Abstract
Human intestinal organoids are three-dimensional tissue structures with a high degree of cellular and architectural complexity similar to the intestine. The identification of Lgr5-expressing adult intestinal stem cells was a seminal advance in the establishment of adult intestinal organoids (also called enteroids), which can be derived from patient biopsies and used to model human diseases. A second type of intestinal organoid that is generated via the directed differentiation of human pluripotent stem cells (hPSCs), into induced human intestinal organoids (iHIOs), has also proven useful in modeling developmental and postnatal diseases of the GI tract. Here we describe the various intestinal organoids systems, and how they have been used to study epithelial cell biology in the context of inflammatory bowel disease (IBD). In addition, we examine the advantages and disadvantages of the different organoid systems. Finally, we discuss emerging technologies and how they may be used in the future for drug development and as therapeutics.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Inflammatory Bowel Disease
- Inflammatory Bowel Disease Patient
- Paneth Cell
- Intestinal Stem Cell
- Human Pluripotent Stem Cell
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Introduction
Inflammatory bowel disease presents with chronic inflammation which damages normal intestinal mucosa. Because the disease is an inflammatory disease, much of the research on IBD has focused on the role of immune cells. However, it has become clear that the epithelial integrity as well as factors involved in healing of mucosa may also play a role in the pathogenesis of the disease. Genome-wide association studies of patients with Crohn’s and colitis have identified genes involved in epithelial biology such as CDH1, HNF4, and SATB2 [1]. Thus, examining epithelial biology is critical for understanding how barrier function and dysfunction in IBD impacts epithelial health and regeneration. Regeneration is important because “mucosal healing” predicts long-term remission in patients treated for IBD.
In the past, models utilizing intestinal epithelial monolayers have been used to study epithelial mechanisms of IBD and in the development of therapies. Despite providing some insight into IBD mechanisms, these two-dimensional in vitro models have several disadvantages . (1) They typically require the use of transformed or cancer cell lines that lack normal physiological properties of intestinal epithelium. (2) They are largely enterocyte models and lack the other differentiated cell types and thus do not reflect intestinal physiology. (3) Mesenchymal cells are also absent in these models. The presence of multiple cell types is particularly important for accurate modeling of disease since IBD could have effects on multiple cell types. For example, depletion of goblet cells is often observed in biopsies from ulcerative colitis (UC) patients and Muc2 deficient mice develop spontaneous colitis, suggesting a role of goblet cells in pathogenesis. Glucagon like peptide-2, a product of enteroendocrine cells (EECs ), has been shown to ameliorate experimental colitis in mice, suggesting that EECs may play a role in regeneration. In addition, Lgr5 positive stem cells are depleted in sites of injury in DSS treated mice [2]. Taken together these observations suggest that most differentiated cell types found in the intestine are involved in the pathology of IBD. Thus an organoid system containing multiple differentiated and stem cell types would more accurately mimic intestinal physiology.
In the past few years the identification of adult intestinal stem cell markers and the ability to isolate and culture these cells have led to significant advances in our understanding of intestinal stem cell function, differentiation, and gastrointestinal cancer [3–9]. Adult intestinal organoids (also called enteroids ) can be grown in 3D culture from single Lgr5+ cells. These intestinal organoids display remarkable similarity to intestinal epithelium in vivo . Intestinal organoids contain a central lumen surrounded by a single cell layer of polarized epithelial cells. In addition, this single cell layer is organized into crypt-villus like domains containing the main differentiated cell types. Lgr5+ stem cells , Paneth cells, and proliferative progenitors localize in the crypt-like structures. Polarized enterocytes line the central lumen, while goblet cells and enteroendocrine cells are scattered throughout the organoid.
