, Roberson ED, Fellows RE (1988) Identification of an insulin-like factor in astrocyte conditioned medium. Brain Res 460(1):60–67
Kanbar R, Stornetta RL, Cash DR, Lewis SJ, Guyenet PG (2010) Photostimulation of Phox2b medullary neurons activates cardiorespiratory function in conscious rats. Am J Respir Crit Care Med 182(9):1184–1194. doi:10.1164/rccm.201001-0047OC
Kang BJ, Chang DA, Mackay DD, West GH, Moreira TS, Takakura AC, Gwilt JM, Guyenet PG, Stornetta RL (2007) Central nervous system distribution of the transcription factor Phox2b in the adult rat. J Comp Neurol 503(5):627–641. doi:10.1002/cne.21409
Kasymov V, Larina O, Castaldo C, Marina N, Patrushev M, Kasparov S, Gourine AV (2013) Differential sensitivity of brainstem versus cortical astrocytes to changes in pH reveals functional regional specialization of astroglia. J Neurosci 33(2):435–441. doi:10.1523/JNEUROSCI.2813-12.2013
Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA (1990) Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci 10(5):1583–1591
Kinney HC, Filiano JJ, Sleeper LA, Mandell F, Valdes-Dapena M, White WF (1995) Decreased muscarinic receptor binding in the arcuate nucleus in sudden infant death syndrome. Science 269(5229):1446–1450
Krause KL, Forster HV, Davis SE, Kiner T, Bonis JM, Pan LG, Qian B (2009) Focal acidosis in the pre-Botzinger complex area of awake goats induces a mild tachypnea. J Appl Physiol 106(1):241–250. doi:10.1152/japplphysiol.90547.2008 (1985)
Krzan M, Stenovec M, Kreft M, Pangrsic T, Grilc S, Haydon PG, Zorec R (2003) Calcium-dependent exocytosis of atrial natriuretic peptide from astrocytes. J Neurosci 23(5):1580–1583
Kuwaki T, Li A, Nattie E (2010) State-dependent central chemoreception: a role of orexin. Respir Physiol Neurobiol 173(3):223–229. doi:10.1016/j.resp.2010.02.006
Lee MG, Hassani OK, Jones BE (2005) Discharge of identified orexin/hypocretin neurons across the sleep-waking cycle. J Neurosci 25(28):6716–6720. doi:10.1523/JNEUROSCI.1887-05.2005
Li A, Nattie EE (1995) Prolonged stimulation of respiration by brain stem metabotropic glutamate receptors. J Appl Physiol 79(5):1650–1656
Li A, Nattie E (2002) CO2 dialysis in one chemoreceptor site, the RTN: stimulus intensity and sensitivity in the awake rat. Respir Physiol Neurobiol 133(1–2):11–22
Li A, Nattie E (2008) Serotonin transporter knockout mice have a reduced ventilatory response to hypercapnia (predominantly in males) but not to hypoxia. J Physiol 586(9):2321–2329. doi:10.1113/jphysiol.2008.152231
Li A, Nattie E (2010) Antagonism of rat orexin receptors by almorexant attenuates central chemoreception in wakefulness in the active period of the diurnal cycle. J Physiol 588(Pt 15):2935–2944. doi:10.1113/jphysiol.2010.191288
Li A, Randall M, Nattie EE (1999) CO(2) microdialysis in retrotrapezoid nucleus of the rat increases breathing in wakefulness but not in sleep. J Appl Physiol 87(3):910–919
Li A, Zhou S, Nattie E (2006) Simultaneous inhibition of caudal medullary raphe and retrotrapezoid nucleus decreases breathing and the CO2 response in conscious rats. J Physiol 577(Pt 1):307–318
Li N, Li A, Nattie E (2013) Focal microdialysis of CO(2) in the perifornical-hypothalamic area increases ventilation during wakefulness but not NREM sleep. Respir Physiol Neurobiol 185(2):349–355. doi:10.1016/j.resp.2012.09.007
