Abstract
This chapter provides an overview of the materials used for manufacturing IPMC actuators and sensors. Recently, considerable effort has been put into investigating various electrode materials and ionic polymer membranes to increase the actuation performance of IPMC and overcome some of the shortcomings to improve their reliability and stability. Various metallic and nonmetallic electrode materials with notable electrochemical and electromechanical properties have been considered for IPMC electrodes. Herein, some of the more commonly used noble metal-based electrodes and recently introduced nonmetallic conductive material (such as transition metal oxide and various carbon derivatives)-based electrode designs along with specific manufacturing approaches are introduced, highlighting their key aspects and design challenges. Also, several representative ionic polymer membranes used for IPMC fabrication such as sulfonated aromatic hydrocarbon, block copolymers, biopolymers, and nanocomposites capable of providing higher electro-chemo-mechanical properties have been investigated. Herein, more recently developed membrane materials including self-assembled sulfonated polyimide block copolymers, functional cellulose-based biopolymers, and graphene-reinforced nanocomposites are introduced, considering their main advantages, facile synthesis process (such as freeze drying method, all-solution process, and electrospinning technique) and actuation performance.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Carbide-derived carbons (CDCs)
- Degree-of-sulfonation (DOS)
- Direct assembly method
- Electrode materials
- Metallic electrode materials
- Nonmetallic electrode materials
- Freeze-dried bacterial cellulose (FDBC)
- Graphene-Nafion polymer actuator
- Impregnation-reduction method
- Ionic polymer-metal composites (IPMCs)
- Biopolymer membrane materials
- Electromechanical properties of
- Metallic electrode materials
- Nanocarbon-composite membrane materials
- Nonmetallic electrode materials
- Sulfonated block copolymer membrane materials
- Sulfonated hydrocarbon backbone membranes
- Pendent sulfonated chitosan (PSC)
- Polyaniline (PANI)
- Sulfonated poly(amic acid) (SPAA)
- Sulfonated poly(styrene-ran-ethylene) (SPSE)
- Sulfonated polyimide (SPI)
1 Electrode Materials
Electrodes have a significant role in the electromechanical coupling of IPMC. Their physical properties such as electric conductivity, mechanical durability, and surface morphology can strongly affect the actuation performance and reliability of IPMC material. Proper electrodes for IPMC should be highly electrically conductive, mechanically compliant, durable against cyclic deformations, and electrochemically inert for operation in corrosive environment (e.g., water) in the presence of electric potential (~4 V). These design requirements limit considerably the choice of available materials. Various types of electrically conductive materials have been investigated for use in IPMC electrodes, including metals (primarily noble metals), transition metal oxides, and various carbon derivatives such nanotubes, graphene, and nanoporous-activated and carbide-derived carbons. This chapter intends to provide an overview of these developments and current challenges.
1.1 Metallic Electrode Materials
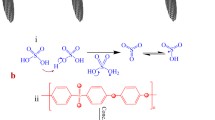
Since the introduction of IPMC materials, the platinum and gold have been most widely used electrode materials for IPMC fabrication due to their high electrochemical stability and excellent electrical conductivity. These materials are fabricated into electrodes using chemical deposition method, also known as impregnation-reduction method or electroless plating (Shahinpoor and Kim 2001; Fujiwara et al. 2000). This method can be used with a wide range of ionic polymer membranes that have ability to selectively exchange cations or anions. The state-of-the-art manufacturing process of IPMC via electroless plating consists of three main steps. First, the ionic polymer membrane is impregnated with desired metal salt by soaking it in respective metal complex solution, such as tetraammineplatinum(II) chloride hydrate (Pt(NH3)4Cl2 · H2O). The metal complex cations diffuse into the polymer through ion-exchange process as illustrated in Fig. 1. Secondly, the platinum complex cations in the polymer are reduced to metallic form at the membrane surface by a chemical reduction process in a solution containing sodium borohydride (NaBH4). The reduction process occurs according the following reaction:
The described impregnation-reduction steps are typically repeated 1–3 times in order to increase the thickness and electric conductivity of the electrodes. The third step is the secondary plating (or surface electroding) process to deposit additional Pt at the outer surface of the electrode to further increase the electrode surface conductivity. The composite is immersed in the Pt complex solution, and by using reducing agents such as hydroxylamine hydrochloride and hydrazine monohydrate, the platinum is deposited on the top of the initial Pt electrode layer. A detailed description of the fabrication process can be found in (Kim and Shahinpoor 2003).
