Abstract
Background
Cerebral radiation necrosis (RN) is a severe complication of radiotherapy for cerebral pathologies. This study discusses the radiographic and pathological features of 12 patients with RN and investigates the management strategy.
Methods
Eleven patients with brain tumors, and one with cerebral cavernous angioma, treated by surgical resection or Gamma Knife alone before radiotherapy developed RN during follow-up. Surgical resection for the cerebral RN was performed in nine patients, and the other three patients received medical treatment. The clinical features, magnetic resonance imaging (MRI), surgical findings, and pathological sections are reviewed.
Results
The diagnosis of RN was confirmed by histological study in all the patients; those with surgical and medical treatment recovered.
Conclusion
As a major complication of radiotherapy, from the clinical and neuroradiological points of view, RN may simulate tumor recurrence. Due to the increasing number of patients with RN who will need to be treated in future years, the definite diagnosis and appropriate treatment of RN remain critical.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
Introduction
Radiotherapy is an important modality option for treating brain tumors, arteriovenous malformations, and head and neck cancers. Clinically, there are three forms of radiation injuries: acute, early delayed, and late delayed reactions [10, 14]. The main manifestation of a late delayed reaction is radiation necrosis (RN), which was first described in 1930 [8]. Since then, numerous reports have documented RN as a major complication of radiotherapy to the brain. The exact incidence of RN after radiotherapy for brain tumors and arteriovenous malformations remains undetermined. Risk factors associated with the development of RN include total radiation dose, fraction size, treatment time, and radiation field and volume. Although late cerebral RN has been seen at a radiotherapy dose of less than 50 Gy, generally, it more often develops with a higher irradiation dose, larger fraction size, and when radiation is combined with chemotherapy [35].
The diagnosis of RN has been challenging since the necrosis and tumor recurrence share similar manifestations, both in clinical presentation and in imaging appearance. Therefore, biopsy and pathological study have been recommended as the diagnostic gold standard. Making a definite diagnosis, which is directly related to the choice of treatment, is of great importance.
Twelve patients, previously treated for brain tumor and arteriovenous malformation, with a neuropathological diagnosis of cerebral RN, were included in this study. The aim of this study was to compare the radiographic and pathological features of RN and report our experience in the management of late cerebral RN by focusing on the therapeutic options of medical and surgical therapy.
Material and Methods
This study was approved by the XinHua Hospital Medical Ethics Committee.
Twelve patients, 7 males and 5 females, ranging in age from 41 to 73 years (mean age 55.8 years) were retrospectively reviewed. Between January 2005 and December 2011, 11 of the 12 patients were surgically treated in our Department. Pathological examinations were performed by two certified neuropathologists and the diagnosis was glioblastoma multiforme (GBM) in 3 cases (27 %) and glioma in 8 (73 %).
One patient, who had a cavernous angioma, found by MRI, preferred stereotactic Gamma Knife radiosurgery to surgical resection. The lesion sites were as follows: 3 temporal; 2 frontal; 3 parietal; 2 frontotemporal; 1 occipital, and 1 parieto-occipital All the patients underwent external-beam fractionated radiation therapy; 1.8–2.0 Gy per day was administered with a 6-MV linear accelerator for 5 consecutive days, for a total dose of 45–60 Gy. Seven patients also received temozolomide as chemotherapy (Table 1).
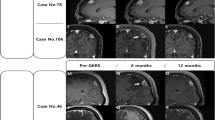
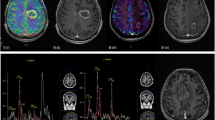
Magnetic resonance imaging (MRI) was performed at 3- to 6-month intervals after completion of the radiation therapy. When clinical deterioration occurred, MRI was performed. All the patients, including the one with cavernous angioma, developed a newly formed lesion mimicking tumor recurrence (Fig. 1). The mean time between the administration of radiation therapy and the appearance of the newly formed lesion was 18 months (range 8–33 months). Nine patients underwent surgical resection of the lesion to alleviate the severe symptoms, while three patients received only medical treatment after stereotactic biopsy, as they had relatively mild symptoms. Pathological study of the specimens showed RN (Fig. 2). MRI or computed tomography (CT) scans of the brain were then performed at 3- to6 -month intervals during the follow-up. The mean duration of follow-up was 16 months (range 4–36 months) (Table 1).
