Abstract
Cancer cells and coagulation system are strictly connected. General prothrombotic mechanisms are related both to the host response to cancer and to the procoagulant activity of cancer cells (Fig. 4.1). Host-related factors include the acute-phase reaction, paraprotein production, inflammation, necrosis, and hemodynamic disorders. Malignant cells can activate blood coagulation in several ways. They can produce: procoagulant factors as tissue factor (TF) and cancer procoagulant factor (CP), which are the most powerful procoagulant observed; microparticles (MP); inflammatory cytokines such as tumor necrosis factor (TNF) and interleukin-1 (IL-1); pro-angiogenic factors as vascular endothelial growth factor (VEGF), which promote both endothelial prothrombotic alterations and angiogenesis. VEGF is an indirect procoagulant that increases microvascular permeability, reprograms gene expression, and promotes the survival of endothelial cells; the resulting increased vascular density plays a key role in the pathophysiology of many cancers. The same procoagulant factors contribute to tumor progression. Also platelets, endothelial cells, and neutrophils of host cells are stimulated to express procoagulant activity. Thus, thromboembolism frequently complicates the course of malignancy and can be the first symptom of cancer [1, 2].
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
1 Introduction
Cancer cells and coagulation system are strictly connected. General prothrombotic mechanisms are related both to the host response to cancer and to the procoagulant activity of cancer cells (◘ Fig. 4.1). Host-related factors include the acute-phase reaction, paraprotein production, inflammation, necrosis, and hemodynamic disorders. Malignant cells can activate blood coagulation in several ways. They can produce: procoagulant factors as tissue factor (TF) and cancer procoagulant factor (CP), which are the most powerful procoagulant observed; microparticles (MP); inflammatory cytokines such as tumor necrosis factor (TNF) and interleukin-1 (IL-1); pro-angiogenic factors as vascular endothelial growth factor (VEGF), which promote both endothelial prothrombotic alterations and angiogenesis. VEGF is an indirect procoagulant that increases microvascular permeability, reprograms gene expression, and promotes the survival of endothelial cells; the resulting increased vascular density plays a key role in the pathophysiology of many cancers. The same procoagulant factors contribute to tumor progression. Also platelets, endothelial cells, and neutrophils of host cells are stimulated to express procoagulant activity. Thus, thromboembolism frequently complicates the course of malignancy and can be the first symptom of cancer [1, 2].
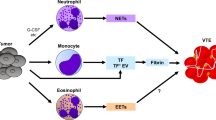
Cancer-hemostatic system interactions. Tumor cells can activate the hemostatic system in multiple ways. Tumor cells release procoagulant activities, and microparticles (MP), by which the coagulation cascade is activated. Tumor cells also activate the host hemostatic cells (endothelial cells, leukocytes, and platelets), by either release of soluble factors or direct adhesion contact, thus eliciting the expression of a procoagulant phenotype of these cells. In addition, the neutrophils can release neutrophil extracellular trap (NETs), and the adhesion of a large quantity of NETs to the vasculature may initiate thrombosis by providing a scaffold for platelet adhesion, activation, and thrombin generation. Reproduced with permission from [1]
2 Clinical Aspects
-
The more common events are venous thromboembolism (VTE), including deep venous thrombosis (DVT) and pulmonary embolism (PE). VTE is the second-leading cause of death in patients with malignancy [3].
-
The arterial thrombotic events (ATE) in cancer are common after treatment with antiangiogenic drugs, cisplatin, and hormonal therapies [4, 5].
-
The cardiovascular risk factors associated with the hypercoagulability of cancer contribute to the precipitating of thrombotic events. VTE incidence is significantly different (P < 0.0001) for the different types of cancer, with the higher rate observed in patients with pancreatic cancer (19.2 %) and the lowest in patients with bladder cancer (8.2 %) [6]. Although DVT is more common in patients with pancreatic cancer (12.6 %), the PE is more common in lung (3.6 %) and gastric cancers (3.3 %).
-
VTE is also associated with recurrent VTE as well as bleeding, both at significantly higher rates than seen in non-cancer patients [7].
-
Systemic chemotherapy increases the risk of VTE sixfold–sevenfold, and the rise in cancer-associated VTE over recent decades may have been caused in part by the introduction of therapies with direct effects on the vascular endothelium [6].
-
Clinically apparent VTE occurs in as many as 10 % of patients with cancer, but autoptic studies have described higher rates of thrombosis in some subgroups: for example the patients who died of pancreatic cancer, [1, 7–9].
-
VTE is associated with a threefold increase in hospitalizations and an increased healthcare resource utilization and costs. It is vital to adopt appropriate strategies for prevention and treatment of venous thromboembolism in order to reduce its impact in patients with cancer and health care system. [10].
3 Screening for Occult Cancer in Patients with Idiopathic VTE
An “idiopathic” VTE may be the first clinical sign of a tumor; up to 10 % of patients with unprovoked VTE receive a cancer diagnosis within the first year subsequent to the event, and more than 60 % of occult cancers are diagnosed shortly after the diagnosis of idiopathic VTE [2, 11]. The utility of using an extensive screening for the purpose of early identification of an occult neoplasm is controversial. In the absence of specific guidelines, clinical practice is very variable and differs depending on the prevailing beliefs of the individual centers. A recently published study compared a limited occult cancer screening (basic blood testing, chest radiography, age screening for breast, cervical, and prostate cancer) to limited occult-cancer screening in combination with computed tomography (CT) of the abdomen and pelvis. There was no significant difference between the two study groups in the mean time to a cancer diagnosis (4.2 months in the limited-screening group and 4.0 months in the limited-screening-plus-CT group, P = 0.88) or in cancer-related mortality (1.4 and 0.9 %, P = 0.75) at 1-year follow-up [12].
