Abstract
In the present chapter, the basics of thermal analysis and the most appropriate thermal investigation methods of gels, glasses, and powders obtained by sol‐gel procedure are presented. Representative data obtained by applying these methods for the mono- and polycomponent gels and powders obtained both by alcoholic and aqueous route are given. The differences that occur between the thermal properties of the materials obtained by the two methods of preparation are underlined. In both cases, it was established that the thermal methods corroborated with other structural investigations (XRD, FT-IR, Raman) allow obtaining information not only about the thermal behavior but also on the chemical composition of the as-prepared gels and powders. Information given by the thermal analysis methods used in order to establish the experimental conditions to transform the gels into (bulk) glasses and to determine their glass transition and crystallization temperature were also discussed.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Introduction
A comprehensive definition of sol‐gel method assumes that the process represents the formation of an inorganic polymeric network by reactions in the solution at low temperatures. In the second step, by adequate thermal treatments, the conversion of the inorganic amorphous polymers takes place either into glasses or into crystalline materials. In the case of glass formation, the temperatures are far lower than the melting temperature of the corresponding oxides (usually around the vitreous transition temperature) (Schmidt 1988).
As it is well established, alkoxides were the first and are mainly used reagents in the sol‐gel method, but inorganic or organic salts are also approached. Based on the type of the precursors and the reaction medium used, two types of sol‐gel processes were developed: in alcoholic (organic) or in aqueous medium.
The aqueous sol‐gel method has also two pathways: the colloidal route (Iler1979) and the aqueous route using different chelating agents (Livage et al. 1988; Brinker and Scherer 1990; Pierre 1998).
In order to establish the adequate temperatures required to transform the different gels into glasses or crystallized materials, the thermal analysis (TA) methods are relevant and are generally used.
In this chapter, the basic of thermal analysis and the most appropriate thermal investigation methods, as well as typical examples of their application for gels, glasses, and powders characterization are presented.
Basic of Thermal Analysis
According to both International Union of Pure and Applied Chemistry (IUPAC) (McNaught and Wilkinson 1997) and International Confederation for Thermal Analysis and Calorimetry (ICTAC) (ICTAC 1991) definitions, the thermal analysis (TA) represents a group of techniques in which a physical property of substance and/or its reaction product(s) is measured as a function of temperature while the substance is subjected to a controlled temperature program. The analysis is performed in a controlled atmosphere: oxidizing, reducing, inert, or in vacuum. The materials are analyzed in a broad range of temperatures under selected dynamic or isothermal temperature program (Malič et al. 2013; Segal et al. 2013).
The physical properties and the methods of thermal analysis associated to these properties are presented in Table 1.
Among the physical properties presented in Table 1, the most frequently investigated to characterize the gels and their transition to glasses or crystalline nanomaterials are the mass established by thermogravimetry (TG) or thermogravimetric analysis (TGA), mass change using derivative thermogravimetry (DTG), temperature difference between the sample and an inert reference material by differential thermal analysis (DTA), and heat or enthalpy change between the sample and an inert reference material determined by differential scanning calorimetry (DSC).
The dilatometry (DIL) represents a technique in which changes in dimension(s) of a substance are measured as function of temperature. The record represents the dilatometric curve.
The TG and DTA are usually measured simultaneously, in single equipment leading to a complex method in thermal analysis, the so-known derivatography (Paulik et al. 1966).
Likewise, simultaneous thermal analysis (STA) generally refers to the simultaneous application of TGA and DSC to the same sample in a single instrument.
Selected physical, electrical, mechanical, etc. properties as function of temperature and/or time could be also established by focused measurements mentioned in Table 1.
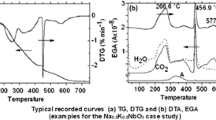
For exemplification, the TG, DTG, and EGA curves of calcium oxalate hydrate (CaC2O4.H2O), used as reference material, were summarized in Fig. 1, while in Fig. 2, the DTA and DSC curves of the same compound are presented.
The basics of all thermal analysis methods of interest for gels, glasses, and powders characterization are discussed in details in the following subchapters.
Thermogravimetric Analysis (TGA) and Derivative Thermogravimetry (DTG)
Thermogravimetric analysis (TGA) or thermogravimetry (TG) is a method of thermal analysis in which changes in the mass of the samples are measured as a function of increasing temperature (with constant heating rate), or as a function of time (with constant temperature and/or constant mass loss) (Coats and Redfern 1963).
TGA is commonly used to determine selected characteristics of materials that present either mass loss due to decomposition, loss of volatiles (such as moisture), or mass gain due to oxidation. Common applications of TGA are (1) materials characterization through analysis of specific decomposition patterns, (2) determination of organic content in a sample, (3) determination of inorganic content in a sample, and (4) studies of degradation mechanisms and reaction kinetics.
In the DTG, the derivative of the mass change is measured as a function of temperature or time. The area under the curve is proportional to the mass loss. The DTG allows quantitative determination of the rate of the mass change and allows establishing overlapping processes.
The basic equipment, called thermobalance, includes an analytical balance inside of a furnace. The maximum temperature may reach over 2000 °C; however, more commonly the investigations are performed at temperatures below 1550 °C or even 1200 °C, especially in the case of sol‐gel-derived samples. The temperature inside of the furnace is controlled by a thermocouple. This is selected based on the maximum temperature of the furnace. The sample is positioned inside an inert crucible, typically consisting of platinum or alumina. Up-to-date equipments use sample masses in the range of few mg or a few tens of mg. Such low masses allow better heat transfer upon analysis.
Before analysis, the thermobalance should be calibrated for temperature and mass, and the baseline of the balance should be recorded mainly to take into account the buoyancy effect as a consequence of the change in the density of the working gas upon heating and cooling (Wedelant 1986; Haines 2002; Malič et al. 2013).
Differential Thermal Analysis (DTA)
In differential thermal analysis (DTA), the temperature difference between a substance and a reference material is measured as function of temperature. The substance and reference material, generally Al2O3, are subjected to a controlled temperature program. The difference in the temperature of the sample and the reference materials is measured as the voltage difference of the thermocouples, positioned in the walls of the sample and reference crucibles. The area of the DTA peak is proportional either to the released heat (in the case of an exothermic reaction upon heating) or adsorbed heat (in the case of an endothermic reaction upon heating). The results of DTA are presented as the temperature difference expressed depending on the type of equipment, usually in μV/mg versus temperature.
Both DTA and DSC provide similar information. DTA measures the difference in temperature between the sample and the reference when they are both put under the same heat, while DSC measures the energy required to keep both the reference and the sample at the same temperature.
Differential Scanning Calorimetry (DSC)
The differential scanning calorimetry (DSC) is a thermoanalytical technique in which as mentioned above the difference in the amount of heat required to increase the temperature of a sample and of a reference is measured as a function of temperature. Both the sample and the reference are maintained at the same temperature during the experiment. Generally, in the DSC analysis, the temperature of the sample holder increases linearly as a function of time. The reference sample should have a well-defined heat capacity over the range of temperatures to be scanned.
The result of a DSC experiment is a curve of heat flow versus temperature or versus time. This heat flow is proportional to the area under the curve. This curve can be used to calculate enthalpies of transitions, by integrating the peak corresponding to a given transition.
It can be shown that the enthalpy of transition can be expressed using the following equation:
where ΔH is the enthalpy of transition, K is the calorimetric constant, and A is the area under the curve. The calorimetric constant will vary from instrument to instrument and can be determined by analyzing a well-characterized sample with known enthalpies of transition.
Physical and chemical changes detected by DTA or DSC include phase transitions as desorption, reduction, dehydration, and decomposition reactions within/with solids, liquids, or with gases, as, for example, oxidation (Dean 1995; Pungor 1995).
