Abstract
The phosphatidylinositol 3-kinase (PI3K) pathway is frequently activated in human cancer and aberrant activation promotes transformation. Major efforts are currently being aimed at pharmacologically targeting the pathway for cancer therapy. Early results have yielded more limited success than has been seen for other selective inhibitors of mutant oncoprotein drivers. In this chapter, we review the biological reasons that may account for such intrinsic and acquired resistance to PI3K-targeted therapy. Mechanisms of resistance commonly seen in models include: (1) relief of negative feedback regulatory programs resulting in induction of upstream pathway signaling, which counteracts drug action and attenuates efficacy and (2) coincident activation of alternate growth signaling pathways. The understanding of how tumors with oncogenic PI3K pathway activation circumvent pharmacologic inhibition suggests that combination therapy will be necessary in many contexts in order to see durable responses. Indeed, more recent clinical efforts testing PI3K-directed therapy have been designed under the assumption that up front combination therapy will be necessary for efficacy. These clinical trials are increasingly employing state-of-the-art correlative analyses including next generation sequencing to determine predictors of sensitivity. Clinical data paired with these correlative analyses will be indispensible in determining the optimal agents, combinations, and genotypes to treat.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Introduction
The phosphatidylinositol 3-kinase (PI3K) pathway, which includes the PI3K holoenzyme and the downstream effector kinases, AKT and the mammalian target of rapamycin (mTOR), is critical for cancer cell growth, proliferation, and survival. Aberrant activation of this pathway promotes transformation [1] and is frequently observed through various mechanisms including activation of upstream receptor tyrosine kinases (e.g., HER2 amplification), activating mutations in pathway components (e.g., accessory and kinase domain mutations in PIK3CA) and loss of function of regulatory components (e.g., inactivation of PTEN). Among the PI3Kinases, the class I family has been most implicated in cancer. Class IA PI3K is composed of a catalytic (p110) and a regulatory (p85) subunit.
PIK3CA, the gene which encodes the alpha-isoform of p110, is among the most frequently mutated genes in human cancer. The finding of frequent activation of this pathway and demonstration of its key functions in transformation and tumor maintenance has spawned an enormous effort to pharmacologically target the pathway for cancer therapy. For the earliest generation of inhibitors, a significant disconnect was observed between antitumor effects in laboratory models and clinical benefit. This was due in part to the lack of selectivity and poor pharmacologic properties of several of these drugs. More recently, highly selective and potent inhibitors of class I PI3Ks, AKT, and mTOR have been developed. While these drugs have not overcome all issues related to effective inhibition of the target kinase, they have allowed a more detailed study of biologic reasons for intrinsic and acquired resistance to inhibition of the pathway in tumors where the pathway is aberrantly activated. The present chapter aims to summarize our current understanding of key biologic mechanisms of resistance to PI3K–AKT–mTOR inhibition in tumors featuring genetic activation of the pathway.
The PI3K–AKT–mTOR Pathway
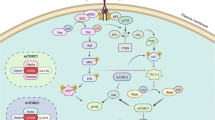
A simplified cartoon illustration of key members of the PI3K pathway is presented in Fig. 5.1 and provides a framework for understanding some of the relationships of the components. PI3K is a cytoplasmic lipid kinase activated by membrane-bound receptor tyrosine kinases (RTKs) such as HER3 and IGFR1 often via SH2 domain containing docking molecules such as IRS1. The lipid kinase activity of PI3K is induced upon binding of the regulatory subunit (p85) to the receptor complex, relieving negative interactions with the kinase domain containing subunit (p110). Upon activation of PI3K, PIP2 is phosphorylated to generate the second messenger PIP3. Phosphatase and tensin homolog (PTEN) is a phosphatase, dephosphorylating PIP3 to PIP2 to inhibit signaling through the PI3K pathway. PIP3 binds to the pleckstrin homology domain (PHD) of AKT, thereby recruiting this downstream effector to the membrane. Interaction with PIP3 provides access to key residues, T308 and S473, which can then be phosphorylated by the PDK1 and mTORC2 kinases respectively. These phosphorylations facilitate the full activation of the Ser/Thr directed AKT kinase toward its many substrates. Among its substrates are several members that can promote activation of the mTOR kinase. Activated AKT directly phosphorylates PRAS40, which otherwise binds to and inhibits mTORC1. AKT is also shown to phosphorylate TSC2, which otherwise negatively regulates the RHEB GTPase that activates mTORC1. Finally, AKT has been shown to directly phosphorylate and promote mTOR activity. Thus, PIP3-mediated activation of AKT provides multiple routes for activation of mTORC1. Downstream effectors of mTORC1, including the ribosomal protein S6 kinase 1 (S6K1) and the translational repressor protein eukaryotic initiation factor 4E (elF-4E) binding protein 1 (4EBP1), are involved in control of cell size and protein translation among other processes key for cell proliferation.
The PI3K-Akt-mTOR pathway. Key components of the PI3K pathway are depicted here. Upon activation, for example by dimerization, membrane-bound RTKs activate PI3K by binding to the PI3K regulatory subunit, p85. This relieves inhibitory interactions with the kinase domain subunit, p110 and results in PI3K activation. PI3K phosphorylates PIP2 to generate PIP3. PTEN serves as a phosphatase, dephosphorylating PIP3 to PIP2 to inhibit signaling through the PI3K pathway. PIP3 binds to the pleckstrin homology domain of AKT, recruiting this effector to the membrane. AKT is phosphorylated by PDK1 and mTORC2 at T308 and S473, respectively, resulting in full AKT activation. AKT activation results in mTORC1 activation, and cell survival and proliferation ultimately results
The pathway as schematized depicts a relatively simple set of processive steps to allow signal transmission. However, physiologic activation, as with all signaling systems, is expected to involve numerous components whose function is to attenuate or downregulate the signal. Such negative feedback regulation is pervasive and selected for in complex systems [2] (see Fig. 5.2). In tumors, the pathway is hyperactivated through mutational events. We and others have observed that the negative feedback is similarly hyperactivated in most of these systems [3–6]. This presents a potential hurdle to nearly any drug designed to inhibit oncoprotein activated signaling. Inhibition of the single node will result in activation of upstream signaling through the loss of this hyperactivated negative feedback. This set of newly active signals represents a means for the cancer cell to adapt to the drug and ultimately resist its effects. This form of resistance appears to play a major role in modulating the effects of PI3K-directed therapies.