As can be seen in Fig. 2, the metallic platinum particles concentrate predominantly near the interface boundaries, typically within 5–20 μm range from the surface. The plating conditions such as soaking time, concentrations, and temperatures are critical to the plating process and determine the penetration depth and conductivity of electrodes that affect the performance of IPMC (Kim and Shahinpoor 2003; Nemat-Nasser 2002). One of the concerns associated with Pt electrodes are developing microcracks under prolonged cyclic operation, leading to decrease in the electrode surface conductivity (Punning et al. 2007). Gold electrodes are more elastic and offer better mechanical stability and higher electrical conductivity, but on the other hand, their fabrication involves more complex processing (Fujiwara et al. 2000).
Two SEM images (top) showing the cross section (left) and close-up (right) of a typical IPMC. The bottom graph shows an X-ray line scan of Pt. As can be seen, Pt is dense at the surface (Shahinpoor and Kim 2001. © IOP Publishing. Reproduced by permission of IOP Publishing. All rights reserved.)
Several alternative materials and their combinations have been researched for IPMC electrodes. Using palladium as a supporting layer underneath the platinum layer has shown improved mechanical stability and higher transduction performance compared to the conventional platinum electrodes (Kim and Kim 2008; Palmre et al. 2014). In this electrode configuration, the Pd is deposited at the inner surface of the ionomer membrane prior to Pt surface layer using electroless plating method. With appropriate control of the plating conditions, highly dispersed Pd particles can be created not only at the ionomer surface, but deep in the polymer membrane (Fig. 3), thereby increasing notably the specific surface area of the electrodes (Palmre et al. 2014). This highly capacitive Pd electrode interface provides significant increase in the blocking force (Fig. 4) and mechanoelectrical output of IPMC. However, the actuation response is slower compared to regular Pt electrodes.
SEM micrograph of the cross sections of 1 mm thick Pd-Pt IPMC (top), and corresponding EDS line scan profile for elements of Pt and Pd (bottom) (Reproduced from Palmre et al. 2014)
Blocking force response in time for IPMCs with Pt and Pd-Pt electrodes at 4 V DC input (Reproduced from Palmre et al. 2014)
IPMC electrodes composed of copper and platinum have been reported (Johanson et al. 2008), in which the reversible electrochemical processes – Cu dissolution and subsequent reduction of Cu2+ ions at the cathode upon actuation can maintain electrical conductivity between the platinum particles during the deformation. The drawbacks of this electrode configuration are the copper layer oxidation and growth of copper dendrites at the electrodes. Also, silver nanopowder and nickel have been used as cost-effective electrode materials (Chung et al. 2006; Siripong et al. 2006). However, the low electrochemical stability of these materials can limit the cycle life of IPMC.
From the aforementioned materials, the noble metals (Pt, Au, or Pd) are usually a preferred choice for IPMC electrodes due to their high electrical conductivity and electrochemical stability and availability in cation complex form that can be effectively used with electroless plating method.
