Abstract
This work constitutes a general description of the results obtained in native aquatic plant species from Portugal (South Western Europe), for the assessment of the potential for phytofiltration techniques of multielement contaminated water. Uptake patterns for metal(loid)s studied indicate the following features: Uranium accumulation in the range of 243–4979 mg/kg (DW) in Fontinalis antipyretica, Callitriche stagnalis, Callitriche hamulata, Callitriche lusitanica, and Ranunculus peltatus; Arsenic concentration from 346 to 2346 mg/kg in C. lusitanica, C. brutia, Lemna minor, Azolla caroliniana, R. trichophyllus, C. stagnalis, and F. antipyretica; Lead from 90.5 to 1104 mg/kg in R. trichophyllus, rhizomes/roots of Typha latifolia, L. minor, Spirodela polyrrhiza, and Myriophyllum spicatum; Copper from 81.8 to 161 mg/kg in C. lusitanica, C. hamulata, R. trichophyllus, and C. stagnalis; and Zinc from 900 to 34,162 mg/kg in L. minor, Lemanea fluviatilis, C. lusitanica, C. brutia, R. trichophyllus, F. antipyretica, and C. stagnalis. The abundance of some of these plant species, their biomass, relatively high bioproductivity, and their ability to accumulate several toxic elements collectively indicates their potential for the development of phytofiltration methodologies, either in monoculture systems or in combined systems representing natural ecosystems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bioaccumulation
- Bioindication
- Biosorption
- Heavy metals
- Macrophytes
- Metalloids
- Phytoextraction
- Phytoremediation
- Portugal
- Wastewater treatment
1 Introduction
All biosphere compartments are vulnerable to pollution , including our freshwater sources—both lentic and lotic. These ecosystems are subjected to constant internal and external changes, both of natural or anthropogenic origin. Anthropogenic influences are often the cause of irreparable damage to some of these ecosystems like mountain streams which are very sensitive. For example, mining activities are well known for their potential deleterious effects on the environment, namely, the contamination of soils, sediments, and waters due to uncontrolled runoff, leaching, and/or aeolian deposition. In fact, leading anthropogenic sources of heavy metals and metalloids pollution are mining and milling operations worldwide. Particularly in the case of abandoned mines where there is no control and monitoring. Lead (Pb), copper (Cu), zinc (Zn), uranium (U), and arsenic (As) are some of the metal(loid)s most frequently reported to have the highest impact on organisms.
Water management in mining areas requires a strategic use of technology, since they imply long-term treatment approaches. The high metal(loid)s content, and the pH/Eh variation of the permanent low flow rate seepage waters, creates major difficulties to the design of efficient and affordable remediation projects [1]. However, water remediation techniques, as well as public awareness campaigns about the risks of exposure to toxic heavy metals and metalloids , should be adopted in contaminated areas, especially where the population still uses private wells as a drinking water source.
Contaminated water can be treated by several methods. Currently, preference is being given to in situ and passive methods that are less environmentally disruptive and more economical. In this context, biotechnology offers phytoremediation techniques as a suitable alternative. In this chapter, results of the last two decades of investigations are presented in the light of phytotechnological potential evaluation, incorporating approaches like phytoremediation, phytoextraction , phytofiltration , biosorption , and bioindication , of the Portuguese native aquatic flora found in waters contaminated with metal(loid)s .
2 Phytoremediation Technology
Phytoremediation is the use of plants (trees, shrubs, grasses, and aquatic plants) and their associated microorganisms in order to remove, degrade, or isolate toxic substances from the environment (e.g., [2–12]). The word phytoremediation derives from the Greek phyton, meaning “plant,” and the Latin remedium, which means “to remedy” or “to correct.” Substances that may be subjected to phytoremediation include metals (Pb, Zn, Cd, Cu, Ni, Hg), metalloids (As, Sb), inorganic compounds (NO3 −, NH4 +, PO4 3-), radioactive chemical elements (U, Cs, Sr), petroleum hydrocarbons (BTEX), pesticides and herbicides (atrazine, bentazon, chlorinated, and nitroaromatic compounds), explosives (TNT, DNT), chlorinated solvents (TCE, PCE) and industrial organic wastes (PCPs, PAHs), and others (e.g., [3, 9, 13–19]). Phytoremediation techniques include different modalities, depending on the chemical nature and properties of the contaminant (if it is inert, volatile, or subject to degradation in the plant or in the soil) and the plant characteristics. Thus, phytoremediation essentially comprises six different strategies, though more than one may be used by the plant simultaneously [8].
2.1 Phytodegradation (Phytotransformation)
Organic contaminants are degraded (metabolized) or mineralized inside plant cells by specific enzymes that include nitroreductases (degradation of nitroaromatic compounds), dehalogenases (degradation of chlorinated solvents and pesticides), and laccases (degradation of anilines). Populus species and Myriophyllum spicatum L. are examples of plants that have these enzymatic systems [20, 21].
2.2 Phytostabilization (Phytoimmobilization)
Contaminants, organic or inorganic, are incorporated into the lignin of the cell wall of roots cells or into humus. Metals are precipitated as insoluble forms by direct action of root exudates and subsequently trapped in the soil matrix. The main objective is to avoid mobilization of contaminants and limit their diffusion in the soil [5, 22–24]. Species of genera Haumaniastrum , Eragrostis , Ascolepis , Gladiolus , and Alyssum are examples of plants cultivated for this purpose .
2.3 Phytovolatilization
This technique relies on the ability of some plants to absorb and volatilize certain metal(loid)s . Some element ions of the groups IIB, VA, and VIA of the periodic table (specifically Hg, Se, and As) are absorbed by the roots, converted into nontoxic forms, and then released into the atmosphere. As example, the species Astragalus bisulcatus (Hook.) A. Gray and Stanleya pinnata (Pursh) Britton for Se or transgenic plants (with bacterial genes) of Arabidopsis thaliana (L.) Heynh., Nicotiana tabacum L., Liriodendron tulipifera L., or Brassica napus L. for Hg can be mentioned [24–28]. This technique can also be used for organic compounds.
2.4 Phytoextraction (Phytoaccumulation, Phytoabsorption, or Phytosequestration)
This involves the absorption of contaminants by roots followed by translocation and accumulation in the aerial parts. It is mainly applied to metals (Cd, Ni, Cu, Zn, Pb) but can also be used for other elements (Se, As) and organic compounds. This technique preferentially uses hyperaccumulator plants that have the ability to store high concentrations of specific metals in their aerial parts (0.01–1 % dry weight, depending on the metal). Elsholtzia splendens Nakai ex F. Maek., Alyssum bertolonii Desv., Noccaea caerulescens (J. Presl & C. Presl) F.K. Mey. (Thlaspi caerulescens J. Presl & C. Presl), and Pteris vittata L. are known examples of hyperaccumulator plants for Cu, Ni, Zn/Cd, and As, respectively [29–36].
2.5 Phytofiltration and Rhizofiltration
When plants absorb, concentrate, and/or precipitate contaminants, particularly heavy metals or radioactive elements, from an aqueous medium through their root system or other submerged organs (e.g., [4, 19, 24, 37–39]). The plants are kept in a hydroponic system, whereby the effluents pass and are “filtered” by the roots (rhizofiltration), or other organs that absorb and concentrate contaminants. Plants with high root biomass, or high absorption surface, with more accumulation capacity (aquatic hyperaccumulators) and tolerance to contaminants achieve the best results. Promising examples include Helianthus annuus L.; Brassica juncea (L.) Czern.; Phragmites australis (Cav.) Trin. ex Steud.; Eichhornia crassipes (Mart.) Solms; Spirodela punctata (G. Mey.) C.H. Thomps.; Fontinalis antipyretica Hedw.; and several species of Salix, Populus, Lemna, and Callitriche [5, 40–44].
2.6 Rhizodegradation and Phytostimulation
Growing roots promote the proliferation of degrading rhizosphere microorganisms which utilize exudates and metabolites of plants as a source of carbon and energy. In addition, plants may exude biodegrading enzymes themselves. The application of rhizodegradation/phytostimulation is limited to organic contaminants [5, 37]. The microbial community in the rhizosphere is heterogeneous due to variable spatial distribution of nutrients; however, species of the genus Pseudomonas are the predominant organisms associated with roots [24, 45, 46].
There are other strategies also, which are considered categories of phytoremediation by some authors, but actually, they are mixed techniques or variations of the earlier mentioned strategies [8].
2.7 Hydraulic Barriers
Some large trees, particularly those with deep roots (e.g., Populus sp.), remove large quantities of groundwater during transpiration. Contaminants in this water are metabolized by plant enzymes and vaporized together with water or simply sequestered in plant tissues [5, 47].
2.8 Vegetation Covers (Vegetative Caps or Phytocovers)
Herbs (usually grasses), eventually shrubs or trees, establish on landfills or tailings, are used to minimize the infiltration of rain water, and contain the spread of pollutants. The roots increase soil aeration thus, promoting biodegradation, evaporation, and transpiration [6, 12, 48–50].
2.9 Constructed Wetlands
These are ecosystems consisting of organic soils, microorganisms, algae, and vascular aquatic plants in areas where the water level is at/near the surface, at least part of the year. All the components work together in the treatment of effluents, through the combined actions of filtration, ion exchange, adsorption, and precipitation (e.g., [37, 51–54]). It is the oldest method of wastewater treatment and is not regarded as proper phytoremediation, since it is based on the contributions of the entire system [5, 55]. Depending on how it processes the water circulation, the wetland is classified as horizontal or surface flow and vertical or subsurface flow. In the latter case ensures a greater reactivity of the influent with the substrate. Good cleaning efficiency, low cost of construction along with easy operation and maintenance are the main advantages. It is widely applied in the treatment of domestic, agricultural, and industrial waste water (e.g., [56, 57]) but has proved to be suitable also for treating acid mine drainages (e.g., [58–60]).
2.10 Phytodesalination
This is a recently reported [24, 61] emerging technique that utilizes halophytes to remove excess salts from saline soils. The potential of Suaeda maritima (L.) Dumort and Sesuvium portulacastrum L. in removal and accumulation of NaCl, from highly saline soils, has been demonstrated [62]. Having own peculiarities, this technique is a modality of phytoextraction .
3 Phytofiltration of Contaminated Waters
As previously referred, there are two main divisions in the aquatic phytoremediation technology that involve [63, 64] (Fig. 11.1): 1—purely aquatic plants, which remove metals from water by roots, leaves, and stems ( phytofiltration ); 2—submersion of the roots of terrestrial plants in order to remove pollutants from the water ( rhizofiltration ). In addition, we may consider a third division [40]: 3—using young plant seedlings growing in aerated water (aquacultured) to remove toxic metals from water ( blastofiltration ).
In the present study dealing with phytofiltration potential of the Portuguese native aquatic flora, the term phytofiltration is used to describe the remediation of metal(loid)-contaminated water through uptake and bioaccumulation of metal(loid)s into organs of aquatic plants. Freshwater vascular plants (or simply aquatic plants) comprise mainly angiosperms with a few fern species, when combined with macroscopic algae are known collectively as macrophytes [63]. By definition, aquatic plants are those that complete their biological life cycle in aquatic environments and include diversified forms such as free-floating, submerged, and emergent life forms [38, 65, 66]. In a more specific way, the aquatic macrophytes can be classified as follows [57, 63, 67–70] (Fig. 11.2).
3.1 Rooted Emergent Plants
These macrophytes are rooted in bottom sediments/submerged soils or in aerial soils at which the water table is about 0.5 m below the soil. These are sometimes referred as semiaquatic plants . They are generally rhizomatous or cormous perennials (e.g., Typha spp., Phragmites spp., Scirpus spp.).