Growth factor activation of signaling and negative feedback. The first panel depicts major elements of the EGFR signaling transduction apparatus in a disassembled state in the absence of growth factor stimulation. In the second panel, addition of growth factor ligand triggers conformational change, dimerization, and transphosphorylation of the receptor. Binding of adaptor proteins follows, resulting in activation of kinase cascades, and stimulation of cellular programs involved in transformation (cell cycle progression, evasion from apoptosis, motility and invasion, increases in cell size, stimulation of protein translation). In the third panel, negative feedback programs are depicted including FOXO mediated repression of expression of receptor tyrosine kinases such as HER3, and mTOR mediated destabilization of the IRS1 adaptor protein via S6K activation
Drug-Induced Relief of Feedback Results in Pathway Reactivation
mTORC1 Inhibition Promotes AKT Activation
During physiologic activation such as IGF stimulation, mTORC1 phosphorylates and activates S6K1. Phosphorylated S6K1 in turn phosphorylates the insulin receptor substrate-1 (IRS1) adaptor protein, inducing its degradation and thereby allowing signal attenuation [7–9] (see Fig. 5.3). O’Reilly et al. examined the significance of this negative feedback pathway in tumors, studying the effect of the allosteric mTORC1 inhibitor, rapamycin on upstream signaling. They demonstrated that treatment of PI3K-hyperactivated cell lines with rapamycin caused an increase in S473 phosphorylation and AKT kinase activity [8]. They further demonstrated that this AKT kinase activation was associated with increased phosphorylation of endogenous AKT substrates including the FoxO1a, FoxO3a, and FoxO4 transcription factors, confirming functional activation of AKT kinase by mTOR inhibition. Using quantitative immunohistochemical assessments of paired pretreatment and on-treatment tumor biopsies, increased pAKT following treatment with the mTOR inhibitor everolimus was confirmed in patients. Furthermore, using a human monoclonal inhibitory antibody to the IGF1R, they demonstrated the significance of this induction of AKT activity. They showed that in cells dependent on IGF1R for proliferation, the induction of AKT activity was also IGF1R dependent and IGF1R inhibition could sensitize cells to the antiproliferative effects of rapamycin, underscoring the relevance of the feedback induction of PI3K/AKT signaling. These findings were corroborated in a study by Shi et al. in a myeloma model where rapamycin resulted in enhanced AKT activity, phosphorylation, and PI3K activity that were IGF1R dependent [9]. Li et al. proposed an alternative mechanism for rapamycin-induced AKT phosphorylation in addition to feedback mediated alterations in IRS1 and GRB10 adaptors [10]. Utilizing small molecule inhibitors, viral inactivators, and siRNA cell-based models, they demonstrated that rapamycin treatment was associated with inhibition of protein phosphatase 2A (PP2A) and this caused reduced activation of DNA-dependent protein kinase (DNA-PK), and consequently reduced phosphorylation of AKT. While mTORC2 is thought to be the predominant kinase for AKT S473 phosphorylation, DNA-PK might also play a role in certain contexts and thus this represents yet another means by which mTOR ultimately feedback regulates AKT signaling.
Negative feedback regulation of PI3K/AKT/mTOR signaling. Depicted in the top panel is negative feedback regulation of PI3K/AKT/mTOR signaling through two major pathways from mTOR and AKT. mTOR regulates adaptor proteins such as IRS1 while AKT regulates the expression of receptor tyrosine kinases (RTK) through the FOXO transcription factors. The consequence of drug inhibition of AKT is shown in the bottom left panel with inhibition of AKT causing loss of negative feedback on RTK expression and so inducing RTK expression. In addition, AKT in many cells activates mTOR and so drug inhibition of AKT leads to inhibition of mTOR leading to loss of negative feedback on IRS1. The sum consequence of AKT inhibition is to activate RTK function through adaptors and increases in RTK expression. Depicted in the right panel is mTORC1 inhibition with rapamycin which predominantly impacts RTK function through the effects on adaptor proteins without the effects on RTK expression
The clinical significance of this finding has been further highlighted in recent years. In a phase I study of single-agent everolimus in patients with advanced solid tumors, immunohistochemical analysis of paired pre-therapy and on-therapy tumor and skin biopsies demonstrated that while downstream markers of mTOR inhibition including pS6 and p4EBP1 were reduced with everolimus, there was an overall statistically significant increase in AKT phosphorylation in tumors and in skin. This increase in pAKT was not attributable to changes in protein expression [11]. A neoadjuvant study of everolimus in PTEN-deficient glioblastoma similarly showed that rapamycin treatment resulted in AKT activation in seven of ten treated patients, and this activation was significantly associated with shorter time to progression during postsurgical maintenance rapamycin therapy [12], evidencing the clinical relevance of this inhibition-induced relief of negative feedback.
mTORC1 Inhibition Relieves GRB10-Mediated PI3K Suppression
In addition to the effect of S6K on IRS1 stability, two simultaneously published reports demonstrated an additional link between mTOR activation and PI3K activity via the GRB10 adaptor protein [13, 14]. The groups found that GRB10 was directly phosphorylated by mTORC1 and thereby promoted its stability. GRB10 functions as a negative regulator of PI3K activity and thus, similar net results would be expected as with IRS1 with mTORC1 inhibition resulting in PI3K activation. These studies illustrate the complexity of feedback regulation of signaling in having redundant means of downregulating the same node.
mTOR Kinase Inhibition Causes Feedback-Dependent Biphasic Regulation of AKT Signaling
Evidence discussed above demonstrates that mTORC1 inhibition causes relief of negative feedback mechanisms and thus an increase in upstream PI3K signaling [8, 9, 11–14]. In the presence of mTORC1 inhibition, this signaling promotes a mTORC2-dependent increase in AKT, with accordant attenuation of therapeutic effects. Therefore, Rodrik-Outmezguine and colleagues studied an mTOR kinase inhibitor which, by binding to the ATP pocket of mTOR, blocks both mTORC1 and mTORC2 [15]. They found that mTOR kinase inhibition blocked mTORC2-mediated phosphorylation of AKT-S473 leading to destabilization of AKT-T308 phosphorylation in cell lines. However, surprisingly, the effect on T308 was transient. Levels of T308 phosphorylation increased within several hours of drug treatment even while S473 phosphorylation remained suppressed. As a result of T308 phosphorylation, AKT activity was induced as evidenced by phosphorylation of its downstream products including FOXO. This effect was not dependent on drug concentration and occurred despite potent mTORC1 inhibition. The basis for these effects appeared to again return to the concepts of relief of feedback. The reactivation of T308 was shown to be coincident and dependent on an induction of RTK signaling.
Active site mTOR kinase inhibitors, initially designed to inhibit mTORC2 in addition to mTORC1, were found to inhibit phosphorylation of 4EBP more completely than the mTORC1 inhibitor rapamycin [16]. Thus, the ATP competitive inhibitors of mTOR appear to be more potent inhibitors of mTORC1 complex than rapalogs. In addition to inhibition-induced relief of negative feedback demonstrated by AKT upregulation, the limited efficacy of rapamycin may in part be due to inherent pharmacologic limitations of FKBP12-dependent allosteric inhibition.
AKT Feedback Regulation of RTK Expression
To understand mechanistically how the PI3K pathway feedback regulates upstream signaling, Chandarlapaty et al. examined the effects of selective AKT and mTORC1 inhibition on RTK signaling [17]. They found that AKT inhibition, but not mTORC1 inhibition, induced the RNA expression of a conserved set of RTKs, including HER3, IGF1R, and insulin receptor, all of which are well-known direct activators of PI3K. The link between RTK expression and AKT activation was found in the FOXO transcription factors that are direct AKT substrates thus explaining the specificity of the effect for AKT and not mTORC1 inhibition (see Fig. 5.3). The induction of RTK expression was shown to have major implications for the tumor cells as monotherapy with the AKT inhibitor was shown to only cause tumor stasis or tumor growth delay, whereas combined inhibition of AKT and the feedback induced RTKs promoted tumor regressions. It was expected and indeed observed that direct inhibitors of PI3K, to the extent that they result in AKT inhibition, also result in a similar induction of RTK expression [17, 18]. The details of the survival signals provided by the induced RTKs are still being examined. The authors show or imply that the induced receptors can both activate other pathways like the RAF/MEK/ERK pathway or simply reactivate the PI3K/AKT pathway and in effect make the drug less potent. The implications of these results are profound and have led to several studies examining combination therapy to more effectively antagonize activated PI3K signaling in tumors. Indeed, such combined targeting has met with early clinical success [19, 20].