Since the discovery of Lgr5 as a marker of intestinal stem cells, several other markers of intestinal stem cells have been identified including Bmi1, Sox9, HopX, Lrig, and mTert [10–14]. Several of these markers are present in label retaining cells located at the +4 position in the crypt. These cells cycle slowly and are now believed to be a reserve stem cell population. Following damage of the intestine, these cells lose their quiescence and contribute to regeneration of the intestine while giving rise to new Lgr5+ stem cells which will maintain homeostasis [15–17]. It is likely that the quiescent stem cell contributes to regeneration in mouse models of colitis since Lgr5+ stem cells are lost during DSS induced colitis. Thus studies using organoid models could elucidate mechanisms by which the intestinal epithelium can regenerate following damage since these organoids contain reserve stem cells [18].
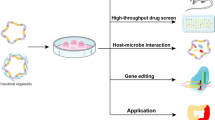
In this chapter we review the available intestinal organoid models and how they have been used in the study of epithelial biology and IBD (Fig. 16.1). For clarity we use three different terms to describe the different “organoid” types. Whole organoid units are derived from adult intestine and contain epithelial, mesenchymal, and neuronal cell types. Enteroids (as designated by the NIH Intestinal Stem Cell Consortium ) are derived from adult intestinal stem cells or crypts and are purely epithelial organoids [19]. Induced human intestinal organoids (iHIOs) are derived from embryonic and induced pluripotent stem cells (collectively called PSCs) and contain both epithelial and mesenchymal cell types. In addition, we discuss the advantages and disadvantages of each model. Finally, we discuss emerging technologies and how they will contribute to understanding the molecular basis of IBD.
Overview of Intestinal Organoid systems . Intestinal organoids can be derived by a variety of methods. Intestinal crypts and LGR5-expressing stem cells can be isolated from IBD patient biopsies and grown as human intestinal enteroids, which contain epithelium only. Thus these human intestinal enteroids can be used to study epithelial functions and mutations can be corrected using CRISPR\Cas9 gene editing technology . In addition, human intestinal organoids can be grown from whole intestinal tissue which will include stromal cells and enteric nerves. These human intestinal organoids can be used to study the interaction between epithelial, mesenchymal, and neuronal cell types. Induced human intestinal organoids (iHIOs) can be generated from human pluripotent stem cells (hPSCs) which are either from human embryonic stem cells or from induced pluripotent stem cells (iPSCs) generated from any somatic cell (blood and urine are easily obtained sources of somatic cells). Mutations in hPSCs can also be corrected using CRISPR/CAS9. iHIOs can be used to study organ development, tissue–tissue interactions, and to model intestinal disease. iHIOs resemble human fetal intestine when grown in vitro. However, iHIOs can be transplanted in vivo under the mouse kidney capsule to generate more mature, functional human intestine
Organoid Models
Whole Organoid Units
One organoid model system was developed by Ootani et al. [20], whereby pieces of whole intestine or colon (including mesenchymal cells and enteric nerves) are grown in a liquid–air interface, which allows for long-term growth of organoid structures. These organoids contain the major differentiated intestinal cell types found in vivo. Furthermore, the presence of a mesenchyme within these organoids allows growth of the organoids without supplementation with niche factors such as Noggin and R-spondin. Thus, this system would be advantageous for examining the effects of epithelial–mesenchymal interactions in the context of inflammation or regeneration; however, to date it has not been used to study IBD. However, the system has been used to effectively investigate the effects of oncogene activation and inactivation of tumor suppressor genes in mouse models and could be used to study these changes in the context of IBD. For example, loss of the APC tumor suppressor gene allows for faster recovery in response to DSS-mediated epithelial injury [21].
Lgr5-Based Mouse Enteroids
The most commonly used intestinal organoid model was developed in the lab of Hans Clevers [9], and these will be referred to as enteroids because they are purely epithelial. Sato and colleagues demonstrated that single Lgr5+ stem cells could be grown into 3D enteroids in Matrigel culture in medium containing EGF, Noggin (BMP antagonist), and R-spondin (ENR). Interestingly enteroids were able to proliferate and differentiate spontaneously despite the lack of any mesenchymal cells , suggesting that ENR media was sufficient to replace the signals derived from supporting mesenchymal cell types. In addition, this system was used to demonstrate that the Paneth cells act as an ISC niche cell by supplying Wnt3a [5]. Although there are numerous studies that have used the mouse enteroid system to address questions about epithelial biology in an IBD context, we focus on several key examples.