3.2 Rooted Submerged Plants
These are rooted in bottom sediments and with leaves under water (e.g., Potamogeton pectinatus L., Myriophyllum spicatum L., Chara spp.).
3.3 Rooted Floating-Leaved Plants
These are rooted in bottom sediments at water depths from about 0.5 to 3 m but with floating leaves. In heterophyllous species submerged leaves accompany the floating leaves. Floating leaves are on short petioles from long ascending stems (e.g., Potamogeton natans L.), or with long and flexible petioles (e.g., Nymphaea spp.) and reproductive organs are floating or aerial.
3.4 Free-Floating Emergent Plants
These are not rooted in sediments but live unattached in water. Forming a highly varied group, they range from long plants with rosettes of aerial and floating leaves and well-developed submerged roots (e.g., Eichhornia crassipes, Pistia stratiotes L.) to minute surface floating plants with few or no roots (e.g., Lemna spp., Azolla spp., Salvinia spp.).
3.5 Free-Floating Submerged Plants
These are submerged, nonrooted aquatic plants (e.g., Ceratophyllum demersum L., Utricularia vulgaris L., Utricularia intermedia Hayne, Utricularia gibba L.).
The first extensive review on the ability of aquatic plants to accumulate chemical elements from the aquatic environment was carried out by Hutchinson [71]. The author reported a set of aquatic plant species with the ability to accumulate cadmium (Cd), lead (Pb), and mercury (Hg) in concentrations that are many higher than the ambient environment. Since then, the number of studies involving aquatic macrophyte communities have been on the rise as a consequence of the growing importance of the water management. Phytofiltration of metal(loid)-contaminated waters involves both abiotic (water and sediments) and biotic (organisms living in the contaminated environment), and the specific process of phytofiltration depends not only on plant traits but also on certain physicochemical and geochemical processes. At natural sites, metal(loid)s removal also depends on factors that include microbial biofilms on abiotic substrates and the growth of periphyton [72–77]. Thus, the metal(loid)s removal from water in natural aquatic environments involves integrated physical, chemical, and biological processes, such as those reported by Hedin et al. [78], Sobolewski [58], and ITRC [51] in wetlands, namely the following (Fig. 11.3).
3.6 Filtration of Suspended Solids
Including adsorbed metal(loid)s .
3.7 Sorption onto Organic Matter
Several metals have a high affinity to bind to organic matter forming stable complexes.
3.8 Oxidation and Hydrolysis
Al, Fe, and Mn can form insoluble compounds—oxides, oxyhydroxides, and hydroxides—through hydrolysis and/or oxidation.
3.9 Formation of Carbonates
Some metals can form carbonates when concentrations of bicarbonate in water are high.
3.10 Formation of Insoluble Sulfides
Anaerobic conditions promote the growth of sulfate-reducing bacteria, which convert sulfates into hydrogen sulfide; metal(loid)s such Ag, As, Cd, Cu, Hg, Pb, and Zn react with hydrogen sulfide to form highly insoluble sulfides.
3.11 Binding to Iron and Manganese Oxides
Several metal(loid)s have a high affinity to bind to Fe and Mn oxides through the adsorption or coprecipitation phenomena.
3.12 Reduction to Nonmobile Forms by Bacterial Activity
Metal(loid)s such as Cr, Cu, Se, and U can be reduced into nonmobile forms—e.g., metallic forms—through processes governed by bacterial activity or physicochemical factors such as Eh–pH and hydrogen sulfide concentrations.
3.13 Biological Methylation and Volatilization
Some metal(loid)s (e.g., Hg, Se, and As) may be converted into nontoxic forms and then released into the atmosphere through volatilization by direct release from plants or by microbial or chemical activity in the water and sediments. These elements can also be biomethylated by plant roots and microorganisms — e.g., under anaerobic sediment conditions, Hg ions are biomethylated by anaerobic microorganisms to methyl mercury; methylation processes made these metal(loid)s highly toxic and available to the entire food chain through biomagnification, creating additional environmental problems.
3.14 Plant Uptake
Metal(loid)s are absorbed from water and/or sediment by roots or other submerged organs followed by translocation and accumulation in the tissues.
Metal(loid) uptake by aquatic plants depends on the type of plant, with direct absorption from the water column to the plant surface followed by passive or active transport across membranes and, to a lesser extent, root uptake [63, 79, 80]. These processes have been observed not only in submerged species (with poorly developed root systems) but also in free-floating plants [63]. However, root uptake in plants with developed root systems can also be effective, as endorsed by Eleocharis dulcis (Burm.f.) Trin. ex Hensch. with higher U levels in roots compared to stems. It has been suggested that the low accumulation in stems has an important advantage because metal cycling and resuspension following the decay of stems are minimized [81]. In another study, Entry et al. [82] demonstrated sunflower ( Helianthus annuus ) seems to be very effective in recovering U from contaminated water. Uranium accumulated mainly in the roots, with concentrations 5000–10,000 times greater than that of the ambient water.
Plants growing near mining sites or in trace element-rich substrates are able to accumulate or exclude toxic metal(loid)s and can therefore tolerate the imposed stress. Some of these accumulating plant species reveal the mineral composition of those substrates, for example, in the soil, sediment, and water. This ability can be used in mineral prospecting, in contamination bioindication or, if the biomass and bioproductivity are high, in phytoremediation. Certain aquatic plants, such as algae, bryophytes, and angiosperms, are considered to be indicators of trace element pollution and have been successfully used as diagnostic tools for monitoring environmental pollution [83, 84]. In fact, the ability of plants to accumulate metal(loid)s from water, which may not be essential for their growth and development, has been observed in several studies performed in natural wetlands where the metal(loid) concentration in aquatic plants is manifold higher than the ambient water. This evidence has led to the generalized idea that metal(loid) hyperaccumulation in aquatic plants is not as rare as in terrestrial plants and that suitable and sustainable remediation strategies could be developed based on this characteristic [63, 79, 80, 85, 86]. This function is of considerable significance in the emerging areas of wastewater treatment and in the establishment of constructed treatment wetlands (e.g., [70]). In recent years, several studies have been performed on metal(loid)s accumulation by aquatic plants, including possible phytofiltration applications.
The accumulation of metal(loid)s in aquatic plants may occur due to absorption, adsorption, and/or other retention mechanisms. Holistically, these physicochemical processes generally fall under the term “bioaccumulation”, when performed by living organisms. Another term, “biosorption”, is used to describe the set of mechanisms for the removal of substances from solution by biological materials (living or dead biomass), including absorption, adsorption, ion exchange, surface complexation, and precipitation (e.g., [64, 87–96]). However, some of these authors only consider the term “biosorption ” in the case of dead biomass.
Several studies have shown that many factors affect the “bioaccumulation ” and/or “biosorption ” of metals in aquatic ecosystems. Among the physicochemical factors, pH is possibly the most important (e.g., [93, 97–101]). This can be attributed to the influence of pH on the solution chemistry of metals and the activity of the functional groups of the biomass [102]. Phytofiltration and rhizofiltration efficiencies are determined by the ability of plants to accumulate metal(loid)s and the biomass production. Thus, phytofiltration and rhizofiltration potential can be estimated by calculation of bioconcentration factor (or biological absorption coefficient) and translocation factor. The bioconcentration factor (BCF ) , defined as the ratio of the total concentration of elements in the plant tissue (C plant) to its concentration in the water in which the plant was growing (C water), is calculated as follows (e.g., [103–105]):
\( \mathrm{B}\mathrm{C}\mathrm{F}=\frac{C_{\mathrm{plant}}}{C_{\mathrm{water}}} \)
Translocation factor (TF) , defined as the ratio of the total concentration of elements in the aerial parts of the plant (C shoot) to the concentration in the root (C root), is calculated as follows (e.g., [104, 106]):
Using both the BCF and the TF it is possible to assess the phytofiltration or rhizofiltration capacity of the plants. A high root-to-shoot translocation (TF) of metal(loid)s is a fundamental characteristic for a plants to be classified as effectively used in rhizofiltration. The commercial efficiency of phytofiltration and rhizofiltration can be estimated by the rate of metal(loid) accumulation and biomass production. After harvesting, biomass may be processed for extraction and recovery of metals with commercial value—phytomining [48, 49, 107, 108]. The commercial value of metals such as Ni, Zn, Cu, or Co may encourage the phytoremediation process. Alternatively, thermal, physical, chemical, or microbiological processes can be used to reduce the volume/weight of biomass .
The earlier referred metal(loid)s removal processes naturally occurring in aquatic ecosystems, constitute a form of natural attenuation of contamination (e.g., [109]). The natural attenuation can be defined as the natural processes of dilution, dispersion, precipitation, sorption, biodegradation, bioaccumulation , volatilization, and/or chemical and biochemical stabilization of contaminants occurring in aquatic environments that effectively reduce contaminant mobility, bioavailability, toxicity, or concentration to levels that are not too harmful on the human health and ecosystems (Fig. 11.4). Thus, human intervention to remediate waters and sediments and restoring aquatic ecosystems can be based on these natural processes. These processes can be replicated in a more complex manner in constructed wetlands, or in a more simply way in phytofiltration and rhizofiltration systems.
4 Phytofiltration Potential of Aquatic Plants
The ability of aquatic plants to accumulate metal(loid)s from water and/or from water–sediment interface has been observed in several studies performed both in field or laboratory conditions (e.g., [63, 80, 85, 86, 110–119]). It has been generally observed that the metal concentration in aquatic plants is several times higher than the concentration in surrounding water. Thus, several aquatic plant species have been identified as accumulators of metal(loid)s and as a result they might prove useful in biomonitoring and phytofiltration .
4.1 Natural Phytofiltration of Metal(loid)-Contaminated Water in Portugal
A few studies to assess the indigenous aquatic plant species of diverse contaminated areas and evaluate their potential for phytofiltration have been performed in Portugal (e.g., [41, 42, 112, 120–129]). In this chapter, important findings have been presented from several studies to evaluate the phytofiltration potential of native aquatic flora grown in waters enriched with metal(loid)s in distinct areas of Portugal.
4.1.1 Study Areas
The studied areas are located in Portugal (South Western Europe), including the uraniferous regions of Nisa (Southern Portugal), Beiras (Central Portugal), and Horta da Vilariça (Northern Portugal). Uraniferous deposits are located in the hercynian granites, in the metasediment enclaves and in the metamorphism contact haloes. In the Beiras region, several deposits have been exploited either by underground or surface mining. The main mineral processing method used was lixiviation, especially during the last active working phase (the last mine closed in 2001). Many of the sites were left in different stages of degradation. However, several programs for the environmental restoration of some of these old mines have been developed. Nisa and Horta da Vilariça regions were recognized for uranium deposits. However, these deposits have never been target for mining.
4.1.2 Material and Methods
Both plant and water samples were collected from selected sites in the vicinity of the uranium mineralized areas. The plants included submerged, free-floating, and rooted emergent species. Plant material was washed thoroughly with fresh water to remove sediment and other foreign objects. The preparation of plant material included, where appropriate, separation into roots and aerial tissues. After drying in an oven at 60 °C for 72 h, plant samples were ground into a homogeneous powder for further analysis. Water pH was determined using a pH meter (WQC-24, DKK-TOA) in the field. HCO3 concentrations were also measured in situ using the titration method. The water samples were filtered through 0.45 μm Millipore cellulose membrane filters and cooled to 4 °C immediately after collection. For determination of metals and As, samples were acidified to pH < 2 with 65 % HNO3 (V/V).