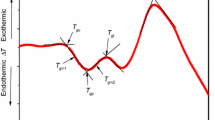
DSC may also be used to observe more sensitive physical changes, such as glass transitions. When the temperature of an amorphous solid is increased, glass transitions may occur. These transitions appear as a step in the baseline of the recorded DSC signal. This is due to the sample undergoing a change in heat capacity; no formal phase change occurs (Dean 1995; Skoog et al. 1998).
As the temperature increases, the amorphous solid becomes less viscous. At some point, the molecules may obtain enough freedom of motion to spontaneously arrange themselves into a crystalline form. This is known as the crystallization temperature (Tc). This transition from amorphous solid to crystalline solid is an exothermic process and results in a peak in the DSC signal. As the temperature increases, the sample could reach its melting temperature (Tm). The melting process results in an endothermic peak in the DSC curve. The ability to determine transition temperatures and enthalpies makes DSC a valuable tool in establishing phase diagrams for various chemical systems (Dean 1995).
It should be noted that reversible processes, such as phase transitions, have different sings of DTA or DSC signals upon heating and cooling (Dean 1995; Pungor 1995; Skoog et al. 1998).
Two types of DSC equipments are known, namely, the power compensated DSC that keeps power supply constant and heat flux DSC that maintains heat flux constant.
The temperature ranges for majority DSC instruments range from about −150 °C (cooling by liquid nitrogen) to 500–700 °C, depending on the type of the chamber, thermocouples, and crucibles. High-temperature heat flux DSC reaches up to 1500–1700 °C (Malič et al. 2013).
Evolved Gas Analysis (EGA)
Evolved gas analysis (EGA) is a method used to study the gas evolved from a heated sample that undergoes decomposition or desorption and analyzes explicitly which gases was evolved. Different analytical methods can be employed such as mass spectrometry, gas chromatography, or Fourier transform spectroscopy.
By coupling the thermal analysis instrument, e.g., TG (thermogravimetry) or DSC (differential scanning calorimetry), with a fast quadrupole mass spectrometer (QMS), the detection of gas separation and identification of the separated components are possible in exact time correlation with the other thermal analysis signals (Price et al. 1980; Barnes 1987). The TG-QMS/DSC or TG-QMS methods yield information on the composition (mass numbers of elements and molecules) of the evolved gases. It allows fast and easy interpretation of atomic/inorganic vapors and standard gases like H2, H2O, CO2, etc. Fragmentation interpretation of the organic molecules is sometimes difficult.
The TG/DSC-FTIR or TG-FTIR methods yield information on the composition (absorption bands) of the evolved gases (bonding conditions). The advantage is an easy interpretation (spectra data bases) of organic vapors without fragmentation. Symmetrical molecules cannot be detected (Mullens et al. 1992).
Dilatometry (DIL)
The dilatometry (DIL) represents a technique in which changes in dimension(s) of a substance are measured as function of temperature in a controlled atmosphere. The results of this analysis are the dilatometric curve. The method allows establishing the temperature dependence of the expansion coefficient α, or the corresponding shrinkage.
The structure of the material and the type of chemical bonding influence also the expansion coefficient, in the following order: compounds with molecular bonds > metals > compounds with covalent bonds and ionic bonds.
The method allows to identify the solid-state reactions, solid decomposition of the solids, polymorphic transitions, crystallization processes, sintering, densification, and so on (Segal et al. 2013).
Combined Methods of Thermal Analysis
The simultaneous methods refer to applying more thermal analysis techniques in the same time. The main advantage of using coupled techniques is represented by obtaining multiples information, more precise and more complete on the thermal events. Moreover, the time required for the experiments is reduced, and the experimental variations that could occur between multiple investigations are eliminated.
The most frequently used combination of simultaneous technique is that between the method that measure the weight variation (TGA) and one of which identify the thermal effect, mainly the DTA, for the derivatography, and/or DSC, for the simultaneous thermal analysis (STA).
The test conditions are perfectly identical for the TG and DTA or DSC signals (same atmosphere, gas flow rate, vapor pressure of the sample, heating rate, thermal contact to the sample crucible and sensor, radiation effect, and so on).
The TG and/or DTA systems could be coupled with the analysis of gaseous products evolved (EGA). The method allows the measurement of gas volume, mass fragments by mass spectrometry (MS), functional groups by Fourier transform infrared spectrometry (FTIR), gas conductivity, or other selected properties.
Focused Thermal Analysis Methods
The focused thermal analysis methods presented in Table 1 are not discussed in detail, being less used in the characterization of the gel, glasses, and powders obtained by sol‐gel method.
Applications of Thermal Analysis
The main advantages of the sol‐gel process compared to other methods of preparation are the high homogeneity of the reagents at molecular level and the possibility to prepare solids with predetermined structure and shape as gels (bulk), films, fibers, and powders by varying the experimental conditions.
For the polycomponent systems, the main advantage consists in the fact that most commonly chemical bonds are achieved between the oxides’ precursors in solution during the sol‐gel process leading to formation of monophasic gel not of mixture of phases.
The thermal behavior of monocomponent, polycomponent gels, and powders obtained by both sol‐gel method, in alcoholic or/and in aqueous media, will be discussed further.
The influence of some important experimental parameters involved in the sol‐gel method upon the thermal properties of the resulted samples will be taken into consideration. Examples of the influence of the type of the alkoxides used, type of the resulted products (gel or powder), the presence of templating agents in the reaction mixture, the influence of enhanced the sol‐gel process (microwaves), and so on will be analyzed.
Taking into considerations that thermal analysis is mandatory for every sol‐gel synthesis in order to establishing the adequate thermal treatment required to transform the gels or powders into glasses or crystallized nanomatrials, the selection of the representative systems for case studies represents a challenge.
Gels, Glasses, and Powders Obtained by Alcoholic Sol‐Gel Method
Silica-Based Gels, Glasses, and Powders
Silica-based nanomaterials were the first synthesized by alcoholic sol‐gel method, and the chemistry of the process was studied mainly for reactions starting with Si-alkoxides. The rather low reactivity of the silica-based alkoxides made possible such investigations.
As it is also well known, one of the greatest advantages of the sol‐gel method is the possibility to obtain glasses by adequate thermal treatments of the synthesized gels even outside of the usual composition domain of glass formation. However, the transition from gel to glasses in which the amorphous character should be preserved is more difficult than the process of gels crystallization.
The amorphous gels differ from the glasses only by their texture. The dehydrated gels are essentially porous materials, and their conversion into nonporous solids takes place by a densification process.
The theoretical evaluation of the transition of the gels to glasses was given by Zarzycki (1982) who established that the possibility to convert gels into glasses represents a competition between the phenomena that lead to densification and those that initiate crystallization.
It was also established that the thermal treatment became more sensitive when the composition of the gels becomes more complex.
SiO2-Based Glasses
SiO2 glasses. Many scientists have approached the systematic experimental studies regarding the transition from SiO2 gels to glasses; among them, the contributions belonging to Nogami and Moriya (1980), Klein and Garvey (1982), Klein et al. (1984), Brinker et al. (1982), and Brinker and Scherer (1985) could be mentioned.
Vitreous SiO2 has been prepared through thermal treatment of gels synthesized by the hydrolysis–polycondensation of organometallic compounds. As catalyst of the hydrolysis reaction, HCl and NH4OH were chosen. Using HCl catalyst, transparent gels were obtained, while in the presence of NH4OH opaque gels resulted (Nogami and Moriya 1980; Brinker et al. 1982).
Gel behavior during the conversion from gels to glasses was monitored by TG, DTA, and shrinkage, establishing the chemical and structural transformation that occurs during this process.
