Abstract
Paper microfluidics play an increasing role in biomedical research. This interest is attributed to the technological simplicity, the considerably lower costs compared to other fabrication approaches, as well as good compatibility with many bioanalytical procedures. A paper-based analytical device can be developed and modified relatively fast; its fabrication typically does not require any sophisticated facilities and highly skilled personnel. Therefore, paper microfluidics can be well applicable in resource-limited settings, complementing and, in some cases, even replacing the traditional bioanalytical tools.
This chapter presents a short overview of the progress made by paper microfluidics in recent years, reviews the basic design and operational principles as well as discusses the application areas of paper-based devices.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Low cost, paper-based point-of-care diagnostics, often also referred as, paper microfluidics and paper lab-on-a-chip is a new, emerging class of microfluidics devices. This chapter summarizes recent advances in the field of paper microfluidics, describes the underlying physical concepts, design and fabrication guidelines, and presents various examples of application of this technology. In this chapter, we mainly focused on the works published in the period 2010–2016. We apologize in advance if any original works and reviews were not mentioned or overlooked due to the large amount of material that has been published in this topic.
Multiple reviews have been published: reviews giving overview over all aspects of paper devices [1–5], reviews which are mainly focused on paper-based biosensors with EC detection [6], functionality of paper devices [7], detection [7, 8], chemical measurements using microfluidic paper-based analytical device (μPAD) [9], bio-sensing techniques and, specifically, use of nanoparticles [10], working principles and reaction mechanisms [11], history of development [12], thorough review of properties of paper material [13], paper devices for biomarkers and bacterial detection [14], infectious disease [15], paper devices in resource-limited settings for diagnostics and education [16].
Traditionally paper microfluidics has been seen as a low cost diagnostic tool for the developing world. There are still big hopes associated with this technology, mainly that it can help people in low and middle-income countries in areas such as public health, environmental monitoring, agriculture, water and food safety and many others [17]. At the moment, according to the World Bank data, more than 5 billion people are living in the areas that can be characterized as low and middle-income. These people might live in low-resource, hard-to-reach areas with a very limited access to primary healthcare and nearby medical institutions. Access to affordable diagnostic tools can be a very positive change in these communities. Another aspect of the same problem: people might lack basic medical education in these areas. If diagnostic tools are easy to use and interpret, many people would be able to apply them to real life problems it e.g. a community health officer at a remote rural village with limited medical education and not only highly educated practitioners could do the initial diagnostic screenings. With time, when reaching technological maturity, this type of technology could be very useful for rapid diagnostics of e.g. non-communicable diseases (e.g. sickle cell, or daily monitoring of blood glucose levels in diabetic patients), but also for communicable (infectious) disease diagnosis (e.g. malaria or ebola). Monitoring quality of drinking water is another application area that still needs attention worldwide, as some regional discrepancies as well as differences between rural and urban regions persist. According to the World Health Organization, 8 out of 10 people living in rural areas do not have access to high quality, clean drinking water sources. Integration of low cost solutions for water quality monitoring would have positive economic effect and improve well-being of people in those regions. Managing the above-mentioned challenges will be more important over the years as the world population will continue to grow. These ideas are few of the core motivating factors behind the development of paper-based microfluidic diagnostic devices.
As the research community on this technology rapidly developed, the field also started receiving a commercial attention as well as interest from non-profit organizations. This is seen following the numerous patents filed in this area in recent times with a few examples referred to here [18–20]. DFA (Diagnostics for All), for example, is a non-profit organization aiming to deliver low-cost, easy-to-use, point-of-care diagnostic devices designed specifically for the developing world [21], and is one of the successful examples of bringing this technology into use. If paper-based devices will be able to overcome such shortcomings of the lateral flow based devices as lack of quantification, specificity and sensitivity, they may potentially revolutionize the field of low cost diagnostics.
The paper microfluidics device works by wicking liquid sample between separate compartments containing assay reagents. The device may consist solely of patterned chromatography paper or may combine several other materials (e.g. polymers, conductive materials, functional nanoparticles, etc.) used in point-of-care. Typically, a paper device has hydrophobic patterns, and hydrophilic areas which are used as chambers and channels, and performing various kinds of fluidic operations. If, for example, a colorimetric assay has been adapted, the result can be read by a phone, or photographed and sent for further analysis to a centralized lab, where a specialized medical practitioner will be making an informed judgement. Time duration of assays on paper is mainly limited by the time needed to wick paper channels of given dimensions and design and by the inherit properties of the assay itself, and typically is within 30 min, which is well sufficient for many analytical applications. Some applications are particularly suitable for paper diagnostics. These are situations where e.g. qualitative results are of primary interests, the analytes are fairly easy to obtain, some device-to-device variations due to fabrication processes are acceptable and assays can be easily transferred for use on paper substrates [22].
Paper-based devices are so attractive due to these factors:
-
Low cost,
-
Operating on low volumes,
-
Abundant supply,
-
Easy to construct devices,
-
Suitable for multiplexing, thus multiple samples can be analyzed simultaneously,
-
Reagent storage in dry form,
-
Facile interpretation of the results (produced signal can be read by eye),
-
Do not require additional instrumentation for liquid transport (without pump),
-
Can be designed not to require additional instrumentation to read test results [23],
-
Possible to combine with portable readout systems [24, 25], smart phones [26–28]
-
Highly-skilled staff is not required,
-
Light-weight material,
-
Does not produce bio-hazardous waste,
-
Devices can be mass-produced,
-
Portable format of devices, and many others.
Factors that are often mentioned in a positive context can be, however, seen as drawbacks when applied in different settings. In fact, this depends on the specifications to device and the application area.
The following drawbacks are often mentioned:
-
Limited sensitivity of many assays,
-
Lack of quantitation in existing assay formats,
-
Limited number of detection methods can be applied, i.e. colorimetric, electrochemical,
-
Often not self-sustained, i.e. to be able to obtain quantitative results paper-based devices need to be integrated with external read-out system, which increases the costs and complexity [22].
Significant progress has been made in increasing functionality of paper-based fluidic devices. At the moment, many fluidic operations are possible including fluidic timers, valves, sophisticated detection units and even power sources (batteries)—therefore, potentially it is possible to design complex assays in single paper-based device. If, some of the promising design concepts could be mass-produced in paper at a price comparable to commercially available rapid tests, they may be commercialized in the near future.
2 Application Areas
The idea of low-cost microfluidics responding to global public health needs has been around before paper microfluidics started developing as an individual field, and, undoubtedly, there was and still is an actual need for monitoring devices used in resource-poor settings [29]. The world Health Organization (WHO) provides the guidelines (“ASSURED” concept) to how devices should be better suitable for being used in developing countries. Paper devices meet such requirements as being affordable, sensitive (sufficient sensitivity for some applications), specific, user-friendly, rapid and robust, equipment-free, portable and target-delivered (to those who in need of the technology) [1]. Essentially, paper devices can be applied to analysis of various bodily fluids and their synthetic substitutes (whole blood [30], human serum [31, 32], artificial serum, synthetic urine, urine, saliva). Detection of a variety of biomarkers has been demonstrated i.e. uric acid and glucose [24, 31], cholesterol [33], simultaneous detection of glucose, lactate and uric acid in urine, ketones [34], salivary nitrite [34], proteins [34], lactate [35], triglyceride [36], nitrates [34].
An overview of the history of the development of this field can be found elsewhere [1]. An important step in growing awareness of this technology was introduction of paper-based ELISA and the first demonstration of the colorimetric glucose assay on paper, which is still used by many researchers as a model application system [27, 37].