Drug-Induced Adaptive/Compensatory Activation of Parallel Signaling Pathways in the Network
Activation of ERK Signaling
Many of the signaling components upstream of PI3K are multivalent and can activate other signaling pathways upon induction. For instance, receptors like IGF1R and HER3 bind to adaptor proteins that can then activate RAS signaling. RAS itself is able to activate both PI3K and ERK signaling. Given the findings that upstream signaling is induced by inhibitors of the PI3K pathway, it was of interest to determine whether ERK signaling was altered by any of these drugs, in what contexts, and to what biologic impact. Among the first studies, Carracedo and colleagues reported their finding of activation of ERK signaling in response to mTORC1 inhibition and demonstrated a benefit in antitumor activity of combined blockade of mTORC1 and MAPK signaling in vitro and in vivo [21]. The authors established the dependency for the induction of MAPK by RAD001 (everolimus) on the activity of both PI3K and RAS as inhibitors of PI3K and dominant negative RAS could abrogate the effects. The data suggested a role for the known S6K-IRS feedback mechanism as this serves as one route (among several) to activate PI3K. Importantly, the authors examined paired tumor biopsies from patients receiving RAD001 in the Phase I setting and corroborated their results showing a marked induction of pERK in the on-treatment biopsies compared to the pretreatment. These data confirmed the clinical relevance of the findings and led them to investigate combined MEK and mTORC1 inhibition and find a marked increase in apoptosis for the combination.
More recently, as several groups have identified profound effects of inhibition of mTOR and AKT on RTK signaling, the consequences of this on the RAS pathway have been investigated. In agreement with the findings by Carracedo, inhibition of both mTOR and AKT in tumors caused a marked upregulation in ERK signaling [15, 17, 22]. In cancer models featuring ErbB signaling activation, the induction was shown to be dependent on EGFR/HER2 as kinase inhibitors could prevent the induction and, like MEK inhibitors, promote apoptosis when given in combination. Taken together, the data do not point to a singular mechanism whereby inhibitors of AKT/mTOR induce ERK signaling, but suggest multiple routes of feedback activation of RTK signaling that could ultimately induce ERK and promote resistance. The data on the effects of direct PI3K inhibitors on the ERK pathway appear to be less clear as some studies have pointed to the requirement for PI3K activity for the ERK induction while others have pointed to the opposite with inhibitors of PI3K causing the ERK induction. A more detailed study using selective PI3K inhibitors will be needed to fully establish the relationship between PI3K activity and ERK activation.
Coordinated Activation of RAS/RAF Signaling in Resistance
As noted earlier, alterations in the PI3K pathway are frequently coincident with lesions that alter the RAS/RAF/MEK/ERK pathway. The specific aspects of transformation that activation of each pathway confers vary considerably based on cell lineage and type of alteration. We and others have observed that in one RAS driven model, MEK inhibition alone may be sufficient to impair tumor growth and even cause regressions, while in another, PI3K pathway inhibition is necessary for full antitumor effects [23]. A general conclusion from these studies is that in many tumor types featuring concurrent RAS or BRAF activation with PI3K pathway alteration, PI3K pathway inhibition alone is insufficient to cause tumor regressions. In most cases, coordinate downregulation of both pathways is necessary but in select ones only one pathway is essential. It has been more commonly observed that such tumors are dominantly dependent on the RAS/RAF/MEK/ERK pathway for survival and thus it has often been considered that PI3K activation serves as a marker of resistance to RAF or MEK inhibition [23]. Conversely, Kras activation may serve as a marker for resistance to PI3K inhibition. In a mouse model of Kras-driven lung tumors, PI3K inhibition did not cause tumor regression despite effective PI3K inhibition [24]. Consistent with this, De Nicolantonio and colleagues demonstrated that KRAS mutations are a negative prognostic marker for sensitivity to mTORC1 inhibition with rapalogs [25]. The authors studied a panel of cell lines derived from multiple disease types including glioblastoma and carcinomas of the breast, ovary, prostate, colon, and uterus known to have PIK3CA or PTEN genetic alterations. They found that everolimus-resistant tumor cells displayed mutations in both PIK3CA and KRAS/BRAF, while everolimus-sensitive cell lines had PI3K pathway mutations but wild-type KRAS/BRAF. Examination of the sensitivity of isogenic cells was consistent with this result with RAS/BRAF mutant models being resistant to rapamycin.
In human tumor xenografts, Ihle and colleagues observed that RAS mutations were associated with marked resistance to PI3K inhibition [26]. Going further, this group examined PIK3CA, KRAS and BRAF mutations in a cohort of cancer patients who had received single-agent everolimus as part of single-institution phase I and phase II studies. They demonstrated with statistical significance that cancer patients whose tumors harbored PIK3CA kinase domain mutations or PTEN loss of function could benefit from everolimus treatment, except in the presence of concomitant KRAS/BRAF mutations. Furthermore, KRAS mutations negatively and significantly affected clinical benefit of everolimus when examined in univariate analysis.
Overall, the above studies seem to corroborate an assumption that the main set of signals downstream of mutant KRAS and BRAF is the MEK/ERK pathway and thus targeted inhibition of PI3K pathway components alone is unlikely to be effective for most of these tumors.
Feedback Regulation of Nuclear Hormone Signaling
For many years it has been known that breast and prostate cancers characterized by nuclear hormone dependence demonstrate coincident mutational activation of the PI3K pathway [27–30]. This finding led to investigations into how the pathways might participate in cross talk. A major question Carver and colleagues sought to understand was how activation of the PI3K pathway affected androgen receptor (AR) signaling. To study this type of crosstalk, they utilized murine and cell line models as well as analyses of human prostate cancers [31]. They focused attention on tumors characterized by activation of the PI3K pathway via loss of PTEN that is frequently seen in castrate resistant prostate cancer.
An analysis of one hundred and six primary prostate tumor specimens for gene expression showed that a signature of AR activation was highly enriched in those tumors with wild-type PTEN status compared to those with PTEN deficiency. Then, examining the effect of acute pathway inhibition using PI3K pathway directed drugs, they found the inverse held as inactivation of PI3K signaling in laboratory models of prostate cancer resulted in increases in AR and AR target gene expression. Given the findings on how PI3K and AKT feedback regulate RTK expression, they examined the role of RTK induction in this phenomenon and observed that increases in RTK activity were necessary for the induction in AR signaling. The mechanisms through which the induced RTKs altered AR activity are yet to be fully elucidated, but it has long been known that activation of ErbB signaling can hyperstimulate AR signaling [32]. Interestingly, the authors further show that there is feedback in the opposite direction as well with inhibition of AR signaling causing an activation of PI3K signaling through an increase in AKT activity [31]. These data provide a strong logic for testing of combinations of inhibitors of the PI3K pathway with AR antagonists that are now under way.
In further support of these ideas, the estrogen receptor (ER) seems to show similar patterns in its relationship to PI3K signaling in breast cancer. Creighton and colleagues used gene expression and proteomic profiling data to create molecular signatures of PI3K activity and found an inverse correlation between PI3K activity and ER protein expression [33]. In breast cancer cell lines, PI3K pathway stimulation resulted in reduction in ER mRNA in a dose-responsive manner, and pathway inhibition increased expression of ER and ER-inducible target genes. Similar to the study by Carver and colleagues [31], this study suggested a role for the PI3K pathway in escape from endocrine therapy in ER driven disease again supporting combination therapy currently under clinical testing.