For patients with a first episode of unprovoked VTE, a limited testing for cancer, including a history and physical examination, complete blood count, serum chemistries, liver function tests and urinalysis, routine age-appropriate cancer screening, and chest radiography, is actually suggested [13].
4 Primary Thromboprophylaxis and Identification of High-Risk Outpatients
4.1 Surgical Prophylaxis
-
After abdominal or pelvic surgery for cancer, thromboprophylaxis with either unfractionated heparin (UFH) or low molecular weight heparin (LMWH) reduces the risk of deep vein thrombosis by about 15 %.
-
UFH versus LMWH.
-
In the ENOXACAN study (designed to compare the efficacy and safety of LMWH enoxaparin 40 mg/day versus UFH at low doses, in the prophylaxis in cancer abdominal or pelvic surgery), the incidence of DVT was 18.2 % in patients with UFH and 14.7 % in the group with enoxaparin [14].
-
a meta-analysis that included 16 clinical trials with 12,890 patients with cancer found no major differences between the perioperative thromboprophylaxis with LMWH compared with UFH in their effects on mortality, VTE, and bleeding in patients with cancer.
-
The advantage of single daily dosing, the most favorable pharmacological profile, and the lower association with heparin-induced thrombocytopenia make the most used LMWH compared with UFH [15, 16].
-
-
The benefit of a longer duration of postoperative anticoagulation than typically used for individuals without cancer was shown in three separate trials that randomized patients undergoing major cancer surgery to 1 week versus 4 weeks of a LMWH.
-
These trials all showed a significant reduction in the incidence of VTE after 4 weeks of anticoagulation compared with 1 week (5 versus 12 %, 7 versus 16 %, and 13 versus 10 %) [17–19].
-
None of the trials showed increased bleeding in the prolonged anticoagulation group. Although it remains to be confirmed that extended thromboprophylaxis improves survival or is cost-effective after surgery for cancer, extended prophylaxis up to 4 weeks after surgery for cancer is recommended by consensus guidelines.
-
-
As regards the laparoscopic surgery, a significant difference in the incidence of postoperative VTE was not observed when compared to traditional open surgery [20].
4.2 High-Risk Cancer Outpatients Identification for Primary Thromboprophylaxis
A recent systematic review estimates the overall risk of VTE to be 13 per 1000 person-years (95% CI, 7-23). The risk of VTE was 68 per 1000 person-years (95% CI, 48 to 96) in patients considered at high risk for metastatic disease or high-risk treatments.The highest risk is in patients with brain cancer (200 per 1000 personyears; 95% CI, 162-247).
-
An appropriate risk stratification of patients is therefore crucial to highlight which patients should receive prophylaxis [21].
Clinical risk factors, biomarkers, or combinations of the two can be used to estimate VTE risk.
-
Clinical risk factors include tumor type, location, stage, and time since diagnosis influence VTE risk, along with patient comorbidities and therapeutic interventions (◘ Table 4.1).
-
Factors related to treatment are: surgery, chemotherapy, hormonal therapy, anti-angiogenic agents (thalidomide, lenalidomide, bevacizumab), central venous catheters, erythropoiesis-stimulating agents (ESAs), blood transfusions.
-
VTE risk after surgery is 2 times higher in patients with known cancer than in the general population; 1/3 of events occur after discharge, and mortality is more than six times higher in patients with VTE compared to those without VTE (8 versus 1.2 %) [23].
Table 4.1 Risk factors for thromboembolism -
-
Individuals with cancer should be considered high risk for development of postoperative VTE. This increased risk is reflected in the Caprini score for VTE in surgical patients, which assigns two points for the presence of malignancy.
-
Chemotherapy with cisplatin and fluorouracil may induce thrombogenic effects through multiple mechanisms that include the secretion by the tumor cells of pro-angiogenic and immunomodulatory cytokines, increase of TF on endothelial cells, direct toxicity on the endothelium, and reduced synthesis of C protein.
-
Erythropoiesis-stimulating agents (ESA) reduce the need for transfusions and improve quality of life but cause an increased incidence of VTE and mortality [23].
-
The advent of novel “targeted” anticancer therapies has not reduced the risk of VTE. Indeed, drugs targeting angiogenesis such as bevacizumab, sunitinib, sorafenib, and the multi-targeted tyrosine kinase inhibitor ponatinib have been associated with arterial thromboembolism.
-
Immunomodulatory agents such as thalidomide and lenalidomide have been associated with very high rates of VTE, and anti-epidermal growth factor antibodies such as cetuximab and panitumumab have also recently been associated with VTE [24–26].
-
-
A particularly high risk has been reported in patients receiving chemotherapy in combination with antiangiogenic agents. The highest incidence of VTE in myeloma occurs during treatment with thalidomide associated with anthracyclines (12–28 %), thalidomide with dexamethasone at high doses (17–26 %), lenalidomide, and high dose of dexamethasone (18–26 %) [27–30].