While a killer-application, that is only possible on paper, is still missing, a wide range of interesting applications has been reported:
-
Medical diagnostics
Paper fluidics can be successfully implemented in medical diagnostics. Especially it is useful for systematic, routine diagnosis, analysis of patients’ samples in places distant from hospitals, analysis of asymptomatic diseases, and evaluation of disease progression [1]. Examples include early cancer detection [38] using multiple biomarkers, among those are r-fetoprotein (AFP), carcinoma antigen 125 (CA125), carcinoma antigen 199 (CA199) and carcinoembryonic antigen (CEA) [32], isolation of extracellular vesicles [39], blood typing [30, 40], drug monitoring (example of induced liver failure [41]), diagnostics of non-communicable diseases (including cardiovascular disease and cancer) [42].
-
Veterinary diagnostics
This application area mainly addresses infectious and viral diseases passed from animals to humans. A wide array of communicable diseases can be transmitted from livestock or wildlife to humans in various ways. Some of these disease can potentially become pandemics [1].
-
Food safety and control, agricultural field
Determination of toxic agents with main examples including salmonellosis and campylobacteriosis infections (via eggs, poultry, and unpasteurised milk), enterohaemorrhagic Escherichia coli (O157:H7 serotype) and cholera (via water, rice, vegetables, seafood) [1], water analysis by electronic tongue device [43], detection of pesticides [44, 45], foods analysis e.g. wine and beer [46].
-
Environmental monitoring
Today, very few developing countries have adapted testing routines for water supplies in rural regions. Contamination due to industrial and agricultural activities, as well as environmental pollution monitoring is still challenging and needs to be tackled. Examples of monitoring of these factors include detection of heavy metals [47], low cost monitoring of environmental pollutants in air [48], measuring the metals (Fe, Cu, Ni, and Cr) content in welding fumes [49, 50], biosensor for organic pollutants in water (e.g. L-DOPA and catechol [51]), bacterial detection (Salmonella and Escherichia Coli) [28].
-
Energy storage and generation
In fact, paper as a substrate has entered other fields, besides traditional point-of-care diagnostics. Batteries and other types of energy storage devices can be constructed based on paper [52], fuel cell harvesting electricity from bacterial metabolism [53], devices generating power when liquid sample is applied [54].
-
Pharmaceuticals
Detection of pharmaceuticals has been demonstrated on paper [55].
-
Forensics
Detection of explosives is a new area of application [56].
3 Physical Principles
The ability to control the flow rates of liquids in paper channels is necessary for successful operation of the device. This section gives an overview of basic physical principles behind flow in paper. For more details, one can refer to the informative articles on this topic [57, 58].
3.1 Flow Through Paper
Darcy’s law describing flow through porous media is the basis for estimation of liquid flow through a paper channel. For the constant width of channels fluid flow can be expressed as
Where Q is the volumetric flow rate, k—permeability of the paper, η—viscosity, WH—area of the channel perpendicular to flow, ΔP—pressure difference over the length of channel L.
When the width of channel is varied:
WiHi is the area perpendicular to flow, Li is the length in the direction of flow, and ΔP is the pressure difference across the length of the channel.
Using parallels between fluidic and electrical resistance, i.e. liquid flow Qi is an equivalent for current, ΔPi pressure drop along the channel is equivalent to voltage drop, and μLi/(kWiHi) is an equivalent to fluidic resistance of each individual channels, one can estimate total flux. If several fluidic elements are connected in series or in parallel, the total flux through this network will follow analogy of Ohm’s law, i.e. sum of individual fluxes when connected in series, and reciprocals when connected in parallel.
3.2 Spreading of Wax and Width of Patterned Channel
Spreading of the molten wax and width of final channels can be predicted using the Washburn equation :
Where L—distance covered by the wax front, η—viscosity (function of time and temperature to which device was exposed during bake), γ—effective surface tension , D—average pore diameter, t—time. The same equation can be used to predict transport of the fluid front in the channel.
The final inner width of the channel formed by wax can be defined as
W C = W P − 2L, where
WC—inner width of the hydrophobic channel, WP—inner width of the printed channel, L—the additional distance that the wax spreads perpendicular to the length of the channel. L is a function of time, heat and the structural properties of paper.
3.3 Transport Time
Transport time through a multi-segment geometry can be calculated using the modified Darcy’s law equation:
Where V is the volume of the geometry, ΔP is pressure difference and Req expressed as
If we assume that permeability, viscosity, and pressure difference are constant, differences in the transport times in two channels would be only due to geometrical differences
This means that all channels of the same length would have the same transport time regardless to other geometrical dimensions.
Of course, in reality, channels often have several sections characterized by different length and width. For a channel that has two sections, both characterized by constant length and width (and same heights), the equation for transport can be modified to
3.4 Signal Visibility
The visibility of the signal is a function of the thickness and the opacity of paper:
4 Main Formats of Paper Devices
Many design concepts in paper microfluidics are inspired by bio-sensing principles and structure of the lateral rapid tests and dipsticks [59]. Examples of the commercial point-of-care tests are shown in Fig. 7.1. If focus in lateral flow assays and dipsticks was their robustness and ease of interpretation (often yes/no answer), paper microfluidics explored various ways to enhance the functionality, increase design complexity while still trying to keep the costs down. Current status on fluidic operations in paper can be found elsewhere [60]. Design concepts of paper fluidic devices are constantly under development. Currently several groups succeeded in integration of sample preparation with simultaneous detection of a biomarker of interest using the same paper device [61].
Commercial point-of-care tests. (a) DetermineTM HIV 1/2 Ag/Ab Combo, (b) DetermineTM TB LAM Ag test, (c) QuickVue Influenza A + B test, (d) Clearview1 Malaria P.f. Test, (e) DirectigenTM EZ Flu A + B, (f) ICON HP (g) ImmunoCard STAT!1 E. coli O157 Plus, (h) A multiplex lateral-flow assay. RAIDTM 5 for the determination of biological threat agents. Adapted from [1] with permission of Royal Society of Chemistry
Transport of liquid through paper is equivalent to transport of liquid through the porous media. It occurs as soon as paper is brought in contact with liquid reagents and therefore has to be confined and directed. To gain some understanding on correlation between the shape of channels and liquid transport, one can also refer to [58]. Most of the channels have rectangular shape, but there is also experimental work with channels with varied width [58]. Typical width of the hydrophilic channel is 0.5–4 mm, hydrophobic barrier can be about 1 mm.
All paper devices can be classified by these three main formats:
-
2D format for paper networks
Two-dimensional paper networks were the first microfluidic designs. A few typical examples are shown in Fig. 7.2. These are mostly channels fully made out of paper, which can either be cut or patterned with a liquid-repelling material to a required shape. The channels may vary in width, length, may contain several segments varying in size for storage of liquid and dry reagents. Modifying channel length and width allows controlling time and spatial distribution of reagents and samples.
Fig. 7.2 2D paper channels of various designs: (a) channels of different width (liquid introduced via the same inlet of the same width) shows faster transport in the strip of smaller width. Images were taken at 2, 10, 50, and 210 s after introduction of fluid to the inlet (Adapted with permission [65] Royal Society of Chemistry); (b) Y-shaped channel with dissolvable barrier can be used to create a delay in the transport time of a fluid along the channel (Adapted with permission [65] Royal Society of Chemistry), (c) Slit-shaped channels and correponding differences in liquid transport (Adapted with permission [58] Royal Society of Chemistry); (d) A simple 2D paper network. The arrival time of multiple reagents at the “detection region” of the paper strip is staggered by placing 3 inlets along the common segment of the device. The geometry of the inlets results in the sequential arrival of fluid from each of the three inlets. (Adapted with permission [65] Royal Society of Chemistry); (e) One of the first paper microfluidic designs with inlet, transport channel and reaction zone. (Adapted from [1] with permission of Royal Society of Chemistry)
To prevent contamination between individual fluidic compartments hydrophobic barriers , absorbing pads or physical separation of reacting zones can be applied. Flow in these channels follows the relationships discussed in the previous sections. Some of the early publications discuss various scenarios of liquid transport in 2D networks such as: Y-shaped and T-shaped devices, structures for hydrodynamic focusing, size-based separation, mixing and dilution [57, 62–65], hydrophobic barriers for time-controlled transport of liquid reagents [66]. The majority of the newly published works are using this design principle probably due to its simplicity.
