Abstract
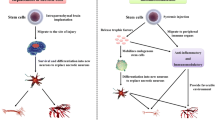
Neuroinflammation modulates brain injury and recovery after stroke. The spleen, as the largest secondary lymphoid organ, contributes to this neuroinflammation, and therefore influences stroke-induced brain injury. When stroke occurs, pro-inflammatory cytokines in the ischemic brain are produced that stimulate inflammatory brain receptors, which initiate communication between the brain and the spleen via the sympathetic nervous system (SNS), parasympathetic nervous system (PNS), and hypothalamus-pituitary adrenal (HPA) axis. The spleen contains various immune cells, including T cells, B cells, monocytes, macrophages, NK, and NKT cells. SNS activity leads to the rapid release of immune cells from the spleen, transient increases of released immune cells into the blood circulation, and their migration to the ischemic brain, thus exacerbating neuroinflammation in the ischemic region and enlarging the infarction. Direct evidence that the spleen contributes to brain injury is the fact that splenectomy robustly reduces infarct size. In addition, reductions in spleen size are correlated with the severity of stroke. Furthermore, immune cells in the spleen are found in the ischemic brain. Nevertheless, although it is well established that the spleen contributes to brain injury, the underlying mechanisms remain unclear. Future studies will reveal how the spleen modulates brain injury, which immune cell types in the spleen play the most important roles, and whether the spleen has a long-term effect on brain recovery after stroke.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Stroke
- Cerebral ischemia
- Splenectomy
- Spleen
- Infarction
- Inflammation
- Monocytes
- Macrophages
- Lymphocytes
- T cells
1 Introduction
Despite numerous extensive studies on stroke therapy in the past decades, few have successfully translated to the clinic and many limitations and potential problems remain. One major limitation is that the brain has been studied in isolation, as if tissue injury in the brain is unrelated to what occurs in peripheral organs. We now realize that stroke not only injures the brain, but it also affects multiple organs, including the skeletal muscle [1–3], heart [4], liver [5], lung [6], and peripheral immune system, including the spleen [7–9]. As a result, it has been speculated that modulating peripheral organs may protect against brain injury. And in fact, we have reported that repetitive ischemia performed in the hind limbs of rats reduces brain infarction after focal ischemia—a phenomenon defined as remote preconditioning [10]. Other important facts are that the spleen interacts with the ischemic brain, as stroke results in spleen atrophy, while spleen removal (splenectomy) performed before stroke onset robustly reduces post-stroke brain injury [11, 12]. Therefore, the spleen may be an alternative avenue or target for stroke treatment. In this Chapter, we discuss how the spleen and its immune cells contribute to brain injury induced by stroke.
2 The Structure and Function of the Spleen
The spleen is a unique organ belonging to the super immune system, which consists of many lymphatic organs and multiple immune cells [13–15]. The immune system includes primary and secondary lymphoid organs. The primary lymphoid organs mainly include the bone marrow and thymus, which are the sites of lymphocyte development and maturation. The secondary lymphoid tissues include the spleen, lymph nodes, and mucosa- and skin-associated lymphoid tissues, within which mature lymphocytes exert their immune response, such as antigen recognition and activation, clonal selection and proliferation, as well as phagocytosis [16–19].
The spleen, as the largest secondary lymphoid organ, plays important roles in the immune response. It is composed of red and white pulp with distinct morphologies and functions [16–19]. Blood circulation in the spleen is supplied by a single artery called the splenic artery, which branches into a network of smaller arteries that travel through the spleen, forming arterioles, and connecting with sinuses [14]. The small arterioles travel to and end in a venous sinusoidal system in the red pulp, which serves as a blood filter by trapping old or damaged red blood cells that are phagocytosed by red pulp macrophages. Another major function of the red pulp is for iron recycling [14]. White pulp is a lymphoid tissue surrounding the small branches of arterials, and is composed of T cell, B cell (follicle), and marginal zones, where the immune response occurs [16–19].
Taken together, the primary function of the spleen is to monitor circulating blood, filter blood by trapping damaged or old red blood cells, and initiate the immune response against pathogens found in the circulation. Both innate and adaptive immune responses can be easily mounted in the spleen, as the unique pattern of blood supply and structure or organization of the immune compartments allow various immune responses to occur. However, the spleen also releases lymphocytes into the blood circulation in response to stress and inflammation, which travel to other organs, including the brain. Therefore, the spleen is also an important organ for modulating brain functions and neuroinflammation.
