Abstract
Minimal invasive surgical techniques have led to renewed interest in unicompartmental arthroplasty (UKA), and various new technologies have been introduced to further improve surgical techniques and patient outcomes. Computer tomogram (CT)-derived implants and instruments address the shortcomings of off-the-shelf (OTS) implants: incomplete bone coverage, nonanatomic match of both femoral and tibial surfaces, and symmetric implants for asymmetric surfaces. Furthermore, custom bi-compartmental arthroplasty (BKA) allow the reconstruction of both the tibio-femoral and the femoral-patellar joint for the medial and lateral side. Custom UKA and BKA offer a bone-sparing resurfacing alternative in combination with the preservation of both cruciate ligaments and solve the short-comings of OTS implants.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Unicompartmental osteoarthritis
- Bicompartmental osteoarthritis
- Custom unicompartmental knee arthroplasty
- Custom bicompartmental arthroplasty
- Single-use 3D printed instrumentation
- Custom knee arthroplasty
Minimal invasive techniques in unicompartmental knee arthroplasty (UKA) have been available for over a decade and are based on three principles: minimization of the joint damage, minimal interference with patient’s lifestyle, and no interference with consequential future treatment options as popularized by John Repicci [1]. The introduction of minimally invasive techniques to UKA has led to renewed interest in UKA. Further, growth rates have tripled compared to the rate of total knee replacement (TKR) over the past decade [2], fueled by the introduction of new technology like mobile-bearing UKA, haptic guidance system, and custom UKA with 3D printed single-use instruments.
The concept of unicompartmental TKR is an attractive alternative to tibial osteotomy or TKR for unicompartmental osteoarthritis [3, 4]. Compared with osteotomy and TKR, unicompartmental arthroplasty has a higher initial success rate and fewer early complications [5]. Additionally, recovery is quicker and more patients going home than patients who have received a TKA but a lower postoperative morbidity [6]. A randomized prospective study comparing patients with unicompartmental osteoarthritis who had received either a UKA or TKR for 15 years showed a survival rate of 89 % for UKA and 79 % for TKR. Further, patients who received the UKA had better range of motion, were more satisfied, and were more active, with a comparable functioning score. The failure rate at 15 years of UKA was not higher than that seen with TKR [7].
Patient Selection
The widespread use of arthroscopic treatment for osteoarthritis of the knee without large mechanical relevant meniscal tears has been questioned [8–10] and is not recommended. Osteotomy remains the procedure of choice for young, athletic, active patients with unicompartmental osteoarthritis who want to return to high impact sport activities. Internal derangement and its treatment in the younger subpopulation are not contraindications to osteotomy and may need an arthroscopic procedure before or after the osteotomy is performed. However, additional studies are needed to clarify the effectiveness of knee arthroscopy in this population. Subluxation and extreme angular deformity are contraindications to both osteotomy and unicompartmental replacement.
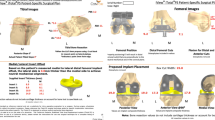
What are the potential benefits of custom unicompartmental arthroplasty? The geometry of the articulating surfaces of the knee is rather complex, and it is unclear whether or not an off-the-shelf (OTS) implant designed for the medial tibiofemoral joint in varying sizes while simultaneously flipping a left medial UKA to use for the lateral tibiofemoral joint of the right knee or vice versa is sufficient. The knee has three compartments with three different J-curves on the femoral side and two tibial surfaces with a more teardrop-shaped and concave medial plateau and a rounder but convex lateral plateau. On the femoral side, the medial and lateral condyles are different: comparing the medial to the lateral J-curves, the posterior radius is similar, but the anterior radius is much larger for the lateral condyle [11]. The average anterior medial radius is 37.5 mm (±3.3 mm) and 43.4 mm (±4.2 mm) for the lateral condyle. Furthermore, the posterior lateral condyle is narrower and the anterior condyle is longer and straighter. The medial condyle is slightly externally rotated and more curved (Fig. 1). Therefore, the question has to be raised whether it makes sense to use one OTS femoral component with different magnifications and use it for both the medial and for the lateral condyle while ignoring the different geometries of both the femoral and tibial compartments.
Besides the shortcomings of not matching the geometries of OTS implants, the wide range of sizes is insufficient: we compared the femoral and tibial dimensions of 48 knee computer tomography (CT) scans and studied how many components would fit within 2 mm. We selected the widest femoral component of OTS UKA available in the USA and compared its width to the widths of the medial and lateral condyles. For the medial condyle, 67 % did not fit within 2 mm in males and 8 % did not fit within 2 mm in females. For the lateral condyle, 88 % did not fit within 2 mm for males and 21 % did not fit within 2 mm for females [12]. While all female tibial (medial and lateral tibial plateau) knee dimensions fell within ± 2 mm of the range of available components, males are not as lucky: taking the longest and widest tibial component, 8 % had anteroposterior (AP) dimensions that were 2 mm longer and 13 % had wider dimensions (>2 mm) on the medial side. On the lateral tibial plateau, 5 % had longer (>2 mm) plateaus, but 29 % had wider dimensions (>2 mm) [12]. Even if OTS UKA improves their design for the tibial component and optimizes the geometry of the tibial implant OTS, tibial implants would not cover more than 76 % of the cortical rim [13]. Other authors have described the mismatch of implants and tibial anatomy and suggested that surgeons should be aware of the fact that some implants fit better than others [14, 15]. These shortcomings are addressed with custom-designed implants, not only for each patient but also designed for each individual compartment of the knee, whether it is for the femoral or tibial side.
The ideal candidate for custom unicompartmental replacement is the unicompartmental osteoarthritic patient with intact cruciate and collateral ligaments, preserved range of motion, and a correctable deformity, but no inflammatory arthritis. There is no difference on patient selection criteria when deciding to use an off-the-shelf (OTS) or a custom UKA. Whether age, weight, and activity should be considered as part of the selection, criteria for UKA have been a reoccurring controversial topic in the literature for decades. The strict narrow indications discussed 20 years ago [3] have been softened and widened. Most surgeons make their final decision on whether to use a UKA or a TKA at the time of surgery after arthrotomy [3]. However, authors question this practice given the limited access to the contralateral side with the use of minimal invasive techniques through a minimal invasive medial approach: it is difficult to judge the integrity of the lateral tibial plateau, and the same is true for the medial compartment through a minimal invasive lateral approach. The practice of judging the contralateral compartment intraoperatively is not recommended in the case of a custom implant: from a cost perspective, it makes no sense to produce several different custom implants like a partial and a total knee replacement and discard the unused implant at the time of surgery.
So how can you optimize the selection process of patients qualifying for UKA and make sure that the correct implant is picked? There are two important steps in the selection process of custom implants. The first step is a general screening for uni-, bi-, or tri-compartmental osteoarthritis with a series of standard X-rays (Fig. 2). Standing posteroanterior bilateral knee views in extension and flexion (Rosenberg views) are good screening tools for unicompartmental medial or lateral osteoarthritis. The standing lateral X-ray helps to identify an anteromedial wear pattern. Furthermore, it is helpful in predicting whether the anterior cruciate ligament (ACL) is intact [16]. Skyline views may demonstrate patellofemoral (PF) osteoarthritis since PF eburnated bone is generally considered a contraindication to UKA, but could be addressed with a custom bicompartmental knee arthroplasty (BKA) . There is one exception: in varus knees with bare bone in the medial tibiofemoral joint and bare bone in the lateral PF joint, the author would not recommend BKA but TKA. With the correction of the varus deformity, the Q angle is increased affecting PF tracking, and sometimes these knees have some form of distal trochlear dysplasia with a pump toward the lateral condyle, which is not addressed with a custom BKA. Interestingly, most patients with isolated PF Osteoarthritis (OA) on plain skyline views may be candidates for patellofemoral arthroplasty (PFA) but have tibiofemoral arthritic changes that are detected in the second part of the screening process: all custom UKA or custom BKA are CT based and can be combined with an arthrogram to map the cartilage in all three compartments. The integrity of both the ACL and posterior cruciate ligament (PCL) can be verified, too. This study (Fig. 3) allows for the identification of small defects or even fissures in the contralateral compartment that would not be detected with plain stress X-rays and potentially could lead to progression of osteoarthritis of the contralateral compartment, which is known to be a frequent failure mechanism in UKA (Fig. 4). The use of a CT arthrogram avoids additional tests or even procedures, such as magnetic resonance imaging or diagnostic knee arthroscopies.
Custom UKA is derived from a preoperative CT scan using a special protocol, including some slices of the hip and ankle to include the mechanical axis of both femur and tibia into the design process (Fig. 5). While the neutral axis in UKA is not restored, the femoral component is designed perpendicular to the femoral mechanical axis. The tibial component, with the use of the custom 3D printed instrumentation, is placed perpendicular to the tibial mechanical axis. The implants and the individualized 3D printed nylon instruments are delivered sterilized in one small container (Fig. 6).
Surgical Technique
For the lateral UKA, the author prefers a slightly different positioning of the knee. The medial UKA is positioned in 90° of knee flexion, but for the lateral UKA, 70° of flexion diminishes the tension of the patella using a lateral para-patellar approach and allows the patella to be moved medial to facilitate exposure to the lateral femoral-patellar compartment. For medial UKA, a short medial minimal invasive approach is recommended. The length of the incision varies depending on the size of the knee, the muscularity, and how easily the patella can be moved. It is more important to protect the wound edges from maceration and to gain sufficient access to the joint without any struggle to expose the knee.
For the medial approach, it is important to avoid any release of the medial collateral ligament (MCL). The author uses a narrow Z-retractor around the medial tibia to remove the osteophytes without getting into the MCL. This allows the Z-retractor to be positioned around the tibia to protect the MCL, while the tibia is cut, without release of the semimembranosus tendon insertion (Fig. 7).
It is easier to cut the femur first to gain more space for the tibial cut. After the linea terminalis is marked the femoral condylar cartilage is removed including the top of the notch (Fig. 8). The medial osteophytes are completely taken off. Care needs to be taken to avoid incomplete osteophyte removal, since the femoral cutting block would be seated slightly more medial and ultimately would move the femoral component 1 or 2 mm more medial in relation to the center of the medial tibial component during range of motion. This may lead to increased shear forces and should be avoided. The femoral cutting block is then placed and pinned and the planned amount of bone is removed off the posterior condyle (Fig. 9). The cartilage needs to be removed before the accurate amount of bony resection is measured, including the saw thickness. The femoral cutting block should be moved 1–2 mm more anteriorly to resect 1–2 mm additional bone to match the planned resection thickness and to avoid flexion tightness. Enough space is now created to remove the cartilage of the anterior two-thirds of the tibia, specifically toward the tibial eminentia. This step is important since this system is the only UKA on the market where the soft tissue balancing is done prior to resecting the tibia. Incomplete removal of the cartilage would either result in a varus/valgus positioning of the tibial component or not enough tibial resection. This is done with special balancing chips (Fig. 10), which are custom fitted to the tibial surface and come in four thicknesses with 1-mm increments. In flexion, the balancing chips are placed on the tibia (Fig. 10a) and its custom fit is verified. If there are some additional osteophytes, they need to be removed. The knee is brought into slight flexion of about 10° starting with the thinnest balancing chip (Fig. 10a) to find the optimal tension of the medial and lateral collateral ligament (Fig. 10b). The majority of knees are balanced with opening between 1–2 mm on the medial side and 2–3 mm on the lateral side using the B or C balancing chip. If the thinnest A chip is used and the knee is not opening at all, incomplete cartilage or osteophyte removal may be the reason and should be readdressed, before cutting the tibia. If the D balancing chip is necessary to balance the knee or if it is still too loose, MCL insufficiency should be considered which cannot be treated with a UKA. Care should be taken to avoid overstuffing of the compartment, which will result in overcorrection. The opening under varus or valgus stress will match the opening after the implants are placed using the default plastic thickness of 6 mm. Figures 11 and 12 show 3-foot films of a varus and valgus knee pre- and post-op with slight under-correction of the deformity.
The femoral cutting block is placed on femoral condyle. The planned amount of bone excluding the cartilage needs to be measured to verify correct amount of resected bone and match planned amount. If not enough bone resection, the femoral cutting block is moved more anteriorly until total planned amount is resected of the posterior condyle to place femoral component “anatomically”
Balancing the knee: first (a) verify anatomic placement of balancing chip in flexion. Bring knee in 10° of flexion (b) and open compartment with valgus or varus stress. If too much opening, increase thickness of balancing chip. Try to avoid using the thinnest chip and verify first if all cartilage of the tibial plateau and osteophytes have been removed, since the thinnest chip removes the most tibial bone stock. Also be careful not to use the D chip, since laxity is most likely an insufficiency of the collateral ligament and will result in overcorrection. After selecting the best thickness of the balancing chip, attach the tibial cutting block including alignment rod and verify varus-valgus alignment and slope (c)
The tibial cutting block is now connected to the balancing chip in extension, and the alignment is verified using the extramedullary rod (Fig. 10c). While some surgeons try to match the individual slope, the author prefers to avoid a tibial slope of more than 7° on the medial side to reduce the strain on the ACL [17]. The slope of the lateral tibial component is matched individually (Fig. 10c). The tibial cutting block is pinned. One pin is in general enough to achieve sufficient fixation. The medial pin in medial UKA should not be used. It would create an additional stress riser of the medial tibial plateau [18, 19] and has been described in cases with tibial plateau fractures following medial UKA. First, the horizontal cut is made. The MCL needs to be protected and undercutting of the eminentia should be avoided. The author leaves the saw blade in situ to protect the posterior cortex from the sagittal cut, since a cut into the posterior cortex may increase the risk of a tibial plateau fracture [20].
After the tibia is cut, the geometry of the resected bone is compared to the geometry of the tibial implant. Insufficient resection or malrotation can be recognized and corrected. After resecting reminiscent meniscal tissue, removing osteophytes from the posterior condyle, the final preparation of the tibial plateau is completed using the drill bit and the puncher for the tibial keel. On the femoral side, the anterior trough is prepared to create space for the anterior tapered femoral component (Fig. 13). The transition to the posterior condyle is smoothened, and multiple drill holes are placed to allow better cement penetration into the femoral condyle. After trialing, the bony surfaces are washed and the components are cemented, starting with the tibia. Mixing the bone cement under vacuum increases the fatigue properties [21]. Both the implants and bony surfaces should be covered with bone cement, and a small suction tip is used to apply a vacuum to the bone [22]. It has been shown that a cement mantle of more than 3 mm improves the fixation and shear strength of the bond but should be less than 5 mm to avoid thermal necrosis [23]. Medium viscosity cement achieves deeper bone penetration than high-viscosity bone cement [24]. Using a trial insert will allow access to any posterior extruded cement. Others prefer to insert the original PE before cementing the femoral component (Fig. 14). Wound closure is performed in standard fashion and most surgeons prefer periarticular injections prior to closure.
Results
Radiographs demonstrate good fit of the components for medial or lateral UKA and BKA in both planes (Figs. 15, 16, 17, and 18). In a prospective multicenter center study of 120 patients (110 medial and 10 lateral), 118 UKA patients improved from baseline scores across all collected scores: average KSS, KSS function, scaled WOMAC, and VAS pain scores. At the 2-year follow-up, 99 % reported satisfaction and 89 % reported that the movement of their knee felt natural, and scores compared favorably to published scores of OTS UKA [25].
Early results of lateral custom UKA are encouraging: In a prospective comparison between 33 custom UKA and 20 OTS UKA, survivorship was 97 % for the custom UKA with a mean follow-up of 37 months versus 85 % with a mean of 32 months in the OTS UKA group [26] on the lateral side.
Early results of custom bicompartmental knee arthroplasty are also encouraging and do not reproduce the reported early high failure rates of OTS bicompartmental knee replacements (BKA) [27, 28]. Thirty-one patients with 34 bicompartmental osteoarthritis were treated with 26 medial and 8 lateral custom BKA and prospectively followed. Patient satisfaction, pain assessment, and survival analysis were conducted with a mean of 30-month follow-up. There were no revisions; 91 % rated their results good or excellent and 97 % indicated that they would have the surgery again.
One study compared the knee kinematics during walking between custom bicompartmental knee replacement (BKA to TKA and healthy controls). There were no significant differences in walking speed, peak knee extensor moment, peak knee power absorption, and knee peak knee power production between custom BKA and healthy controls. TKR showed slower walking speed, less peak knee extensor moment, and less peak knee power absorption and production [29].
Postoperative Rehabilitation
Perioperative multimodal pain management in combination with periarticular infiltration of local anesthetics can facilitate a quick recovery with good pain control. In general, patients are allowed to bear weight as tolerated and to walk with an assistive device within the first day. Rehabilitation is less painful and quicker than with TKR.
Summary
Custom uni- or bicompartmental knee replacements offer an attractive alternative to OTS implants using a minimal medial or lateral approach to the knee. Given the increased numbers of patients in need of joint replacement, UKA is an important treatment with clinical results that are similar to those of TKA at 10 and 15 years. Custom UKA and BKA offer a bone-sparing alternative along with preservation of both cruciate ligaments. It is feasible to consider UKA and BKA as the first prosthetic treatment for osteoarthritis in middle-aged patients. Custom partial knee replacements address the anatomic differences between the medial and lateral tibiofemoral geometries and have been a helpful addition to surgeon’s armamentarium in the last decade. Long-term studies are needed to demonstrate that custom anatomic implants outpace the results of OTS UKA implants, which are designed exclusively for the medial tibiofemoral joint.
References
Repicci JA. Mini-invasive knee unicompartmental arthroplasty: bone-sparing technique. Surg Technol Int. 2003;11:282–6.
Riddle DL, Jiranek WA, McGlynn FJ. Yearly incidence of unicompartmental knee arthroplasty in the United States. J Arthroplasty. 2008;23(3):408–12.
Kozinn SC, Scott R. Unicondylar knee arthroplasty. J Bone Joint Surg Am. 1989;71(1):145–50.
Borus T, Thornhill T. Unicompartmental knee arthroplasty. J Am Acad Orthop Surg. 2008;16(1):9–18.
Jackson M, Sarangi PP, Newman JH. Revision total knee arthroplasty. Comparison of outcome following primary proximal tibial osteotomy or unicompartmental arthroplasty. J Arthroplasty. 1994;9(5):539–42.
Brown NM, Sheth NP, Davis K, Berend ME, Lombardi AV, Berend KR, Della Valle CJ. Total knee arthroplasty has higher postoperative morbidity than unicompartmental knee arthroplasty: a multicenter analysis. J Arthroplasty. 2012;27(8):86–90.
Newman J, Pydisetty RV, Ackroyd C. Unicompartmental or total knee replacement: the 15-year results of a prospective randomised controlled trial. J Bone Joint Surg. 2009;91(1):52–7.
Kirkley A, Birmingham TB, Litchfield RB, Giffin JR, Willits KR, Wong CJ, Feagan BG, Donner A, Griffin SH, D’Ascanio LM, Pope JE, Fowler PJ. A randomized trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2008;359(11):1097–107.
Moseley JB, O’Malley K, Petersen NJ, Menke TJ, Brody BA, Kuykendall DH, Hollingsworth JC, Ashton CM, Wray NP. A controlled trial of arthroscopic surgery for osteoarthritis of the knee. N Engl J Med. 2002;347(2):81–8.
Katz JN, Losina E. Surgery versus physical therapy for meniscal tear and osteoarthritis. N Engl J Med. 2013;369(7):677–8.
Mensch JS, Amstutz HC. Knee morphology as a guide to knee replacement. Clin Orthop Relat Res. 1975;112:231–41.
Fitz W, Bliss R, Losina E. Current fit of medial and lateral unicompartmental knee arthroplasty. Acta Orthop Belg. 2013;79(2):191–6.
Fitzpatrick C, FitzPatrick D, Lee J, Auger D. Statistical design of unicompartmental tibial implants and comparison with current devices. Knee. 2007;14(2):138–44.
Servien E, Saffarini M, Lustig S, Chomel S, Neyret P. Lateral versus medial tibial plateau: morphometric analysis and adaptability with current tibial component design. Knee Surg Sports Traumatol Arthrosc. 2008;16(12):1141–5.
Surendran S, Kwak DS, Lee UY, Park SE, Gopinathan P, Han SH, Han CW. Anthropometry of the medial tibial condyle to design the tibial component for unicondylar knee arthroplasty for the Korean population. Knee Surg Sports Traumatol Arthrosc. 2007;15(4):436–42.
Keyes GW, Carr AJ, Miller RK, Goodfellow JW. The radiographic classification of medial gonarthrosis. Correlation with operation methods in 200 knees. Acta Orthop Scand. 1992;63(5):497–501.
Hernigou P, Deschamps G. Posterior slope of the tibial implant and the outcome of unicompartmental knee arthroplasty. J Bone Joint Surg Am. 2004;86(3):506–11.
Brumby SA, Carrington R, Zayontz S, Reish T, Scott RD. Tibial plateau stress fracture: a complication of unicompartmental knee arthroplasty using 4 guide pinholes. J Arthroplasty. 2003;18(6):809–12.
Van Loon P, de Munnynck B, Bellemans J. Periprosthetic fracture of the tibial plateau after unicompartmental knee arthroplasty. Acta Orthop Belg. 2006;72(3):369–74.
Clarius M, Haas D, Aldinger PR, Jaeger S, Jakubowitz E, Seeger JB. Periprosthetic tibial fractures in unicompartmental knee arthroplasty as a function of extended sagittal saw cuts: an experimental study. Knee. 2010;17(1):57–60.
Lindén U. Fatigue properties of bone cement. Comparison of mixing techniques. Acta Orthop Scand. 1989;60(4):431–3.
Stannage K, Shakespeare D, Bulsara M. Suction technique to improve cement penetration under the tibial component in total knee arthroplasty. Knee. 2003;10(1):67–73.
Vanlommel J, Luyckx JP, Labey L, Innocenti B, De Corte R, Bellemans J. Cementing the tibial component in total knee arthroplasty. J Arthroplasty. 2011;26(3):492–6.
Rey RM, Paiement GD, McGann WM, Jasty M, Harrigan TP, Burke DW, Harris WH. A study of intrusion characteristics of low viscosity cement Simplex-P and Palacos cements in a bovine cancellous bone model. Clin Orthop Relat Res. 1987;215:272–8.
Sinha R, Burkhardt J, Martin G, Mack D, Dauphine R, Levine M, Barnes L, editors. Customized, individually made unicondylar knee replacement: a prospective, multicenter study of 2-year clinical outcomes. Boston: 44th Annual Advances in Arthroplasty Course at Harvard Medical School 2014.
Demange MK, Von Keudell A, Probst C, Yoshioka H, Gomoll AH. Patient-specific implants for lateral unicompartmental knee arthroplasty. Int Orthop. 2015;39(8):1519–26.
Müller M, Matziolis G, Falk R, Hommel H. The bicompartmental knee joint prosthesis Journey Deuce: failure analysis and optimization strategies. Orthopade. 2012;41(11):894–904.
Palumbo BT, Henderson ER, Edwards PK, Burris RB, Gutiérrez S, Raterman SJ. Initial experience of the Journey-Deuce bicompartmental knee prosthesis: a review of 36 cases. J Arthroplasty. 2011;26(6):40–5.
German Congress of Orthopaedics and Traumatology (DKOU), Trans. Differences in knee mechanics between customized, individually made BKR and off-the-shelf TKR patients during walking. Berlin; 2015.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this entry
Cite this entry
Fitz, W. (2016). Custom Unicompartmental Knee Arthroplasty. In: Scuderi, G., Tria, A. (eds) Minimally Invasive Surgery in Orthopedics. Springer, Cham. https://doi.org/10.1007/978-3-319-34109-5_121
Download citation
DOI: https://doi.org/10.1007/978-3-319-34109-5_121
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-34107-1
Online ISBN: 978-3-319-34109-5
eBook Packages: MedicineReference Module Medicine