Loeschcke HH (1982) Central chemosensitivity and the reaction theory. J Physiol 332:1–24
Lorier AR, Huxtable AG, Robinson DM, Lipski J, Housley GD, Funk GD (2007) P2Y1 receptor modulation of the pre-Botzinger complex inspiratory rhythm generating network in vitro. J Neurosci 27(5):993–1005. doi:10.1523/JNEUROSCI.3948-06.2007
Makara JK, Petheö GL, Tóth A, Spät A (2001) pH-sensitive inwardly rectifying chloride current in cultured rat cortical astrocytes. Glia 34:52–58
Mallios VJ, Lydic R, Baghdoyan HA (1995) Muscarinic receptor subtypes are differentially distributed across brain stem respiratory nuclei. Am J Physiol 268:L941–L949
Marina N, Abdala AP, Trapp S, Li A, Nattie EE, Hewinson J, Smith JC, Paton JF, Gourine AV (2010) Essential role of Phox2b-expressing ventrolateral brainstem neurons in the chemosensory control of inspiration and expiration. J Neurosci 30(37):12466–12473. doi:10.1523/JNEUROSCI.3141-10.2010
Martino PF, Davis S, Opansky C, Krause K, Bonis JM, Pan LG, Qian B, Forster HV (2007) The cerebellar fastigial nucleus contributes to CO2-H+ ventilatory sensitivity in awake goats. Respir Physiol Neurobiol 157(2–3):242–251. doi:10.1016/j.resp.2007.01.019
Meigh L, Greenhalgh SA, Rodgers TL, Cann MJ, Roper DI, Dale N (2013) CO(2)directly modulates connexin 26 by formation of carbamate bridges between subunits. Elife 2:e01213. doi:10.7554/eLife.01213
Mercure L, Tannenbaum GS, Schipper HM, Phaneuf D, Wainberg MA (1996) Expression of the somatostatin gene in human astrocytoma cell lines. Clin Diagn Lab Immunol 3(2):151–155
Messier ML, Li A, Nattie EE (2002) Muscimol inhibition of medullary raphe neurons decreases the CO2 response and alters sleep in newborn piglets. Respir Physiol Neurobiol 133(3):197–214
Messier ML, Li A, Nattie EE (2004) Inhibition of medullary raphe serotonergic neurons has age-dependent effects on the CO2 response in newborn piglets. J Appl Physiol 96(5):1909–1919
Mitchell RA, Loeschcke HH, Massion WH, Severinghaus JW (1963) Respiratory responses mediated through superficial chemosensitive areas on the medulla. J Appl Physiol 18(3):523–533
Monteau R, Morin D, Hilaire G (1990) Acetylcholine and central chemosensitivity: in vitro study in the newborn rat. Respir Physiol 81:241–254
Morgado-Valle C, Feldman JL (2007) NMDA receptors in preBotzinger complex neurons can drive respiratory rhythm independent of AMPA receptors. J Physiol 582(Pt 1):359–368. doi:10.1113/jphysiol.2007.130617
Mulkey DK, Stornetta RL, Weston MC, Simmons JR, Parker A, Bayliss DA, Guyenet PG (2004) Respiratory control by ventral surface chemoreceptor neurons in rats. Nat Neurosci 7(12):1360–1369
Mulkey DK, Mistry AM, Guyenet PG, Bayliss DA (2006) Purinergic P2 receptors modulate excitability but do not mediate pH sensitivity of RTN respiratory chemoreceptors. J Neurosci 26(27):7230–7233. doi:10.1523/JNEUROSCI.1696-06.2006
Murphy S, Minor RL Jr, Welk G, Harrison DG (1990) Evidence for an astrocyte-derived vasorelaxing factor with properties similar to nitric oxide. J Neurochem 55(1):349–351