To define the chemical characteristics of the stream water as well as the occurrence of heavy metal and other elemental contamination, several parameters were measured using current analytical methods, including Atomic Absorption Spectrometry (AAS, SOLAAR M Series equipment from Thermo–Unicam) for Ca, Mg, Na, and K; coupled graphite furnace AAS for Fe, Mn, Cu, Zn, Cd, Co, Ni, Bi, Cr, Pb, and As; and the High Performance Anion Exchange Chromatography method for Cl−, NO3 −, and PO4 3−. Plants were prepared by microwave digestion with an HNO3–H2O2 mixture in closed Teflon vessels (Multiwave 3000, Anton Paar). The analysis was performed in the same way as stated for water. Fluorometry was adopted for the determination of the U content in the water and plants using a “Fluorat-02-2 Manalyzer” (Lumex, Russia).
Water data quality control was performed by inserting reagent blanks and duplicate samples into each batch. Analytical precision, defined as the percent relative variation at the 95 % confidence level, ranged from 2 to 6 %, depending on the concentration levels. Certified reference material from the National Water Research Institute of Canada (reference TMDA-62) was also used to validate the results. For the plants, the analytical methods were assessed using a Polish certified reference material, Virginia Tobacco Leaves (CTA-VTL-2), which was included in the triplicate analyses. The agreement between the certified reference values and the values determined by the analytical method was in the range of 85.5–110.2 %.
4.1.3 Phytofiltration of Arsenic-Contaminated Water
Arsenic (As) is a metalloid that is ubiquitous in nature and is found in minerals and rocks, soils, natural waters, the atmosphere, and organisms (e.g., [130–132]). More than 245 minerals contain As, and the principal source of As is geological. However, human activities such as mining, pesticide application, and burning of fossil fuels also cause As pollution (e.g., [132]). Arsenic can occur in the environment in a variety of chemical forms with different mobility, bioavailability, and toxicity. Arsenic exists in inorganic and organic complexes such as arsenate [As(V)], arsenite [As(III)], monomethylarsonic acid (MMA), dimethylarsinic acid (DMA), trimethylarsine (TMA), arsenocholine (AsC), arsenobetaine (AsB), arsenosugars, and others, and can occur in four oxidation states: +V (arsenate), +III (arsenite), 0 (arsenic), and −III (arsine) (e.g., [104]). However, in natural waters, As is found mostly in the inorganic form as oxyanions of trivalent arsenite [As(III)] or pentavalent arsenate [As(V)] (e.g., [131, 132]). These forms, which are also the most biologically important species, are interchangeable depending on the redox status of the environment [104].
In the natural environment, redox potential (Eh) and pH are the most important factors controlling As speciation. Under oxidizing conditions (high Eh values), H2AsO4 − is dominant at low pH (less than approximately pH 6.9), while at higher pH, HAsO4 2− becomes dominant. In the pH range from 2 to 11, both H2AsO4 − and HAsO4 2− species exist. The species H3AsO4 0 and AsO4 3− may be present under extremely acidic and alkaline conditions, respectively. Under reducing conditions (low Eh values), H3AsO3 is the predominant inorganic As species (e.g., [131, 132]).
Concentrations of As in unpolluted surface water and groundwater typically vary from 1 to 10 μg/L (e.g., [132]). The highest concentrations of As are found in groundwater as a result of water–rock interactions and the tendency for favorable physical and geochemical conditions for As mobilization and accumulation (e.g., [131]). In fresh water, the variation is in the range of 0.15–0.45 μg/L [132] or 0.1–0.8 μg/L but can range up to 2 μg/L [131]. In this oxic water, arsenate is the predominant species , and both arsenate and arsenite are bioavailable to the plants in aquatic systems (e.g., [133]).
Although no evidence exists that As is essential for plant nutrition, As is naturally present in plants but in concentrations that rarely exceed 1 mg/kg (e.g., [130]). However, plants vary considerably in their tolerance of As and in the amount of As that they can take up from soils and water.
In the context of constructed wetlands, García et al. [134] reported that the direct uptake and accumulation of As in plants appears to play insignificant role in As removal. Similar findings were drawn by Singhakant et al. [135], who reported that only 0.5–1 % of the total As input was accumulated in plant tissues. There are also studies indicating that wetland plants have a remarkable effect on As retention [136, 137]. Several studies have shown that roots accumulate more As than shoots (e.g., [138, 139]). Except in hyperaccumulator plants, the typical ratios of shoot to root As concentrations are <1. The As distribution, in general, decreases from root to stem and leaf to fruit [130]. Different reasons may explain why As remains mostly in plant roots, such as limited translocation of As from roots to shoots (e.g., [140]), the presence of Fe and S (e.g., [141]), the effect of As speciation in the mechanism of translocation and its relationship to the phosphate transporter (e.g., [142]), and the formation of As(III)-phytochelation (PC) complexes in roots and subsequent sequestration in root vacuoles (e.g.,[105]). Arsenic speciation appears to play an important role in the uptake mechanism and further translocation. Due to the chemical similarity between arsenate and phosphate, arsenate is presumed to be taken up by the same transporters of phosphate in the roots. However, the form of As that is translocated to shoots is not known nor is how this translocation occurs [142, 143].
Reay [144] reported that the species Ceratophyllum demersum , a free-floating submergent plant, has been shown to accumulate As with a 20,000-fold concentration factor. Meanwhile, several studies have identified aquatic plants with high As content: Lagarosiphon major (Ridl.) Moss (300 mg/kg, in dry weight, DW [63]), Egeria densa Planch. (>1000 mg/kg DW [145]), C. demersum (>1000 mg/kg DW [145]), and Lemna gibba L. (1021.7 ± 250.8 mg/kg DW [146]). Some species of submerged macrophytes such as Callitriche stagnalis Scop. and Myriophyllum propinquum A. Cunn. have revealed high potential to accumulate As and therefore show potential for phytofiltration of As-contaminated water [147]. Other plants reported to accumulate As with some potential for phytofiltration of As-contaminated water are as follows: Lepidium sativum L. [148], Rorippa nasturtium-aquaticum (L.) Hayek [147], Spirodela polyrrhiza (L.) Schleid. [133], Eichhornia crassipes (Mart.) Solms and Lemna minor L. [149], Hydrilla verticillata (L.f.) Royle [105], Eleocharis acicularis (L.) Roem. & Schult. [103], and Arundo donax L. [104], Callitriche lusitanica Schotsman [42], Micranthemum umbrosum (J.F. Gmel.) S.F. Blake [150]; Pistia stratiotes [151], and Vallisneria natans (Lour.) H. Hara. [152].
The pH may affect the bioavailability of As and the consequent uptake by plants. For example, Wells and Richardson [153] reported a decrease in arsenate uptake in the moss Hylocomium splendens (Hedw.) Schimp. with increasing pH. In this moss, arsenate uptake was optimal at pH 5, where H2AsO4 − was the dominant form in solution. As the pH increased to pH 8, where HAsO4 2− was the dominant anion, arsenate uptake decreased. Maximum As uptake rates occurring at pH 6.5 was observed by Mukherjee and Kumar [154] in aquatic plant Pistia stratiotes . The accumulation of As therefore depends on the type of plant (e.g., [141, 143]). The potential of some aquatic plants to accumulate As has been well demonstrated, and thus strongly supports their possible use in phytofiltration of As-contaminated water (e.g., [105, 155]).
In the studied areas As was detected in the surface waters at a range of concentrations between 0.15 and 40.2 μg/L (Fig. 11.5). The pH of the water ranged between 4.9 and 8.6. According to Smedley and Kinniburgh [131] and Sharma and Sohn [132], under oxidizing conditions, both H2AsO4 − and HAsO4 2− inorganic As species exist in the pH range found in the waters that were studied, although the form H2AsO4 − may be predominant. At 28 of the sites sampled, the As concentration exceeded the limit (10 μg/L) established by the World Health Organization [156] for drinking water (Fig. 11.5).
Arsenic concentration relative to pH for stream waters of the studied areas. The World Health Organization [156] drinking-water provisional guideline value of 10 μg/L is indicated for reference
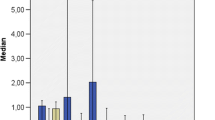
The results of the chemical analysis of As on the most representative aquatic plant species in the studied areas revealed that As is highly accumulated by some species (Figs. 11.6, 11.7 and 11.8). High bioaccumulation levels were observed in several species at a magnitude much higher than the concentration in the surrounding water. The highest concentrations of As were found in the submerged species (Fig. 11.7) Callitriche lusitanica (2346 mg/kg, in dry weight, DW), Ranunculus tripartitus DC. (1463 mg/kg DW), Callitriche brutia Petagna (523 mg/kg DW), Callitriche stagnalis (354 mg/kg DW), Ranunculus trichophyllus Chaix ex Vill. (354 mg/kg DW), Callitriche hamulata Kütz. ex W.D.J. Koch (190 mg/kg DW), Ranunculus peltatus subsp. saniculifolius (Viv.) C.D.K. Cook (120 mg/kg DW), in the free-floating species (Fig. 11.6) Lemna minor (430 mg/kg DW), Azolla caroliniana Willd. (397 mg/kg DW), in the bryophyte Fontinalis antipyretica (346 mg/kg DW), and in the rooted emergent species (Fig. 11.8) Montia fontana L. (305 mg/kg DW), Galium palustre L. (247 mg/kg DW), and Oenanthe crocata L. (158 mg/kg DW). The measured concentrations in the remaining rooted emergent plants, such as Apium nodiflorum (L.) Lag., Typha latifolia L., and Juncus effusus L. were significantly lower when compared with the previous species, even in the rhizomes/roots.
The species Callitriche stagnalis and Callitriche lusitanica showed average BCFs of 1.1 × 104 and 1.8 × 104, respectively [42], revealing a great phytofiltration ability. The highest concentrations of As were therefore found in the plants from the Callitrichaceae family. Similar behavior was reported by Robinson et al. [147] in the Taupo Volcanic Zone of New Zealand, where As concentrations of 4215 mg/kg (DW) in Callitriche stagnalis and 422 mg/kg (DW) in Callitriche petriei R. Mason was found in waters with high As concentration (mean of 90 μg/L). The submerged species Ranunculus trichophyllus and Ranunculus peltatus subsp. saniculifolius also showed a high BCF , with averages of 7.5 × 103 and 1.1 × 104, respectively [42], and showed a very highly significant (P < 0.001) positive correlation with the As present in the water [42]. Therefore, this species may serve as an indicator of As pollution.
The free-floating species Lemna minor and Azolla caroliniana showed good ability to accumulate As in a similar way with average BCFs of 6.1 × 103 and 5.5 × 103 [42]. They therefore have a great potential for As accumulation in fresh waters. Lemna minor also showed a very significant (P < 0.001) positive correlation with the As present in the waters [42]. Therefore, this species may serve as a good indicator of As pollution. The bryophyte Fontinalis antipyretica showed a strong ability to accumulate large amounts of As, displaying a high BCF of approximately 1.2 × 104 [42]. In general, bryophytes have a great potential for rapid accumulation and exhibit seasonal fluctuations depending upon the environmental contaminants [84]. As this species does not have a root system, uptake occurs through the rhizoids as an ionic exchange between the environment and the basal portion of the plant.