(a, b) Axial magnetic resonance (MR) images of patient with primary glioma. The solid portion of radionecrosis and the perilesional edema in the left temporal lobe have low signal intensity on axial T1-MRI and high signal intensity on axial T2-MRI (c, d) axial MR images of patient with primary cavernous angioma. The solid portion of radionecrosis and the perilesional edema in the frontal lobe show iso-to-hypointense signal intensity on T1 Fluid attenuated inversion recovery (FLAIR) image; the lesion was irregularly enhancement. Hyperintense signal with patches of a hypointense signal area are shown on T2-MR image
Results
In our series the median preoperative Karnofsky performance score (KPS) was 80. Apparent total surgical removal of the lesion was performed in nine patients, all of whom had a significant reduction in intracranial pressure within a few days postoperatively. No major complications occurred.
Two patients presented with a severe motor deficit of the left arm and two had postoperative seizures. Transient dysphasia was observed in two more patients. After surgery, brain edema progressively resolved in all the patients within 3 weeks, allowing a reduction or suspension of corticosteroid therapy by that time.
Three patients developed mild neurological symptoms a few weeks postoperatively. MRI showed a worsening of cerebral edema, which recovered after corticosteroid administration.
Discussion
The treatment of brain tumors remains challenging, although neurosurgery, radiotherapy, and chemotherapy are the current options, and they can be integrated. However, prolongation of survival can be accompanied by the appearance of new features, such as RN, which has increased in incidence since radiotherapy started to be considered an outstanding treatment opportunity for brain tumors, arteriovenous malformations, and some head and neck cancers [7].
The primary goal of brain radiotherapy is to deliver a therapeutic dose of radiation, sparing the surrounding normal brain tissue; in fact, irradiation occasionally affects the normal tissue, damaging normal brain tissue near the tumor site [13, 36]. The tolerance of normal tissue has been a limiting factor in the radiation therapy of cerebral pathologies. Patients vary in their individual responses to radiotherapy: some may develop severe adverse reactions, while others receiving comparable radiation doses for similar pathologies in similar locations do not. The exact reason for this variability in response remains unclear, although several researchers have tried to address the issues of intrinsic tissue sensitivity over the past two decades [1, 9, 20, 28, 33], and a median dose of 20 Gy in a single fraction has been advocated to obtain an optimal balance between therapeutic efficacy and the risk of complications [17, 23].
Currently, the mechanisms of RN are still an open question. Theories of vascular injury; glial injury; autoimmune reactions; and oxyradical damage of cell membrane lipids have been advanced so far (15, 21). These mechanisms may generally coexist. The main target of RN is neuroglial cells, especially oligodendroglial and endothelial cells, rather than neurons [31]. Endothelial cell damage caused by abnormal microvascular circulation, “nutritional” insufficiency, and disruption of the blood-brain barrier, promoted by the activated immunological system, contribute to the development of gliosis, vascular injury, and progressive necrosis of the surrounding brain parenchyma [4, 6, 19, 22, 26, 29, 34].
As previously stated, efforts have long been made by a great many investigators to apply radiographic imaging studies to the differential diagnosis of recurrent tumor and RN [5, 24, 32], which can be a radiologic dilemma, since they share the following features at CT or MRI: the original tumor site, mass effect, and contrast enhancing with surrounding edema, and may increase in size over time. Other techniques, such as spectroscopy and perfusion MR, perfusion CT, positron emission tomography (PET) and single-photon emission CT (SPECT) have also been widely used. Nevertheless, so far no evidence has been provided that any of these investigations is apparently superior to any other modalities in terms of diagnostic sensitivity or specificity [2, 5, 6, 21, 25]. With no best option recommended, the decision to use one or more imaging techniques always depends on a series of factors, such as the availability of various imaging modalities at an institution, the location and size of the tumor, the neurological findings, and the cost.
A differential diagnosis is very important to illuminate the appropriate management: recurrent tumor might be treated with surgical resection, radiation, or chemotherapy, while RN may benefit from corticosteroids, other medical therapies, or surgery [5, 30]. The definitive diagnosis of RN requires pathological studies, despite the existence of sampling error from a stereotactic biopsy, due to the frequent mixed area of tumoral cells and necrosis. However, in patients with previously irradiated tumor or other pathologies in whom RN or tumor recurrence was clinically or radiographically suspected, results from stereotactic biopsy or surgical biopsy could be used to differentiate tumor recurrence, RN, a mixture of both lesions, and radiation-induced tumor [10].
Spontaneous resolution of cerebral RN may happen, but in most patients symptoms would develop and can be progressive, calling for treatment to provide symptomatic relief [11]. It is reported that resolution or improvement may be obtained following medical treatment with high-dose corticosteroids [16]. However, Gutin and colleagues reported that the effect of steroids for acute episodes in patients with lateral cerebral RN proved to be doubtful. The steroid level at radiation is reported to have an adverse effect on the outcome, owing to severe systemic complications, and, as a consequence, increased susceptibility to RN [12, 18]. According to our personal experience, although no definitive conclusion can currently be drawn, we suggest that the management of patients with RN is predominantly surgical in those with elevated intracranial pressure, or if symptoms require prompt control or they progress with conservative treatment. Moreover, surgical intervention may also provide a biopsy specimen and confirm the diagnosis. This statement seems to be in accord with the literature [15, 16, 22, 27, 34].