The identified phenomena by TG/DTA could be summarized as follows:
-
(1)
The physical desorption of water and organic solvents from the micropores (between 100 °C and 150 °C)
-
(2)
Decomposition (between 200 °C and 300 °C) and burning (between 275 °C and 400 °C) of the organic residual groups
-
(3)
Condensation-polymerization
-
(4)
Volume relaxation
-
(5)
Viscous sintering
It results that the chemical processes are dominant at low temperature, while the physical processes are predominant at higher temperatures. There is also a domain of temperatures, between 400 °C and 700 °C, in which the two types of processes are overlapping.
The shrinkage measurements show that for the acid-catalyzed gels, the temperatures corresponding to the onset of rapid shrinkage vary from about 600 to about 800 °C, while for the base-catalyzed gels the shrinkage rate increases to about 900 °C due to their different structures.
Taking into account the domains of temperatures mentioned above and the structure of the prepared gels, for each studied case, specific thermal treatment procedure were established. Usually very low heating rate (0.5–1 °C/min.) and prolonged plateaus in the domains of temperature where the mentioned chemical and physical processes take place are recommended.
SiO2–B2O5 glasses. Glasses of the SiO2–B2O3 system containing up to about 50 mol% B2O3 were prepared from Si(OC2H5)4 and B(OCH3)3 through hydrolysis, gelling, and heating (Nogami and Moriya 1982). The transparent monolithic gels were obtained by hydrolyzing the solution of B(OCH3)3, reacted with the previously partially hydrolyzed Si(OC2H5)4. On heating the gels, the network structure was strengthened accompanying the formation of Si-O-B bonds. The gels were converted into the glasses by heating above the critical temperature which corresponded to about 0.7–0.8 of the liquid temperature.
The gel to glass transition was investigated by TGA, thermal shrinkage, and bulk density measurements. The results obtained for the sample with molar composition of 30B2O3.70SiO2 (B30) are presented in Fig. 3.
Change in thermal shrinkage (A), TG (B), and bulk density (C) of 30B2O3.70SiO2 (B30) sample with increasing temperature (Nogami and Moriya 1982) (Published with permission from Elsevier)
A large weight loss due to evaporation of water is observed around 100 °C, followed by a gradual and slight decrease in weight with increasing temperature. The gel shrinks gradually from 200 °C and abruptly above 700 °C. Bulk density of the gel increases sigmoidally and reaches a constant value above 800 °C. These behaviors on heating are consistent with those observed on the conversion of the gel prepared from Si(OC2H5)4 into the glass (Nogami and Moriya 1980). Therefore, the gels of the SiO2–B2O3 system are also considered converted into the glasses by heating above the temperature at which the shrinkage is finished.
The confirmation that glasses were thus obtained is given by the fact they show glass transition temperatures, as presented in Fig. 4.
Thermal expansion curves: Tg, glass transition temperature; Td, dilatometric softening temperature of the glasses with different content of B2O3. Note: B15, B30, B50 indicates the amount of B in the composition of the glasses (Nogami and Moriya 1982) (Reproduced with the permission of Elsevier)
The glass transition temperature, Vickers hardness, and density of the glasses prepared by the sol‐gel process were almost the same as those of the conventional glasses. The B-O-B bonds were not found in the infrared spectra of the glasses which contained B2O3 below about 33 mol%.
Similar results were obtained latter by Villegas and Fernandez-Navarro (1988).
More complex gels could be also obtained by sol‐gel method and transformed into glasses by adequate thermal treatment.
Er-doped SiO2–Y2O3 glasses. Ranganathan and Klein (2008) prepared erbium-doped yttrium silicate glasses and glass–ceramics by the sol‐gel process starting with tetraethyl orthosilicate as silica reagent and different amount of yttrium nitrate (between 0.2 and 20 mol% and the disilicate composition). For doping erbium acetate was used. The solutions gelled in 2–7 days and then were dried for 2 weeks. The densification of the samples to transparent or translucent monolith was performed by multi-step heat treatment up to 1000 °C design based on the result obtained by differential thermal analysis. The TG/DTA curves show two weight loss regions, 120–130 °C and 320–330 °C corresponding first to the removal of surface water and second to the decomposition and removal of residual organics. At higher temperatures, there was a gradual weight loss due to further removal of solvent and water from the pores and the decomposition of acetates, nitrates, and silanols. There was little or no weight loss at temperatures higher than 700 °C. The compositions with ⩾80 mol% SiO2 produce translucent glass–ceramics, and those that contain ⩾90 mol% SiO2 produce largely transparent glass–ceramics. Above 1000 °C, samples crystallized to yttrium disilicate and cristobalite. Phase separation before crystallization influenced the formation of the crystal phases.
SiO2-Based Gels and Powders
In the case when substituted Si-alkoxides were used as precursors in the sol‐gel method, during the hydrolysis–polycondensation reactions, the Si-C bonds are not broken and permanently bonded organic radicals on the inorganic -Si-O-Si- network are obtained, resulting in the inorganic–organic hybrid materials.
A comparative study of the thermal stability of silica gel and SiO2-based inorganic–organic hybrid materials starting with tetraethoxysilane (TEOS), methyltriethoxysilane (MTEOS), vinyltriethoxysilane (VTEOS), and methyltrimethoxysilane (MTMOS) was previously reported (Zaharescu et al. 1999). In all cases, the starting solutions had the same molar ratio: Si-alkoxide/H2O/EtOH = 1/1/1.75.
The TG/DTA curves of the synthesized gels are presented in Fig. 5. The differences in their thermal behavior are correlated to the molecular structure of the obtained gels. From the data presented in Table 2, it could be observed that based on the type of the alkoxide used significantly different molecular species were determined by gas chromatography coupled with mass spectrometry (GC-MS) (Zaharescu et al. 1999).
TG/DTA curves of gels obtained at different aging periods starting with (a) TEOS, (b) MTEOS, (c) VTEOS, (d) MTMOS (Zaharescu et al. 1999) (Published with the permission from Springer)
For the silica powder obtained using the TEOS, a continuous mass loss is observed that is characteristic for the homogeneous amorphous materials obtained by sol‐gel method, confirming the behavior mentioned by the previous studies (Nogami and Moriya 1980; Brinker et al. 1982; Zaharescu et al. 1989).
Among the substituted alkoxides, the gel obtained starting with MTEOS has shown the highest thermal stability, while the gel obtained using VTEOS presented lowest one. A particular behavior was noticed for the gel obtained starting with MTMOS that decomposes in four steps. This behavior could be assigned to the presence in the gel of four predominant types of molecular species (dimmers, trimmers, tetramers, and pentamers) (see Table 2), in similar amount. These might have different thermal stability that could lead to the four-step decomposition.
Silica-based hybrid inorganic–organic “melting gels.” Among the silica-based hybrid inorganic–organic gels, the so-called melting gels present a high interest, for several applications involving pattern transfer and imprinting. They have the ability to soften and flow at around 100 °C for some combinations of mono- and disubstituted alkoxysiloxanes, where substitutions are either all aromatic or all aliphatic.
In a recent study, Jitianu et al. (2015) prepared melting gels starting with phenyltriethoxysilane (PhTES) and dimethyldiethoxysilane (DMDES), meaning both an aromatic and aliphatic substitution. Thermal gravimetric analysis (TGA) coupled with differential thermal analysis (DTA) was performed to measure weight loss, while differential scanning calorimetry (DSC) was performed to identify glass transition temperatures.
The TG and DTA results of the melting gels in the PhTES–DMDES system are presented in Fig. 6, while the results of the DSC investigations are shown in Fig. 7.
Thermogravimetric (TG) curves and differential thermal analysis (DTA) curves for the fresh melting gels recorded under oxidative condition (Jitianu et al. 2015) (Published with the permission from John Wiley & Sons)
Differential scanning calorimetry (DSC) analysis, performed under nitrogen, for the fresh studied melting gels (Jitianu et al. 2015) (Published with the permission from John Wiley & Sons)
From the data presented in Fig. 6, it was established that below 450 °C, the gels lost little weight, while around 600 °C the weight loss dropped. This temperature is lower than the temperature for gels prepared with only aromatic substitutions, but higher than that for gels prepared with only aliphatic substitutions.