-
3D format for paper networks,
Three-dimensionality was an important step in developing device complexity. In these devices, liquid transport occurs in both vertical and lateral directions [32, 67–71]. 3D devices, shown in Figs. 7.3 and 7.4, have two main advantages (1) better suitable for multiplexing, a higher number of tests can be simultaneously integrated; (2) more complex assays can be integrated as three-dimensionality (liquid flow can now be controlled in both lateral x-y and vertical z directions) allows for more complex fluidic operations and more suitable for multi-step assays[72, 73]. Since liquid flow can be transported vertically, the distribution times between various reaction zones can be significantly reduced, so the required sample volume.
Fig. 7.3 Example of an assay based on 3D origami immunodevice. (a) The filter tab was folded above the test pad; (b) the blood sample was added into each paper microzone on the folded filter tab with the aid of Folder-I; (c) the filter tab was removed and one waste tab was folded below the test pad; (d) the washing buffer was added into each immunozone on the test pad to wash the immunozones with the aid of the used Folder-I; (e) the used waste tab was removed and a solution of AgNPs-luminol/Ab2 was added into each immunozone on the test pad; (f) the remaining waste tab was folded below the reversed test pad and the device was washed again with the aid of Folder-II; (g) the used waste tab was removed and the reagent tab was folded above the test pad; (h) the hydrogen peroxide solution was added into the reagent cell to trigger the CL reactions with the aid of a new Folder-I. Reproduced from [72] with permission of from Royal Society of Chemistry
Fig. 7.4 Example of a process flow for fabrication of 3D microPADs using a toner. (a) Schematic of the four steps of device fabrication. (b) The top of the device after addition of aqueous dyes to the sample inlets and wicking across the channels for 2 min. (c) The bottom of the device after addition of aqueous dyes. (d) Cross-section of the device showing the layers of paper and toner. The toner is used for bonding of the layers of paper together, it prevents fluids in channels from mixing. Ports in the toner layer allow wicking between layers of paper. Reproduced from [73] with permission of Royal Society of Chemistry
-
Centrifugal, i.e. paper-disc format,
Flow in 2D and 3D devices are governed by capillary force. Centrifugal paper-based systems are operating on principles of interplay of centrifugal and capillary forces [36, 74, 75]. This adds more possibilities to time and spatial time control as reagents can be recirculated within the same channel multiple times and the flow rate through the paper can be well controlled. Compared to this, in 2D and 3D networks, the flow through channel is typically constant and reverse flow is not possible. Two examples of centrifugal systems are shown in Fig. 7.5.
Fig. 7.5 Centrifugal paper-disc devices: (a) whole paper disc is used in this device assembly and fluidic structures are patterned in paper by wax, (b) paper inserts with wax patterns are cut and integrated into polymeric parts. Adapted from [75] with permission of IEEE
-
Various hybrid formats, that cannot be strictly assigned to either of the above mentioned categories.
Combination of paper devices with other materials and the advances in other areas of microfluidic research gave rise to some interesting hybrid device concepts. Various efforts for hybrid integration of electrodes into paper has been demonstrated: electrical circuit used for electrical readout can be attached externally [53], functionalized paper can be placed on top of screen-printed electrode [76], or electrodes can be incorporated as a part of the fluidic network directly in paper [43, 77]. Since patterning of electrodes on paper or hybrid integration with external devices are relatively established process, paper microfluidics can be combined with other areas such as e.g. digital microfluidics, which allows development of some complex assays [78]. These areas can potentially profit strongly from each other as low power and flexibility of operational control of digital microfluidics can be well combined with suitability for rapid prototyping of paper fluidics. Fabrication of electrodes for digital microfluidic devices in a classic clean room would be significantly more expensive. Another example is a combination of paper with hydrogels , where hydrogels are used for liquid storage and upon application of external stimulus release liquid into paper [79]. Sugars were applied to paper for programmable time-delays, that can be used for sequential reagent delivery [80].
5 Types of Paper and Its Functionalization
Traditionally, rapid point-of-care tests use nitrocellulose membranes (e.g. membranes available from Millipore). The term “membrane” is exclusively used to describe nitrocellulose in the lateral flow format [1]. Nitrocellulose-based technologies are in close relationship to paper microfluidic devices. Attempts to replace nitrocellulose in the diagnostics industry with other materials (nylon, polyvinylidene fluoride and Fusion 5 from Whatman) were not successful due to high-costs, manufacturing challenges and need for additional optimization of chemistry. In order to replace current manufacturing practices, to which industries are typically reluctant, paper-based devices have to compete in manufacturing price, signal-to-noise ratio, robustness and functionality. The term “paper” refers to cellulosic materials (i.e. filter paper and chromatography paper used in microfluidic devices). 90 % of paper is produced from wood stock, however for diagnostic purposes a paper from cotton is desired (to eliminate interferences from lignin coming from wood). Unlike nitrocellulose membranes exhibiting hydrophobic properties due to its cellulose acetate blends, filter and chromatographic papers are hydrophilic and do not require the deposition of surfactants to improve wetting properties. Additionally, there are several other requirements to paper material: suitability for processing of biological samples in small volumes and within short times (e.g. wicking time between reaction zones), specificity and sensitivity comparable to commercial rapid tests [1].
Another crucial factor is sufficient protein binding to allow for the formation of sharp and intense capture zones while keeping the signal from nonspecific background low.
There is no universal type of paper that will suit all applications. Choice of design is highly dependent on the type and structure of paper. Structural properties, insights into physics and chemistry of paper [81] can be found in dedicated reviews. Utilization of different papers, and different channel width were explored by some researchers earlier [36, 82]. Some experimental work on papers of different grades can be found in [75]. Commercial paper grades differ in flow rate, pore size and porosity, thickness, color, particle retention and other properties. Ideally, in paper microfluidic devices there should be high consistency (within single device as well as batch-to-batch) in surface pore size, thickness, protein binding capacity (irreversible capture of reagents at the detection zones), flow characteristics, magnitude of obtained signal during detection and stability during storage. Binding characteristics of selected paper should be tested during the development stage of device. Protein binding capability will dependent on the paper surface area available for capture. It should also be verified that binding capabilities are not altered during the manufacturing process or when assay is run [13].
Paper requires chemical activation to immobilize antibodies. Many researchers address that it is suitable for variety of (bio)functionalization procedures [13]. Techniques for functionalization of paper, and main factors influencing functionalization procedures such as structure and surface chemistry are discussed [82, 83]. Early works describing strategies for treatment of paper surface with DNA, both physical adsorption and covalent binding [84], salinization [45], in situ polymerization of molecular imprinted polymer on paper [45], modification of paper with poly (vinyl pyrrolidone) and polyaniline [33]. Functionalization with polymers gives numerous active sites to build up a sensitive detection method [85, 86].
There are, however, some concerns associated with possible integration of paper in diagnostic devices. For example, colloidal gold and latex labels, used in the industry, require more open pore materials such as glass fibre and polyester for optimized stabilization and release from it during the assay run. Surface quality is another key parameter in optimizing the performance of paper-based microfluidics. Paper has relatively rough surface characteristics, which might cause various challenges in reproducibility of quantitative measurements. Recently, a novel class of materials, so-called synthetic paper, with controlled porosity characteristics, has been introduced and may have potential for integration in point-of-care devices [87].