Regarding the patient with cavernous angioma in our series, we strongly supported surgery to remove both the RN and the remaining lesion, since cavernous angioma can re-bleed and support epileptic seizures; in our opinion such a surgery it is not an especially demanding operation, particularly when the nidus is located on the convexity. Similarly to treatment in those patients with long-term recurrent epilepsy unresponsive to antiepileptic drugs, surgery should be resolutely carried out in order to remove the nidus, and to prevent massive hemorrhage and expansion of the epileptic focus. Moreover, surgery should also be considered in the following conditions: (1) acute or progressive functional nervous damage; (2) a single nidus in a non-eloquent area or in the eloquent area in cases of hemorrhage; and (3) a serious focal symptom arising out of multiple encephalic pathological changes [3].
In our study, surgical treatment appeared to be a favorable strategy in patients with good KPS and accessible location of the necrotic mass. We believe that the number of patients requiring treatment of RN will rise in the coming years with the increasing population receiving radiotherapy. Accordingly, prospective randomized, multicenter studies and even complete guidelines are necessary for the management of RN.
Conclusion
As a major complication of radiotherapy, RN may simulate tumor recurrence in both its clinical features and on MRI. The definite diagnosis and appropriate treatment of RN are critical, and will be even more important, given that there will be increasing numbers of patients with RN to be treated in the coming years.
References
Alter BP (2002) Radiosensitivity in Fanconi’s anemia patients. Radiother Oncol : J Eur Soc Therape Radiol Oncol 62:345–347
Barajas RF, Chang JS, Sneed PK, Segal MR, McDermott MW, Cha S (2009) Distinguishing recurrent intra-axial metastatic tumor from radiation necrosis following gamma knife radiosurgery using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. AJNR Am J Neuroradiol 30:367–372
Bertalanffy H, Benes L, Miyazawa T, Alberti O, Siegel AM, Sure U (2002) Cerebral cavernomas in the adult. Review of the literature and analysis of 72 surgically treated patients. Neurosurg Rev 25:1–53; discussion 54–55
Coderre JA, Morris GM, Micca PL, Hopewell JW, Verhagen I, Kleiboer BJ, van der Kogel AJ (2006) Late effects of radiation on the central nervous system: role of vascular endothelial damage and glial stem cell survival. Radiat Res 166:495–503
Dequesada IM, Quisling RG, Yachnis A, Friedman WA (2008) Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery 63:898–903; discussion 904
Dropcho EJ (2010) Neurotoxicity of radiation therapy. Neurol Clin 28:217–234
Eisele SC, Dietrich J (2015) Cerebral radiation necrosis: diagnostic challenge and clinical management. Rev Neurol 61:225–232
Eyster EF, Nielsen SL, Sheline GE, Wilson CB (1974) Cerebral radiation necrosis simulating a brain tumor. Case report. J Neurosurg 40:267–271
Gatti RA (2001) The inherited basis of human radiosensitivity. Acta Oncol 40:702–711
Giglio P, Gilbert MR (2003) Cerebral radiation necrosis. Neurologist 9:180–188
Glass JP, Hwang TL, Leavens ME, Libshitz HI (1984) Cerebral radiation necrosis following treatment of extracranial malignancies. Cancer 54:1966–1972
Gutin PH, McDermott MW, Ross G, Chan PH, Chen SF, Levin KJ, Babuna O, Marton LJ (1990) Polyamine accumulation and vasogenic oedema in the genesis of late delayed radiation injury of the central nervous system (CNS). Acta Neurochir Suppl 51:372–374
Hopewell JW, Millar WT, Ang KK (2007) Toward improving the therapeutic ratio in stereotactic radiosurgery: selective modulation of the radiation responses of both normal tissues and tumor. J Neurosurg 107:84–93
Iwai Y, Yamanaka K, Oda J, Tsuyuguchi N, Ochi H (2001) Tracer accumulation in radiation necrosis of the brain after thallium-201 SPECT and [11C]methionine PET – case report. Neurol Med Chir 41:415–418
Kano H, Kondziolka D, Zorro O, Lobato-Polo J, Flickinger JC, Lunsford LD (2009) The results of resection after stereotactic radiosurgery for brain metastases. J Neurosurg 111:825–831