-
A variety of biomarkers have also been associated with VTE in malignancy. These include platelet count ≥350,000 before chemotherapy, white blood cell count >11,000 before chemotherapy, hemoglobin value <10 g/dL, elevated tissue factor expression on the surface of cancer cells, high activity of circulating tissue factor, elevated d-dimer, elevated soluble P-selectin, thrombin generation, and levels of TF bearing microparticles (TFMP) [31–33].
A validated model to guide clinical decisions on thromboprophylaxis in patients treated with chemotherapy has been developed by Khorana. The Khorana score is calculated by assigning points for clinical parameters available for patients (i.e., site of primary tumor, hmatologic parameters, and body mass index) [34] (◘ Table 4.2).
-
This risk score was originally derived from a development cohort of 2701 patients and then validated in an independent cohort of 1365 patients, stratified into three risk groups to predict the development of VTE. The cumulative incidence of VTE at 2.5 months ranged from 0.3 to 6.7 % in patients with the fewest and most risk factors, respectively.
Table 4.2 Predictive model for VTE. Points for the risk model are based on the regression coefficients obtained from the final model and divided the population into 3 risk categories based on the score from the risk model: low (score 0), intermediate (score 1-2), and high (score 3; not present) -
Subsequently, the score was externally validated prospectively, and modified version of the score was used in an observational cohort study: the Vienna Cancer and Thrombosis Study (CATS). The modified score included:
-
Additional high-risk tumor types (brain, myeloma, kidney).
-
Two additional laboratory values: soluble P-selectin and d-dimer levels.
-
In a retrospective analysis, the cumulative incidence rates of VTE at 6 months were 1 % for the lowest risk group (0 points) and 35 % for the highest risk group (≥5 points) [35].
Recently, the same working group has explored the utility of soluble plasma VEGF-A (sVEGF) as a biomarker for the prediction of VTE in patients with cancer, and elevated sVEGF is proved to be associated with an increased risk of VTE in patients with cancer [36].
In the PROTECHT score, the inclusion of chemotherapy with platinum (carboplatin, cisplatin) or gemcitabine over the five variables identified high-risk cancer patients in a post hoc analysis of the PROTECHT study. Patients with higher absolute risk of VTE would derive the most benefit as demonstrated by the analysis of subgroups of two largest randomized trials.. Rates of VTE in high-risk patients enrolled in PROTECHT were 11.1 % in the placebo arm and 4.5 % in the nadroparin arm (NNT = 15 versus 77 for low and intermediate-risk patients) [37].
In the subgroup analysis of SAVE-ONCO, rates of VTE in patients with Khorana risk score ≥3 were 5.4 % in the placebo arm and 1.5 % in the semuloparin arm (NNT = 25 for high-risk patients to 333 for low-risk patients) [38].
Anticoagulant prophylaxis has been shown to reduce the risk of VTE in medical patients, but none of the trials reported the rates of symptomatic venous thromboembolic events or major bleeding episodes according to cancer status; recommendations are therefore made based upon extrapolation of data from randomized trials that included only a small minority of cancer patients [39].
4.3 Multiple Myeloma
VTE incidence is about 5 % in myeloma patients treated with conventional chemotherapy. The anti-angiogenic agents for the treatment of multiple myeloma, such as thalidomide and lenalidomide, are known to activate endothelial cells and platelets, and to damage the vascular endothelium, increasing the thrombotic risk. VTE incidence is particularly high when thalidomide or lenalidomide is combined with high-dose dexamethasone (480 mg/month), with doxorubicin or with polichemotherapy. For patients with multiple myeloma, a risk assessment model has been proposed from the Myeloma Working Group. It takes into account individual risk factors, factors linked to the disease and therapy [27].
Special Recommendations
-
Aspirin is recommended for patients with 0–1 risk factors.
-
Enoxaparin 40 mg per day or full-dose warfarin (target INR 2–3) are indicated both for patients with two or more individual risk factors related to myeloma, both for patients treated with thalidomide/lenalidomide associated with high-dose dexamethasone and/or doxorubicin.
-
A randomized study compared the effectiveness of low dose aspirin, 1.5 mg/day dose warfarin, and enoxaparin 40 mg in patients with newly diagnosed myeloma treated with thalidomide-based regimens, cortisone and melphalan or bortezomib. Similar efficacy of therapeutic regimens in reducing VTE in elderly patients was observed; however, enoxaparin showed greater efficacy compared to warfarin in reducing major thromboembolic events, acute cardiovascular events, and sudden deaths [40].
-
Another study compared low-dose aspirin with enoxaparin 40 mg in patients treated with lenalidomide.
-
The incidence of VTE was 2.27 % in the aspirin group and 1.2 % in the LMWH group, suggesting that aspirin is an effective therapeutic alternative for patients at “standard” risk [41].
-
-
It was also noted that the treatment with bortezomib reduces the 2 % risk of VTE for an antithrombotic effect due to the synthesis of nitric oxide which would result in a reduced platelet activation [42].
-
All patients with malignancies undergoing major surgery should be considered for both pharmacological thromboprophylaxis with UFH or with LMWH, unless contraindicated due to active bleeding or high risk of bleeding.
-
Once daily LMWH, UFH three times a day or fondaparinux is recommended to prevent postoperative VTE in patients with cancer.
-
Pharmacological prophylaxis should be started 12–2 h before surgery and continued for at least 7–10 days. Use of fondaparinux must be made 6 h after surgery.