1.2 Nonmetallic Electrode Materials
Recently, various nonmetallic conductive materials have gained interest as electrode materials for IPMCs. For instance, transition metal oxide powders (such as RuO2) (Akle et al. 2007b), carbon nanotubes (Akle and Leo 2008; Lee et al. 2007), graphene (Kim et al. 2014b), highly porous-activated and carbide-derived carbons (Palmre et al. 2009), and carbon aerogels (Palmre et al. 2011) have been investigated for IPMC electrodes. The powder materials can be assembled into electrodes by physical heat-pressing using direct assembly method (Akle et al. 2007a) or casting technique (Fukushima et al. 2005). The direct assembly method consists of first mixing the conductive powder in Nafion-alcohol dispersion and then applying the conductor/ionomer mixture directly by painting on the surface of ionic polymer membrane (Fig. 5). Finally, the obtained composite is laminated between two gold foil layers by heat-pressing to conjugate the electrodes layers with the membrane (Fig. 6). Alternatively, the casting technique is based on casting the conductive powder/polymer mixture into individual electrode films and assembling them together by heat-pressing. Compared to the noble metals, the conductive powders are less expensive and have a large specific surface area, which is desired for creating high charge density at polymer-electrode interface. For instance, the specific surface area of carbon nanotubes can be up to 1000 m2/g, while the highly porous carbide-derived carbons (CDCs) typically range from 1000 to 2000 m2/g. CDCs are produced by extraction of metal ions from carbide precursor by chlorination at elevated temperatures (Presser et al. 2011). CDCs are also unique for their precisely controllable synthesis process that allows fine-tuning the pore dimensions according to electrolyte properties (Gogotsi et al. 2003). However, the mentioned powder materials have generally insufficient electric conductivity. Therefore, the electrode surface is covered additionally with a conductive gold foil layer (Akle et al. 2007a; Palmre et al. 2009). The added metallic layer makes the manufacturing process often more complicated and can cause problems due to the delamination under cyclic deformation (Akle et al. 2007b). The porous carbon materials, particularly activated carbons and carbide-derived carbons prepared at low chlorination temperatures, also tend to have poor electrical conductivity within the electrode cross section (Palmre et al. 2012). This results in a slow electrical double-layer charging and a slow electromechanical response of an actuator. Also, it has been noted that activated carbons, despite having a large specific surface area, exhibit a limited capacitance due to their low mesoporosity and resulting poor electrolyte accessibility (Lu et al. 2011). Therefore, conductive additives such as nanotubes, carbon black, and polyaniline (PANI) have been added in the carbon electrodes to increase the mesoporosity as well as electric conductivity, thereby improving considerably the performance of the electrodes (Lu et al. 2011; Sugino et al. 2011).
Schematic showing the four steps of the direct assembly process for building IPMC materials (Reproduced from Akle et al. 2007a, with kind permission from Springer Science and Business Media.)
(a) Schematic of IPMC assembled using direct assembly process. (b) SEM image of the upper high surface area RuO2 electrode (Akle et al. 2007b. © IOP Publishing. Reproduced by permission of IOP Publishing. All rights reserved)
Several studies have indicated that the accumulation of electrolyte charges in the vicinity of electrodes is the key to producing a high strain and force output of IPMC (Akle et al. 2006; Wallmersperger et al. 2008; Pugal et al. 2011). Therefore, designing the electrodes with high specific surface area is gaining momentum in the development of IPMC materials (Torop et al. 2011; Palmre et al. 2011, 2014; Sugino et al. 2011). Promising electrode materials for this purpose are the mentioned highly porous carbons and carbon nanotubes. The CDC-based IPMC electrodes have shown one of the best performances among nonmetallic electrodes, exceeding the peak strain and strain rate of RuO2 electrodes more than twice (Table 1) (Palmre et al. 2009). However, there are still challenges to overcome such as insufficient electric conductivity and limited electrolyte diffusion in the carbon electrode structure. Also, it is important to note that the nonmetallic powder materials require physical assembly by heat-pressing and therefore are mainly suited for fabricating “dry-type” actuators based on nonvolatile electrolytes such as ionic liquids (Akle et al. 2007b). One of the challenges with using the ionic liquids as electrolytes is the lower response speed compared to the water-solvated membranes (Bennett and Leo 2004). Therefore, water-based IPMCs manufactured by electroless plating of noble metals that offer one of the fastest strain responses and ability to be operated in aqueous environment are more suited for underwater robotic applications.