Wnt-β-Catenin Cooperation with PI3K Signaling
One of the major downstream effects of activated PI3K signaling is to suppress the FOXO transcription factors that play key roles in cell cycle control and survival. The FOXO family of transcription factors has additionally been implicated in Wnt-β-catenin signaling as β-catenin interacts with the FOXO family of transcription factors and acts as a transcriptional co-activator, enhancing expression of FOXO-target genes [34].
Tenbaum and colleagues investigated the physiologic significance of this Wnt and PI3K pathway interaction in colorectal tumor maintenance and progression [35]. They found that when β-catenin and FOXO co-localized in the nucleus through Wnt activation and PI3K pathway inhibition, respectively, metastatic outgrowth was induced. They demonstrated that activation of β-catenin conferred resistance to AKT inhibitor-induced apoptosis. Moreover, they demonstrated that activation of β-catenin or inhibition of AKT had minimal effect on finding metastasis, but together sharply increased the frequency of metastases from multiple organ sites. Moreover, the authors demonstrated that reduction of β-catenin content could potentially reverse this form of resistance by restoring the apoptotic response to PI3K/AKT inhibition. This study has clear implications for the potential efficacy of PI3K/AKT inhibitors in tumors with activated Wnt signaling and the phenomenon will be followed closely as drugs targeting the PI3K pathways are examined in PI3K activated tumors.
Myc Amplification
Myc amplification is among the most common alterations promoting tumor formation in all of cancer and has been specifically identified in many of the same malignancies that also feature PI3K activation. Liu et al. examined the question of mechanisms of resistance to PI3K utilizing a transgenic murine models of PIK3CA driven mammary tumors [36]. In this model, enforced overexpression of mutant PIK3CA resulted in tumor formation. They examined resistance by taking established tumors and removing mutant expression by doxycycline withdrawal. This was shown to cause sustained tumor regression followed by approximately two-thirds of tumors resuming growth in the absence of mutant PIK3CA expression. The tumors were analyzed and showed Myc amplification in 2 of the tumors and concurrent c-Met overexpression in another set of tumors. The Myc-amplified tumors were shown to be Myc dependent using knockdown and PI3K-independent using pharmacologic studies.
In further support, indirect data from a synthetic lethal screen identified NOTCH pathway activation with consequent Myc activation as potentially of relevance in PI3K pathway inhibitor resistance [37]. In addition, activation of Myc was further shown by a group in response to rapalogs through an effect of inducing PDK activity [38]. The relevance of these different routes of activating Myc in PI3K activated and driven tumors has yet to be fully ascertained in human tumors but represent important areas for investigation among patients being treated with PI3K directed therapies. Specifically, looking for Myc concurrent amplification and examining tumoral Myc levels on drug would be of great interest.
JAK2/STAT5 Inhibition Circumvents PI3K Resistance
The Janus family of kinases (JAKs) and the associated signal transducers and activators of transcription factors (STATs) are well-described inducers of oncogenic phenotypes in numerous lineages. JAK kinases are activated upon binding of ligands (including hormones and cytokines) to their receptors, or by mutational activation, as in the JAK2 V617F mutation in myeloproliferative disorders. In malignant states, activating ligands can be secreted by cancer cells and/or may be present in the tumor microenvironment. There is particular interest in the possible roles of activated JAK/STAT signaling in inflammatory and aggressive triple negative breast cancers which also feature activation of the PI3K pathway via loss of PTEN. Britschgi et al. investigated the effects of PI3K pathway inhibition on JAK/STAT signaling in triple-negative breast cancer [39]. In cell line and murine models, they identified upregulation of JAK2 and STAT5 in response to both PI3K and mTOR inhibitors. The basis for this induction was found to involve both the already described feedback induction of IGF1R signaling and its interaction with JAK/STAT signaling as well a secondary mechanism involving upregulation of IL8-CXCR1. They determined the significance of this upregulation utilizing the JAK2 selective inhibitor, NVP-BSK805 and showed this promoted apoptosis when given in combination with the PI3K pathway inhibitor. This ultimately translated to improved survival for murine models administered the combination over the single agents.
Additional Implicated Mechanisms of PI3K Resistance, Mechanisms not yet Elucidated
mTORC1 Inhibition Is Required for Sensitivity to PI3K-Alpha Inhibition
Many of the mechanisms described above involve induction of upstream or parallel signaling pathways that ultimately cause activation of new oncogenic signals, while a few involve pathway reactivation. The latter type of phenomenon has been more commonly implicated in resistance to targeted therapy of cancer and consistent with this was a recent report demonstrating that mTORC1 inhibition is required for sensitivity to PI3K p110α inhibitors in a panel of PIK3CA-mutant breast cancer cell lines [40]. In this report, the authors examined biomarkers of sensitivity to PI3K alpha inhibition among PIK3CA mutant cell lines. They identified degree of inhibition of phosphorylation of residues 240/4 of S6 as the best indicator of sensitivity to BYL719, a highly selective PI3K alpha inhibitor. Among the sensitive models, resistance to PI3K inhibition was induced by mTORC1 activation, while among the resistant models, increased apoptosis was seen in cells treated with combination mTORC1 and p110α inhibition. Most provocatively, the authors studied paired tumor biopsies of patients treated with a p110α inhibitor on a phase I clinical trial and found that the magnitude of inhibition of S6 phosphorylation correlated with clinical response, and that reactivation of pS6 correlated with development of acquired resistance. This study emphasized the critical role of mTORC1 inhibition in conferring and maintaining sensitivity to PI3K pathway inhibitors and suggested the possibility that addition of rapamycin to selective PI3K inhibitors may be rational while also raising questions as to what factors cause uncoupling of mTORC1 from PIK3CA.
Inactivating PTEN Mutations Result in PI3K-Beta Hyperactivation
As mentioned previously, PTEN is a tumor suppressor that negatively regulates the PI3K pathway by dephosphorylating PIP3 to PIP2 as well as serving as a phosphatase for IRS-1 [41], thereby inhibiting AKT activation and downstream pathway activation. Loss of function mutations in PTEN comprise a known mechanism of constitutive PI3K pathway activation. Interestingly, PTEN deficient cancers have been previously shown to depend largely on pathway activation via the PI3K beta isoform [42–44]. It logically follows that inactivating mutations in PTEN may serve as a mechanism of intrinsic and acquired resistance to PI3K inhibitors that selectively antagonize PI3K alpha. Indeed emerging data is consistent with this [45]. Castel, Baselga and colleagues sequenced the DNA of multiple metastatic tumors from a patient with a PIK3CA mutant breast tumor who initially responded to an alpha-selective PI3K inhibitor with subsequent progression of disease in several metastatic sites. Unlike the pretreatment tumor sample, PTEN loss and a missense mutation were identified in multiple progressing metastases, while a periaortic lesion that was responding at the time of death did not show evidence of PTEN loss. The authors went on to generate a patient derived xenograft from one of the non-responding sites and showed that addition of a beta-selective PI3K inhibitor to the alpha-selective inhibitor could cause tumor growth inhibition unlike the alpha-selective inhibitor alone. These data powerfully illustrate the dynamic nature of therapeutic resistance and argue for genomic evaluation of tissue at progression as an invaluable correlate of ongoing clinical trials.