Finally, note that it is possible to combine nitrocellulose, filter, and chromatographic paper in one device where positive sides of each type of these materials can be utilized. All these materials can be cut by laser or PC-controlled knife plotter, and assembled in a single process.
6 Existing Fabrication Technologies
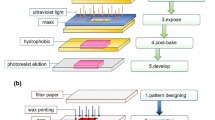
This section reviews technologies that are applied for fabrication of paper-based devices. Because paper is a very flexible material, the following techniques are often applied (Fig. 7.6): inkjet, wax, flexography, or screen-printing all use non-toxic reagents. In the majority of the works a paper device needs to go through two main fabrication stages, i.e. patterning of hydrophobic channels for liquid confinement and assembly [88]. On the laboratory level, some deviations from the described low-cost methods are possible, but due to the limited space we only provide general descriptions.
Examples of paper-based structures fabricated by (a) wax-printing (Adapted from [92] with permission of Royal Society of Chemistry), (b) screen-printing (Adapted from [99] with permission of Royal Society of Chemistry), (c) inkjet-printing (Adapted from [104] with permission of John Wiley and Sons) and (d) photolithography assisted method (FLASH) (Reproduced from [108] with permission of Royal Society of Chemistry)
6.1 Technologies for Patterning of Hydrophobic Barriers
Paper is a flexible material; therefore, various printing techniques are well suited to form a pattern. Physical blocking of pores in paper with hydrophobic material is a widely used method. The rise of this technology goes back to 2007 when a description of the process for creating hydrophobic pattern in paper and using hydrophilic channels for pumpless liquid transport was reported by Whitesides and co-workers [89]. Hydrophobic barriers can be made using wax, SU-8 and other photoresist materials, PDMS , alkenyl ketene dimer [5], polystyrene , ethyl cellulose, silicones, rosin, paraffin, printer varnish, cellulose esters, hydrophobic gels [90] and possibly others. All these materials are impervious to water and allow for implementation of various structures for transport and storage of liquids. Width of channel structures that can be achieved by these methods varies and can go down to 200-300 μm with some optimization.
6.1.1 Wax Printing
Wax printing [91, 92] is the most commonly used technique. It is easy, fast, and low cost, and can be easily applied for small-scale manufacturing. Typically, the channel width is in the range of 1–5 mm. This technology is very straightforward: a design is sent to a printer and printed directly on a sheet of paper, this paper can be sandwiched between a metal foil and placed on a hot-plate at ca. 70 °C for 1 min. For better penetration of wax into paper it can be baked from both sides. Baking temperature, baking time and printing mode are parameters that can be used for optimization in each specific case. Speading of wax in paper after bake can be predicted (see description in the earlier section). Reproducibility of channel dimensions and printing resolution will depend on quality of the printer and homogeneity of heat applied during the bake. Wax printing has been widely applied to 2D, 3D fluidic devices as well as in centrifugal paper-based systems. Details of the method for fabrication of 3D μPADs by wax printing can be found elsewhere [93]. Permeability of the paper is another parameter describing flow through it. Permeability has been alternated using papers that were impregnated with wax [94]. Accurate control over the penetration depth of melted wax, printed on both sides of a paper substrate allows formation of multilayers of patterned channels in the substrate [95].
6.1.2 Screen-Printing
Screen-printing [96, 97] is a versatile technique where liquid material is transferred onto substrate via a screen (a grid with a stencil attached or formed directly on it) manually or using an automatic tool, which regulates pressure applied on substrate and amount of printed material. After applying the material, the paper substrate is allowed to dry, and maybe subjected to heat or other treatments. What is characteristic for this method is that much thicker layers of printed material can be applied compared to wax printing by a commercial printer. There are also less strict requirements to size, planarity, shape and thickness of the selected paper substrate. Advantages of screen-printing technique [98] are fast fabrication times, low costs, flexibility (different materials can be printed) and capability of mass production. Several instrument-free, single-step screen-printing methods on chromatographic paper were demonstrated, e.g. patterning of PDMS solution with minimum channel width of ca. 600 μm [99], patterning of polystyrene through a patterned screen [100] with minimum channel width of ca. 670 μm (minimum width of hydrophobic barrier was ca. 380 μm). Another variation of this technique utilizing spraying of material through a pre-defined micro stencil instead of squeezing through a screen as in conventional screen-printing, i.e. a mask containing pattern to be transferred [101]. This technique is also widely applied to form electrodes in paper [77].
6.1.3 Inkjet-Printing
Inkjet-printing is also one of the early methods applied for fabrication of paper-based devices [102–104]. Review of inkjet-printed technologies applied on paper can be found elsewhere [105, 106]. In short, this technology is based on transferring material directly into paper via a nozzle (nozzle is activated e.g. piezo-electrically or thermally). Material is jetted in a close proximity from paper surface and follows a required pattern. Printed lines are formed from hydrophobic inks, e.g. PDMS , which was also adapted to roll-to-roll technology [103, 107]. Besides the setup of the inkjet-printer (jetting pressure applied via the nozzle), the chemistry and wetting properties of the printed material is probably the most crucial optimization parameter to achieve good quality of the printed image.
6.1.4 Flexographic Printing
Flexographic printing is method utilizing a flexible plate containing a pattern which is transferred by applying a liquid ink . It is another variation of the screen-printing technique, which is widely applied in industrial settings. It is suitable for various inks and substrates. One example is flexographic printing of polystyrene applied using the roll-to-roll technology. Several layers need to be applied to achieve hydrophobic channels where width of channels is typically 1 mm [107].
6.1.5 Photolithography -Assisted Methods
Conventional UV lithography can be used to produce pattern on photo-curable polymer that was brought in contact with paper substrate by one or other method. Paper can be impregnated with the photoresist via dipping prior to patterning using a UV lamp through a glass or polymeric mask containing desired patterns. The UV-assisted methods, i.e. FLASH method, was one of the first methods introduced for patterning of paper-based devices [108]. UV lithography can aid very defined and reproducible structures but fabrication costs are high and not every lab has access to photolithography facilities. A variety of hybrid fabrication methods were demonstrated, for example, an interesting method where photolithographic patterning of Parafilm® was subsequently followed by embossing of the film into paper [109].
6.1.6 Plasma Treatment
Definition of hydrophilic and hydrophobic regions can also be achieved by exposure to plasma [110]. Oxygen plasma can also be used to introduce new fluidic functionalities in paper, e.g. multiple-use valves [111].
6.2 Technologies for Assembly of Devices
After paper devices were patterned with hydrophobic materials to form channels and reaction zones, they still need to be assembled. Common methods include cutting, stacking several layers of paper together, shaping of paper by cutting or folding (origami-like technique [71]) with or without use of adhesive tapes [70, 112]. Paper can also be laminated prior to cutting and stacking by methods similar to production of ID cards [113, 114]. Cutting can be done by knife plotter controlled by PC (down to 0.5–0.8 mm with some optimization of cutting parameters), or CO2 laser can be used for cutting cellulose (down to 1–1.5 mm). Maintaining contact between hydrophilic features in each layer of a 3D paper-based microfluidic device is the key fabrication challenge for these devices. Dry-stored reagents are deposited onto individual layers before assembling the final 3D device [1].
6.3 Technologies for Fabrication of Electrodes in Paper
Many detection methods applied on paper require reproducible fabrication of paper electrodes. Electrodes, typically conductive inks (carbon, Ag/AgCl), can be formed by various techniques, including those discussed above: screen-printing [96, 97], electrospraying [33], painting or dipping paper in conductive ink , or e.g. solution of carbon nanotubes [115, 116], combination of direct printing of Au and Ag stripes and subsequent electrochemical deposition of AgCl layer, and even by using a regular pencil [44]. Combination of fluidic networks in paper printed over electrodes allows for many applications involving measurement of electrical signals [107] such as e.g. digital microfluidics [117]. Functionality of paper can be extended by modifying its properties or hybridly combining it with conductive materials for various electronics applications [118].
