Abstract
Research traditionally outstrips regulation leading to a lag between scientific advances and regulatory frameworks. This is nowhere more apparent than in the arena of nanomaterials (NMs) safety testing. Here, regulatory focus has been on assessing the suitability of existing regulatory regimes and standardised assays for use with NMs. Meanwhile scientific focus has moved towards an acceptance of the fact that as-made or so-called pristine NMs do not exist in real products or the environments as a result of physical, chemical, biological and binding-related transformations which drive the NMs towards lower surface energy states. Thus, in parallel with the move towards alternative test methods, there is a need to support regulatory authorities in understanding the relevant species to test in the case of NMs risk assessment and how to best incorporate such new knowledge into regulation. This chapter appraises some of the steps that could support such a transition, including looking for precedent in contiguous regulatory models for assessing transformed variants (e.g. pesticide metabolites), considering grouping and read-across strategies for likely NMs transformations, and validating standard tests for NMs ageing. Finally, it will consider the legal issues surrounding manufacturer’s responsibility for providing safety data for materials that are no longer the as-produced materials. As there is an essentially infinite array of uses/formulations for NMs, all of which can transform the NM from its original form and composition; where does and should a manufacturer’s responsibilities end?
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Sewage Sludge
- Natural Organic Matter
- Plant Protection Product
- Sewage Sludge Amendment
- OECD Test Guideline
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Nanotechnology is a rapidly evolving enabling technology with the potential to revolutionise modern life. The global market for nanomaterials (NMs) is estimated at a market value of €20 billion, with the current direct employment in the NM sector estimated at up to 400,000 in Europe alone.Footnote 1 An estimated 20,000 different NMs are under development around the world, expected to total 11 million tonnes annual production (see Footnote 1). However, an increasing body of scientific evidence would suggest that some materials in their nanoform may induce harmful biological or environmental effects through a variety of potential mechanisms linked specifically to their nanoproperties, not all of which are fully understood or quantified as yet. A key confounding factor is that NMs, unlike conventional chemicals, are highly affected by their surroundings, transforming chemically, agglomerating and/or acquiring an evolving coating of environmental or biological macromolecules, which provides them with an ‘environmental’ or ‘biological’ identity that is distinct from their initial ‘synthetic’ identity (Fadeelet al., 2013; Lynch et al.,, 2014; Walczyk et al., 2010). Indeed, NMs are at the boundary between molecules and solid state behaviour, meaning that they can often display new and unusual properties, linked to both their small size (e.g. quantum confinement effects and access to biological receptors facilitating active internalisation by cells) and enormous surface area-to-volume ratios leading to highly reactive surfaces and enormous capacity for adsorbing molecules from their surroundings. Indeed it is the presence of such qualities and capacities that has driven research in nanotechnology and the development of products containing NMs. Factoring this context- and time-dependent evolution into assessment of the fate, behaviour and impacts of NMs is essential to move forward in terms of ensuring the safe implementation of nanotechnologies, and science-based regulation of new materials and the products that these enable (Valsami-Jones & Lynch, 2015).
There is, for instance, a clear need to increase the ‘environmental realism’ in the design and understanding of nano-(eco)safety assessments to account for the non-static nature of NMs in the environment, with the environment here including also human exposure (i.e. changes to NMs as a result of contact with skin, airways etc.). Increasing the realism of nanosafety studies includes, for example: use of relevant NM forms; consideration of the appropriate exposure medium (e.g. in light of the ongoing debate as to the ethics (Brunner et al., 2010) and relevance of the 10 % serum conditions used for in vitro studies to the in vivo situation (ESAC, 2008) and the potential for differential protein binding under the different conditions (Monopoli et al., 2011)) which can manifest as different uptakes and toxicities under the different conditions (Kim et al., 2014); testing of environmentally relevant (e.g. appropriately transformed, see below) chemistries and longer term and lower-dose exposures, again based on the physicochemical aspects of the properties of the NM driving their environmental fate. Given this complexity, the rapid pace of development of science, the cross-disciplinarity and cross-sectoral span of knowledge required, and the lack of skilled professionals in this area, there is a clear and pressing need to train a cohort of professionals to bridge academic and policy/regulatory/industry approaches to risk assessment of NMs.
A recent review of the (environmental) transformations of NMs categorised the types of transformation reactions undergone by NMs as chemical (e.g. photooxidation and photoreduction), physical (e.g. agglomeration or dissolution), biological (e.g. oxidation and carboxylation) and interactions with biomolecules, including proteins, polysaccharides, lipids and natural organic matter, all of which ultimately influence the NMs’ persistence, bioavailability/biouptake, reactivity and toxicity. Natural organic matter (NOM; originating from the decay of plant and animal matter) is a complex polydisperse polymeric mixture, whose properties echo their structural diversity as well as their state of aggregation, conformation and surface charge distribution (Lynch et al., 2014). The observed interactions of NMs and NOM are analogous to the interactions with proteins and the formation of ‘protein coronas’ in biological systems; the behaviour and impacts of NMs depends on the types and amounts of these biological and environmental molecules attached to their surfaces. Collectively these interactions provide a contextual or ‘environmental identity’ to the NMs that has to be taken into account when, for example effects are assessed in the environment (Cerrillo et al., 2015; Lynch et al., 2014; Nasser & Lynch, 2015).
There has been considerable debate worldwide as to whether existing regulatory approaches are sufficient to assess the human and environmental implications of NMs (Frater et al. 2006). Indeed, it is the dual role of REACH,Footnote 2 protecting both health and safety and industrial competitiveness, that is at the heart of much of the debate surrounding the applicability of REACH regulation for NMs, as industry are among the strongest voices saying that current regulations are sufficient to capture any potential risks of NMs, while the scientific community continue to call for additional research to answer this question (Malkiewicz et al., 2011; Lee and Vaughan 2010). The identification and mitigation of potential human and environmental risks is vital for consumer confidence and the continued growth of the nanotechnology sector.
A 2012 study by the Center for International Environmental Law in Switzerland, ‘Just Out of REACH’ identified four key gaps for NMs in the registration phase of REACH, an essential step that requires chemical manufacturers and importers to provide key health and safety information (Azoulay, 2012), namely:
-
REACH does not define NMs, and contains no nano-specific provisions
-
Most NMs evade registration until 2018; yet, they can still enter the EU market
-
REACH’s schedule for registration hinges on the number of tonnes of a chemical, essentially missing all NMs, which are generally produced in far smaller quantities
-
REACH test guidelines fail to consider the special properties of NMs.
The authors explored possible remedies to close these loopholes, but rejected the possibility of renegotiating REACH to add specific provisions on nanotechnology, as this would be practically challenging and could invite further weakening of the current regulation, in favour of developing a stand-alone regulation, carefully aligned with the chemical rules, but specifically tailored to NMs, with sufficient flexibility to allow for future adjustments as NMs are better understood, without requiring additional changes to REACH (Azoulay, 2012). This was preferred to amendments to the technical guidance, as it was suggested these would fall short of bridging the existing legal gaps (Azoulay, 2012).
