Abstract
Plants have three genomes in their cells: the nuclear genome, the mitochondrial genome (mtDNA), and the chloroplast genome (cpDNA). They replicate independently on cell division and independently of the DNA located in the nucleus. There are few mitochondrion genome sequences until now owing to high structure variation and abundant repeat sequences found in the mitochondrion genome when compared to chloroplast genome. In this chapter, we outline some general features of the complete chloroplast genome sequences of Actinidia chinensis var. chinensis and Actinidia chinensis var. deliciosa obtained through de novo assembly of Illumina paired-end reads and produced by total DNA sequencing. The cp genomes share the typical quadripartite cp structure, with a pair of inverted repeats each separated by a small-single-copy region and a large-single-copy region. Gene content is typical for the plastomes of dicotyledonous angiosperms. In Actinidia, only mitochondria are inherited from the mother, while chloroplasts are inherited from the father, with very rare cases of maternal or biparental inheritance. These patterns of inheritance allow interspecies relationships within the genus Actinidia to be investigated using mt and cp DNA sequences.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Inverted Repeat
- Chloroplast Genome
- Inverted Repeat Sequence
- Inverted Repeat Region
- Biparental Inheritance
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
4.1 The Cytoplasmic Genomes
Plants have three genomes in their cells: the nuclear genome, the mitochondrial genome (mtDNA), and the chloroplast genome (cpDNA). Mitochondrial and chloroplast genomes are collectively known as cytoplasmic genomes. They replicate independently on cell division and independently of the DNA located in the nucleus. Both organelles have an endosymbiont origin and are descended, with little modification, from ancient, free-living bacteria. However, there are few mitochondrion genome sequences until now owing to high structure variation and abundant repeat sequences found in the mitochondrion genome when compared to chloroplast genome.
4.2 The Chloroplast Genome in Actinidia
Typically, chloroplast genomes in angiosperms are highly conserved and have a circular DNA sequence ranging from 115 to 165 kb in length and consisting of a large-single-copy region (LSC; 80–90 kb) and a small-single-copy region (SSC; 16–27 kb), separated by an inverted repeat (IR) (Palmer 1991; Raubeson and Jansen 2005). In contrast to nuclear and mitochondrial genomes, chloroplast genomes are largely conserved in gene content, organization, and structure (Raubeson and Jansen 2005). However, mutations, duplications, losses, and rearrangements of genes have been observed in several angiosperm lineages (Lee et al. 2007). The first complete plastid genome sequence of a vascular plant was that of tobacco (Shinozaki et al. 1986). Advances in next-generation sequencing technologies have enabled the rapid acquisition of whole cp genome sequences at low cost. At present, over 600 cp genome sequences are deposited at the National Center for Biotechnology Information (NCBI) including genomes of all the major lineages of the plant kingdom. Chloroplast sequences have proven useful for resolving phylogenetic relationships at lower taxonomic levels in plants owing to high interspecific variability (Soltis and Soltis 1998). Thus, information on the complete chloroplast genome sequence is valuable for molecular systematics.
4.2.1 Organization of the Chloroplast Genome
The nucleotide sequences of Actinidia chinensis var. chinensis (2x), A. chinensis var. chinensis (4x), Actinidia chinensis var. deliciosa (4x), and A. chinensis var. deliciosa (6x) range from 156,346 bp in A. chinensis (2x) to 157,375 bp in A. chinensis var. deliciosa (6x). All four cp genomes share the typical quadripartite cp structure, with a pair of inverted repeats (IRs 24,013─24,391 bp) each separated by a SSC (20,332─20,336 bp) and a LSC (87,984─88,337 bp) (Table 4.1). This structure agrees well with that based on restriction mapping of Hudson and Gardner (1988), although the total lengths slightly differ.