The rooted emergent species Montia fontana , Galium palustre , and Oenanthe crocata also showed a significant As accumulation in their aerial organs and high BCF (with averages of 5.2 × 104, 1.4 × 104, and 4 × 104, respectively [42]). The remaining rooted emergent species Apium nodiflorum , Typha latifolia , and Juncus effusus , in spite of their high biomass and bioproductivity, did not show a significant As accumulation in their aerial organs. In these species, only the leaves of Juncus effusus showed a very highly significant (P < 0.001) positive correlation with the As found in the water [42]. Among the studied rooted emergent species, only in Typha latifolia and Juncus effusus the aerial parts (leaves) and the underground parts (rhizomes/roots) were separated. The As concentrations are significantly higher in the underground parts, and, in general, the TF values are below 1 [42]. Further studies on the rooted emergent species are therefore needed to investigate the mechanism of As uptake, translocation, and accumulation, considering both the water and the sediment and taking into account the relationship with Fe, S, and phosphate and determining the As species present.
4.1.4 Phytofiltration of Uranium-Contaminated Water
In the natural environment, U occurs almost entirely as 238U in its hexavalent state (U6+), and in minor quantities as 235U, and in trace quantities as 234U. In aqueous systems U reacts with oxygen to form uranyl ion UO2 2+ which is highly stable and soluble, which determines its toxicity [157–160]. Other soluble forms are UF6, UO2(NO3)2, UO2Cl2, UO2F2, uranyl acetates, sulfates, and carbonates [161]. Several physicochemical and biological variables may influence the U speciation, bioavailability, uptake, and toxicity in fresh surface waters, including pH, water hardness, natural organic matter, and microbial activity [94, 98, 102, 162–164]. The average global concentration of U in river water is ~0.3 μg/L [165], which is within the range 0.2–0.6 μg/L suggested by Palmer and Edmond [166].
The potential of some aquatic plants to accumulate U has been well demonstrated, supporting their possible use in phytofiltration of U-contaminated water. Among aquatic plants, algae are of considerable interest to ecological engineers due to their ability to sequester U as evidenced by the fact that many algae can survive in abundance under extreme environmental conditions (e.g., [119, 158]). Algae grow in a wide spectrum of contaminated waters from alkaline environments (Chara, Nitella) to acidic mine drainage wastewaters (Mougeotia, Ulothrix). Therefore, Kalin et al. [158] suggested that algae could provide a simple and long-term solution for removing U in waste streams through the combined processes of adsorption, reduction, and transformation .
Pettersson et al. [167] identified the water lily ( Nymphaea violacea Lehm.) as an accumulator of several radionuclides when they observed high levels of U and Th series radionuclides in plant roots, rhizomes, and foliage in the vicinity of the Ranger Uranium Mine (Australia). At the same mine, an attempt to phytoremediate mine runoff water was tested using Eleocharis dulcis [81]. Members of Lemnacea are the most favored plants for phytoremediation and have been intensively described in literature as duck weeds including Lemna, Spirodella, Wolffia, and Wolffiella [133, 168]. Lemna gibba (612.36 ± 143.6 mg/kg DW) is an example of a U accumulator plant [169]. Other aquatic plants suggested as U accumulators are Zostera japonica Ascherson & Graebner and Zostera marina L. [170], Phragmites australis (Cav.) Trin ex. Steud. [171, 172], Hydrilla verticillata (L.f.) Royle [94], Callitriche stagnalis and Fontinalis antipyretica [41, 129], and Spirodela punctata [44].
Many microbial organisms, including bacteria, lichens, fungi, and algae, have been studied for their U-binding capacity, and the maximum U uptake was most frequently observed between pH 4 and 5 [98, 102, 173]. Similar results have been found in several other plant materials, such as dried roots [174] or coir pith [175]. Srivastava et al. [94] also observed in the aquatic plant Hydrilla verticillata that the maximum U uptake rates occurred at pH 5. Pratas et al. [41] found a very highly significant (P < 0.001) negative correlation between water pH and U concentration in Callitriche stagnalis .
The results of chemical analysis of the stream water samples at the studied sites show that U was detected in the surface waters at concentrations ranging from 0.23 to 1217 μg/L (Fig. 11.9). From a geographical interpretation of the sampling locations, it was clear that mine effluents contributed significantly to aquatic U contamination. Mean U concentration was higher in streams directly fed by mine drainage due to two locations with high concentrations, near Cunha Baixa and Urgeiriça mine sites [129].
Uranium concentration relative to pH for stream waters of the studied areas. The World Health Organization [156] drinking-water provisional guideline value of 30 μg/L is indicated for reference
According to Wang et al. [102], in an acidic environment (pH < 4.0), U(VI) occurs predominately as UO2 2+, whereas at higher pH ranges (4.0 < pH < 7.0) composite hydrolyzed ionic species yield compounds such as UO2OH+, (UO2)2(OH)2 2+ and (UO2)3(OH)5+. When the pH is above 7.0, U(VI) precipitates easily. The pH of water samples ranged between 4.9 and 8.6 and reveals a complex behavior due to the wide hydrochemical variability. The mean U concentration (11.1 μg/L) is significantly higher than the range values estimated by Palmer and Edmond [166] as global riverine U flux. At 9 of the sites sampled, the U concentration exceeded the provisional guideline value (30 μg/L) indicated by the World Health Organization [156] for drinking water (Fig. 11.9). At these points, U concentrations could be directly linked to mining activities since these streams were directly fed by mine drainage.
The analysis of plants from the studied areas revealed high U bioaccumulation levels in several species and in magnitudes much higher than the ambient water concentrations (Figs. 11.10, 11.11 and 11.12). However, the water samples represent a “snapshot” while the U in the plant tissue may reflect more of an average of the U over time as the water U content could change after a rainfall or prolonged drought. In general, highest concentrations of U were observed in the submerged species and the lowest in the free-floating species. Among the submerged species highest concentrations of U were found in Fontinalis antipyretica (4979 mg/kg, DW), Callitriche stagnalis (1963 mg/kg DW) followed by Callitriche hamulata (379 mg/kg DW), Ranunculus peltatus subsp. saniculifolius (243 mg/kg DW), Callitriche lusitanica (218 mg/kg DW), and Ranunculus trichophyllus (65.8 mg/kgDW) (Fig. 11.11).
The hydrophyte moss Fontinalis antipyretica showed a strong ability to accumulate large amounts of U, displaying a high mean BCF of approximately 1.0 × 104 [129] and a highly significant (P < 0.001) positive correlation with the U present in water [129]. Therefore, this species may serve as an indicator of U pollution. Accumulation of other metal(loid)s has also been demonstrated in Fontinalis antipyretica, such as As, Se, Cd, Cr, Cu, Pb, and Zn (e.g., [42, 176–185]). The species Callitriche stagnalis , Callitriche hamulata , and Callitriche lusitanica showed average BCFs of 3.0 × 103, 7.5 × 103, and 5.9 × 103, respectively [129], revealing a great phytofiltration ability. Furthermore, Callitriche stagnalis showed a highly significant (P < 0.001) positive correlation with the U present in the water [129]. Therefore, this species also serves as an excellent indicator of U pollution. High BCFs were also seen in Ranunculus peltatus subsp. saniculifolius and Ranunculus trichophyllus , with averages of 1.6 × 104 and 3.2 × 103, respectively [129].
Among the free-floating species (Fig. 11.10), Lemna minor showed good ability to accumulate U (42.5 mg/kg DW) as previously observed in Lemna gibba [169], with average BCF of 1.4 × 103 [129]. This plant belongs to the Lemnaceae family much studied in phytoremediation (e.g., [41, 128, 133, 149, 168, 186]). According to these studies, the fast growth rate, widespread distribution in natural wetlands, total independence from sediment, and adaptation to stress conditions like mine waters, makes such species a good option for water treatment technologies, in spite of the constant need for biomass removal. Lemna minor also showed a very significant (P < 0.001) positive correlation with the U present in the waters [129]. However, the U contents in free-floating plants probably cannot reflect their corresponding water U concentrations as they are moved with running river water. Nevertheless, Lemna minor also showed a very significant (P < 0.001) positive correlation with the U present in the standing water [41]. Therefore, this species may serve as a good indicator of U pollution.
Among the rooted emergent species high U concentrations were observed in rhizomes/roots of Typha latifolia (380 mg/kg DW), and Juncus effusus (132 mg/kg DW), and in the aerial parts of Myosotis secunda Al. Murray (188 mg/kg DW), Juncus effusus (99.9 mg/kg DW), Apium nodiflorum (64.5 mg/kg DW), Galium palustre (62.4 mg/kg DW), Oenanthe crocata (42.2 mg/kg DW), and Rorippa sylvestris (L.) Besser (33.8 mg/kg DW) (Fig. 11.12). Myosotis secunda, Rorippa sylvestris, Juncus effusus, Apium nodiflorum, Galium palustre, and Oenanthe crocata also showed high BCFs (mean BCF : 2.2 × 104, 4.8 × 103, 2.6 × 103, 3.6 × 103, 6.6 × 103, and 1.1 × 103, respectively) [129]. Only Rorippa nasturtium-aquaticum showed a significant (P < 0.05) positive correlation with the U content of water. More studies are therefore needed on these emergent species taking into consideration the mechanism of U uptake and accumulation, partitioning of U between stems and roots, water column, and sediment.
Among the species studied, U concentrations are higher in the underground parts (significantly more in Juncus effusus and Typha latifolia ), except for Baldellia ranunculoides (L.) Parl., Cyperus eragrostis Lam., Mentha pulegium L., and Rorippa sylvestris. In these species the mean TF values are above 1 suggesting better partitioning in the aerial parts. Pettersson et al. [167] identified the water lily, Nymphaea violacea , as an accumulator of several radionuclides when they observed high levels of 234U, 238U, 228Th, 230Th, 232Th, 226Ra, 210Pb, and 210Po in the plant, waters, and sediments in the vicinity of the Ranger Uranium mine (Australia). Higher levels of these contaminants were detected in the roots and rhizomes suggesting root uptake from sediment as the main uptake mechanism .
Preferential partitioning of U in roots may be attributed to the effect of U speciation in the mechanism of translocation and its complexation with phosphate. Studies have shown that among the physicochemical factors, pH is possibly the most important contributing factor [162, 163]. This can be attributed to the influence of pH on the speciation and bioavailability of metals and the activity of the functional groups of the biomass [102]. Other physicochemical variables which may influence the U speciation, bioavailability, and toxicity in fresh surface waters are water hardness, alkalinity, and natural organic matter [164]. In the studied areas, water pH varied from 4.9 to 8.6; this range favors the occurrence of U composite hydrolyzed ionic species. Variation in pH and the effect on U speciation may have affected the results. Only the species Myosotis secunda had a significant (P < 0.05) negative correlation with the pH of the water. On the other hand, the species Azolla caroliniana showed a significant (P < 0.05) positive correlation with the U present in the water [129]. Species with high U uptake, such as Fontinalis antipyretica , Callitriche hamulata , Ranunculus peltatus subsp. saniculifolius, Callitriche lusitanica , Typha latifolia (rhizomes/roots), Juncus effusus , and Myosotis secunda, may also be used in phytofiltration devices either in monoculture systems or in combined systems resembling the natural systems.
4.1.5 Phytofiltration of Lead-, Copper-, and Zinc-Contaminated Water
Among heavy metals, Pb is one of the most hazardous pollutants, due to its impact on human health and environment (e.g., [113]). The main sources of Pb pollution are mining and smelting, industrial effluents, fertilizers, pesticides, and municipal sewage sludge (e.g., [113, 187]). In plants, Pb toxicity leads to reduction in cell division and inhibition of photosynthesis [188], decreases in seed germination, as well as growth, dry biomass of roots and shoots, and disruption of mineral nutrition [187]. Lead bioaccumulation potential and the effect of Pb stress have been studied recently in various aquatic plant species, including Fontinalis antipyretica [180], Ceratophyllum demersum L. [79, 189], Potamogeton pectinatus L. and Potamogeton malaianus Miq. [190], Wolffia arrhiza (L.) Horkel ex. Wimm. [191], Lemna minor [117, 186], Lemna gibba [117], Najas indica (Willd.) Cham. [192], Typha latifolia [193, 194], Eichhornia crassipes (Mart.) Solms [113, 195], and Callitriche cophocarpa Sendtn. [196].