7 Detection Methods
7.1 Colourimetric
Colourimetric method has been widely applied in paper for quantification of concentration of analytes [35, 51]. Colourimetric detection may include or not an enzymatic stage. The method is very common for medical laboratories and, as a result, many assays are based on it. It is also a convenient method for multiplexing [119] and can be combined with other detection methods, e.g. electrochemical [120]. Readout of colourimetric signals can be achieved with a smartphone or dedicated readout system.
7.2 Electrochemical
Detection methods which are using change in electric current or potential as a result of biochemical reaction occurring in a paper device are called electrochemical [38, 44, 121, 122]. Cyclic voltammetry is a very common measurement technique [97, 103], as well as potentiometric [103, 123]. There are also more exotic variations of these such as streaming potential measurements [124].
7.3 Chemiluminescence and Electrochemiluminescence
Detection of electromagnetic radiation as light emitted during chemical reaction is the basis for chemiluminescence [125, 126]. Electrochemiluminescence method is luminescence generated by electrochemical reactions [47, 127].
7.4 Fluorescence
Fluorescence-based assays also have been integrated into paper [128, 129]. Overall, although fluorescence sensing brings new opportunities to paper-based detection, cost reduction and miniaturization of fluorescence readers are still on-going issues [1].
7.5 Nanoparticles
Many detection practices using nanoparticles are coming from the lateral flow assays. Antibody-conjugated gold nanoparticles and monodisperse latex, coupled with fluorescent dyes, are widely utilized in commercial rapid point-of-care tests. In general, colloidal gold particles are a preferred option due to higher colour intensity compared with coloured latex particles; they also can be dispersed in higher density as they typically ten times smaller than monodisperse latex particles [1, 130].
7.6 Other Methods
There are also a few methods, which are less popular and cannot be strictly assigned to any of the methods described above. Paper-based microfluidic calorimeter has been proposed [131]. This method would only suit for assays producing or consuming sufficient amount of heat to be detected as the method is not highly sensitive. Distance-based detection method where distance travelled by analyte producing colorimetric response is used as an analytical signal [50, 132]. Similarly, time to obtain response in the reference area of the device can be used as readout [23, 114].
Various efforts were also made to achieve visual detection just by naked eye which is a very resource-poor settings inspired idea. Specific designs aiming at solely visual determination of test result, e.g. counting bars that turned colorful after analyte has reached it were demonstrated [23]. Electrical measurements were applied for monitoring of bacterial movement through the paper, which both has fundamental interest (measurement of speed of movement of bio-entities through paper network is challenging) and potentially interesting applications [133]. Raman spectroscopy [55] was also applied as a detection method, and although this technique might be very feasible in certain applications, it still remains a sophisticated and expensive technique.
References
Yetisen AK, Akram MS, Lowe CR (2013) Paper-based microfluidic point-of-care diagnostic devices. Lab Chip 13:2210–2251. doi:10.1039/c3lc50169h
Liana DD, Raguse B, Gooding JJ, Chow E (2012) Recent advances in paper-based sensors. Sensors 12:11505–11526. doi:10.3390/s120911505
Martinez AW, Phillips ST, Whitesides GM (2010) Diagnostics for the developing world: microfluidic paper-based analytical devices. Anal Chem 82:3–10
Martinez AW, Phillips ST, Butte MJ, Whitesides GM (2013) Patterned paper as a platform for inexpensive, low volume, portable bioassays. Angew Chem Int Ed Engl 46:1318–1320. doi:10.1002/anie.200603817.Patterned
Li X, Ballerini DR, Shen W (2014) A perspective on paper-based microfluidics: current status and future trends. Biomicrofluidics 011301:1–13. doi:10.1063/1.3687398
Liu B, Du D, Hua X et al (2014) Paper-based electrochemical biosensors: from test strips to paper-based microfluidics. Electroanalysis 26:1214–1223. doi:10.1002/elan.201400036
Ballerini DR, Li X, Shen W (2012) Patterned paper and alternative materials as substrates for low-cost microfluidic diagnostics. Microfluid Nanofluid 13:769–787. doi:10.1007/s10404-012-0999-2
Santhiago M, Nery EW, Santos GP, Kubota LT (2014) Microfluidic paper-based devices for bioanalytical applications. Bioanalysis 6:89–106. doi:10.4155/bio.13.296
Cate DM, Adkins JA, Mettakoonpitak J, Henry CS (2015) Recent developments in paper-based microfluidic devices. Anal Chem 87:19–41. doi:10.1021/ac503968p
Ge X, Mohamed A, Du D et al (2014) Nanomaterial-enhanced paper based biosensors. Trends Anal Chem 58:31–39. doi:10.1016/j.trac.2014.03.008
Hu J, Wang S, Wang L et al (2014) Advances in paper-based point-of-care diagnostics. Bioelectronics 54:585–597. doi:10.1016/j.bios.2013.10.075
Lisowski P, Zarzycki PK (2013) Microfluidic paper-based analytical devices (microPADs) and micro total analysis systems (microTAS): development, applications and future trends. Chromatographia 76:1201–1214. doi:10.1007/s10337-013-2413-y
Pelton R (2009) Bioactive paper provides a low-cost platform for diagnostics. Trends Analyt Chem 28:925–942. doi:10.1016/j.trac.2009.05.005
Shah P, Zhu X, Li C (2013) Development of paper-based analytical kit for point-of-care testing. Expert Rev Mol Diagn 13:83–91. doi:10.1586/erm.12.130
Rozand C (2014) Paper-based analytical devices for point-of-care infectious disease testing. Eur J Clin Microbiol Infect Dis 33:147–156. doi:10.1007/s10096-013-1945-2
Smith S, Moodley K, Govender U et al (2015) Paper-based smart microfluidics for education and low-cost diagnostics. S Afr J Sci 111:0–10. doi:10.17159/sajs.2015/20140358
Mao X, Jun T (2012) Lab on chip microfluidic diagnostics for the developing world. Lab a chipchip 12:1412–1416. doi:10.1039/c2lc90022j
Dickey M, Martinez A, Phillips S, et al. (2014) Paper-based microfluidic systems. US Patent #8,921,118
Carrilho E, Martinez AW, Whitesides GM (2012) Methods of micropatterning paper-based microfluidics. US Patent #0,198,684
Whitesides GM, Phillips ST, Martinez AW, et al. (2015) Lateral flow and flow-through bioassay devices based on patterned porous media, methods of making same, and methods of using same. US patent #9,193,988
Volpatti LR, Yetisen AK (2014) Commercialization of microfluidic devices. Trends Biotechnol 32:347–350. doi:10.1016/j.tibtech.2014.04.010