-
The extended prophylaxis for 4 weeks in patients with a high risk of VTE (pelvic and abdominal surgery) and low bleeding risk for patients undergoing abdominal or pelvic surgery for cancer with high-risk features such as reduced mobility, obesity, history of VTE, or other risk factors is recommended.
-
The use of LMWH for the prevention of VTE in cancer patients undergoing laparoscopic surgery is recommended as for laparotomy.
-
Mechanical methods are used in association with drug therapy in high-risk patients but are not recommended as monotherapy except when pharmacological prophylaxis is contraindicated for active bleeding or high risk of bleeding.
5 Appropriate Immediate and Long-Term Treatment for Patients with Acute Thromboembolism and the Role of NOACs
The goal of therapy is to resolve the acute episode and to prevent recurrence, extension, and embolism while minimizing the risk of bleeding. However, treatment of VTE in cancer is complicated due to higher rates of recurrent VTE as well as a higher risk of bleeding with anticoagulation treatment.
5.1 Acute Treatment (First 10 Days)
-
The therapeutic options for TVE in the acute phase include UFH, LMWH, and Fondaparinux.
-
Few studies have directly compared the anticoagulant therapy for the initial treatment of VTE in cancer patients. In a meta-analysis of 16 randomized controlled trials in cancer patients treated with anticoagulants for VTE, LMWH compared to UHF was associated with a reduction in mortality at 3 months (RR 0.71; 95 % CI 0.52, 0.98) without an increased risk of bleeding [43].
-
LMWH offers other advantages over UFH: cost, ease of dosing and a lower risk of heparin-induced heparin (HIT).
-
There are no studies in the literature of direct comparison between fondaparinux and LMWH for the initial treatment of VTE.
-
UFH can be used in patients with severe renal impairment (CrCl < 30 mL/min) because the liver is a main site of heparin biotransformation. Other advantages of UFH are the short half-life and the reversibility of effect with protamine sulfate.
-
The initial dose of UFH for VTE treatment is based on weight, with the recommended dose of 80 units/kg bolus followed by 18 units/kg/h infusion.
-
UFH is contraindicated in patients with HIT, and in this situation fondaparinux or a thrombin inhibitor represents a better alternative.
-
Fondaparinux is not the first choice in patients with cancer because of the long half-life, the absence of an effective antidote, and the renal clearance of 100 %.
-
Analysis of Matisse-DVT trial subgroup showed higher rates of recurrent VTE in the fondaparinux group than in the enoxaparin group: 12.7 % compared with 5.4 % with no differences in mortality and bleeding [44].
-
Currently, the use of LMWH for the initial treatment of VTE patients with cancer is recommended as treatment of choice.
5.2 Long-Term (First 3 Months) and Extended Therapy (No Planned Stop Date)
-
The minimum duration of anticoagulant therapy for VTE is usually 3 months; this period of treatment is referred to as “long-term therapy.” For long-term therapy, the possible options are vitamin K antagonists (VKA), LMWHH, Fondaparinux, and new oral anticoagulants (NOACs) ad dabigatran, rivaroxaban, apixaban.
-
A meta-analysis of seven randomized clinical trials involving cancer patients with VTE showed a significant reduction of recurrent VTE in patients treated with LMWH compared with those treated with VKA [45].
-
LMWH are preferred to VKA therapy in patients with cancer for several reasons: there is a substantial rate of recurrent VTE in patients with VTE and cancer who are treated with VKA; benefits of LMWH compared to VKA therapy are greater in patients with metastatic disease treated with aggressive chemotherapy [46]; it is often harder to keep patients with cancer who are on VKA in the therapeutic range; LMWH is reliable in patients who have difficulty with oral therapy; and LMWH is easier to withhold or adjust than VKA if invasive interventions are required or thrombocytopenia develops.
-
In the CLOT trial, the efficacy and safety of immediate dalteparin (200 units/kg daily for 5–7 days) followed by chronic (6 months) therapy with an oral coumarin derivative are compared versus chronic dalteparin therapy (200 units/kg daily for 1 month followed by 150 units/kg for months 2–6) in patients with cancer (most of patients had metastatic disease) after diagnosis of acute proximal DVT, PE, or both. Full dose dalteparin was given for 1 month followed by a reduced dose (75–80 %) for 2–6 months.
-
Prolonged treatment with LMWH for 6 months reduced thromboembolic recurrence from 17 to 9 % (P = 0.0017), compared to standard therapy without increasing the risk of bleeding [46].
-
The CATCH study evaluated the efficacy and safety of tinzaparin in patients with active cancer and symptomatic VTE. The patients were randomized to receive tinzaparin 175 IU/kg once a day for 6 months or initial tinzaparin 175 IU/kg once a day for 5–10 days followed by warfarin for 6 months.
-
Tinzaparin significantly reduced the risk of recurrent symptomatic DVT, nonfatal and clinically relevant bleeding [47].
-
Fondaparinux seems to have a similar safety and efficacy profile as LMWH [48].
-
Studies focused on the use of NOAC in cancer patients are still lacking. Preliminary results of the subgroup analyses and meta-analysis of randomized clinical trials suggest that they have efficacy and safety comparable to anticoagulation with VKA and could represent an alternative for oral anticoagulant therapy.