2 Ionic Polymer Membranes
Ionic polymer membranes play an important role in determining actuator performances. Especially, their electro-chemo-mechanical properties, such as ion transport properties (ionic-exchange capacity (IEC), liquid electrolyte uptake and ionic conductivity, etc.) and the mechanical properties (tensile modulus, strength, and elongation, etc.), must be evaluated carefully to enhance the actuator performances. As the most popular materials used for IPMC membranes, perfluorinated polymers, such as Nafion and Flemion with ionic sulfonate or carboxylate groups, respectively, are widely investigated. Nafion is a perfluorinated sulfonic acid ionomer membrane consisting of a Teflon-like backbone and short side chains terminated by hydrophilic sulfonic acid groups. However, these perfluorinated ionomer membranes suffer from significant drawbacks, such as low actuation bandwidth, low blocking force and durability, environmentally unfriendliness of fluorinated polymer, and high cost of fabrication, which curtail their practical applications. To overcome these problems, many synthetic ionic polymers have been proposed as leading candidates. As one of alternative ionic polymers, sulfonated hydrocarbon polymers have received significant amount of attention due to their cost-effectiveness, easy fabrication, tunable stiffness, and high ion transport properties, resulting from their controllable monomer composition, especially via manipulation of block copolymers. Another solution is to utilize naturally abundant functional biopolymers, such as cellulose-derivatives and chitosan with high ionic conductivity, environmental friendliness, low cost, and uniform film formation. Yet another solution is to reinforce functional nanoparticles in a polymer matrix, resulting in a high-performance nanocomposite membrane. This section intends to provide an overview of the recent research developments in ionic polymer membranes.
2.1 Sulfonated Hydrocarbon Backbone Membranes
A great deal of effort has been invested in the development of sulfonated aromatic or aliphatic hydrocarbon backbone polymers by adopting the post sulfonation process, blending method, and cross-linking procedure to control hydrophilic-hydrophobic morphology and electro-chemo-mechanical properties. For instance, sulfonated poly(styrene-ran-ethylene) (SPSE , Wang et al. 2010a) with aliphatic hydrocarbon backbone, cross-linked SPSE (XSPSE, Wang et al. 2010c) with better microphase-separated morphology by UV irradiation, and sulfonated aromatic PEI (polyetherimide) (SPEI, Rajagopalan et al. 2010) with controllable stiffness have been investigated as alternative IPMC membranes. Also, several ionic networking membranes have been developed through blending and cross-linking methods. Poly(styrene-alt-maleimide) (PSMI)-incorporated poly(vinylidene fluoride) (PVDF) (PSMI/PVDF, Lu et al. 2008a, b) exhibited several times larger bending performance than the Nafion counterpart due to unique hydrophilic nanochannels. Sulfonated poly(ether ether ketone)-incorporated PVDF (SPEEK/PVDF, Jeon et al. 2009) showed excellent electromechanical responses due to the tailored stiffness and nanochannels inside the ionic networking matrix. Cross-linked PVA/SPTES (Wang et al. 2010b) was developed by physical cross-linking between SPTES (sulfonated poly(arylenethioethersulfone)) copolymer and PVA (polyvinyl alcohol), resulting in an absence of back-relaxation and a dramatic increase in bending deformation. Different types of hydrocarbon backbone membranes have also been presented and compared (Jo et al. 2013). However, these hydrocarbon series still have limitations that do not satisfy the desired electro-chemo-mechanical properties and fail to deliver better actuation performances beyond those of Nafion (Fig. 7).
