Abstract
Plastids are ubiquitously present in plants and are the organelles for carotenoid biosynthesis and storage. Based on their morphology and function, plastids are classified into various types, i.e. proplastids, etioplasts, chloroplasts, amyloplasts, and chromoplasts. All plastids, except proplastids, can synthesize carotenoids. However, plastid types have a profound effect on carotenoid accumulation and stability. In this chapter, we discuss carotenoid biosynthesis and regulation in various plastids with a focus on carotenoids in chromoplasts. Plastid transition related to carotenoid biosynthesis and the different capacity of various plastids to sequester carotenoids and the associated effect on carotenoid stability are described in light of carotenoid accumulation in plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Plastids are ubiquitously found in higher plants. They are essential organelles that are believed to have evolved from the endosymbiosis of an ancestral cyanobacterial progenitor. During evolution, most of the ancestor genes are transferred to the nucleus and only a small number was left in the plastid genome. Although plastids of a plant possess the same plastid genome, they vary greatly in their morphology and function within the plant following the import of many nucleus-encoded proteins. Based on their morphology and function, plastids are classified into a number of subtypes, i.e. proplastids, etioplasts , chloroplasts , leucoplasts, and chromoplasts (Lopez-Juez and Pyke 2005).
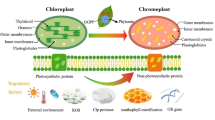
Major plastid types and their interconversion in plant. Proplastid found in meristematic tissues can differentiate into all kinds of other plastids. Proplastid is small, undifferentiated plastid and does not have the capacity to synthesize carotenoids. Etioplast occurs during dark growth of plants and is regarded as a transient stage in chloroplast formation (Fig. 10.1). Etioplast is characterized with membranous pro-lamellar and synthesizes low level of carotenoids. Amyloplast is filled with starch in storage tissues and of great agricultural importance. Amyloplast can be converted into chloroplast or chromoplast, and synthesizes low to mediate level of carotenoids. Chloroplast is the most prominent plastid in green tissue and has distinctive thylakoid discs. Chloroplast can interconvert into amyloplast or chromoplast during ripening and synthesizes high level of carotenoids coordinately with chlorophyll synthesis. Chromoplast is loaded with carotenoid pigments. Chromoplast is classified into globular, crystalline, membranous and tubular types based on the carotenoid-lipoprotein sequestering structures. Chromoplast possesses unique mechanisms to synthesize and accumulate massive amounts of carotenoids, giving the vivid orange, red and yellow color in many flowers, fruits and vegetables (Fig. 10.1)
Various plastids are enriched in different tissues with unique properties. Proplastids are small, colorless, and undifferentiated plastids abundant in cells of meristematic tissues of apical shoot, root tips, and developing young fruits as well as of reproductive tissues. All plastids are derived from proplastids. Despite of their importance, not much is known about proplastid biology due to the difficulty in isolating them. Electron microscopy analyses provide an estimate of 10–20 proplastids per meristematic cell (Lopez-Juez and Pyke 2005). Etioplasts occur during dark growth of plants and are regarded as an intermediary stage in the chloroplast formation from proplastids. Etioplasts are characterized by the presence of a unique inner membrane network of the prolamellar body and by the absence of chlorophylls. While the etioplast stage is frequently transient under natural conditions, etioplasts are believed to be the plastids in some special tissues such as cabbage heads (Solymosi and Schoefs 2010). Chloroplasts are the plastids that define plants. Chloroplasts contain the green pigment chlorophylls and have distinctive internal thylakoid discs. They are found in green leaves and other green tissues. Chloroplasts are the best characterized plastids due to their importance to the overall plant metabolism and plant survival. Leucoplasts are the general term for a group of differentiated and colorless plastids, which include amyloplasts , elaioplasts, and proteoplasts. Amyloplasts are of great economic and agricultural importance as they accumulated starch, which provides the major human energy. Amyloplasts are enriched in many storage tissues, such as in the endosperm of many seeds particularly of cereal seeds, in tubers, in root vegetables, and in some fruits. Amyloplasts generally arise from proplastids but also can be derived from other plastid types (Lopez-Juez and Pyke 2005). Elaioplasts and proteoplasts store lipids and proteins, respectively. Elaioplasts are filled with numerous globules or plastoglobules and enriched in oil seeds. Proteoplasts harbor crystalline, fibrillary, or amorphous masses of proteins. Both elaioplasts and proteoplasts are much less studied plastids in plants. Chromoplasts are characterized with high level accumulation of carotenoid pigments. The vivid red, orange and yellow colors in many flowers, fruits, and vegetables own their hue to the presence of carotenoids in chromoplasts. Chromoplasts are often derived from chloroplasts during green fruit ripening and also arise directly from proplastids or other non-colored plastids during fruit development and root growth (Li and Yuan 2013; Yuan et al. 2015).
Plastids fulfil various important roles in plant metabolism. Apart from their well-known functions, such as photosynthesis in chloroplasts, carotenoid metabolism in chromoplasts, and starch biosynthesis in amyloplasts , plastids are also the major sites for synthesis of many primary and secondary metabolites, and assimilation of nitrogen and sulfur (Kuntz and Rolland 2012; Lopez-Juez 2007; Neuhaus and Emes 2000). Proplastids as undifferentiated plastids in meristematic tissues are able to synthesize amino acids for protein synthesis, fatty acids for membrane lipid proliferation, and nucleotide precursors for DNA and RNA synthesis (Brautigam and Weber 2009). Although etioplasts are characterized by the absence of chlorophylls, etioplasts synthesize chlorophyll precursor (pchlide), carotenoids, and metabolites to be ready for the establishment of photosynthesis once illumination with light (Solymosi and Schoefs 2010). Chloroplasts serve not only the site of fixation of CO2 for photosynthesis, but are also responsible for the synthesis of amino acids, fatty acids, and many secondary metabolites, as well as for the storage of starch and oil compounds (Jensen and Leister 2014; Linka and Weber 2012). Similarly, chromoplasts are found to contain the enzymes involved in the synthesis and metabolism of many plastid metabolites such as amino acids and fatty acids in add to carotenoid biosynthesis (Egea et al. 2010; Li and Yuan 2013). The same is true for amyloplasts although they are primarily responsible for the synthesis and storage of starch (Dupont 2008). While the photosynthetic chloroplasts are autotrophic, the other non-photosynthetic plastids are heterotrophic. All plastids are surrounded by double envelope membranes and retain a semi-autonomous character. In addition, plastid types are inter-convertible in response to developmental or environmental cues.
Plastids are the site for carotenoid biosynthesis and storage. Carotenoids as a group of widely distributed pigments are de novo synthesized in all types of plastids except proplastids (Howitt and Pogson 2006; Shumskaya and Wurtzel 2013). As carotenoid biosynthetic enzymes are either membrane associated or membrane proteins, carotenoid biosynthesis is believed to be carried out in plastid membranes (Cazzonelli and Pogson 2010; Li and Yuan 2013; Nisar et al. 2015; Ruiz-Sola and Rodriguez-Concepcion 2012; Yuan et al. 2015). Proteomics research reveals that plastoglobules isolated from chromoplasts also contain carotenoid biosynthesis enzymes (Ytterberg et al. 2006). These studies suggest ability of plastoglobules for carotenoid biosynthesis in addition to their functions in carotenoid sequestration and storage.
Different types of plastids possess different capacity to synthesize and sequester carotenoids. Thus, the type of plastids exerts profound effect on carotenoid accumulation and stability (Li and Yuan 2013; Ruiz-Sola and Rodriguez-Concepcion 2012). Due to the negative regulation of phytoene synthase (PSY) gene expression by phytochrome-interacting factors abundant in the etiolated tissues (Toledo-Ortiz et al. 2010), etioplasts in the etiolated tissues exhibit low biosynthetic activity and contain very low levels of carotenoids. The majority of the carotenoids synthesized are lutein and violaxanthin (Rodriguez-Villalon et al. 2009; Welsch et al. 2000), which give yellowish color of etioplasts and the etiolated tissues. Many storage tissues of important crops, such as wheat, maize, potato, and cassava synthesize and accumulate low to mediate levels of carotenoids in amyloplasts of seeds and roots (Howitt and Pogson 2006). In many cases, xanthophylls (i.e. lutein, zeaxanthin, and violaxanthin) are the major accumulated carotenoids to yield yellow color. Carotenoids are found in the amyloplast envelope membranes (Lopez et al. 2008). The lack of appropriate lipoprotein sequestering substructures in the amyloplasts may restrict their capacity to significantly accumulate and stably store carotenoids (Li et al. 2012). Carotenoids are essential components of photosynthetic complexes in chloroplasts of leaves and other green tissues. Carotenoid biosynthesis is believed to occur in the envelope and the thylakoid membranes in chloroplasts (Cazzonelli and Pogson 2010; Ruiz-Sola and Rodriguez-Concepcion 2012). The formation of chlorophyll and carotenoid lipoprotein complex in thylakoid membranes enables to sequester and store the synthesized carotenoids for high level of accumulation in chloroplasts. Chromoplasts as carotenoid accumulating plastids develop unique mechanisms to synthesize and accumulate massive amounts of carotenoids. The carotenoid lipoprotein sequestering substructures and/or plastoglobules within chromoplasts promotes continuous carotenoid biosynthesis as well as sequestration and stable storage of synthesized carotenoids in chromoplasts (Li and Yuan 2013; Yuan et al. 2015). These unique features of chromoplasts enable massive accumulation of carotenoids found in many flowers, fruits and vegetables.