Phillips ST, Lewis GG (2014) The expanding role of paper in point-of-care diagnostics. Expert Rev Mol Diagn 14:123–125. doi:10.1586/14737159.2014.887445
Lewis GG, Ditucci MJ, Phillips ST (2012) Quantifying analytes in paper-based microfluidic devices without using external electronic readers. Angew Chem Int Ed 51:12707–12710. doi:10.1002/anie.201207239
Zhao C, Thuo MM, Liu X (2015) Corrigendum: A microfluidic paper-based electrochemical biosensor array for multiplexed detection of metabolic biomarkers (2013 Sci. Technol. Adv. Mater. 14 054402). Sci Technol Adv Mater 16:049501. doi:10.1088/1468-6996/16/4/049501
Nie Z, Deiss F, Liu X et al (2010) Integration of paper-based microfluidic devices with commercial electrochemical readers. Lab Chip 10:3163–3169. doi:10.1039/c0lc00237b
Park TS, Yoon J (2015) Smartphone detection of Escherichia coli from field water samples on paper microfluidics. IEEE Sens J 15:1902–1907
Chun HJ, Park YM, Han YD et al (2014) Paper-based glucose biosensing system utilizing a smartphone as a signal reader. BioChip J 8:218–226. doi:10.1007/s13206-014-8308-7
Park TS, Li W, Mccracken KE, Yoon J (2013) Smartphone quantifies Salmonella from paper microfluidics. Lab Chip 13:4832–4840. doi:10.1039/c3lc50976a
Yager P, Edwards T, Fu E et al (2006) Microfluidic diagnostic technologies for global public health. Nature 442:412–418. doi:10.1038/nature05064
Su J, Al-Tamimi M, Garnier G (2012) Engineering paper as a substrate for blood typing. Cellulose 19:1749–1758. doi:10.1007/s10570-012-9748-7
Chen X, Chen J, Wang F et al (2012) Determination of glucose and uric acid with bienzyme colorimetry on microfluidic paper-based analysis devices. Biosen Bioelectron 35:363–368. doi:10.1016/j.bios.2012.03.018
Ge L, Yan J, Song X et al (2012) Three-dimensional paper-based electrochemiluminescence immunodevice for multiplexed measurement of biomarkers and point-of-care testing. Biomaterials 33:1024–1031. doi:10.1016/j.biomaterials.2011.10.065
Ruecha N, Rangkupan R, Rodthongkum N (2014) Novel paper-based cholesterol biosensor using graphene/polyvinylpyrrolidone/polyaniline nanocomposite. Biosens Bioelectron 52:13–19. doi:10.1016/j.bios.2013.08.018
Klasner SA, Price AK, Hoeman KW et al (2010) Paper-based microfluidic devices for analysis of clinically relevant analytes present in urine and saliva. Anal Bioanal Chem 397:1821–1829. doi:10.1007/s00216-010-3718-4
Dungchai W, Chailapakul O, Henry CS (2010) Use of multiple colorimetric indicators for paper-based microfluidic devices. Anal Chim Acta 674:227–233. doi:10.1016/j.aca.2010.06.019
Godino N, Vereshchagina E, Gorkin R, Ducree J (2013) Centrifugal automation of a triglyceride bioassay on a low-cost hybrid paper-polymer device. Microfluid Nanofluid 16:895–905. doi:10.1007/s10404-013-1283-9
Elisa P, Cheng C, Martinez AW et al (2010) Paper-based ELISA. Angew Chem Int Ed Engl 49:4771–4774. doi:10.1002/anie.201001005
Su M, Ge L, Ge S et al (2014) Paper-based electrochemical cyto-device for sensitive detection of cancer cells and in situ anticancer drug screening. Anal Chim Acta 847:1–9
Chen C, Lin B-R, Wang H-K et al (2014) Paper-based immunoaffinity devices for accessible isolation and characterization of extracellular vesicles. Microfluid Nanofluid 16:849–856. doi:10.1007/s10404-014-1359-1
Li M, Tian J, Al-tamimi M, Shen W (2012) Paper-based blood typing device that reports patient’s blood type “in writing.”. Angew Chem Int Ed 51:5497–5501. doi:10.1002/anie.201201822
Pollock NR, Rolland JP, Kumar S et al (2013) A paper-based multiplexed transaminase test for low-cost, point-of-care liver function testing. Sci Transl Med 4:1521129. doi:10.1126/scitranslmed.3003981.A
Warren AD, Kwong GA, Wood DK et al (2014) Point-of-care diagnostics for noncommunicable diseases using synthetic urinary biomarkers and paper microfluidics. Proc Natl Acad Sci U S A 111:3671–3676. doi:10.1073/pnas.1314651111
Nery EW, Guimares JA, Kubota LT (2015) Paper-based electronic tongue. Electroanalysis 27:1–7. doi:10.1002/elan.201500054
Santhiago M, Henry CS, Kubota LT (2014) Low cost, simple three dimensional electrochemical paper-based analytical device for determination of p-nitrophenol. Electrochim Acta 130:771–777
Wang S, Ge L, Li L et al (2013) Molecularly imprinted polymer grafted paper-based multi-disk micro-disk plate for chemiluminescence detection of pesticide. Biosen Bioelectron 50:262–268. doi:10.1016/j.bios.2013.07.003
Witkowska Nery E, Kubota LT (2016) Integrated, paper-based potentiometric electronic tongue for the analysis of beer and wine. Anal Chim Acta 918:60–68. doi:10.1016/j.aca.2016.03.004
Zhang M, Ge L, Ge S et al (2013) Three-dimensional paper-based electrochemiluminescence device for simultaneous detection of Pb 2+ and Hg 2+ based on potential-control technique. Biosens Bioelectron 41:544–550. doi:10.1016/j.bios.2012.09.022
Henry CS, Kim Y, Mettakoonpitak J, Guerrero T (2014) Multifunctional paper microfluidic devices for environmental analysis. In: 18th International Conference on Miniaturized Systems for Chemistry and Life Sciences. San Antonio, 26–30 Oct 2014, pp 45–47
Cate DM, Nanthasurasak P, Riwkulkajorn P et al (2014) Rapid detection of transition metals in welding fumes using paper-based analytical devices. Ann Occup Hyg 58:413–423. doi:10.1093/annhyg/met078
Cate DM, Noblitt SD, Henry CS (2015) Lab on a chip of metals using distance-based detection †. Lab Chip 15:2808–2818. doi:10.1039/C5LC00364D
Oktem HA, Senyurt O, Eyidogan FI et al (2012) Development of a laccase based paper biosensor for the detection of phenolic. J Food Agric Environ 10:1030–1034
Nguyen TH, Fraiwan A, Choi S (2014) Paper-based batteries: a review. Biosens Bioelectron 54:640–649. doi:10.1016/j.bios.2013.11.007
Fraiwan A, Lee H, Choi S (2014) A Multianode paper-based microbial fuel cell: a potential power source for disposable biosensors. IEEE Sens J 14:3385–3390
Thom NK, Yeung K, Pillion MB, Phillips ST (2012) ‘Fluidic batteries’ as low-cost sources of power in paper-based microfluidic devices. Lab Chip 12:1768–1770. doi:10.1039/c2lc40126f
Craig D, Mazilu M, Dholakia K (2015) Quantitative detection of pharmaceuticals using a combination of paper microfluidics and wavelength modulated Raman spectroscopy. Plus One 10:1–10. doi:10.1371/journal.pone.0123334
Blanes L, Taudte R V, Roux C, Doble P (2014) Using paper-based microfluidics and lab on a chip technologies for the rapid analysis of trinitro aromatic explosives. In: 18th International Conference on Miniaturized Systems for Chemistry and Life Sciences. San Antonio, 26–30 Oct, pp 1581–1582
Fu E, Ramsey SA, Kauffman P et al (2011) Transport in two-dimensional paper networks. Microfluid Nanofluid 10:29–35. doi:10.1007/s10404-010-0643-y