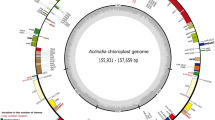
The Actinidia cp genomes contain 113 unique genes including 79 protein-coding genes, 30 tRNA genes, and four rRNA-coding genes when duplicated genes are counted only once (Fig. 4.1 and Table 4.1). Four protein-coding, four rRNA-coding, and eight tRNA genes are duplicated and located in the IR regions. Fourteen of the protein-coding genes and eight of the tRNA genes contain introns, 16 of which contain a single intron, whereas one (ycf3) has two introns (Table 4.2). The gene rps12 is trans-spliced, the 5′-end exon is located in the LSC region, and the 3′-end exon and intron are duplicated and located in the IR regions. Gene content is typical for the plastomes of the dicotyledonous angiosperms, being most similar to those of Camellia sinensis (Theaceae) (Yang et al. 2013) as compared with those of sequenced land plants. Most protein-coding genes have the standard AUG as the initiator codon (Raubeson and Jansen 2005), but ndhD has an initiator codon of ACG. The trnfM-CAU genes are duplicated in the LSC of the Actinidia chloroplast genome and separated by 14 bp with the same orientation. The overall GC content of the Actinidia chloroplast genome is 37.2 %. The GC content of the Actinidia cp genome is close to that of Ardisia polysticta (37.07 %) and other asterids (Ku et al. 2013).
Gene maps of the Actinidia plastid genome. Genes shown on the outside of the map are transcribed clockwise, while genes on the inside are transcribed counterclockwise. Genes belonging to different functional groups are color-coded (Yao et al. 2015b)
4.2.2 Repeat Structure
A total of 34 pairs of repeats (30 bp or longer) with a sequence identity greater than 90 % were identified in the Actinidia cp genome using the software REPuter (Kurtz et al. 2001), of which 29 are forward repeats and 5 inverted repeats (Table 4.3). Searches for shorter and/or more divergent repeats would probably identify many additional repeated sequences. The repeats range from 30 to 79 bp in length and are repeated from two to nine times. Most of the repeated sequences occur in the regions of noncoding DNA, whereas some were found in protein-coding regions (e.g., ycf2).
4.2.3 ClpP Lost in the Actinida cp Genome
Gene loss and gene transfer to the nucleus is a common feature of cp genomes (Stegemann et al. 2003; Kleine et al. 2009). For instance, the rpl22 gene of the Fagaceae (Jansen et al. 2011) and the Fabaceae (Gantt et al. 1991), the infA in rosids (Millen et al. 2001), the rpl32 gene in Populus and the Salicaceae (Cusack and Wolfe 2007; Ueda et al. 2007), and accD in Trifolium (Magee et al. 2010) have been transferred to the nuclear genome. The clpP gene, which encodes the proteolytic subunit of Clp protease with over 200 amino acids, is widely distributed among the cp genome of various land plant species (Wicke et al. 2011). The clpP gene encoded in the C. sinensis chloroplast genome is absent in the Actinida cp genome (Yang et al. 2013). The close relationship of these two plant families indicates that this difference reflects a relatively recent event, either gene loss or functional transfer to the nucleus.
Among the angiosperm plastids investigated thus far, clpP is also partially or completely missing from the plastid genome of Passiflora (Passifloraceae, Jansen et al. 2007), Trachelium caeruleum (Fabaceae, Haberle et al. 2008), Scaevola (Goodeniaceae, Wicke et al. 2011), and Vaccinium macrocarpon (Ericaceae, Fajardo et al. 2013). The availability of the sequenced A. chinensis var. chinensis nuclear genome gave us the opportunity to search for these missing genes by using the C. sinensis chloroplast-encoded proteins. Fourteen gene fragments that encode the ATP-dependent clp protease proteolytic subunit were annotated in the kiwifruit genome (http://bioinfo.bti.cornell.edu/cgi-bin/kiwi/home.cgi). In this study, two fragments (135 and 198 bp) from kiwifruit genome CDS sequences were found to have 77 and 89 % similarity to clpP encoded in the cpDNA of C. sinensis, which suggests that clpP has probably been replaced by a copy of the same gene that was transferred to the nucleus during chloroplast evolution, but this needs to be further explored.