Lorenzo ND, Nolletti A, Palma L (1978) Late cerebral radionecrosis. Surg Neurol 10:281–290
Maldaun MV, Aguiar PH, Lang F, Suki D, Wildrick D, Sawaya R (2008) Radiosurgery in the treatment of brain metastases: critical review regarding complications. Neurosurg Rev 31:1–8; discussion 8–9
Morris JG, Grattan-Smith P, Panegyres PK, O’Neill P, Soo YS, Langlands AO (1994) Delayed cerebral radiation necrosis. Q J Med 87:119–129
Pena LA, Fuks Z, Kolesnick RN (2000) Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res 60:321–327
Roberts SA, Spreadborough AR, Bulman B, Barber JB, Evans DG, Scott D (1999) Heritability of cellular radiosensitivity: a marker of low-penetrance predisposition genes in breast cancer? Am J Hum Genet 65:784–794
Ross DA, Sandler HM, Balter JM, Hayman JA, Archer PG, Auer DL (2002) Imaging changes after stereotactic radiosurgery of primary and secondary malignant brain tumors. J Neurooncol 56:175–181
Ruben JD, Dally M, Bailey M, Smith R, McLean CA, Fedele P (2006) Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys 65:499–508
Shehata MK, Young B, Reid B, Patchell RA, St Clair W, Sims J, Sanders M, Meigooni A, Mohiuddin M, Regine WF (2004) Stereotatic radiosurgery of 468 brain metastases < or =2 cm: implications for SRS dose and whole brain radiation therapy. Int J Radiat Oncol Biol Phys 59:87–93
Soffietti R, Sciolla R, Giordana MT, Vasario E, Schiffer D (1985) Delayed adverse effects after irradiation of gliomas: clinicopathological analysis. J Neurooncol 3:187–192
Sundgren PC (2009) MR spectroscopy in radiation injury. AJNR Am J Neuroradiol 30:1469–1476
Szeifert GT, Massager N, DeVriendt D, David P, De Smedt F, Rorive S, Salmon I, Brotchi J, Levivier M (2002) Observations of intracranial neoplasms treated with gamma knife radiosurgery. J Neurosurg 97:623–626
Takeuchi J, Hanakita J, Abe M, Handa H (1976) Brain necrosis after repeated radiotherapy. Surg Neurol 5:89–93
Taylor AM, Harnden DG, Arlett CF, Harcourt SA, Lehmann AR, Stevens S, Bridges BA (1975) Ataxia telangiectasia: a human mutation with abnormal radiation sensitivity. Nature 258:427–429
Torcuator R, Zuniga R, Mohan YS, Rock J, Doyle T, Anderson J, Gutierrez J, Ryu S, Jain R, Rosenblum M, Mikkelsen T (2009) Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol 94:63–68
Truong MT, St Clair EG, Donahue BR, Rush SC, Miller DC, Formenti SC, Knopp EA, Han K, Golfinos JG (2006) Results of surgical resection for progression of brain metastases previously treated by gamma knife radiosurgery. Neurosurgery 59:86–97; discussion 86–97
Tsui EY, Chan JH, Leung TW, Yuen MK, Cheung YK, Luk SH, Tung SY (2000) Radionecrosis of the temporal lobe: dynamic susceptibility contrast MRI. Neuroradiology 42:149–152
van Dellen JR, Danziger A (1978) Failure of computerized tomography to differentiate between radiation necrosis and cerebral tumour. S Afr Med J 53:171–172
West CM, Elliott RM, Burnet NG (2007) The genomics revolution and radiotherapy. Clin Oncol 19:470–480
Wong ST, Loo KT, Yam KY, Hung WM, Fok KF, Yuen SC, Fong D (2010) Results of excision of cerebral radionecrosis: experience in patients treated with radiation therapy for nasopharyngeal carcinoma. J Neurosurg 113:293–300
Yoshii Y (2008) Pathological review of late cerebral radionecrosis. Brain Tumor Pathol 25:51–58
Yu JB, Schulder M, Knisely J (2012) Radiosurgical dose selection for brain metastasis. Prog Neurol Surg 25:139–147
Conflict of Interest Statement
The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this paper
Cite this paper
Liao, C., Visocchi, M., Zhang, W., Yang, M., Zhong, W., Liu, P. (2017). Management of Cerebral Radiation Necrosis: A Retrospective Study of 12 Patients. In: Visocchi, M., Mehdorn, H.M., Katayama, Y., von Wild, K.R.H. (eds) Trends in Reconstructive Neurosurgery. Acta Neurochirurgica Supplement, vol 124. Springer, Cham. https://doi.org/10.1007/978-3-319-39546-3_30
Download citation
DOI: https://doi.org/10.1007/978-3-319-39546-3_30
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-39545-6
Online ISBN: 978-3-319-39546-3
eBook Packages: MedicineMedicine (R0)