-
A meta-analysis of six RCTs assessed the efficacy and safety of NOACs in patients with cancer-associated VTE. NOACs were shown to be as effective (OR 0.63, 95 % CI 0.37–1.10) and as safe (OR 0.77, 95 % CI 0.41–1.44) as warfarin [49]. The risk reduction for recurrent VTE with the NOACs compared to LMWH has not been assessed but, based on indirect comparisons, LMWH may be more effective that the NOACs in patients with VTE and cancer.
-
However, the small number of patients with cancer (5–8 %) enrolled in each study and the use of warfarin or placebo rather than LMWH in the control do not support yet their use for this population [22, 49–61].
-
Furthermore, there are interactions with some chemotherapeutic agents [49].
-
NOACs have not been compared with VKA in a broad spectrum of patients with VTE and cancer, and indirect comparisons have not shown convincingly different outcomes with different NOACs (◘ Table 4.3).
Table 4.3 Key points of most recent guidelines -
A preference for one NOAC over another or for either a NOAC or VKA in patients with cancer cannot presently be expressed.
See ◘ Tables 4.3 and 4.4 for further details on indications and dose of anticoagulant as prophylaxis and therapy, according to different guidelines.
5.3 Treatment of Catheter-Related DVT
Permanent central venous catheters (CVC) are usually regularly washed with heparin to maintain their patency. However, symptomatic catheter-related venous thrombosis may occur in 4–8 % of patients. Clinical symptoms include edema, pain, and erythema of the affected limb; most of the CVC are inserted in the superior vena cava: thus, there may be swelling of the neck, supraclavicular area, or face. The thrombosis can develop acutely or over a more prolonged period of time. In case of thrombosis, the choice is influenced by the need of maintaining the device for further antineoplastic treatments of the patient [61, 62].
-
The removal is recommended if the device is no longer required or when patency cannot be restored by thrombolysis and/or anticoagulation; there are contraindications to anticoagulation (active bleeding, platelet count <50,000/μL, or recent central nervous system bleeding or surgery), if the catheter is infected or dysfunctional.
-
Before removing the device, a short time (5–7 days) of anticoagulant therapy is recommended to reduce the risk of embolization. Therefore evaluation of the likelihood and consequences of embolization according to the size and location of the thrombus should be carried out.
-
Patients with cancer who have had their CVCs removed and then replaced without anticoagulation often experience recurrent thrombosis.
-
-
Anticoagulant therapy with LMWH is recommended if the catheter must remain in situ for a period of at least 3 months up to 6 months [62].
-
Thrombolytic therapy is justified in cases of thrombosis of the superior vena cava with caval syndrome poorly tolerated or complete occlusion of the CVC. Instillation of 2 mg of t-PA or Urokinase 10,000 IU infused in each catheter lumen for 4 h once a week may restore catheter patency.
-
In case of symptomatic CVC thrombosis, anticoagulant treatment is recommended for a minimum of 3 months.
-
In this setting, LMWHs are suggested. Oral VKA can also be used, in the absence of direct comparisons of these two types of anticoagulants in this setting.
-
The CVC can be kept in place if it is functional, well-positioned, and noninfected with good resolution of symptoms under close surveillance.
5.4 Vena Cava Filters
-
The implantation of inferior vena cava (IVC) filters should be restricted to patients with VTE and/or PE and contraindications to anticoagulation [63].
-
Active bleeding.
-
High risk of bleeding.
-
Undergoing surgery at high risk of bleeding (as major abdominal surgery).
-
-
Their use in patients with recurrent thrombotic events despite anticoagulant therapy does not appear logical because the filters do not treat the condition of thrombosis, and the presence of an intravascular foreign body is likely to promote the formation of a thrombus proximal or distal to the thrombus filter.
-
IVC thrombotic occlusion is the presence of an occluding thrombus in the IVC after filter insertion can be symptomatic or asymptomatic and remains a serious complication of IVC filtration. The reported incidence ranges from 2 to 30 %.
-
Renal failure secondary to renal vein thrombosis has been reported after suprarenal filter placement. Suprarenal filters should be avoided in patients with a single functional kidney, renal insufficiency, or previous renal vein thrombosis.
-
-
The possible complications of inferior vena cava filters are [64]:
-
Penetration of the vein wall by a filter strut or anchor device with transmural incorporation, with secondary lesion to the neighbouring structures.
-
Filter embolization, defined as post-deployment movement of the filter or its components to a distant anatomic site (heart and pulmonary tree).
-
Filter fracture (i.e., breakage or separation); the reported incidence of filter fracture is as high as 2–10 %.
-
-
Retrievable devices should be used, and possibly be removed, re-starting the anticoagulant therapy, as soon as possible [63, 64].
Special Recommendations
-
Brain tumor in itself is not a contraindication to anticoagulation therapy in VTE. For the treatment of VTE in patients with brain tumor, it is preferable to use LMWH for 6 months in all patients except those with tumors that have a high rate of intracranial hemorrhage (metastases from melanoma, choriocarcinoma, thyroid carcinoma, and renal cell carcinoma).
-
There is no evidence to recommend LMWH or UFH over another in elderly patients with active malignancy. Tinzaparin might have a favorable biologic profile using therapeutic dosing in the setting of renal insufficiency.
-
In patients with renal insufficiency and Ccr < 30 mL/min, enoxaparin might have a less favorable biologic and the dosage should be reduced a 1 mg/kg sc every 24 h or factor Xa will be monitored UFH, tinzaparin and dalteparin are preferred as agents.
-
In cancer patients who are pregnant, standard prophylaxis should be implemented.