This chapter will describe carotenoid biosynthesis and storage in various plastids with a focus on carotenoids in chromoplasts. The plastid transition and carotenoid stability in different plastids will be discussed in light of carotenoid accumulation for crop nutritional quality improvement.
2 Carotenoids in Etioplasts
Etioplasts are considered as an intermediary stage in chloroplast formation under natural condition although most of the studies are carried out in dark-grown seedlings (Solymosi and Schoefs 2010). Etioplasts in the etiolated tissues de novo synthesize a set of carotenoids similar to that of green tissues with lutein and violaxanthin as the predominant carotenoids (Park et al. 2002; Welsch et al. 2000). These carotenoids accumulated in etioplasts are believed to be critical for the dark grown seedlings to optimize transition to photosynthetic development upon illumination and contribute to the adaptation of soil-emerging seedlings to sunlight (Rodriguez-Villalon et al. 2009). This is supported by the observation of Arabidopsis ccr2 mutations, which cause delayed greening during photomorphogenesis (Park et al. 2002). The dark-grown ccr2 mutants lack lutein and violaxanthin and do not produce the prolamellar bodies that define etioplasts (Cuttriss et al. 2007; Park et al. 2002). The findings also establish the specific role of these xanthophylls in prolamellar body assembly in etioplasts.
Carotenoid biosynthetic genes and enzymes are expressed in etioplasts in dark grown plants. However, they are regulated differently in etiolated tissues to control carotenoid biosynthesis. Phytoene synthase (PSY) is the key enzyme in the carotenoid biosynthetic pathway and the major determent of carotenoid level in plants (Cazzonelli and Pogson 2010; Nisar et al. 2015; Ruiz-Sola and Rodriguez-Concepcion 2012; Yuan et al. 2015). In etiolated tissues, PSY transcript level is low. Examination of its expression in responses to different light shows that PSY expression is mediated by phytochrome (von Lintig et al. 1997). More recent work reveals that it is phytochrome-interacting factors that negatively suppress PSY gene expression in dark-grown seedlings (Toledo-Ortiz et al. 2010). The phytochrome-interacting factors directly binds to the promoter of the PSY gene, resulting in suppressing PSY expression. In addition to transcriptional regulation under phytochome control, the topological localization of PSY protein also regulates its capacity in controlling carotenoid biosynthesis at enzymatic activity level. In etiolated seedlings, most of PSY protein are located within the prolamellar bodies and exhibit low enzymatic activity because of lacking competent membranes (Welsch et al. 2000). In contrast to PSY, the expression of phytoene desaturase (PDS), the next enzyme in the pathway, is not regulated by phytochrome and the PDS enzyme activity does not show topological redistribution at protein level. The transcript level of PDS along with geranylgeranyl pyrophosphate synthase (GGPS) is consistent in response to various light treatment (von Lintig et al. 1997). PDS protein abundance remains relatively constant in the membrane even following expose to light (Welsch et al. 2000). As PSY is the first and rate-limiting enzyme in the carotenoid biosynthetic pathway, the low transcript level plus its topological localization within the prolamellar bodies are responsible for the low level of carotenoid accumulation in etioplasts.
Etioplasts are differentiated into chloroplasts during de-etiolation when dark-grown seedlings are exposed to light. During the transition, a strong increase in carotenoid content along with chlorophyll content leads to the formation of functional photosynthetic apparatus. The increased carotenoid accumulation is associated with up-regulation of PSY gene expression, protein level, and enzyme activity (Botella-Pavia et al. 2004; von Lintig et al. 1997; Welsch et al. 2000). The light induced PSY expression is uncovered to be due to a rapid de-repression of PSY gene expression following light-triggered degradation of the negative regulators phytochrome-interacting factors (Toledo-Ortiz et al. 2010). In addition, PSY activity is localized to the thylakoids membrane of chloroplasts following the establishment of photosynthetic apparatus (Welsch et al. 2000), which greatly enhances its capacity for carotenoid biosynthesis. Unlike PSY, the other carotenoid biosynthetic enzymes, i.e. GGPS and PDS, are constitutively expressed even carotenoid and chlorophyll contents increase greatly during the transition (von Lintig et al. 1997; Welsch et al. 2000). They are not regulated by the phytochrome-interacting factors (Toledo-Ortiz et al. 2010). However, a strong and coordinated increase in carotenoid production and the expression of DXS and HDR, two genes in the upstream of carotenoid biosynthetic pathway, are observed during deetiolation from etioplasts to chloroplasts (Botella-Pavia et al. 2004).
3 Carotenoids in Chloroplasts
Chloroplasts along with all other plastids are surrounded by two envelope membranes. In addition to these membranes, chloroplasts are unique in containing thylakoids, which are the sites for the light reaction of photosynthesis . Carotenoids serve as light-harvesting pigments and are ubiquitous protective agent in the membranes of chloroplasts. Carotenoid composition in photosynthetic leaf tissue of plants is remarkably conserved with lutein, β-carotene , violaxanthin, and neoxanthin occurring in decreased order (Pogson et al. 1998). The majority of carotenoids in photosynthetic tissues are localized in the thylakoid membranes with a small fraction of non-protein bound carotenoids acting as antioxidants in photosynthetic apparatus (Domonkos et al. 2013; Havaux et al. 2004). While β-carotene is part of the reaction center subunits of photosystem I and II, the xanthophylls are accessory pigments and structural units of light-harvesting complexes (Nelson and Ben-Shem 2004). In addition to serve as the essential structural components of photosynthetic complexes, carotenoids are critical for photoprotection of chloroplasts and for the assembly and stabilization of protein complexes in the thylakoid membranes. The formation of lipoprotein pigment complexes and the build-up of thylakoid membranes along with the development of plastoglobuli in chloroplasts promote sequestration and storage of the synthesized carotenoids, resulting in high level of accumulation in chloroplasts.
While the majority of carotenoids are localized in thylakoids, the chloroplast envelope membranes are evidenced by chloroplast proteomics analysis to be the major site for carotenoid biosynthesis and metabolism, and the thylakoids appear to be restricted to xanthophyll cycle (Joyard et al. 2009). The suborganelle localization of carotenoid enzyme proteins is supported by data from biochemical, genetic and other studies (Ruiz-Sola and Rodriguez-Concepcion 2012). However, how carotenoids synthesized in the chloroplast envelope membranes are transported into the thylakoids remains unknown. Carotenoid biosynthesis in chloroplasts of green tissues occurs in a coordinated manner with other cellular processes for assembly of the photosynthetic apparatus. Thus, it is not surprising to find that defects in chlorophyll biosynthesis and chloroplast development result in reduced levels of carotenoid accumulation observed in many cases (Casanova-Sáez et al. 2014; Fambrini et al. 2004; Yu et al. 2012; Zhang et al. 2016). It is well established that carotenoid steady-state regulatory mechanisms are pronounced in green leaf tissues for maintaining optimal photosynthesis and photoprotection due to that the carotenoid combination is the most functionally adaptive (Dall’Osto et al. 2007; Kim et al. 2009; Pogson et al. 1996; Tian et al. 2003). As a result, overexpression of PSY, the rate-limiting enzyme in the carotenoid biosynthetic pathway, generally does not significantly alter carotenoid content in leaves (Maass et al. 2009). The increased phytoene production by the enhanced PSY activity is likely to be rapidly converted into downstream carotenoids and then apocarotenoids (Zhou et al. 2015). Indeed, data from 14CO2 pulse-chase labeling experiments demonstrate continuous turnover of carotenoids in Arabidopsis leaves, much high than expected (Beisel et al. 2010). The regulation of continuous biosynthesis and turnover of pigments takes place as essential part of the maintenance and formation of photosynthetic membranes (Beisel et al. 2010). Constitutive overexpression of PSY in some cases is observed to cause severe dwarf phenotype and change carotenoid content and composition due to the perturbance of gibberellin and other pathways (Busch et al. 2002; Fray et al. 1995). Because of the pronounced regulation of carotenoid biosynthesis in chloroplasts, overexpression of other carotenoid biosynthetic genes, such as PDS, also exerts little effect on carotenoid accumulation and the expression of other carotenogenic genes (Busch et al. 2002).