3 The Spleen Is Involved in Brain Infarction Induced by Stroke
It is well known that bidirectional effects occur between the injured brain and peripheral immune system after stroke, and the spleen has a unique role in such bidirectional effects. On one hand, stroke causes peripheral immune suppression [9], which results in post-stroke infection and mortality [20–23]. The immune suppression is manifested by a reduction in lymphocyte activation and spleen atrophy after stroke [9]. As a major secondary lymphocyte organ, the spleen functions to maintain lymphocyte and erythrocyte populations. Splenic atrophy is a typical marker of the immune suppression induced by stroke [9]. On the other hand, the peripheral immune system increases local brain inflammation via recruitment and infiltration of circulating neutrophils [24–27], monocytes/macrophages [28, 29], and T cells [30–35], thus exacerbating ischemic injury.
The most direct evidence that spleen is involved in brain injury is shown in splenectomy studies. Ajmo and colleagues showed in their first study that splenectomy performed 2 weeks before stroke reduced infarction by 80 % [11, 12]. This study is supported by Li et al., who found that splenectomy performed immediately before traumatic brain injury decreases animal mortality and improves cognitive function in rats [36, 37]. In addition, Ostrowski et al. reported that acute splenic irradiation, which results in lymphocyte death in the spleen, reduces brain injury in rat focal cerebral ischemia [38]. They found that the therapeutic time window extends to 3–4 h after stroke onset, suggesting that spleen modulation post-stroke can provide neuroprotection [38]. More recently, Seifert et al. and Zhang et al. also confirmed the neuroprotective effect of splenectomy against stroke [39–41]. In contrast, Kim et al. failed to see any neuroprotective effect of splenectomy immediately before stroke on reducing infarction, although splenectomy did reduce the accumulation of inflammatory macrophages in the ischemic brain [42]. The explanation for this controversial result is unknown. Although the authors argue that the timing of splenectomy immediately before stroke is a factor [42], others have shown that spleen irradiation performed even 3–4 h after stroke can generate protection [38].
In addition to the protective effects of splenectomy against stroke, evidence exists to suggest that the spleen modulates brain injury induced by stroke. First, there is a correlation between reductions in spleen size after stroke and the extent of infarction in animal studies, as well as between spleen size and brain injury in stroke patients [43]. Second, a number of studies suggest that neuroprotectants attenuate the reduction in spleen size and splenocyte numbers. As we reported, moderate hypothermia attenuates the reduction in spleen size and lymphocyte numbers, and this correlates with the robust protective effects of hypothermia on brain infarction [44]. In addition, cord blood injection inhibited brain injury, which was associated with less reduced spleen sizes [45]. Taken together, the spleen contributes to brain injury induced by stroke.
Nevertheless, the underlying mechanisms involved in the cross talk between the spleen and post-stroke ischemic brain, and how immune cells derived from the spleen modulate ischemic brain injury are unclear. In the next two sections I will identify potential mechanisms based on previous studies about spleen function, as well as the possible roles of splenic immune cells in neuroinflammation.
4 Communication Between the Spleen and Brain
Bidirectional communication between the spleen and brain occurs via the immune system and central nervous system (CNS). The peripheral lymphoid organs, including the spleen, are hardwired to the autonomous nervous system [46]. In addition, lymphocytes in the spleen and other peripheral lymphoid organs express receptors for neurotransmitters released from the nervous system [23]. Other sensors, such as cytokine receptors in the CNS, relay information from the CNS to peripheral organs. This information is mainly processed in the brain by the frontal premotor cortex, hypothalamus, pituitary, and brain stem, which forms three major pathways: the hypothalamus–pituitary–adrenal (HPA) axis, the sympathetic nervous system (SNS), and the parasympathetic nervous system (PNS) [23]. These three major systems bridge the peripheral lymphoid organs, including the spleen, with the brain.
The SNS innervates almost all lymphoid organs, including the bone marrow, thymus, lymphoid nodes, and spleen [23]. In addition, almost all leukocytes express adrenergic receptors, which react with the neurotransmitter catecholamine released from the SNS [47–51]. Activation of the SNS due to inflammatory stimulation in the brain results in a large release of catecholamine, which influences immune functions in the peripheral lymphoid organs [23]. It is known that catecholamine releases from the SNS results in a transient and rapid release of leukocytes from the lymphoid organs, including the spleen [52, 53]. This results in a rapid increase of leukocytes into the circulation, which migrate to the brain and exacerbate the brain’s inflammatory response [52]. Indeed, Ajmo and colleagues demonstrated that treatment with either prazosin, an α1 receptor blocker, or carvedilol, a pan adrenergic receptor blocker, prevented the reduction in spleen size, and carvedilol significantly reduced infarct volume, suggesting that SNS-mediated catecholamines regulate the splenic response to stroke through the activation of adrenergic receptors [12].