Nakamura A, Zhang W, Yanagisawa M, Fukuda Y, Kuwaki T (2007) Vigilance state-dependent attenuation of hypercapnic chemoreflex and exaggerated sleep apnea in orexin knockout mice. J Appl Physiol 102(1):241–248. doi:10.1152/japplphysiol.00679.2006 (1985)
Nattie E (1999) CO2, brainstem chemoreceptors and breathing. Prog Neurobiol 59(4):299–331
Nattie EE (2001) Central chemosensitivity, sleep, and wakefulness. Respir Physiol 129(1–2):257–268
Nattie E (2011) Julius H. Comroe, Jr., distinguished lecture: central chemoreception: then … and now. J Appl Physiol 110(1):1–8. doi:10.1152/japplphysiol.01061.2010
Nattie E, Li A (1990) Ventral medulla sites of muscarinic receptor subtypes involved in cardiorespiratory control. J Appl Physiol 69(1):33–41
Nattie EE, Li A (1994) Retrotrapezoid nucleus lesions decrease phrenic activity and CO2 sensitivity in rats. Respir Physiol 97(1):63–77
Nattie EE, Li A (1995) Rat retrotrapezoid nucleus iono- and metabotropic glutamate receptors and the control of breathing. J Appl Physiol 78(1):153–163
Nattie E, Li A (2000) Muscimol dialysis in the retrotrapezoid nucleus region inhibits breathing in the awake rat. J Appl Physiol 89(1):153–162
Nattie EE, Li A (2001) CO2 dialysis in the medullary raphe of the rat increases ventilation in sleep. J Appl Physiol 90(4):1247–1257
Nattie EE, Li A (2002a) CO2 dialysis in nucleus tractus solitarius region of rat increases ventilation in sleep and wakefulness. J Appl Physiol 92(5):2119–2130
Nattie EE, Li A (2002b) Substance P-saporin lesion of neurons with NK1 receptors in one chemoreceptor site in rats decreases ventilation and chemosensitivity. J Physiol 544(Pt 2):603–616
Nattie E, Li A (2006) Central chemoreception 2005: a brief review. Auton Neurosci 126–127:332–338
Nattie G, Li A (2008) Multiple central chemoreceptor sites: cell types and function in vivo. Adv Exp Med Biol 605:343–347
Nattie E, Li A (2009) Central chemoreception is a complex system function that involves multiple brain stem sites. J Appl Physiol 106(4):1464–1466. doi:10.1152/japplphysiol.00112.2008
Nattie E, Li A (2010) Central chemoreception in wakefulness and sleep: evidence for a distributed network and a role for orexin. J Appl Physiol 108(5):1417–1424. doi:10.1152/japplphysiol.01261.2009
Nattie E, Li A (2012) Central chemoreceptors: locations and functions. Compr Physiol 2(1):221–254. doi:10.1002/cphy.c100083
Nattie EE, Wood J, Mega A, Goritski W (1989) Rostral ventrolateral medulla muscarinic receptor involvement in central ventilatory chemosensitivity. J Appl Physiol 66(3):1462–1470
Nattie EE, Fung ML, Li A, St John WM (1993a) Responses of respiratory modulated and tonic units in the retrotrapezoid nucleus to CO2. Respir Physiol 94(1):35–50
Nattie EE, Gdovin M, Li A (1993b) Retrotrapezoid nucleus glutamate receptors: control of CO2-sensitive phrenic and sympathetic output. J Appl Physiol 74(6):2958–2968
Nattie EE, Li A, Mills J, Huang Q (1994) Retrotrapezoid nucleus muscarinic receptor subtypes localized by autoradiography. Respir Physiol 96(2–3):189–197
Nattie EE, Li A, Richerson GB, Lappi DA (2004) Medullary serotonergic neurones and adjacent neurones that express neurokinin-1 receptors are both involved in chemoreception in vivo. J Physiol 556(Pt 1):235–253. doi:10.1113/jphysiol.2003.059766