In contrast to the Pb, certain heavy metals are required for the metabolic processes in plants. However, despite this, some of these metals, including Cu and Zn, become toxic at elevated levels (e.g., [197, 198]). Several aquatic plant species have been identified as accumulators of multi metals, including Cu and Zn, and as a result they might prove useful in phytofiltration technique. Some examples are as follows: Ceratophyllum demersum L. for Cu, Cr, Fe, and Mn [79]; Fontinalis antipyretica for Zn, Cu, and Cd [180, 181]; Callitriche palustris L. for Cu [199]; Myriophyllum aquaticum (Vell.) Verdc., Ludwigina palustris (L.) Ell., and Mentha aquatica L. for Cu, Fe, Hg, and Zn [200]; Lemna minor for Cu, Cd, Pb, and Zn [117, 201]; Potamogeton pectinatus L. and Potamogeton malaianus Miq. for Cd, Mn, Zn, and Cu [190]; Lemna gibba for Cu, Pb, and Zn [117, 202]; Elodea canadensis Michx. and Elodea nuttallii (Planch.) H. St. John for Cd, Cr, Cu, Mn, Ni, Pb, Zn, and Fe [203]; Eichhornia crassipes (Mart.) Solms for Cu, Zn, Cd, and Cr [195, 204–206]; and Callitriche cophocarpa Sendtn. for Tl, Cd, Zn, and Cr [196]. In the studied areas Pb, Cu, and Zn were detected in the surface waters at a concentration ranges of 0.1–13.4 μg/L, 0.45–125 μg/L, and 1.00–441 μg/L, respectively (Figs. 11.13, 11.14, and 11.15). Only at two of the sites sampled the Pb concentration exceeded the provisional guideline value (10 μg/L) indicated by the World Health Organization [156] for drinking water (Fig. 11.13).
Lead concentration relative to pH for stream waters of the studied areas. The World Health Organization [156] drinking-water provisional guideline value of 10 μg/L is indicated for reference
The analytical results on the most representative aquatic plant species in the studied areas revealed the following significative uptake patterns: Pb from 90.5 to 1104 mg/kg in Ranunculus trichophyllus ; rhizomes/roots of Typha latifolia , Lemna minor , Spirodela polyrrhiza (L.) Schleid.; and Myriophyllum spicatum (Figs. 11.16, 11.17, and 11.18); Cu from 81.8 to 161 mg/kg in Callitriche lusitanica , Callitriche hamulata , Ranunculus trichophyllus, and Callitriche stagnalis (Figs. 11.19, 11.20 and 11.21); and Zn from 900 to 34,162 mg/kg in Lemna minor, Lemanea fluviatilis , Callitriche lusitanica, Callitriche brutia, Ranunculus trichophyllus, Fontinalis antipyretica , and Callitriche stagnalis (Figs. 11.22, 11.23, and 11.24).
5 Conclusion
The studied aquatic plant species exhibited ability to accumulate several metal(loid)s , namely As, U, Pb, Cu, and Zn, in concentrations that are orders of magnitude higher than the surrounding water. This ability, reveled by several species, confirmed their high potential for the phytofiltration of contaminated waters. In general, submerged plants exhibited higher metal(loid) content. The highest U concentrations were observed in the bryophyte Fontinalis antipyretica and members of the monogeneric family Callitrichaceae. In the rooted emergent species, U seemed to be preferentially partitioned in rhizome/roots; maximum U content was observed in Typha latifolia rhizomes. The highest concentrations of As were found in the representatives of Callitrichaceae family. Other species with high As concentrations were Lemna minor , Azolla caroliniana , Ranunculus trichophyllus , Fontinalis antipyretica, Montia fontana , and Galium palustre .
The accumulation patterns of U and As of some of the aforementioned plants may also make them potential tools as bioindicators for trace elements in limnetic environment. Any adverse ecological impact in the aquatic food chain of these metal(loid)s by studied aquatic plants would be considered for future research. The abundance of Fontinalis antipyretica and Callitrichaceae family members, their biomass, relatively high bioproductivity, and ability to accumulate several toxic elements at the same time make them promising candidates for the development of phytofiltration methodologies. Other species with high metal(loid) uptake such as Lemna minor , Azolla caroliniana , Ranunculus trichophyllus , Montia fontana , and Galium palustre can also be used in phytofiltration applications either in monoculture systems or in a combined systems representing the natural systems.
References
Lottermoser BG (2003) Mine wastes: Characterization, treatment and environmental impacts. Springer, Berlin, p 400
Chaney RL, Malik M, Li YM, Brown SL, Angle JS, Baker AJM (1997) Phytoremediation of soil metals. Curr Opin Biotechnol 8:279–284
Ensley BD (2000) Rationale for use of phytoremediation. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals: Using plants to clean up the environment. Wiley, New York, pp 3–11
ITRC (2001) Phytotechnology technical and regulatory guidance document. Interstate Technology & Regulatory Council, Phytotechnologies Work Team, Washington, DC, p 124
Prasad MNV (2004) Phytoremediation of metals and radionuclides in the environment: The case for natural hyperaccumulators, metal transporters, soil-amending chelators and transgenic plants. In: Prasad MNV (ed) Heavy metal stress in plants: from biomolecules to ecosystems, 2nd edn. Springer, Berlin, pp 345–391
Mendez MO, Maier RM (2008) Phytoremediation of mine tailings in temperate and arid environments. Rev Environ Sci Biotechnol 7:47–59
Dickinson NM, Baker AJM, Doronila A, Laidlaw S, Reeves RD (2009) Phytoremediation of inorganics: realism and synergies. Int J Phytoremediation 11:97–114
Favas PJC, Pratas J, Varun M, D’Souza R, Paul MS (2014) Phytoremediation of soils contaminated with metals and metalloids at mining areas: Potential of native flora. In: Hernández-Soriano MC (ed) Environmental risk assessment of soil contamination. InTech, Rijeka, pp 485–517
El-Ramady HR, Abdalla N, Alshaal T, Elhenawy AS, Shams MS, Faizy SE-DA et al (2015) Giant reed for selenium phytoremediation under changing climate. Environ Chem Lett 13:359–380
Varun M, D’Souza R, Favas PJC, Pratas J, Paul MS (2015) Utilization and supplementation of phytoextraction potential of some terrestrial plants in metal-contaminated soils. In: Ansari AA, Gill SS, Gill R, Lanza GR, Newman L (eds) Phytoremediation: Management of environmental contaminants, vol 1, Springer. Cham, Heidelberg, pp 177–200
Roy M, Giri AK, Dutta S, Mukherjee P (2015) Integrated phytobial remediation for sustainable management of arsenic in soil and water. Environ Int 75:180–198
Favas PJC, Pratas J, Chaturvedi R, Paul MS, Prasad MNV (2016) Tree crops on abandoned mines for environmental remediation and industrial feedstock. In: Prasad MNV (ed) Bioremediation and Bioeconomy. Elsevier, Amsterdam, pp 219–249
ITRC (2009) Phytotechnology technical and regulatory guidance and decision trees, revised. PHYTO-3. Interstate Technology & Regulatory Council, Phytotechnologies Team, Washington, DC, p 204
Nwoko CO (2010) Trends in phytoremediation of toxic elemental and organic pollutants. Afr J Biotechnol 9(37):6010–6016
Bose S, Rai V, Bhattacharya S, Chaudhuri P, Bhattacharyya AK (2011) Phytoremediation: A promising technology of bioremediation for the removal of heavy metal and organic pollutants from the soil. In: Golubev IA (ed) Handbook of phytoremediation. Nova Science, New York, pp 263–296
Gaur N, Flora G, Yadav M, Tiwari A (2014) A review with recent advancements on bioremediation-based abolition of heavy metals. Environ Sci Processes Impacts 16(2):180–193
Yavari S, Malakahmad A, Sapari NB (2015) A review on phytoremediation of crude oil spills. Water Air Soil Pollut 226(8):279
Zhang X, Wang J, Liu X, Gu L, Hou Y, He C et al (2015) Potential of Sagittaria trifolia for phytoremediation of diesel. Int J Phytoremediat 17:1220–1226
He Y, Chi J (2016) Phytoremediation of sediments polluted with phenanthrene and pyrene by four submerged aquatic plants. J Soils Sediments 16:309–317
Schnoor JL, Licht LA, McCutcheon SC, Wolfe NL, Carreira LH (1995) Phytoremediation of organic and nutrient contaminants. Environ Sci Technol 29:318A–323A
Rylott EL, Bruce NC (2008) Plants disarm soil: engineering plants for the phytoremediation of explosives. Trends Biotechnol 27(2):73–81
Berti WR, Cunningham SD (2000) Phytostabilization of metals. In: Ensley BD (ed) Raskin I. Wiley, Phytoremediation of toxic metals. Using plants to clean up the environment. New York, pp 71–88
Domínguez MT, Madrid F, Marañón T, Murillo JM (2009) Cadmium availability in soil and retention in oak roots: potential for phytostabilization. Chemosphere 76:480–486
Ali H, Khan E, Sajad MA (2013) Phytoremediation of heavy metals: Concepts and applications. Chemosphere 91:869–881
Brooks RR (1998) Phytoremediation by volatilisation. In: Brooks RR (ed) Plants that hyperaccumulate heavy metals: their role in phytoremediation, microbiology, archaeology, mineral exploration and phytomining. CAB International, New York, pp 289–312
Pilon-Smits E, Pilon M (2000) Breeding mercury-breathing plants for environmental cleanup. Trends Plant Sci 5(6):235–236
Ruiz ON, Daniell H (2009) Genetic engineering to enhance mercury phytoremediation. Curr Opin Biotechnol 20:213–219
Pilon-Smits EAH, LeDuc DL (2009) Phytoremediation of selenium using transgenic plants. Curr Opin Biotechnol 20:207–212
McGrath SP (1998) Phytoextraction for soil remediation. In: Brooks RR (ed) Plants that hyperaccumulate heavy metals: their role in phytoremediation, microbiology, archaeology, mineral exploration and phytomining. CAB International, New York, pp 261–287
Blaylock MJ, Huang JW (2000) Phytoextraction of metals. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals: Using plants to clean up the environment. Wiley, New York, pp 53–70
Ma LQ, Komar KM, Tu C, Zhang W, Cai Y, Kennelley ED (2001) A fern that hyperaccumulates arsenic. Nature 409:579
McGrath SP, Zhao FJ (2003) Phytoextraction of metals and metalloids from contaminated soils. Curr Opin Biotechnol 14:277–282
Hernández-Allica J, Becerril JM, Garbisu C (2008) Assessment of the phytoextraction potential of high biomass crop plants. Environ Pollut 152:32–40
Pedron F, Petruzzelli G, Barbafieri M, Tassi E (2009) Strategies to use phytoextraction in very acidic soil contaminated by heavy metals. Chemosphere 75:808–814
Xie QE, Yan XL, Liao XY, Li X (2009) The arsenic hyperaccumulator fern Pteris vittata L. Environ Sci Technol 43(22):8488–8495