Mechanisms of Resistance Common to Cellular Signaling Pathways
Ligand-Mediated Activation of Distinct, Non-inhibited Kinases with Shared Downstream Targets
Wilson et al. discussed a broadly relevant mechanism of resistance to inhibitors of growth factor-driven kinases. They described that in the presence of an inhibitor to a specific kinase, RTK ligands are able to mediate activation of distinct, non-inhibited kinases that share critical downstream targets with the kinase being inhibited [46]. To demonstrate this, the authors exposed kinase-“addicted” and inhibited human cancer cell lines to various, widely expressed RTK ligands including hepatocyte growth factor (HGF) and epidermal growth factor (EGF). They found that exposure of these kinase-addicted and inhibited cells to ligands could “rescue” them from the effect of kinase inhibition. Co-targeting the secondary activated kinase blocked ligand rescue, whereas targeting the secondary activated kinases alone did not impact cell proliferation. These findings indicated that the “rescue” effect seen was in fact associated with ligand-mediated activation of other RTKs which stimulated redundant, pro-survival signaling pathways, on which tumor cells became reliant in the setting of inhibition of a primary growth pathway. Additionally, the RTK profile of tumor cells prior to treatment correlated with ligand-induced rescue, generating the hypothesis that the RTK profile of tumors prior to treatment could have relevance in determining optimal therapeutic combinations in a given patient.
As a specific example of this phenomenon, the authors demonstrated that HGF conferred resistance to the BRAF inhibitor vemurafenib in BRAF-mutant melanoma cells. This effect correlated with MET expression, was delayed by concomitant MET inhibition, and involved MAPK reactivation. After xenograft confirmation, the authors next investigated these findings in a clinical context. Pretreatment HGF levels were assessed in 126 patients with BRAF-mutant metastatic melanoma treated with vemurafenib on the BRIM2 clinical trial. Plasma HGF levels correlated with outcome in a continuous manner, and suggested that HGF may have a role in the response to BRAF inhibition in patients with this disease. Overall, this concept of ligand-mediated resistance fits in well with the finding of the signaling network being adapted to the inhibitor by negative feedback and thus very susceptible to reactivation by such mechanisms. Indeed, this idea that the network has increased “signalability,” or transduction of signals from activated RTKs, was further demonstrated by Lito et al. again examining BRAF inhibition in melanoma [3]. They demonstrated that in BRAF-mutant melanomas, high levels of ERK-dependent feedback reduce signaling from activated RTKs, but that ERK inhibition relieves this feedback and as an adaptation to inhibition, enhances the ability of ligands to activate signaling. This resultant ligand-mediated signaling may attenuate the antitumor effects of the inhibitor.
Elkabets et al. examined the role of ligands in resistance to PI3K-alpha inhibition more specifically. They used a high-throughput platform to screen for secreted ligands that opposed antiproliferative effects of a p110α inhibitor in otherwise sensitive cell lines [40]. They identified insulin-like growth factor 1 (IGF1) and neuregulin 1 (NRG1) as ligands which were able to reverse the effects of the p110α inhibitor and promote growth. They then validated these findings through the exogenous supplementation of IGF1 and NRG1 to PIK3CA-mutant cell lines treated with p110α inhibition. They found that with supplementation of IGF1 and NRG1 to these cell lines, an increase in downstream PI3K pathway effectors, including pS6, was seen. This effect was reversed by the addition of an mTORC1 inhibitor, suggesting that the resistance-conferring ligands activated mTORC1 in a p110α-independent manner, again emphasizing the importance of redundancy and feedback within signaling networks.
Tumor Microenvironment May Confer Targeted Therapy Resistance
Although the majority of studies investigating targeted therapy resistance focus on mechanisms of resistance relating to molecular events within a cell, Straussman and colleagues described the significance of the tumor microenvironment [47]. The authors developed a coculture system utilizing green fluorescent protein (GFP)-labeled tumor cells in culture with stromal cells. When they examined interactions between cancer cells, stromal cells and anticancer drugs, they found that drugs that induce tumor apoptosis are sometimes rendered ineffective in the presence of stromal cells. Furthermore, this effect was significantly more evident when the drugs used were targeted agents as opposed to cytotoxic chemotherapy. In further support of the importance of ligand activation of signaling, they showed that stromal cell secretion of HGF resulted in activation of the HGF receptor MET, MAPK, and PI3K signaling, and resistance to RAF inhibition. This resistance to RAF inhibition correlated with HGF expression. Dual inhibition, either with RAF and HGF neutralizing antibodies, or RAF and MET inhibitors, was able to overcome this drug resistance. Although there was not a specific discussion of PI3K inhibitor resistance, this study generates an important and understudied concept relating to targeted therapy resistance: elements, such as secreted ligands, of a tumor microenvironment can confer therapy resistance. A fuller understanding the mechanisms behind specific resistance-conferring microenvironments may inform rational drug combinations.
Drug-Resistance-Conferring Oncogene Alterations
Intrinsic and acquired point mutations in protein kinase inhibitors have been noted to confer resistance to targeted therapy, for example, as in the case of the EGFR T790M mutation conferring resistance to erlotinib. Zunder and colleagues studied whether or not point mutations in PIK3CA confer resistance to PI3K inhibitors [48]. They used an Saccharomyces cerevisiae screen, comprised of mutagenized residues lining the affinity pocket, and multiple PI3K inhibitors. A potential hotspot for resistance mutations, I800, was identified with two potential resistance-conferring mutations: I800L and I800M. However, resistance mutations at the “gatekeeper” residue, which controls access to a large hydrophobic pocket in which most kinase inhibitors bind, were not identified. Following the publication of this study in 2008, to our knowledge, no definitive literature has highlighted the clinical importance of inhibitor resistance-conferring mutations though assessing for resistance mutations has not either been a major component of correlative studies accompanying the clinical investigation of PI3K pathway inhibitors.
Huw and colleagues examined PIK3CA mutant, HER2-amplified cell pools and single cell clones that were able to grow in the presence of high concentrations of the pan-class I PI3K inhibitor GDC-0941 [49]. Using genome-wide copy number analyses they found high-level amplification of the PIK3CA locus. Knockdown of PIK3CA in the resistant cells decreased pathway activation and restored sensitivity to PI3K inhibitor, confirming that resistance to PI3K inhibition in these cells was likely due to this specific amplification event. Whether overexpression or amplification of PI3K mediates resistance in patients is unknown, however it has been previously seen with resistance to other kinase inhibitors such as in the case of BRAF amplification in BRAF V600E mutant tumors [50].
Conclusion and Future Directions
The frequent deregulation of the PI3K signaling pathway in human malignancy reflects its crucial role in cell growth, survival and proliferation. Despite the strong scientific rationale supporting therapeutic inhibition of components of this pathway, early studies on pharmacologic agents targeting this pathway among patients with activated tumors have met with less success than has been seen for other selective inhibitors of mutant oncoprotein drivers. Above, we have reviewed the substantial and expanding body of literature investigating reasons why this might be the case. First, it is evident that under physiologic conditions, the PI3K pathway is regulated by numerous, homeostatic negative feedback loops; under conditions of oncogenic activation, negative feedback is similarly hyperactivated. Pharmacologic inhibition as a therapy for such cancers relieves this hyperactive feedback and thus results in activation of upstream signaling. The consequence of this induced signaling is that it can transmit oncogenic signals and thus promote drug resistance. The two major ways by which this can occur is through (1) reactivation of the same signaling network—that is, making the drug less effective at inhibiting its target, and (2) induction of other signaling network that deliver new oncogenic signals. These feedback signals make the case for combination therapy directed specifically at the most relevant/dominant feedback pathway. Complicating such efforts is the layers of feedback present and thus the difficulty of determining which is most important to target.
A second and related means of resistance relates to coincident alternate pathway activation. Tumors may harbor hits in both the PI3K pathway and, for instance, the RAS/RAF pathway and thus have a more limited dependence on either alone. This similarly argues for genotype-directed combination therapy. Complicating these efforts has been the challenge of trying to combine inhibitors of such fundamental cell signaling pathways; this ultimately results in compromises in drug dosing which likely limit efficacy. Despite these challenges, the need for such complexity in therapeutic decision-making is obvious. The PI3K pathway is a key driver of transformation, tumor maintenance, and tumor progression in a large fraction of cancers. PI3K pathway inhibitors alone are unlikely to be sufficiently effective or to produce sustained responses, but combinations do hold the possibility of durable efficacy in the metastatic setting and curative therapy in the adjuvant setting. Ongoing preclinical and clinical efforts are thus being aimed at detecting, anticipating, and therapeutically manipulating pathways that promote PI3K inhibitor resistance [20, 51] (Table 5.1). Currently, the majority of clinical trials examining pharmacologic PI3K inhibition involve not only a PI3K inhibitor, but also chemotherapeutic, targeted, or endocrine therapy. Correlative analyses of these trials are increasingly employing phosphoproteomic studies to identify biomarkers indicative of pathway inhibition and activation (Table 5.1). With the continued incorporation of next generation sequencing techniques, genetic predictors of therapeutic resistance and sensitivity are being increasingly explored as well. We thus anticipate a major landscape change in the coming years for incorporation of PI3K pathway inhibitors into clinical practice—we simply do not anticipate it to be typically as a single agent.
References
Vivanco I, Sawyers CL (2002) The phosphatidylinositol 3-kinase AKT pathway in human cancer. Nat Rev Cancer 2(7):489–501. doi:10.1038/nrc839
Chandarlapaty S (2012) Negative feedback and adaptive resistance to the targeted therapy of cancer. Cancer Discovery 2(4):311–319. doi:10.1158/2159-8290.CD-12-0018
Lito P, Pratilas CA, Joseph EW, Tadi M, Halilovic E, Zubrowski M, Huang A, Wong WL, Callahan MK, Merghoub T, Wolchok JD, de Stanchina E, Chandarlapaty S, Poulikakos PI, Fagin JA, Rosen N (2012) Relief of profound feedback inhibition of mitogenic signaling by RAF inhibitors attenuates their activity in BRAFV600E melanomas. Cancer Cell 22(5):668–682. doi:10.1016/j.ccr.2012.10.009
Montero-Conde C, Ruiz-Llorente S, Dominguez JM, Knauf JA, Viale A, Sherman EJ, Ryder M, Ghossein RA, Rosen N, Fagin JA (2013) Relief of feedback inhibition of HER3 transcription by RAF and MEK inhibitors attenuates their antitumor effects in BRAF-mutant thyroid carcinomas. Cancer Discovery 3(5):520–533. doi:10.1158/2159-8290.CD-12-0531
Pratilas CA, Taylor BS, Ye Q, Viale A, Sander C, Solit DB, Rosen N (2009) (V600E)BRAF is associated with disabled feedback inhibition of RAF-MEK signaling and elevated transcriptional output of the pathway. Proc Natl Acad Sci USA 106(11):4519–4524. doi:10.1073/pnas.0900780106
Ercan D, Xu C, Yanagita M, Monast CS, Pratilas CA, Montero J, Butaney M, Shimamura T, Sholl L, Ivanova EV, Tadi M, Rogers A, Repellin C, Capelletti M, Maertens O, Goetz EM, Letai A, Garraway LA, Lazzara MJ, Rosen N, Gray NS, Wong KK, Janne PA (2012) Reactivation of ERK signaling causes resistance to EGFR kinase inhibitors. Cancer Discovery 2(10):934–947. doi:10.1158/2159-8290.CD-12-0103
Haruta T, Uno T, Kawahara J, Takano A, Egawa K, Sharma PM, Olefsky JM, Kobayashi M (2000) A rapamycin-sensitive pathway down-regulates insulin signaling via phosphorylation and proteasomal degradation of insulin receptor substrate-1. Mol Endocrinol 14(6):783–794
O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL, Baselga J, Rosen N (2006) mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates AKT. Cancer Res 66(3):1500–1508. doi:10.1158/0008-5472.CAN-05-2925
Shi Y, Yan H, Frost P, Gera J, Lichtenstein A (2005) Mammalian target of rapamycin inhibitors activate the AKT kinase in multiple myeloma cells by up-regulating the insulin-like growth factor receptor/insulin receptor substrate-1/phosphatidylinositol 3-kinase cascade. Mol Cancer Ther 4(10):1533–1540. doi:10.1158/1535-7163.MCT-05-0068
Li Y, Wang X, Yue P, Tao H, Ramalingam SS, Owonikoko TK, Deng X, Wang Y, Fu H, Khuri FR, Sun SY (2013) Protein phosphatase 2A and DNA-dependent protein kinase are involved in mediating rapamycin-induced AKT phosphorylation. J Biol Chem 288(19):13215–13224. doi:10.1074/jbc.M113.463679
Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, Martinelli E, Ramon Y, Cajal S, Jones S, Vidal L, Shand N, Macarulla T, Ramos FJ, Dimitrijevic S, Zoellner U, Tang P, Stumm M, Lane HA, Lebwohl D, Baselga J (2008) Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. Journal of clinical oncology. Off J Am Soc Clin Oncol 26(10):1603–1610. doi:10.1200/JCO.2007.14.5482
Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown K, Dang J, Zhu S, Hsueh T, Chen Y, Wang W, Youngkin D, Liau L, Martin N, Becker D, Bergsneider M, Lai A, Green R, Oglesby T, Koleto M, Trent J, Horvath S, Mischel PS, Mellinghoff IK, Sawyers CL (2008) Antitumor activity of rapamycin in a Phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLoS Med 5(1):e8. doi:10.1371/journal.pmed.0050008
Hsu PP, Kang SA, Rameseder J, Zhang Y, Ottina KA, Lim D, Peterson TR, Choi Y, Gray NS, Yaffe MB, Marto JA, Sabatini DM (2011) The mTOR-regulated phosphoproteome reveals a mechanism of mTORC1-mediated inhibition of growth factor signaling. Science 332(6035):1317–1322. doi:10.1126/science.1199498
