Abstract
MicroRNAs (miRNAs) are a group of small noncoding RNA molecules that play a major role in posttranscriptional regulation of gene expression and are expressed in an organ-specific manner. One miRNA can potentially regulate the expression of several genes, depending on cell type and differentiation stage. miRNAs are differentially expressed in the male and female gonads and have an organ-specific reproductive function. Exerting their affect through germ cells and gonadal somatic cells, miRNAs regulate key proteins necessary for gonad development. The role of miRNAs in the testes is only starting to emerge though they have been shown to be required for adequate spermatogenesis. Widely explored in the ovary, miRNAs were suggested to play a fundamental role in follicles’ assembly, growth, differentiation, and ovulation. In this chapter, we focus on data obtained from mice in which distinct proteins that participate in the biosynthesis of miRNAs were conditionally knocked out from germ cells (spermatogonial cells or oocytes) or gonadal somatic cells (Sertoli or granulosa cells). We detail recent advances in identification of particular miRNAs and their significance in the development and function of male and female gonads. miRNAs can serve as biomarkers and therapeutic agents of pathological conditions; thus, elucidating the branched and complex network of reproduction-related miRNAs will aid understanding of gonads’ physiology and managing reproduction disorders.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
12.1 miRNAs Biogenesis
MicroRNAs (miRNAs) are a group of small noncoding RNA sequences, ranging in size from 18 to 25 nucleotides (nt) and playing a major role in posttranscriptional gene expression regulation by several mechanisms. There are two distinct subclasses of miRNAs, which differ by their biogenesis: the canonical and the noncanonical pathways. Canonical miRNAs are initially transcribed as long primary RNA transcripts (pri-miRNAs) that fold to form hairpin structure (Quick-Cleveland et al. 2014). Pri-miRNAs are recognized by DGCR8 (DiGeorge Syndrome Critical Region 8), a double-strand RNA binding protein that directs DROSHA, an RNase III enzyme, to cleave the pri-miRNA; the resulting product is a short stem-loop precursor miRNA (pre-miRNA; Nguyen et al. 2015). The pre-miRNA is then transported to the cytoplasm where it is cleaved by the RNase III enzyme DICER to a double-stranded mature miRNA (Fig. 12.1; reviewed by Ha and Kim 2014). Biogenesis of noncanonical miRNAs includes several pathways that bypass components of the machinery used for the synthesis of canonical miRNAs (Fig. 12.1; Yang and Lai 2011). Whereas most of the noncanonical miRNAs are DICER dependent and DROSHA or DGCR8 independent (Chong et al. 2010; Ender et al. 2008; Ruby et al. 2007), some are DICER independent (Cheloufi et al. 2010; Havens et al. 2012).
Biogenesis of miRNAs. Canonical miRNAs pathway: canonical miRNAs are transcribed in the nucleus from a miRNA gene or intron to long primary transcripts (Pri-miRNAs) that form a hairpin structure. Pri-miRNAs are processed to 60–70 nt precursor miRNAs (pre-miRNAs) by the DROSHA-DGCR8 complex. The Pre-miRNAs are exported to the cytoplasm where they are cleaved by DICER to double-stranded mature miRNAs, which are then loaded onto Argonaute proteins (AGO1–4). Noncanonical miRNAs pathways: these include DICER-independent and DROSHA-DGCR8-dependent as well as DICER-dependent and DROSHA-DGCR8-independent pathways. The latter constitute the majority of the noncanonical miRNAs and include several distinct pathways: (a) Mirtrons are transcribed from short introns, spliced and debranched into a product that is recognized by Dicer, hence bypassing Drosha cleavage. (b) Mirtron-like miRNAs are transcribed from long introns or independent genes. (c) snoRNAs, shRNAs, and tRNAs-derived miRNAs require the activity of Dicer but are independent of DROSHA-DGCR8
Generally, once a mature miRNA duplex is formed, one strand is selected and incorporated into one of the four Argonaute (AGO) proteins, forming the core of the RNA-induced silencing complex (RISC; Gregory et al. 2005; Hutvagner and Simard 2008). Within the RISC complex, the miRNA binds to its target mRNA by sequence complementarity between the seed sequence of the miRNA (nt 2–8) and the 3′UTR of the target mRNA. Inhibition of target mRNA expression can be executed by deadenylation of mRNA leading to its degradation, by mRNA cleavage, by translation inhibition, or by localization to sub-cytoplasmic compartments, known as P-bodies, where the mRNA can be either stored or degraded (reviewed by Stefani and Slack 2008; Inui et al. 2010). Based on current database information, there are more than 3000 miRNAs in the mouse genome; many of them are expressed in the gonadal somatic and germ cells and are assumed to have key roles in the development and physiological function of both female and male gonads.
12.2 miRNAs and Sex Determination
Expression of miRNAs is known to be diversely regulated in different organs during various stages of differentiation. Mishima et al. (2008) used cloning analysis to demonstrate different expression profiles of miRNAs in adult ovaries and testes, indicating that miRNAs have organ-specific reproductive functions. Comparison between prenatal XY and XX gonads, during the period of sex determination, revealed characteristic miRNA signatures in the developing ovaries and testes, implying that miRNAs play a role in the development of female and male gonads, already at early stages (Rakoczy et al. 2013).
The development of a gonad into an ovary or testis is dependent on the presence or absence of the Sry gene (located on Y chromosome), which is expressed in pre-Sertoli cells of 10.5–12.5 days post coitum (dpc) mouse embryos (Ewen and Koopman 2010). A critical target of SRY is the SRY-related HMG-box gene 9 transcription factor, known as SOX9 (reviewed by Ungewitter and Yao 2012), which is expressed at low levels in the developing testes and ovaries. Once the expression of SRY is upregulated, the expression of SOX9 in the testes increases, and in the ovaries it becomes undetectable (Kobayashi et al. 2005).
Ectopic expression of SOX9 in mice XX gonads leads to the development of XX testes (Vidal et al. 2001), whereas lack of SOX9 in XY mice leads to the development of XY ovaries (Barrionuevo et al. 2006). Recently, SOX9 was shown to regulate the expression of miR-202-5p and miR-202-3p, two sexually dimorphic miRNAs that are predominantly expressed in the somatic cells of XY gonads at the time of sex determination (Wainwright et al. 2013). The expression of miR-124 is similar in 11.5 dpc (prior to sexual differentiation) XY and XX gonads and increases dramatically only in XX gonads at 13.5 dpc (post sex determination). Knockdown of miR-124 in 13.5 dpc embryonic XX gonads resulted in elevated SOX9 expression, implying that miR-124 have a role in downregulation of SOX9 in XX gonad from the stage of sex determination onward (Real et al. 2013).
12.3 miRNAs in the Testis
Of the many miRNAs present in mouse testes, some are preferential or are exclusively expressed in the testes (Ro et al. 2007). Furthermore, testicular miRNAs are differentially expressed during mouse spermatogenesis (Buchold et al. 2010; Ro et al. 2007; Sree et al. 2014; Yan et al. 2007). The following chapter details data obtained from experiments in models of knockout (KO) mice in which components of the miRNA biogenesis machinery have been conditionally knocked out (cKO) from either Sertoli cells or spermatogonial cells. The role of specific miRNAs in the testes function will be briefly summarized.
12.3.1 miRNAs in Sertoli Cell
MiRNAs hold a great potential in regulating physiological and pathological processes in the male reproductive system (Kotaja 2014) and are highly abundant in Sertoli cells, compared to other noncoding RNAs (Tan et al. 2014). Nevertheless, their role in Sertoli cells is only starting to emerge. The expression of many miRNAs in adult Sertoli cells is testosterone dependent; the expression of some is differentially regulated during the first wave of spermatogenesis (Panneerdoss et al. 2012), indicating that androgens regulate spermatogenesis through a miRNAs-dependent mechanism.
KO mouse model was used to gain insight into the involvement of miRNAs in the function of Sertoli cells and their contribution to spermatogenesis. According to this model, Dicer1 is conditionally deleted from Sertoli cells using the Cre-loxP recombination system (Schwenk et al. 1995) under regulation of the Anti-Müllerian hormone (Amh) promoter (Kim et al. 2010a; Papaioannou et al. 2009). Because DICER is essential for the biogenesis of canonical and most noncanonical miRNAs, Dicer1 cKO cells exhibit dramatic changes in their miRNAs’ profile (reviewed by Abdelfattah and Choi 2015). Papaioannou et al. (2009) found that deletion of Dicer1 from Sertoli cells caused a drastic reduction in mice testes size already 15 days postpartum (dpp), leading to infertility. In testes of 15 dpp mice, a significant proportion of both Sertoli and spermatogonial cells have undergone apoptosis, inferring that miRNAs of Sertoli cells are required for spermatogenesis (Papaioannou et al. 2009). These findings are in agreement with another study that showed an increased rate of apoptosis in Sertoli cells and spermatogonial cells, in the testes of Dicer1 cKO mice, already at 3 dpp (Kim et al. 2010a). Whereas morphological changes caused by deletion of Dicer1 were observed in testes of 3 dpp mice, transcriptome analysis of testes from Dicer1 cKO mice indicated alterations in gene expression already at 0 dpp (Kim et al. 2010a). The proteomic profile of Dicer1 cKO mice was also dramatically altered already at the time of birth (Papaioannou et al. 2011).
12.3.2 miRNAs in Spermatogonial Cells
As mentioned, miRNAs are differentially expressed during mouse spermatogenesis (Buchold et al. 2010; Ro et al. 2007; Sree et al. 2014; Yan et al. 2007). Interestingly, miRNAs that are differentially expressed during spermatogenesis have a nonrandomized chromosomal distribution; an enrichment of miRNAs derived from chromosome 12 was found in testes of 7 dpp old mice and from chromosomes 2 and X in testes of 14 dpp old mice (Buchold et al. 2010). This was further supported by Sree et al. who found dramatic changes in the expression of miRNAs in testes of 8 and 16 dpp old mice, with many of the differentially expressed miRNAs mapped to X chromosome (Sree et al. 2014). This chromosomal localization of many of the miRNAs that are preferentially expressed in testes at the time of meiotic sex chromosome inactivation (MSCI) is somewhat surprising. However, a significant percentage of the X-linked miRNAs is transcribed in pachytene spermatocytes (12–14 dpp), in which MSCI is ongoing. Of these, some miRNAs remained upregulated in round spermatids, indicating that they also escape post-meiotic sex chromatin (PMSC), thus implying that X-linked miRNAs can escape MSCI and PMSC and may have a functional role in spermatogenesis (Song et al. 2009).
As in Sertoli cells, the general role of miRNAs in the spermatogonial cells was initially evaluated using cKO mice in which Dicer1 was deleted from spermatogonial cells using the Cre-loxP recombination system (Schwenk et al. 1995). However, as opposed to Sertoli cells, several models have been employed to conditionally delete Dicer1 from spermatogonial cells, by utilizing different promoters to regulate the expression of Cre recombinase (reviewed by Kotaja 2014). Accordingly, deletion of Dicer1 was achieved at different stages of spermatogenesis, yielding various phenotypic results. Using the Tissue nonspecific alkaline phosphatase (Tnap) promoter, Cre recombinase is preferentially expressed in primordial germ cells (PGCs) from 10 dpc onward (Lomelí et al. 2000). Testes of 12.5 dpc Dicer1 cKO mice had a decreased number of germ cells due to attenuated PGCs proliferation (Hayashi et al. 2008); their differentiated elongated spermatids exhibited abnormal morphology (Maatouk et al. 2008). When Vasa/Ddx4 promoter is used to drive the expression of Cre recombinase, deletion of Dicer1 begins at 12.5 dpc and reaches ~90 % by 18 dpc (Gallardo et al. 2007). These Dicer1 cKO mice are non-fertile and exhibit decreased-size testes and abnormal structure of the seminiferous tubules, due to increased apoptosis that started around 15 dpp and impaired spermiogenesis (Liu et al. 2012; Romero et al. 2011; Zimmermann et al. 2014). To evaluate whether the time of Dicer1 deletion has an impact on mice fertility, Liu et al. used two promoters: Stimulated by retinoic acid 8 (Stra8) and Phosphoglycerate kinase 2 (Pgk2) to drive the expression of Cre recombinase. Deletion of Dicer1 using Stra8 promoter occurred at 3 dpp; the cKO male mice were sub-fertile and exhibited phenotypes similar to Vasa/Ddx4-mediated Dicer1-deleted mice, though to a lesser degree (Liu et al. 2012). However, using a similar method (Stra8 promoter), other male cKO mice were completely non-fertile (Greenlee et al. 2012; Wu et al. 2012). On the contrary to the Stra8 promoter, deletion of Dicer1 is achieved only in spermatocytes and spermatids using the Pgk2 promoter and these cKO mice had no prominent phenotype (Liu et al. 2012). Selective deletion of Dicer1 using neurogenin 3 (Ngn3) promoter takes place in spermatogonial cell only from 5 dpp onward, causing defects in relatively progressive stages of spermatogenesis, indicated by normal appearance of early meiotic cells, defects in differentiation of haploid cells, and increased apoptosis (Korhonen et al. 2011). Chang et al. used the protamine 1 (Prm1) promoter to drive the expression of Cre recombinase (O’Gorman et al. 1997), causing deletion of Dicer1 at 18 dpp when the first wave of round spermatids appears (Bellvé et al. 1977). According to this model, male cKO mice exhibited increased apoptosis of round spermatids, various sperms morphological abnormalities, and improper chromatin compaction and spermiation failure. Surprisingly, the fertility of cKO males was not completely abolished; they sired fewer litters with reduced litter sizes (Chang et al. 2012). Taken together, it appears that DICER is required throughout male germ cell differentiation. The diverse severity of the different cKO mice model phenotypes may be attributed both by the time in which deletion of Dicer1 is achieved and by incomplete deletion of Dicer1.
DICER is required for the biogenesis of miRNAs and of small-interference RNAs (siRNAs; reviewed by Carthew and Sontheimer 2009). To distinguish between the contribution of miRNAs and siRNAs to the phenotype of Dicer1 cKO mice, several models have been employed in which different proteins, specific for the biogenesis of miRNAs, have been conditionally knocked out in spermatogonial cells (reviewed by Kotaja 2014). Deletion of Drosha interfered with processing of pri-miRNAs into mature miRNAs, resulting in a decreased expression of canonical miRNAs but retaining an intact siRNA pathway (Abdelfattah and Choi 2015). Specific deletion of Drosha in spermatogonial cells, using Stra8 promoter to drive the expression of Cre recombinase, resulted in male sterility due to disrupted spermatogenesis (Wu et al. 2012). Interestingly, Drosha cKO mice exhibited more severe spermatogenic disruptions than Stra8-mediated Dicer1 cKO mice. miRNAs sequencing in pachytene spermatocytes and round spermatids identified several upregulated miRNAs in Drosha cKO cells that were downregulated or absent in Dicer1 cKO cells, and vice versa, representing distinct subgroups of noncanonical miRNAs (Wu et al. 2012). DROSHA needs to form a complex with DGCR8 for successfully processing pri-miRNAs into mature miRNAs (Nguyen et al. 2015). Zimmermann et al. compared the phenotype of Dicer1 cKO mice to the phenotype of Dgcr8 cKO mice, using Vasa/Ddx4 promoter to drive the expression of Cre recombinanse. cKO mice of both phenotypes displayed similar defects, though the Dgcr8 phenotype was milder (Zimmermann et al. 2014). These findings reinforce the importance of miRNAs in the developing testes and emphasize the role of siRNAs. When Dicer1 or Dgcr8 were conditionally deleted by the Vasa/Ddx4 promoter, cKO spermatocytes exhibited drastic alterations in the morphology of sex chromosomes and failed to progress through meiosis (Modzelewski et al. 2015). This phenotype is likely to be a result of dysregulation of Ataxia telangiectasia mutated (ATM) kinase that possesses many potential sites for miRNA binding. Of these, miR-18a/b, miR-183, and miR-16 are all expressed in spermatocytes and are depleted in Dgcr8 and Dicer cKO cells (Modzelewski et al. 2015). miR-18a/b, miR-183, and miR-16 are differentially expressed during the first wave of spermatogenesis, though they constitute only a small percentage of the miRNAs expressed in spermatogonial cells during this period (Buchold et al. 2010). The chromosomal localization of these miRNAs is heterogenic and only miR-18b is mapped to X chromosome (Buchold et al. 2010). Furthermore, miR-18a and miR-18b are part of the miR-17/92 and miR-106a/363 clusters, respectively (Mogilyansky and Rigoutsos 2013). The first cluster is highly expressed in PGCs and spermatogonia cells (Hayashi et al. 2008) and is downregulated during retinoic acid (RA)-induced spermatogonial cell differentiation (Tong et al. 2012).
In pachytene spermatocytes, AGO4 is localized to sites of asynapsis and the transcriptionally silenced X and Y chromosome subdomain (sex body; Modzelewski et al. 2012), thus adding another level of complexity to the relationship between miRNAs and spermatogonial cell sex chromosomes. The morphology of the sex body in Ago4-null male mice was abnormal, leading to influx of RNA Polymerase II and failure in MSCI, indicated by expression of many sex-linked mRNA transcripts. The expression of many miRNAs was also reduced in Ago4-null spermatocytes, a reduction that was not consistent among different miRNA families, indicating some degree of miRNA specificity for AGO4. Interestingly, the expression of all miRNAs encoded on X chromosomes was significantly decreased (Modzelewski et al. 2012).
Of the AGO family of proteins, AGO4 and AGO3 are highly expressed in the testes whereas AGO1 and AGO2 are more uniformly expressed in meiotic and non-meiotic tissues (Modzelewski et al. 2012). Some of the functions of mammalian AGO proteins in miRNAs’ silencing overlap, implying a possible compensation among them (Su et al. 2009). Thus, it is not surprising that the expression of AGO3 was upregulated in testes of Ago4-null mice (Modzelewski et al. 2012). To date, AGO2 is the only member of the AGO family of proteins reported to possess a slicer catalytic activity, in both mouse (Cifuentes et al. 2010) and human (Meister et al. 2004). Furthermore, though all four AGO proteins can contribute to repression of siRNAs targets, AGO2 is more effective in siRNA-mediated gene silencing (Wu et al. 2008). In contrast, the contribution of AGO2 to repression of miRNAs targets is similar to that of the other three AGO proteins (Ma et al. 2014). Spermatogenesis was not hampered in cKO mice in which Ago2 was deleted from germ cells, using TNAP promoter to drive the expression of Cre recombinase (Hayashi et al. 2008). However, as explained above, redundancy of AGO1–4 can account for the lack of phenotype in Ago2 cKO mice.
12.4 Specific miRNAs and Their Contribution to Testes Functionality
Several miRNAs have been implicated to regulate spermatogonial stem cells’ (SSCs) self-renewal; among them are miR-21, miR-20, and miR-106a, which are preferentially expressed in SSCs than in testicular somatic cells (He et al. 2013; Niu et al. 2011). Inhibition of miR-21 increased the number of germ cells undergoing apoptosis (Niu et al. 2011), whereas overexpression of miR-20 or miR-106a increased the proliferation rate of SSCs (He et al. 2013).
Adult mice SSCs undergo a series of mitotic divisions, producing undifferentiated spermatogonia cells (de Rooij and Russell 2000). RA regulates initiation of spermatogonia cell differentiation (Snyder et al. 2010) as well as entry into meiosis (Barrios et al. 2010). Exposure of spermatogonia to RA causes alteration in the expression of miRNAs, including the miR-17/92 and miR-106a/363 clusters (Tong et al. 2012), miR-221/222 cluster (Yang et al. 2013a), and miR-146 (Huszar and Payne 2013)—all are downregulated by RA and necessary for maintaining spermatogonia cells in an undifferentiated state. In contrast, RA increases the expression of members of the let-7 family miRNAs in spermatogonia cells, through suppression of LIN28 (Tong et al. 2011).
Overexpression of miR-184 promoted spermatogonia cell proliferation and caused a decrease in the expression of Nuclear receptor corepressor 2 (NCOR2) by targeting its 3′UTR region. miR-184 is also detected through the stages of primary and secondary spermatocytes and till the stage of round spermatids (Wu et al. 2011). The miR-449 cluster is upregulated in the testes upon meiotic initiation, as well as in testes of adult mice. In situ hybridization indicated that this cluster is expressed specifically in spermatocytes and spermatids, implying a possible role of the miR-449 cluster in initiation of meiosis and spermiogenesis (Bao et al. 2012), though mice lacking the miR-449 cluster exhibit normal spermatogenesis and fertility. This conflict could be explained by a functional redundancy of miR-449 cluster and miR-34b/c, which shares a cohort of target genes with the miR-449 cluster and possesses a similar expression pattern during male germ cell development (Bao et al. 2012).
Ample data exist concerning the importance of specific miRNAs during late stages of spermiogenesis. Conversion of nucleosomal chromatin to compact non-nucleosomal chromatin in elongating spermatids requires time-restricted regulation of the expression of “chromatin packing proteins,” including protamines (PRM1, PRM2; Miller et al. 2010) and transition proteins (TNP1, TNP2; Adham et al. 2001; Meistrich et al. 2003). Spermatozoa of Heat shock transcription factor 2 (HSF2)-deficient mice have an increased rate of mild head abnormalities and altered levels of PRM1, PRM2, and TNP2 (Åkerfelt et al. 2008). The expression of HSF2 in the testes is regulated post-transcriptionally by miR-18 that belongs to the miR-17/92 cluster. Whereas miR-18 is expressed predominantly in spermatocytes, HSF2 is highly expressed in spermatogonia, decreases in spermatocytes, and reappears in round and elongating spermatids, while the level of miR-18 decreases (Björk et al. 2010). Thus, miR-18 may have a role in regulating the time-restricted expression of “chromatin packing proteins” through HSF2. Other miRNAs are known to regulate the expression of “chromatin packing proteins”: miR-469, which binds to the coding region of TNP2 and PRM2 mRNAs and inhibits translation (Dai et al. 2011), and miR-122a which binds to the 3′UTR region of TNP2, causing cleavage of the mRNA (Yu et al. 2005).
12.5 miRNAs in the Ovary
The general involvement of miRNAs in the ovary was studied using mice with cKO in key proteins that participate in the biogenesis of miRNAs, either in granulosa (follicular) cells or in oocytes. In the following section, we detail data obtained from these KO models and evaluate the comprehensive contribution of miRNAs to ovarian functionality. The role of specific miRNAs in the ovary will be discussed later.
12.5.1 miRNAs in Granulosa Cells
The possible role of miRNAs in the ovary was demonstrated by cKO of Dicer1 from granulosa cells using the Anti-Müllerian hormone receptor type 2 promoter to drive the expression of Cre recombinase (Jamin et al. 2002). A study by Nagaraja et al. (2008) demonstrated that folliculogenesis was not hampered in ovaries of adult Dicer1 cKO mice, with the exception of increased follicular atresia. Upon controlled super-ovulation, Dicer1 cKO mice yielded half the number of oocytes ovulated by control mice and histological examination of the ovaries showed oocytes trapped within luteinized follicles. When mated with wild-type males, fewer fertilized oocytes progressed to the two-cell stage, suggesting that the expression of Dicer1 in granulosa cells affects also oocyte quality indirectly (Nagaraja et al. 2008). These findings were supported by other studies that showed increased follicular atresia (Lei et al. 2010) and lower ovulation rate (Hong et al. 2008) in Dicer1 cKO mice. However, because DICER does not participate only in the biogenesis of miRNAs but also in the biogenesis of siRNAs, it cannot be determined whether miRNAs are solely responsible for this phenotype. Study by Otsuka et al. (2008) indicated that global reduction of Dicer1 expression by a hypomorphic mutation causes infertility due to defects in ovarian angiogenesis and corpus luteum (CL) insufficiency. It is likely that these effects are miRNA dependent because in vivo overexpression of miR-17-5p and let-7b allowed partial vascular recovery in the CL of these Dicer1 hypomorphic mice. Other ovarian functions such as folliculogenesis, oocyte maturation, and ovulation were not hampered. However, the investigators stated that it cannot be excluded that miRNAs regulate other ovarian functions, but the development and function of the CL appear to be more sensitive to the reduced expression of DICER (Otsuka et al. 2008).
12.5.2 miRNAs in Oocytes
To explore the role of miRNAs in oocytes, several models have been employed (see hereinafter). In cKO mouse model in which Dicer1 was deleted, using the zona pellucida glycoprotein 3 (Zp3) promoter to drive the expression of Cre recombinase, oocyte growth and development were not hampered (Murchison et al. 2007). However, the majority of Dicer1 cKO oocytes did not complete the first meiotic division, either in vivo or in vitro, and were arrested at the first metaphase (MI). Oocytes that completed the first meiotic division and extruded the first polar body (PBI) frequently exhibited multiple spindles with misaligned chromosomes, a phenotype that was also observed in Dicer1 cKO MI oocytes (Murchison et al. 2007). Microarrays revealed differences in the expression levels of mRNA transcripts from Dicer1 cKO oocytes and control oocytes matured in vitro. A strong correlation was found between the mRNA transcripts whose level was increased in Dicer1 cKO oocytes to mRNAs that are selectively degraded during meiotic maturation of wild-type oocytes (Murchison et al. 2007; Su et al. 2007). Furthermore, several putative miRNA target sites were identified within the upregulated mRNA transcripts, though many were not related to any known mouse miRNAs (Murchison et al. 2007). This was further supported by another study that showed an association between miRNA binding sites and specific transcript isoforms during the transition of oocytes from germinal vesicle (GV) stage to metaphase of the second meiotic division (MII) and degradation of maternal mRNA (Salisbury et al. 2009).
A similar method (ZP3 promoter-mediated expression of Cre recombinase) was used to generate Ago2 cKO mouse oocytes (Kaneda et al. 2009). These oocytes developed normally and matured in vivo. However, when ovulated, they exhibited abnormal spindles and their chromosomes were misaligned, similar to the phenotype of Dicer1 cKO oocytes. Moreover, their mRNA expression profile was radically different than that of WT oocytes, concomitant with a decrease in the expression of most miRNAs, by more than 80 % (Kaneda et al. 2009). Like DICER, AGO2 is also common to the miRNA and siRNA biogenesis pathways. Hence, the phenotype of Dicer1- or Ago2-deficient oocytes can also be attributed to differences in the expression of siRNAs.
To distinguish between the role of miRNAs and siRNAs in mouse oocytes, Suh et al. (2010) used the ZP3 promoter–Cre recombinase system to generate cKO mice with deleted Dgcr8, which is specific to the biogenesis of canonical miRNAs. Surprisingly, though the levels of their miRNAs were decreased, the oocytes matured normally and produced healthy-appearing offspring when fertilized in vitro with wild-type sperm. The deletion of Dgcr8 had a negative impact on fecundity because Dgcr8-deficient mothers produced fewer offspring than control mothers. The investigators did not detect prominent changes in the mRNA expression profile between Dgcr8 cKO oocytes and control oocytes (Suh et al. 2010), unlike the case in Dicer1 cKO and Ago2 cKO oocytes (Kaneda et al. 2009; Murchison et al. 2007). The lack of a profound phenotype of Dgcr8 cKO oocytes may be partially explained by the presence of noncanonical miRNAs, specifically those that are DGCR8 independent. One of these noncanonical pathways, the mirtrons (short RNA duplexes mapped to short hairpin introns), is DICER dependent and DGCR8 independent (reviewed by Yang and Lai 2011), meaning that lack of mirtrons in Dicer1 cKO oocytes and its presence in the Dgcr8 cKO oocytes may be held responsible for the differences between the phenotypes of the Dicer1 and Dgcr8 cKO oocytes. To exclude this possibility, the contribution of mirtrons to the changes observed in mRNA transcripts of Dicer1 KO was evaluated (Suh et al. 2010). However, no association was found between two examined mirtrons and mRNA transcripts that are upregulated in Dicer1 KO oocytes. In contrast, siRNAs were mapped to many of the upregulated transcripts (Suh et al. 2010).
These findings imply that the phenotype of Dicer1 cKO oocytes is a result of decreased expression of siRNAs rather than of miRNAs. However, several aspects need to be considered. Deletion of Dgcr8 by ZP3 promoter that drives the expression of Cre recombinase occurs only when the oocytes start growing (Epifano et al. 1995), leaving a window for synthesis of miRNAs that are necessary for their further growth and maturation. Moreover, several novel subgroups of noncanonical miRNAs that require the activity of DICER but not that of DGCR8 were discovered recently (Fig. 12.1). One biogenesis pathway shares similarity with the mirtron pathway, though distinguished from them as these noncanonical miRNAs are transcribed from long noncoding introns or independent genes, rather than short introns (Chong et al. 2010). Other pathways include miRNAs derived from small-nuclear RNAs (snoRNAs), endogenous short-hairpin RNAs (shRNAs), and tRNAs (reviewed by Miyoshi et al. 2010). These noncanonical miRNAs may contribute to the differences between the phenotype of the Dicer1 and Dgcr8 cKO oocytes.
It seems that when evaluating the activity of oocytes miRNAs, it is of great importance to distinguish between growing and fully grown GV oocytes. Ma et al. (2010) used the luciferase reporter assay to examine the activity of two endogenous miRNAs, let-7a and miR-30c, which are abundant in the oocyte (Tang et al. 2007). Several reporter constructs were used, each containing 2–4 bulged miRNA binding sites or one perfect complementary site (Ma et al. 2010). In growing oocytes, both let-7a and miR-30c suppressed the expression of the reporter luciferase, when under the regulation of either the bulged sites or the perfect complementary site. In fully grown oocytes, only miR-30c inhibited the expression of both bulged and perfect site reporters, whereas let-7a suppressed only the expression of the perfect site reporter (Ma et al. 2010). These findings imply that miRNA activity is slightly suppressed in fully grown GV oocytes, compared to growing oocytes, though miRNAs remain functional in fully grown GV oocytes.
Growing and fully grown GV oocytes differ in the lack of P-bodies within fully grown oocytes. P-bodies are involved in RNA-mediated silencing and translational inhibition. Liu et al. (2005) used reporter mRNAs that contain binding sites for endogenous and exogenous miRNAs and demonstrated that localization of mRNAs to P-bodies is miRNA dependent. It was further shown that the AGO family of proteins, including AGO2, are localized to P-bodies (Liu et al. 2005). Flemr et al. (2010) showed that P-bodies disperse as the oocytes grow, concomitantly with the formation of mRNA-containing aggregates in the subcortical zone of fully grown oocytes. These mRNA-containing aggregates do not contain AGO2 protein and disappear in mature MII oocytes (Flemr et al. 2010). However, Swetloff et al. (2009) detected P-body-like foci in fully grown mouse oocytes and their presence was sensitive to depletion of Dicer1, implying a possible occurrence of miRNA-mediated gene silencing in the P-body-like foci of fully grown GV oocytes. Like the abovementioned mRNA-containing aggregates, these P-body-like foci also disappeared in mature oocytes. Taken together, alterations in the localization of mRNAs, miRNAs, and RNA-binding proteins may partially explain the differences in the activity of miRNAs found between growing and fully grown mouse oocytes.
The activity of miRNAs is affected also by editing of adenosine to inosine (A–I), which can cause a decreased activity, retargeting of miRNAs, or degradation of pre-miRNA (reviewed by Nishikura 2006). García-López et al. (2013) examined the editing profile of several miRNAs that are expressed in mouse oocytes and early embryos and are edited in other cell types. The majority of these miRNAs were unedited, with only few mature edited forms expressed in oocytes and zygotes. On the contrary, edited miRNAs are relatively highly expressed in two-cell embryos and remain so in blastocysts (García-López et al. 2013). The susceptibility of other miRNAs expressed in fully grown oocytes to A–I editing needs to be explored in order to evaluate the possible role of A–I editing in regulating miRNA activity in oocytes.
Adenylation is also involved in the regulation of miRNA activity and stability, though the frequency of miRNA adenylation is usually very low (reviewed by Kim et al. 2010b). Lee et al. (2014) found that maternal miRNAs are adenylated in mouse MII oocytes, reaching a total of ~30 % and dropping down to 5 % in 8–16 cell embryos. However, the rate of adenylated miRNAs in growing and fully grown GV oocytes is yet to be determined.
12.6 Specific miRNAs and Their Contribution to Ovarian Functionality
Lately, a progress has been made in identifying the role of specific miRNAs and their target genes in the ovary, specifically in granulosa cells and oocytes. Herein, we discuss data regarding the involvement of these miRNAs in distinct processes occurring throughout folliculogenesis, oocyte maturation, and ovulation.
12.6.1 Assembly of Primordial Follicles
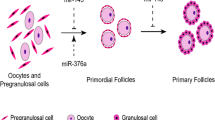
Mouse primordial germ cells migrate to the genital ridge and undergo several mitotic divisions, forming clusters of oogonia, or what is termed cysts. At 13.5 dpc, oogonia enter the first meiosis and from then on are referred to as oocytes (reviewed by Pepling 2012). Oocytes will further progress through meiosis and arrest at the diplotene stage of the first prophase. This arrest takes place between 17.5 dpc and 5 dpp when the last oocytes reach the diplotene stage (reviewed by Pepling 2012). During this period approximately two-thirds of mouse oocytes will die, accompanied by cysts breakdown. The surviving oocytes become enclosed within a single layer of flattened pre-granulosa cells, forming the primordial follicles (Pepling and Spradling 2001; Fig. 12.2).
MiRNA regulation of primordial follicle formation in the mouse. A scheme illustrating events that occur during early stages of mouse ovarian development is presented. Germline cysts are formed between 10.5 and 13.5 dpc, accompanied by multiple mitotic divisions. Around 13.5 dpc, first oocytes enter first meiosis and arrest at the diplotene stage between 17.5 dpc and 5 dpp. During this time (17.5 dpc–5 dpp), breakdown of germline cysts occurs followed by primordial follicle assembly. Overexpression of miR-143 in 15.5 dpc ovaries inhibits the formation of primordial follicles by suppressing proliferation of pre-granulosa cells. Ovaries of 18.5 dpc mice transfected with miR-376a have an increased number of primordial follicles. Several miRNAs modulate the secretion of E2, an inhibitor of primordial follicle formation (miR-320, miR-133, miR-383), or the activity of activin, an inducer of primordial follicle formation (miR-145, miR-181a)
It is believed that apoptosis is the major mechanism involved in the loss of oocytes during cyst breakdown and primordial follicle assembly (reviewed by Tiwari et al. 2015). Xu et al. (2011) showed that proliferating cell nuclear antigen (PCNA) is differentially expressed in oocytes during the critical time of cyst breakdown and primordial follicle assembly. The expression of PCNA decreases from 13.5 to 18.5 dpc and increases from 18.5 dpc to 5 dpp. RNA interference blocks the increase of PCNA expression in cultured 18.5 dpc mouse ovaries, thus dramatically reducing oocytes’ apoptosis and increasing primordial follicles assembly (Xu et al. 2011). In another study conducted by the same group, miR-376a was found to downregulate PCNA expression and its expression was reciprocally correlated with that of PCNA mRNA in fetal mouse ovaries. Furthermore, cultured 18.5 dpc ovaries transfected with miR-376a exhibited reduced apoptosis of oocytes and an increase in the number of primordial follicles; this effect was mediated by miR-376a-inhibition of PCNA (Zhang et al. 2014a). These findings imply that by inhibiting oocytes’ apoptosis miR-376a acts as a positive regulator of primordial follicles assembly.
The effect of miRNAs on primordial follicles assembly is not confined to the oocytes. miR-143 is predominantly expressed in pre-granulosa cells of prenatal mouse ovaries, as indicated by in situ hybridization; its expression increases during the time of primordial follicle assembly, from 15.5 dpc to 1 dpp, and remains elevated till 4 dpp (Zhang et al. 2013a). Overexpression of miR-143 in 15.5 dpc ovaries inhibits the formation of primordial follicles by suppressing proliferation of pre-granulosa cells. Accordingly, inhibition of miR-143 in ovaries of the same age resulted in an increase in the number of primordial follicles as well as in proliferation of pre-granulosa cells, thus implying that miR-143 acts as a negative regulator of primordial follicle formation (Zhang et al. 2013a).
To date, there is limited evidence supporting the involvement of specific miRNAs in primordial follicle assembly through regulation of gene expression in mouse pre-granulosa cells. However, several miRNAs have been implicated as participants in the regulation of estradiol (E2) production in granulosa cells, increasing E2 level by targeting Forkhead L2 (Foxl2; Dai et al. 2013) or RNA-binding motif, single-stranded interacting protein 1 (RBMS1; Yin et al. 2012), and decreasing E2 level by targeting the E2F1 and steroidogenic factor 1 (SF1) transcription factors (Yin et al. 2014). Because estrogen negatively regulates cyst breakdown and assembly of primordial follicles, it is possible that these miRNAs contribute to the development of a relatively low estrogenic environment, permissive of primordial follicle assembly (Kezele and Skinner 2003; Chen et al. 2007, 2009).
Unlike estrogen, activin A, a member of the TGF-β superfamily, causes an increase in the number of primordial follicles when administered to neonatal mice during the period of cyst breakdown and primordial follicle assembly (Bristol-Gould et al. 2006). The inverse effect of these two signaling pathways is further supported by the presence of multi-oocytic follicles (MOF) in ovaries of neonatal estrogen-treated mice (Kipp et al. 2007) and of mice models in which activin expression or its downstream signaling was suppressed (Bristol-Gould et al. 2005; Pangas et al. 2007). Furthermore, ovaries of neonatal mice treated with diethylstilbestrol (DES) or E2 had lower level of activin subunits and an attenuated activin signaling, as determined by the level of phosphorylated Smad-2 (Kipp et al. 2007), implying that the estrogen and activin signaling pathways act in a reciprocal manner and are linked together as well. Interestingly, both miR-145 and miR-181a inhibit activin function in granulosa cells by targeting activin receptor IB (ActRIB) and IIA (ActRIIA), respectively, thereby suppressing proliferation of granulosa cells (Yan et al. 2012; Zhang et al. 2013b). Hence, miRNAs might have a role in maintaining a delicate balance between estrogen and activin, thus enabling cyst breakdown and primordial follicle assembly in a time-restricted manner.
12.6.2 Primordial to Primary Follicle Transition
Primordial follicles remain dormant for a period of time that varies between follicles. In the mouse, activation of few primordial follicles to start growing begins shortly after the formation of all primordial follicles. Most of the primordial follicles will be gradually recruited to the growing pool throughout postnatal life (Fortune et al. 2000; reviewed by Hirshfield 1991).
The involvement of miRNAs in the process of primordial follicle activation is largely obscure. Yang et al. (2013b) showed that 24 miRNAs are differentially expressed in mouse ovaries during the developmental period of primordial follicles into primary follicles. Whereas some miRNAs were downregulated, others were upregulated, implying they might have a role in maintaining the dormancy of primordial follicles or in initiating their development. Gene ontology analysis revealed many signaling pathways that are modulated by these differentially expressed miRNAs (Yang et al. 2013b); many of these signaling pathways are involved in initiation of primordial follicle development (Skinner 2005). The upregulated miR-145 is predominantly expressed in granulosa cells of primary follicles and is undetectable in pre-granulosa cells of primordial follicles (Yang et al. 2013b). Inhibition of miR-145 in whole ovaries during the time of primordial to primary transition causes a significant decrease in the number of primordial follicles, coincident with an increase in the number of primary follicles, with no effect on the total number of follicles (Yang et al. 2013b). Accelerated primordial follicle activation caused by anti-miR-145 is dependent on TGF-β receptor type 2, a direct target of miR-145 (Yang et al. 2013b), that belongs to the TGF-β superfamily, known to be involved in the development of primordial follicles (Trombly et al. 2009).
The possible involvement of other differentially expressed miRNAs during the primordial to primary transition is of great interest. One of these miRNAs, miR-125a-3p, is upregulated in primary follicles compared to primordial follicles (Yang et al. 2013b). We recently found that miR-125a-3p post-transcriptionally regulates the expression of Fyn, a Src-family kinase (SFK), in granulosa cells (Grossman et al. 2015). SFKs can either synergize or operate apart and can also compensate one another (Bock and Herz 2003; Zhang et al. 2014b). Of the SFKs, Src is essential for activation of primordial follicle by regulating the PI3K-PKC-ERK1/2 pathway (Du et al. 2012). Along with the increase in miR-125a-3p during the time of primordial follicles activation, it may point at a possible involvement of miR-125a-3p in this process.
Though yet to be established, other miRNAs are suspected to be involved in activation of primordial follicles due to their proven ability to regulate key factors in this process. One of these miRNAs is miR-133b, which downregulates the expression of Foxl2 in granulosa cells of growing follicles (Dai et al. 2013). The fundamental role of Foxl2 in differentiation of granulosa cells is demonstrated by the block of the transition from squamous pre-granulosa cells to cuboidal granulosa cells in the absence of FOXL2 (Schmidt et al. 2004). Hence, as a negative regulator of Foxl2, miR-133b is expected to remain low during activation of primordial follicles. Currently there is no data regarding the expression pattern of miR-133b during these early stages of follicular development.
12.6.3 Follicular Growth
At early stages of folliculogenesis, granulosa cells of single-layered primary follicles undergo mitotic divisions to form multilayered secondary follicles surrounded with a layer of theca cells. As the small secondary follicles grow to become large pre-antral follicles, proliferation of granulosa and theca cells continues and is coordinated with oocyte growth. The early folliculogenesis stages are modulated primarily by intraovarian signaling molecules, acting in both autocrine and paracrine manners, including activins (reviewed by Oktem and Urman 2010).
Expression of activin βA and βB subunits as well as activin receptors (ActRIB, ActRIIA, and ActRIIB) was detected already in somatic cells and oocytes of early follicles from postnatal mouse ovaries (Bristol-Gould et al. 2006). Several studies have demonstrated that activins play an important role in the proliferation and differentiation of granulosa cells (Guzel et al. 2014; Smitz et al. 1998). Interestingly, miR-145 was found to inhibit granulosa cell proliferation; this effect is dependent upon the ability of miR-145 to inhibit the expression of ActRIB. miR-145 also targets and inhibits the expression of cyclin D2, whereas activin A exerts an opposite effect on the expression of cyclin D2 and causes a decrease in the expression of miR-145 (Yan et al. 2012). These data point at miR-145 as a dominant regulator of granulosa cell proliferation and demonstrate a feedback loop between miR-145 and the activin pathway.
Activin A also causes a decrease in the level of miR-181a in mouse primary granulosa cells (Zhang et al. 2013b). The expression of miR-181a is high in primary follicles and decreases dramatically in pre-antral and antral follicles, concomitant with an increase in the level of ActRIIA and granulosa cell proliferation. Furthermore, miR-181a inhibits proliferation of granulosa cells, by decreasing the expression of ActRIIA through direct binding to ActRIIA 3′UTR (Zhang et al. 2013b).
Activins are also well known for their ability to induce expression of FSH receptor (Nakamura et al. 1993) and potentiate FSH effect in granulosa cells by increasing the level of cAMP (El-Hefnawy and Zeleznik 2001). Though the exact stage in which follicles become FSH dependent is controversial, it is well established that antrum formation and growth of early antral to preovulatory antral follicles are dependent upon FSH and its interaction with intraovarian factors (Kumar et al. 1997; Sánchez et al. 2010). Many miRNAs are under the regulation of FSH, in both mouse and rat granulosa cells (Yao et al. 2009, 2010b), implying that miRNAs might participate in FSH signaling in granulosa cells. Furthermore, a specific miRNA, expressed in human granulosa cells and predicted to regulate ActRIIB and Smad-2, aligns with the intronic region of the FSH-receptor gene (Velthut-Meikas et al. 2013), adding another level of complexity to the involvement of miRNAs in modulating FSH signaling.
FSH induces aromatization of androgen to estrogen and expression of LH receptor in granulosa cells (Hillier 1994). In turn, estrogen induces proliferation of granulosa cells and facilitates the activity of FSH. Normal cyclic levels of estrogen and estrogen receptors are important for proper folliculogenesis as well as ovulation (reviewed by Drummond and Findlay 1999). Several miRNAs have been demonstrated to regulate secretion of estrogen from granulosa cells by inhibiting the expression of steroidogenesis key members. Recently, a feedback loop between two miRNAs that modulate E2 production differentially was demonstrated in mouse granulosa cells. Transactivation of miR-383 by SF1 causes an increase in E2 secretion (Yin et al. 2012). Overexpression of miR-383 promotes the transcription of pri-miR-320, causing an increase in the expression of mature miR-320, which in turn inhibits the expression of SF1, causing a decrease in E2 secretion and granulosa cell proliferation (Yin et al. 2012, 2014). Both miRNAs are downregulated by TGF-β1 and FSH, or its analog, PMSG (Yao et al. 2010a). On the contrary, TGF-β1 upregulates the expression of miR-224, which facilitates secretion of E2 from granulosa cells (Yao et al. 2010a). The expression of miR-224 is unaffected by overexpression of miR-383 (Yin et al. 2014), implying that miRNAs are involved, via different pathways, in regulation of E2 production in granulosa cells.
Granulosa cell function is also regulated by androgens, already from early stages of folliculogenesis. Both testosterone and dihydrotestosterone have a prosurvival effect on mouse primary granulosa cells. Sen et al. demonstrated that this effect of testosterone and dihydrotestosterone is mediated by an increase in the levels of expression of miR-125b, followed by a decrease in the expression of pro-apoptotic proteins (Sen et al. 2014). Overall miRNAs appear to have a prominent role in mediating granulosa cells function, proliferation, and survival in response to intraovarian and extraovarian factors.
12.6.4 Oocyte Maturation and Early Embryogenesis
Starting at embryonic life and all throughout oocytes’ growth, they remain arrested at the prophase of the first meiotic division and are characterized by a prominent nucleus, the GV. LH surge induces oocytes within selected antral follicles to resume the first meiotic division and undergo oocyte maturation, manifested by chromosome condensation, GV breakdown (GVBD), spindle formation and migration toward the oocyte cortex, segregation of homologous chromosomes, extrusion of the first polar body (PBI), and an arrest at metaphase of the second meiotic division (MII). Ovulated MII oocytes resume their second meiotic division upon fertilization. The process of oocyte maturation is highly coordinated both in time and space and involves many signaling pathways (reviewed by Brunet and Maro 2005; Solc et al. 2010).
It is well established that fully grown GV mouse oocytes are trancriptionally quiescent. Because transcription activity begins only at late zygote stage, oocytes rely on pre-synthesized mRNA transcripts for the processes of maturation, fertilization, and early embryogenesis (Bouniol-baly et al. 1999; Zeng and Schultz 2005). Thus, posttranscriptional regulation of existing transcripts is of great importance. Several mechanisms of posttranscriptional regulation are indicated in mouse oocytes (reviewed by Kang and Han 2011); the one operated by maternal miRNAs is the most controversial mechanism, mainly, as discussed above, due to differences in the phenotypes of Dicer1 and Dgcr8 cKO oocytes (Suh et al. 2010). Though Suh et al. (2010) found that miRNAs are not essential for oocyte maturation and early embryo development, several studies suggest that miRNAs play a role in their regulation (elaborated hereinafter). miR-335-5p is highly expressed in GV oocytes throughout maturation and in MII oocytes, and a decrease in its expression is detected shortly after fertilization. Microinjection of miR-355-5p mimic or miR-355-5p inhibitor into GV oocytes caused high incidence of GVBD or MI-arrested oocytes that exhibit spindle abnormalities. Many of the MII oocytes that completed maturation had a two-cell-like morphology, indicating failure in asymmetric cell division. When injected into zygotes, both miR-355-5p mimic and miR-355-5p inhibitor had no visible effect on embryo development till the blastocyst stage. miR-355-5p inhibits the expression of dishevelled-associated activator of morphogenesis 1 (Daam1; Cui et al. 2013), which modulates actin microfilaments (Liu et al. 2008), implying that the effect of miR-335-5p on oocyte maturation is mediated by regulation of the actin cytoskeleton.
The expression of the noncanonical miR-320 (Kim and Choi 2012), which is detected in human follicular fluids, is correlated with the quality of day 3 embryos (Feng et al. 2015). Knockdown of miR-320 in mouse MII oocytes causes a decrease in the number of two-cell embryos and blastocysts, as well as an aberration in the expression of components of the WNT signaling pathway (Feng et al. 2015). Though the WNT signaling pathways were studied in oocytes mainly in regard to early embryonic development, the canonical WNT signaling pathway is active in mouse oocytes from the stage of secondary follicle; its activity increases as the follicle grows, concomitantly with an increase in oocyte diameter, but it is undetectable in mature MII oocytes (Usongo et al. 2012). Along with the possible role of WNT in modulating the actin cytoskeleton (Akiyama and Kawasaki 2006) and its cross talk with the PI3K/AKT signaling pathway (reviewed by Boyer et al. 2010), it is of great interest to explore the involvement of miR-320 in oocyte maturation and emphasize its role as a regulator of WNT signaling.
The role of miRNAs in oocytes should not be limited to a direct effect on maternal miRNAs, but should rather include indirect effects mediated via granulosa cell miRNAs. Kim et al. (2013) showed that four miRNAs (let-7b, let-7c, miR-27a, and miR-322) are differentially expressed in mouse granulosa cells derived from in vitro cultured, ovulation-induced antral follicles that contain either MII or MI oocytes (Kim et al. 2013). Maturation rate of oocytes in in vitro cultured antral follicles after transfection of granulosa cells with miR-27a mimic is decreased, but ovulation and fertilization rates are not affected. Transfection with miR-27a inhibitor had an inverse effect, causing an increase in the rate of oocyte maturation. Similar effects were obtained upon transfection with let-7c and miR-322 inhibitors, whereas their mimic sequences had no effect on all examined criteria (Kim et al. 2013). These findings imply that miRNAs expressed within granulosa cells are involved in specific regulation of oocyte maturation.
12.6.5 Ovulation and Formation of the Corpus Luteum
When exposed to ovulatory dose of LH/hCG, both mural and cumulus granulosa cells undergo massive changes in gene expression pattern, emphasizing the importance of transcription and posttranscriptional modifications during the peri-ovulatory period (reviewed by Stocco et al. 2007). In mouse ovaries, miRNAs regulate several processes occurring during the peri-ovulatory period, by targeting key proteins necessary for cumulus expansion, follicular wall rupture, luteinization, and cell survival.
During their expansion, cumulus cells synthesize and secrete PTX3, which then localizes to the matrix where it regulates the organization of hyaluronan (Salustri et al. 2004). The expression of PTX3 is downregulated directly by miR-224. Administration of hCG to equine chorionic-gonadotropin (eCG)-primed mice inhibits the expression of miR-224 in mural granulosa cells and cumulus–oocyte complexes (COCs), whereas the expression of PTX3 increases. Overexpression of miR-224, in in vitro cultured COCs, decreases the rate of cumulus expansion in response to EGF (Yao et al. 2014). Furthermore, when miR-224 is overexpressed in vivo, less COCs are recovered from the oviducts (Yao et al. 2014). As previously mentioned, miR-224 also facilitates proliferation of granulosa cells and E2 production (Yao et al. 2010a), demonstrating a distinct role played by the same miRNA in the follicle, depending on the stage of follicular development.
Several miRNAs are under the regulation of LH/hCG in mural granulosa cells; among them are miR-132 and miR-212, both driven from a single pri-miRNA and upregulated upon in vivo administration of hCG (Fiedler et al. 2008). In vitro studies revealed that both miR-132 and miR-212 regulate the expression of the C-terminal binding protein, CTBP1 (Fiedler et al. 2008), hence holding a great potential in mediating LH/hCG-induced changes in global genes transcription. Another miRNA, miR-21, is also upregulated by hCG. Knockdown of miR-21 in in vitro cultured primary granulosa cells causes an increase in the level of cleaved caspase-3 and in cell apoptosis (Carletti et al. 2010). Furthermore, knockdown of miR-21 in ovaries overrides the anti-apoptotic effect of hCG triggering, causing increased apoptosis of granulosa cells and impaired ovulation (Carletti et al. 2010).
Contrary to miRNAs that are upregulated by hCG and transduce its effect in granulosa cells (Carletti et al. 2010; Fiedler et al. 2008), we have recently found that the expression of miR-125a-3p is transiently downregulated in mural granulosa cells exposed to hCG (Grossman et al. 2015). This miRNA directly downregulates the expression of Fyn kinase, thus inhibiting hCG-induced Fyn upregulation and granulosa cell migration. Overexpression of miR-125a-3p in ovaries of PMSG-hCG-treated mice decreased ovulation rate, similar to the effect of Fyn si-RNA, inferring that the decrease in miR-125a-3p in response to hCG, accompanied by increased Fyn level, supports proper ovulation. The ratio between miR-125a-3p and Fyn is higher in ovaries of anovulatory mice, treated prenatally with dihydrotestosterone, than in ovaries of control mice, thus implying that dysregulation of the expression of miR-125a-3p may contribute to anovulatory pathologies (Grossman et al. 2015).
Aside from its anti-migratory properties, miR-125a-3p has a pro-apoptotic effect in several cell lines (Jiang et al. 2013; Ninio-Many et al. 2014; Yin et al. 2015). It is well established that apoptosis must be inhibited to allow the formation and function of the corpus luteum (CL; reviewed by Stocco et al. 2007). We, therefore, examined whether the expression of miR-125a-3p in mouse luteinized granulosa cells remains low after in vivo administration of hCG. Our results present that it decreased 4 h after hCG administration, returned to control value 4 h later (Grossman et al. 2015), decreased again 16 h after hCG administration, and remained low for another 16 h (Fig. 12.3). We also found that overexpression of miR-125a-3p in immortalized human granulosa cell line (SVOG; Lie et al. 1996) causes a dose-dependent decreased secretion of amphiregulin (AREG; Fig. 12.4), a prosurvival factor of human CL (Ben-Ami et al. 2009). These findings imply that, in addition to its role in ovulation, miR-125a-3p might participate in the regulation of CL formation and maintenance.
MiR-125a-3p expression in luteinized mouse granulosa cells. Seven-weeks-old mice were administered with 5 IU PMSG and either killed 48 h later (control group; 0) or injected with 7 IU hCG and killed 16 or 32 h later. Ovaries were excised, and mural granulosa cells were isolated and subjected to quantitative PCR analysis. The expression of miR-125a-3p in granulosa cells of each mouse was normalized to the level of U6 snRNA expression; the average ratio for each treatment group is presented. Data were analyzed by one-way ANOVA. Bars are mean ± SEM (n = 5). *P < 0.05
Secretion of amphiregulin from miR-125a-3p overexpressing cells. SVOG cells were transfected with 10 nM scramble miR (negative control; NC), or with two concentrations of miR-125a-3p mimic (10 or 30 nM), using lipofectamine 2000. Cells were incubated for 12 h, culture media were refreshed, and cells were incubated for additional 24 h. Conditioned media were collected, centrifuged at 1200 rpm for 5 min and subjected to ELISA assay for the detection of amphiregulin. Experiment was repeated twice. Bars are mean (indicated on top) ± SEM
Not surprisingly, the effect of miRNAs in ovulation is not confined to locally acting miRNAs but is also at the pituitary level. Hasuwa et al. (2013) showed that female mice lacking miR-200b and miR-429 (both share the same seed sequence) are anovulatory. These miRNAs are highly expressed in the pituitary, where they regulate the expression of the ZEB1 transcription factor. Elimination of miR-200b and miR-429 results in increased expression of ZEB1, followed by inhibition of the β subunit of LH and hence low concentration of circulating LH and a failure to release the LH surge (Hasuwa et al. 2013).
12.7 Conclusion
Gonad development is comprised of a series of events occurring at the gonadal level and regulated by a panel of hormones secreted from the hypothalamic–pituitary–gonadal axis. miRNAs have prominent roles in gonad development, exerting their effect either directly through silencing the expression of key proteins in gonadal somatic or germ cells or indirectly acting at the hypothalamus–pituitary level. Taking into account that one miRNA can regulate the expression of many genes depending on cell type, state of differentiation, and environment, the task of fully understanding the role of miRNAs in gonad development should be challenging.
References
Abdelfattah AM, Choi MY (2015) Update on non-canonical microRNAs. Biomol Concepts 5:275–287
Adham IM, Nayernia K, Burkhardt-Gottges E et al (2001) Teratozoospermia in mice lacking the transition protein 2 (Tnp2). Mol Hum Reprod 7:513–520
Åkerfelt M, Henriksson E, Laiho A et al (2008) Promoter ChIP-chip analysis in mouse testis reveals Y chromosome occupancy by HSF2. Proc Natl Acad Sci U S A 105:11224–11229
Akiyama T, Kawasaki Y (2006) Wnt signalling and the actin cytoskeleton. Oncogene 25:7538–7544
Bao J, Li D, Wang L et al (2012) MicroRNA-449 and microRNA-34b/c function redundantly in murine testes by targeting E2F transcription factor-retinoblastoma protein (E2F-pRb) pathway. J Biol Chem 287:21686–21698
Barrionuevo F, Bagheri-Fam S, Klattig J et al (2006) Homozygous inactivation of Sox9 causes complete XY sex reversal in mice. Biol Reprod 74:195–201
Barrios F, Filipponi D, Pellegrini M et al (2010) Opposing effects of retinoic acid and FGF9 on Nanos2 expression and meiotic entry of mouse germ cells. J Cell Sci 123:871–880
Bellvé AR, Cavicchia JC, Millette CF et al (1977) Spermatogenic cells of the prepuberal mouse. Isolation and morphological characterization. J Cell Biol 74:68–85
Ben-Ami I, Armon L, Freimann S et al (2009) EGF-like growth factors as LH mediators in the human corpus luteum. Hum Reprod 24:176–184
Björk JK, Sandqvist A, Elsing AN et al (2010) miR-18, a member of Oncomir-1, targets heat shock transcription factor 2 in spermatogenesis. Development 137:3177–3184
Bock HH, Herz J (2003) Reelin activates SRC family tyrosine kinases in neurons. Curr Biol 13:18–26
Bouniol-Baly C, Hamraoui L, Guibert J et al (1999) Differential transcriptional activity associated with chromatin configuration in fully grown mouse germinal vesicle oocytes. Biol Reprod 587:580–587
Boyer A, Goff AK, Boerboom D (2010) WNT signaling in ovarian follicle biology and tumorigenesis. Trends Endocrinol Metab 21:25–32
Bristol-Gould SK, Hutten CG, Sturgis C et al (2005) The development of a mouse model of ovarian endosalpingiosis. Endocrinology 146:5228–5236
Bristol-Gould SK, Kreeger PK, Selkirk CG et al (2006) Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol 298:132–148
Brunet S, Maro B (2005) Cytoskeleton and cell cycle control during meiotic maturation of the mouse oocyte: integrating time and space. Reproduction 130:801–811
Buchold GM, Coarfa C, Kim J et al (2010) Analysis of microRNA expression in the prepubertal testis. PLoS One 5:e15317
Carletti MZ, Fiedler SD, Christenson LK (2010) MicroRNA 21 blocks apoptosis in mouse periovulatory granulosa cells. Biol Reprod 83:286–295
Carthew RW, Sontheimer EJ (2009) Origins and mechanisms of miRNAs and siRNAs. Cell 136:642–655
Chang Y-F, Lee-Chang JS, Imam JS et al (2012) Interaction between microRNAs and actin-associated protein Arpc5 regulates translational suppression during male germ cell differentiation. Proc Natl Acad Sci U S A 109:5750–5755
Cheloufi S, Dos Santos CO, Chong MMW, Hannon GJ (2010) A dicer-independent miRNA biogenesis pathway that requires Ago catalysis. Nature 465:584–589
Chen Y, Jefferson WN, Newbold RR et al (2007) Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology 148:3580–3590
Chen Y, Breen K, Pepling ME (2009) Estrogen can signal through multiple pathways to regulate oocyte cyst breakdown and primordial follicle assembly in the neonatal mouse ovary. J Endocrinol 202:407–417
Chong MMW, Zhang G, Cheloufi S et al (2010) Canonical and alternate functions of the microRNA biogenesis machinery. Genes Dev 24:1951–1960
Cifuentes D, Xue H, Taylor DW et al (2010) A novel miRNA processing pathway independent of Dicer requires Argonaute2 catalytic activity. Science 328:1694–1698
Cui XS, Sun SC, Kang YK, Kim NH (2013) Involvement of microRNA-335-5p in cytoskeleton dynamics in mouse oocytes. Reprod Fertil Dev 25:691–699
Dai L, Tsai-Morris C-H, Sato H et al (2011) Testis-specific miRNA-469 up-regulated in gonadotropin-regulated testicular RNA helicase (GRTH/DDX25)-null mice silences transition protein 2 and protamine 2 messages at sites within coding region: implications of its role in germ cell development. J Biol Chem 286:44306–44318
Dai A, Sun H, Fang T et al (2013) MicroRNA-133b stimulates ovarian estradiol synthesis by targeting Foxl2. FEBS Lett 587:2474–2482
de Rooij DG, Russell LD (2000) All you wanted to know about spermatogonia but were afraid to ask. J Androl 21:776–798
Drummond AE, Findlay JK (1999) The role of estrogen in folliculogenesis. Mol Cell Endocrinol 151:57–64
Du X-Y, Huang J, Xu L-Q et al (2012) The proto-oncogene c-src is involved in primordial follicle activation through the PI3K, PKC and MAPK signaling pathways. Reprod Biol Endocrinol 10:58
El-Hefnawy T, Zeleznik AJ (2001) Synergism between FSH and activin in the regulation of proliferating cell nuclear antigen (PCNA) and cyclin D2 expression in rat granulosa cells. Endocrinology 142:4357–4362
Ender C, Krek A, Friedländer MR et al (2008) A Human snoRNA with MicroRNA-Like Functions. Mol Cell 32:519–528
Epifano O, Liang LF, Familari M et al (1995) Coordinate expression of the three zona pellucida genes during mouse oogenesis. Development 121:1947–1956
Ewen KA, Koopman P (2010) Mouse germ cell development: from specification to sex determination. Mol Cell Endocrinol 323:76–93
Feng R, Sang Q, Zhu Y et al (2015) MiRNA-320 in the human follicular fluid is associated with embryo quality in vivo and affects mouse embryonic development in vitro. Sci Rep 5:8689
Fiedler SD, Carletti MZ, Hong X, Christenson LK (2008) Hormonal regulation of MicroRNA expression in periovulatory mouse mural granulosa cells. Biol Reprod 79:1030–1037
Flemr M, Ma J, Schultz RM, Svoboda P (2010) P-body loss is concomitant with formation of a messenger RNA storage domain in mouse oocytes. Biol Reprod 82:1008–1017
Fortune JE, Cushman RA, Wahl CM, Kito S (2000) The primordial to primary follicle transition. Mol Cell Endocrinol 163:53–60
Gallardo T, Shirley L, John GB, Castrillon DH (2007) Generation of a germ cell-specific mouse transgenic Cre line, Vasa-Cre. Genesis 417:413–417
García-López J, Hourcade JDD, Del Mazo J (2013) Reprogramming of microRNAs by adenosine-to-inosine editing and the selective elimination of edited microRNA precursors in mouse oocytes and preimplantation embryos. Nucleic Acids Res 41:5483–5493
Greenlee AR, Shiao M-S, Snyder E et al (2012) Deregulated sex chromosome gene expression with male germ cell-specific loss of Dicer1. PLoS One 7:e46359
Gregory RI, Chendrimada TP, Cooch N, Shiekhattar R (2005) Human RISC couples microRNA biogenesis and posttranscriptional gene silencing. Cell 123:631–640
Grossman H, Chuderland D, Ninio-Many L et al (2015) A novel regulatory pathway in granulosa cells, the LH/human chorionic gonadotropin-microRNA-125a-3p-Fyn pathway, is required for ovulation. FASEB J 29(8):3206–3216
Guzel Y, Nur Şahin G, Sekeroglu M, Deniz A (2014) Recombinant activin A enhances the growth and survival of isolated preantral follicles cultured three-dimensionally in extracellular basement matrix protein (matrigel) under serum-free conditions. Gynecol Endocrinol 30:388–391
Ha M, Kim VN (2014) Regulation of microRNA biogenesis. Nat Rev Mol Cell Biol 15:509–524
Hasuwa H, Ueda J, Ikawa M, Okabe M (2013) MiR-200b and miR-429 function in mouse ovulation and are essential for female fertility. Science 341:71–74
Havens MA, Reich AA, Duelli DM, Hastings ML (2012) Biogenesis of mammalian microRNAs by a non-canonical processing pathway. Nucleic Acids Res 40:4626–4640
Hayashi K, Chuva de Sousa Lopes SM, Kaneda M et al (2008) MicroRNA biogenesis is required for mouse primordial germ cell development and spermatogenesis. PLoS One 3:e1738
He Z, Jiang J, Kokkinaki M et al (2013) MiRNA-20 and MiRNA-106a Regulate Spermatogonial Stem Cell Renewal at the Post-transcriptional Level via Targeting STAT3 and Ccnd1. Stem Cells 31:1–22
Hillier SG (1994) Current concepts of the roles of follicle stimulating hormone and luteinizing hormone in folliculogenesis. Hum Reprod 9:188–191
Hirshfield AN (1991) Development of follicles in the mammalian ovary. Int Rev Cytol 124:43–101
Hong X, Luense LJ, McGinnis LK et al (2008) Dicer1 is essential for female fertility and normal development of the female reproductive system. Endocrinology 149:6207–6212
Huszar JM, Payne CJ (2013) MicroRNA 146 (Mir146) modulates spermatogonial differentiation by retinoic acid in mice. Biol Reprod 88:15
Hutvagner G, Simard MJ (2008) Argonaute proteins: key players in RNA silencing. Nat Rev Mol Cell Biol 9:22–32
Inui M, Martello G, Piccolo S (2010) MicroRNA control of signal transduction. Nat Rev Mol Cell Biol 11:252–263
Jamin SP, Arango NA, Mishina Y et al (2002) Requirement of Bmpr1a for Müllerian duct regression during male sexual development. Nat Genet 32:408–410
Jiang L, Chang J, Zhang Q et al (2013) MicroRNA hsa-miR-125a-3p activates p53 and induces apoptosis in lung cancer cells. Cancer Invest 31:538–544
Kaneda M, Tang F, O’Carroll D et al (2009) Essential role for Argonaute2 protein in mouse oogenesis. Epigenetics Chromatin 2:9
Kang MK, Han SJ (2011) Post-transcriptional and post-translational regulation during mouse oocyte maturation. BMB Rep 44:147–157
Kezele P, Skinner MK (2003) Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology 144:3329–3337
Kim B-M, Choi MY (2012) Non-canonical microRNAs miR-320 and miR-702 promote proliferation in Dgcr8-deficient embryonic stem cells. Biochem Biophys Res Commun 462:183–189
Kim G, Georg INA, Scherthan H et al (2010a) Dicer is required for Sertoli cell function and survival. Int J Dev Biol 875:867–875
Kim Y-K, Heo I, Kim VN (2010b) Modifications of small RNAs and their associated proteins. Cell 143:703–709
Kim YJ, Ku S-Y, Kim YY et al (2013) MicroRNAs transfected into granulosa cells may regulate oocyte meiotic competence during in vitro maturation of mouse follicles. Hum Reprod 28(11):3050–3061
Kipp JL, Kilen SM, Bristol-Gould S et al (2007) Neonatal exposure to estrogens suppresses activin expression and signaling in the mouse ovary. Endocrinology 148:1968–1976
Kobayashi A, Chang H, Chaboissier M-C et al (2005) Sox9 in testis determination. Ann N Y Acad Sci 1061:9–17
Korhonen HM, Meikar O, Yadav RP et al (2011) Dicer is required for haploid male germ cell differentiation in mice. PLoS One 6:e24821
Kotaja N (2014) MicroRNAs and spermatogenesis. Fertil Steril 101:1552–1562
Kumar TR, Wang Y, Lu N, Matzuk MM (1997) Follicle stimulating hormone is required for ovarian follicle maturation but not male fertility. Nat Genet 15:201–204
Lee M, Choi Y, Kim K et al (2014) Adenylation of maternally inherited microRNAs by Wispy. Mol Cell 56:696–707
Lei L, Jin S, Gonzalez G et al (2010) The regulatory role of Dicer in folliculogenesis in mice. Mol Cell Endocrinol 315:63–73
Lie BL, Leung E, Leung PC, Auersperg N (1996) Long-term growth and steroidogenic potential of human granulosa-lutein cells immortalized with SV40 large T antigen. Mol Cell Endocrinol 120:169–176
Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R (2005) MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat Cell Biol 7:719–723
Liu W, Sato A, Khadka D et al (2008) Mechanism of activation of the Formin protein Daam1. Proc Natl Acad Sci U S A 105:210–215
Liu D, Li L, Fu H et al (2012) Biochemical and biophysical research communications inactivation of Dicer1 has a severe cumulative impact on the formation of mature germ cells in mouse testes. Biochem Biophys Res Commun 422:114–120
Lomelí H, Ramos-Mejia V, Nagy A (2000) Targeted insertion of Cre recombinase into the TNAP gene: excision in primordial germ cells. Genesis 26:8–10
Ma J, Flemr M, Stein P et al (2010) MicroRNA activity is suppressed in mouse oocytes. Curr Biol 20:265–270
Ma H, Zhang J, Wu H (2014) Designing Ago2-specific siRNA/shRNA to avoid competition with endogenous miRNAs. Mol Ther Nucleic Acids 3:e176
Maatouk DM, Loveland KL, McManus MT et al (2008) Dicer1 is required for differentiation of the mouse male germline. Biol Reprod 79:696–703
Meister G, Landthaler M, Patkaniowska A et al (2004) Human Argonaute2 mediates RNA cleavage targeted by miRNAs and siRNAs. Mol Cell 15:185–197
Meistrich ML, Mohapatra B, Shirley CR, Zhao M (2003) Roles of transition nuclear proteins in spermiogenesis. Chromosoma 111:483–488
Miller D, Brinkworth M, Iles D (2010) Paternal DNA packaging in spermatozoa: more than the sum of its parts? DNA, histones, protamines and epigenetics. Reproduction 139:287–301
Mishima T, Takizawa T, Luo SS et al (2008) MicroRNA (miRNA) cloning analysis reveals sex differences in miRNA expression profiles between adult mouse testis and ovary. Reproduction 136:811–822
Miyoshi K, Miyoshi T, Siomi H (2010) Many ways to generate microRNA-like small RNAs: non-canonical pathways for microRNA production. Mol Genet Genomics 284:95–103
Modzelewski AJ, Holmes RJ, Hilz S et al (2012) AGO4 regulates entry into meiosis and influences silencing of sex chromosomes in the male mouse germ line. Dev Cell 23:251–264
Modzelewski AJ, Hilz S, Crate EA et al (2015) Dgcr8 and Dicer are essential for sex chromosome integrity during meiosis in males. J Cell Sci 128:2314–2327
Mogilyansky E, Rigoutsos I (2013) The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ 20:1603–1614
Murchison EP, Stein P, Xuan Z et al (2007) Critical roles for Dicer in the female germline. Genes Dev 21:682–693
Nagaraja AK, Andreu-Vieyra C, Franco HL et al (2008) Deletion of Dicer in somatic cells of the female reproductive tract causes sterility. Mol Endocrinol 22:2336–2352
Nakamura M, Minegishi T, Hasegawa Y et al (1993) Effect of an activin A on follicle-stimulating hormone (FSH) receptor messenger ribonucleic acid levels and FSH receptor expressions in cultured rat granulosa cells. Endocrinology 133:538–544
Nguyen TA, Jo MH, Choi Y-G et al (2015) Functional anatomy of the human microprocessor. Cell 161:1374–1387
Ninio-Many L, Grossman H, Levi M et al (2014) MicroRNA miR-125a-3p modulates molecular pathway of motility and migration in prostate cancer cells. Oncoscience 1:250–261
Nishikura K (2006) Editor meets silencer: crosstalk between RNA editing and RNA interference. Nat Rev Mol Cell Biol 7:919–931
Niu Z, Goodyear SM, Rao S et al (2011) MicroRNA-21 regulates the self-renewal of mouse spermatogonial stem cells. Proc Natl Acad Sci U S A 108:12740–12745
O’Gorman S, Dagenais NA, Qian M, Marchuk Y (1997) Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc Natl Acad Sci U S A 94:14602–14607
Oktem O, Urman B (2010) Understanding follicle growth in vivo. Hum Reprod 25:2944–2954
Otsuka M, Zheng M, Hayashi M et al (2008) Impaired microRNA processing causes corpus luteum insufficiency and infertility in mice. J Clin Invest 118:1944–1954
Pangas SA, Jorgez CJ, Tran M et al (2007) Intraovarian activins are required for female fertility. Mol Endocrinol 21:2458–2471
Panneerdoss S, Chang Y, Buddavarapu KC et al (2012) Androgen-responsive microRNAs in mouse Sertoli cells. PLoS One 7:e41146
Papaioannou MD, Pitetti J, Ro S et al (2009) Sertoli cell Dicer is essential for spermatogenesis in mice. Dev Biol 326:250–259
Papaioannou MD, Lagarrigue M, Vejnar CE et al (2011) Loss of dicer in Sertoli cells has a major impact on the testicular proteome of mice. Mol Cell Proteomics 10:1–14
Pepling ME (2012) Follicular assembly: mechanisms of action. Reproduction 143:139–149
Pepling ME, Spradling AC (2001) Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol 234:339–351
Quick-Cleveland J, Jacob JP, Weitz SH et al (2014) The DGCR8 RNA-binding heme domain recognizes primary microRNAs by clamping the hairpin. Cell Rep 7:1994–2005
Rakoczy J, Fernandez-Valverde SL, Glazov EA et al (2013) MicroRNAs-140-5p/140-3p modulate Leydig cell numbers in the developing mouse testis. Biol Reprod 88:143
Real FM, Sekido R, Lupiáñez DG et al (2013) A microRNA (mmu-miR-124) prevents Sox9 expression in developing mouse ovarian cells. Biol Reprod 89:78
Ro S, Park C, Sanders KM et al (2007) Cloning and expression profiling of testis-expressed microRNAs. Dev Biol 311:592–602
Romero Y, Meikar O, Papaioannou MD et al (2011) Dicer1 depletion in male germ cells leads to infertility due to cumulative meiotic and spermiogenic defects. PLoS One 6:e25241
Ruby JG, Jan CH, Bartel DP (2007) Intronic microRNA precursors that bypass Drosha processing. Nature 448:83–86
Salisbury J, Hutchison KW, Wigglesworth K et al (2009) Probe-level analysis of expression microarrays characterizes isoform-specific degradation during mouse oocyte maturation. PLoS One 4:1–11
Salustri A, Garlanda C, Hirsch E et al (2004) PTX3 plays a key role in the organization of the cumulus oophorus extracellular matrix and in in vivo fertilization. Development 131:1577–1586
Sánchez F, Adriaenssens T, Romero S, Smitz J (2010) Different follicle-stimulating hormone exposure regimens during antral follicle growth alter gene expression in the cumulus-oocyte complex in mice. Biol Reprod 83:514–524
Schmidt D, Ovitt CE, Anlag K et al (2004) The murine winged-helix transcription factor Foxl2 is required for granulosa cell differentiation and ovary maintenance. Development 131:933–942
Schwenk F, Baron U, Rajewsky K (1995) A cre-transgenic mouse strain for the ubiquitous deletion of IoxP-flanked gene segments including deletion in germ cells. Nucleic Acids Res 23:5080–5081
Sen A, Prizant H, Light A et al (2014) Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc Natl Acad Sci U S A 111:3008–3013
Skinner MK (2005) Regulation of primordial follicle assembly and development. Hum Reprod Update 11:461–471
Smitz J, Cortvrindt R, Hu Y, Vanderstichele H (1998) Effects of recombinant activin A on in vitro culture of mouse preantral follicles. Mol Reprod Dev 50:294–304
Snyder EM, Small C, Griswold MD (2010) Retinoic acid availability drives the asynchronous initiation of spermatogonial differentiation in the mouse. Biol Reprod 83:783–790
Solc P, Schultz RM, Motlik J (2010) Prophase I arrest and progression to metaphase I in mouse oocytes: comparison of resumption of meiosis and recovery from G2-arrest in somatic cells. Mol Hum Reprod 16:654–664
Song R, Ro S, Michaels JD et al (2009) Many X-linked microRNAs escape meiotic sex chromosome inactivation. Nat Genet 41:488–493
Sree S, Radhakrishnan K, Indu S, Kumar PG (2014) Dramatic changes in 67 miRNAs during initiation of first wave of spermatogenesis in Mus musculus testis: global regulatory insights generated by miRNA-mRNA network analysis. Biol Reprod 91:69
Stefani G, Slack FJ (2008) Small non-coding RNAs in animal development. Nat Rev Mol Cell Biol 9:219–230
Stocco C, Telleria C, Gibori G (2007) The molecular control of corpus luteum formation, function, and regression. Endocr Rev 28:117–149
Su YQ, Sugiura K, Woo Y et al (2007) Selective degradation of transcripts during meiotic maturation of mouse oocytes. Dev Biol 302:104–117
Su H, Trombly MI, Chen J, Wang X (2009) Essential and overlapping functions for mammalian Argonautes in microRNA silencing. Genes Dev 23:304–317
Suh N, Baehner L, Moltzahn F et al (2010) MicroRNA function is globally suppressed in mouse oocytes and early embryos. Curr Biol 20:271–277
Swetloff A, Conne B, Huarte J et al (2009) Dcp1-bodies in mouse oocytes. Mol Biol Cell 20:4951–4961
Tan T, Zhang Y, Ji W, Zheng P (2014) miRNA signature in mouse spermatogonial stem cells revealed by high-throughput sequencing. Biomed Res Int 2014:154251
Tang F, Kaneda M, O’Carroll D et al (2007) Maternal microRNAs are essential for mouse zygotic development. Genes Dev 21:644–648
Tiwari M, Prasad S, Tripathi A et al (2015) Apoptosis in mammalian oocytes: a review. Apoptosis 20:1019–1025
Tong M-H, Mitchell D, Evanoff R, Griswold MD (2011) Expression of Mirlet7 family microRNAs in response to retinoic acid-induced spermatogonial differentiation in mice. Biol Reprod 85:189–197
Tong M-H, Mitchell DA, McGowan SD et al (2012) Two miRNA clusters, Mir-17-92 (Mirc1) and Mir-106b-25 (Mirc3), are involved in the regulation of spermatogonial differentiation in mice. Biol Reprod 86:72
Trombly DJ, Woodruff TK, Mayo KE (2009) Roles for transforming growth factor beta superfamily proteins in early folliculogenesis. Semin Reprod Med 27:14–23
Ungewitter EK, Yao HHC (2012) How to make a gonad: cellular mechanisms governing formation of the testes and ovaries. Sex Dev 7:7–20
Usongo M, Rizk A, Farookhi R (2012) β-Catenin/Tcf signaling in murine oocytes identifies nonovulatory follicles. Reproduction 144:669–676
Velthut-Meikas A, Simm J, Tuuri T et al (2013) Research resource: small RNA-seq of human granulosa cells reveals miRNAs in FSHR and aromatase genes. Mol Endocrinol 27:1128–1141
Vidal VP, Chaboissier MC, de Rooij DG, Schedl A (2001) Sox9 induces testis development in XX transgenic mice. Nat Genet 28:216–217
Wainwright EN, Jorgensen JS, Kim Y et al (2013) SOX9 regulates microRNA miR-202-5p/3p expression during mouse testis differentiation. Biol Reprod 89:34
Wu L, Fan J, Belasco JG (2008) Importance of translation and nonnucleolytic ago proteins for on-target RNA interference. Curr Biol 18:1327–1332
Wu J, Bao J, Wang L et al (2011) MicroRNA-184 downregulates nuclear receptor corepressor 2 in mouse spermatogenesis. BMC Dev Biol 11:64
Wu Q, Song R, Ortogero N et al (2012) The RNase III enzyme DROSHA is essential for microRNA production and spermatogenesis. J Biol Chem 287:25173–25190
Xu B, Hua J, Zhang Y et al (2011) Proliferating cell nuclear antigen (PCNA) regulates primordial follicle assembly by promoting apoptosis of oocytes in fetal and neonatal mouse ovaries. PLoS One 6:e16046
Yan N, Lu Y, Sun H et al (2007) A microarray for microRNA profiling in mouse testis tissues. Reproduction 134:73–79
Yan G, Zhang L, Fang T et al (2012) MicroRNA-145 suppresses mouse granulosa cell proliferation by targeting activin receptor IB. FEBS Lett 586:3263–3270
Yang J-S, Lai EC (2011) Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol Cell 43:892–903
Yang Q-E, Racicot KE, Kaucher AV et al (2013a) MicroRNAs 221 and 222 regulate the undifferentiated state in mammalian male germ cells. Development 140:280–290
Yang S, Wang S, Luo A et al (2013b) Expression patterns and regulatory functions of microRNAs during the initiation of primordial follicle development in the neonatal mouse ovary. Biol Reprod 89:126
Yao N, Lu C-L, Zhao J-J et al (2009) A network of miRNAs expressed in the ovary are regulated by FSH. Front Biosci 14:3239–3245
Yao G, Yin M, Lian J et al (2010a) MicroRNA-224 is involved in transforming growth factor-beta-mediated mouse granulosa cell proliferation and granulosa cell function by targeting Smad4. Mol Endocrinol 24:540–551
Yao N, Yang B-Q, Liu Y et al (2010b) Follicle-stimulating hormone regulation of microRNA expression on progesterone production in cultured rat granulosa cells. Endocrine 38:158–166
Yao G, Liang M, Liang N et al (2014) MicroRNA-224 is involved in the regulation of mouse cumulus expansion by targeting Ptx3. Mol Cell Endocrinol 382:244–253
Yin M, Lü M, Yao G et al (2012) Transactivation of microRNA-383 by steroidogenic factor-1 promotes estradiol release from mouse ovarian granulosa cells by targeting RBMS1. Mol Endocrinol 26:1129–1143
Yin M, Wang X, Yao G et al (2014) Transactivation of microRNA-320 by microRNA-383 regulates granulosa cell functions by targeting E2F1 and SF-1 proteins. J Biol Chem 289:18239–18257
Yin F, Zhang JN, Wang SW et al (2015) MiR-125a-3p regulates glioma apoptosis and invasion by regulating Nrg1. PLoS One 10:e0116759
Yu Z, Raabe T, Hecht NB (2005) MicroRNA Mirn122a reduces expression of the posttranscriptionally regulated germ cell transition protein 2 (Tnp2) messenger RNA (mRNA) by mRNA cleavage. Biol Reprod 73:427–433
Zeng F, Schultz RM (2005) RNA transcript profiling during zygotic gene activation in the preimplantation mouse embryo. Dev Biol 283:40–57
Zhang J, Ji X, Zhou D et al (2013a) miR-143 is critical for the formation of primordial follicles in mice. Front Biosci 1:588–597
Zhang Q, Sun H, Jiang Y et al (2013b) MicroRNA-181a suppresses mouse granulosa cell proliferation by targeting activin receptor IIA. PLoS One 8:e59667
Zhang H, Jiang X, Zhang Y et al (2014a) MicroRNA 376a regulates follicle assembly by targeting Pcna in fetal and neonatal mouse ovaries. Reproduction 148:43–54
Zhang X, Simerly C, Hartnett C et al (2014b) Src-family tyrosine kinase activities are essential for differentiation of human embryonic stem cells. Stem Cell Res 13:379–389
Zimmermann C, Romero Y, Warnefors M et al (2014) Germ cell-specific targeting of DICER or DGCR8 reveals a novel role for endo-siRNAs in the progression of mammalian spermatogenesis and male fertility. PLoS One 9:e107023
Grant Support
This work was supported by a grant from the Israel Science Foundation (ISF 470/14 to R.S.).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Grossman, H., Shalgi, R. (2016). A Role of MicroRNAs in Cell Differentiation During Gonad Development. In: Piprek, R. (eds) Molecular Mechanisms of Cell Differentiation in Gonad Development. Results and Problems in Cell Differentiation, vol 58. Springer, Cham. https://doi.org/10.1007/978-3-319-31973-5_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-31973-5_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-31971-1
Online ISBN: 978-3-319-31973-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)