Studies of carotenoid mutants in chloroplasts contribute greatly to our understanding of carotenoid metabolism and the functional roles of carotenogenic genes and enzymes in plants. Lutein is the most abundant carotenoid found in leaf tissue of plants. Characterization of lut1, lut2, and lut5 mutants defective in lutein biosynthesis in Arabidopsis reveals novel carotenoid hydroxylases and the preferred pathway for lutein synthesis in plants (Kim and DellaPenna 2006; Pogson et al. 1996; Tian and DellaPenna 2001). ccr2 disrupts carotenoid biosynthesis and causes a reduction in lutein under light due to the defective in carotenoid isomerase (CRITISO) (Park et al. 2002). A defect in CRTISO is also found to be responsible for the orange head Chinese cabbage (Zhang et al. 2015). Investigation of the ccr2 mutant along with the tangerine mutant of tomato reveals light-induced isomerization in chloroplasts of green tissues, but not in chromoplasts of fruit and flowers (Isaacson et al. 2002; Park et al. 2002). Further studies of ccr1 mutant that causes reduced CRTISO expression shows an epigenetic mechanism that contributes to the regulation of carotenoid isomerization (Cazzonelli et al. 2009; Cazzonelli et al. 2010). Zeaxanthin and violaxanthin form xanthophyll cycle to dissipate excess light energy in the light-harvesting complexes and protect the photosynthetic apparatus from high light intensity (Niyogi 1999). The Arabidopsis npq1 mutant unable to convert violaxanthin to zeaxanthin shows nonphotochemical quenching (NPQ) reduction, establishing a central role of xanthophyll cycle in the regulation of photosynthetic energy conversion (Niyogi et al. 1998). Neoxanthin is essential components of photosynthetic complexes in chloroplasts. The Arabidopsis aba4 mutant affects the conversion of violaxanthin to neoxanthin and shows more sensitive to oxidative stress (North et al. 2007). Furthermore, the aba4 mutant contains reduced level of ABA, demonstrating that neoxanthin serves as precursor for ABA biosynthesis. In addition to produce plant hormones ABA and strigolactones , carotenoid-derived apocarotenoids are shown to function as signaling molecules in regulating early chloroplast and leaf development through investigation of ζ-carotene desaturase mutants in Arabidopsis (Avendano-Vazquez et al. 2014), and as stress signal in inducing defense mechanisms against reactive oxygen species induced by high light in chloroplasts (Havaux 2014; Ramel et al. 2012).
Some recent studies in Arabidopsis plants provide additional new insights into the posttranscriptional regulation of carotenoid metabolism in plants. The upstream deoxy-D-xylulose 5-phosphate synthase (DXS) is important for carotenoid flux control. Its activity is found to be posttranscriptionally regulated by J20 and Hsp70 chaperones in Arabidopsis leaves, as mutant defective in plastidial J20 protein results in reduced DXS enzyme activity with increased level of enzymatically inactive DXS protein (Pulido et al. 2013). PSY protein level and enzyme activity are discovered to be posttranscriptionally regulated by the OR family proteins in a positive manner (Zhou et al. 2015). High level of the OR proteins increases PSY protein expression, and low expression reduces PSY protein level, concomitant with altered carotenoid levels.
Chloroplasts are differentiated into chromoplasts as often seen the color change from green to red during green fruit ripening , such as in tomato and pepper. This process is characterized by complete degradation of chlorophyll and breakdown of thylakoid structures of chloroplasts, accompanied with remodeling of internal membrane systems and dramatic accumulation of carotenoids (Bian et al. 2011; Egea et al. 2010; Li and Yuan 2013). As a result, carotenoids become localized in plastoglobuli and/or various carotenoid lipoprotein substructures within chromoplasts. During the transition, photosynthesis-related genes are pronouncedly down-regulated (Kahlau and Bock 2008). Accordingly, strong decrease in the abundance of proteins involved in photosynthesis, Calvin cycle and photorespiration along with carbohydrate metabolism is observed (Barsan et al. 2012). Plastids gradually acquire new biosynthetic capacities, particularly the ability to massively synthesize and accumulate carotenoids. The expression of upstream genes of carotenoid metabolites accumulated is enhanced, while that of downstream genes is suppressed. For example, during fruit ripening from chloroplasts to chromoplasts, the accumulation of lycopene in tomato is accompanied with increased transcript levels of DXS, PSY, and PDS, and decreased expression of Lcye and Lcyb (Lois et al. 2000; Ronen et al. 1999). The accumulation of capsanthin and capsorubin in pepper is paralleled by up-regulation of DXS, GGPS, BCH and CCS during plastid transition (Bouvier and Camara 2007; Bouvier et al. 1994). Similarly, the accumulation of β-cryptoxanthin, zeaxanthin, and violaxanthin in some citrus fruits is accompanied with a simultaneous increase in the expression of upstream genes of PSY, PDS, ZDX, LycB, BCH, and ZEP (Kato et al. 2004). Apart from the increased capacity of biosynthesis, the remodeling of the internal membrane systems during the transition from chloroplasts to chromoplasts distinguishes their capacities in sequestrating and accumulating newly synthesized carotenoid end products. This is demonstrated in tobacco where expression of a β-carotene ketolase under control of tomato PDS promoter results in only trace amounts of ketocarotenoid production in chloroplasts in leaves, but produces high levels of them in chromoplasts of nectaries of the transgenic plants (Mann et al. 2000).
4 Carotenoids in Amyloplasts
Amyloplasts as starch enriched organelles are agronomically important because they are abundance in cereal seeds of major staple crops (e.g. rice, maize, wheat, and sorghum), roots (e.g. cassava), and tubers (e.g. potato). A general low to mediate levels of carotenoids are synthesized and accumulated in amyloplasts in majority of these staple crops (Wurtzel et al. 2012). Unlike carotenoid constituents in chloroplasts of green tissues, carotenoid composition and content found in amyloplasts of storage organs vary to give colors ranging from white to yellow with lutein or zeaxanthin as the major carotenoid (Howitt and Pogson 2006). None of natural rice germplasm contains carotenoids in endosperm due to lack of functional endosperm specific PSY (Chaudhary et al. 2010; Welsch et al. 2008). Thus, rice endosperm with carotenoid accumulation such as Golden Rice could be only accomplished with biotechnology approaches. Maize exhibits significant variation in carotenoid content and composition (Harjes et al. 2008). While the yellow varieties contain mainly lutein and/or zeaxanthin, there are orange varieties with relative abundance of β-carotene (Berardo et al. 2009; Yan et al. 2010). Limited natural variation exists in wheat endosperm and a general low level of carotenoids is observed with lutein as the main carotenoid (Howitt et al. 2009). Yellow sorghum varieties contain zeaxanthin as the most abundant carotenoid followed by lutein with low level of β-carotene , but their carotenoid contents are significantly lower than yellow maize (Kean et al. 2007). Similarly, cassava roots are generally white and the yellow-rooted cultivars contain low level of β-carotene (Adewusi and Bradbury 1993; Kimura et al. 2007). Potato tuber flesh colors range from white to dark yellow. While the common cultivated varieties contain low level of carotenoids with lutein and/or β-cryptoxanthin as the main carotenoid, the dark yellow ones accumulate zeaxanthin as the primary carotenoid (Brown et al. 1993; Morris et al. 2004; Zhou et al. 2011). The roles of low levels of carotenoids in these storage organs are suggested to be associated with ABA production for dormancy and they contribute to the antioxidant system to protect membranes (Howitt and Pogson 2006).
Studies of carotenoid accumulation in amyloplasts also reveal new information for our understanding of carotenoid metabolism in plants. In the grass family such as maize, rice, and sorghum, multiple PSYs share tissue-specific regulation of carotenoid metabolic flux (Li et al. 2008b). PSY1 encoded by the yellow1 (y1) locus is required for carotenoid accumulation in maize endosperm (Li et al. 2008a). PSY1 transcript levels are shown to exist statistically significant correlation with total endosperm carotenoid content in a maize germplasm diversity collection (Li et al. 2008a). In contrast, PSY3 influences root carotenoid biosynthesis and is induced in response to abiotic stress to interfere with stress-induced ABA accumulation in both maize and rice (Li et al. 2008b; Welsch et al. 2008). Interestingly, the three maize PSY enzyme proteins exist in distinctive plastid compartments when they are transiently expressed in leaves. While PSY2 and PSY3 are localized to plastoglobules of chloroplasts , PSY1 shows altered localization depending on its allelic variant (Shumskaya et al. 2012). All maize varieties with yellow endosperm have the same specific amino acid variant of PSY1, which appears to be a soluble protein in plastids. This variant happens to be the one used for the production of Golden Rice 2 (Shumskaya and Wurtzel 2013). In contrast, the mutated variant of PSY1 with a specific amino acid replacement promotes the formation of fibrils, characteristic for high levels of carotenoid accumulation in some chromoplasts (Shumskaya et al. 2012). It has been suggested that the different suborganellar localization of PSY1 isozyme could affect carotenoid biosynthesis and stability (Shumskaya and Wurtzel 2013). In addition, in cassava a single nucleotide polymorphism of PSY is observed in yellow-rooted cultivars; the resulting change of a single amino acid in a highly conserved region of PSY has an increased catalytic activity and enhanced carotenoid production (Welsch et al. 2010). Investigation of the maize y9 locus identifies a previously unknown pathway enzyme, ζ-carotene isomerase (Z-ISO), for carotenoid biosynthesis. Z-ISO isomerizes the 15-cis double bond in 9,15,9’-tri-cis-ζ-carotene to 9,9’-di-cis-ζ-carotene. It is required for carotenoid biosynthesis in seed endosperm and suggested to play an important role in adaption of environmental stress (Chen et al. 2010).