-
Heparin-induced thrombocytopenia (HIT), due to the formation of antibodies against a complex platelet factor 4 (PF4)/heparin, is a consumptive thrombocytopenia associated with a serious pro-thrombotic state. The treatment of HIT is based on the use of direct thrombin inhibitors, such as lepirudin (0.08 mg/kg/h, to be halved in case of kidney failure), argatroban, and bivalirudin. Fondaparinux may be an alternative, but its use is not consolidated and is considered off-label.
6 Management of Recurrent VTE on Anticoagulation Therapy [55, 63–67]
-
In patients with thrombosis occurring during treatment with oral anticoagulant, a relapse with INR not in the therapeutic range requires a dose adjustment in order to obtain INR in the range between 2 and 3.
-
For recurrent VTE on a non-LMWH anticoagulant, LMWHs are suggested.
-
If recurrence occurs in the course of LMWH at subtherapeutic doses (75–80 %), the re-administration of the LMWH at full dose may be effective in more than 90 % of patients and for recurrent VTE on full dose of LMWH; the dose may be increased by about 25 %.
-
If the patient is taking medications that increase the risk of thrombosis such as estrogens or chemotherapy, these treatments should be possibly withdrawn.
7 Treatment Strategy in Patients with Thrombocytopenia
-
In patients with thrombocytopenia, full doses of anticoagulation can be used for VTE treatment if the platelet count is ≥50,000, and there is no evidence of bleeding.
-
For patients with a platelet count of 20,000–50,000, a 50 % of full dose must be employed.
-
Stop anticoagulants for a count <20,000 always adapting the decision to the individual case.
-
If severe cancer or chemotherapy-induced thrombocytopenia is present, platelet transfusions may be used to allow anticoagulation; an experience of few cases reported in the literature suggests that the use of prophylactic doses of LMWH can be tolerated in patients with platelet counts ≤20 × 109/L with associated resolution of thrombotic symptoms [67].
References
Falanga A, Marchetti M, Russo L. The mechanisms of cancer-associated thrombosis. Thromb Res. 2015;135 Suppl 1:S8–11.
Prandoni P, Falanga A, Piccioli A. Cancer and venous thromboembolism. Lancet Oncol. 2005;6:401–10.
Khorana AA, Francis CW, Culakova E, et al. Thromboembolism is a leading cause of death in cancer patients receiving outpatient chemotherapy. J Thromb Haemost. 2007;5(3):632–4.
Sanon S, Lenihan DJ, Mouhayar E. Peripheral arterial ischemic events in cancer patients. Vasc Med. 2011;16:119–30.
Di Nisio M, Ferrante N, Feragalli B, et al. Arterial thrombosis in ambulatory cancer patients treated with chemotherapy. Thromb Res. 2011;127:382–3.
Khorana AA, Dalal M, Lin J, Connolly GC. Incidence and predictors of venous thromboembolism (VTE) among ambulatory high-risk cancer patients undergoing chemotherapy in the United States. Cancer. 2013;119:648–55.
Prandoni P, Lensing AW, Piccioli A, et al. Recurrent venous thromboembolism and bleeding complications during anticoagulant treatment in patients with cancer and venous thrombosis. Blood. 2002;100:3484–8.
Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712.
Sproul EE. Carcinoma and venous thrombosis: the frequency of association of carcinoma in the body or tail of the pancreas with multiple venous thrombosis. Am J Cancer. 1938;34:566.
Khorana AA, Dalal MR, Lin J, Connolly GC. Health care costs associated with venous thromboembolism in selected high risk ambulatory patients with solid tumors undergoing chemotherapy in the United States. Clinicoecon Outcomes Res. 2013;5:101–8.
White RH, Chew HK, Zhou H, et al. Incidence of venous thromboembolism in the year before the diagnosis of cancer in 528,693 adults. Arch Intern Med. 2005;165:1782–7.
Carrier M, Lazo-Langner A, Shivakumar S, SOME Investigators, et al. Screening for occult cancer in unprovoked venous thromboembolism. N Engl J Med. 2015;373(8):697–70.
Khorana AA, Carrier M, Garcia DA, Lee AY. Guidance for the prevention and treatment of cancer-associated venous thromboembolism. J Thromb Thrombolysis. 2016;41(1):81–91. doi:10.1007/s11239-015-1313-4.
ENOXACAN Study Group. Efficacy and safety of enoxaparin versus unfractionated heparin for prevention of deep vein thrombosis in elective cancer surgery: a double-blind randomized multicentre trial with venographic assessment. Br J Surg. 1997;84:1099–103.
Akl EA, Kahale L, Sperati F, et al. Low molecular weight heparin versus unfractionated heparin for perioperative thromboprophylaxis in patients with cancer. Cochrane Database Syst Rev. 2014;6:CD009447. doi:10.1002/14651858.CD009447.pub2.
Akl EA, Kahale LA, Schünemann HJ. Association between perioperative low-molecular-weight heparin vs unfractionated heparin and clinical outcomes in patients with cancer undergoing surgery. JAMA. 2015;313:1364–5.
Bergqvist D, Agnelli G, Cohen AT, ENOXACAN II Investigators, et al. Duration of prophylaxis against venous thromboembolism with enoxaparin after surgery for cancer. N Engl J Med. 2002;346:975.