Van der Ent A, Baker AJM, Reeves RD, Pollard AJ, Schat H (2013) Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 362:319–334
Frers C (2009) El uso de plantas acuáticas en el tratamiento de aguas residuales. El Planeta Azul, Carmen de Areco
Dhote S, Dixit S (2009) Water quality improvement through macrophytes—a review. Environ Monit Assess 152:149–153
Krishna R, Fulekar MH, Pathank B (2012) Rhizofiltration: A green technology for remediation of heavy metals. Int J Innov BioSci 2(4):193–199
Dushenkov S, Kapulnik Y (2000) Phytofiltration of metals. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals: Using plants to clean up the environment. Wiley, New York, pp 89–106
Pratas J, Favas PJC, Paulo C, Rodrigues N, Prasad MNV (2012) Uranium accumulation by aquatic plants from uranium-contaminated water in Central Portugal. Int J Phytoremediat 14:221–234
Favas PJC, Pratas J, Prasad MNV (2012) Accumulation of arsenic by aquatic plants in large-scale field conditions: Opportunities for phytoremediation and bioindication. Sci Total Environ 433:390–397
Rezania S, Ponraj M, Talaiekhozani A, Mohamad SE, Din MFM, Taib SM et al (2015) Perspectives of phytoremediation using water hyacinth for removal of heavy metals, organic and inorganic pollutants in wastewater. J Environ Manage 163:125–133
Nie X, Dong F, Liu N, Liu M, Zhang D, Kang W et al (2015) Subcellular distribution of uranium in the roots of Spirodela punctata and surface interactions. Appl Surf Sci 347:122–130
Crowley DE, Alvey S, Gilbert ES (1997) Rhizosphere ecology of xenobiotic-degrading microorganisms. In: Kruger EL, Anderson TA, Coats JR (eds) Phytoremediation of soil and water contaminants. ACS Symposium Series, Washington, pp 20–36
Khan MS, Zaidi A, Wani PA, Oves M (2009) Role of plant growth promoting rhizobacteria in the remediation of metal contaminated soils. Environ Chem Lett 7:1–19
Schnoor JL (2000) Phytostabilization of metals using hybrid poplar trees. In: Raskin I, Ensley BD (eds) Phytoremediation of toxic metals: Using plants to clean up the Environment. Wiley, New York, pp 133–150
Brooks RR, Chambers MF, Nicks LJ, Robinson BH (1998) Phytomining. Trends Plant Sci 3:359–362
Brooks RR, Chiarucci A, Jaffré T (1998) Revegetation and stabilization of mine dumps and other degraded terrain. In: Brooks RR (ed) Plants that hyperaccumulate heavy metals: Their role in phytoremediation, microbiology, archaeology, mineral exploration and phytomining. CAB International, New York, pp 227–247
Prasad MNV (2015) Engineered phyto-covers as natural caps for containment of hazardous mine and municipal solid waste dump sites – possible energy sources. In: Öztürk M, Ashraf M, Aksoy A, Ahmad MSA (eds) Phytoremediation for green energy. Springer, Dordrecht, pp 55–68
ITRC (2003) Technical and regulatory guidance document for constructed treatment wetlands. Interstate Technology & Regulatory Council, Wetlands Team, p 199
Vymazal J (2009) The use constructed wetlands with horizontal sub-surface flow for various types of wastewater. Ecol Eng 35:1–17
Olguín EJ, Sánchez-Galván G (2010) Aquatic phytoremediation: Novel insights in tropical and subtropical regions. Pure Appl Chem 82(1):27–38
Fonder N, Headley T (2013) The taxonomy of treatment wetlands: A proposed classification and nomenclature system. Ecol Eng 51:203–211
Horne AJ (2000) Phytoremediation by constructed wetlands. In: Terry N, Bañuelos G (eds) Phytoremediation of contaminated soil and water. Lewis Publishers, New York, pp 13–39
Kadlec RH, Wallace SD (2008) Treatment wetlands, 2nd edn. CRC Press, Boca Raton, FL, p 366
Vymazal J, Kröpfelová L (2008) Wastewater treatment in constructed wetlands with horizontal sub-surface flow. Springer, Dordrecht, p 566
Sobolewski A (1999) A review of processes responsible for metal removal in wetlands treating contaminated mine drainage. Int J Phytoremediat 1(1):19–51
Nyquist J, Greger M (2009) A field study of constructed wetlands for preventing and treating acid mine drainage. Ecol Eng 35:630–642
Adams A, Raman A, Hodgkins D (2013) How do the plants used in phytoremediation in constructed wetlands, a sustainable remediation strategy, perform in heavy-metal contaminated mine sites? Water Environ J 27(3):373–386
Zorrig W, Rabhi M, Ferchichi S, Smaoui A, Abdelly C (2012) Phytodesalination: a solution for salt-affected soils in arid and semi-arid regions. J Arid Land Stud 22:299–302
Ravindran KC, Venkatesan K, Balakrishnan V, Chellappan KP, Balasubramanian T (2007) Restoration of saline land by halophytes for Indian soils. Soil Biol Biochem 39:2661–2664
Brooks RR, Robinson BH (1998) Aquatic phytoremediation by accumulator plants. In: Brooks RR (ed) Plants that hyperaccumulate heavy metals: their role in phytoremediation, microbiology, archaeology, mineral exploration and phytomining. CAB International, New York, pp 203–226
Kikuchi T, Tanaka S (2012) Biological removal and recovery of toxic heavy metals in water environment. Crit Rev Environ Sci Technol 42:1007–1057
Bracamonte SC, Domingo LM (2002) Plantas aquáticas de las lagunas y humedales de Castilla-La Mancha. Real Jardín Botánico, Junta de Comunidades de Castilla-La Mancha, Madrid, p 340
Dhir B (2013) Phytoremediation: Role of aquatic plants in environmental clean-up. Springer, New Delhi, p 111
Arber A (1920) A study of aquatic angiosperms. Cambridge Univ. Press, Cambridge, p 436
Sculthorpe CD (1967) The biology of aquatic vascular plants. St. Martin’s Press, New York, p 610
Outridge PM, Noller BN (1991) Accumulation of toxic trace elements by freshwater vascular plants. Rev Environ Contam Toxicol 121:1–63
Rai PK (2009) Heavy metal phytoremediation from aquatic ecosystems with special reference to macrophytes. Crit Rev Environ Sci Technol 39:697–753
Hutchinson GE (1975) A treatise on limnology, Vol 3: Limnological Botany. John Wiley & Sons, New York, p 672
Gray BR, Hill WR, Stewart AJ (2001) Effects of development time, biomass and ferromanganese oxides on nickel sorption by stream periphyton. Environ Pollut 112:61–71
Bradac P, Wagner B, Kistler D, Traber J, Behra R, Sigg L (2010) Cadmium speciation and accumulation in periphyton in a small stream with dynamic concentration variations. Environ Pollut 158:641–648
Stout L, Nüsslein K (2010) Biotechnological potential of aquatic plant-microbe interactions. Curr Opin Biotechnol 21:339–345
Krawczyk-Bärsch E, Lünsdorf H, Arnold T, Brendler V, Eisbein E, Jenk U, Zimmermann U (2011) The influence of biofilms on the migration of uranium in acid mine drainage (AMD) waters. Sci Total Environ 409:3059–3065
Castro MCR, Ureea G, Guasch H (2015) Influence of the interaction between phosphate and arsenate on periphyton's growth and its nutrient uptake capacity. Sci Total Environ 503–504:122–132
Srivastava S, Bhargava A (2016) Biofilms and human health. Biotechnol Lett 38:1–22
Hedin RS, Nairn RW, Kleinmann RLP (1994) The passive treatment of coal mine drainage. U.S. Bureau of Mines Information, Circular 9389, p 35
Rai UN, Sinha S, Tripathi RD, Chandra P (1995) Wastewater treatability potential of some aquatic macrophytes: Removal of heavy metals. Ecol Eng 5(1):5–12
Nyquist J, Greger M (2007) Uptake of Zn, Cu, and Cd in metal loaded Elodea canadensis. Environ Exp Bot 60:219–226
Overall RA, Parry DL (2004) The uptake of uranium by Eleocharis dulcis (Chinese water chestnut) in the Ranger Uranium Mine constructed wetland filter. Environ Pollut 132:307–320
Entry JA, Vance NC, Hamilton MA, Zabowski D, Watrud LS, Adriano DC (1996) Phytoremediation of soil contaminated with low concentrations of radionuclides. Water Air Soil Pollut 88:167–176
Markert B (1993) Plants as biomonitors: indicators for heavy metals in the terrestrial environment. VCH, Weinheim, p 645
Cenci RM (2000) The use of aquatic moss (Fontinalis antipyretica) as monitor of contamination in standing and running waters: limits and advantages. J Limnol 60(Suppl 1):53–61
Fritioff Å, Greger M (2006) Uptake and distribution of Zn, Cu, Cd, and Pb in an aquatic plant Potamogeton natans. Chemosphere 63:220–227
Rahman MA, Hasegawa H (2011) Aquatic arsenic: Phytoremediation using floating macrophytes. Chemosphere 83:633–646
Tsezos M, Volesky B (1981) Biosorption of uranium and thorium. Biotechnol Bioeng 23:583–604
Volesky B, Holan ZR (1995) Biosorption of heavy metals. Biotechnol Prog 11:235–250
Abia AA, Horsfall M Jr, Didi O (2003) The use of chemically modified and unmodified cassava waste for the removal of Cd, Cu and Zn ions from aqueous solution. Bioresource Technol 90(3):345–348
Gamez G, Gardea-Torresdey JL, Tiemann KJ, Parsons J, Dokken K, Yacaman MJ (2003) Recovery of gold(III) from multi-elemental solutions by alfalfa biomass. Adv Environ Res 7(2):563–571
Raize O, Argaman Y, Yannai S (2004) Mechanisms of biosorption of different heavy metals by brown marine macroalgae. Biotechnol Bioeng 87:451–458
Gardea-Torresdey JL, De La Rosa G, Peralta-Videa JR (2004) Use of phytofiltration technologies in the removal of heavy metals: A review. Pure Appl Chem 76(4):801–813
Gadd GM (2009) Biosorption: critical review of scientific rationale, environmental importance and significance for pollution treatment. J Chem Technol Biotechnol 84:13–28
Srivastava S, Bhainsa KC, D’Souza SF (2010) Investigation of uranium accumulation potential and biochemical responses of an aquatic weed Hydrilla verticillata (L.f.) Royle. Bioresource Technol 101:2573–2579
Olguín EJ, Sánchez-Galván G (2012) Heavy metal removal in phytofiltration and phycoremediation: The need to differentiate between bioadsorption and bioaccumulation. New Biotechnol 30(1):3–8
Ungureanu G, Santos S, Boaventura R, Botelho C (2015) Arsenic and antimony in water and wastewater: Overview of removal techniques with special reference to latest advances in adsorption. J Environ Manage 151:326–342
Ebbs SD, Brady DJ, Kochian LV (1998) Role of uranium speciation in the uptake and translocation of uranium by plants. J Exp Bot 49:1183–1190
Li PF, Mao ZY, Rao XJ, Wang XM, Min MZ, Qiu LW, Liu ZL (2004) Biosorption of uranium by lake-harvested biomass from a cyanobacterium bloom. Bioresource Technol 94:193–195