Neusch C, Papadopoulos N, Muller M, Maletzki I, Winter SM, Hirrlinger J, Handschuh M, Bahr M, Richter DW, Kirchhoff F, Hulsmann S (2006) Lack of the Kir4.1 channel subunit abolishes K+ buffering properties of astrocytes in the ventral respiratory group: impact on extracellular K+ regulation. J Neurophysiol 95(3):1843–1852. doi:10.1152/jn.00996.2005
Newton K, Malik V, Lee-Chiong T (2014) Sleep and breathing. Clin Chest Med 35(3):451–456. doi:10.1016/j.ccm.2014.06.001
Nichols NL, Wilkinson KA, Powell FL, Dean JB, Putnam RW (2009) Chronic hypoxia suppresses the CO2 response of solitary complex (SC) neurons from rats. Respir Physiol Neurobiol 168(3):272–280. doi:10.1016/j.resp.2009.07.012
North RA (2002) Molecular physiology of P2X receptors. Physiol Rev 82:1013–1067
Oberheim NA, Takano T, Han X, He W, Lin JH, Wang F, Xu Q, Wyatt JD, Pilcher W, Ojemann JG, Ransom BR, Goldman SA, Nedergaard M (2009) Uniquely hominid features of adult human astrocytes. J Neurosci 29(10):3276–3287. doi:10.1523/JNEUROSCI.4707-08.2009
Oberheim NA, Goldman SA, Nedergaard M (2012) Heterogeneity of astrocytic form and function. Methods Mol Biol 814:23–45. doi:10.1007/978-1-61779-452-0_3
Ohno K, Sakurai T (2008) Orexin neuronal circuitry: role in the regulation of sleep and wakefulness. Front Neuroendocrinol 29(1):70–87. doi:10.1016/j.yfrne.2007.08.001
Onimaru H, Homma I (2006) Point:Counterpoint: The parafacial respiratory group (pFRG)/pre-Botzinger complex (preBotC) is the primary site of respiratory rhythm generation in the mammal. Point: the PFRG is the primary site of respiratory rhythm generation in the mammal. J Appl Physiol 100(6):2094–2095
Onimaru H, Kumagawa Y, Homma I (2006) Respiration-related rhythmic activity in the rostral medulla of newborn rats. J Neurophysiol 96(1):55–61
Onimaru H, Ikeda K, Kawakami K (2008) CO2-sensitive preinspiratory neurons of the parafacial respiratory group express Phox2b in the neonatal rat. J Neurosci 28(48):12845–12850. doi:10.1523/JNEUROSCI.3625-08.2008
Onimaru H, Ikeda K, Kawakami K (2009) Phox2b, RTN/pFRG neurons and respiratory rhythmogenesis. Respir Physiol Neurobiol 168(1–2):13–18. doi:10.1016/j.resp.2009.03.007
Oyamada Y, Ballantyne D, Muckenhoff K, Scheid P (1998) Respiration-modulated membrane potential and chemosensitivity of locus coeruleus neurones in the in vitro brainstem-spinal cord of the neonatal rat. J Physiol (London) 513(Pt 2):381–398
Paixao S, Klein R (2010) Neuron-astrocyte communication and synaptic plasticity. Curr Opin Neurobiol 20(4):466–473. doi:10.1016/j.conb.2010.04.008
Parpura V, Basarsky TA, Liu F, Jeftinija K, Jeftinija S, Haydon PG (1994) Glutamate-mediated astrocyte-neuron signalling. Nature 369(6483):744–747. doi:10.1038/369744a0
Paterson DS, Thompson EG, Kinney HC (2006) Serotonergic and glutamatergic neurons at the ventral medullary surface of the human infant: Observations relevant to central chemosensitivity in early human life. Auton Neurosci 124(1–2):112–124. doi:10.1016/j.autneu.2005.12.009
Pattyn A, Morin X, Cremer H, Goridis C, Brunet JF (1999) The homeobox gene Phox2b is essential for the development of autonomic neural crest derivatives. Nature 399(6734):366–370. doi:10.1038/20700
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA 91(22):10625–10629
Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M, Costalat R, Magistretti PJ (2007) Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55(12):1251–1262. doi:10.1002/glia.20528
Perea G, Araque A (2010) GLIA modulates synaptic transmission. Brain Res Rev 63(1–2):93–102. doi:10.1016/j.brainresrev.2009.10.005
Perea G, Sur M, Araque A (2014) Neuron-glia networks: integral gear of brain function. Front Cell Neurosci 8:378. doi:10.3389/fncel.2014.00378
Ramanantsoa N, Hirsch MR, Thoby-Brisson M, Dubreuil V, Bouvier J, Ruffault PL, Matrot B, Fortin G, Brunet JF, Gallego J, Goridis C (2011) Breathing without CO2 chemosensitivity in conditional Phox2b mutants. J Neurosci 31(36):12880–12888. doi:10.1523/JNEUROSCI.1721-11.2011
Ray RS, Corcoran AE, Brust RD, Kim JC, Richerson GB, Nattie E, Dymecki SM (2011) Impaired respiratory and body temperature control upon acute serotonergic neuron inhibition. Science 333(6042):637–642. doi:10.1126/science.1205295
Ray RS, Corcoran AE, Brust RD, Soriano LP, Nattie EE, Dymecki SM (2013) Egr2-neurons control the adult respiratory response to hypercapnia. Brain Res 1511:115–125. doi:10.1016/j.brainres.2012.12.017
Richerson GB (1995) Response to CO2 of neurons in the rostral ventral medulla in vitro. J Neurophysiol 73(3):933–944
Richter DW, Spyer KM (2001) Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci 24(8):464–472
Richter DW, Camerer H, Sonnhof U (1978) Changes in extracellular potassium during the spontaneous activity of medullary respiratory neurones. Pflugers Arch 376(2):139–149
Ritucci NA, Erlichman JS, Leiter JC, Putnam RW (2005) Response of membrane potential and intracellular pH to hypercapnia in neurons and astrocytes from rat retrotrapezoid nucleus. Am J Physiol 289:R851–R861. doi:10.1152/ajpregu.00132.2005.-We
Rodriguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A (2015) Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience. doi:10.1016/j.neuroscience.2015.01.007
Rosenberg D, Kartvelishvily E, Shleper M, Klinker CM, Bowser MT, Wolosker H (2010) Neuronal release of D-serine: a physiological pathway controlling extracellular D-serine concentration. FASEB J 24(8):2951–2961. doi:10.1096/fj.09-147967
Ruggiero DA, Giuliano R, Anwar M, Stornetta R, Reis DJ (1990) Anatomical substrates of cholinergic-autonomic regulation in the rat. J Comp Neurol 292(1):1–53. doi:10.1002/cne.902920102
Sakurai T (2014) The role of orexin in motivated behaviours. Nat Rev 15(11):719–731. doi:10.1038/nrn3837
Schell MJ, Molliver ME, Snyder SH (1995) D-serine, an endogenous synaptic modulator: localization to astrocytes and glutamate-stimulated release. Proc Natl Acad Sci USA 92(9):3948–3952
Schmitt BM, Berger UV, Douglas RM, Bevensee MO, Hediger MA, Haddad GG, Boron WF (2000) Na/HCO3 cotransporters in rat brain: expression in glia, neurons, and choroid plexus. J Neurosci 20(18):6839–6848
Schnell C, Fresemann J, Hulsmann S (2011) Determinants of functional coupling between astrocytes and respiratory neurons in the pre-Botzinger complex. PLoS One 6(10):e26309. doi:10.1371/journal.pone.0026309
Selmaj KW, Farooq M, Norton WT, Raine CS, Brosnan CF (1990) Proliferation of astrocytes in vitro in response to cytokines. A primary role for tumor necrosis factor. J Immunol 144(1):129–135