Final heat treatment was carried out at 150 °C for the gel with 80% PhTES–20% DMDES (in mol%). The consolidation temperature increased with increasing DMDES content up to 205 °C for the gel with 50% PhTES–50% DMDES. After this heat treatment, the melting gels no longer soften.
The DSC data presented in Fig. 7 have shown that the glass transition temperatures (Tg) ranged between −61 °C and +5.6 °C. The mentioned temperatures are situated between the values in the methyl only system, where all Tg values are less than 0 °C, (Jitianu et al. 2012) and the values in the phenyl only system, where Tg values are greater than 0 °C. The Tg decreased with an increase in the DMDES fraction.
Based on the obtained results, it could be concluded that the temperature of softening and consolidation can be changed in melting gels by changing the ratio between the monosubstituted and disubstituted siloxanes. In the same time, the range between softening and consolidation can be adjusted by changing the substituted groups from aromatic with aliphatic. For a given composition, the mentioned substitutions determine the glass transition temperature, the consolidation temperature, and the difference between them.
1D SiO2 nanostructures. The interest for SiO2-based nanotubes is directly connected to their different application in various fields such as biochemistry, separation science, drug delivery, or nanotechnology (Anastasescu et al. 2010). Possible applications of SiO2 nanotubes in catalysis and photocatalysis were also established (Anastasescu et al. 2009, 2012).
Although there are increasing numbers of studies on the synthesis of multifunctional SiO2 nanomaterials with one-dimensional structure, their preparation by sol‐gel method, as a pure phase, is still a challenge.
The influence of the experimental conditions of the sol‐gel process in order to obtain either pure hollow SiO2 tubes or SiO2 nanoparticles was studied by Anastasescu et al. (2010). The synthesis of SiO2 tubes and nanospheres was accomplished starting with tetraethyl orthosilicate (TEOS) in the following molar ratio: [Tartaric acid: H2O:ROH]:TEOS:NH4OH = [1:0.04:25]:1:16. The only difference between the experimental conditions for SiO2 tubes or nanosphere formation consisted in the type of tartaric acid used as templating agent, namely, DL-tartaric acid for nanotubes and meso-tartaric acid for nanopowders.
The TEM images of the as-synthesized tubes and nanospheres are presented in Fig. 8, and the evolution of DTA/DTG curves for the tubular and spherical SiO2 powders is presented in Fig. 9. The samples did not display any significant differences. Only the adsorbed water evolution, accompanied by an endothermic effect with the maximum at about 65 °C and a continuous weight loss following up to 1200 °C, can be observed. However, in the case of SiO2 nanotubes, a small and broad endothermic effect was noticed at 375 °C that could be assigned to the elimination of both the organic residues and the structural hydroxyls from the nano-/microtubes. This observation, in correlation with the FT-IR data, confirms the presence of a higher amount of structural hydroxyls strongly bonded in the structure of tubular samples.
TEM images of the SiO2 nanotubes (a) and nanopowders (b) obtained in the same experimental conditions, but using different templating agent (DL-tartaric acid in the first case (a) and meso-tartaric acid in the second case (b)) (Anastasescu et al. 2010) (Reproduced with permission from Elsevier)
DTA/TG curves of the tubular (T) and spherical (S) SiO2 (Anastasescu et al. 2010) (Published with permission from Elsevier)
Nakamura and Matsui (1995) have established that thermal treatment in air, higher than 800 °C (800–1000 °C), changes the as-prepared amorphous tubes into tubular SiO2 glasses.
The dried and thermally treated SiO2 nanotubes have shown similar structure and thermal behavior with the SiO2 nanopowders, but presented unexpected, morphology-dependent photocatalytic, properties (Anastasescu et al. 2009).
Other Monocomponent Oxide Systems
Sol‐gel processes allow synthesis of powders with a more elaborate structure from point of view of composition, purity, size, and size distribution. For the powders, the sol‐gel refers to processing in a liquid medium to obtain a solid matter which does not settle under gravity—that means they do not precipitate. In the water presence, the precursors undertake the following succession of transformation: hydrolysis →polymerization→ nucleation → growth (Pierre 1998).
Al2O3, TiO2, MgO, and Fe2O3 sol‐gel monocomponent powders have been obtained by Crisan et al. (Crisan et al. 2000). Such powders may be used as row materials for advanced polycrystalline ceramics preparation, as pigments, catalysts, or support in chromatography.
As reagents, Al(O-iC3H7)3, Ti(O-C2H5)4, Mg(O-C2H5)2, and Fe(acac)3 were used. The hydrolysis of aluminum and titanium alkoxides took place in uncatalyzed reaction mixture (Yoldas 1975; Chappell et al. 1990). Base catalysis was used for preparation of magnesium and iron powders after proper procedure. The hydrolysis of aluminum and iron alkoxides took place with a high water excess due to the slower evolution of their hydrolyses–polycondensation reactions. The increased reactivity of titanium alkoxides justified the performing the reaction at room temperature in this case.
It is well known that powders obtained by sol‐gel method contain always adsorbed and structural water, as well as organic residues. The evolution of the mentioned components at different temperature, obtained by TG/DTA, is presented in Fig. 10.
TG/DTA results for all samples studied (Al2O3, TiO2, MgO, Fe2O3 precursors powders) (Crisan et al. 2000) (Published with permission of INOE Publishing House)
In all cases, the endothermal effects in the 20–160 °C temperature range are assigned to the evolution of adsorbed water and alcohol and in the range of 150–400 °C to the removal of residual OH groups. The water elimination in the case of aluminum oxide takes place in two steps, the second one being accompanied by the endothermal effect at 460 °C. The thermal behavior of aluminum oxide powder is characteristic to pseudoboehmite decomposition. The exothermal effect, without mass loss, at about 675 °C, noticed in the DTA/TGA curve of TiO2 powder is assigned to the anatase → rutile transformation.
For Mg-containing powder, the Mg(OH)2 decomposition occurs with a significant mass loss simultaneously with MgO crystallization, being accompanied by strong endothermal effect at 405 °C.
The exothermic effect at about 542 °C for the iron-containing powder could be assigned to the crystallization of α-Fe2O3.
The thermal stability of the obtained powders is different and increase in the order TiO2 < MgO < Fe2O3 < Al2O3. The as-prepared TiO2 powder losses the waters and organic residues up to 300 °C, while other gels need, in order to eliminate the mentioned molecular species, an annealing up to 450 °C.
The thermal treatment of the oxide powders does not change the particles size but decreases their tendency to agglomerate.
Doped oxide system. Among the oxides obtained by sol‐gel method, TiO2 is one of the most highly studied due to its specific properties having applications in various fields such as photocatalysis (Tahir and Amin 2013, gas sensors, dye-sensitized solar cells (O’Regan and Gratzel 1991; Dumbrava et al. 2008), optical coatings, antimicrobial materials (Wei et al. 2014), and pigments.
To improve the properties of TiO2 nanopowders, several dopants are used, depending on the desired properties. As dopants various metals, nonmetals, and co-doping were used (Akpan and Hameed 2010). Among metals, different transition metal ions, Pt4+, Mo5+, Mo+6, V5+, V4+, Fe3+, Ru3+, Co3+, Ag+, and Al3+; rare earth elements, La3+, Ce3+, Er3+, Pr3+, Gd3+, Nd3+, and Sm3+; or alkaline earth metals were used as dopants. Moreover, various researchers have co-doped titanium dioxide with metal–metal systems, metal–nonmetal systems or nonmetal–nonmetal system. The amount of dopant varies from 0.002 to 10 wt%. (Stanciu et al. 2014).