In addition to the SNS, PNS and HPA are also involved in the crosstalk between the CNS and peripheral lymphoid organs [23]. PNS activity modulated by the release of acetylcholine promotes the anti-inflammatory response, and reduces cytokine production, such as IL-1β and TNFα, in the peripheral organs [54]. In addition, HPA activity leads to the release of glucocorticoids, which are also anti-inflammatory and immune-suppressive [55, 56]. Nevertheless, whether or not PNS and HPA activity results in leukocyte release from the spleen is not clear.
Although it is clear that the spleen contributes to brain injury induced by stroke, and communication between the spleen and CNS is modulated by the three pathways previously discussed, the role of the SNS, PNS, and HPA in the worsening effects of spleen on brain injury has not been well studied. It is likely that stroke results in neuroinflammation in the ischemic brain, which produces various inflammatory cytokines, such as IL-1β, IL-6, and TNF α [23]. These released cytokines may secret into the CSF or diffuse within the ischemic brain, thus stimulating the SNS and resulting in the release of leukocytes from the spleen. Another possibility is that inflammatory cytokines are released from the ischemic brain into the circulation, and these cytokines act directly on the spleen via the circulating blood [23]. The released leukocytes could then infiltrate into the ischemic brain, and result in a larger infarction.
5 Neuroinflammation and Spleen Immune-Cell Trafficking to the Brain After Stroke
Inflammation plays several critical roles in stroke and stroke-induced brain injury. For example, systemic infection-induced inflammation correlates with stroke [57–63], and surgery-induced inflammation also increases the risk of stroke [64–66]. Second, after ischemic stroke there is an immediate onset of neuroinflammation, which involves multiple facets, including brain damage, tissue clearance, and functional recovery [67]. The spleen may be involved in these aforementioned neuroinflammation and stroke, although there is no clear evidence indicating how spleen immune cells modulate stroke, and the role of splenocytes on post-stroke brain recovery has not been studied.
Upon stroke, cerebral blood vessel(s) is occluded and become hypoxic, causing reduced nitric oxide and further constriction of blood vessels, which increases production of reactive oxygen species and platelet activity [67]. These intravascular changes cause the adhesion of leukocytes to the blood vessel walls, and increases in blood–brain barrier permeability and leukocyte infiltrate to the ischemic brain. Therefore, in addition to resident microglia in the brain, which are activated and transformed into macrophages, other leukocytes from circulating blood, including neutrophils, monocytes/macrophages, B cells, T cells, NK, and NKT cells, play important roles in neuroinflammation and brain injury induced by stroke [67].
It is known that T cells modulate brain injury [31, 32, 67]. T cells include the subsets of CD4 T cells, CD8 T cells, and γδT cells. Functionally, activated CD4 T cells during immune responses can be further differentiated into Th1, Th2, and Th17 subsets. It has been reported that the lack of total T cells, or a subset of CD4, CD8, or γδT cells, results in a smaller infarction compared with immune intact animals [68]. In addition, the lack of Th1 cells results in neuroprotection, while the lack of Th2 cells exacerbates brain injury [31]. We also observed that the lack of Th17 was neuroprotective against stroke (unpublished observation). Th17 cells function by secreting IL-17. A previous study has shown that neutralization of IL-17 attenuates neuroinflammation [69]. Among these cell types, CD4+CD25+ regulatory T cells (Treg) have been the most widely studied, with results showing that Treg cells have anti-inflammatory functions and their activity attenuates delayed brain injury induced by stroke [68, 70, 71]. B cells have been less studied in stroke. The first study on B cells suggested that B cells do not have an effect on brain injury induced by stroke [33]. More recently, regulatory B cells were found to inhibit brain injury [72].
Monocytes/macrophages are also important for brain injury induced by stroke. Macrophages can be derived from both brain resident microglia and peripheral blood monocytes. The latter can be released from bone marrow and other secondary lymphoid organs, including the spleen. Functionally, macrophages are polarized into M1 and M2 phenotypes, with pro- and anti-inflammatory roles, respectively [73, 74]. It has been reported that the M1 phenotype is detrimental, while M2 phenotype is beneficial, in ischemic stroke [75–77]. In particular, M2 macrophages may play a critical role in promoting brain repair and recovery after stroke [75].