Shao XM, Feldman JL (2005) Cholinergic neurotransmission in the preBotzinger Complex modulates excitability of inspiratory neurons and regulates respiratory rhythm. Neuroscience 130(4):1069–1081
Shao XM, Feldman JL (2009) Central cholinergic regulation of respiration: nicotinic receptors. Acta Pharmacol Sin 30(6):761–770. doi:10.1038/aps.2009.88
Shao XM, Tan W, Xiu J, Puskar N, Fonck C, Lester HA, Feldman JL (2008) Alpha4* nicotinic receptors in preBotzinger complex mediate cholinergic/nicotinic modulation of respiratory rhythm. J Neurosci 28(2):519–528. doi:10.1523/JNEUROSCI.3666-07.2008
Shinoda H, Marini AM, Cosi C, Schwartz JP (1989) Brain region and gene specificity of neuropeptide gene expression in cultured astrocytes. Science 245(4916):415–417
Sidoryk-Wegrzynowicz M, Wegrzynowicz M, Lee E, Bowman AB, Aschner M (2011) Role of astrocytes in brain function and disease. Toxicol Pathol 39(1):115–123. doi:10.1177/0192623310385254
Smith JC, Ellenberger H, Ballanyi K, Richter DW, Feldman JL (1991) Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science 254(5032):726–729
Sobrinho CR, Wenker IC, Poss EM, Takakura AC, Moreira TS, Mulkey DK (2014) Purinergic signalling contributes to chemoreception in the retrotrapezoid nucleus but not the nucleus of the solitary tract or medullary raphe. J Physiol 592(Pt 6):1309–1323. doi:10.1113/jphysiol.2013.268490
Solomon IC (2003) Focal CO2/H+ alters phrenic motor output response to chemical stimulation of cat pre-Botzinger complex in vivo. J Appl Physiol 94(6):2151–2157
Solomon IC, Edelman NH, O’Neal MH 3rd (2000) CO(2)/H(+) chemoreception in the cat pre-Botzinger complex in vivo. J Appl Physiol 88(6):1996–2007
Spyer KM, Dale N, Gourine AV (2004) ATP is a key mediator of central and peripheral chemosensory transduction. Exp Physiol 89(1):53–59
Stipursky J, Romao L, Tortelli V, Neto VM, Gomes FC (2011) Neuron-glia signaling: Implications for astrocyte differentiation and synapse formation. Life Sci 89(15–16):524–531. doi:10.1016/j.lfs.2011.04.005
Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG (2006) Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 26(40):10305–10314. doi:10.1523/JNEUROSCI.2917-06.2006
Stornetta RL, Macon CJ, Nguyen TM, Coates MB, Guyenet PG (2013) Cholinergic neurons in the mouse rostral ventrolateral medulla target sensory afferent areas. Brain Struct Funct 218(2):455–475. doi:10.1007/s00429-012-0408-3
Sunanaga J, Deng BS, Zhang W, Kanmura Y, Kuwaki T (2009) CO2 activates orexin-containing neurons in mice. Respir Physiol Neurobiol 166(3):184–186. doi:10.1016/j.resp.2009.03.006
Swanson RA, Graham SH (1994) Fluorocitrate and fluoroacetate effects on astrocyte metabolism in vitro. Brain Res 664(1–2):94–100
Szoke K, Hartel K, Grass D, Hirrlinger PG, Hirrlinger J, Hulsmann S (2006) Glycine transporter 1 expression in the ventral respiratory group is restricted to protoplasmic astrocytes. Brain Res 1119(1):182–189. doi:10.1016/j.brainres.2006.08.089
Takakura AC, Moreira TS, Stornetta RL, West GH, Gwilt JM, Guyenet PG (2008) Selective lesion of retrotrapezoid Phox2b-expressing neurons raises the apnoeic threshold in rats. J Physiol 586(Pt 12):2975–2991. doi:10.1113/jphysiol.2008.153163 jphysiol.2008.153163[pii]