In our case study, Fe3+ was selected as dopant, using as precursor Fe(NO3)3.9H2O reagent and rather low alcohol/Ti(alkoxide) molar ratio, namely, 21.0 or 36.5, respectively, as compared to the much higher ratio, most frequently mentioned in the literature (Crişan et al. 2015).
When TiO2 was doped with 2% Fe2O3, using a reaction mixture with alcohol/Ti-alkoxide = 21.0 molar ratio, gel was obtained, while in the case of 36.5 molar ratio powders resulted. In order to establish the thermal behavior of the gel and powders, their TG/DTA analysis was performed. The obtained results are presented in Figs. 11 and 12.
It could be noticed that the decomposition occurs with a closed weight loss for the gel and powder. In the range 20–150 °C, in both cases physical adsorbed water and solvent was eliminated.
At higher temperature, in the case of Fe-doped TiO2 gel, stepwise decomposition is noticed assigned to the decomposition and elimination of organic residues and nitrated species from dopant sources, accompanied with an exothermal effect at 228 °C. The exothermal effects at about 326 °C and 423 °C are assigned to the burning out of the organic residues and to the crystallization of TiO2 as anatase.
For the Fe-doped TiO2 powder, a continuous weight loss is noticed that could be assigned mainly to the adsorbed water and structural hydroxyls elimination. At temperatures higher than 200 °C, namely, 420 °C, a small exothermic effect is noticed that is assigned to the anatase crystallization.
Based on the results of the thermal analysis, both samples were thermally treated for 1 h at 450 °C, and the obtained TEM images are presented as inserts in Figs. 11 and 12.
The TEM results show that nanosized powders resulted in both cases but with different size that could influence also their specific surface area and their properties.
Polycomponent Oxide Systems
MgO–TiO2 system is the first selected case study for polycomponent systems.
Among the oxide nanopowders, the monocomponent as well as bicomponent ones belonging to the MgO–TiO2 system present increased interest in the field of environmental protection and decontamination, many studies being focused on their preparation by several wet chemical methods.
The monocomponent and binary powders were prepared starting with titanium ethoxide (Ti(OC2H5)4) and magnesium nitrate (Mg(NO3)2⋅6H2O) as reagents, the ethyl alcohol (C2H5OH) as solvent, H2O as hydrolysis reagent, and the ammonia (NH4OH) as catalyst.
The thermal behavior of the as-prepared powders is presented in Figs. 13 and 14.
Thermogravimetric curves of as-prepared powders (Todan et al. 2014) (Published with permission from Springer)
Thermodifferential profiles of the as-prepared powders (Todan et al. 2014) (Published with the permission from Springer)
For all studied samples, the mass loss and the corresponding endothermic effects were assigned to the evolution of adsorbed water and structural hydroxyl groups. The dehydration of the monocomponent powders takes place below 100 °C, while the structural hydroxyls are eliminated in the 150–400 °C temperature range.
In the case of titania-based powder, a continuous mass loss is observed that is characteristic for the homogeneous amorphous materials obtained by sol‐gel method. The exothermic effect at 403 °C noticed in the case of the TiO2-based powder could be assigned to the anatase phase crystallization. In this temperature range (150–400 °C), the MgO-based powder presents a change of the slope of the mass loss accompanied with a well-defined endothermic effect at 342 °C.
In the case of the TiO2–MgO binary powder, the mass loss takes place stepwise being accompanied by corresponding endothermic effects, assigned to water, structural hydroxyls, and organic residue elimination. The exothermic effect at 531 °C shows the crystallization of the resulted powders. The effect is accompanied by a small mass loss that could be correlated with the decomposition of the MgCO3 intermediate formed due to the reaction of MgO with CO2 from atmosphere. According to the literature data, the phase formation and crystallization of several MgTiO3, Mg2TiO4, and MgTi2O5 compounds could take place (Hsiang and Lin 2004; Filipovic et al. 2010).
It can be seen that the main decomposition effects of the binary MgO–TiO2 powder do not overlap with the thermal effects of the monocomponent powders, allowing to conclude that the binary system does not represent a physical mixture of the individual oxides, but chemical bonds were formed during the sol‐gel process between the TiO2 and MgO precursors. It was observed that TiO2 powder thermally treated at 300 °C has anatase structure and exhibit high surface area. For the MgO powder, by thermal treatment, periclase structure was obtained with a low surface area. The MgO–TiO2 powders crystallized at 600 °C as a mixture of magnesium dititanate with pseudobrookite structure and very low amount of anatase.
The adsorption capacity of the studied samples depends mainly of their composition rather than of their structure and morphology, the greatest tendency for adsorption of CO2 from atmosphere being established for MgO powders.
Sodium potassium niobate. Lead-free piezoelectric ceramics have been intensively studied since the early 2000s due to the increased interest to replace the toxic PbTiO3 and Pb(Ti,ZrO)3 piezoelectric and ferroelectric compounds that contain high amount of lead (Muralt 2000; Scott 2007; Roedel et al. 2009).
Among the alternatives, which could replace lead-based perovskites (Egerton and Dillon 1959; Zhang et al. 2007), the stoichiometric sodium potassium niobate (K0.5Na0.5)NbO3 (KNN), with the alkali metal molar ratio equal to 1, has been one of the most intensively studied.
Many attempts were done to prepare (K0.5Na0.5)NbO3 (KNN), using several methods; among them the sol‐gel process plays an important role.
Kupec et al. (2014) prepared (K0.5Na0.5)NbO3 (KNN) by sol‐gel method starting with potassium acetate (KO2C2H3, 99 + %), sodium acetate (NaO2C2H3, 99.5%) and Nb-ethoxide (Nb(OCH2CH3)5, 99.99%) dissolved in 2-methoxyethanol, either in a stoichiometric ratio or with 5% or 10% of potassium or sodium acetate excess.
The TG/DTA/EGA of the as-dried precursors in air, presented in Fig. 15, revealed that the thermal decomposition occurred gradually. Upon heating to 200 °C, evaporation of water and residual solvent occurred as confirmed by a broad endothermal peak. By further heating from 200 °C to 480 °C, the evolution of water, carbon dioxide, and traces of acetone (A) confirmed the oxidation of organic species, i.e., alkoxide and acetate groups, with almost 200 °C below the onset of the chemical decomposition of alkali acetates. This fact indicates that the resulted gel is not a mixture of phases, but a heterometallic complex was formed in solution, in agreement with FTIR analysis (not presented here). Both the amount and to a lesser extent the choice of alkali acetate excess influenced the thermal decomposition of the organic groups predominantly in the temperature interval between ~200 °C and ~400 °C.
(a) TG and DTG and (b) DTA and EGA curves of the stoichiometric KNN precursor. Curve A in the figure denotes evolution of acetone (Kupec et al. 2014) (Reproduced with the permission of Slovenian Chemical Society)
From 480 °C to 630 °C for the stoichiometric (KNN) and the KNN with 5% potassium excess (5 K-KNN) and to 700 °C for the KNN with 10% potassium excess (10 K-KNN) samples, the decomposition of carbonaceous residues occurred. Similar thermal decomposition pathways have been reported also for other acetate alkoxide-based precursors, such as PbTiO3 or Pb(Zr,Ti)O3 (Kupec et al. 2014).
Based on the TG results and preliminary experiments, the pyrolysis and crystallization temperatures of the KNN gels were set to 300 °C and 750 °C, respectively.
Gels and Powders Obtained by Aqueous Sol‐Gel Method
According to Pierre (1998) in both alkoxide and aqueous sol‐gel routes, the precursors undertake the following succession of transformations in the presence of water:
In the case of alkoxide-based route (non-ionized precursors), the reactions that occur are the following:
while the dissociated precursors (metallic salts in aqueous solutions) react as follows:
where A = chelating agent
The reaction (5) clearly indicates that the chelating agent is an important reagent in the aqueous gel formation.