As we discussed, the spleen contains most of the cell types in the immune system, including T cells, B cells, monocytes, and macrophages, and stroke results in the contraction of the spleen, which leads to the release of leukocytes into the blood circulation. The released leukocytes then migrate to the brain modulating acute brain injury. Nevertheless, as discussed, leukocytes recruited into the ischemic brain include those released not only from the spleen and other secondary lymphoid nodes, but also from the primary lymphoid organs, such as bone marrow. It still remains unclear how much the spleen contributes to or modulates brain injury induced by stroke among these various lymphoid organs.
Although the spleen contributes to brain injury induced by stroke, few studies have examined the exact roles of the individual cell types that make up splenocytes or the underlying mechanisms of spleen-induced brain injury. Nevertheless, several lines of evidence from previous studies indeed suggest that the involvement of spleen in ischemic brain injury is associated with splenocytes and neuroinflammation. First, one recent study suggests that spleen contraction induced by stroke correlates with reduced cell numbers of monocytes in the spleen, including pro-inflammatory Ly6Chi and anti-inflammatory Ly6clow monocytes [42]. The study further showed that the deployment of these monocyte subsets coincided with respective increases in the ischemic brain [42]. In contrast, splenectomy reduced leukocyte infiltrations into the ischemic brain [42], suggesting that monocytes are released from the spleen and migrate to the ischemic brain. Second, there is additional direct evidence that splenocytes infiltrate into the ischemic brain. In one study, splenocytes were labeled with CFSE, and it was found that CFSE-positive cells were released into the blood from the spleen, including T cells, neutrophils, and monocytes. The presence of CFSE-positive monocytes and NK cells in the cerebral blood vessels of the ischemic brain [39] suggests that splenocytes migrate into the ischemic brain. Third, splenocyte infiltrations are associated with the expression of inflammatory factors. Splenectomy results in reductions in T cells, neutrophils, and macrophages in the ischemic brain [41], and this is associated with decreases in pro-inflammatory cytokines, such as IL-1β and TNFα, and with increases in anti-inflammatory cytokines, including IL-10, in the brain [41]. Another study suggests that the protective effect of splenectomy is associated with IFNγ, as IFNγ was found to be increased in the spleen in early stroke followed by increases in the brain [40]. Fourth, the protective effects of neuroprotectants are linked to spleen functions. For instance, agmatine treatment reduced the contraction of white pulp, and inhibited the accumulation of CD11b macrophages and Treg cells in the spleen [78]. In addition, MFX treatment attenuated Ly6C expression in pro-inflammatory macrophage subsets and CCR2 expression in the spleen tissues [79]. Furthermore, the injection of cord blood reversed the reduction in spleen size and concomitant reductions in CD8 T cells after stroke, as well as increased IL-10 while inhibiting IFN-gamma [45].
6 Problems and Future Research Directions
It is well established that the spleen contributes to acute brain injury after stroke, but there are many questions raised from previous studies. First, the underlying mechanisms of the spleen’s contribution to brain injury are not understood. Future studies can address the following questions: How are the nerve pathways, including SNS, PNS, and HPA involved in the interaction between the spleen and brain after stroke? How are immune cells released from the spleen? Which splenocytes migrate to the ischemic brain, and which cell types play the most important roles? Second, the spleen is only one of the immune organs, and how much it contributes to ischemic brain injury is unknown. Therefore, one may ask, what is the relative contribution of the spleen, in comparison to the other lymphoid organs, to brain injury? Third, previous studies have focused on studying the relationship between acute brain injury and the spleen; whether the spleen plays an important role in brain repair and recovery has not been studied. More scientific issues include: Does the spleen play a critical role in brain recovery after stroke? If so, what is the underlying mechanism?
7 Conclusions
Stroke results in the release of inflammatory factors that stimulate nerve tissue sensors inside and outside of the brain as well as sensors expressed on peripheral organs, such as the spleen. The contraction of the spleen after stroke leads to the release of splenocytes, which migrate to the ischemic brain and exacerbate brain injury. Taken together, the spleen plays an important role in acute brain injury induced by stroke, but the underlying mechanisms require more study, and the importance of the spleen to brain repair and recovery is not clear.
References
Knops M, Werner CG, Scherbakov N, Fiebach J, Dreier JP, Meisel A, Heuschmann PU, Jungehulsing GJ, von Haehling S, Dirnagl U, Anker SD, Doehner W. Investigation of changes in body composition, metabolic profile and skeletal muscle functional capacity in ischemic stroke patients: the rationale and design of the Body Size in Stroke Study (BoSSS). J Cachex Sarcopenia Muscle. 2013;4:199–207.
Scherbakov N, von Haehling S, Anker SD, Dirnagl U, Doehner W. Stroke induced sarcopenia: muscle wasting and disability after stroke. Int J Cardiol. 2013;170:89–94.