Kakkar VV, Balibrea JL, Martínez-González J, Prandoni P, CANBESURE Study Group. Extended prophylaxis with bemiparin for the prevention of venous thromboembolism after abdominal or pelvic surgery for cancer: the CANBESURE randomized study. J Thromb Haemost. 2010;8:1223–9.
Rasmussen MS, Jorgensen LN, Wille-Jørgensen P, FAME Investigators, et al. Prolonged prophylaxis with dalteparin to prevent late thromboembolic complications in patients undergoing major abdominal surgery: a multicenter randomized open-label study. J Thromb Haemost. 2006;4:2384–90.
Cui G, Wang X, Yao W, Li H. Incidence of postoperative venous thromboembolism after laparoscopic versus open colorectal cancer surgery: a meta-analysis. Surg Laparosc Endosc Percutan Tech. 2013;23:128–34.
Horsted F, West J, Grainge MJ. Risk of venous thromboembolism in patients with cancer: a systematic review and meta-analysis. PLoS Med. 2012;9:e1001275.
Lyman GH, Bohlke K, Khorana AA, American Society of Clinical Oncology, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American society of clinical oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33(6):654–6.
Merkow RP, Bilimoria KY, McCarter MD, et al. Post-discharge venous thromboembolism after cancer surgery: extending the case for extended prophylaxis. Ann Surg. 2011;254:131.
Choueiri TK, Schutz FA, Je Y, Rosenberg JE, Bellmunt J. Risk of arterial thromboembolic events with sunitinib and sorafenib: a systematic review and meta-analysis of clinical trials. J Clin Oncol. 2010;28:2280–5.
Cavo M, Zamagni E, Cellini C, et al. Deep-vein thrombosis in patients with multiple myeloma receiving first-line thalidomide-dexamethasone therapy. Blood. 2002;100:2272–3.
Petrelli F, Cabiddu M, Borgonovo K, Barni S. Risk of venous and arterial thromboembolic events associated with anti-EGFR agents: a meta-analysis of randomized clinical trials. Ann Oncol. 2012;23:1672–9.
Palumbo A, Rajkumar SV, Dimopoulos MA, International Myeloma Working Group, et al. Prevention of thalidomide- and lenalidomide-associated thrombosis in myeloma. Leukemia. 2008;22:414.
Zangari M, Anaissie E, Barlogie B, et al. Increased risk of deep-vein thrombosis in patients with multiple myeloma receiving thalidomide and chemotherapy. Blood. 2001;98:1614.
Rajkumar SV, Blood E. Lenalidomide and venous thrombosis in multiple myeloma. N Engl J Med. 2006;354:2079.
Rajkumar SV, Jacobus S, Callender N, et al. Phase III trial of lenalidomide plus high-dose dexamethasone versus lenalidomide plus low-dose dexamethasone in newly diagnosed multiple myeloma (E4A03): a trial coordinated by the Eastern Cooperative Oncology Group (abstract). Clin Oncol. 2007;25:968s.
Mackman N. Role of tissue factor in hemostasis, thrombosis, and vascular development. Arterioscler Thromb Vasc Biol. 2004;24:1015–22.
Khorana AA, Liebman HA, White RH, et al. The risk of venous thromboembolism in patients with cancer. Alexandria, VA: American Society of Clinical Oncology, ASCO Educational Book; 2008. p. 240–8.
Carrier M, Le Gal G, Wells PS, et al. Systematic review: the Trousseau syndrome revisited: should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149:323–33.
Khorana AA, Kuderer NM, Culakova E, et al. Development and validation of a predictive model for chemotherapy-associated thrombosis. Blood. 2008;111:4902–7.
Ay C, Dunkler D, Marosi C, et al. Prediction of venous thromboembolism in cancer patients. Blood. 2010;116:5377.
Posch F, Thaler J, Zlabinger GJ, et al. Soluble vascular endothelial growth factor (sVEGF) and the risk of venous thromboembolism in patients with cancer: results from the Vienna cancer and thrombosis study (CATS). Clin Cancer Res. 2016;22:200–6.
Verso M, Agnelli G, Barni S, et al. A modified Khorana risk assessment score for venous thromboembolism in cancer patients receiving chemotherapy: the Protecht score. Intern Emerg Med. 2012;7:291–2.
George D, Agnelli G, Fisher W, et al. Poster presentation: venous thromboembolism (VTE) prevention with semuloparin in cancer patients initiating chemotherapy: benefit risk assessment by VTE risk in SAVE-ONCO. https://ash.confex.com/ash/2011/webprogram/Paper39639.
Carrier M, Khorana AA, Moretto P, et al. Lack of evidence to support thromboprophylaxis in hospitalized medical patients with cancer. Am J Med. 2014;127(1):82–6.
Palumbo A, Cavo M, Bringhen S, et al. Aspirin, warfarin, or enoxaparin thromboprophylaxis in patients with multiple myeloma treated with thalidomide: a phase III, open-label, randomized tri. J Clin Oncol. 2011;29:986–9381.
Larocca A, Cavallo F, Bringhen S, et al. Aspirin or enoxaparin thromboprophylaxis for patients with newly diagnosed multiple myeloma treated with lenalidomide. Blood. 2012;119:933–9.
Zangari M, Fink L, Zhan F, Tricot G. Low venous thromboembolic risk with bortezomib in multiple myeloma and potential protective effect with thalidomide/lenalidomide-based therapy: review of data from phase 3 trials and studies of novel combination regimens. Clin Lymphoma Myeloma Leuk. 2011;11:228–36.