Several hydrocarbon backbone membranes for preparing IPMC membrane materials (SSEBS, Wang et al. 2007. © 2007 Elsevier Ltd. Reproduced by permission of Elsevier BV.; SPSE, Wang et al. 2010a. © 2010 Society of Chemical Industry. Reproduced by permission of John Wiley & Sons, Inc.; SSPSE, Wang et al. 2010c. © 2010 Elsevier Ltd. Reproduced by permission of Elsevier BV; SPEI, Rajagopalan et al. 2010. © 2010 Elsevier Ltd. Reproduced by permission of Elsevier BV.; PSMI/PVDF, Lu et al. 2008a. © 2008 WILEY-VCH Verlag GmbH & Co. Reproduced by permission of John Wiley & Sons, Inc.; SPI, Cheedarala et al. 2014. © 2014 WILEY-VCH Verlag GmbH & Co. Reproduced by permission of John Wiley & Sons, Inc. All rights reserved.)
2.2 Sulfonated Block Copolymer Membrane Materials
Several sulfonated block copolymers such as SSEBS (sulfonated poly (styrene-b-ethylene-co-butylene-b-styrene)) block copolymer (Wang et al. 2007), ABA-Triblock copolymer (Imaizumi et al. 2012), sulfonated pentablock ABCBA copolymers (Gao et al. 2012), and sulfonated pentablock ionomer (PBI)-based copolymers (Vargantwar et al. 2012), which contain hydrophilic nanochannels and well-organized nanostructure networks with micro-/nanomorphology, have been investigated to enhance actuator performance. Unfortunately, these sulfonated block copolymer membranes have difficulties in fabrication, which include a complex synthesis procedure and low mass production, and deficiencies in their electro-chemo-mechanical properties. Therefore, an alternative synthetic approach is needed to develop novel and simple block copolymers with intriguing nanostructures in a polymer matrix. As one of promising block copolymers, sulfonated polyimide (SPI) block copolymers contain alternate hydrophobic and hydrophilic multiblocks having aliphatic and aromatic segments and display high thermal stability, high ionic conductivity, reliable mechanical properties, and low price. Recently SPI-based actuator with well-defined silver electrodes was developed via an in situ self-metallization (Song et al. 2011). The total fabrication procedure involves the synthesis of a sulfonated poly(amic acid) (SPAA) membrane precursor for SPI derived from BTDA, ODA, and lithium-containing BDSA as a dianhydride and diamine, respectively. After the ionic-exchange process with the Ag salt, the SPAA-Ag+ membrane was subjected to thermal treatment, resulting in imidization of SPAA and simultaneous reduction of silver cations to silver metal layer on both sides of the membrane as shown in Fig. 8a, b.
(a) Chemical structure of SPI with self-metallized silver electrodes, (b) schematic illustration of self-metallization process, (c) step responses of self-metallized SPI actuator and Nafion-based IPMC actuator under 0.5 V DC voltage (Song et al. 2011. © 2011 WILEY-VCH Verlag GmbH & Co. Reproduced by permission of John Wiley & Sons, Inc. All rights reserved.)
Compared with a Nafion-based actuator, the self-metallized SPI actuator with highly conductive silver electrodes showed a much larger tip displacement without the back-relaxation phenomenon under low 0.5 V DC voltage (Fig. 8c). But further investigation for high-performance actuator is needed due to its moderate electro-active performance and poor durability, resulting from the oxidation of silver layers under higher voltage stimulation. Furthermore, SPI-based actuators from a combination of ionic liquid, polyimide, and carbon electrode materials were reported (Imaizumi et al. 2013). But, until now, SPI-based polymer actuators, which have relatively high mechanical properties, low actuation performance, and inferior durability due to densely packed polymer matrix and low compatibility with metallic electrodes fabricated by electroless plating, have not been improved.