Springer J, Schust S, Peske K, Tschirner A, Rex A, Engel O, Scherbakov N, Meisel A, von Haehling S, Boschmann M, Anker SD, Dirnagl U, Doehner W. Catabolic signaling and muscle wasting after acute ischemic stroke in mice: indication for a stroke-specific sarcopenia. Stroke. 2014;45:3675–83.
Ishikawa H, Tajiri N, Vasconcellos J, Kaneko Y, Mimura O, Dezawa M, Borlongan CV. Ischemic stroke brain sends indirect cell death signals to the heart. Stroke. 2013;44:3175–82.
Ottani A, Giuliani D, Mioni C, Galantucci M, Minutoli L, Bitto A, Altavilla D, Zaffe D, Botticelli AR, Squadrito F, Guarini S. Vagus nerve mediates the protective effects of melanocortins against cerebral and systemic damage after ischemic stroke. J Cereb Blood Flow Metab. 2009;29:512–23.
Wu S, Fang CX, Kim J, Ren J. Enhanced pulmonary inflammation following experimental intracerebral hemorrhage. Exp Neurol. 2006;200:245–9.
Offner H. Modeling immunity and inflammation in stroke: don’t be afraid of mice? Stroke. 2014;45:e181–2.
Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–65.
Offner H, Subramanian S, Parker SM, Wang C, Afentoulis ME, Lewis A, Vandenbark AA, Hurn PD. Splenic atrophy in experimental stroke is accompanied by increased regulatory T cells and circulating macrophages. J Immunol. 2006;176:6523–31.
Ren C, Yan Z, Wei D, Gao X, Chen X, Zhao H. Limb remote ischemic postconditioning protects against focal ischemia in rats. Brain Res. 2009;1288:88–94.
Ajmo Jr CT, Vernon DO, Collier L, Hall AA, Garbuzova-Davis S, Willing A, Pennypacker KR. The spleen contributes to stroke-induced neurodegeneration. J Neurosci Res. 2008;86:2227–34.
Ajmo Jr CT, Collier LA, Leonardo CC, Hall AA, Green SM, Womble TA, Cuevas J, Willing AE, Pennypacker KR. Blockade of adrenoreceptors inhibits the splenic response to stroke. Exp Neurol. 2009;218:47–55.
Chaplin DD. Regulation of spleen white pulp structure and function by lymphotoxin. Adv Exp Med Biol. 2002;512:49–56.
Mebius RE, Kraal G. Structure and function of the spleen. Nat Rev Immunol. 2005;5:606–16.
Scothorne RJ. The spleen: structure and function. Histopathology. 1985;9:663–9.
Bronte V, Pittet MJ. The spleen in local and systemic regulation of immunity. Immunity. 2013;39:806–18.
den Haan JM, Kraal G. Innate immune functions of macrophage subpopulations in the spleen. J Innate Immun. 2012;4:437–45.
Hey YY, O’Neill HC. Murine spleen contains a diversity of myeloid and dendritic cells distinct in antigen presenting function. J Cell Mol Med. 2012;16:2611–9.
Zhao L, Liu L, Guo B, Zhu B. Regulation of adaptive immune responses by guiding cell movements in the spleen. Front Microbiol. 2015;6:645.
Braun JS, Prass K, Dirnagl U, Meisel A, Meisel C. Protection from brain damage and bacterial infection in murine stroke by the novel caspase-inhibitor Q-VD-OPH. Exp Neurol. 2007;206:183–91.
Engel O, Meisel A. Models of infection before and after stroke: investigating new targets. Infect Disord Drug Targets. 2010;10:98–104.
Meisel A, Meisel C, Harms H, Hartmann O, Ulm L. Predicting post-stroke infections and outcome with blood-based immune and stress markers. Cerebrovasc Dis. 2012;33:580–8.
Meisel C, Schwab JM, Prass K, Meisel A, Dirnagl U. Central nervous system injury-induced immune deficiency syndrome. Nat Rev Neurosci. 2005;6:775–86.
Arakawa K, Yasuda S, Hao H, Kataoka Y, Morii I, Kasahara Y, Kawamura A, Ishibashi-Ueda H, Miyazaki S. Significant association between neutrophil aggregation in aspirated thrombus and myocardial damage in patients with ST-segment elevation acute myocardial infarction. Circ J. 2009;73:139–44.
Ikegame Y, Yamashita K, Hayashi S, Yoshimura S, Nakashima S, Iwama T. Neutrophil elastase inhibitor prevents ischemic brain damage via reduction of vasogenic edema. Hypertens Res. 2010;33:703–7.