One study by Günther and coworkers examined the role of caspase 8 in epithelial cell death and inflammation of the ileum [22]. This work was based on the observation that mice deficient in caspase 8 within the intestinal epithelium (Casp8ΔIEC) have depleted Paneth and goblets cells and develop spontaneous ileitis. Examination of enteroids derived from the intestines of Wild Type (WT) and Casp8ΔIEC mice revealed no differences in the number of Paneth cells, suggesting that a factor present in vivo affected the differentiation or survival of this cell type. To further explore this possibility, the authors examined the effect of TNF-α stimulation on organoids from WT and Casp8ΔIEC mice. Interestingly, 24 h after stimulation, WT enteroids appeared normal while Casp8ΔIEC enteroids underwent nec-1-dependent necrosis. In addition, the authors presented evidence that RIP-mediated necroptosis of Paneth cells was also present in samples from patients with Crohn’s disease and suggested that this may be the cause of the defective antimicrobial defense that is observed in these patients.
In another study, Farin and Karthaus et al. used enteroids to examine the effect of bacterial antigens on Paneth and goblet cells [23]. Interestingly these antigens had little effect on these cell types regardless of whether they were administered apically or basolaterally. However, when inflammatory cytokines were applied to these organoids, only IFN-γ caused degranulation of Paneth cells. In addition, IFN-γ stimulation led to loss of mucus containing goblet cells and mature enterocytes. As a consequence of the loss of Paneth cells, organoid growth was severely compromised likely due to the role of Paneth cells as a niche cell for intestinal stem cells. Taken together, these studies demonstrate how this organoid system can be used to interrogate the effect of inflammatory cytokines on various cell types.
Human Enteroids
Human enteroids (as designated by the NIH Intestinal Stem Cell Consortium [19]) can be grown from isolated intestinal crypts or CD44+CD166+CD24lo single cells in Matrigel based 3D culture [9, 24]. In the case of colon, single cells can be grown into colonoids by FACS sorting based on the expression of ephrin type-B receptor 2 (EPHB2) [7]. Enteroids have been generated from small and large intestine and require a unique set of growth factors. Cultured human intestinal enteroids (and colonoids) require Wnt3a, gastrin, nicotinamide, A-83-01 (Alk4/5/6 inhibitor) and SB202190 (p38 inhibitor) in addition to basic growth media containing EGF, Noggin, and R-spondin-1 [8]. These enteroids display remarkable similarity to intestinal tissue in vivo. Enteroids self-organize into structures containing a central lumen surrounded by a single cell layer of polarized epithelial cells. In addition, this single cell layer is organized into crypt-villus like structures which can differentiate into the main differentiated cell types following withdrawal of Wnt3a, nicotinamide, and SB202190. Interestingly, like their mouse counterparts, these organoids lack mesenchymal cells, suggesting that growth factors produced by mesenchymal cells (rather than physical interaction with these cells) are necessary for maintenance of the intestinal stem cell niche.
Directed Differentiation of Pluripotent Stem Cells into Induced Human Intestinal Organoids (iHIOs )
Another method for deriving intestinal organoids is through directed differentiation of human pluripotent stem cells in a manner that approximates embryonic development of the intestines [25–27]. First pluripotent stem cells are differentiated into definitive endoderm by treatment with Activin A. Subsequent activation of Wnt and FGF pathways promotes a posterior endoderm fate and induces morphogenesis into mid/hingut spheroids. Once formed, these midgut/hindgut spheroids can be grown into iHIOs in three-dimensional culture conditions that favor intestinal growth [9, 26]. Moreover, growth of these spheroids into iHIOs recapitulates developmental events that occur in vivo. Midgut/hindgut spheroids transition from a simple cuboidal epithelium into a pseudostratified epithelium , which then undergoes cytodifferentiation and transitions into a polarized epithelium which contains zones of differentiation and proliferation. When compared to developing mouse intestine, these intestinal organoid cultures undergo strikingly similar transitions [28]. In addition, iHIOs also contain a layer of mesenchymal cells which also mature along with the epithelium, giving rise to fibroblasts, smooth muscle cells, and subepithelial fibroblasts.
Transplantation of iHIOs in vivo allows for substantial growth maturation of the intestinal tissue [29]. Transplanted iHIOs form crypts and villi as well as circular and longitudinal muscle layers. Furthermore, these tissues are functional as demonstrated by brush border activity, mucus secretion and polypeptide uptake. Since iHIOs generated from IPSCs can also grow and mature in vivo, IBD patient specific organoids could be generated and transplanted and immune cells from the patients could be injected to study inflammatory responses of the intestinal tissue. This would allow for a humanized mouse model of IBD that could aid in personalized drug development.
One example of the use of the iHIO system for IBD research is work by Xue and colleagues [30]. In this work, iHIOs were used to model inflammatory hypoxia. When iHIOs were grown in 1 % oxygen, they increased their expression of TNF-α when compared to organoids grown in ambient oxygen. When the hypoxia inducible factor EPAS1 was inhibited by a chemical antagonist, the increase in TNF-α expression was inhibited. This work suggests that iHIOs are capable of expressing proinflammatory cytokines in the context of hypoxia . However, it was unclear if TNF-α expression was initiated by the epithelium or mesenchyme of the iHIOs.
Choosing an Organoid Model
Because of the variety of organoid models available, the advantages and disadvantages of each model should be taken into consideration. The use of the right model is dependent on the biological question to be addressed and cell type(s) to be examined. Here we discuss the advantages and disadvantages of the three main organoid models.
Enteroids from mouse intestine are a great model system for many reasons. First, these organoids are strictly epithelial and can be used to study epithelial biology directly. Second, genetic tools are available to examine lineage tracing, generating conditional knockouts and for visualizing epithelial architecture in live organoids. The availability of these tools is advantageous especially in the context of gene knockouts which may affect organoid viability. In this case, organoids can be grown and the gene of interested can be excised using an inducible Cre. Another advantage of this system is that organoids maintain zones of proliferation and differentiation. This is especially important when examination of multiple cell types is desired. There are several properties of this system that may limit its utility for human IBD studies, for example as a murine system it may not reflect human pathobiology. Second, enteroids being epithelial only, can not be used to study the role of mesenchymal/stromal cell types in IBD. Lastly, one limitation of all organoids is that there are no immune cells present; however, addition of exogenous immune cells is possible.
Human intestinal enteroids offer an alternative to mouse enteroids. The most obvious advantage is that human intestinal enteroids are derived from humans and thus may more accurately reflect human intestinal epithelial cell biology. Furthermore, human intestinal enteroids can be generated from normal and diseased patients in order to examine epithelial biology within a disease context. In addition, enteroids are amenable to CRISPR\Cas9 mediated gene editing , which can allow for correction of mutated genes [31] or to modify tumor suppressor and oncogenes for modeling colorectal cancer [32, 33]. This system does have some disadvantages. First, generation of these enteroids requires human patient samples which may be difficult to obtain. Second, the majority of surgical samples are from diseased patients so these organoids may not reflect normal intestinal physiology. Moreover, enteroids do not maintain proliferation and differentiation to the extent that mouse organoids do. Thus, this system may be impractical when interrogating questions which require the presence of the main intestinal call types. As with mouse enteroids, human enteroids are only epithelial and therefore mesenchymal interactions cannot be studied.
Induced Human intestinal organoids derived from human pluripotent stem cells also have unique advantages and disadvantages. First, these organoids are human and they can be generated from human pluripotent stem cells, which can be grown and expanded infinitely. Second, these organoids contain a mesenchymal layer that matures with the epithelium and thus can be used to study epithelial–mesenchymal interactions . This is especially important when examining a susceptibility gene like NKX2-3 which is expressed in the intestinal mesenchyme [1, 34]. Furthermore, human pluripotent stem cells are amenable to viral based transgene delivery as well as CRISPR\Cas9 mediated gene editing [26, 35]. Lastly, as describe above, human intestinal organoids can be grown in vivo, whereby they mature and form crypts from which enteroids can be derived [29]. This allows for patient-specific derivation of organoids and enteroids without the need for surgical acquisition of intestinal tissues. However, iHIOs have limitations. First, in vitro grown organoids are fetal in nature, which inhibits the examination of mature differentiated cell types. Second, these organoids lack regional specificity, which may be crucial for accurate modeling since IBD often presents in specific regions of the small and large intestine.