The composition of the gels obtained by the two mentioned methods (alcoholic and aqueous) will be different, and it is expected to present different thermal behavior.
Monocomponent Oxide Systems
ZnO gel and powder. Nanostructured ZnO materials have received increased interest due to their remarkable performances in electronics, optics, and photonics, being used as dye-sensitized solar cells, photocatalyst, or gas sensors (Mihaiu et al. 2013).
Among the methods used for ZnO nanostructure preparation, the solution chemical routes become promising options for large-scale production, being faster and less expensive.
Synthesis of ZnO in aqueous media using different experimental conditions was performed by Mihaiu et al. (2013). The starting reagents for the precursor solution preparation were zinc nitrate tetrahydrate-Zn(NO3)2 · 4H2O, hexamethylenetetramine-(CH2)6N4 (HMTA), and deionized water.
If the solution of Zn(NO3)2.4H2O:HMTA with 4:1 molar ratio was reacted and stored at room temperatures, gel was obtained (sample P1). By heating the same solution at 80 °C for 48 h, powder resulted (P2 sample).
In order to establish the transformation of the gel and powder into nanocrystallized ZnO, their thermal behavior was studied.
The simultaneous TG/DTA curves of the P1 (gel) and P2 (powder) samples in air are presented in Fig. 16.
TG/DTA analysis of the P1 (gel) and P2 (powder) samples (Mihaiu et al. 2013) (Published with permission of Revue Roumaine de Chimie)
The TG curves of the P1 (gel) and P2 (powder) samples show, more or less, a similar behavior. The weight loss is greater in the case of P1 (gel) sample, probably, due to higher amount of partially reacted or residual organic components embedded in the gel. TG curves show several temperature regions for both samples which initially contain Zn(OH)(NO3)(H2O) (zinc hydroxide nitrate hydrate), NH4NO3 (ammonium nitrate), and more or less amount of organic gel-type material, respectively.
Auffredic and Louёr (1983) reported that Zn(OH)NO3.H2O compound decomposes to ZnO in a two-step process via a Zn3(OH)4(NO3)2 phase. More recently, Malecka et al. (2003) observed from TG analysis that Zn3(OH)4(NO3)2 decomposes to ZnO also in two steps. They proposed, therefore, that the first decomposition step of Zn3(OH)4(NO3)2 corresponds to the formation of amorphous anhydrous zinc nitrate, which then decomposes to ZnO in the second step.
According to the TG/DTA results, the samples were isothermally treated at 300 °C for 1 h leading to the ZnO formation.
Polycomponent Systems
Li2O–CoO system. The sol‐gel processes in aqueous media of the transitional metal ions were extensively studied by Livage et al (1988). In spite of the fact that this pathway presents numerous scientific and economic advantages, this was less explored in the last years.
The importance of LiCoO2 was growing fast and becomes one of the most common storage materials for commercial lithium-ion batteries due to the ease of preparation, excellent cycle performance, and good C-rate capability. More recently LiCoO2 was employed in applications such as electric (Wang et al. 2008) and hybrid electric vehicles (Zheng et al. 2014).
The first method used to prepare LiCoO2 was the ceramic method involving solid-state reactions at high temperature (900 °C and higher). To avoid the use of the high temperature approach, a variety of low temperature processes has been developed, such as precipitation method, alcoholic and water-based sol‐gel methods, and hydrothermal and new combined methods such as sol‐gel-based electrospinning or microwave-assisted hydrothermal synthesis (Predoana et al. 2015a).
Studies regarding the preparation of the LiCoO2 powders by sol‐gel method in aqueous media, in the presence of citric acid (CA), were reported by Predoana et al. (2015a).
The lithium cobalt citrate gel precursor, denoted (Li-Co-CA) with Li:Co:CA = 1.1:1:1 molar ratio, was obtained using Li(NO3).6H2O and Co(NO3)2.6H2O or Li(CH3COO).2H2O and Co(CH3COO)2.4H2O as reagents and citric acid (CA) as chelating agent. The gelation took place at 80 °C for 16 h. The monometal Li-citric acid gel, denoted (Li-CA), and Co-citric acid gel, denoted (Co-CA), were obtained by a similar procedure.
The resulting gels and powders, prepared from the solutions obtained using metal nitrates, were labeled (N) or (A) in the case of acetate.
In order to identify the thermal process required for elimination of the organic residues and obtaining crystallized powders, the gels were subjected to TG/DTA investigations.
The thermal decomposition of both Li-Co-CA nitrate- and acetate-based gels, in comparison with those of the gels obtained in the monometal ion systems, was approached, and the results are presented in Fig. 17.
Thermal decomposition of mono and binary gels, (a) starting with nitrates (N), (b) starting with acetates (A) (Predoana et al. 2015a) (Published with the permission from Springer)
For the Li-Co-CA nitrate binary gel (Fig. 17a), the main exothermic effect was identified at 394 °C and was assigned to the binary gel decomposition. While for the monometal gel systems, the main exothermic effects were identified at 320 °C for Co-CA (N) and at 408 °C for Li-CA (N). The main decomposition effect of the binary Li-Co-CA nitrate gel lies between the main decomposition effects of the monometal gels. This observation allows concluding that the binary metal–ion system is not physical mixture of phases, but complex coordinative gels.
The Li-Co-CA acetate binary gel (Fig. 17b) presented a broad exothermal peak that exhibits two maxima at 357 °C and 398 °C. These two maxima occur between the temperatures of decomposition of the monometal gels Co-CA (A) at 345 °C and Li-CA (A) at 500 °C. Consequently, for Li-Co-CA (A) on may conclude that the two maxima correspond to the decomposition of a gel which consists of two heteropolynuclear complexes with different thermal stability (Predoana et al. 2015b).
In order to bring more information and to confirm the assumptions mentioned on the composition of the resulted gels, their thermal behavior was investigated also by TG/DTA/EGA (Predoana et al. 2015b).
The results obtained by TG/DTA/EGA are discussed only for the Li-Co-CA (A) gel, and the corresponding curves are presented in Fig. 18, revealing in all cases only the evolution of H2O and CO2 with the maxima almost overlapping with the DTA peaks.
Comparing the TG/EGA curves of the Li-Co-CA (A) gel with the corresponding curves of the monometallic gels may assume that the first peak (357 °C) is closed to that corresponding to the decomposition of Co-CA (A) gel as is presented in Fig. 17. The second peak (402 °C) lies between the temperatures of decomposition of the Co- and Li-gels and should be assigned to the decomposition of the binary Li-Co-coordination complex gel. The result obtained by TG/EGA investigations have confirmed those obtained by TG/DTA bringing in this way information about the composition of the resulted gels.
Based on the results of the thermal analysis, the coordination complex gels were heated up to 400 °C with the heating rate of 1 °C min−1 and held at 400 °C for 1 h and heated up to 700 °C with the heating rate of 5 °C min−1 and held at 700 °C for 6 h. For both studied systems, a single pure R-3 m-layered hexagonal phase of lithium cobalt oxide was obtained (JCPDS 01-075-3201).
Oxide Nanopowders Obtained by Enhanced Sol‐Gel Methods
In recent years, the use of microwave energy to obtain nanoparticles has attracted attention, following the successful application of microwaves in organic synthesis (Kappe et al. 2013; Dudley et al. 2015).
The use of microwave in the nanoparticles synthesis may result in increasing the reaction rate, narrowing particle size distribution, increasing purity, and inducing fewer surface defects and high uniformity of shape and morphology (Baghbanzadeh et al. 2011). More importantly, the behavior of the crystallization can be controlled by adjusting the reaction temperature, time, and composition of the synthesis system used (Zhu and Chen 2014).