Multiple control points have been identified to regulate carotenoid amount and composition in amyloplasts. PSY as a key regulator of carotenoid biosynthesis plays an important role for carotenoid accumulation in staple crops, such as in maize kernel (Li et al. 2008a), wheat endosperm (Howitt et al. 2009), and cassava root (Welsch et al. 2010). In maize, PSY1 accounts for up to 27.2 % of phenotypic variation in seed carotenoid content in a QTL analysis (Chander et al. 2008). β-carotene hydroxylase (BCH) is found to be responsible for potato tuber flesh color (Brown et al. 2006; Kloosterman et al. 2010; Zhou et al. 2011). The high level of zeaxanthin accumulation in potato tubers is controlled by a combination of the dominant BCH2 allele and homozygous recessive ZEP allele (Wolters et al. 2010) and associated with low expression of CCD4 (Campbell et al. 2010). BCH also likely represents another control point for β-carotene level in crops. Hydoxylase3 locus was found to contribute to β-carotene level in maize (Vallabhaneni et al. 2009). Lycopene ε-cylase (LcyE) is critical in directing metabolic flux from one branch to another branch within the carotenoid biosynthetic pathway. It is a main regulator in determination of the β-carotene/α-carotene ratio and underlies a major β-carotene QTL in maize kernel (Bai et al. 2009; Harjes et al. 2008; Yan et al. 2010).
Carotenoid biosynthesis in the major staple crops is believed to occur in the membranes of amyloplasts. At least some of the carotenoid biosynthetic pathway enzyme proteins are found to be associated with amyloplast envelope membranes (Wurtzel et al. 2012), such as PSY1 is localized in the amyloplast membranes in maize endosperm (Li et al. 2008a). Because amyloplasts are lack of carotenoid lipoprotein sequestering substructures within the plastids (Lopez et al. 2008), carotenoids synthesized in amyloplasts are known to be more vulnerable for degradation during both plant growth and postharvest storage (Li and Yuan 2013). Carotenoid content increases during early maize endosperm development, but decreases before seed mature (Farre et al. 2013). A general loss of carotenoids has been observed during storage of many staple crops (http://www.harvestplus.org/). A range between 30–56 % of provitamin A carotenoids are lost during storage of a number of commercial high-carotenoid yellow maize (Dua et al. 1965). Similarly, between 20 and 48 % of total carotenoids in yellow wheat are lost during storage at 20 °C (Hidalgo and Brandolini 2008).
Amyloplasts are shown to be able to directly differentiate into chromoplasts. The color in saffron deep-red stigmas of flowers is due to high level of carotenoid accumulation in chromoplasts. The deep-red stigmas start from the colorless ovary along the style up. Electron microscope analysis of plastid development in the pistil reveals that the saffron chromoplasts in the deep-red stigma originate from amyloplasts since amyloplasts are the only plastids existing in the colorless basal portion of style and the plastid transition is via a unique amylo-chromoplast plastid type (Grilli Caiola and Canini 2004). Similarly, in developing tobacco floral nectaries as they change to orange color, amyloplasts are converted into chromoplasts via amylo-chromoplasts, resulting in the accumulation of carotenoids with bright orange nectary (Horner et al. 2007). The conversion of amyloplasts into chromoplasts leads to enhanced capacity to massively synthesize and accumulate carotenoids.
5 Carotenoids in Chromoplasts
Chromoplasts as carotenoid enriched plastids are mostly found in maturing fruits and some vegetables. Chromoplasts can be differentiated or converted from a number of plastid types. As discussed above, chromoplasts are derived from fully developed chloroplasts as seen during fruit ripening from green to red or yellow fruits. In the fruit peel of tomato, pepper, mango, and sweet orange, chloroplasts differentiate into photosynthetically inactive chromoplasts; this conversion process emerges in early ripening stages and accompanies with markedly increased accumulation of carotenoids in colored chromoplasts in subsequent fruit maturation (Frey-Wyssling and Kreutzer 1958; Rosso 1968; Vísquez-Caicedo et al. 2006). Chromoplasts also arise from non-photosynthetic plastids. Amyloplasts are shown to be differentiate into chromoplasts in saffron red-stigmas of flowers (Grilli Caiola and Canini 2004) and in developing tobacco floral nectary as it changes to orange color (Horner et al. 2007). Similarly, colorless proplastids and leucoplasts can also directly differentiate into chromoplasts as the case in carrot root (Kim et al. 2010), in orange curd cauliflower mutant (Lu et al. 2006), and in papaya and berries (Knoth et al. 1986; Schweiggert et al. 2011). While most studies on chromoplasts have been focused on the biosynthesis of carotenoids (Egea et al. 2010; Bian et al. 2011; Li and Yuan 2013), little is known about the molecular mechanisms underlying chromoplast biogenesis (Li and Yuan 2013; Yuan et al. 2015). The Or gene isolated from cauliflower orange curd mutant represents the only known gene that acts as a bona fide molecular switch to trigger chromoplast biogenesis (Giuliano and Diretto 2007; Lu et al. 2006). However, how Or initiates chromoplast differentiation remains an enigma.
Chromoplasts exist with various sequestering substructures such as crystalline, globular, tubular, and membranous structures, and are classified based on these morphologies (Camara et al. 1995; Egea et al. 2010; Li and Yuan 2013). Generally, there are more than one pigment-bearing substructures within a chromoplast or in a crop species. For example, crystalline bodies are observed in chromoplasts of tomato, but globular- and membrane-shaped structures also coexist (Harris and Spurr 1969). The typical saffron stigma chromoplasts have reticulo-tubular structure, contain a mix of tubules, vesicles and plastoglobules (Grilli Caiola and Canini 2004). A more recent study of chromoplast structures in nine carotenoid-rich fruits and vegetables also reveals the coexistence of more than one substructure within a chromoplast (Jeffery et al. 2012). However, some crops appear to be predominant with chromoplasts containing a particular type of pigment-bearing substructures. For example, carrot , tomato, and red papaya are enriched with crystal chromoplasts; mango contains high concentration of plastoglobuli in chromoplasts; and watermelon, mango and butternut are prominent with membranous chromoplasts (Jeffery et al. 2012).
Unlike other type of plastids, chromoplasts synthesize and accumulate diverse carotenoid compounds in different fruits and vegetables. In some cases, the specific carotenoids accumulated appear to be associated with the formation of specific carotenoid sequestering substructures within chromoplasts. By examining diverse chili pepper fruits with various carotenoid chemical composition, it establishes a linkage between the unique carotenoids accumulated with various specific chromoplast architecture as determined by chromatographic methods (Kilcrease et al. 2013). Crystalline bodies observed in chromoplasts of carrots, papaya, and tomato are found to predominantly consist of β-carotene or lycopene (Harris and Spurr 1969; Schweiggert et al. 2011). Globular and/or tubular-globular chromoplasts described for yellow fruits such as from kiwi and yellow papaya contained lutein or β-cryptoxanthin as major carotenoid (Montefiori et al. 2009; Schweiggert et al. 2011). The highly heterogeneous nature of the substructures in chromoplasts contributes to the various profiles of carotenoid accumulation found in fruits, vegetables, and roots.
The specific feature of chromoplasts with various carotenoid-lipoprotein sequestering substructures is responsible for massive amount of carotenoid accumulation in chromoplasts. Indeed, a strong association between proliferation of carotenoid-lipoprotein sequestering substructures and increase of carotenoid accumulation is observed (Al Babili et al. 1996; Li et al. 2012; Rabbani et al. 1998). The increased carotenoid content during long term storage of transgenic OR potato tubers directly correlates with increased amount of pigment-bearing substructures released from chromoplasts (Li et al. 2012). Additionally, the development of chromoplasts with high level of carotenoid accumulation during tomato fruit ripening is accompanied with large amounts and considerably enlargement of plastoglobules (Bian et al. 2011). Plastoglobules are well-known for their function in carotenoid sequestration and storage. Carotenoids are highly stable in plastoglobules. These carotenoid-lipoprotein sequestering substructures are believed to serve two roles for the high capacity of chromoplasts in promoting carotenoid biosynthesis and accumulation. One is to facilitate the sequestration of newly synthesized carotenoids to avoid the overloading of end products at the site of carotenoid biosynthesis in chromoplast membranes for continuous biosynthesis; and the other one is to serve as deposition sink for stable storage of carotenoids (Li and Van Eck 2007; Li and Yuan 2013; Vishnevetsky et al. 1999).