Elizalde E, Urteaga R, Berli CLA (2015) Rational design of capillary-driven flows for paper-based microfluidics. Lab Chip 15:2173–2180. doi:10.1039/c4lc01487a
Li C, Vandenberg K, Prabhulkar S et al (2011) Paper based point-of-care testing disc for multiplex whole cell bacteria analysis. Biosen Bioelectron 26:4342–4348. doi:10.1016/j.bios.2011.04.035
Toley BJ, Wang JA, Gupta M et al (2015) A versatile valving toolkit for automating fluidic operations in paper microfluidic devices. Lab Chip 15:1432–1444. doi:10.1039/C4LC01155D
Songjaroen T, Dungchai W, Chailapakul O, Henry S (2012) Blood separation on microfluidic paper-based analytical devices. Lab Chip 12:3392–3398. doi:10.1039/c2lc21299d
Osborn JL, Lutz B, Fu E et al (2010) Microfluidics without pumps: reinventing the T-sensor and H-filter in paper networks. Lab Chip 10:2659–2665. doi:10.1039/c004821f
Fu E, Kauffman P, Lutz B, Yager P (2010) Chemical signal amplification in two-dimensional paper networks. Sensors Actuators B Chem 149:325–328. doi:10.1016/j.snb.2010.06.024
Lutz BR, Trinh P, Ball C et al (2011) Two-dimensional paper networks: programmable fluidic disconnects for multi-step processes in shaped paper. Lab Chip 11:4274–4278. doi:10.1039/c1lc20758j
Fu E, Lutz B, Kauffman P, Yager P (2010) Controlled reagent transport in disposable 2D paper networks. Lab Chip 10:918–920. doi:10.1039/b919614e
Apilux A, Ukita Y, Chikae M et al (2013) Development of automated paper-based devices for sequential multistep sandwich enzyme-linked immunosorbent assays using inkjet printing. Lab Chip 13:126–135. doi:10.1039/c2lc40690j
Wu L, Ma C, Zheng X et al (2015) Paper-based electrochemiluminescence origami device for protein detection using assembled cascade DNA—carbon dots nanotags based on rolling circle ampli fi cation. Biosens Bioelectron 68:413–420. doi:10.1016/j.bios.2015.01.034
Liu H, Crooks RM (2011) Three-dimensional paper microfluidic devices assembled using the principles of origami. J Am Chem Soc 133:17564–17566
Deraney RN, Rolland P, Mace CR (2014) A device architecture for three-dimensional, patterned paper immunoassays. Lab Chip 14:4653–4658. doi:10.1039/C4LC00876F
Martinez AW, Phillips ST, Whitesides GM (2008) Three-dimensional microfluidic devices fabricated in layered paper and tape. Proc Natl Acad Sci U S A 105:19606–19611
Kirby AE, Wheeler AR (2013) Microfluidic origami: a new device format for in-line reaction monitoring by nanoelectrospray ionization mass spectrometry. Lab Chip 13:2533–2540. doi:10.1039/c3lc41431k
Ge L, Wang S, Song X, Yu J (2012) 3D Origami-based multifunction-integrated immunodevice: low-cost and multiplexed sandwich chemiluminescence immunoassay on microfluidic paperbased analytical device. Lab Chip 12:3150–3158. doi:10.1039/c2lc40325k
Schilling KM, Jauregui D, Martinez AW (2013) Paper and toner three-dimensional fluidic devices: programming fluid flow to improve point-of-care diagnostics. Lab Chip 13:628–631. doi:10.1039/c2lc40984d
Hwang H, Kim S, Kim T et al (2011) Paper on a disc: balancing the capillary-driven flow with a centrifugal force. Lab Chip 11:3404–3406. doi:10.1039/c1lc20445a
Vereshchagina E, Bourke K, Meehan L, et al. (2013) Multi-material paper-disc devices for low cost biomedical diagnostics. In: 26th International Conference on Micro Electro Mechanical Systems. pp 1049–1052
Shiong C, Lawrence K, Ngin S, Zerda C (2014) A “green” cellulose paper based glucose amperometric biosensor. Sensors Actuators B Chem 193:536–541
Godino N, Gorkin R, Bourke K, Ducre J (2012) Fabricating electrodes for amperometric detection in hybrid paper/polymer lab-on-a-chip devices. Lab Chip 12:3281–3284. doi:10.1039/c2lc40223h
Fobel R, Kirby AE, Ng AHC et al (2014) Paper microfluidics goes digital. Adv Mater 26:2838–2843. doi:10.1002/adma.201305168
Niedl RR, Beta C (2015) Hydrogel-driven paper-based microfluidics. Lab Chip 15:2452–2459. doi:10.1039/c5lc00276a
Lutz B, Liang T, Fu E et al (2014) Dissolvable fluidic time delays for programming multi-step assays in instrument-free paper diagnostics. Lab Chip 13:2840–2847. doi:10.1039/c3lc50178g.Dissolvable
Alava M, Niskanen K (2006) The physics of paper. Rep Prog Phys 69:669–723. doi:10.1088/0034-4885/69/3/R03
Alexander B, Carstens F, Trieb C et al (2014) Engineering microfluidic papers: effect of fiber source and paper sheet properties on capillary-driven fluid flow. Microfluid Nanofluid 16:789–799. doi:10.1007/s10404-013-1324-4
Kong F, Hu YF (2012) Biomolecule immobilization techniques for bioactive paper fabrication. Anal Bioanal Chem 403:7–13. doi:10.1007/s00216-012-5821-1
Su S, Nutiu R, Filipe CDM et al (2007) Adsorption and covalent coupling of ATP-Binding DNA aptamers onto cellulose. Langmuir 23:1300–1302
Wu Y, Xue P, Hui KM, Kang Y (2014) A paper-based microfluidic electrochemical immunodevice integrated with ampli fi cation-by-polymerization for the ultrasensitive multiplexed detection of cancer biomarkers. Biosens Bioelectron 52:180–187. doi:10.1016/j.bios.2013.08.039
Hosseini S, Azari P, Farahmand E et al (2015) Polymethacrylate coated electrospun PHB fibers: an exquisite outlook for fabrication of paper-based biosensors. Biosens Bioelectron 69:257–264. doi:10.1016/j.bios.2015.02.034
Hansson J, Yasuga H, Haraldsson T, van der Wijngaart W (2016) Synthetic microfluidic paper: high surface area and high porosity micropillar arrays. Lab Chip 16:298–304. doi:10.1039/c5lc01318f
Mace CR, Deraney RN (2014) Manufacturing prototypes for paper-based diagnostic devices. Microfluid Nanofluid 16:801–809. doi:10.1007/s10404-013-1314-6
Martinez AW, Phillips ST, Butte MJ, Whitesides GM (2007) Patterned paper as a platform for inexpensive, low-volume, portable bioassays. Angew Chem Int Ed 46:1318–1320. doi:10.1002/anie.200603817
Wang J, Monton RN, Zhang X et al (2014) Hydrophobic sol–gel channel patterning strategies for paper-based microfluidics. Lab Chip 14:691–695. doi:10.1039/c3lc51313k
Lu Y, Shi W, Qin J, Lin B (2010) Fabrication and characterization of paper-based microfluidics prepared in nitrocellulose membrane by wax printing. Anal Chem 82:329–335. doi:10.1021/ac9020193
Carrilho E, Martinez AW, Whitesides GM (2009) Understanding wax printing: a simple micropatterning process for paper-based microfluidics. Anal Chem 81:7091–7095. doi:10.1021/ac901071p
Renault C, Koehne J, Ricco AJ, Crooks RM (2014) Three-dimensional wax patterning of paper fluidic devices. Langmuir 30:7030–7036
Jang I, Song S (2015) Facile and precise flow control for a paper-based. Lab Chip 15:3405–3412. doi:10.1039/C5LC00465A
Li X, Liu X (2014) Fabrication of three-dimensional microfluidic channels in a single layer of cellulose paper. Microfluid Nanofluid 16:819–827. doi:10.1007/s10404-014-1340-z