In order to establish the influence of the microwaves irradiation of the solutions during the sol‐gel process and on the thermal behavior of the resulted TiO2-based gels, the TG/DTA/EGA comparative analysis of the obtained gels was performed on TiO2- and V-doped TiO2 samples.
TiO2-based powders. The use of vanadium as a doping agent seems to have beneficial influence on the properties of titanium dioxide by reducing the band gap energy, increasing the absorption of visible light, and increasing specific surface area, properties that are reflected in the increasing of photocatalytic activity. The optimal amount of V doping TiO2 is a controversial subject and depends on the different synthesis conditions, the precursors and catalysts used, which can lead to different structural characteristics.
The composition of the studied samples was TiO2- and V-doped TiO2 (TVO) powders with TiO2:V2O5 = 98:2 and 99.95:0.05 molar ratios and the starting reagents were tetraethyl orthotitanate, vanadyl acetylacetonate, and ethanol as solvent. The starting solutions were homogenized by stirring for 2 h at room temperature or were exposed to microwave (MW) for 5 min at 300 W and a frequency of 2.45 GHz.
The influence of the microwaves on the thermal stability of the resulted gels was established by TG/DTA/EGA measurements and will be discussed only for the samples doped with 2% V. The recorded TG/DTA/EGA curves are presented in Fig. 19.
In both cases, sol‐gel and microwave-assisted sol‐gel methods, the same gases were evolved, but at different temperatures.
In the case of Ti-V 2% SG samples, the EGA curves have shown that the evolution of H2O is noticed at low temperature about 100 °C and around 300 °C, while CO2 is evolved only around 300 °C. In the 230–330 °C range, the ratio between H2O and CO2 is rather similar. In this way one may assume that molecular species with similar size and composition are formed by this method of preparation.
For the Ti-V 2% MW samples, the EGA curves have shown that the H2O evolution is noticed especially around 100 °C and 230 °C. At 350 °C, a lower H2O elimination was observed. The CO2 evolution takes place with three exothermal maxima, at 250 °C, 350 °C and over 400 °C, being displaced as compared with the water evolution.
It results that in the case of the microwave assisted sol‐gel method, a more complex thermal decomposition is observed, assigned to the higher number of molecular species formed in the gel.
It is expected that the different molecular species present in the gels obtained by microwave-assisted sol‐gel method will influence not only the thermal behavior but also their properties.
Conclusions
In the present chapter, the basics of thermal analysis and the most appropriate thermal investigation methods of gels, glasses, and powders obtained by sol‐gel procedure are presented.
Representative data (case studies) obtained by applying these methods in the case of mono- and polycomponent gels and powders obtained both by alcoholic and aqueous route, respectively, are given.
In the case of alcoholic route, the thermal behavior of monocomponent systems of gels and powders as well as polycomponent ones was presented. In the same time, studies regarding the transformation of the gels into bulk glasses are discussed.
For the aqueous route, some case studies for the thermal behavior of monocomponent as well as for binary powders and gels are given.
The influence of the microwaves on the sol‐gel process was also studied, and its effect on the thermal behavior of the resulted gels was established.
In both cases, it was established that the thermal methods corroborated with other structural investigations (XRD, FT-IR, Raman) allow obtaining information not only about the thermal behavior but also on the chemical composition of the as-prepared gels and powders.
References
Akpan UG, Hameed BH. The advancements in sol–gel method of doped-TiO2 photocatalysts. Appl Catal Gen. 2010;375:1–11.
Anastasescu C, Zaharescu M, Balint I. Unexpected photocatalytic activity of simple and platinum modified tubular SiO2 for the oxidation of oxalic acid to CO2. Catal Lett. 2009;132:81–6.
Anastasescu C, Anastasescu M, Teodorescu VS, Gartner M, Zaharescu M. SiO2 nanospheres and tubes obtained by sol–gel method. J Non-Cryst Solids. 2010;356:2634–40.
Anastasescu C, Anastasescu M, Zaharescu M, Balint I. Platinum-modified SiO2 with tubular morphology as efficient membrane-type microreactors for mineralization of formic acid. J Nanopart Res. 2012;14:1198 (12 pages).
Auffredic JP, Louёr D. Etude thermodynamique de la décomposition thermique des hydroxynitrates de zinc. J Solid State Chem. 1983;46:245–52.
Baghbanzadeh M, Carbone L, Cozzoli PD, Kappe CO. Microwave-assisted synthesis of colloidal inorganic nanocrystals. Angew Chem Int Ed. 2011;50:11312–59.
Barnes PA. Applications of new methods and instrumentation in thermal analysis. Thermochim Acta. 1987;114:1–13.
Brinker CJ, Scherer GW. Sol–gel-glass: I. Gelation and gel structure. J Non-Cryst Solids. 1985;70:301–22.
Brinker CJ, Scherer GW. Sol–gel science. The physics and chemistry of sol–gel processing. Boston: Academic; 1990.
Brinker CJ, Keefer KD, Schaffer DW, Ashley C. Sol–gel transition in simple silicates. J Non-Cryst Solids. 1982;42:47–64.
Chappell JS, Procopio LJ, Birchall JD. Observations on modifying particle formation in the hydrolysis of titanium (IV) tetra-ethoxide. J Mater Sci Lett. 1990;9:1329–31.
Coats AW, Redfern JP. Thermogravimetric analysis: a review. Analyst. 1963;88:906–24.
Crisan M, Jitianu A, Crisan D, Balasoiu M, Dragan N, Zaharescu M. Sol–gel monocomponent nano-sized oxide powders. J Optoelectron Adv Mat. 2000;2:339–44.
Crişan M, Răileanu M, Drăgan N, Crişan D, Ianculescu A, Niţoi I, Oancea P, Şomăcescu S, Stănică N, Vasile B, Stan C. Sol–gel iron-doped TiO2 nanopowders with photocatalytic activity. Appl Catal A-Gen. 2015;504:130–42.
Dean JA. The analytical chemistry handbook. New York: McGraw Hill; 1995. p. 15.1–5.
Dudley GB, Richert R, Stiegman AE. On the existence of and mechanism for microwave-specific reaction rate enhancement. Chem Sci. 2015;6:2144–52.
Dumbrava A, Georgescu A, Damache G, Badea C, Enache I, Oprea C, Gartu MA. Dye-sensitized solar cells based on nanocrystalline TiO2 and natural pigments. J Optoelectron Adv Mat. 2008;10:2996–3002.
Egerton L, Dillon DM. Piezoelectric and dielectric properties of ceramics in the system potassium-sodium niobate. J Am Ceram Soc. 1959;42:438–42.
Filipovic S, Obradovic N, Pavlovic VB, Markovic S, Mitric M, Ristic MM. Influence of mechanical activation on microstructure and crystal structure of sintered MgO-TiO2 system. Sci Sinter. 2010;42:143–51.
Haines PJ. Principles of thermal analysis and calorimetry. Cambridge: Royal Society of Chemistry; 2002.
Hsiang HI, Lin SC. Effects of aging on the phase transformation and sintering properties of TiO2 gels. Mater Sci Eng A. 2004;380:67–72.
ICTAC. For better thermal analysis and calorimetry. 3rd ed. Bundoora: International Confederation for Thermal Analysis; 1991.
Iler RK. The chemistry of silica. Chichester: Wiley; 1979.
Jitianu A, Lammers K, Arbuckle-Kiel GA, Klein LC. Thermal analysis of organically modified siloxane melting gels. J Therm Anal Calorim. 2012;107:1039–45.
Jitianu A, Gonzalez G, Klein LC. Hybrid sol–gel glasses with glass-transition temperatures below room temperature. J Am Ceram Soc. 2015;98:3673–9.