Yu Y, Yoon SO, Poulogiannis G, Yang Q, Ma XM, Villen J, Kubica N, Hoffman GR, Cantley LC, Gygi SP, Blenis J (2011) Phosphoproteomic analysis identifies GRB10 as an mTORC1 substrate that negatively regulates insulin signaling. Science 332(6035):1322–1326. doi:10.1126/science.1199484
Rodrik-Outmezguine VS, Chandarlapaty S, Pagano NC, Poulikakos PI, Scaltriti M, Moskatel E, Baselga J, Guichard S, Rosen N (2011) mTOR kinase inhibition causes feedback-dependent biphasic regulation of AKT signaling. Cancer Discovery 1(3):248–259. doi:10.1158/2159-8290.CD-11-0085
Feldman ME, Apsel B, Uotila A, Loewith R, Knight ZA, Ruggero D, Shokat KM (2009) Active-site inhibitors of mTOR target rapamycin-resistant outputs of mTORC1 and mTORC2. PLoS Biol 7(2):e38. doi:10.1371/journal.pbio.1000038
Chandarlapaty S, Sawai A, Scaltriti M, Rodrik-Outmezguine V, Grbovic-Huezo O, Serra V, Majumder PK, Baselga J, Rosen N (2011) AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 19(1):58–71. doi:10.1016/j.ccr.2010.10.031
Chakrabarty A, Sanchez V, Kuba MG, Rinehart C, Arteaga CL (2012) Feedback upregulation of HER3 (ErbB3) expression and activity attenuates antitumor effect of PI3K inhibitors. Proc Natl Acad Sci USA 109(8):2718–2723. doi:10.1073/pnas.1018001108
Gajria D, King T, Pannu H, Sakr R, Modi S, Drullinsky P, Syldor A, Patil S, Seidman A, Norton L, Rosen N, Hudis C, Chandarlapaty S (2012) Tolerability and efficacy of targeting both mTOR and HER2 signaling in trastuzumab-refractory HER2 + metastatic breast cancer. Cancer Res 72(24 Suppl 3). doi:10.1158/0008-5472.SABCS12-P5-18-04
Morrow PK, Wulf GM, Ensor J, Booser DJ, Moore JA, Flores PR, Xiong Y, Zhang S, Krop IE, Winer EP, Kindelberger DW, Coviello J, Sahin AA, Nunez R, Hortobagyi GN, Yu D, Esteva FJ (2011) Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol Off J Am Soc Clin Oncol 29(23):3126–3132. doi:10.1200/JCO.2010.32.2321
Carracedo A, Ma L, Teruya-Feldstein J, Rojo F, Salmena L, Alimonti A, Egia A, Sasaki AT, Thomas G, Kozma SC, Papa A, Nardella C, Cantley LC, Baselga J, Pandolfi PP (2008) Inhibition of mTORC1 leads to MAPK pathway activation through a PI3K-dependent feedback loop in human cancer. J Clin Invest 118(9):3065–3074. doi:10.1172/JCI34739
Serra V, Scaltriti M, Prudkin L, Eichhorn PJ, Ibrahim YH, Chandarlapaty S, Markman B, Rodriguez O, Guzman M, Rodriguez S, Gili M, Russillo M, Parra JL, Singh S, Arribas J, Rosen N, Baselga J (2011) PI3K inhibition results in enhanced HER signaling and acquired ERK dependency in HER2-overexpressing breast cancer. Oncogene 30(22):2547–2557. doi:10.1038/onc.2010.626
Halilovic E, She QB, Ye Q, Pagliarini R, Sellers WR, Solit DB, Rosen N (2010) PIK3CA mutation uncouples tumor growth and cyclin D1 regulation from MEK/ERK and mutant KRAS signaling. Cancer Res 70(17):6804–6814. doi:10.1158/0008-5472.CAN-10-0409
Engelman JA, Chen L, Tan X, Crosby K, Guimaraes AR, Upadhyay R, Maira M, McNamara K, Perera SA, Song Y, Chirieac LR, Kaur R, Lightbown A, Simendinger J, Li T, Padera RF, Garcia-Echeverria C, Weissleder R, Mahmood U, Cantley LC, Wong KK (2008) Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med 14(12):1351–1356. doi:10.1038/nm.1890
Di Nicolantonio F, Arena S, Tabernero J, Grosso S, Molinari F, Macarulla T, Russo M, Cancelliere C, Zecchin D, Mazzucchelli L, Sasazuki T, Shirasawa S, Geuna M, Frattini M, Baselga J, Gallicchio M, Biffo S, Bardelli A (2010) Deregulation of the PI3K and KRAS signaling pathways in human cancer cells determines their response to everolimus. J Clin Invest 120(8):2858–2866. doi:10.1172/JCI37539
Ihle NT, Lemos R Jr, Wipf P, Yacoub A, Mitchell C, Siwak D, Mills GB, Dent P, Kirkpatrick DL, Powis G (2009) Mutations in the phosphatidylinositol-3-kinase pathway predict for antitumor activity of the inhibitor PX-866 whereas oncogenic ras is a dominant predictor for resistance. Cancer Res 69(1):143–150. doi:10.1158/0008-5472.CAN-07-6656
Bachman KE, Argani P, Samuels Y, Silliman N, Ptak J, Szabo S, Konishi H, Karakas B, Blair BG, Lin C, Peters BA, Velculescu VE, Park BH (2004) The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther 3(8):772–775
Samuels Y, Wang Z, Bardelli A, Silliman N, Ptak J, Szabo S, Yan H, Gazdar A, Powell SM, Riggins GJ, Willson JK, Markowitz S, Kinzler KW, Vogelstein B, Velculescu VE (2004) High frequency of mutations of the PIK3CA gene in human cancers. Science 304(5670):554. doi:10.1126/science.1096502
Stemke-Hale K, Gonzalez-Angulo AM, Lluch A, Neve RM, Kuo WL, Davies M, Carey M, Hu Z, Guan Y, Sahin A, Symmans WF, Pusztai L, Nolden LK, Horlings H, Berns K, Hung MC, van de Vijver MJ, Valero V, Gray JW, Bernards R, Mills GB, Hennessy BT (2008) An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res 68(15):6084–6091. doi:10.1158/0008-5472.CAN-07-6854
Taylor BS, Schultz N, Hieronymus H, Gopalan A, Xiao Y, Carver BS, Arora VK, Kaushik P, Cerami E, Reva B, Antipin Y, Mitsiades N, Landers T, Dolgalev I, Major JE, Wilson M, Socci ND, Lash AE, Heguy A, Eastham JA, Scher HI, Reuter VE, Scardino PT, Sander C, Sawyers CL, Gerald WL (2010) Integrative genomic profiling of human prostate cancer. Cancer Cell 18(1):11–22. doi:10.1016/j.ccr.2010.05.026
Carver BS, Chapinski C, Wongvipat J, Hieronymus H, Chen Y, Chandarlapaty S, Arora VK, Le C, Koutcher J, Scher H, Scardino PT, Rosen N, Sawyers CL (2011) Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 19(5):575–586. doi:10.1016/j.ccr.2011.04.008
Mellinghoff IK, Vivanco I, Kwon A, Tran C, Wongvipat J, Sawyers CL (2004) HER2/neu kinase-dependent modulation of androgen receptor function through effects on DNA binding and stability. Cancer Cell 6(5):517–527. doi:10.1016/j.ccr.2004.09.031
Creighton CJ, Fu X, Hennessy BT, Casa AJ, Zhang Y, Gonzalez-Angulo AM, Lluch A, Gray JW, Brown PH, Hilsenbeck SG, Osborne CK, Mills GB, Lee AV, Schiff R (2010) Proteomic and transcriptomic profiling reveals a link between the PI3K pathway and lower estrogen-receptor (ER) levels and activity in ER + breast cancer. Breast Cancer Res BCR 12(3):R40. doi:10.1186/bcr2594