Dungchai W, Chailapakul O, Henry CS (2011) Paper microfluidics using wax screen-printing. Analyst 136:77–82. doi:10.1039/c0an00406e
Metters JP, Houssein SM, Kampouris DK, Banks CE (2013) Paper-based electroanalytical sensing platforms. Anal Methods 5:103–110. doi:10.1039/c2ay26396c
Yafia M, Shukla S, Najjaran H (2015) Fabrication of digital microfluidic devices on flexible paper-based and rigid substrates via screen printing. J Micromech Microeng 25:57001. doi:10.1088/0960-1317/25/5/057001 (11 pp)
Mohammadi S, Maeki M, Mohamadi RM et al (2015) An instrument-free, screen-printed paper microfluidic device that enables bio and chemical sensing. Analyst 140:6493–6499. doi:10.1039/c5an00909j
Sameenoi Y, Nongkai N, Nouanthavong S, Charles S (2014) One-step polymer screen-printing for microfluidic paper-based analytical device (μPAD) fabrication. Analyst 139:6580–6588. doi:10.1039/C4AN01624F
Tao H, Chieffo LR, Brenckle MA et al (2011) Metamaterials on paper as a sensing platform. Adv Mater 23:3197–3201. doi:10.1002/adma.201100163
Abe K, Suzuki K, Citterio D (2008) Inkjet-printed microfluidic multianalyte chemical sensing paper. Anal Chem 80:6928–6934
Määttänen A, Vanamo U, Ihalainen P et al (2013) A low-cost paper-based inkjet-printed platform for electrochemical analyses. Sensors Actuators B Chem 177:153–162
Ko H, Lee J, Kim Y et al (2014) Active digital microfluidic paper chips with inkjet-printed patterned electrodes. Adv Mater 26:2335–2340. doi:10.1002/adma.201305014
Le HP (1998) Ink-jet printing technology. J Imaging Sci Technol 42:1998
Yamada K, Henares TG, Suzuki K, Citterio D (2015) Paper-based inkjet-printed microfluidic analytical devices. Angew Chem Int Ed 54:5294–5310. doi:10.1002/anie.201411508
Määttänen A, Fors D, Wang S et al (2011) Paper-based planar reaction arrays for printed diagnostics. Sensors Actuators B Chem 160:1404–1412. doi:10.1016/j.snb.2011.09.086
Martinez AW, Phillips ST, Wiley BJ et al (2008) FLASH: a rapid method for prototyping paper-based microfluidic devices †‡. Lab Chip 8:2146–2150. doi:10.1039/b811135a
Yu SL, Shi ZZ (2015) Microfluidic paper-based analytical devices fabricated by low-cost photolithography and embossing of Parafilm®. Lab Chip 15:1642–1645. doi:10.1039/C5LC00044K
Li X, Tian J, Nguyen T, Shen W (2008) Paper-based microfluidic devices by plasma treatment. Anal Chem 80:9131–9134. doi:10.1021/ac801729t
Salentijn GIJ, Hamidon NN, Verpoorte E (2016) Solvent-dependent on/off valving using selectively permeable barriers in paper microfluidics. Lab Chip 16:1013–1021. doi:10.1039/C5LC01355K
Siegel BAC, Phillips ST, Dickey MD et al (2010) Foldable printed circuit boards on paper substrates. Adv Funct Mater 20:28–35. doi:10.1002/adfm.200901363
Cassano CL, Fan ZH (2013) Laminated paper-based analytical devices (LPAD): fabrication, characterization, and assays. Microfluid Nanofluid 15:173–181. doi:10.1007/s10404-013-1140-x
Lewis GG, Robbins JS, Phillips ST (2013) Point-of-care assay platform for quantifying active enzymes to Femtomolar levels using measurements of time as the readout. Anal Chem 85:10432–10439
Pozuelo M, Blondeau P, Novell M et al (2013) Paper-based chemiresistor for detection of ultralow concentrations of protein. Biosens Bioelectron 49:462–465. doi:10.1016/j.bios.2013.06.007
Fong K, Lee K, Yang S (2012) Fabrication of carbon nanotube-based pH sensor for paper-based microfluidics. Microelectron Eng 100:1–5. doi:10.1016/j.mee.2012.07.113
Abadian A, Jafarabadi-Ashtiani S (2014) Paper-based digital microfluidics. Microfluid Nanofluid 16:989–995. doi:10.1007/s10404-014-1345-7
Tobjörk D, Österbacka R (2011) Paper electronics. Adv Mater 23:1935–1961. doi:10.1002/adma.201004692
Peters KL, Corbin I, Kaufman LM et al (2014) Simultaneous colorimetric detection of improvised explosive compounds using micro fl uidic paper- based analytical devices (m PADs). Anal Methods 7:63–70. doi:10.1039/C4AY01677G
Rattanarat P, Dungchai W, Cate D et al (2014) Multilayer paper-based device for colorimetric and electrochemical quantification of metals. Anal Chem 86:3555–3562. doi:10.1021/ac5000224
Nie Z, Nijhuis CA, Gong J et al (2010) Electrochemical sensing in paper-based microfluidic devices †. Lab Chip 10:477–483. doi:10.1039/b917150a
Cunningham JC, Kogan MR, Tsai Y-J et al (2016) Paper-based sensor for electrochemical detection of silver nanoparticle labels by galvanic exchange. ACS Sensors 1:40–47. doi:10.1021/acssensors.5b00051
Szucs J, Gyurcsanyi RI (2012) Towards protein assays on paper platforms with potentiometric detection. Electroanalysis 24:146–152. doi:10.1002/elan.201100522
Leung V, Shehata AM, Filipe CDM, Pelton R (2010) Streaming potential sensing in paper-based microfluidic channels. Colloids Surfaces A Physicochem Eng Asp 364:16–18. doi:10.1016/j.colsurfa.2010.04.008
Wang S, Ge L, Song X et al (2012) Paper-based chemiluminescence ELISA: Lab-on-paper based on chitosan modified paper device and wax-screen-printing. Biosens Bioelectron 31:212–218. doi:10.1016/j.bios.2011.10.019
Yu J, Ge L, Huang J et al (2011) Microfluidic paper-based chemiluminescence biosensor for simultaneous determination of glucose and uric acid. Lab Chip 11:1286–1291. doi:10.1039/c0lc00524j
Liu R, Zhang C, Liu M (2015) Open bipolar electrode-electrochemiluminescence imaging sensing using paper-based microfluidics. Sensors Actuators B Chem 216:255–262
He M, Liu Z (2013) Paper-based microfluidic device with upconversion fluorescence assay. Anal Chem 85:11691–11694. doi:10.1021/ac403693g
Xu S, Dong B, Zhou D et al (2016) Paper-based upconversion fluorescence resonance energy transfer biosensor for sensitive detection of multiple cancer biomarkers. Sci Rep 2:1–9. doi:10.1038/srep23406
Kumar A, Hens A, Arun K, Chatterjee M (2015) A paper based microfluidic device for easy detection of uric acid using positively charged gold nanoparticles. Analyst 140:1817–1821. doi:10.1039/C4AN02333A
Davaji B, Hoon C (2014) A paper-based calorimetric micro fl uidics platform for bio-chemical sensing. Biosens Bioelectron 59:120–126. doi:10.1016/j.bios.2014.03.022
Cate DM, Dungchai W, Cunningham JC, Henry CS (2013) Simple, distance-based measurement for paper analytical devices. Lab Chip 13:2397–2404. doi:10.1039/c3lc50072a
Choi G, Choi S (2015) Bacterial cell transportation in paper-based microfluidics. In: Transducers. Anchorage, 21–25 June 2015, pp 3–6
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Vereshchagina, E. (2016). Paper Microfluidics. In: Dixit, C., Kaushik, A. (eds) Microfluidics for Biologists. Springer, Cham. https://doi.org/10.1007/978-3-319-40036-5_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-40036-5_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-40035-8
Online ISBN: 978-3-319-40036-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)