Takakura AC, Barna BF, Cruz JC, Colombari E, Moreira TS (2014) Phox2b-expressing retrotrapezoid neurons and the integration of central and peripheral chemosensory control of breathing in conscious rats. Exp Physiol 99(3):571–585. doi:10.1113/expphysiol.2013.076752
Tatehata T, Shiosaka S, Wanaka A, Rao ZR, Tohyama M (1987) Immunocytochemical localization of the choline acetyltransferase containing neuron system in the rat lower brain stem. J Hirnforsch 28(6):707–716
Taylor NC, Li A, Nattie EE (2006) Ventilatory effects of muscimol microdialysis into the rostral medullary raphe region of conscious rats. Respir Physiol Neurobiol 153(3):203–216
Teppema LJ, Veening JG, Kranenburg A, Dahan A, Berkenbosch A, Olievier C (1997) Expression of c-fos in the rat brainstem after exposure to hypoxia and to normoxic and hyperoxic hypercapnia. J Comp Neurol 388(2):169–190
Terada J, Nakamura A, Zhang W, Yanagisawa M, Kuriyama T, Fukuda Y, Kuwaki T (2008) Ventilatory long-term facilitation in mice can be observed during both sleep and wake periods and depends on orexin. J Appl Physiol 104(2):499–507. doi:10.1152/japplphysiol.00919.2007 (1985)
Thomas T, Spyer KM (2000) ATP as a mediator of mammalian central CO2 chemoreception. J Physiol (London) 523(Pt 2):441–447
Toyama S, Sakurai T, Tatsumi K, Kuwaki T (2009) Attenuated phrenic long-term facilitation in orexin neuron-ablated mice. Respir Physiol Neurobiol 168(3):295–302. doi:10.1016/j.resp.2009.07.025
Turovsky E, Karagiannis A, Abdala AP, Gourine AV (2015) Impaired CO2 sensitivity of astrocytes in a mouse model of Rett syndrome. J Physiol 593(14):3159–3168. doi:10.1113/JP270369
von Euler C (1986) Brain stem mechanisms for generation and control of breathing pattern. In: Cherniack NS, Widdicombe JG (eds) American physiological society: handbook of physiology, vol 2., control of breathingWilliams & Wilkins Co., Baltimore, Maryland, pp 1–68
Wang W, Richerson GB (1999) Development of chemosensitivity of rat medullary raphe neurons. Neuroscience 90(3):1001–1011
Wang W, Pizzonia JH, Richerson GB (1998) Chemosensitivity of rat medullary raphe neurones in primary tissue culture. J Physiol (London) 511(Pt 2):433–450
Wang S, Shi Y, Shu S, Guyenet PG, Bayliss DA (2013) Phox2b-expressing retrotrapezoid neurons are intrinsically responsive to H+ and CO2. J Neurosci 33(18):7756–7761. doi:10.1523/JNEUROSCI.5550-12.2013
Wenker IC, Kreneisz O, Nishiyama A, Mulkey DK (2010) Astrocytes in the retrotrapezoid nucleus sense H+ by inhibition of a Kir4.1–Kir5.1-like current and may contribute to chemoreception by a purinergic mechanism. J Neurophysiol 104(6):3042–3052. doi:10.1152/jn.00544.2010
Wenker IC, Sobrinho CR, Takakura AC, Moreira TS, Mulkey DK (2012) Regulation of ventral surface CO2/H+ -sensitive neurons by purinergic signalling. J Physiol 590(Pt 9):2137–2150. doi:10.1113/jphysiol.2012.229666
Westergaard N, Sonnewald U, Schousboe A (1994) Release of alpha-ketoglutarate, malate and succinate from cultured astrocytes: possible role in amino acid neurotransmitter homeostasis. Neurosci Lett 176(1):105–109
Wickstrom R, Hokfelt T, Lagercrantz H (2002) Development of CO(2)-response in the early newborn period in rat. Respir Physiol Neurobiol 132(2):145–158