Akl EA, Kahale L, Neumann I, et al. Anticoagulation for the initial treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014; 19;(6):CD006649.
Van Doormaal FF, Raskob GE, Davidson BL, et al. Treatment of venous thromboembolism in patients with cancer: subgroup analysis of the Matisse clinical trials. Thromb Haemost. 2009;101:762–9.
Akl EA, Kahale L, Barba M, et al. Anticoagulation for the long-term treatment of venous thromboembolism in patients with cancer. Cochrane Database Syst Rev. 2014;7:CD006650.
Lee AYY, Levine MN, Baker RI, et al. Low-molecular-weight heparin versus a coumarin for the prevention of recurrent venous thromboembolism in patients with cancer. N Engl J Med. 2003;349:146–1539.
Lee AY, Kamphuisen PW, Meyer G, CATCH Investigators, et al. Tinzaparin vs warfarin for treatment of acute venous thromboembolism in patients with active cancer: a randomized clinical trial. JAMA. 2015;314(7):677–8.
Pesavento R, Amitrano M, Trujillo-Santos J, et al. Fondaparinux in the initial and long-term treatment of venous thromboembolism. Thromb Res. 2015;135:311–7.
Bauersachs R, Berkowitz SD, Brenner B, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510.
Buller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–97.
Buller HR, Decousus H, Grosso MA, et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–15.
Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808.
Vedovati MC, Germini F, Agnelli G, Becattini C. Direct oral anticoagulants in patients with VTE and cancer: a systematic review and meta-analysis. Chest. 2015;147:475–83.
Franchini M, Bonfanti C, Lippi G. Cancer-associated thrombosis: investigating the role of new oral anticoagulants. Thromb Res. 2015;135:777–81.
Lee AY, Peterson EA. Treatment of cancer-associated thrombosis. Blood. 2013;122:2310–7.
Carrier M, Cameron C, Delluc A, et al. Efficacy and safety of anticoagulant therapy for the treatment of acute cancer-associated thrombosis: a systematic review and meta-analysis. Thromb Res. 2014;134:1214–9.
Lyman GH, Khorana AA, Kuderer NM, et al. American society of clinical oncology clinical practice. J Clin Oncol. 2013;31:2189–204.
Streiff MB, Holmstrom B, Ashrani A, et al. Cancer-associated venous thromboembolic disease, version 1.2015. J Natl Compr Canc Netw. 2015;13:1079–95.
Watson HG, Keeling DM, Laffan M, et al. British Committee for Standards in Haematology Guideline on aspects of cancer-related venous thrombosis Guideline on aspects of cancer-related venous thrombosis. Br J Haematol. 2015;170:640–8.
Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–52.
Debourdeau P, Farge D, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. J Thromb Haemost. 2013;11:71–80.
Schiffer CA, Mangu PB, Wade JC, et al. Central venous catheter care for the patient with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31:1357–70.
Abtahian F, Hawkins BM, Ryan DP, et al. Inferior vena cava filter usage, complications, and retrieval rate in cancer patients. Am J Med. 2014;127:1111–7.
Casanegra AI, Landrum LM, Tafur AJ. Retrievable inferior vena cava filters in patients with cancer: complications and retrieval success rate. Int J Vasc Med. 2016;2016:6413541.
Carrier M, Le Gal G, Cho R, et al. Dose escalation of low molecular weight heparin to manage recurrent venous thromboembolic events despite systemic anticoagulation in cancer patients. J Thromb Haemost. 2009;7:760–5.
Heit JA, Mohr DN, Silverstein MD, et al. Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study. Arch Intern Med. 2000;160:761–8.
Herishanu Y, Misgav M, Kirgner I, et al. Enoxaparin can be used safely in patients with severe thrombocytopenia due to intensive chemotherapy regimens. Leuk Lymphoma. 2004;45:1407–11. Review.
Suggested Reading: Society Guidelines
Streiff MB, Holmstrom B, Ashrani A, et al. Cancer-associated venous thromboembolic disease, version 1.2015. J Natl Compr Canc Netw. 2015;13(9):1079–9554.
Lyman GH, Khorana AA, Kuderer NM, et al. American society of clinical oncology clinical practice. J Clin Oncol. 2013;31(17):2189–20455.
Lyman GH, Bohlke K1, Khorana AA, American Society of Clinical Oncology, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: American society of clinical oncology clinical practice guideline update 2014. J Clin Oncol. 2015;33:654–6.
Watson HG, Keeling DM, Laffan M, et al. British Committee for Standards in Haematology Guideline on aspects of cancer-related venous thrombosis. Guideline on aspects of cancer-related venous thrombosis. Br J Haematol. 2015;170:640–857.
Kearon C, Akl EA, Ornelas J, et al. Antithrombotic therapy for VTE disease: CHEST guideline and expert panel report. Chest. 2016;149:315–5258.
Debourdeau P, Farge D, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. J Thromb Haemost. 2013;11:71–8059.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Bisceglia, I., Maurea, N. (2017). Thromboembolic Disorders as a Consequence of Cancer. In: Lestuzzi, C., Oliva, S., Ferraù, F. (eds) Manual of Cardio-oncology. Springer, Cham. https://doi.org/10.1007/978-3-319-40236-9_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-40236-9_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-40234-5
Online ISBN: 978-3-319-40236-9
eBook Packages: MedicineMedicine (R0)