Perez-de-Puig I, Miro-Mur F, Ferrer-Ferrer M, Gelpi E, Pedragosa J, Justicia C, Urra X, Chamorro A, Planas AM. Neutrophil recruitment to the brain in mouse and human ischemic stroke. Acta Neuropathol. 2015;129:239–57.
Zhao X, Sun G, Zhang H, Ting SM, Song S, Gonzales N, Aronowski J. Polymorphonuclear neutrophil in brain parenchyma after experimental intracerebral hemorrhage. Transl Stroke Res. 2014;5:554–61.
Hammond MD, Taylor RA, Mullen MT, Ai Y, Aguila HL, Mack M, Kasner SE, McCullough LD, Sansing LH. CCR2+ Ly6C(hi) inflammatory monocyte recruitment exacerbates acute disability following intracerebral hemorrhage. J Neurosci. 2014;34:3901–9.
Joo SP, Xie W, Xiong X, Xu B, Zhao H. Ischemic postconditioning protects against focal cerebral ischemia by inhibiting brain inflammation while attenuating peripheral lymphopenia in mice. Neuroscience. 2013;243:149–57.
Dziennis S, Mader S, Akiyoshi K, Ren X, Ayala P, Burrows GG, Vandenbark AA, Herson PS, Hurn PD, Offner HA. Therapy with recombinant T-cell receptor ligand reduces infarct size and infiltrating inflammatory cells in brain after middle cerebral artery occlusion in mice. Metab Brain Dis. 2011;26:123–33.
Gu L, Xiong X, Zhang H, Xu B, Steinberg GK, Zhao H. Distinctive effects of T cell subsets in neuronal injury induced by cocultured splenocytes in vitro and by in vivo stroke in mice. Stroke. 2012;43:1941–6.
Gu L, Xiong X, Wei D, Gao X, Krams S, Zhao H. T cells contribute to stroke-induced lymphopenia in rats. PLoS One. 2013;8:e59602.
Hurn PD, Subramanian S, Parker SM, Afentoulis ME, Kaler LJ, Vandenbark AA, Offner H. T- and B-cell-deficient mice with experimental stroke have reduced lesion size and inflammation. J Cereb Blood Flow Metab. 2007;27:1798–805.
Ren X, Akiyoshi K, Vandenbark AA, Hurn PD, Offner H. CD4 + FoxP3+ regulatory T-cells in cerebral ischemic stroke. Metab Brain Dis. 2011;26:87–90.
Xiong X, Gu L, Zhang H, Xu B, Zhu S, Zhao H. The protective effects of T cell deficiency against brain injury are ischemic model-dependent in rats. Neurochem Int. 2013;62:265–70.
Chu W, Li M, Li F, Hu R, Chen Z, Lin J, Feng H. Immediate splenectomy down-regulates the MAPK-NF-kappaB signaling pathway in rat brain after severe traumatic brain injury. J Trauma Acute Care Surg. 2013;74:1446–53.
Li M, Li F, Luo C, Shan Y, Zhang L, Qian Z, Zhu G, Lin J, Feng H. Immediate splenectomy decreases mortality and improves cognitive function of rats after severe traumatic brain injury. J Trauma. 2011;71:141–7.
Ostrowski RP, Schulte RW, Nie Y, Ling T, Lee T, Manaenko A, Gridley DS, Zhang JH. Acute splenic irradiation reduces brain injury in the rat focal ischemic stroke model. Transl Stroke Res. 2012;3:473–81.
Seifert HA, Hall AA, Chapman CB, Collier LA, Willing AE, Pennypacker KR. A transient decrease in spleen size following stroke corresponds to splenocyte release into systemic circulation. J Neuroimmune Pharmacol. 2012;7:1017–24.
Seifert HA, Leonardo CC, Hall AA, Rowe DD, Collier LA, Benkovic SA, Willing AE, Pennypacker KR. The spleen contributes to stroke induced neurodegeneration through interferon gamma signaling. Metab Brain Dis. 2012;27:131–41.
Zhang BJ, Men XJ, Lu ZQ, Li HY, Qiu W, Hu XQ. Splenectomy protects experimental rats from cerebral damage after stroke due to anti-inflammatory effects. Chin Med J (Engl). 2013;126:2354–60.
Kim E, Yang J, Beltran CD, Cho S. Role of spleen-derived monocytes/macrophages in acute ischemic brain injury. J Cereb Blood Flow Metab. 2014;34:1411–9.
Sahota P, Vahidy F, Nguyen C, Bui TT, Yang B, Parsha K, Garrett J, Bambhroliya A, Barreto A, Grotta JC, Aronowski J, Rahbar MH, Savitz S. Changes in spleen size in patients with acute ischemic stroke: a pilot observational study. Int J Stroke. 2013;8:60–7.
Gu LJ, Xiong XX, Ito T, Lee J, Xu BH, Krams S, Steinberg GK, Zhao H. Moderate hypothermia inhibits brain inflammation and attenuates stroke-induced immunodepression in rats. CNS Neurosci Ther. 2014;20:67–75.
Golden JE, Shahaduzzaman M, Wabnitz A, Green S, Womble TA, Sanberg PR, Pennypacker KR, Willing AE. Human umbilical cord blood cells alter blood and spleen cell populations after stroke. Transl Stroke Res. 2012;3:491–9.
Huston JM, Ochani M, Rosas-Ballina M, Liao H, Ochani K, Pavlov VA, Gallowitsch-Puerta M, Ashok M, Czura CJ, Foxwell B, Tracey KJ, Ulloa L. Splenectomy inactivates the cholinergic antiinflammatory pathway during lethal endotoxemia and polymicrobial sepsis. J Exp Med. 2006;203:1623–8.
Galant SP, Underwood S, Allred S, Hanifin JM. Beta adrenergic receptor binding on polymorphonuclear leukocytes in atopic dermatitis. J Invest Dermatol. 1979;72:330–2.
Heijnen CJ, Rouppe van der Voort C, Wulffraat N, van der Net J, Kuis W, Kavelaars A. Functional alpha 1-adrenergic receptors on leukocytes of patients with polyarticular juvenile rheumatoid arthritis. J Neuroimmunol. 1996;71:223–6.
Kozlik R, Kramer HH, Wicht H, Bircks W, Reinhardt D. Beta-adrenoceptor density on mononuclear leukocytes and right atrial myocardium in infants and children with congenital heart disease. Klin Wochenschr. 1991;69:910–6.
Nwosu UC, Rice PJ. Beta-adrenoceptor changes in mononuclear leukocytes during pregnancy. Proc Soc Exp Biol Med. 1995;209:157–62.
Prengel AW, Lindner KH, Anhaupl T, Trunk E, Georgieff M, Lurie KG. Regulation of beta 2-adrenergic receptors on mononuclear leukocytes in patients with acute ischemic heart disease. Crit Care Med. 1997;25:646–51.
Hanna RN, Hedrick CC. Stressing out stem cells: linking stress and hematopoiesis in cardiovascular disease. Nat Med. 2014;20:707–8.
Scheiermann C, Kunisaki Y, Lucas D, Chow A, Jang JE, Zhang D, Hashimoto D, Merad M, Frenette PS. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37:290–301.
Cheyuo C, Jacob A, Wu R, Zhou M, Coppa GF, Wang P. The parasympathetic nervous system in the quest for stroke therapeutics. J Cereb Blood Flow Metab. 2011;31:1187–95.
Melief J, de Wit SJ, van Eden CG, Teunissen C, Hamann J, Uitdehaag BM, Swaab D, Huitinga I. HPA axis activity in multiple sclerosis correlates with disease severity, lesion type and gene expression in normal-appearing white matter. Acta Neuropathol. 2013;126:237–49.
Silverman MN, Sternberg EM. Glucocorticoid regulation of inflammation and its functional correlates: from HPA axis to glucocorticoid receptor dysfunction. Ann N Y Acad Sci. 2012;1261:55–63.
Bova IY, Bornstein NM, Korczyn AD. Acute infection as a risk factor for ischemic stroke. Stroke. 1996;27:2204–6.
Emsley HC, Tyrrell PJ. Inflammation and infection in clinical stroke. J Cereb Blood Flow Metab. 2002;22:1399–419.
Galante A, Pietroiusti A, Silvestrini M, Stanzione P, Bernardi G. Leukocyte aggregation: a possible link between infection and ischemic stroke. Stroke. 1992;23:1533.
Jeerakathil T, Lo W. Infection as cause of stroke: a contagious idea that may explain racial disparity. Neurology. 2014;82:908–9.
Mattila KJ, Valtonen VV, Nieminen MS, Asikainen S. Role of infection as a risk factor for atherosclerosis, myocardial infarction, and stroke. Clin Infect Dis. 1998;26:719–34.
Palm F, Urbanek C, Grau A. Infection, its treatment and the risk for stroke. Curr Vasc Pharmacol. 2009;7:146–52.
Worthmann H, Tryc AB, Deb M, Goldbecker A, Ma YT, Tountopoulou A, Lichtinghagen R, Weissenborn K. Linking infection and inflammation in acute ischemic stroke. Ann N Y Acad Sci. 2010;1207:116–22.
Dacey LJ, Likosky DS, Leavitt BJ, Lahey SJ, Quinn RD, Hernandez Jr F, Quinton HB, Desimone JP, Ross CS, O'Connor GT, Northern New England Cardiovascular Disease Study Group. Perioperative stroke and long-term survival after coronary bypass graft surgery. Ann Thorac Surg. 2005;79:532–6. discussion 537.
McAlister FA, Jacka M, Graham M, Youngson E, Cembrowski G, Bagshaw SM, Pannu N, Townsend DR, Srinathan S, Alonso-Coello P, Devereaux PJ. The prediction of postoperative stroke or death in patients with preoperative atrial fibrillation undergoing non-cardiac surgery: a VISION sub-study. J Thromb Haemost. 2015;13(10):1768–75.
Wu JC, Chen YC, Liu L, Chen TJ, Huang WC, Thien PF, Cheng H, Lo SS. The risk of stroke after spinal fusion surgery: a national cohort study. Spine J. 2012;12:492–9.
Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808.
Kleinschnitz C, et al. Regulatory T cells are strong promoters of acute ischemic stroke in mice by inducing dysfunction of the cerebral microvasculature. Blood. 2013;121:679–91.
Gelderblom M, Weymar A, Bernreuther C, Velden J, Arunachalam P, Steinbach K, Orthey E, Arumugam TV, Leypoldt F, Simova O, Thom V, Friese MA, Prinz I, Holscher C, Glatzel M, Korn T, Gerloff C, Tolosa E, Magnus T. Neutralization of the IL-17 axis diminishes neutrophil invasion and protects from ischemic stroke. Blood. 2012;120:3793–802.
Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, Giese T, Veltkamp R. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–9.
Planas AM, Chamorro A. Regulatory T cells protect the brain after stroke. Nat Med. 2009;15:138–9.
Ren X, Akiyoshi K, Dziennis S, Vandenbark AA, Herson PS, Hurn PD, Offner H. Regulatory B cells limit CNS inflammation and neurologic deficits in murine experimental stroke. J Neurosci. 2011;31:8556–63.
Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32:593–604.
Van Dyken SJ, Locksley RM. Interleukin-4- and interleukin-13-mediated alternatively activated macrophages: roles in homeostasis and disease. Annu Rev Immunol. 2013;31:317–43.
Jin Q, Cheng J, Liu Y, Wu J, Wang X, Wei S, Zhou X, Qin Z, Jia J, Zhen X. Improvement of functional recovery by chronic metformin treatment is associated with enhanced alternative activation of microglia/macrophages and increased angiogenesis and neurogenesis following experimental stroke. Brain Behav Immun. 2014;40:131–42.
Pan J, Jin JL, Ge HM, Yin KL, Chen X, Han LJ, Chen Y, Qian L, Li XX, Xu Y. Malibatol a regulates microglia M1/M2 polarization in experimental stroke in a PPARgamma-dependent manner. J Neuroinflammation. 2015;12:51.
Suenaga J, Hu X, Pu H, Shi Y, Hassan SH, Xu M, Leak RK, Stetler RA, Gao Y, Chen J. White matter injury and microglia/macrophage polarization are strongly linked with age-related long-term deficits in neurological function after stroke. Exp Neurol. 2015;272:109–19.
Uranchimeg D, Kim JH, Kim JY, Lee WT, Park KA, Batbaatar G, Tundevrentsen S, Amgalanbaatar D, Lee JE. Recovered changes in the spleen by agmatine treatment after transient cerebral ischemia. Anat Cell Biol. 2010;43:44–53.
Bao Y, Kim E, Bhosle S, Mehta H, Cho S. A role for spleen monocytes in post-ischemic brain inflammation and injury. J Neuroinflammation. 2010;7:92.
Acknowledgement
I wish to thank Ms. Cindy H. Samos for editorial assistance. This study was supported by NINDS grants 2R56NS06413606, 2 R01 NS064136-06.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Zhao, H. (2016). The Role of Spleen-Derived Immune Cells in Ischemic Brain Injury. In: Chen, J., Zhang, J., Hu, X. (eds) Non-Neuronal Mechanisms of Brain Damage and Repair After Stroke. Springer Series in Translational Stroke Research. Springer, Cham. https://doi.org/10.1007/978-3-319-32337-4_10
Download citation
DOI: https://doi.org/10.1007/978-3-319-32337-4_10
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32335-0
Online ISBN: 978-3-319-32337-4
eBook Packages: MedicineMedicine (R0)