Essers MA, de Vries-Smits LM, Barker N, Polderman PE, Burgering BM, Korswagen HC (2005) Functional interaction between beta-catenin and FOXO in oxidative stress signaling. Science 308(5725):1181–1184. doi:10.1126/science.1109083
Tenbaum SP, Ordonez-Moran P, Puig I, Chicote I, Arques O, Landolfi S, Fernandez Y, Herance JR, Gispert JD, Mendizabal L, Aguilar S, Ramon Y, Cajal S, Schwartz S, Jr., Vivancos A, Espin E, Rojas S, Baselga J, Tabernero J, Munoz A, Palmer HG (2012) Beta-catenin confers resistance to PI3K and AKT inhibitors and subverts FOXO3a to promote metastasis in colon cancer. Nat Med 18(6):892–901. doi:10.1038/nm.2772
Liu P, Cheng H, Santiago S, Raeder M, Zhang F, Isabella A, Yang J, Semaan DJ, Chen C, Fox EA, Gray NS, Monahan J, Schlegel R, Beroukhim R, Mills GB, Zhao JJ (2011) Oncogenic PIK3CA-driven mammary tumors frequently recur via PI3K pathway-dependent and PI3K pathway-independent mechanisms. Nat Med 17(9):1116–1120. doi:10.1038/nm.2402
Muellner MK, Uras IZ, Gapp BV, Kerzendorfer C, Smida M, Lechtermann H, Craig-Mueller N, Colinge J, Duernberger G, Nijman SM (2011) A chemical-genetic screen reveals a mechanism of resistance to PI3K inhibitors in cancer. Nat Chem Biol 7(11):787–793. doi:10.1038/nchembio.695
Tan J, Li Z, Lee PL, Guan P, Aau M, Lee ST, Feng M, Lim CZ, Lee EY, Wee ZN, Lim YC, Karuturi RK, Yu Q (2013) PDK1 signaling towards PLK1-Myc activation confers oncogenic transformation and tumor initiating cell activation and resistance to mTOR-targeted therapy. Cancer Discovery. doi:10.1158/2159-8290.CD-12-0595
Britschgi A, Andraos R, Brinkhaus H, Klebba I, Romanet V, Muller U, Murakami M, Radimerski T, Bentires-Alj M (2012) JAK2/STAT5 inhibition circumvents resistance to PI3K/mTOR blockade: a rationale for cotargeting these pathways in metastatic breast cancer. Cancer Cell 22(6):796–811. doi:10.1016/j.ccr.2012.10.023
Elkabets M, Vora S, Juric D, Morse N, Mino-Kenudson M, Muranen T, Tao J, Campos AB, Rodon J, Ibrahim YH, Serra V, Rodrik-Outmezguine V, Hazra S, Singh S, Kim P, Quadt C, Liu M, Huang A, Rosen N, Engelman JA, Scaltriti M, Baselga J (2013) mTORC1 inhibition is required for sensitivity to PI3K p110α inhibitors in PIK3CA-mutant breast cancer. Sci Trans Med 5(196):196–199. doi:10.1126/scitranslmed.3005747
Shi Y, Wang J, Chandarlapaty S, Cross J, Thompson C, Rosen N, Jiang X (2014) PTEN is a protein tyrosine phosphatase for IRS1. Nat Struct Mol Biol 21(6):522–527. doi:10.1038/nsmb.2828
Jia S, Liu Z, Zhang S, Liu P, Zhang L, Lee SH, Zhang J, Signoretti S, Loda M, Roberts TM, Zhao JJ (2008) Essential roles of PI(3)K-p110beta in cell growth, metabolism and tumorigenesis. Nature 454(7205):776–779. doi:10.1038/nature07091
Torbett NE, Luna-Moran A, Knight ZA, Houk A, Moasser M, Weiss W, Shokat KM, Stokoe D (2008) A chemical screen in diverse breast cancer cell lines reveals genetic enhancers and suppressors of sensitivity to PI3K isoform-selective inhibition. Biochem J 415(1):97–110. doi:10.1042/BJ20080639
Wee S, Wiederschain D, Maira SM, Loo A, Miller C, deBeaumont R, Stegmeier F, Yao YM, Lengauer C (2008) PTEN-deficient cancers depend on PIK3CB. Proc Natl Acad Sci USA 105(35):13057–13062. doi:10.1073/pnas.0802655105
Castel P, Juric D, Won H, Ainscough B, Ellis H, Ebbesen S, Griffith M, Griffith O, Iyer G, Sgroi D, Isakoff S, Mardis E, Solit D, Lowe S, Quadt C, Peters M, Berger M, Scaltriti M, Baselga J (2014) Loss of PTEN leads to clinical resistance to the PI3Kα inhibitor BYL719 and provides evidence of convergent evolution under selective therapeutic pressure (abstract) abstract LB-327. In: Proceedings of the annual meeting of the American Association for Cancer Research, 8 April 2014
Wilson TR, Fridlyand J, Yan Y, Penuel E, Burton L, Chan E, Peng J, Lin E, Wang Y, Sosman J, Ribas A, Li J, Moffat J, Sutherlin DP, Koeppen H, Merchant M, Neve R, Settleman J (2012) Widespread potential for growth-factor-driven resistance to anticancer kinase inhibitors. Nature 487(7408):505–509. doi:10.1038/nature11249
Straussman R, Morikawa T, Shee K, Barzily-Rokni M, Qian ZR, Du J, Davis A, Mongare MM, Gould J, Frederick DT, Cooper ZA, Chapman PB, Solit DB, Ribas A, Lo RS, Flaherty KT, Ogino S, Wargo JA, Golub TR (2012) Tumour micro-environment elicits innate resistance to RAF inhibitors through HGF secretion. Nature 487(7408):500–504. doi:10.1038/nature11183
Zunder ER, Knight ZA, Houseman BT, Apsel B, Shokat KM (2008) Discovery of drug-resistant and drug-sensitizing mutations in the oncogenic PI3K isoform p110α. Cancer Cell 14(2):180–192. doi:10.1016/j.ccr.2008.06.014
Huw LY, O’Brien C, Pandita A, Mohan S, Spoerke JM, Lu S, Wang Y, Hampton GM, Wilson TR, Lackner MR (2013) Acquired PIK3CA amplification causes resistance to selective phosphoinositide 3-kinase inhibitors in breast cancer. Oncogenesis 2:e83. doi:10.1038/oncsis.2013.46
Shi H, Moriceau G, Kong X, Lee MK, Lee H, Koya RC, Ng C, Chodon T, Scolyer RA, Dahlman KB, Sosman JA, Kefford RF, Long GV, Nelson SF, Ribas A, Lo RS (2012) Melanoma whole-exome sequencing identifies (V600E)B-RAF amplification-mediated acquired B-RAF inhibitor resistance. Nat Commun 3:724. doi:10.1038/ncomms1727
Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN (2012) Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med 366(6):520–529. doi:10.1056/NEJMoa1109653
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this chapter
Cite this chapter
Shah, P.D., Chandarlapaty, S. (2016). Resistance to PI3K Pathway Inhibition. In: Dey, N., De, P., Leyland-Jones, B. (eds) PI3K-mTOR in Cancer and Cancer Therapy. Cancer Drug Discovery and Development. Humana Press, Cham. https://doi.org/10.1007/978-3-319-34211-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-34211-5_5
Published:
Publisher Name: Humana Press, Cham
Print ISBN: 978-3-319-34209-2
Online ISBN: 978-3-319-34211-5
eBook Packages: MedicineMedicine (R0)