It is thus not surprising that biogenesis of chromoplasts exerts strong effect on carotenoid content in crops. The cauliflower Or causes the normal white curd tissue to accumulate high levels of β-carotene , producing a striking orange curd mutant phenotype. The Or gene, which encodes a protein containing DnaJ cysteine-rich zinc finger domain, is found to induce carotenoid accumulation by triggering the differentiation of non-colored plastids into chromoplasts (Li et al. 2001; Lu et al. 2006). Ectopic expression of the Or transgene in both white cauliflower and potato tubers leads to the formation of chromoplasts with enhanced carotenoid content (Lopez et al. 2008; Lu et al. 2006). Similarly, increase of chromoplast compartment size and number also results in enhanced carotenoid levels. In the tomato high pigment mutants as well as in the tomato flacca and sitiens mutants, the high levels of carotenoids are found to be directly linked to an increased plastid number and/or compartment size (Galpaz et al. 2008; Kolotilin et al. 2007; Liu et al. 2004).
Since the prominent role of chromoplasts is involved in synthesis and accumulation of carotenoid pigments, carotenoid metabolism and regulation are best investigated in chromoplasts (Egea et al. 2010; Li and Yuan 2013; Yuan et al. 2015). Although the carotenoid biosynthetic enzyme protein capsanthin/capsorubin synthase represents the most abundant protein in pepper chromoplasts (Siddique et al. 2006; Wang et al. 2013), the other core enzyme proteins are generally present at low abundance in plants. Expression of carotenoid biosynthetic genes has been subjected to extensive studies in many chromoplast-containing tissues. Transcriptional regulation of carotenoid biosynthesis prevails in chromoplasts during fruit ripening from green to red or yellow fruits in tomato and pepper as described above. Similar transcriptional regulation also occurs in red-flesh orange mutant of “Hong Anliu” when compared to wide type “Anliu” (Liu et al. 2007). However, the transcriptional control has been found not to be always the primary regulatory mechanism in some other cases, especially in chromoplasts derived from white tissues (Yuan et al. 2015). No correlation between carotenogenic gene expression and carotenoid accumulation is observed in orange cauliflower mutant, squash, and carrot root (Clotault et al. 2008; Li et al. 2001; Zhang et al. 2014). Thus, carotenoid biosynthesis and accumulation in chromoplasts is under complex regulation.
Because chromoplasts accumulate high levels of carotenoids that are essential for nutritional and sensory quality of agricultural products, chromoplast proteomes from a number of carotenoid enriched crops have been investigated to provide insights into the general metabolism and function of chromoplasts. A large number of chromoplast proteins from various crop species such as watermelon, tomato, carrot , orange cauliflower, red papaya, citrus, and red bell pepper have been identified (Barsan et al. 2010; Siddique et al. 2006; Wang et al. 2013; Zeng et al. 2011). Examination of these chromoplast proteomes identifies relative abundance of early core carotenoid pathway proteins, which may suggest an important role of them for metabolic flux into carotenoid biosynthetic pathway (Wang et al. 2013). In addition, a number of key metabolic and cellular processes appear to be crucial for chromoplast biogenesis and development, which include lipid metabolism for plastid membrane proliferation, carbohydrate metabolism to provide precursors for the biosynthesis of carotenoids and many other metabolites within chromoplasts, and chaperones for protein modification and translocation, as well as redox system and reactive oxygen species, and energy production and import into chromoplasts (Egea et al. 2010; Li and Yuan 2013).
While carotenoids in amyloplasts of staple crops are more vulnerable for degradation, carotenoids in chromoplasts appear to be more stable due to sequestering in carotenoid lipoprotein substructures including plastoglobules. In transgenic potato tubers, the induction of chromoplast formation by the Or transgene has been found to not only help retain carotenoids, but also stimulate continuous carotenoid accumulation during long term storage (Li et al. 2012). The enhanced carotenoid stability and accumulation are closely associated with amount of colored carotenoid lipoprotein sequestering substructures formed. Carotenoids in chromoplasts of some other fruits and vegetable are also known to be more stable. Carrot retains carotene content and continuous accumulation during storage (Booth 1951; Imsic et al. 2010; Kopas-Lane and Warthesen 1995). Similarly, storage of butternut leads to continuously increased carotenoid content. The increased accumulation has been shown to be associated with the transition of amylochromoplasts into chromoplasts (Zhang et al. 2014).
Chromoplasts provide a plastid-localized sink, which not only facilitates active carotenoid biosynthesis , but also enhances stable storage of the synthesized products with increased carotenoid stability. The specific characteristics of chromoplasts provide potential for high capacity of carotenoid biosynthesis and stable accumulation. Vitamin A plays vital role in human health and its deficiency leads to eye damage, growth retardation, and reduced immune responses. Worldwide, some 250 million preschool children suffer from vitamin A deficient, which is caused primarily by simple diets of staple crops with low levels of provitamin A carotenoids. Many important staple crops such as wheat, rice, barley, maize, potato, and cassava synthesize and store low levels of carotenoids in the membranes of amyloplasts in the edible seeds or roots. In addition to low content, retention of these carotenoids in staple crops has been a major concern during post-harvest storage and over 50 % of provitamin A carotenoids can be lost during storage. Because of the ability and capacity of chromoplasts to actively synthesize and effectively sequester carotenoids, induction of chromoplast formation or conversion of some amyloplasts into chromoplasts in the edible organs likely facilitates carotenoid synthesis and stable storage in these major staple crops with improved nutritional quality.
References
Adewusi SRA, Bradbury JH (1993) Carotenoids in cassava: comparison of open-column and HPLC methods of analysis. J Sci Food Agric 62:375–383
Al Babili S, Lintig J,V, Haubruck H, Beyer P (1996) A novel, soluble form of phytoene desaturase from Narcissus pseudonarcissus chromoplasts is Hsp70-complexed and competent for flavinylation, membrane association and enzymatic activation. Plant J 9:601–612
Avendano-Vazquez AO, Cordoba E, Llamas E, San RC, Nisar N, De la Torre S, Ramos-Vega M, Gutierrez-Nava MD, Cazzonelli CI, Pogson BJ, Leon P (2014) An Uncharacterized apocarotenoid-derived signal generated in zeta-carotene desaturase mutants regulates leaf development and the expression of chloroplast and nuclear genes in Arabidopsis. Plant Cell 26:2524–2537
Bai L, Kim EH, DellaPenna D, Brutnell TP (2009) Novel lycopene epsilon cyclase activities in maize revealed through perturbation of carotenoid biosynthesis. Plant J 59:588–599
Barsan C, Sanchez-Bel P, Rombaldi C, Egea I, Rossignol M, Kuntz M, Zouine M, Latche A, Bouzayen M, Pech JC (2010) Characteristics of the tomato chromoplast revealed by proteomic analysis. J Exp Bot 61:2413–2431
Barsan C, Zouine M, Maza E, Bian W, Egea I, Rossignol M, Bouyssie D, Pichereaux C, Purgatto E, Bouzayen M, Latche A, Pech JC (2012) Proteomic analysis of chloroplast-to-chromoplast transition in tomato reveals metabolic shifts coupled with disrupted thylakoid biogenesis machinery and elevated energy-production components. Plant Physiol 160:708–725