Williams RH, Jensen LT, Verkhratsky A, Fugger L, Burdakov D (2007) Control of hypothalamic orexin neurons by acid and CO2. Proc Natl Acad Sci USA 104(25):10685–10690. doi:10.1073/pnas.0702676104
Wolosker H (2011) Serine racemase and the serine shuttle between neurons and astrocytes. Biochim Biophys Acta. doi:10.1016/j.bbapap.2011.01.001
Wu J, Xu H, Shen W, Jiang C (2004) Expression and coexpression of CO2-sensitive Kir channels in brainstem neurons of rats. J Membrane Biol 197:179–191. doi:10.1007/s00232-004-0652-4
Wu Z, Zhang J, Nakanishi H (2005) Leptomeningeal cells activate microglia and astrocytes to induce IL-10 production by releasing pro-inflammatory cytokines during systemic inflammation. J Neuroimmunol 167(1–2):90–98. doi:10.1016/j.jneuroim.2005.06.025
Xu F, Frazier DT (1995) Medullary respiratory neuronal activity modulated by stimulation of the fastigial nucleus of the cerebellum. Brain Res 705(1–2):53–64
Xu F, Zhang Z, Frazier DT (2001) Microinjection of acetazolamide into the fastigial nucleus augments respiratory output in the rat. J Appl Physiol 91(5):2342–2350
Xu G, Wang W, Kimelberg HK, Zhou M (2010) Electrical coupling of astrocytes in rat hippocampal slices under physiological and simulated ischemic conditions. Glia 58(4):481–493. doi:10.1002/glia.20939
Yao ST, Barden JA, Finkelstein DI, Bennett MR, Lawrence AJ (2000) Comparative study on the distribution patterns of P2X1-P2X6 receptor immunoreactivity in the brainstem of the rat and the common marmoset (Callithrix jacchus): association with catecholamine cell groups. J Comp Neurol 427:485–507
Young JK, Dreshaj IA, Wilson CG, Martin RJ, Zaidi SI, Haxhiu MA (2005a) An astrocyte toxin influences the pattern of breathing and the ventilatory response to hypercapnia in neonatal rats. Respir Physiol Neurobiol 147(1):19–30. doi:10.1016/j.resp.2005.01.009
Young JK, Wu M, Manaye KF, Kc P, Allard JS, Mack SO, Haxhiu MA (2005b) Orexin stimulates breathing via medullary and spinal pathways. J Appl Physiol 98(4):1387–1395. doi:10.1152/japplphysiol.00914.2004 (1985)
Yudkoff M, Daikhin Y, Nissim I, Pleasure D, Stern J (1994) Inhibition of astrocyte glutamine production by alpha-ketoisocaproic acid. J Neurochem 63(4):1508–1515
Zwicker JD, Rajani V, Hahn LB, Funk GD (2011) Purinergic modulation of preBotzinger complex inspiratory rhythm in rodents: the interaction between ATP and adenosine. J Physiol 589(Pt 18):4583–4600. doi:10.1113/jphysiol.2011.210930
Acknowledgments
Support from Grants Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT) #1130874 (JE), Comisión Nacional de Ciencia y Tecnología (CONICYT) #21140669 (MJ Olivares), Comisión Nacional de Ciencia y Tecnología (CONICYT) #21120594 (S Beltrán-Castillo). DICYT-USACH (JE).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Eugenín León, J., Olivares, M.J., Beltrán-Castillo, S. (2016). Role of Astrocytes in Central Respiratory Chemoreception. In: von Bernhardi, R. (eds) Glial Cells in Health and Disease of the CNS. Advances in Experimental Medicine and Biology, vol 949. Springer, Cham. https://doi.org/10.1007/978-3-319-40764-7_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-40764-7_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-40762-3
Online ISBN: 978-3-319-40764-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)