Translation of Organoid Models of IBD
Organoid model systems have revolutionized the field of gastrointestinal biology by fostering the interrogation of biological question in a complex ex vivo model which recapitulates many aspects of normal intestinal physiology. Importantly, human organoid systems can eliminate concerns about species differences where human pathology is not adequately modeled in murine systems. So how can these systems be translated into medical applications? In this section we discuss how emerging technologies advance the use of organoid systems in medicine.
Because IBD is a multifactorial disease, epithelium only organoid systems may not fully recapitulate aspects of disease. As mentioned previously, some IBD susceptibility genes are expressed specifically in the mesenchyme. To study mesenchymal factors, the iHIO system, which contains mesenchyme, would allow interrogation of the role of mesenchymal factors in IBD. The microbiota constitute another system that is perturbed in IBD but has not been studied in an organoid system. Although the microbiota exist in an anoxic environment, the ability of organoids to grow in low oxygen, and the relatively hypoxic nature of the organoid lumen open the possibility for incorporation of microbiota. Finally incorporation of immune cells into organoid cultures would be essential for elucidating mechanisms of communication between these cells and the intestinal epithelium. Given that immune cells are largely housed in the stroma, whole organoid units and iHIOs, both containing mesenchyme, might be good systems to start with for incorporation of immune cells. A comparison of enteroids and mesenchyme-containing systems would allow for systematic analysis of how different cell types interact with immune cells.
With the advent of CRISPR\Cas9 technology , an efficient method for gene editing, it is now possible to generate genetically modified organoid systems containing cell reporters for live cell imaging and monitoring organoid function. With regard to IBD research, CRISPR\Cas9 technology could be used to generate organoids that contain mutations associated with IBD susceptibility. Alternatively, enteroids or organoids could be generated from IBD patients to examine the effects of mutations on epithelial biology. CRISPR\Cas9 technology could then be used to correct the mutation to determine if phenotype can be reversed. Proof of concept for this approach has been demonstrated in enteroids from cystic fibrosis patients which could be corrected using CRISPR\Cas9 technology [31].
Regenerative medicine is another possible therapeutic use of organoid systems. Intestinal tissue generated ex vivo could be used to replace damaged intestinal tissue in IBD patients. Proof of concept for this approach has been shown in mice. Colonoids generated from single Lrg5+ colonic stem cells are able to engraft into the colon of mice with chemically induced mucosal lesions [36]. Generation of enteroids from IBD patient biopsies or generation of iHIOs from patient derived induced pluripotent stem cells could be used to generate tissue for transplantation. In addition, patient mutations could be corrected using gene editing technology. Despite the promise of regenerative medicine, vastly improved methods to efficiently and safely incorporate engineered intestinal tissue will need to be developed before tissues could be used therapeutically.
Lastly, intestinal organoids hold promise for the field of personalized medicine. Patient derived enteroids or organoids could be used to screen drugs that may be effective in treating IBD. This approach has been used, whereby a biobank of human colorectal cancers was used for drug screening [37]. Such studies could be aided further by new high-throughput microfluidic technologies that allow screening of thousands of organoids [18].
Conclusions and Future Directions
The development of organoid methodologies has led to an expanded knowledge of intestinal epithelial cell biology. Organoid systems are well suited for use in personalized medicine and regenerative medicine. Important improvements to organoid-based systems could include incorporation of additional cellular complexity, such as immune cells , which would allow for better modeling of IBD. Being able to manipulate cell types, genetically or via the culture conditions, should allow for a mechanistic dissection of the cellular and molecular mechanisms that participate in the pathogenesis of IBD. With the evolution of new technologies for gene editing and high-throughput analysis of organoids using microfluidic platforms , as well as the feasibility of whole-genome sequencing, the use of organoid systems for personalized medicine should be greatly expedited.
References
McGovern DP, Gardet A, Torkvist L, Goyette P, Essers J, Taylor KD, et al. Genome-wide association identifies multiple ulcerative colitis susceptibility loci. Nat Genet. 2010;42:332–7.
Davidson LA, Goldsby JS, Callaway ES, Shah MS, Barker N, Chapkin RS. Alteration of colonic stem cell gene signatures during the regenerative response to injury. Biochim Biophys Acta. 2012;1822:1600–7.
Barker N, Ridgway RA, van Es JH, van de Wetering M, Begthel H, van den Born M, et al. Crypt stem cells as the cells-of-origin of intestinal cancer. Nature. 2009;457:608–11.
Barker N, van Es JH, Kuipers J, Kujala P, van den Born M, Cozijnsen M, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–7.
Sato T, van Es JH, Snippert HJ, Stange DE, Vries RG, van den Born M, et al. Paneth cells constitute the niche for Lgr5 stem cells in intestinal crypts. Nature. 2011;469:415–8.
Snippert HJ, van der Flier LG, Sato T, van Es JH, van den Born M, Kroon-Veenboer C, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–44.
Jung P, Sato T, Merlos-Suarez A, Barriga FM, Iglesias M, Rossell D, et al. Isolation and in vitro expansion of human colonic stem cells. Nat Med. 2011;17:1225–7.
Sato T, Stange DE, Ferrante M, Vries RG, Van Es JH, Van den Brink S, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett's epithelium. Gastroenterology. 2011;141:1762–72.
Sato T, Vries RG, Snippert HJ, van de Wetering M, Barker N, Stange DE, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459:262–5.
Gracz AD, Ramalingam S, Magness ST. Sox9 expression marks a subset of CD24-expressing small intestine epithelial stem cells that form organoids in vitro. Am J Physiol Gastrointest Liver Physiol. 2010;298:G590–600.
Montgomery RK, Carlone DL, Richmond CA, Farilla L, Kranendonk ME, Henderson DE, et al. Mouse telomerase reverse transcriptase (mTert) expression marks slowly cycling intestinal stem cells. Proc Natl Acad Sci U S A. 2011;108:179–84.
Powell AE, Wang Y, Li Y, Poulin EJ, Means AL, Washington MK, et al. The pan-ErbB negative regulator Lrig1 is an intestinal stem cell marker that functions as a tumor suppressor. Cell. 2012;149:146–58.
Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–20.
Takeda N, Jain R, LeBoeuf MR, Wang Q, Lu MM, Epstein JA. Interconversion between intestinal stem cell populations in distinct niches. Science. 2011;334:1420–4.
Metcalfe C, Kljavin NM, Ybarra R, de Sauvage FJ. Lgr5+ stem cells are indispensable for radiation-induced intestinal regeneration. Cell Stem Cell. 2014;14:149–59.
Tian H, Biehs B, Warming S, Leong KG, Rangell L, Klein OD, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478:255–9.
Yan KS, Chia LA, Li X, Ootani A, Su J, Lee JY, et al. The intestinal stem cell markers Bmi1 and Lgr5 identify two functionally distinct populations. Proc Natl Acad Sci U S A. 2012;109:466–71.
Gracz AD, Williamson IA, Roche KC, Johnston MJ, Wang F, Wang Y, et al. A high-throughput platform for stem cell niche co-cultures and downstream gene expression analysis. Nat Cell Biol. 2015;17:340–9.
Stelzner M, Helmrath M, Dunn JC, Henning SJ, Houchen CW, Kuo C, et al. A nomenclature for intestinal in vitro cultures. Am J Physiol Gastrointest Liver Physiol. 2012;302:G1359–63.
Ootani A, Li X, Sangiorgi E, Ho QT, Ueno H, Toda S, et al. Sustained in vitro intestinal epithelial culture within a Wnt-dependent stem cell niche. Nat Med. 2009;15:701–6.
Li X, Nadauld L, Ootani A, Corney DC, Pai RK, Gevaert O, et al. Oncogenic transformation of diverse gastrointestinal tissues in primary organoid culture. Nat Med. 2014;20:769–77.
Gunther C, Buchen B, He GW, Hornef M, Torow N, Neumann H, et al. Caspase-8 controls the gut response to microbial challenges by TNF-alpha-dependent and independent pathways. Gut. 2015;64:601–10.
Farin HF, Karthaus WR, Kujala P, Rakhshandehroo M, Schwank G, Vries RG, et al. Paneth cell extrusion and release of antimicrobial products is directly controlled by immune cell-derived IFN-gamma. J Exp Med. 2014;211:1393–405.
Wang F, Scoville D, He XC, Mahe MM, Box A, Perry JM, et al. Isolation and characterization of intestinal stem cells based on surface marker combinations and colony-formation assay. Gastroenterology. 2013;145:383–95. e1–21.
McCracken KW, Howell JC, Wells JM, Spence JR. Generating human intestinal tissue from pluripotent stem cells in vitro. Nat Protoc. 2011;6:1920–8.
Spence JR, Mayhew CN, Rankin SA, Kuhar MF, Vallance JE, Tolle K, et al. Directed differentiation of human pluripotent stem cells into intestinal tissue in vitro. Nature. 2011;470:105–9.
Wells JM, Spence JR. How to make an intestine. Development. 2014;141:752–60.
Spence JR, Lauf R, Shroyer NF. Vertebrate intestinal endoderm development. Dev Dyn. 2011;240:501–20.
Watson CL, Mahe MM, Munera J, Howell JC, Sundaram N, Poling HM, et al. An in vivo model of human small intestine using pluripotent stem cells. Nat Med. 2014;20:1310–4.
Xue X, Ramakrishnan S, Anderson E, Taylor M, Zimmermann EM, Spence JR, et al. Endothelial PAS domain protein 1 activates the inflammatory response in the intestinal epithelium to promote colitis in mice. Gastroenterology. 2013;145:831–41.
Schwank G, Koo BK, Sasselli V, Dekkers JF, Heo I, Demircan T, et al. Functional repair of CFTR by CRISPR/Cas9 in intestinal stem cell organoids of cystic fibrosis patients. Cell Stem Cell. 2013;13:653–8.
Drost J, van Jaarsveld RH, Ponsioen B, Zimberlin C, van Boxtel R, Buijs A, et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature. 2015;521:43–7.
Matano M, Date S, Shimokawa M, Takano A, Fujii M, Ohta Y, et al. Modeling colorectal cancer using CRISPR-Cas9-mediated engineering of human intestinal organoids. Nat Med. 2015;21:256–62.
Pabst O, Schneider A, Brand T, Arnold HH. The mouse Nkx2-3 homeodomain gene is expressed in gut mesenchyme during pre- and postnatal mouse development. Dev Dyn. 1997;209:29–35.
McGrath PS, Watson CL, Ingram C, Helmrath MA, Wells JM. The basic helix-loop-helix transcription factor NEUROG3 is required for development of the human endocrine pancreas. Diabetes. 2015;64:2497–505.
Yui S, Nakamura T, Sato T, Nemoto Y, Mizutani T, Zheng X, et al. Functional engraftment of colon epithelium expanded in vitro from a single adult Lgr5(+) stem cell. Nat Med. 2012;18:618–23.
van de Wetering M, Francies HE, Francis JM, Bounova G, Iorio F, Pronk A, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161:933–45.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing AG
About this chapter
Cite this chapter
Múnera, J.O., Wells, J.M. (2017). Stem Cells and Organoids to Study Epithelial Cell Biology in IBD. In: Baumgart, D. (eds) Crohn's Disease and Ulcerative Colitis. Springer, Cham. https://doi.org/10.1007/978-3-319-33703-6_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-33703-6_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-33701-2
Online ISBN: 978-3-319-33703-6
eBook Packages: MedicineMedicine (R0)