Beisel KG, Jahnke S, Hofmann D, Koppchen S, Schurr U, Matsubara S (2010) Continuous turnover of carotenes and chlorophyll a in mature leaves of Arabidopsis revealed by (CO2)-C-14 pulse-chase labeling. Plant Physiol 152:2188–2199
Berardo N, Mazzinelli G, Valoti P, Lagana P, Redaelli R (2009) Characterization of maize germplasm for the chemical composition of the grain. J Agric Food Chem 57:2378–2384
Bian W, Barsan C, Egea I, Purgatto E, Chervin C, Zouine M, Latche A, Bouzayen M, Pech JC (2011) Metabolic and molecular events occurring during chromoplast biogenesis. J Bot 2011. doi:10.1155/2011/289859
Booth VH (1951) Chromogenesis in stored carrots. J Agric Food Chem 2:353–358
Botella-Pavia P, Besumbes O, Phillips MA, Carretero-Paulet L, Boronat A, Rodriguez-Concepcion M (2004) Regulation of carotenoid biosynthesis in plants: evidence for a key role of hydroxymethylbutenyl diphosphate reductase in controlling the supply of plastidial isoprenoid precursors. Plant J 40:188–199
Bouvier F, Camara B (2007) The role of plastids in ripening fruits. In: Wise RP, Hoober JK (eds) The Structure and Function of Plastids. Springer, Dordrecht, pp 419–432
Bouvier F, Hugueney P, D’Harlingue A, Kuntz M M, Camara B (1994) Xanthophyll biosynthesis in chromoplasts: isolation and molecular cloning of an enzyme catalyzing the conversion of 5,6-epoxycarotenoid into ketocarotenoid. Plant J 6:45–54
Brautigam A, Weber AP (2009) Proteomic analysis of the proplastid envelope membrane provides novel insights into small molecule and protein transport across proplastid membranes. Mol Plant 2:1247–1261
Brown CR, Edwards CG, Yang CP, Dean BB (1993) Orange flesh trait in potato: inheritance and carotenoid content. J Am Soc Hortic Sci 118:145–150
Brown CR, Kim TS, Ganga Z, Haynes K, De Jong D, Jahn M, Paran I, De Jong W (2006) Segregation of total carotenoid in high level potato germplasm and its relationship to beta-carotene hydroxylase polymorphism. Am J Potato Res 83:365–372
Busch M, Seuter A, Hain R (2002) Functional analysis of the early steps of carotenoid biosynthesis in tobacco. Plant Physiol 128:439–453
Camara B, Hugueney P, Bouvier F, Kuntz M, Moneger R (1995) Biochemistry and molecular biology of chromoplast development. Int Rev Cyt 163:175–247
Campbell R, Ducreux LJ, Morris WL, Morris JA, Suttle JC, Ramsay G, Bryan GJ, Hedley PE, Taylor MA (2010) The metabolic and developmental roles of carotenoid cleavage dioxygenase4 from potato. Plant Physiol 154:656–664
Cazzonelli CI, Pogson BJ (2010) Source to sink: regulation of carotenoid biosynthesis in plants. Trends Plant Sci 15:266–274
Cazzonelli CI, Cuttriss AJ, Cossetto SB, Pye W, Crisp P, Whelan J, Finnegan EJ, Turnbull C, Pogson BJ (2009) Regulation of carotenoid composition and shoot branching in Arabidopsis by a chromatin modifying histone methyltransferase, SDG8. Plant Cell 21:39–53
Cazzonelli CI, Roberts AC, Carmody ME, Pogson BJ (2010) Transcriptional control of SET DOMAIN GROUP 8 and CAROTENOID ISOMERASE during Arabidopsis development. Mol Plant 3:174–191
Chander S, Guo YQ, Yang XH, Zhang J, Lu XQ, Yan JB, Song TM, Rocheford TR, Li JS (2008) Using molecular markers to identify two major loci controlling carotenoid contents in maize grain. Theor Appl Genet 116:223–233
Chaudhary N, Nijhawan A, Khurana J, Khurana P (2010) Carotenoid biosynthesis genes in rice: structural analysis, genome-wide expression profiling and phylogenetic analysis. Mol Genet Genomics 283:13–33
Chen Y, Li F, Wurtzel ET (2010) Isolation and characterization of the Z-ISO gene encoding a missing component of carotenoid biosynthesis in plants. Plant Physiol 153:66–79
Clotault J, Peltier D, Berruyer R, Thomas M, Briard M, Geoffriau E (2008) Expression of carotenoid biosynthesis genes during carrot root development. J Exp Bot 59:3563–3573
Cuttriss AJ, Chubb AC, Alawady A, Grimm B, Pogson BJ (2007) Regulation of lutein biosynthesis and prolamellar body formation in Arabidopsis. Funct Plant Biol 34:663–672
Dall’Osto L, Fiore A, Cazzaniga S, Giuliano G, Bassi R (2007) Different roles of alpha- and beta-branch xanthophylls in photosystem assembly and photoprotection. J Biol Chem 282:35056–35068
Domonkos I, Kis M, Gombos Z, Ughy B (2013) Carotenoids, versatile components of oxygenic photosynthesis. Prog Lipid Res 52:539–561
Dua PN, Day EJ, Grogan CO (1965) Loss of carotenoids in stored commercial and high-carotenoid yellow corn, Zea mays L. Agron J 57:501–502
Dupont FM (2008) Metabolic pathways of the wheat (Triticum aestivum) endosperm amyloplast revealed by proteomics. BMC Plant Biol 8:39. doi:10.1186/1471-2229-8-39
Egea I, Barsan C, Bian W, Purgatto E, Latché A, Chervin C, Bouzayen M, Pech JC (2010) Chromoplast differentiation: current status and perspectives. Plant Cell Physiol 51:1601–1611
Fambrini M, Castagna A, Vecchia FD, Degl’Innocenti E, Ranieri A, Vernieri P, Pardossi A, Guidi L, Rascio N, Pugliesi C (2004) Characterization of a pigment-deficient mutant of sunflower (Helianthus annuus L.) with abnormal chloroplast biogenesis, reduced PS II activity and low endogenous level of abscisic acid. Plant Sci 167:79–89
Farre G, Maiam RS, Alves R, Vilaprinyo E, Sorribas A, Canela R, Naqvi S, Sandmann G, Capell T, Zhu C, Christou P (2013) Targeted transcriptomic and metabolic profiling reveals temporal bottlenecks in the maize carotenoid pathway that may be addressed by multigene engineering. Plant J 75:441–455
Fray RG, Wallace A, Fraser PD, Valero D, Hedden P, Bramley PM, Grierson D (1995) Constitutive expression of a fruit phytoene synthase gene in transgenic tomatoes causes dwarfism by redirecting metabolites from the gibberellin pathway. Plant J 8:693–701
Frey-Wyssling A, Kreutzer E (1958) The submicroscopic development of chromoplasts in the fruit of Capsicum annuum L. J Ultrastruct Res 1:397–411
Galpaz N, Wang Q, Menda N, Zamir D, Hirschberg J (2008) Abscisic acid deficiency in the tomato mutant high-pigment 3 leading to increased plastid number and higher fruit lycopene content. Plant J 53:717–730
Giuliano G, Diretto G (2007) Of chromoplasts and chaperones. Trends Plant Sci 12:529–531
Grilli Caiola M, Canini A (2004) Ultrastructure of chromoplasts and other plastids in Crocus sativus L. (Iridaceae). Plant Biosyst 138:43–52
Harjes CE, Rocheford TR, Bai L, Brutnell TP, Kandianis CB, Sowinski SG, Stapleton AE, Vallabhaneni R, Williams M, Wurtzel ET, Yan JB, Buckler ES (2008) Natural genetic variation in lycopene epsilon cyclase tapped for maize biofortification. Science 319:330–333
Harris WM, Spurr AR (1969) Chromoplasts of tomato fruits. I. Ultrastructure of low-pigment and high-beta mutants. Carotene analyses. Am J Bot 56:369–379
Havaux M (2014) Carotenoid oxidation products as stress signals in plants. Plant J 79:597–606
Havaux M, Dall’Osto L, Cuine S, Giuliano G, Bassi R (2004) The effect of zeaxanthin as the only xanthophyll on the structure and function of the photosynthetic apparatus in Arabidopsis thaliana. J Biol Chem 279:13878–13888
Hidalgo A, Brandolini A (2008) Kinetics of carotenoids degradation during the storage of Einkorn (Triticum monococcum L. ssp. monococcum) and bread wheat (Triticum aestivum L. ssp. aestivum) flours. J Agric Food Chem 56:11300–11305
Horner HT, Healy RA, Ren G, Fritz D, Klyne A, Seames C, Thornburg RW (2007) Amyloplast to chromoplast conversion in developing ornamental tobacco floral nectaries provides sugar for nectar and antioxidants for protection. Am J Bot 94:12–24
Howitt CA, Pogson BJ (2006) Carotenoid accumulation and function in seeds and non-green tissues. Plant Cell Environ 29:435–445
Howitt C, Cavanagh C, Bowerman A, Cazzonelli C, Rampling L, Mimica J, Pogson B (2009) Alternative splicing, activation of cryptic exons and amino acid substitutions in carotenoid biosynthetic genes are associated with lutein accumulation in wheat endosperm. Funct Integr Genomic 9:363–376
Imsic M, Winkler S, Tomkins B, Jones R (2010) Effect of storage and cooking on beta-carotene isomers in carrots (Daucus carota L. cv. Stefanoa). J Agric Food Chem 58:5109–5113
Isaacson T, Ronen G, Zamir D, Hirschberg J (2002) Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of β-carotene and xanthophylls in plants. Plant Cell 14:333–342
Jeffery J, Holzenburg A, King S (2012) Physical barriers to carotenoid bioaccessibility. Ultrastructure survey of chromoplast and cell wall morphology in nine carotenoid-containing fruits and vegetables. J Sci Food Agric 92:2594–2602
Jensen PE, Leister D (2014) Chloroplast evolution, structure and functions. F1000Prime Rep 6:40
Joyard J, Ferro M, Masselon C, Seigneurin-Berny D, Salvi D, Garin J, Rolland N (2009) Chloroplast proteomics and the compartmentation of plastidial isoprenoid biosynthetic pathways. Mol Plant 2:1154–1180
Kahlau S, Bock R (2008) Plastid transcriptomics and translatomics of tomato fruit development and chloroplast-to-chromoplast differentiation: chromoplast gene expression largely serves the production of a single protein. Plant Cell 20:856–874
Kato M, Ikoma Y, Matsumoto H, Sugiura M, Hyodo H, Yano M (2004) Accumulation of carotenoids and expression of carotenoid biosynthetic genes during maturation in citrus fruit. Plant Physiol 134:824–837
Kean EG, Ejeta G, Hamaker BR, Ferruzzi MG (2007) Characterization of carotenoid pigments in mature and developing kernels of selected yellow-endosperm sorghum varieties. J Agric Food Chem 55:2619–2626
Kilcrease J, Collins AM, Richins RD, Timlin JA, O’Connell MA (2013) Multiple microscopic approaches demonstrate linkage between chromoplast architecture and carotenoid composition in diverse Capsicum annuum fruit. Plant J 76:1074–1083
Kim J, DellaPenna D (2006) Defining the primary route for lutein synthesis in plants: the role of Arabidopsis carotenoid beta-ring hydroxylase CYP97A3. Proc Natl Acad Sci U S A 103:3474–3479
Kim J, Smith JJ, Tian L, DellaPenna D (2009) The evolution and function of carotenoid hydroxylases in Arabidopsis. Plant Cell Physiol 50:463–479
Kim JE, Rensing KH, Douglas CJ, Cheng KM (2010) Chromoplasts ultrastructure and estimated carotene content in root secondary phloem of different carrot varieties. Planta 231:549–558
Kimura M, Kobori CN, Rodriguez-Amaya DB, Nestel P (2007) Screening and HPLC methods for carotenoids in sweetpotato, cassava and maize for plant breeding trials. Food Chemist 100:1734–1746
Kloosterman B, Oortwijn M, uitdeWilligen J, America T, de Vos R, Visser RGF, Bachem CWB (2010) From QTL to candidate gene: genetical genomics of simple and complex traits in potato using a pooling strategy. BMC Genomics 11:158
Knoth R, Hansmann P, Sitte P (1986) Chromoplasts of Palisota barteri, and the molecular structure of chromoplast tubules. Planta 168:167–174
Kolotilin I, Koltai H, Tadmor Y, Bar-Or C, Reuveni M, Meir A, Nahon S, Shlomo H, Chen L, Levin I (2007) Transcriptional profiling of high pigment-2dg tomato mutant links early fruit plastid biogenesis with its overproduction of phytonutrients. Plant Physiol 145:389–401
Kopas-Lane LM, Warthesen JJ (1995) Carotenoid photostability in raw spinach and carrots during cold storage. J Food Sci 60:773–776
Kuntz M, Rolland N (2012) Subcellular and sub-organellar proteomics as a complementary tool to study the evolution of the plastid proteome. In: Bullerwell CE (ed) Organelle genetics, 10th edn. Springer, New York, pp 217–238
Li L, Van Eck J (2007) Metabolic engineering of carotenoid accumulation by creating a metabolic sink. Transgenic Res 16:581–585
Li L, Yuan H (2013) Chromoplast biogenesis and carotenoid accumulation. Arch Biochem Biophys 539:102–109
Li L, Paolillo DJ, Parthasarathy MV, DiMuzio EM, Garvin DF (2001) A novel gene mutation that confers abnormal patterns of β-carotene accumulation in cauliflower (Brassica oleracea var. botrytis). Plant J 26:59–67
Li F, Vallabhaneni R, Yu J, Rocheford T, Wurtzel ET (2008a) The maize phytoene synthase gene family: overlapping roles for carotenogenesis in endosperm, photomorphogenesis, and thermal stress tolerance. Plant Physiol 147:1334–1346
Li F, Vallabhaneni R, Wurtzel ET (2008b) PSY3, a new member of the phytoene synthase gene family conserved in the Poaceae and regulator of abiotic stress-induced root carotenogenesis. Plant Physiol 146:1333–1345
Li L, Yang Y, Xu Q, Owsiany K, Welsch R, Chitchumroonchokchai C, Lu S, Van EJ, Deng XX, Failla M, Thannhauser TW (2012) The Or gene enhances carotenoid accumulation and stability during post-harvest storage of potato tubers. Mol Plant 5:339–352
Linka M, Weber A (2012) Evolutionary integration of chloroplast metabolism with the metabolic networks of the cells. In: Burnap R, Vermaas W (eds) Functional genomics and evolution of photosynthetic systems, 33rd edn. Springer, Dordrecht, pp 199–224
Liu Y, Roof S, Ye Z, Barry C, van Tuinen A, Vrebalov J, Bowler C, Giovannoni J (2004) Manipulation of light signal transduction as a means of modifying fruit nutritional quality in tomato. Proc Natl Acad Sci U S A 101:9897–9902
Liu Q, Xu J, Liu Y, Zhao X, Deng X, Guo L, Gu J (2007) A novel bud mutation that confers abnormal patterns of lycopene accumulation in sweet orange fruit (Citrus sinensis L. Osbeck). J Exp Bot 58:4161–4171
Lois LM, Rodriguez-Concepcion M, Gallego F, Campos N, Boronat A (2000) Carotenoid biosynthesis during tomato fruit development: regulatory role of 1-deoxy-D-xylulose 5-phosphate synthase. Plant J 22:503–513
Lopez AB, Van Eck J, Conlin BJ, Paolillo DJ, O’Neill J, Li L (2008) Effect of the cauliflower Or transgene on carotenoid accumulation and chromoplast formation in transgenic potato tubers. J Exp Bot 59:213–223
Lopez-Juez E (2007) Plastid biogenesis, between light and shadows. J Exp Bot 58:11–26
Lopez-Juez E, Pyke KA (2005) Plastids unleashed: their development and their integration in plant development. Int J Dev Biol 49:557–577
Lu S, Van Eck J, Zhou X, Lopex AB, O’Halloran DM, Cosman KM, Conlin B, Paolillo DJ, Garvin DF, Vrebalov J, Kochian L,V, Kupper H, Earle ED, Cao J, Li L (2006) The cauliflower Or gene encodes a DnaJ cysteine-rich domain-containing protein that mediates high-levels of β-carotene accumulation. Plant Cell 18:3594–3605
Maass D, Arango J, Wast F, Beyer P, Welsch R (2009) Carotenoid crystal formation in Arabidopsis and carro troots caused by increased phytoene synthase protein levels. PLoS One 4:e6373
Mann V, Harker M, Pecker I, Hirschberg J (2000) Metabolic engineering of astaxanthin production in tobacco flowers. Nat Biotechnol 18:888–892
Montefiori M, Comeskey DJ, Wohlers M, McGhie TK (2009) Characterization and quantification of Anthocyanins in red Kiwifruit (Actinidia spp.). J Agric Food Chem 57:6856–6861
Morris WL, Ducreux L, Griffths DW, Stewart D, Davies HV, Taylor MA (2004) Carotenogenesis during tuber development and storage in potato. J Exp Bot 55:975–982
Nelson N, Ben-Shem A (2004) The complex architecture of oxygenic photosynthesis. Nat Rev Mol Cell Biol 5:971–982
Neuhaus HE, Emes MJ (2000) Nonphotosynthetic metabolism in plastids. Annu Rev Plant Physiol Plant Mol Biol 51:111–140
Nisar N, Li L, Lu S, Khin NC, Pogson BJ (2015) Carotenoid metabolism in plants. Mol Plant 8:68–82
Niyogi KK (1999) Photoprotection revisited: genetic and molecular approaches. Annu Rev Plant Physiol Plant Mol Biol 50:333–359
Niyogi KK, Grossman AR, Bjorkman O (1998) Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10:1121–1134
North HM, De Almeida A, Boutin JP, Frey A, To A, Botran L, Sotta B, Marion-Poll A (2007) The Arabidopsis ABA-deficient mutant aba4 demonstrates that the major route for stress-induced ABA accumulation is via neoxanthin isomers. Plant J 50:810–824
Park H, Kreunen SS, Cuttriss AJ, DellaPenna D, Pogson BJ (2002) Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell 14:321–332
Pogson BJ, McDonald KA, Truong M, Britton G, Dellapenna D (1996) Arabidopsis carotenoid mutants demonstrate that lutein is not essential for photosynthesis in higher plants. Plant Cell 8:1627–1639
Pogson BJ, Niyogi KK, Bjorkman O, Dellapenna D (1998) Altered xanthophyll compositions adversely affect chlorophyll accumulation and nonphotochemical quenching in Arabidopsis mutants. Proc Natl Acad Sci U S A 95:13324–13329
Pulido P, Toledo-Ortiz G, Phillips MA, Wright LP, Rodriguez-Concepcion M (2013) Arabidopsis J-protein J20 delivers the first enzyme of the plastidial isoprenoid pathway to protein quality control. Plant Cell 25:4183–4194
Rabbani S, Beyer P, Lintig J,V, Hugueney P, Kleinig H (1998) Induced beta-carotene synthesis driven by triacylglycerol deposition in the unicellular alga Dunaliella bardawil. Plant Physiol 116:1239–1248
Ramel F, Birtic S, Ginies C, Soubigou-Taconnat L, Triantaphylides C, Havaux M (2012) Carotenoid oxidation products are stress signals that mediate gene responses to singlet oxygen in plants. Proc Natl Acad Sci U S A 109:5535–5540
Rn C-S, Mateo-Bonmatí E, Kangasjärvi S, Candela H, Micol JL (2014) Arabidopsis ANGULATA10 is required for thylakoid biogenesis and mesophyll development. J Exp Bot 65:2391–2404
Rodriguez-Villalon A, Gas E, Rodriguez-Concepcion M (2009) Phytoene synthase activity controls the biosynthesis of carotenoids and the supply of their metabolic precursors in dark-grown Arabidopsis seedlings. Plant J 60:424–435
Ronen G, Cohen M, Zamir D, Hirschberg J (1999) Regulation of carotenoid biosynthesis during tomato fruit development: expression of the gene for lycopene epsilon-cyclase is down-regulated during ripening and is elevated in the mutant Delta. Plant J 17:341–351
Rosso SW (1968) The ultrastructure of chromoplast development in red tomatoes. J Ultrastruct Res 25:307–322
Ruiz-Sola MA, Rodriguez-Concepcion M (2012) Carotenoid biosynthesis in Arabidopsis: a colorful pathway. Arabidopsis Book 10:e0158
Schweiggert RM, Steingass CB, Heller A, Esquivel P, Carle R (2011) Characterization of chromoplasts and carotenoids of red- and yellow-fleshed papaya (Carica papaya L.). Planta 234:1031–1044
Shumskaya M, Wurtzel ET (2013) The carotenoid biosyntehtic pathway: thinking in all dimensions. Plant Sci 108:58–63
Shumskaya M, Bradbury LM, Monaco RR, Wurtzel ET (2012) Plastid localization of the key carotenoid enzyme phytoene synthase is altered by isozyme, allelic variation, and activity. Plant Cell 24:3725–3741
Siddique MA, Grossmann J, Gruissem W, Baginsky S (2006) Proteome analysis of bell pepper (Capsicum annuum L.) chromoplasts. Plant Cell Physiol 47:1663–1673
Solymosi K, Schoefs B (2010) Etioplast and etio-chloroplast formation under natural conditions: the dark side of chlorophyll biosynthesis in angiosperms. Photosynth Res 105:143–166
Tian L, DellaPenna D (2001) Characterization of a second carotenoid beta-hydroxylase gene from Arabidopsis and its relationship to the LUT1 locus. Plant Mol Biol 47:379–388
Tian L, Magallanes LM, Musetti V, Dellapenna D (2003) Functional analysis of beta- and epsilon-ring carotenoid hydroxylases in Arabidopsis. Plant Cell 15:1320–1332
Toledo-Ortiz G, Huq E, Rodriguez-Concepcion M (2010) Direct regulation of phytoene synthase gene expression and carotenoid biosynthesis by phytochrome-interacting factors. Proc Natl Acad Sci U S A 107:11626–11631
Vallabhaneni R, Gallagher CE, Licciardello N, Cuttriss AJ, Quinlan RF, Wurtzel ET (2009) Metabolite sorting of a germplasm collection reveals the hydroxylase3 locus as a new target for maize provitamin A biofortification. Plant Physiol 151:1635–1645
Vishnevetsky M, Ovadis M, Vainstein A (1999) Carotenoid sequestration in plants: the role of carotenoid-associated proteins. Trends Plant Sci 4:232–235
Vísquez-Caicedo AL, Heller A, Neidhart S, Carle R (2006) Chromoplast Morphology and β-Carotene Accumulation during Postharvest Ripening of Mango Cv. ‘Tommy Atkins’. J Agric Food Chem 54:5769–5776
von Lintig J, Welsch R, Bonk M, Giuliano G, Batschauer A, Kleinig H (1997) Light-dependent regulation of carotenoid biosynthesis occurs at the level of phytoene synthase expression and is mediated by phytochrome in Sinapis alba and Arabidopsis thaliana seedlings. Plant J 12:625–634
Wang YQ, Yang Y, Fei Z, Yuan H, Fish T, Thannhauser TW, Mazourek M, Kochian LV, Wang X, Li L (2013) Proteomic analysis of chromoplasts from six crop species reveals insights into chromoplast function and development. J Exp Bot 64:949–961
Welsch R, Beyer P, Hugueney P, Kleinig H, Von LJ (2000) Regulation and activation of phytoene synthase, a key enzyme in carotenoid biosynthesis, during photomorphogenesis. Planta 211:846–854
Welsch R, Wust F, Bar C, Al-Babili S, Beyer P (2008) A third phytoene synthase is devoted to abiotic stress-induced ABA formation in rice and defines functional diversification of PSYs. Plant Physiol 147:367–380
Welsch R, Arango J, Bar C, Salazar B, Al-Babili S, Js B, Chavarriaga P, Ceballos H, Tohme J, Beyer P (2010) Provitamin A accumulation in cassava (Manihot esculenta) roots driven by a single nucleotide polymorphism in a phytoene synthase gene. Plant Cell 22:3348–3356
Wolters AMA, Uitdewilligen JGAML, Kloosterman BA, Hutten RCB, Visser RGF, van Eck HJ (2010) Identification of alleles of carotenoid pathway genes important for zeaxanthin accumulation in potato tubers. Plant Mol Biol 73:659–671
Wurtzel ET, Cuttriss A, Vallabhaneni R (2012) Maize provitamin a carotenoids, current resources, and future metabolic engineering challenges. Front Plant Sci 3:2–12
Yan J, Kandianis CB, Harjes CE, Bai L, Kim EH, Yang X, Skinner DJ, Fu Z, Mitchell S, Li Q, Fernandez MGS, Zaharieva M, Babu R, Fu Y, Palacios N, Li J, Dellapenna D, Brutnell T, Buckler ES, Warburton ML, Rocheford T (2010) Rare genetic variation at Zea mays crtRB1 increases [beta]-carotene in maize grain. Nat Genet 42:322–327
Ytterberg AJ, Peltier JB, van Wijk KJ (2006) Protein profiling of plastoglobules in chloroplasts and chromoplasts. A surprising site for differential accumulation of metabolic enzymes. Plant Physiol 140:984–997
Yu B, Gruber MY, Khachatourians GG, Zhou R, Epp DJ, Hegedus DD, Parkin IAP, Welsch R, Hannoufa A (2012) Arabidopsis cpSRP54 regulates carotenoid accumulation in Arabidopsis and Brassica napus. J Exp Bot 63:5189–5202
Yuan H, Zhang J, Coimbatore D, Li L (2015) Carotenoid metabolism and regulation in horticultural crops. Hortic Res 2:15036
Zeng Y, Pan Z, Ding Y, Zhu A, Cao H, Xu Q, Deng X (2011) A proteomic analysis of the chromoplasts isolated from sweet orange fruits [Citrus sinensis (L.) Osbeck]. J Exp Bot 62:5297–5309
Zhang MK, Zhang MP, Mazourek M, Tadmor Y, Li L (2014) Regulatory control of carotenoid accumulation in winter squash during storage. Planta 240:1063–1074
Zhang J, Yuan H, Fei Z, Pogson BJ, Zhang L, Li L (2015) Molecular characterization and transcriptome analysis of orange head Chinese cabbage (Brassica rapa L. ssp. pekinensis). Planta 241:1381–1394
Zhang J, Yuan H, Yang Y, Fish T, Lyi SM, Thannhauser T, Zhang L, Li L (2016) Plastid ribosomal protein S5 is invovled in photosynthesis, plant development, and cold stress tolerance in Arabidopsis. J Exp Bot 67:2731–2744. doi:10.1093/jxb/erw106
Zhou X, McQuinn R, Fei Z, Wolters AMA, Van Eck J, Brown CR, Giovannoni JJ, Li L (2011) Regulatory control of high levels of carotenoid accumulation in potato tubers. Plant Cell Environ 34:1020–1030
Zhou X, Welsch R, Yang Y, Alvarez D, Riediger M, Yuan H, Fish T, Liu J, Thannhauser TW, Li L (2015) Arabidopsis OR proteins are the major posttranscriptional regulators of phytoene synthase in controlling carotenoid biosynthesis. Proc Natl Acad Sci U S A 112(11):3558–3563. doi:10.1073/pnas.1420831112
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Li, L., Yuan, H., Zeng, Y., Xu, Q. (2016). Plastids and Carotenoid Accumulation. In: Stange, C. (eds) Carotenoids in Nature. Subcellular Biochemistry, vol 79. Springer, Cham. https://doi.org/10.1007/978-3-319-39126-7_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-39126-7_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-39124-3
Online ISBN: 978-3-319-39126-7
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)