4.2.4 Inverted Repeat (IR) Contraction
The IR boundaries were compared among four families in the asterids, including Actinidia (Actinidiaceae), V. macrocarpon (Ericaceae, Fajardo et al. 2013), A. polysticta (Myrsinaceae, Ku et al. 2013), and C. sinensis (Theaceae, Yang et al. 2013). The cpDNA of Actinidia is collinear with the previously published plastomes of A. polysticta and C. sinensis in gene order and overall homology. Figure 4.2 shows the detailed comparison of the IR/single-copy (SC) boundaries between four representative members of basal asterids (Camellia, Ardisia, Vaccinium, and Actinidia). In angiosperms, the downstream sequences of IRb/SSC are conserved, with the ndhF gene adjacent to it. In the Actinidia, Camellia, and Ardisia cp genomes, the IR expands into the ycf1 gene and inserts the ycf1 pseudogene at the IRb/SSC border, whereas ycf1 is lost in Vaccinium. For Camellia and Ardisia, the IRa/LSC junction is found within rps19 and pseudogenes of rps19 are located at the IRa/LSC boundary. However, in Actinidia, the rps19 gene does not extend into the IR region, and thus, the rps19 pseudogene is not observed. The IR extends into the psbA gene and inserts a short psbA pseudogene at the IRb/LSC border, which is similar to that of Vaccinium.
Comparison of the border positions of LSC, SSC, and IR regions in four basal asterid species. Boxes above the main line represent the genes at the IR/SC borders. The pseudogenes at the borders are shown by ψ (Yao et al. 2015b)
Compared to other species, the length of the IRs in the kiwifruit cp genome is fairly short; for example, in Theaceae, the size of the IR regions ranges from 26,025 to 26,057 bp (Yang et al. 2013); in the basal asterids, such as the Ericaceae, the length of the IRs is 34,232 bp (Fajardo et al. 2013). The IR sequence in Actinidia is about 2 kb smaller than that in Ardisia (Ku et al. 2013). Hence, there has also been contraction of the IR in Actinidia at the IR/SSC boundary relative to the IRs in Camellia and Ardisia. However, size variation by contractions within IR sequences contributes little to overall size variation between cp genomes of different taxa in asterids.
4.3 Cytoplasmic DNA and Its Inheritance in Actinidia
The cytoplasmic genomes are inherited uniparentally, that is, from a single parent without the recombination due to the crossing over which occurs in the nuclear genome.
In gymnosperms , mitochondria are inherited from the mother and chloroplasts from the father, while in angiosperms , both organelles are inherited from the mother, with a few exceptions so far documented in the literature (reviewed in Hagemann 2004). One of these exceptions is represented by the genus Actinidia, where only mitochondria are inherited from the mother, while chloroplasts are inherited from the father, as in gymnosperms (Cipriani et al. 1995; Testolin and Cipriani 1997; Chat et al. 1999). This kind of inheritance, unusual for the angiosperms, is shown in Fig. 4.1 and is apparently general in kiwifruit species, with very rare cases of maternal or biparental inheritance (Chat 2003; Li et al. 2013), the latter being a generalized low-frequency paternal leakage of plastids via pollen that could be underestimated in angiosperms and could contradict the common assumption of strict maternal inheritance of plastids (Reboud and Zey 1994; Azhagiri and Maliga 2007).
4.4 Cytoplasmic DNA and Phylogenetic Reconstructions in Actinidia
The genus Actinidia has 54 species and 21 botanical varieties (Li et al. 2007a). Most of these species intercross very easily as it has been demonstrated by large crossing experiments (Hirsch et al. 2001; Li et al. 2013). Therefore, two species that occur together in nature may have produced natural hybrids during the evolution of the genus. This is indeed the case in kiwifruit and has been recognized as one of the main obstacles to consistent phylogenetic reconstruction of the genus (Huang and Ferguson 2007; Huang and Liu 2014; Yao et al. 2015a).
The differential inheritance of chloroplasts and mitochondria offers an invaluable opportunity for identifying the species originating through such interspecific hybridizations, if the two parent species are, taxonomically speaking, sufficiently distant from each other to have accumulated mutations at both chloroplast and mitochondrial DNA sequences. Once interspecific hybrids have been identified, a phylogenetic reconstruction limited to the primary species would be easier.
Over the past three decades, numerous phylogenetic studies using chloroplast DNA sequence data have contributed to our understanding of the evolutionary relationships within the genus (Li et al. 2007b; Chat et al. 2004). However, interspecies relationships within the genus Actinidia remain largely controversial (Huang 2014). The phylogenetic analyses based on the matK sequence indicated the current infrageneric subdivision of Actinidia based on morphological characters does not reflect the phylogeny of the genus (Fig. 4.3; Li et al. 2002). For instance, A. rufa is misplaced in the section Leiocarpae, but should be in a lineage distinct from that of the other members of section Leiocarpae. The Leiocarpae, which have glabrous ovaries and fruit, are probably monophyletic (see Huang and Ferguson 2007), but the remaining three traditional sections seem artificial.
Left One of seven most parsimonious trees inferred from matK gene sequences (Li et al. 2002). Right One of four most parsimonious trees resulting from a heuristic search of 41 sequences (rbcL and trnL-trnF) and restriction sites (matK and psbC-trnS) of 30 Actinidia species (Chat et al. 2004). LEI, section Leiocarpae; MAC, section Maculatae; STE, section Stellatae; and STR, section Strigosae
Consider the example shown in Fig. 4.4, where the species A. chinensis is close to A. indochinensis in the chloroplast tree, but not in the mitochondrial tree. We need to be careful to draw correct inferences from tree topology, but it is clear that when two species are placed together in one tree and not in the other, one species of the pair would be a hybrid having the second species, or one with a similar organelle genome, as a parent.
The first large phylogenetic reconstruction of chloroplast and mitochondrial trees in Actinidia was carried out by Chat et al. (2004) (Fig. 4.3). The occurrences of possible interspecific hybridizations were high indeed, since as many as 11 of the 41 taxa examined (26 %) originated in such a way if their positions in both chloroplast and mitochondrial phylogenetic trees were considered (Chat et al. 2004). Such extensive reticulate evolution has been confirmed by other authors (Li et al. 2003, 2007a, b; Huang and Liu 2014).
One should consider another case. Sometimes two phenotypically contrasting taxa are placed next to each other in both chloroplast and mitochondrial trees. This is the case of A. chinensis var. chinensis and A. callosa var. strigillosa, considered by Chat (2003). The two species are morphologically distinct. A plausible explanation has been discussed by Chat (2003) and takes into consideration hybrids originating many times and in both directions (female of species A × male of species B and male of species A × female of species B). These hybrids can cross with each other, thus producing progeny with both chloroplasts and mitochondria of the type of only one of the original species. This probably happened many times in the evolution of Actinidia and could explain the difficulty in finding appropriate classification keys based on phenotypic traits as will be discussed later.
4.4.1 The Sequences of Choice
Plant genomes are made up of a number of coding and noncoding sequences that apparently evolve at different rates. Coding sequences show fewer mutations than noncoding ones, because usually they cannot maintain mutations that alter the reading frame, create stop codons, or create other changes that silence the gene or weaken it, all occurrences on which natural selection does not operate in the case of noncoding sequences (introns, intergenic spacers, etc.). For this reason, coding sequences are more useful in taxonomical reconstruction at higher hierarchical levels, while noncoding sequences are more suitable for taxonomical reconstructions at lower hierarchical levels. Often the best results are obtained by a mixture of both kinds of sequence (Demesure et al. 1995; Soltis and Soltis 1998).
The main sequences adopted for taxonomic reconstructions in the genus Actinidia are listed in Table 4.4.
4.4.2 Reconciliation of Morphological and Molecular Data
In spite of the powerful tools offered by the analysis of cytoplasmic DNA sequences, the phylogenetic reconstruction of the genus Actinidia is far from yet being accomplished. Besides the complexity due to natural hybridization and the reticulate evolution of many taxa, further technical problems include the scarce representation of each taxon, sometimes limited to a single individual of one sex, from genotypes exchanged among repositories and originating from seed, that sometimes are the result of natural hybridization between species in collections, where the natural geographic barriers are overcome, and sometimes from a misattribution of samples to a given species. Furthermore, the genus has been subjected to a number of major revisions with many taxa being reduced to synonymy (Li et al. 2007a, b).
The first attempts to make a reconstruction based on the comparison of cp- and mtDNA sequences, even if carried out with relatively few taxa, clearly demonstrated that phylogenetic reconstructions are in conflict with the classification at that date based on morphological traits.
The Leiocarpae is the only monoplyletic group to be identified by several authors, although there is evidence that even that section could be polyphyletic , with the series Solidae being distinct (Cipriani et al. 1998). The other clades group together taxa that belong to different sections and series, thus giving no phylogenetic credit to traits, such as the presence/absence of lenticels (spots) on fruit, the lamellate/solid pith, and the type of hairs (simple/stellate).
The recent review of the genus by Huang and Ferguson (2007) stresses that the geographic distribution of species should be taken more into consideration for the subdivision of the genus. It was suggested that the best and most natural solution might be to put the Solidae as an ancestral group that gave rise to the Leiocarpae. Then, Actinidia should be divided into two sections: Leiocarpae (glabrous fruit with smooth skin) and Maculatae (spotted fruit), the latter then being further divided into four series containing taxa more homogeneous for their geographic origin. The new series would encompass species from the Yangtze Valley, from southeastern, southern, and southwestern China (Li et al. 2003; Huang and Ferguson 2007; Huang 2014).
This hypothesis needs to be confirmed by a more detailed and more comprehensive exploration of the genus at the molecular level, but it would better reconcile molecular data and morphological traits, taking into account the complex reticulation of the genus.
References
Azhagiri AK, Maliga P (2007) Exceptional paternal inheritance of plastids in Arabidopsis suggests that low-frequency leakage of plastids via pollen may be universal in plants. Plant J 52(5):817–823
Chat J (2003) Transmission des génomes cytoplasmiques et phylogénie moléculaire chez Actinidia. Thèse de doctorat, INAPG Paris-Grignon
Chat J, Chalak L, Petit RJ (1999) Strict paternal inheritance of chloroplast DNA and maternal inheritance of mitochondrial DNA in intraspecific crosses of kiwifruit. Theor Appl Genet 99:314–322
Chat J, Jáuregui B, Petit RJ, Nadot S (2004) Reticulate evolution in kiwifruit (Actinidia, Actinidiaceae) identified by comparing their maternal and paternal phylogenies. Am J Bot 91(5):736–747
Cipriani G, Testolin R, Morgante M (1995) Paternal inheritance of plastids in interspecific hybrids of the genus Actinidia revealed by PCR-amplified chloroplast DNA fragments. Mol Gen Genet 247:693–697
Cipriani G, Testolin R, Gardner R (1998) Restriction-site variation of PCR-amplified chloroplast DNA regions and its implication for the evolution and taxonomy of Actinidia. Theor Appl Genet 96(3/4):389–396
Cusack BP, Wolfe KH (2007) When gene marriages don’t work out: divorce by subfunctionalization. Trends Genet 23:270–272
Demesure B, Sodzi N, Petit RJ (1995) A set of universal primers for amplification of polymorphic non-coding regions of mitochondrial and chloroplast DNA in plants. Mol Ecol 4:129–134
Fajardo D, Senalik D, Ames M, Zhu H-Y, Steffan SA, Harbut R et al (2013) Complete plastid genome sequence of Vaccinium macrocarpon: structure, gene content, and rearrangements revealed by next generation sequencing. Tree Genet Genomes 9:489–498
Gantt JS, Baldauf SL, Calie PJ, Weeden NF, Palmer JD (1991) Transfer of rpl22 to the nucleus greatly preceded its loss from the chloroplast and involved the gain of an intron. EMBO J 10:3073–3078
Haberle R, Fourcade HM, Boore JL, Jansen RK (2008) Extensive rearrangements in the chloroplast genome of Trachelium caeruleum are associated with repeats and tRNA genes. J Mol Evol 66:350–361
Hagemann R (2004) The sexual inheritance of plant organelles. In: Daniell H, Chase CD (eds) Molecular biology and biotechnology of plant organelles: chloroplasts and mitochondria. Springer, Dordrecht, pp 93–113
Hirsch AM, Testolin R, Brown S, Chat J, Fortune D, Bureau JM et al (2001) Embryo rescue from interspecific crosses in the genus Actinidia (kiwifruit). Plant Cell Report 20:508–516
Huang H-W (2014) The genus Actinidia, a world monograph. Science Press, Beijing
Huang H-W, Ferguson AR (2007) Genetic resources of kiwifruit: domestication and breeding. Hort Rev 33:1–121
Huang H-W, Liu Y-F (2014) Natural hybridization, introgression breeding, and cultivar improvement in the genus Actinidia. Tree Genet Genomes 10:1113–1122
Hudson KR, Gardner RC (1988) Organisation of the chloroplast genome of kiwifruit (Actinidia deliciosa). Curr Genet 13:339–342
Jansen RK, Cai Z-Q, Raubeson LA, Daniell H, dePamphilis CW, Leebens-Mack J et al (2007) Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. Proc Natl Acad Sci USA 104:19369–19374
Jansen RK, Saski C, Lee S-B, Hansen AK, Daniell H (2011) Complete plastid genome sequences of three rosids (Castanea, Prunus, Theobroma): evidence for at least two independent transfers of rpl22 to the nucleus. Mol Biol Evol 28:835–847
Kleine T, Maier UG, Leister D (2009) DNA transfer from organelles to the nucleus: the idiosyncratic genetics of endosymbiosis. Annu Rev Plant Biol 60:115–138
Ku C, Hu J-M, Kuo C-H (2013) Complete plastid genome sequence of the basal asterid Ardisia polysticta Miq. and comparative analyses of asterid plastid genomes. PLoS ONE 8(4):e62548
Kurtz S, Choudhuri JV, Ohlebusch E, Schleiermacher C, Stoye J, Giegerich R (2001) REPuter: the manifold applications of repeat analysis on a genomic scale. Nucleic Acids Res 29:4633–4642
Lee H-L, Jansen RK, Chumley TW, Kim K-J (2007) Gene relocations within chloroplast genomes of Jasminum and Menodora (Oleaceae) are due to multiple, overlapping inversions. Mol Biol Evol 24:1161–1180
Li J-Q, Huang H-W, Sang T (2002) Molecular phylogeny and infrageneric classification of Actinidia (Actinidiaceae). Syst Bot 27:408–415
Li Z-Z, Huang H-W, Jiang Z-W, Li J-Q, Kubisiak TL (2003) Phylogenetic relationships in Actinidia as revealed by RAPDs and PCR-RFLPs of mtDNA. Acta Hort 610:387–396
Li J-Q, Li X-W, Soejarto DD (2007a) Actinidiaceae. In: Wu Z-Y, Raven PH, Hong D-Y (eds) Flora of China, vol 12. Science Press, Missouri Botanical Garden Press, Beijing, St Louis, pp 334–360
Li ZZ, Kang M, Huang HW, Testolin R (2007b) Phylogenetic relationships in Actinidia as revealed by nuclear DNA genetic markers and cytoplasmic DNA sequence analysis. Acta horticulturae 753:45–58
Li D-W, Qi X-Q, Li X-W, Li L, Zhong C-H, Huang H-W (2013) Maternal inheritance of mitochondrial genomes and complex inheritance of chloroplast genomes in Actinidia Lind.: evidences from interspecific crosses. Mol Genet Genomics 288:101–110
Magee AM, Aspinall S, Rice DW, Cusack BP, Sémon M, Perry AS et al (2010) Localized hypermutation and associated gene losses in legume chloroplast genomes. Genome Res 20:1700–1710
Millen RS, Olmstead RG, Adams KL, Palmer JD, Lao NT, Heggie L et al (2001) Many parallel losses of infA from chloroplast DNA during angiosperm evolution with multiple independent transfers to the nucleus. Plant Cell 13:645–658
Palmer JD (1991) Plastid chromosomes: structure and evolution. In: Bogorad L, Vasil IK (eds) The molecular biology of plastids: cell genetics of plants, vol 7A. Academic Press, San Diego, pp 5–53
Raubeson LA, Jansen RK (2005) Chloroplast genomes of plants. In: Henry RJ (ed) Plant diversity and evolution of plants: genotypic and phenotypic variation in higher plants. CABI, Cambridge, pp 45–68
Reboud X, Zey C (1994) Organelle inheritance in plants. Heredity 72:132–140
Shinozaki K, Ohme M, Tanaka M, Wakasugi T, Hayashida N, Matsubayashi T (1986) The complete nucleotide sequence of tobacco chloroplast genome: its gene organization and expression. EMBO J 5:2043–2049
Soltis DE, Soltis PS (1998) Choosing an approach and an appropriate gene for phylogenetic analysis. In: Soltis DE, Soltis PS, Doyle JJ (eds) Molecular systematics of plants. II DNA sequencing. Kluwer Academic Publishers, Boston, pp 1–42
Stegemann S, Hartmann S, Ruf S, Bock R (2003) High-frequency gene transfer from the chloroplast genome to the nucleus. Proc Natl Acad Sci USA 100:8828–8833
Testolin R, Cipriani G (1997) Paternal inheritance of chloroplast DNA and maternal inheritance of mitochondrial DNA in the genus Actinidia. Theor Appl Genet 94:897–903
Ueda M, Fujimoto M, S-i Arimura, Murata J, Tsutsumi N, K-i Kadowaki (2007) Loss of the rpl32 gene from the chloroplast genome and subsequent acquisition of a preexisting transit peptide within the nuclear gene in Populus. Gene 402:51–56
Wicke S, Schneeweiss GM, dePamphilis CW, Müller KF, Quandt D (2011) The evolution of the plastid chromosome in land plants: gene content, gene order, gene function. Plant Mol Biol 76:273–297
Yang J-B, Yang S-X, Li H-T, Yang J, Li D-Z (2013) Comparative chloroplast genomes of Camellia species. PLoS ONE 8:e73053
Yao X-H, Liu L, Yan M-K, Li D-W, Zhong C-H, Huang H-W (2015a) Exon primed intron-crossing (EPIC) markers reveals natural hybridization and introgression in Actinidia (Actinidiaceae) with sympatric distribution. Biochem Syst Ecol 59:246–255
Yao X-H, Tang P, Li Z-Z, Li D-W, Liu Y-F, Huang H-W (2015b) The first complete chloroplast genome sequences in Actinidiaceae: genome structure and comparative analysis. PLoS ONE 10(6):e0129347
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Yao, X., Huang, H. (2016). Cytoplasmic DNA in Actinidia . In: Testolin, R., Huang, HW., Ferguson, A. (eds) The Kiwifruit Genome. Compendium of Plant Genomes. Springer, Cham. https://doi.org/10.1007/978-3-319-32274-2_4
Download citation
DOI: https://doi.org/10.1007/978-3-319-32274-2_4
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32272-8
Online ISBN: 978-3-319-32274-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)