A report funded by the SKEP ERA-NET (Scientific Knowledge for Environmental Protection) assessing the applicability of REACH to NMs also identified several challenges, including those listed above, as well as questioning the basis for the classification of some NMs as phase-in.Footnote 3 Thus, nanoforms of existing substances (i.e. those with an EINECS number) would, by default, be treated as phase-in substances. Thus, some NMs are considered as phase-in substances (e.g. gold and TiO2), while others are non-phase-in substances (e.g. fullerenes). The report indicates that there is no scientific evidence to suggest that those two groups of NMs (phase-in or non-phase-in) represent a different likelihood of causing a concern, and thus that there is no reason to treat them differently. Among the 22 recommendations in the report was that nanoforms of substances should be treated as different substances from their bulk counterparts and that none of the phase-in provisions should apply (Malkiewicz et al., 2011).
However, despite these and other reports calling for change, the approach chosen by the European Commission and the European Chemicals Agency has been to amend the Technical Guidance annexes to REACH rather than amend REACH itself:
Some needs for adjustments have been identified [by the REACH review report, Footnote 4 February 2013], but balanced against the interest of ensuring legislative stability and predictability, the Commission concludes that changes to the enacting terms of REACH will not be proposed.
The introduction of a major re-focussing of REACH by guidance raises questions of legitimacy given the lack of democratic engagement with such technical revision (Vaughan, 2015). The scope of the revision is focused on the technical aspects related to NMs set out in the REACH annexes. The final version of the amendments to REACH Annexes for NMs is still pending at the time of writing (November 2015), following an extensive consultation as to the costs and benefits of a proposed range of modifications, from business as usual through to introduction of a range of additional data and testing requirements (see Table 9.1), with the questionnaire asking participants to consider the implications of each measure for cost of regulation, safety of NMs and efficiency of the regulatory process. Given the focus on amending the technical annexes, the questionnaire was also of a technical nature and was designed primarily for the informed expert user.
The focus of regulatory research to date has been on assessing the suitability of existing regulatory regimes and standardised assays for control of NMs dispersion and presentation to the test system/organisms. For example, the OECD expert meeting on Physical-Chemical Properties of Manufactured Nanomaterials and Test Guidelines (2013 in Querétaro, Mexico) assessed the applicability of existing OECD Test Guidelines (TG) on Physical-chemical Properties of Manufactured Nanomaterials and identified the need to update current or develop new OECD Test Guidelines and/or OECD Guidance Documents (GD) which are relevant for safety and regulatory decision making (OECD, 2014). The categories of endpoints selected were (a) State of Dispersion, Aggregation and Agglomeration of NMs; (b) Size (and Size Distribution) of NMs; (c) Surface Area and Porosity and (d) Surface Reactivity (OECD, 2014). An ecotoxicology and environmental fate (of NMs) focused expert meeting suggested that tiered approaches or decision trees be established in order to provide guidance on three main steps (a) stock/stem suspension preparation, (b) preparation of exposure suspension and (c) conducting the tests (Kühnel & Nickel, 2014). Among the key recommendations (see Table 9.2 for the sub-set related to environmental fate) were that ‘more knowledge on effects of aged or transformed NM as the environmentally more relevant fraction’ is needed. This was also linked to the widely agreed need for physical chemical characterisation of NM, which is considered essential for all subsequent steps of testing (and thus includes any interactions and transformations) (Kühnel & Nickel, 2014). These expert recommendations are supported by a recent evaluation of the REACH guidance with regard to NM which indicated that REACH guidance was found not to fully cover the specific environmental fate of NM (alterations, dissolution and partitioning) and hence needs adjustments (Meesters et al., 2013). In this context, degradation was defined as changes in the NM surfaces, for example by oxidation processes or changes of coatings while transformation was defined as basic changes in NM composition or form, for example dissolution processes or heteroaggregation (Levard et al., 2012).
Thus, consideration of the dynamic nature of NMs, and their evolution and transformation by their surroundings, is slowly trickling into regulatory consciousness, although is still a long way behind scientific knowledge regarding environmental transformations of NMs. For example, scientific focus has moved towards an acceptance of the fact that as-made or so-called pristine NMs do not exist in real products or the environments as a result of physical, chemical, biological and binding-related transformations which drive the NMs towards lower surface energy states. This is evidenced by the fact that scientific journals are demanding characterisation in the relevant test media as a condition of publication, for example. Additionally, multiple studies are emerging in the literature showing quite different physicochemical properties of pristine versus aged NMs which are often linked to significantly different (eco)toxicological responses; for example, a comparison of the aqueous behaviour between newly purchased commercially manufactured copper nanoparticles (NPs) to NPs that were allowed to sit in the laboratory environment for several years under ambient conditions revealed that the (aged) NPs exhibited unique chemistry including oxide phases that form during storage and surface adsorption properties (Mudunkotuwa et al., 2012). Additionally, the aged NPs exhibited differences in solubility, aggregation and reactivity that can affect the mobility and toxicity of these materials (Mudunkotuwa et al., 2012). The authors of the copper NPs study suggested that having a clear understanding of how these NMs will change upon aging, and consequent alterations in their physicochemical properties will enable establishing reliable structure–activity correlations, a critical step in moving beyond the current case-by-case analysis risk assessment of NMs (Mudunkotuwa et al., 2012). Taking this a step further, Izak-Neu and co-authors assessed the effect of storage time and storage conditions on the observed toxicity of AgNPs and demonstrated that AgNPs’ ‘aging’ during storage (even under optimal conditions) resulted in changes in their cytotoxicity and suggested that a clear and time-resolved understanding of the changes in physicochemical characteristics of any metal NPs occurring under different conditions seems to be crucial for the interpretation of their biological effects (Izak-Nau et al., 2015). The most influential factors of AgNPs’ ‘aging’ were found to be higher temperature and exposure to daylight, with the nature of the capping agent and the stabilisation mechanism also contributing. On the basis of the evidence presented here, one important recommendation for nanosafety assessment studies is to periodically monitor the crucial NMs’ physicochemical parameters such as size/agglomeration, surface charge and dissolution throughout the duration of the study to ensure that any changes can be accounted for in the data interpretation and analysis. It might also be good practice to note the total time period between NM synthesis, characterisation and toxicity testing, with periodic (e.g. monthly) re-testing of parameters such as size distribution to ensure relevance of the characterisation data to the toxicity data (Izak-Nau et al., 2015). Similar impacts of ageing (in Milli Q water) of Zinc oxide NMs on the mutagenicity of the NMs to human–hamster hybrid (AL) cells were found, whereby the ZnO NMs underwent sophisticated physicochemical transformations with aging such as microstructural changes, the formation of hydrozincite (Zn5(CO3)2(OH)6) and the release of free zinc ions (Wang et al., 2015). Interestingly, the aged ZnO NMs resulted in much lower cytotoxicity but a relatively higher degree of mutation than fresh ZnO NMs (Wang et al., 2015).
2 Understanding/Predicting the Relevant Species to Test
Based on current knowledge, predicting the distribution and bioavailability of any NM in the environment is highly speculative, but may depend on a number of the following variables (Malkiewicz et al., 2011):
-
Initial physicochemical characteristics of the NM. Core chemistry, size, particle charge and surface functionality (Jarvie & King, 2010) are of particular relevance.
-
The form in which it is released (free/embedded in a matrix).
-
The environmental compartment into which it is released (air, soil/sediment matrices, freshwater and marine) (Navarro et al., 2008).
-
The interactions that occur with both abiotic and biotic components of the natural environment, and how these may transform the NM (Lowry et al., 2012).
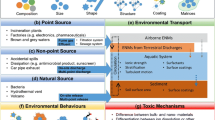
Figure 9.1 shows possible mechanisms of interactions between an idealised NM and abiotic and biotic elements that are predicted to be the most influential in determining NM fate. Research is underway to determine the kinetics of these different transformations, both in the environment generally (Ma et al., 2013), and in cells or organisms specifically (Chen et al., 2013), and which occur under such circumstances (Dale et al., 2015) such that in the future quantitative structure–transformation relationships will be a possibility, linked with the chemistry (Liu et al., 2013) or biochemistry (Prins, 2015) of the surroundings. For example, using water chemistry data from across Europe coupled with data regarding NM agglomeration rates in vitro, it was possible to predict NM stability in the water column (Liu et al., 2013), which provides insights regarding NM transport and bioavailability, for example. Prins (2015) suggested that the multivalent nature of gold NMs in contact with biological systems permits functional roles in biomolecular affinity and signal transduction, as multiple non-covalent interactions with small molecules that enhances affinity, but is also the basis of simple signal transduction pathways and adaptive behaviour (Prins, 2015). These relationships can then be further linked to quantitative structure–activity relationships (QSARs) for toxicity (Gajewicz et al., 2015) utilising either the pristine or transformed forms, depending on which proves to be more predictive of uptake and toxicological effect of NMs (Toropova et al., 2015).
Additionally, due to the enormous surface area to volume ratio, and the high proportion of molecules at the surface, NMs have a high surface energy that they seek to lower by binding to available biomolecules from their surroundings such as components of product formulations, proteins or lipids in living systems, natural organic matter (NOM) components of water or soil or exuded and secreted biomolecules in complex ecosystems (Lynch et al., 2014; Nasser & Lynch, 2015). Formation of a biomolecule corona around NMs is a ubiquitous phenomenon that occurs instantaneously upon contact with available macromolecules. Research to date has focussed on the interactions of NMs with blood proteins (human or animal sera) or lung surfactant proteins to correlate corona composition with NM uptake and impacts on living systems (Albanese et al., 2014; Di Silvio et al., 2015; Duan et al., 2015; Halamoda-Kenzaoui et al., 2015). Environmental interactions to date have focussed on NM–NOM interaction studies, primarily assessing the impact of the humic substances on particle stability/bioavailability (Lynch et al., 2014). Much less work has investigated the potential for NMs to bind the exuded biomolecules central to much of the plant and microorganism world (Nasser & Lynch, 2015), where secretion of biomolecules can be a defensive response to repel insect attack, or an offensive habit to repel other incompatible or competitive plants (Nordlund & Lewis, 1976). Early work in this direction has assessed the binding of proteins secreted by Daphnia magna and their influence on NM uptake and toxicity to Daphnia, illustrating a clear enhancement of NM uptake and a lower EC50 in the presence of the secreted corona (Nasser & Lynch, 2015).
Approaches to predict the reactivity of metals, and thus the transformations that they will undergo in the environment and subsequent uptake by biological organisms include the Hard–soft acid base theory (HSAB theory; also termed Pearson’s acid base theory) (Pearson, 1963). According to the HSAB concept, hard acids prefer binding to the hard bases to give ionic complexes, whereas the soft acids prefer binding to soft bases to give covalent complexes. The HSAB classification, which has been determined empirically, provides an ordering of transition metals according to their preferences for specific organic ligands (Fig. 9.2). For example, soft acids (such as Hg(II), Cu(I), Ag(I) and cadmium(II) (Cd(II))) and borderline acids (such as Co(II), Ni(II), Cu(II) and Zn(II)) tend to associate tightly with soft bases, such as the sulfhydryl (R–SH) groups that are found in proteins. Consequently, the antibacterial toxicity of these metals is approximately proportional to their affinity for soft bases (Workentine et al., 2008), again potentially allowing for development of predictive transformation–activity relationships. Since many of the commonest NMs are composed of elements in the soft acid category (see Fig. 9.2), HSAB is a useful tool to predict environmental transformations in the environment, such as the tendency for AgNMs to be sulphidised in environments containing high sulphur contents, such as fresh or sea water or waste water treatment plants (Kent et al., 2014). While the concept is well established in assessing metal toxicity, including for predicting metal toxicity to microbes (Lemire et al., 2013); for example, it has yet to be applied for assessing or predicting the binding of specific protein sequences (epitopes) to metal or metal oxide NMs or as a means to predict toxicity for NMs. HSAB has been used to predict propensity for covalent binding of electrophiles to biological substrates (Carlson, 1990), and since protein binding is linked with NM uptake (Albanese et al., 2014; Walkey et al., 2014), there is certainly scope for predicting NMs biological and ecological coronas on this basis.
Pearson’s Hard–soft acid base (HSAB) theory (Pearson, 1963) can predict the selectivity of metal ions for biological donor ligands. Hard acids and bases tend to have a smaller ionic radius, a high oxidation state and weak polarisability. By contrast, soft species tend to have a large ionic radius, a low oxidation state and strong polarisability. Hard acids react preferentially with hard bases, and soft acids with soft bases. The affinity of a hard acid for a hard base is mostly ionic in nature, whereas the interaction between a soft acid and soft base is mostly covalent. Acids and bases that have an intermediate character are classified as borderline. This classification scheme is qualitative and can be used to predict the binding preferences of metals even in complex mixtures of donor ligands (Haas & Franz, 2009; Waldron et al., 2009). Electronegativity describes the tendency of an atom to attract electrons towards it. By contrast, polarisability refers to the tendency of the electrons around an atom to be distorted from their regular distribution, typically towards the nucleus of another, more electronegative atom. With permission from (Lemire et al., 2013)
Grouping of substances and read-across is one of the most commonly used alternative approaches for filling data gaps in registrations submitted under REACH.Footnote 5 This approach uses relevant information from analogous (‘source’) substances to predict the properties of ‘target’ substances. If the grouping and read-across approach is applied correctly, experimental testing can be reduced as there is no need to test every target substancee. A recent proposal from the European Centre for Ecotoxicology and Toxicology of Chemicals (ECETOC), ‘Nano Task Force’, for a Decision-making framework for the grouping and testing of NMs (DF4nanoGrouping) identifies four main NMs groups encompassing (1) soluble NMs, (2) biopersistent high aspect ratio NMs, (3) passive NMs and (4) active NMs. Since the exact correlation of intrinsic material properties and apical toxic effect is not yet established, the DF4nanoGrouping uses the ‘functionality’ of NMs for grouping rather than relying on intrinsic material properties alone. However, in light of the transformations that NMs undergo, including in cells and organisms, grouping on the basis of transformed or aged forms may prove more predictive. To illustrate this, Fig. 9.3, adapted from the grouping proposal of Stuber et al. (as the outcome from a Swiss workshop on REACH applicability to NMs), illustrates that grouping NMs according to e.g. their initial (Time 0) or transformed (during exposure) physicochemical properties and linked to their toxicological characteristics would reduce testing efforts.
Adaption of read-across strategy proposed by Studer et al. (2015) for NMs. NMs require additional characterisation in comparison to conventional chemicals. After their characterisation, they can be associated with predefined clouds of similar NMs that require the same testing strategy. Some clouds behave similarly for a particular endpoint, which allows read-across clouds for this specific endpoint (dashed blue oval, in this example endpoint A and B for Cloud 1 and 2). Therefore, testing efforts can be significantly decreased the more information is known. In the example above, Cloud 2 needs only to be tested on Endpoint(s) x, because information from Cloud 1 can be used for endpoint A and B (but not for endpoint x). A prerequisite for an efficient testing strategy is a validated grouping scheme. As an extension to this approach, we also consider that the characterisation during the exposure time might actually be the more relevant one, and thus that read-across should be from Cloud 1 to Cloud 3 (red dotted line) assuming that the transformed NMs characteristics are similar. Adapted from Studer et al. (2015)
3 Understanding Appropriate Timescales and Formats for Testing NMs
Given the dynamic nature of NMs and their transformations in the environment, current (although limited) approaches to long-term exposure and hazard testing may also need to consider the appropriate form of the NM to test. Since many such approaches require replacement of the exposure media periodically, usually with freshly dispersed NMs, the exposure form introduced at the subsequent timepoints will not be representative of the real (continuous) exposure, as a result of re-introduction of the pristine NMs. One possible approach would be to determine the total media amount needed and add the NMs to the media from the outset such that the replacement media contains NMs the same ‘age’ as the organism-exposed media.
At present, only limited information about the potential impact of aging on NM toxicity to organisms is available, although what is published indicates the need to re-assess how we do toxicity studies and what form of the NM is appropriate to test. A study investigated acute (96 h) and chronic (21 days) implications of systematically aged titanium dioxide NMs (nTiO2; ~90 nm) on the standard test species Daphnia magna following the respective test guidelines. The nTiO2 were aged for 0, 1, 3 and 6 days in media with varying ionic strengths (Milli-Q water: approx. 0.00 mmol/L and ASTM: 9.25 mmol/L) in the presence or absence of natural organic matter (NOM). Irrespective of the other parameters, aging in Milli-Q did not change the acute toxicity relative to an unaged control. In contrast, 6 days aged nTiO2 in ASTM without NOM caused a fourfold decreased acute toxicity. Relative to the 0 day aged particles, nTiO2 aged for 1 and 3 days in ASTM with NOM, which is the most environmentally relevant setup used here, significantly increased acute toxicity (by approximately 30 %), while a toxicity reduction (60 %) was observed for 6 days aged nTiO2. Comparable patterns were observed during the chronic experiments. A likely explanation for this phenomenon is that the aging of nTiO2 increases the particle size at the start of the experiment or the time of the water exchange from <100 nm to approximately 500 nm, which is the optimal size range to be taken up by filter feeding D. Magna (Seitz et al., 2015). If subjected to further agglomeration, larger nTiO2 agglomerates, however, cannot be retained by the daphnids’ filter apparatus ultimately reducing their ecotoxicological potential. This non-linear pattern of increasing and decreasing nTiO2-related toxicity over the aging duration highlights the knowledge gap regarding the underlying mechanisms and processes (Seitz et al., 2015).
Another study addressed the relative importance of particle coating, sewage sludge amendment and aging on aggregation and dissolution of manufactured Ag NPs in soil pore water. Ag NPs with citrate (CIT) or polyvinylpyrrolidone (PVP) coatings were incubated with soil or municipal sewage sludge which was then amended to soil (1 % or 3 % sludge (w/w)). Pore waters were extracted after 1 week and 2 and 6 months and analysed for chemical speciation, aggregation state and dissolution. Ag NP coating had profound effects on aggregation state and partitioning to pore water in the absence of sewage sludge, but pre-incubation with sewage sludge negated these effects. This suggests that Ag NP coating does not need to be taken into account to understand fate of Ag NPs applied to soil through biosolids amendment. Aging of soil also had profound effects that depended on Ag NP coating and sludge amendment (Whitley et al., 2013).
4 Manufacturer’s Responsibility Regarding ‘Transformed’ NMs?
Underpinning the REACH regime is the notion that industry is best placed to monitor the chemicals which they place on the market. Manufacturers, importers and downstream users are required to ensure that the chemicals they manufacture, import or use do not adversely affect human health or the environment (Lee & Stokes, 2009). Currently, the onus is on the NM (chemical) manufacturer or importer to ensure safety for proposed applications of their downstream users. In light of NMs and nanotechnologies status as an enabling technology and the vast range of products that incorporate NMs, is it possible for the manufacturer of an NM to foresee the eventualities of use, especially in fast-growing areas such as green energy? Can the person at the beginning of the NMs life-cycle (the manufacturer/importer) foresee all eventualities, including the transformations of the NMs under different exposure scenarios and test for them?
The enormous reactive surface area of NMs confers many NMs the ability to sorb and transform pollutants, a feature that has been exploited for bioremediation applications of, for example heavy metals, pharmaceuticals or pesticides using nanoscale zero valent Iron particles (El-Temsah et al., 2015; Kanel et al., 2006; Machado et al., 2013). Whether the presence of an NM in a polluted environment ameliorates (e.g. influence of carbon nanotubes (CNTs) on pyrene bioaccumulation in earthworms (Petersen et al., 2009)), or intensifies (e.g. increase in uptake of Cu by D. magna in the presence of single-walled CNTs (Kim et al., 2010)), the toxicity of the secondary compound will be dependent on the specific form these interactions take, which in turn depends on the physicochemical properties of the NMs, its chemical composition and the properties of the surrounding medium (ionic strength, pH, etc.) (Lynch et al., 2014; Malkiewicz et al., 2011; Yang et al., 2013). A scenario can also be envisaged whereby an interaction with an NM widens the environmental distribution of a secondary pollutant, for example the aggregation and sedimentation of an NM with a secondary pollutant sorbed from the water column. This dual ability of NM to both elute (e.g. catalyst or other contaminants (Kim et al., 2010)) and sequester and transport potentially toxic materials (known as the Trojan-horse effect (Auffan et al., 2012)) is shown schematically in Fig. 9.4. Such effects, and specifically who is responsible in the legal and regulatory sense for the transformed NMs, needs to be addressed within regulation.
Illustration of some of the new challenges related to regulation of NMs, whereby they can release chemicals to the environment, and also sequester chemicals to them, presenting the adsorbed chemicals in new ways at the NMs surface. Both of these phenomena can lead to increased bioavailability of the chemicals, and to new toxicities not previously regulated for. Additionally, fate and behaviour of the NMs in the environment must be addressed critically, including assessment of their bioaccumulation and biopersistence rate and their metabolism or biotransformation potential in various environmental and biological compartments and species
The question then becomes whether a manufacturer could foresee that his harmless NMs would end up in an environmental compartment where it collected substances from conventional industrial discharge and concentrated them to a degree where the exposure became significant to organisms that encountered/ingested the NMs? Is the manufacturer responsible for providing safety data for materials that are no longer the as-produced NMs? As there is an essentially infinite array of uses/formulations for NMs, all of which can transform the NM from its original form and composition, where does and should a manufacturer’s responsibilities end?
The 27th Report of the Royal Commission on Environmental Pollution (RCEP), which focussed in large part on NMs (Royal Commission on Environmental Protection (RCEP), 2008), also picked up on some issues surrounding manufacturer responsibility within REACH. RCEP warns that substantial amendments will be needed, and considers several options including the extension of product ‘take-back’ requirements, such as those contained in the Waste Electrical and Electronic Equipment (WEEE) Directive, to products containing NMs, with the goal of minimising environmental exposure to potentially hazardous substances at the end of their life by enabling consumers to return a product to the original retailer or manufacturer (Lee & Stokes, 2009).
5 Mechanisms to Support Regulatory Authorities Regarding NMs Risk Assessment
While clarifying uncertainties with regard to existing regulatory frameworks is essential, there is also a need to organise and use the information that is available in a more productive and integrated manner. One approach to doing this is building integrative technology roadmaps for nanotechnology-risk governance, and continuous refinement of the methodology through application via case studies (Malkiewicz et al., 2011).
Pastoor et al. (2014) suggest a comprehensive framework for bringing together knowledge to enable effective decision making (Pastoor et al. 2014). The so-called RISK21 framework is presented as a problem formulation-based, exposure-driven, tiered data acquisition approach that incorporates exposure and toxicity estimates and their respective uncertainties to guide informed human health safety decisions as soon as sufficient evidence is acquired to address the specific problem formulation (Arts et al., 2015; Pastoor et al. 2014). The value of the roadmap, as described by the authors, is its capacity to chronicle the stepwise acquisition of scientific information and display it in a clear concise fashion: detailed exposure and toxicity data can be coalesced into an understandable rendering that can be flexibly revisited as new information is generated. The approach is non-judgemental with regard to the methodological origin of the data, as long as they can be expressed in a common metric (Pastoor et al. 2014).
Meesters et al. propose that incorporation of the specific environmental fate processes of engineered NMs into the environmental-risk assessment framework of REACH requires a pragmatic approach; they identified three major assumptions made in REACH guidance that are not applicable to NMs and suggest prioritisation of efforts accordingly: (1) in REACH, environmental alteration processes are all thought of as removal processes, whereas alterations of NMs in the environment may greatly affect their properties, environmental effects and behaviour; (2) in REACH, chemicals are supposed to dissolve instantaneously and completely on release into the environment, whereas NMs should be treated as non-dissolved nano-sized solids and (3) in REACH, partitioning of dissolved chemicals to solid particles in air, water and soil is estimated with thermodynamic equilibrium coefficients, but in the case of NMs, thermodynamic equilibrium between ‘dispersed’ and ‘attached’ states is generally not expected (Meesters et al., 2013). By focusing on the specific aspects of where NMs differ from classical chemicals, it is possible to rapidly assess where additional or alternative testing approaches are required, such as alterations to the Technical Guidance and/or annexes of REACH. A similar pragmatic approach has been suggested for consideration of a framework for regulation of nano-formulated pesticides, where it was proposed that the nanocomponent only needed to be considered from a regulatory perspective as long as it was associated with the active ingredient and thus could potentially affect its toxicokinetics (rate of uptake) or toxicodynamics (function) (Kookana et al., 2014). Thus, it was recommended to consider the durability of the NM-active ingredient (a.i.) complex and its persistence and mobility in order to identify cases where only the a.i. needed to be tested (in the usual manner as for non-nano a.i.s) versus those cases where only the NM-a.i. complex needed testing due to the fact that the a.i. is never separated from the nanocarrier, or the intermediate scenario where all three species needed to be assessed (Kookana et al., 2014).
A similar strategy, of focusing on the specific aspects or emergent properties that made new hierarchical NMs (called nanohybrids) different from their conventional NM counterparts, has recently been suggested (Saleh et al., 2015). Within the existing regulatory framework, the guiding principle remains to determine the influencing property or properties that will dictate nanohybrid materials’ release, fate and transport, exposure and toxicity. However, when such properties are the result of conjugation or hybridisation, the possible combinations of multiple materials are extremely large and go beyond the challenges around NM size, shape and coatings type that are currently being addressed systematically by the nano safety community. Strategies are needed to rationally narrow down this ever-expanding space, so that comprehensive nano safety evaluation can be performed with reliability and in a timely manner. Central to evaluation of nanohybrids (NH) is an assessment of the stability (integrity) of the ensemble material during environmental transport, transformation and exposure (Saleh et al., 2015). NHs that maintain their unique properties in environmental and biological media could have unique, yet to be studied, environmental health and safety implications; so the stability of these NHs under environmentally relevant conditions needs to be evaluated (Saleh et al., 2015).
6 Regulatory Precedent for Assessing Transformed Variants (E.g. Drug and Pesticide Metabolites)
The European Commission Guidance Document on the Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council Directive 91/414/EEC (2003) provides a framework to assess the relevance of metabolites found in groundwater, the major environmental compartment of concern in the EU. Groundwater was identified as a natural resource which should be protected using a ‘limit value’ for active substances and their relevant metabolites (provided in Annex VI of Directive 91/414/EC (and Directive 98/83/EC)). The guidance document describes a scheme to determine whether a metabolite is relevant or not relevant using criteria of biological activity, genotoxicity and toxicological hazard (Terry et al., 2015). Relevant metabolites are subject to the 0.1 μg/L limit value in groundwater. Non-relevant metabolites are non-genotoxic metabolites without specific hazard properties (toxic, carcinogenic and toxic to reproduction) and with no, or significantly reduced, biological efficacy against pests and are subject to a refined human health-risk assessment when their concentration in groundwater is estimated to be above 0.75 μg/L.
Originally proposed for substances intended for use in food-packaging materials (Frawley, 1967), the threshold of toxicological concern (TTC) describes a level of exposure that is considered to represent negligible risk to humans. TTC was extended (Munro et al., 1996) to develop human exposure thresholds for non-genotoxic chemicals for three structural classes of chemicals based on the Cramer decision tree (Cramer et al., 1978):
-
Class I: structurally simple chemicals that are efficiently metabolised, with low potential for toxicity.
-
Class II: chemicals of intermediate concern that are less innocuous than class I substances but that lack the positive indicators of toxicity that are characteristic of class III chemicals.
-
Class III: chemicals which have structures suggestive of significant toxicity or those which cannot be presumed safe.
TTCs are analogous to chemical-specific reference doses, such as an Acceptable Daily Intakes, but as generic reference values, a TTC can be used to assess the risk from estimated exposures for chemicals with limited toxicity data (EFSA, 2012; Terry et al., 2015).
A recent development of the TTC concept was to introduce the approach of comparative toxicity, which was used to determine the environmental metabolites of a new chemical, sulfoxaflor (X11422208) (Terry et al., 2015). The ultimate aim was to address the human safety of the metabolites with the minimum number of in vivo studies, while at the same time, ensuring that human safety would be considered addressed on a global regulatory scale (Terry et al., 2015). The comparative toxicity component was designed to determine whether the metabolites had the same or similar toxicity profiles to their parent molecule, and also to one another, with the ultimate goal of establishing whether the metabolites had the potential to cause key effects—such as cancer and developmental toxicity, based on mode-of-action (MoA) studies—and to develop a relative potency factor (RPF) compared to the parent molecule (Terry et al., 2015).
Another domain where metabolites are a well-established concern is pharmacology and medicine design. Here, species differences in drug metabolism present challenges that may confound the non-clinical safety assessment of candidate drugs: The first challenge is encountered when metabolites are formed uniquely or disproportionately in humans (Powley et al., 2009). Another challenge is understanding the human relevance of toxicities associated with metabolites formed uniquely or disproportionately in a non-clinical species (Powley et al., 2009). Approaches suggested to overcome this include development of genetically modified organisms (e.g. human P450 expressing models) whose metabolism profiles more closely resemble humans. When compared to the current strategy for handling metabolite challenges (i.e. direct administration of metabolite), identifying an appropriate human P450 expressing model could provide a number of benefits, including improved scientific relevance of the evaluation, decreased resource needs and a possible reduction in the number of animals used. These benefits may ultimately improve the quality and speed by which promising new drug candidates are developed and delivered to patients, and could potentially be adapted for assessment of NMs transformation products.
7 Lessons from Other Areas: What Could Be Adapted for Transformed NMs?
While NMs do present multiple new challenges for regulators, specifically around transformation and ageing from the pristine or as-produced material, they are certainly not alone in this. A well-known example from regulation is the issue of metabolites and degradates of pesticides and their residues in food, and indeed, there has recently been a suggestion that these should be regulated alongside the starting active ingredient in terms of the residue definition for dietary-risk assessment (EFSA Panel on Plant Protection Products and their Residues (PPR), 2012). Thus, while a comprehensive toxicological dossier is developed for parent compounds, prior to approval of substances for use within the EU (Regulation EC (No) 1107/2009), there is often only limited information available about the toxicological properties of metabolites (EFSA Panel on Plant Protection Products and their Residues (PPR), 2012). In light of this, in 2012 the European Food Safety Authority (EFSA) asked its Plant Protection Products and their Residues Panel to develop an opinion on approaches to evaluate the toxicological relevance of metabolites and degradates of pesticide active substances in dietary-risk assessment. A key issue was to determine whether a metabolite would be tested along with the parent compound in laboratory species as part of routine assessment, or whether, due to its formation in vivo in specific plants or livestock following exposure, a specific metabolite was not available for testing. On the basis of this analysis, the panel made a series of recommendations regarding an alternative approach to assessment of pesticide metabolites. The report developed 12 recommendations for pesticide metabolites, summarised in Table 9.3, many of which could also be applicable to aged or transformed NMs.
8 Conclusions
Given the undisputed fact that NMs age, transform and evolve from their pristine state as-produced, through their formulation and use phase, and upon contact with living systems, be that intentional (e.g. nanomedicines, nano-enhanced foods, textiles or cosmetics) or unintentional (e.g. following excretion of nanomedicines into wastewater, washing of textiles, etc.) regulation for NMs needs to evolve to capture these transformed states and assess their toxicity relative to the parent NM. There is emerging regulatory precedent for this in food, pesticide and medicine regulation that could be adapted for NMs in consumer and industrial products. For example, analysis of the toxicity of metabolites produced in human and non-human species is encoded in medicine and pesticide regulation, and the TTC concept has been extended to include comparative toxicity, which has been used to determine the environmental metabolites of a chemical, for example. In all cases, a clear focus on where the NM and the transformed NM differ from conventional chemicals/macroscale particles needs to be centre stage in considering additional testing requirements in order to be pragmatic and not stifle innovation or commercial activity. Thus, if the transformation is to the ionic metal, then classical metal toxicity testing applies, while if the transformation is to an increasingly stable sulphidated form, then the testing should consider NM lifetime, stability in various environments and final environmental sinks, in addition to the types of degradation and metabolites that might result over time in these sinks. A point for clarification remains in terms of manufacturer/importer responsibility for ensuring the safety of environmentally transformed variants of the original NM, which will require further debate and discussion as more data on this topic emerges and fate and behaviour data are more deeply embedded into life cycle approaches and regulatory frameworks.
Notes
- 1.
- 2.
- 3.
NMs will be considered as “phase-in” if they or their base substance are listed on the European Inventory of Existing Commercial Chemical Substances (EINECS) are considered as No-Longer Polymers or have been manufactured in the EU but not placed on the market between 1st of June 1992 and 1st of June 2007.
- 4.
Report from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the regions in accordance with Article 117(4) of REACH and Article 46(2) of CLP, and a review of certain elements of REACH in line with Articles 75(2), 138(2), 138(3) and 138(6) of REACH {COM(2013)0049}. Available: http://www.ipex.eu/IPEXL-WEB/dossier/document/COM20130049.do.
- 5.
Bibliography
Albanese, A., Walkey, C. D., Olsen, J. B., Guo, H., Emili, A., & Chan, W. C. (2014). Secreted biomolecules alter the biological identity and cellular interactions of nanoparticles. ACS Nano, 8, 5515–5526.
Arts, J. H. E., Mackenzie Hadi, M., Irfan, M.-A., Keene, A. M., Kreiling, R., Lyon, D., … Landsiedel, R. (2015). A decision-making framework for the grouping and testing of nanomaterials (DF4nanoGrouping). Regulatory Toxicology and Pharmacology, 71, S1–S27.
Auffan, M., Rose, J., Proux, O., Masion, A., Liu, W., Benameur, L., … Bottero, J. Y. (2012). Is there a Trojan-horse effect during magnetic nanoparticles and metalloid cocontamination of human dermal fibroblasts? Environmental Science and Technology, 46, 10789–10796.
Azoulay, D. (2012). Nanorisk: Nanomaterials “Just Out of REACH” of EU regulations. The Center for International Environmental Law. Retrieved from http://www.ciel.org/Publications/Nano_Reach_Study_Feb2012.pdf
Brunner, D., Frank, J., Appl, H., Schöffl, H., Pfaller, W., & Gstraunthaler, G. (2010). Serum-free cell culture: The serum-free media interactive online database. ALTEX, 27, 53–62.
Carlson, R. M. (1990). Assessment of the propensity for covalent binding of electrophiles to biological substrates. Environmental Health Perspectives, 87, 227–232.
Cerrillo, C., Barandika, G., Igartua, A., Areitioaurtena, O., & Mendoza, G. (2015). Towards the standardization of nanoecotoxicity testing: Natural organic matter ‘camouflages’ the adverse effects of TiO2 and CeO2 nanoparticles on green microalgae. Science of the Total Environment, 543, 95–104.
Chen, S., Goode, A. E., Sweeney, S., Theodorou, I. G., Thorley, A. J., Ruenraroengsak, P., … Porter, A. E. (2013). Sulfidation of silver nanowires inside human alveolar epithelial cells: A potential detoxification mechanism. Nanoscale, 5, 9839–9847.
Cramer, G. M., Ford, R. A., & Hall, R. L. (1978). Estimation of toxic hazard—A decision tree approach. Food and Cosmetics Toxicology, 16, 255–276.
Dale, A. L., Casman, E. A., Lowry, G. V., Lead, J. R., Viparelli, E., & Baalousha, M. (2015). Modeling nanomaterial environmental fate in aquatic systems. Environmental Science and Technology, 49(5), 2587–2893.
Di Silvio, D., Rigby, N., Bajka, B., Mackie, A., & Baldelli Bombelli, F. (2015). Effect of protein corona magnetite nanoparticles derived from bread in vitro digestion on Caco-2 cells morphology and uptake. International Journal of Biochemistry and Cell Biology, S1357–2725, 30044-3.
Duan, G., Kang, S. G., Tian, X., Garate, J. A., Zhao, L., Ge, C., & Zhou, R. (2015). Protein corona mitigates the cytotoxicity of graphene oxide by reducing its physical interaction with cell membrane. Nanoscale, 7, 15214–15224.
EFSA. (2012). EFSA scientific committee; scientific opinion on exploring options for providing advice about possible human health risks based on the concept of threshold of toxicological concern (TTC). EFSA Journal, 10, 2750.
EFSA Panel on Plant Protection Products and their Residues (PPR). (2012). Scientific opinion on evaluation of the toxicological relevance of pesticide metabolites for dietary risk assessment. EFSA Journal, 10, 2799 [2187 pp].
El-Temsah, Y. S., Sevcu, A., Bobcikova, K., Cernik, M., & Joner, E. J. (2015). DDT degradation efficiency and ecotoxicological effects of two types of nano-sized zero-valent iron (nZVI) in water and soil. Chemosphere, 144, 2221–2228.
ESAC. (2008). ESAC statement on the use of FCS and other animal-derived supplements. Retrieved November 30, 2015, from https://eurl-ecvam.jrc.ec.europa.eu/about-ecvam/archive-publications/publication/ESAC28_statement_FCS_20080508.pdf
Fadeel, B., Feliu, N., Vogt, C., Abdelmonem, A. M., & Parak, W. J. (2013). Bridge over troubled waters: Understanding the synthetic and biological identities of engineered nanomaterials. Wiley Interdisciplinary Reviews: Nanomedicine and Nanobiotechnology, 5, 111–129
Frater, L., Stokes, E., Lee, R., & Oriola, T. (2006). An overview of the framework of current regulation affecting the development and marketing of nanomaterials: A report for the DTI. London: Department of Trade & Industry.
Frawley, J. P. (1967). Scientific evidence and common sense as a basis for food-packaging regulations. Food and Cosmetics Toxicology, 5, 293–308.
Gajewicz, A., Schaeublin, N., Rasulev, B., Hussain, S., Leszczynska, D., Puzyn, T., & Leszczynski, J. (2015). Towards understanding mechanisms governing cytotoxicity of metal oxides nanoparticles: Hints from nano-QSAR studies. Nanotoxicology, 9, 313–325.
Haas, K. L., & Franz, K. J. (2009). Application of metal coordination chemistry to explore and manipulate cell biology. Chemistry Review, 109, 4921–4960.
Halamoda-Kenzaoui, B., Ceridono, M., Colpo, P., Valsesia, A., Urbán, P., Ojea-Jiménez, I., … Kinsner-Ovaskainen, A. (2015). Dispersion behaviour of silica nanoparticles in biological media and its influence on cellular uptake. PLoS One, 10, e0141593.
Hassellöv, M., & Kaegi, R. (2009). Analysis and characterization of manufactured nanoparticles in aquatic environments. In J. R. Lead & E. Smith (Eds.), Environmental and human health impacts of nanotechnology. Oxford, England: Wiley.
Izak-Nau, E., Huk, A., Reidy, B., Uggerud, H., Vadset, M., Eiden, S., … Lynch, I. (2015). Impact of storage conditions and storage time on silver: nanoparticles’ physicochemical properties and implications for their biological effects. RSC Advances, 5, 84172–84185.
Jarvie, H. P., & King, S. M. (2010). Just scratching the surface? New techniques show how surface functionality of NPs influences their environmental fate. Nano Today, 5, 248–250.
Kanel, S. R., Greneche, J. M., & Choi, H. (2006). Arsenic(V) removal from groundwater using nano scale zero-valent iron as a colloidal reactive barrier material. Environmental Science and Technology, 40, 2045–2050.
Kent, R. D., Oser, J. G., & Vikesland, P. J. (2014). Controlled evaluation of silver nanoparticle sulfidation in a full-scale wastewater treatment plant. Environmental Science and Technology, 48, 8564–8572.
Kim, K. T., Klaine, S. J., Lin, S., Ke, P. C., & Kim, S. D. (2010). Acute toxicity of copper and single-walled carbon nanotubes to daphnia magna. Environmental Toxicology and Chemistry, 29, 122–126.
Kim, J. A., Salvati, A., Åberg, C., & Dawson, K. A. (2014). Suppression of nanoparticle cytotoxicity approaching in vivo serum concentrations: Limitations of in vitro testing for nanosafety. Nanoscale, 6(23), 14180–14184.
Kim, J. S., Song, K. S., Joo, H. J., Lee, J. H., & Yu, I. J. (2010). Determination of cytotoxicity attributed to multiwall carbon nanotubes (MWCNT) in normal human embryonic lung cell (WI-38) line. Journal of Toxicology and Environmental Health. Part A, 73, 1521–1529.
Kookana, R. S., Boxall, A. B., Reeves, P. T., Ashauer, R., Beulke, S., Chaudhry, Q., … Van den Brink, P. J. (2014). Nanopesticides: Guiding principles for regulatory evaluation of environmental risks. Journal of Agricultural and Food Chemistry, 62, 4227–4240.
Kühnel, D., & Nickel, C. (2014). The OECD expert meeting on ecotoxicology and environmental fate—Towards the development of improved OECD guidelines for the testing of nanomaterials. Science of the Total Environment, 472, 347–353
Lee, R., & Vaughan, S. (2010). REACHing down: Nanomaterials and chemical safety in the EU. Journal of Law, Innovation and Technology, 2(2), 193–217.
Lee, R., & Stokes, E. (2009). Twenty-first century novel: Regulating nanotechnologies. Journal of Environmental Law, 21, 469–482.
Lemire, J. A., Harrison, J. J., & Turner, R. J. (2013). Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nature Reviews Microbiology, 11, 371–384.
Levard, C., Hotze, E. M., Lowry, G. V., & Brown, G. E., Jr. (2012). Environmental transformations of silver nanoparticles: Impact on stability and toxicity. Environmental Science and Technology, 46, 6900–6914.
Liu, J., von der Kammer, F., Zhang, B., Legros, S., & Hofmann, T. (2013). Combining spatially resolved hydrochemical data with in-vitro nanoparticle stability testing: assessing environmental behavior of functionalized gold nanoparticles on a continental scale. Environmental International, 59, 53–62.
Lowry, G. V., Gregory, K. B., Apte, S. C., & Lead, J. R. (2012). Transformations of nanomaterials in the environment. Environmental Science and Technology, 46, 6893–6899.
Lynch, I., Dawson, K. A., Lead, J. R., & Valsami-Jones, E. (2014). Macromolecular coronas and their importance in nanotoxicology and nanoecotoxicology. In J. R. Lead & E. Valsami-Jones E. (Eds.), Nanoscience and the environment (Vol. 7).
Ma, R., Levard, C., Michel, F. M., Brown, G. E., Jr., & Lowry, G. V. (2013). Sulfidation mechanism for zinc oxide nanoparticles and the effect of sulfidation on their solubility. Environmental Science and Technology, 47, 2527–2534.
Machado, S., Stawiński, W., Slonina, P., Pinto, A. R., Grosso, J. P., Nouws, H. P., … Delerue-Matos, C. (2013). Application of green zero-valent iron nanoparticles to the remediation of soils contaminated with ibuprofen. Science of the Total Environment, 461–462, 323–329.
Malkiewicz, K., Pettitt, M., Dawson, K. A., Toikka, A., Hansson, S. O., Hukkinen, J., … Lead, J. (2011). Nanomaterials in REACH—project report (SKEP) 15 August 2011 (final).
Meesters, J. A., Veltman, K., Hendriks, A. J., & van de Meent, D. (2013). Environmental exposure assessment of engineered nanoparticles: Why REACH needs adjustment. Integrated Environmental Assessment and Management, 9, 15–26.
Monopoli, M. P., Walczyk, D., Campbell, A., Elia, G., Lynch, I., Bombelli, F. B., & Dawson, K. A. (2011). Physical-chemical aspects of protein corona: Relevance to in vitro and in vivo biological impacts of nanoparticles. Journal of the American Chemical Society, 133, 2525–2534.