Kappe CO, Pieber B, Dallinger D. Microwave effects in organic synthesis: myth or reality? Angew Chem Int Ed. 2013;52:1088–94.
Klein LC, Garvey GJ. Monolithic dried gels. J Non-Cryst Solids. 1982;48:97–104.
Klein LC, Gallo TA, Garvey GJ. Densification of the monolithic silica gels below 1000 °C. J Non-Cryst Solids. 1984;63:23–33.
Kupec A, Mocioiu OC, Cilenšek J, Zaharescu M, Malič B. Study of thermal decomposition of (K0.5Na0.5)NbO3 thin-films precursors with different amounts of alkali-acetate excess. Acta Chim Slov. 2014;61:548–54.
Livage J, Henry M, Sanchez C. Sol–gel chemistry of transition metal oxides. Prog Solid State Chem. 1988;18:259–341.
Małecka B, Gajerski R, Małecki A, Wierzbicka M, Olszewski P. Mass spectral studies on the mechanism of thermal decomposition of Zn(NO3)2 · nH2O. Thermochim Acta. 2003;404:125–32.
Malič B, Kupec A, Kosec M. Thermal analysis. In: Schneller T, Waser R, Kosec M, Payne D, editors. Chemical solution deposition of functional thin films. Springer: Springer-Verlag Wien; 2013. p. 163–79.
McNaught AD, Wilkinson A. IUPAC compendium of chemical technology. 2nd ed. Oxford: Blakwell; 1997.
Mihaiu S, Madarász J, Pokol G, Szilágyi IM, Kaszás T, Mocioiu OC, Atkinson I, Toader A, Munteanu C, Marinescu VE, Zaharescu M. Thermal behavior of ZnO precursor powders obtained from aqueous solutions. Rev Roum Chim. 2013;58:335–45.
Mullens J, Carleer R, Reggers R, Yperman J, Vanhees J, Van Poucke LC. Coupling between FTIR (Bruker IFS 66) and TG (Du Pont TGA 951–2000). Thermochim Acta. 1992;212:219–25.
Muralt P. Ferroelectric thin films for micro-sensors and actuators: a review. J Micromech Microeng. 2000;10:136–46.
Nakamura H, Matsui Y. Silica gel nanotubes obtained by the sol–gel method. J Am Chem Soc. 1995;117:2651–2.
Nogami M, Moriya Y. Glass formation through hydrolysis of Si(OC2H5)4 with NH4OH and HCl solutions. J Non-Cryst Solids. 1980;37:191–201.
Nogami M, Moriya Y. Glass formation of the SiO2-B2O3 system by the gel process from metal alkoxides. J Non-Cryst Solids. 1982;48:359–66.
O’Regan B, Gratzel M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature. 1991;353:737–40.
Paulik F, Paulik J, Erdey L. Derivatography. A complex method in thermal analysis. Talanta. 1966;13(10):1405–30.
Pierre AC. Introduction to the sol–gel process. Boston: Kluwer Academic; 1998.
Predoana L, Jitianu A, Voicescu M, Apostol N, Zaharescu M. Study of formation of LiCoO2 using a modified Pechini aqueous sol–gel process. J Sol-gel Sci Technol. 2015a;74:406–18.
Predoana L, Jitianu A, Preda S, Malic B, Zaharescu M. Thermal behavior of Li-Co-citric acid water-based gels as precursors for LiCoO2 powders. J Therm Anal Calorim. 2015b;119(1):145–53.
Price D, Dollimore D, Fatemi NS, Whitehead R. Mass spectrometric determination of kinetic parameters for solid state decomposition reactions. Part 1. Method; calcium oxalate decomposition. Thermochim Acta. 1980;42:323–32.
Pungor E. A practical guide to instrumental analysis. Boca Raton: CRC Press; 1995. p. 181–91.
Ranganathan V, Klein LC. Sol–gel synthesis of erbium-doped yttrium silicate glass–ceramics. J Non-Cryst Solids. 2008;354:3567–357.
Roedel J, Jo W, Seifert KTP, Anton EM, Granzow T, Damjanovi D. Perspective on the development of lead-free piezoceramics. J Am Ceram Soc. 2009;92:1153–77.
Schmidt H. Chemistry of material preparation by the sol–gel process. J Non-Cryst Solids. 1988;100:51–64.
Scott JF. Applications of modern ferroelectrics. Science. 2007;315:954–9.
Segal E, Budrugeac P, Carp O, Doca N, Popescu C, Vlase T. Analiza termicᾰ. Fundamente şi aplicaţii (in Romanian). Bucharest: Ed. Academiei Române; 2013.
Skoog DA, Holler FJ, Nieman T. Principles of instrumental analysis. Philadelphia/Orlando: Saunders College Pub/Harcourt Brace College Publishers; 1998.
Stanciu I, Predoana L, Anastasescu C, Culita DC, Preda S, Pandele Cusu J, Munteanu C, Rusu A, Balint I, Zaharescu M. Structure and properties of vanadium doped TiO2 powders prepared by slol-gel method. Rev Roum Chim. 2014;59:919–29.
Tahir M, Amin NAS. Advances in visible light responsive titanium oxide-based photocatalysts for CO2 conversion to hydrocarbon fuels. Energ Conver Manage. 2013;76:194–214.
Todan L, Dascalescu T, Preda S, Andronescu C, Munteanu C, Culita DC, Rusu A, State R, Zaharescu M. Porous nano sized oxide powders in the MgO-TiO2 binary system obtained by sol–gel method. Ceram Int. 2014;40:15693–701.
Villegas MA, Fernandez Navarro JM. Characterization of B2O3-SiO2 glasses prepared via sol–gel. J Mater Sci. 1988;23:2464–78.
Wang QS, Sun JH, Chen CH, Zhou XM. Thermal properties and kinetics study of charged LiCoO2 by TG and C80 methods. J Therm Anal Calorim. 2008;92:563–6.
Wedelant WWM. Thermal analysis. 3rd ed. New York: Wiley; 1986.
Wei X, Yang Z, Tay SL, Gao W. Photocatalytic TiO2 nanoparticles enhanced polymer antimicrobial coating. Appl Surf Sci. 2014;290:274–9.
Yoldas BE. Alumina gels that form porous transparent Al2O3. J Mater Sci. 1975;10:1856–60.
Zaharescu M, Pîrlog C, Crişan M, Sahini M. Structural characterization of vitreous SiO2 made from gels. Rev Roum Chim. 1989;34(2):703–7.
Zaharescu M, Jitianu A, Braileanu A, Badescu V, Pokol G, Madarasz J, Novak Cs. Thermal stability of SiO2-based inorganic-organic hybrid materials. J Therm Anal Calorim. 1999;56:191–8.
Zarzycki J. Gel → glass transition. J Non-Cryst Solids. 1982;48:105–16.
Zhang S, Xia R, Shrout TR. Lead-free piezoelectric ceramics vs. PZT? J Electroceram. 2007;19:251–7.
Zheng J, Li X, Yu Y, Feng X, Zhao Y. Novel high phosphorus content phosphaphenanthrene-based efficient flame retardant additives for lithium-ion battery. J Therm Anal Calorim. 2014;117:319–24.
Zhu YJ, Chen F. Microwave-assisted preparation of inorganic nanostructures in liquid phase. Chem Rev. 2014;114:6462–555.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this entry
Cite this entry
Zaharescu, M., Predoana, L., Pandele-Cusu, J. (2018). Thermal Analysis on Gels, Glasses, and Powders. In: Klein, L., Aparicio, M., Jitianu, A. (eds) Handbook of Sol-Gel Science and Technology. Springer, Cham. https://doi.org/10.1007/978-3-319-32101-1_99
Download citation
DOI: https://doi.org/10.1007/978-3-319-32101-1_99
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-32099-1
Online ISBN: 978-3-319-32101-1
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics