Abstract
Cochlear implantation is carried out to recover the sense of hearing. However, its functional outcome varies highly between patients. In the current work, we present a study to assess the functional outcomes of cochlear implants considering the inter-variability found among a population of patients. In order to capture the cochlear anatomical details, a statistical shape model is created from high-resolution human \(\mu \)CT data. A population of virtual patients is automatically generated by sampling new anatomical instances from the statistical shape model. For each virtual patient, an implant insertion is simulated and a finite element model is generated to estimate the electrical field created into the cochlea. These simulations are defined according to the monopolar stimulation protocol of a cochlear implant and a prediction of the voltage spread over the population of virtual patients is evaluated.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
1 Introduction
Over 5 \(\%\) of the worldwide population over the age of 45 years suffer from severe hearing impairment, being considered eligible for cochlear implantation (CI) surgery [17]. However, there is a high variability in the outcomes of CI due to the influence of patient-specific on the level of hearing restoration. Consequently, an accurate prediction of the surgery outcome of the patient is needed to estimate the performance of the cochlear implant. Although computational models have not been applied as a common technique into the clinical practice of CI, some authors have reported promising results predicting its outcomes [2, 10, 14]. Specifically, we have previously presented in-silico studies with promising results for patient-specific cases, where the outcomes of a personalized CI model were assessed [2, 3, 8]. However, the developed automatic framework has the potential to predict CI outcomes not only for patient-specific cases, but also for a more complete virtual study of the population. This is specially useful to carry out evaluations on the implant performance among a group of patients in order to be able to optimize CI electrode array design to the widest range of the population possible.
In this work, a statistical shape model (SSM) has been created from high-resolution \(\mu \)CT data to capture inter-patient variability and to provide a computational tool for virtual patient sampling. Special attention has been given to the insertion depth of the electrode array of the cochlear implant since it highly contributes to the variability in CI outcomes [12]. We presented a virtual insertion algorithm which physically deform the electrode array according to the geometry of the cochlear anatomy of the patient. It allows controlling surgical insertion parameters, such as the depth of insertion of the electrode array [4, 9]. This virtual insertion approach is included within the automatic framework proposed which allows obtaining a full finite element model of the CI. Finally, the computational electrical simulations are carried out [8].
We have improved the computational method by using a more detailed model of the cochlea with respect to our previous work [2]. In addition, we obtain an accurate insertion by using a surgical simulator software to compute the final position of the electrode. Thus, we believe that a more realistic virtual insertion is achieved and consequently, more accurate results of the electrode stimulation can be obtained. This complete framework allow us to assess the nerve stimulation zones in a group of virtual patients by means of realistic CI models and voltage spread prediction. This provides valuable information for electrode design and stimulation parameters optimization.
2 Generation of Computational Models
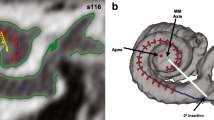
The framework includes a cochlear Statistical Shape Model (SSM), to generate virtual patient anatomies (Fig. 1), a virtual insertion algorithm, to place the electrode array inside the cochlea, and a mesh generation step to create the volumetric finite element (FE) models. Additional background information is found in [8].
Firstly, the SSM is generated from a more suited anatomical reference and with an improved registration procedure [6], allowing the SSM to capture the cochlear population variability in a more satisfactory manner. Most notably, the semi-circular canals are no longer included. The model extends far enough into the vestibule to include the oval and round window. Even though the ending in vestibule is rough and abrupt, the change to the new reference model is motivated and justified by the addition of the well-defined cochlear partition (i.e. a basilar membrane approximation) present in this particular dataset [1]. This provides additional realism to the anatomical model, and facilitates a change to the procedure for virtual placement of the electrode array.
The electrode position of the real cochlear implantation procedure has been computed by means of a planning simulator software. It consists on real-time simulations based on a deformation model which includes the mechanical properties of both electrode and cochlea and a collision model [16]. Afterwards, the virtual insertion algorithm is applied over the original electrode geometry. This algorithm allows obtaining a deformation for the electrode array according to this surgical insertion position [4, 9]. Thus a final electrode mesh is obtained with a realistic placement of the implant for the given patient. This electrode array mesh consisted in a Med-EL Flex28 design, with 12 stimulating channels (electrodes) and a length of 28 mm.
Within the automatic framework, 100 nerve fiber bundles were generated according to the patient’s anatomy and an outer box was created to model as the surrounding bone of the cochlea. Finally all elements were merged and transformed into a single volumetric mesh (Fig. 2). This procedure was repeated in an automatic way for each of the virtual patients sampled from the SSM.
3 Finite Element Simulation: Electrical Model and Stimulation Protocol
For the FE electrical simulation, the static current conduction solver of the open source multiphysics Elmer software has been used [11]. Maxwell’s equations are defined in the quasi-static approximation and the electrical potential is obtained by solving the Poisson equation. Both Dirichlet or Neumann boundary conditions can be used to describe the electric potential, describing the potential and the current values on the boundary, respectively.
Three stimulation protocols can be set up in a cochlear implant according to the electrode configuration. In this work, we have used the monopolar (MP) stimulation (Fig. 3). In this configuration, one electrode is activated emitting current while the bone surrounding the cochlea has been set to ground. For all models, the value of the current stimulation was 1mA [2]. The conductivity parameters of the cochlea structures defined for the electrical simulation were chosen according to [13]. Each simulation was run in steady state formulation and comprised one activated electrode, thus resulting in 12 simulations per virtual patient.
4 Results
A total of 30 virtual patients were sampled randomly from the SSM and studied under a MP stimulation. The electrical simulation framework was run automatically, thus 30 electrical simulations were finally obtained. Since the cochlear shape varies between patients, different lengths of virtual insertion depth were obtained. The length obtained was 25.2 \(\pm \) 1.2 mm with a number of turns of 1.56 \(\pm \) 0.04, corresponding to 563 \(\pm \) 15\(^o \). The virtual insertion algorithm was successfully run in all cases. However, changes in the element area of the electrode array mesh were observed, which prompted us to further quantify these local geometry changes (see Fig. 4). The average changes of element area for all virtual patients evaluated were -4.6 \(\pm \) 3.9\(\%\). The generation of the computational CI model took 228 \(\pm \) 18 seconds, obtaining a volumetric mesh of 1.2\(\times 10^{6}\) \(\pm \) 7\(\times 10^{4}\) of tetrahedral elements with a mesh quality of 0.785 \(\pm \) 0.001. The mesh quality of each model was assessed by computing the aspect ratio of each element, expressed in a range from 0 to 1, corresponding to nearly degenerated mesh element and regular tetrahedral one, respectively [7].
Local changes on the area of each electrode mesh element after the deformation by the virtual insertion. (a) Changes are represented over the surface of the electrode in a scale of -1 to 1, being the maximum decrease and increase, respectively, compared to the area before the deformation. (b) Central mark of the box is the median and its edges the 25th and 75th percentiles of the element area changes of each of the 30 virtual patients.
Potential (V) generated for each stimulation protocol (horizontal axis) in each nerve fibers (vertical axis). (a) Mean and (b) standard deviation of the voltage spread for all virtual patients evaluated. (c) Examples of the voltage spread for a single patient, where differences can be appreciated due to changes in cochlear anatomy. Patient ID shown in (c) are respectively 1,2,9,11,15,17,23 and 30.
12 electrical simulations were run for each model, for a total of 360 runs. Figure 5 shows the electric field for each nerve fiber under the stimulation of the 12 MP stimulation protocols. It can be observed that some zone with a high voltage spread are located far from the perfect diagonal. This implies that each electrode does not exclusively activate the most nearby nerve fiber. This effect is called cross-talk and it is a reason of discrepancy between electrical hearing perceptions in patients with a cochlear implant and normal acoustical hearing. All these virtual patients have in common the cross-talk presented in the apical part of the cochlea. Therein, the nerves located in the basal part are nonspecifically activated by electrodes number 10 to 12 (see Fig. 5). This corresponds to cross-turn stimulation. We show in Fig. 6(a) the mean excitation spread along the spiral ganglion (anatomical structure composed of soma for all neural fibers). Each curve corresponds to one of the 12 MP stimulation protocol. Figure 6(b) shows in detail the excitation spread of the MP stimulation 6 for all 30 patients, where the sixth electrode has been activated.
5 Discussion and Future Work
The main contribution of this work is the CI assessment on a population of virtual patients sampled from a SSM. As far as we know, this is the first population-based study to evaluate the results of a CI electrical simulation. Additionally, we have improved the CI model with respect to our previous work [2], providing a more realistic finite element model based on high-resolution data, real electrode array design and virtual surgical placement. The virtual insertion has proved to be consistent in all cases tested, so a realistic mesh deformation after the virtual insertion is obtained. Simulations have been run successfully in all cases, obtaining results in agreement with previous reported clinical results [15], including the cross-talk zones [5].
Nonetheless, our work has some limitations. The mean excitation spread evaluated along the spiral ganglion has some discrepancies compared to literature [13]. Even though the behaviour is similar and shows a general tendency, we believe that some work needs to be done regarding the geometrical nerve generation since their position could modify the results obtained from the electrode stimulation. Despite this, we do believe that this work is a step closer to the accurate prediction of the nerve activation.
The results obtained help to better explain the behaviour of the excitation spread within a group of patients, observing the variations obtained accounting for the inter-patient anatomy variability. This framework has promising potential to optimize stimulation parameters and electrode placement that better fit the anatomy and level of impairment of each patient. Therefore, we could provide the best functional outcome possible. In future work, other sources of variability will be taken into account. For example, the implant placement or the electrode array configuration which would provide additional valuable information in the process of optimizing the CI.
References
Braun, K., Böhnke, F., Stark, T.: Three-dimensional representation of the human cochlea using micro-computed tomography data: presenting an anatomical model for further numerical calculations. Acta Otolaryngol. 6(132), 603–613 (2012)
Ceresa, M., Mangado Lopez, N., Dejea Velardo, H., Carranza Herrezuelo, N., Mistrik, P., Kjer, H.M., Vera, S., Paulsen, R.R., González Ballester, M.A.: Patient-specific simulation of implant placement and function for cochlear implantation surgery planning. In: Golland, P., Hata, N., Barillot, C., Hornegger, J., Howe, R. (eds.) MICCAI 2014, Part II. LNCS, vol. 8674, pp. 49–56. Springer, Heidelberg (2014)
Ceresa, M., Mangado, N., Andrews, R.J., Ballester, M.A.G.: Computational models for predicting outcomes of neuroprosthesis implantation: the case of cochlear implants. J. Mol. Biol. 52(2), 934–941 (2015)
Duchateau, N., Mangado, N., Ceresa, M., Mistrik, P., Vera, S., González Ballester, M.: Virtual cochlear electrode insertion via parallel transport frame. In: Proceedings of International Symposium on Biomedical Imaging (2015)
Gani, M., Valentini, G., Sigrist, A., Kós, M., Boëx, C.: Implications of deep electrode insertion on cochlear implant fitting. J. Assoc. Res. Otolaryngol. 8, 69–83 (2007)
Kjer, H.M., Vera, S., Fagertun, J., Gil, D., González-Ballester, M.Á., Paulsen, R.: Image registration of cochlear \(\mu \) CT data using heat distribution similarity. In: Paulsen, R.R., Pedersen, K.S. (eds.) SCIA 2015. LNCS, vol. 9127, pp. 234–245. Springer, Heidelberg (2015)
Liu, A., Joe, B.: Relationship between tetrahedron shape measures. BIT Numer. Math. 34(2), 268–287 (1994)
Mangado, N., Ceresa, M., Duchateau, N., Dejea Velardo, H., Kjer, H., Paulsen, R., R., Vera, S., Mistrik, P., Herrero, J., González Ballester, M.: Automatic generation of a computational model for monopolar stimulation of cochlear implants. In: Proceedings of Computer Assisted Radiology and Surgery (2015)
Mangado, N., Duchateau, N., Ceresa, M., Kjer, H., Vera, S., Mistrik, P., Herrero, J., González Ballester, M.: Patient-specific virtual insertion of electrode array for electrical simulations of cochlear implants. In: Proceedings of Computer Assisted Radiology and Surgery (2015)
Nogueira, W.: Finite element study on chochlear implant electrical activity. In: ICBT Proceeding (2013)
Råback, P., Malinen, M., Ruokolainen, J., Pursula, A., Zwinger, T.: Elmer Models Manual. CSC-IT Center for Science, Helsinki (2013)
Roland Jr., J.T.: Cochlear implant electrode insertion. Operative Tech. Otolaryngol.-Head Neck Surg. 16(2), 86–92 (2005)
Saba, R., Elliott, S.J., Wang, S.: Modelling the effects of cochlear implant current focusing. Cochlear Implants Int. 15(6), 318–326 (2014)
Smit, J.E., Hanekom, T., Hanekom, J.J.: Estimation of stimulus attenuation in cochlear implants. J. Neurosci. Methods 180(2), 363–373 (2009)
Vanpoucke, F., Boermans, P., Frijns, J.: Assessing the placement of a cochlear electrode array by multidimensional scaling. IEEE Trans. Biomed. Eng. 59, 307–310 (2012)
Vera, S., Caro, R., Perez, F., Bordone, M., Herrero, J., Kjer, H., Fagertun, J., Paulsen, R., Dhanasingh, A., Barazzetti, L., Reyes.M, González Ballester, M.: Cochlear implant planning, selection and simulation with patient specific data. In: Proceedings of Computer Assisted Radiology and Surgery (2015)
World Health Organization: Deafness and hearing impairment (2012)
Acknowledgement
The research leading to these results received funding from the European Union Seventh Frame Programme (FP7/2007-2013) under grant agreement 304857, HEAR-EU project.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this paper
Cite this paper
Mangado, N. et al. (2016). Monopolar Stimulation of the Implanted Cochlea: A Synthetic Population-Based Study. In: Oyarzun Laura, C., et al. Clinical Image-Based Procedures. Translational Research in Medical Imaging. CLIP 2015. Lecture Notes in Computer Science(), vol 9401. Springer, Cham. https://doi.org/10.1007/978-3-319-31808-0_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-31808-0_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-31807-3
Online ISBN: 978-3-319-31808-0
eBook Packages: Computer ScienceComputer Science (R0)