Mudunkotuwa, I. A., Pettibone, J. M., & Grassian, V. H. (2012). Environmental implications of nanoparticle aging in the processing and fate of copper-based nanomaterials. Environmental Science and Technology, 46, 7001–7010.
Munro, I. C., Ford, R. A., Kenepohl, E., & Sprenger, J. G. (1996). Correlation of structural class with no-observed-effect-levels: A proposal for establishing a threshold of concern. Food and Chemical Toxicology, 34, 829–867.
Nasser, F., & Lynch, I. (2015). Secreted protein eco-corona mediates uptake and impacts of polystyrene nanoparticles on Daphnia magna. Journal of Proteomics, S1874–3919, 30121–30124.
Navarro, E., Baun, A., Behra, R., Hartmann, N. B., Filser, J., Miao, A.-J., … Sigg, L. (2008). Environmental behavior and ecotoxicity of engineered NPs to algae, plants, and fungi. Ecotoxicology, 17, 372–386.
Nordlund, D. A., & Lewis, W. J. (1976). Terminology of chemical releasing stimuli in intraspecific and interspecific interactions. Journal of Chemical Ecology, 2, 211–220.
OECD. (2014). Report of the OECD expert meeting on the pysical chemical properties of manufactured nanomaterials and test guidelines. ENV/JM/MONO(2014)15, environment, health and safety publications series on the safety of manufactured nanomaterials No. 41.
Pastoor, T. P., Bachman, A. N., Bell, D. R., Cohen, S. M., Dellarco, M., Dewhurst, I. C., … Boobis, A. R. (2014). A 21st century roadmap for human health risk assessment. Critical Reviews in Toxicology, 44(Suppl 3), 1–5.
Pearson, R. G. (1963). Hard and soft acids and bases. Journal of the American Chemical Society, 85, 3533–3539.
Petersen, E., Akkanen, J., Kukkonen, J. V. K., & Weber, W. J., Jr. (2009). Biological uptake and depuration of carbon nanotubes by Daphnia magna. Environmental Science and Technology, 43, 2969–2975.
Powley, M. W., Frederick, C. B., Sistare, F. D., & DeGeorge, J. J. (2009). Safety assessment of drug metabolites: implications of regulatory guidance and potential application of genetically engineered mouse models that express human P450s. Chemical Research in Toxicology, 22, 257–262.
Priester, J. H., Ge, Y., Mielke, R. E., Horst, A. M., Moritz, S. C., Espinosa, K., … Holden, P. A. (2012). Soybean susceptibility to manufactured nanomaterials with evidence for food quality and soil fertility interruption. Proceedings of the National Academy of Sciences, 109, 14734–14735
Prins, L. J. (2015). Emergence of complex chemistry on an organic monolayer. Accounts of Chemical Research, 48, 1920–1928.
Royal Commission on Environmental Protection (RCEP). (2008). Novel materials in the environment: The case of nanotechnology (27th Report, RCEP, London 71).
Saleh, N. B., Aich, N., Plazas-Tuttle, J., Lead, J. R., & Lowry, G. V. (2015). Research strategy to determine when novel nanohybrids pose unique environmental risks. Environmental Science: Nano, 2, 11–18.
Seitz, F., Lüderwald, S., Rosenfeldt, R. R., Schulz, P., & Bundschuh, M. (2015). Aging of TiO2 nanoparticles transiently increases their toxicity to the pelagic microcrustacean Daphnia magna. PLoS One, 10, e0126021.
Sokolov, V. I. (2009). Chiral stereochemistry of nanoparticles. Russian Journal of Coordination Chemistry, 35, 553–565.
Studer, C., Aicher, L., Gasic, B., von Goetz, N., Hoet, P., Huwyler, J., … Walser, T (2015). Scientific basis for regulatory decision-making of nanomaterials report on the workshop, 20-21 January 2014, Center of Applied Ecotoxicology, Dübendorf. Chimia (Aarau), 69, 52–56.
Terry, C., Rasoulpour, R. J., Knowles, S., & Billington, R. (2015). Utilizing relative potency factors (RPF) and threshold of toxicological concern (TTC) concepts to assess hazard and human risk assessment profiles of environmental metabolites: A case study. Regulatory Toxicology and Pharmacology, 71, 301–317.
Toropova, A. P., Toropov, A. A., Veselinović, A. M., Veselinović, J. B., Benfenati, E., Leszczynska, D., & Leszczynski, J. (2015). Nano-QSAR: Model of mutagenicity of fullerene as a mathematical function of different conditions. Ecotoxicology and Environmental Safety, 124, 32–36.
Valsami-Jones, E., & Lynch, I. (2015). NANOSAFETY. How safe are nanomaterials? Science, 350, 388–389.
Vaughan, S. (2015). EU chemicals regulation: New governance, hybridity and REACH. Cheltenham, England: Edward Elgar.
Walczyk, D., Bombelli, F. B., Monopoli, M. P., Lynch, I., & Dawson, K. A. (2010). What the cell “sees” in bionanoscience. Journal of the American Chemical Society, 132, 5761–5768.
Waldron, K. J., Rutherford, J. C., Ford, D., & Robinson, N. J. (2009). Metalloproteins and metal sensing. Nature, 460, 823–830.
Walkey, C. D., Olsen, J. B., Song, F., Liu, R., Guo, H., Olsen, D. W., … Chan, W. C. (2014). Protein corona fingerprinting predicts the cellular interaction of gold and silver nanoparticles. ACS Nano, 8, 2439–2455.
Wang, M. M., Wang, Y. C., Wang, X. N., Liu, Y., Zhang, H., Zhang, J. W., … Xu, A. (2015). Mutagenicity of ZnO nanoparticles in mammalian cells: Role of physicochemical transformations under the aging process. Nanotoxicology, 9, 972–982.
Whitley, A. R., Levard, C., Oostveen, E., Bertsch, P. M., Matocha, C. J., von der Kammer, F., & Unrine, J. M. (2013). Behavior of Ag nanoparticles in soil: Effects of particle surface coating, aging and sewage sludge amendment. Environmental Pollution, 182, 141–149.
Workentine, M. L., Harrison, J. J., Stenroos, P. U., Ceri, H., & Turner, R. J. (2008). Pseudomonas fluorescens’ view of the periodic table. Environmental Microbiology, 10, 238–250.
Yang, S. T., Liu, Y., Wang, Y. W., & Cao, A. (2013). Biosafety and bioapplication of nanomaterials by designing protein–nanoparticle interactions. Small, 9, 1635–1653.
Acknowledgement
This chapter is based on concepts developed within EU FP7 Marie Curie Career Integration grant EcofriendlyNano (PCIG14-GA-2013-631612).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Lynch, I., Lee, R.G. (2016). In Support of the Inclusion of Data on Nanomaterials Transformations and Environmental Interactions into Existing Regulatory Frameworks. In: Murphy, F., McAlea, E., Mullins, M. (eds) Managing Risk in Nanotechnology. Innovation, Technology, and Knowledge Management. Springer, Cham. https://doi.org/10.1007/978-3-319-32392-3_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-32392-3_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32390-9
Online ISBN: 978-3-319-32392-3
eBook Packages: Business and ManagementBusiness and Management (R0)