Sawalha MF, Peralta-Videa JR, Duarte-Gardea M, Gardea-Torresdey JL (2008) Removal of copper, lead, and zinc from contaminated water by saltbush biomass. Analysis of the optimum binding, stripping, and binding mechanism. Bioresour Technol 99(10):4438–4444
Marchand L, Mench M, Jacob DL, Otte ML (2010) Metal and metalloid removal in constructed wetlands, with emphasis on the importance of plants and standardized measurements: A review. Environ Pollut 158:3447–3461
Kumar D, Gaur JP (2011) Metal biosorption by two cyanobacterial mats in relation to pH, biomass concentration, pretreatment and reuse. Bioresource Technol 102:2529–2535
Wang J, Hu X, Wang J, Bao Z, Xie S, Yang J (2010) The tolerance of Rhizopus arrihizus to U(VI) and biosorption beaviour of U(VI) onto R. arrihizus. Biochem Eng J 51:19–23
Ha NTH, Sakakibara M, Sano S (2011) Accumulation of indium and other heavy metals by Eleocharis acicularis: An option for phytoremediation and phytomining. Bioresource Technol 102:2228–2234
Mirza N, Pervez A, Mahmood Q, Shah MM, Shafqat MN (2011) Ecological restoration of arsenic contaminated soil by Arundo donax L. Ecol Eng 37:1949–1956
Xue PY, Yan CZ (2011) Arsenic accumulation and translocation in the submerged macrophyte Hydrilla verticillata (L.f.) Royle. Chemosphere 85:1176–1181
Tu S, Ma LQ (2004) Comparison of arsenic and phosphate uptake and distribution in arsenic hyperaccumulating and nonhyperaccumulating fern. J Plant Nutr 27(7):1227–1242
Sheoran V, Sheoran AS, Poonia P (2009) Phytomining: a review. Miner Eng 22:1007–1019
Morais I, Campos JS, Favas PJC, Pratas J, Pita F, Prasad MNV (2015) Nickel accumulation by Alyssum serpyllifolium subsp. lusitanicum (Brassicaceae) from serpentine soils of Bragança and Morais (Portugal) ultramafic massifs: plant-soil relationships and prospects for phytomining. Aust J Bot 63(2):17–30
OECD (2002) Environmental remediation of uranium production facilities. OECD Nuclear Energy Agency, International Atomic Energy Agency, Paris, p 323
Cardwell AJ, Hawker DW, Greenway M (2002) Metal accumulation in aquatic macrophytes from Southeast Queensland, Australia. Chemosphere 48:653–663
Abhilash PC, Pandey VC, Srivastava P, Rakesh PS, Chandran S, Singh N, Thomas AP (2009) Phytofiltration of cadmium from water by Limnocharis flava (L.) Buchenau grown in free-floating culture system. J Hazard Mater 170(2-3):791–797
Pratas J, Paulo C, Favas PJC, Venkatachalam P (2014) Potential of aquatic plants for phytofiltration of uranium-contaminated waters in laboratory conditions. Ecol Eng 69:170–176
Malar S, Vikram SS, Favas PJC, Perumal V (2014) Lead heavy metal toxicity induced changes on growth and antioxidative enzymes level in water hyacinths [Eichhornia crassipes (Mart.)]. Bot Stud 55:54
Malar S, Sahi SV, Favas PJC, Venkatachalam P (2015) Mercury heavy-metal-induced physiochemical changes and genotoxic alterations in water hyacinths [Eichhornia crassipes (Mart.)]. Environ Sci Pollut Res 22:4597–4608
Chowdhury R, Favas PJC, Pratas J, Jonathan MP, Ganesh PS, Sarkar SK (2015) Accumulation of trace metals by mangrove plants in Indian Sundarban Wetland: Prospects for phytoremediation. Int J Phytoremediat 17:885–894
Rodriguez-Hernandez MC, Bonifas I, Alfaro-De la Torre MC, Flores-Flores JL, Bañuelos-Hernández B, Patiño-Rodríguez O (2015) Increased accumulation of cadmium and lead under Ca and Fe deficiency in Typha latifolia: A study of two pore channel (TPC1) gene responses. Environ Exp Bot 115:38–48
Sasmaz M, Topal EIA, Obek E, Sasmaz A (2015) The potential of Lemna gibba L. and Lemna minor L. to remove Cu, Pb, Zn, and As in gallery water in a mining area in Keban, Turkey. J Environ Manage 163:246–253
González CI, Maine MA, Cazenave J, Hadad HR, Benavides MP (2015) Ni accumulation and its effects on physiological and biochemical parameters of Eichhornia crassipes. Environ Exp Bot 117:20–27
Jha VN, Tripathi RM, Sethy NK, Sahoo SK (2016) Uptake of uranium by aquatic plants growing in fresh water ecosystem around uranium mill tailings pond at Jaduguda, India. Sci Total Environ 539:175–184
Paulo C (2006) Selecção de plantas aquáticas e perspectivas na fitorremediação de escorrências uraníferas (Aquatic plant selection and perspective in uranium contaminated water phytoremediation), Master thesis. Instituto Superior Técnico, Lisboa
Favas PJC, Pratas JS (2007) Uptake of heavy metals, and arsenic by an aquatic plant in the vicinity of the abandoned Ervedosa tin mine (NE Portugal). Geochim Cosmochim Acta 71(15):A270–A270
Paulo C, Pratas J (2008) Environmental contamination control of water drainage from uranium mines by aquatic plants. In: Prasad MNV (ed) Trace elements as contaminants and nutrients: Consequences in ecosystems and human health. Wiley, Chichester, pp 623–651
Pratas J, Favas P, Rodrigues N, Prasad M (2010) Arsenic accumulation in aquatic plants (Central Portugal). In: Kallel A, Hassairi A, Bulucea CA, Mastorakis N (eds) Advances in waste management. WSEAS Press, Kantaoui, pp 73–76
Pratas J, Favas P, Rodrigues N, Prasad M, Freitas H (2010) Phytofiltration of uranium by aquatic plants of Central Portugal. In: Kallel A, Hassairi A, Bulucea CA, Mastorakis N (eds) Advances in waste management. WSEAS Press, Kantaoui, pp 77–80
Rodrigues N, Pratas J, Tavares L, Branches A (2010) Natural immobilization of uranium in streams. WSEAS Trans Environ Dev 6(7):539–548
Carvalho FP, Oliveira JM, Malta M (2011) Radionuclides in plants growing on sludge and water from uranium mine water treatment. Ecol Eng 37:1058–1063
Favas PJC, Pratas J, Prasad MNV, D’Souza R, Varun M, Paul M (2013) Potential for phytoremediation of multi-element contaminated water using aquatic plants. Curr Opin Biotech 24S:S128
Teixeira S, Vieira MN, Marques JE, Pereira R (2014) Bioremediation of an iron-rich mine effluent by Lemna minor. Int J Phytoremediat 16(12):1228–1240
Favas PJC, Pratas J, Varun M, D’Souza R, Paul MS (2014) Accumulation of uranium by aquatic plants in field conditions: Prospects for phytoremediation. Sci Total Environ 470–471:993–1002
Adriano D (2001) Trace elements in terrestrial environments: Biogeochemistry, bioavailability and risks of metals. Springer, New York, p 867
Smedley PL, Kinniburgh DG (2002) A review of the source, behaviour and distribution of arsenic in natural waters. Appl Geochem 17:517–568
Sharma VK, Sohn M (2009) Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environ Int 35:743–759
Rahman MA, Hasegawa H, Ueda K, Maki T, Okumura C, Rahman MM (2007) Arsenic accumulation in duckweed (Spirodela polyrhiza L.): A good option for phytoremediation. Chemosphere 69:493–499
García J, Rousseau DPL, Morató J, Lesage E, Matamoros V, Bayona JM (2010) Contaminant removal processes in subsurface-flow constructed wetlands: a review. Crit Rev Environ Sci Technol 40:561–661
Singhakant C, Koottatep T, Satayavivad J (2009) Enhanced arsenic removals through plant interactions in subsurface-flow constructed wetlands. J Environ Sci Health 44:163–169
Sasmaza A, Obek E (2009) The accumulation of arsenic, uranium, and boron in Lemna gibba L. exposed to secondary effluents. Ecol Eng 35:1564–1567
Rahman KZ, Wiessner A, Kuschk P, Afferden M, Mattusch J, Müller RA (2011) Fate and distribution of arsenic in laboratory-scale subsurface horizontal-flow constructed wetlands treating an artificial wastewater. Ecol Eng 37:1214–1224
Hozhina EI, Khramov AA, Gerasimov PA, Kumarkov AA (2001) Uptake of heavy metals, arsenic, and antimony by aquatic plants in the vicinity of ore mining and processing industries. J Geochem Explor 74:153–162
Adhikari AR, Acharya K, Shanahan SA, Zhou X (2011) Removal of nutrients and metals by constructed and naturally created wetlands in the Las Vegas Valley, Nevada. Environ Monit Assess 180:97–113
Wang J, Zhao FJ, Meharg AA, Raab A, Feldmann J, McGrath SP (2002) Mechanisms of arsenic hyperaccumulation in Pteris vittata. Uptake kinetics, interactions with phosphate, and arsenic speciation. Plant Physiol 130:1552–1561
Zhao FJ, McGrath SP, Meharg AA (2010) Arsenic as a food chain contaminant: mechanisms of plant uptake and metabolism and mitigation strategies. Annu Rev Plant Biol 61:535–559
Dhankher OP (2005) Arsenic metabolism in plants: an inside story. New Phytol 168:503–505
Lizama AK, Fletcher TD, Sun G (2011) Removal processes for arsenic in constructed wetlands. Chemosphere 84:1032–1043
Reay PF (1972) Accumulation of arsenic from arsenic-rich natural waters by aquatic plants. J Appl Ecol 9:557–565
Robinson BH, Brooks RR, Outred HA, Kirkman JH (1995) The distribution and fate of arsenic in the Waikato river system, North Island, New Zealand. Chem Spec Bioavailab 7:89–96
Mkandawire M, Taubert B, Dudel EG (2004) Capacity of Lemna gibba L. (duckweed) for uranium and arsenic phytoremediation in mine tailing waters. Int J Phytoremediat 6:347–362
Robinson B, Kim N, Marchetti M, Moni C, Schroeter L, Dijssel C, Milne G, Clothier B (2006) Arsenic hyperaccumulation by aquatic macrophytes in the Taupo Volcanic Zone, New Zealand. Environ Exp Bot 58:206–215
Robinson BH, Duwig C, Bolan NS, Kannathasan M, Saravanan A (2003) Uptake of arsenic by New Zealand watercress (Lepidium sativum). Sci Total Environ 301:67–73
Alvarado S, Guédez M, Lué-Merú MP, Nelson G, Alvaro A, Jesús AC, Gyula Z (2008) Arsenic removal from waters by bioremediation with the aquatic plants Water Hyacinth (Eichhornia crassipes) and Lesser Duckweed (Lemna minor). Bioresource Technol 99:8436–8440
Islam MS, Ueno Y, Sikder MT, Kurasaki M (2013) Phytofiltration of arsenic and cadmium from the water environment using Micranthemum umbrosum (J.F. Gmel) S.F. Blake as a hyperaccumulator. Int J Phytoremediat 15(10):1010–1021
Farnese FS, Oliveira JA, Lima FS, Leão GA, Gusman GS, Silva LC (2014) Evaluation of the potential of Pistia stratiotes L. (water lettuce) for bioindication and phytoremediation of aquatic environments contaminated with arsenic. Braz J Biol 74:S103–S112
Chen G, Liu X, Brookes PC, Xu J (2015) Opportunities for phytoremediation and bioindication of arsenic contaminated water using a submerged aquatic plant: Vallisneria natans (Lour.) H.Hara. Int J Phytoremediat 17:249–255
Wells JM, Richardson DHS (1985) Anion accumulation by the moss Hylocomium splendens: uptake and competition studies involving arsenate, selenate, selenite, phosphate, sulphate and sulphite. New Phytol 101:571–583
Mukherjee S, Kumar S (2005) Arsenic uptake potential of water lettuce (Pistia stratiotes L.). Int J Environ Stud 62:249–258
Srivastava S, Sounderajan S, Udas A, Suprasanna P (2014) Effect of combinations of aquatic plants (Hydrilla, Ceratophyllum, Eichhornia, Lemna and Wolffia) on arsenic removal in field conditions. Ecol Eng 73:297–301
WHO (2011) Guidelines for drinking-water quality, 4th edn. World Health Organization, Geneva, p 564
Malczewska B, Myers O, Shuey C, Lewis J (2003) Recommendations for a uranium health-based ground water standard. Ground Water Quality Bureau, Environment Department, New Mexico, p 71
Kalin M, Wheeler WN, Meinrath G (2005) The removal of uranium from mining waste water using algal/microbial biomass. J Environ Radioact 78:151–177
Schöner A, Noubactep C, Büchel G, Sauter M (2009) Geochemistry of natural wetlands in former uranium milling sites (eastern Germany) and implications for uranium retention. Chem Erde-Geochem 69:91–107
Bhalara PD, Punetha D, Balasubramanian K (2014) A review of potential remediation techniques for uranium(VI) ion retrieval from contaminated aqueous environment. J Environ Chem Eng 2:1621–1634
Durakoviae A (1999) Medical effects of internal contamination with uranium. Croatian Med J 40(1):49–66
Laurette J, Larue C, Llorens I, Jaillard D, Jouneau P-H, Bourguignon J, Carrière M (2012) Speciation of uranium in plants upon root accumulation and root-to-shoot translocation: A XAS and TEM study. Environ Exp Bot 77:87–95
Laurette J, Larue C, Mariet C, Brisset F, Khodja H, Bourguignon J, Carrière M (2012) Influence of uranium speciation on its accumulation and translocation in three plant species: Oilseed rape, sunflower and wheat. Environ Exp Bot 77:96–107
Markich SJ (2013) Water hardness reduces the accumulation and toxicity of uranium in a freshwater macrophyte (Ceratophyllum demersum). Sci Total Environ 443:582–589
Mangini A, Sonntag C, Bertsch G, Mueller E (1979) Evidence for a higher natural uranium content in world rivers. Nature 278:337–339
Palmer MR, Edmond JM (1993) Uranium in river water. Geochim Cosmochim Acta 57:4947–4955
Pettersson HBL, Johnston HA, Murray AS (1993) Uptake of uranium and thorium series radionuclides by the water lily, Nymphaea violacea. J Environ Radioact 19:85–108
Miretzky P, Saralegui A, Cirelli AF (2004) Aquatic macrophytes potential for the simultaneous removal of heavy metals Buenos Aires, Argentina. Chemosphere 57(8):997–1005
Mkandawire M, Dudel EG (2005) Accumulation of arsenic in Lemna gibba L. (duckweed) in tailing waters of two abandoned uranium mining sites in Saxony, Germany. Sci Total Environ 336:81–89
Kondo K, Kawabata H, Ueda S, Hasegawa H, Inaba J, Mitamura O, Seike Y, Ohmomo Y (2003) Distribution of aquatic plants and absorption of radionuclides by plants through the leaf surface in brackish Lake Obuchi, Japan, bordered by nuclear fuel cycle facilities. J Radioanal Nucl Chem 257:305–312
Gerth A, Hebner A, Kiessig G, Zellmer A (2005) Passive treatment of minewater at the Schlema-Alberoda site. In: Merkel B, Hasche-Berge A (eds) Uranium in the environment: Mining impact and consequences. Springer, Berlin, pp 409–414
Černe M, Smodiš B, Štrok M (2011) Uptake of radionuclides by a common reed (Phragmites australis (Cav.) Trin. Ex Steud.) grown in the vicinity of the former uranium mine at Žirovski vrh. Nucl Eng Des 241:1282–1286
Bhat SV, Melo JS, Chaugule BB, D’Souza SF (2008) Biosorption characteristics of uranium(VI) from aqueous medium onto Catenella repens, a red alga. J Hazard Mater 158:628–635
Bhainsa KC, D’Souza SF (2001) Uranium (VI) biosorption by dried roots of Eichhornia crassipes (water hyacinth). J Environ Sci Health 36:1621–1631
Parab H, Joshi S, Shenoy N, Verma R, Lali A, Sudersanan M (2005) Uranium removal from aqueous solution by coir pith: equilibrium and kinetic studies. Bioresource Technol 96:1241–1248
Burton MAS, Peterson PJ (1979) Metal accumulation by aquatic bryophytes from polluted mine streams. Environ Pollut 19:39–46
Say PJ, Harding JPC, Whitton BA (1981) Aquatic mosses as monitors of heavy metal contamination in the river Etherow. Great Britain Environ Pollut Ser B 2:295–307
Mouvet C (1984) Accumulation of chromium and copper by the aquatic moss Fontinalis antipyretica L. ex Hedw. transplanted in a metal-contaminated river. Environ Technol Lett 5:541–548
Goncalves EPR, Boaventura RAR (1998) Uptake and release kinetics of copper by the aquatic moss Fontinalis antipyretica. Water Res 32(4):1305–13
Siebert A, Bruns I, Krauss G-J, Miersch J, Markert B (1996) The use of the aquatic moss Fontinalis antipyretica L. ex Hedw. as a bioindicator for heavy metals: 1. Fundamental investigations into heavy metal accumulation in Fontinalis antipyretica L. ex Hedw. Sci Total Environ 177:137–144
Bruns I, Friese K, Markert B, Krauss G-J (1997) The use of Fontinalis antipyretica L. ex Hedw. as a bioindicator for heavy metals: 2. Heavy metal accumulation and physiological reaction of Fontinalis antipyretica L. ex Hedw. in active biomonitoring in the River Elbe. Sci Total Environ 204:161–176
Vázquez MD, Wappelhorst O, Markert B (2004) Determination of 28 elements in aquatic moss Fontinalis antipyretica Hedw. and water from the upper reaches of the river Nysa (CZ, D), by ICPMS, ICP-OES and AAS. Water Air Soil Pollut 152:153–172
Gapeeva MV, Dolotov AV, Chemeris EV (2010) Prospects of using mosses (Fontinalis antipyretica Hedw. and Pylaisia polyantha (Hedw.) Bruch et al.) as indicators of environmental contamination with heavy metals. Russ J Ecol 41(1):28–31
Díaz S, Villares R, Carballeira A (2012) Uptake kinetics of As, Hg, Sb, and Se in the aquatic moss Fontinalis antipyretica Hedw. Water Air Soil Poll 223:3409–3423
Mechora Š, Germ M, Stibilj V (2012) Selenium and its species in the aquatic moss Fontinalis antipyretica. Sci Total Environ 438:122–126
Uysal Y, Taner F (2009) Effect of pH, temperature, and lead concentration on the bioremoval of lead from water using Lemna minor. Int J Phytoremediat 11:591–608
Sharma P, Dubey RS (2005) Lead toxicity in plants. Braz J Plant Physiol 17:35–52
Ekmekci Y, Tanyolac D, Ayhan B (2009) A crop tolerating oxidative stress induced by excess lead: maize. Acta Physiol Plant 31:319–330
Mishra S, Srivastava S, Tripathi R, Kumar R, Seth C, Gupta D (2006) Lead detoxification by coontail (Ceratophyllum demersum L.) involves induction of phytochelatins and antioxidant system in response to its accumulation. Chemosphere 65:1027–1039
Peng K, Luo C, Lou L, Li X, Shen Z (2008) Bioaccumulation of heavy metals by the aquatic plants Potamogeton pectinatus L. and Potamogeton malaianus Miq. And their potential use for contamination indicators and in wastewater treatment. Sci Total Environ 392:22–29
Piotrowska A, Bajguz A, Godlewska B, Czerpak R, Kaminska M (2009) Jasmonic acid as modulator of lead toxicity in aquatic plant Wolffia arrhiza (Lamnaceae). Environ Exp Bot 66:507–513
Singh R, Tripathi RD, Dwivedi S, Kumar A, Trivedi PK, Chakrabarty D (2010) Lead bioaccumulation potential of an aquatic macropyte Najas indica are related to antioxidant system. Bioresour Technol 101:3025–3032
Alonso-Castro AJ, Carranza-Álvarez C, Alfaro-De la Torre MC, Chávez-Guerrero L, García-De la Cruz RF (2009) Removal and accumulation of cadmium and lead by Typha latifolia exposed to single and mixed metal solutions. Arch Environ Contam Toxicol 57:688–696
Lyubenova L, Pongrac P, Vogel-Mikuš K, Mezek GK, Vavpetič P, Grlj N, Regvar M, Pelicon P, Schröder P (2013) The fate of arsenic, cadmium and lead in Typha latifolia: A case study on the applicability of micro-PIXE in plant ionomics. J Hazard Mater 248–249:371–378
Smolyakov BS (2012) Uptake of Zn, Cu, Pb, and Cd by water hyacinth in the initial stage of water system remediation. Appl Geochem 27:1214–1219
Augustynowicz J, Tokarz K, Baran A, Płachno BJ (2014) Phytoremediation of water polluted by thallium, cadmium, zinc, and lead with the use of macrophyte Callitriche cophocarpa. Arch Environ Contam Toxicol 66:572–581
Alloway BJ (1995) Soil processes and the behavior of metals. In: Alloway BJ (ed) Heavy metals in soils. Blackie, Glasgow, pp 38–57
Succuro JS, McDonald SS, Lu CR (2009) Phytoremediation: The wave of the future. In: Kirakosyan A, Kaufman PB (eds) Recent advances in plant biotechnology. Springer, Dordrecht, pp 119–135
Samecka-Cymerman A, Kempers AJ (2001) Bioindication of heavy metals with aquatic macrophytes: The case of a stream polluted with power plant sewages in Poland. J Toxicol Environ Health 62(1):57–67
Kamal M, Ghaly AE, Mahmoud N, Côté R (2004) Phytoaccumulation of heavy metals by aquatic plants. Environ Int 29:1029–1039
Hou W, Chen X, Song G, Wang Q, Chang CC (2007) Effects of copper and cadmium on heavy metal polluted waterbody restoration by duckweed (Lemna minor). Plant Physiol Biochem 45:62–69
Khellaf N, Zerdaoui M (2009) Phytoaccumulation of zinc by the aquatic plant, Lemna gibba L. Bioresource Technol 100:6137–6140
Thiébaut G, Gross Y, Gierlinski P, Boiché A (2010) Accumulation of metals in Elodea canadensis and Elodea nuttallii: Implications for plant–macroinvertebrate interactions. Sci Total Environ 408:5499–5505
Zhu YL, Zayed AM, Quian JH, De Souza M, Terry N (1999) Phytoaccumulation of trace metals by wetland plants: II. Water hyacinth. J Environ Qual 28:339–344
Hu C, Zhang L, Hamilton D, Zhou W, Yang T, Zhu D (2007) Physiological responses induced by copper bioaccumulation in Eichhornia crassipes (Mart.). Hydrobiologia 579:211–218
Mishra VK, Tripathi BD (2009) Accumulation of chromium and zinc from aqueous solutions using water hyacinth (Eichhornia crassipes). J Hazard Mater 164:1059–1063
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Favas, P.J.C., Pratas, J., Paul, M.S., Sarkar, S.K., Prasad, M.N.V. (2016). Phytofiltration of Metal(loid)-Contaminated Water: The Potential of Native Aquatic Plants. In: Ansari, A., Gill, S., Gill, R., Lanza, G., Newman, L. (eds) Phytoremediation. Springer, Cham. https://doi.org/10.1007/978-3-319-40148-5_11
Download citation
DOI: https://doi.org/10.1007/978-3-319-40148-5_11
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-40146-1
Online ISBN: 978-3-319-40148-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)