Very recently a self-assembled 3D ionic networked SPI polymer actuator with π–π stacked layers and alternate hydrophilic nanochannels have been investigated by introducing simple and strong atom-level regio-specific interaction of hydrophilic and hydrophobic SPI coblocks with anions and cations in the ionic liquid (EMI.Tf2N) (Cheedarala et al. 2014). Moreover, facile and ultrafast all-solution process involving solvent blending, dry casting, and drop casting was developed to make all-organic soft actuators with highly conductive and flexible PEDOT:PSS electrodes (Fig. 9a). This higher bending performance of the newly developed Ntda-SPI-SO3Li-EMI.Tf2N is induced by the high ionic conductivity and tuned mechanical properties, resulting from strong ionic interactions among the SO3Li, ionic liquid and PEDOT:PSS, and π-π stacked 3D-networked polymer matrix with continuous and interconnected ion transport nanochannels (Fig. 9b). Therefore, large peak-to-peak strains of over 0.05 were acquired in all frequency ranges over ±0.5 V as shown in (Fig. 9c).
(a) Schematic illustration of SPI actuator based on a π-π stacked 3D ionic network membrane and ultrafast solution processing, (b) chemical interaction among SPI, ionic liquid, and PEDOT:PSS, (c) peak-to-peak strains at different voltages and frequencies (Cheedarala et al. 2014. © 2014 WILEY-VCH Verlag GmbH & Co. Reproduced by permission of John Wiley & Sons, Inc. All rights reserved.)
2.3 Biopolymer Membrane Materials
Development of high-performance biopolymer membranes has been attempted to solve the problems of conventional synthetic ionic polymers. As one of efforts, well-defined and regular electrospun PANI/CA biopolymer actuators with a good dispersion of chopped PANI (polyaniline) nanoparticles inside the CA (cellulose acetate) nanofibers were reported (Hong et al. 2013). Only a small amount of chopped PANI nanoparticles (0.1 % and 0.5wt%), inducing well-dispersed nanoporous structures (Fig. 10a), can improve the actuation performance of cellulose-based actuators due to the enhanced material properties as presented in Fig. 10b. Moreover, the consumed power of 0.5wt% PANI/CA actuator is lower than that of others because of the effect of highly conductive PANI nanoparticles and their good dispersion in CA matrix (Fig. 10c). Therefore, this investigation suggests that the electrospinning is an extremely practical and effective technique for constructing a series of well-dispersed nanoporous membranes with controllable and repeatable products, unlike conventional casting method.
(a) Schematic diagram of fabrication process for electrospun PANI /CA actuators, (b) harmonic responses, (c) hysteresis responses of electrospun actuators under sinusoidal electrical inputs with peak voltage of 3 V and excitation frequency of 0.1 Hz (Hong et al. 2013. © 2013 Elsevier Ltd. Reproduced by permission of Elsevier BV. All rights reserved.)
To make high-performance air-working actuators, ionic liquids as mobile salts should be embedded in the cellulose membrane. But pure cellulose is not familiar with absorbing much ionic liquid because of its packed structure and high crystallinity. Therefore, freeze-dried bacterial cellulose (FDBC) with sponge-like porous network structure through a simple freeze-dry method at −50 °C for 24 h can be used to solve these problems. Because FDBC with high porosity and large surface area can absorb a large quantity of ionic liquid and exhibit enhanced electrochemical properties. A novel high-performance electro-active biopolymer actuator with a tri-layered sandwich structure that consists of FDBC and poly(3,4-ethylenedioxythiophene) poly(styrenesulfonate) (PEDOT:PSS) face sheet layers was developed as presented in Fig. 11a (Kim et al. 2013). The actuation performance of the developed actuator was significantly improved through the synergistic effects of the electrochemical doping processes of PEDOT:PSS electrode layers and sufficient ion migration of the dissociated ionic liquids inside the FDBC with a sponge form. So the FDBC actuator exhibited a much larger bending deformation than pure bacterial cellulose actuator as shown in Fig. 11b. Also, under sinusoidal excitation with an excitation frequency of 0.1 Hz and peak voltage of 3 V, the EMIM-BF4 absorbed FDBC actuator, inducing much larger bending deformations, reached a peak tip displacement of ±1.511 mm (Fig. 11c).
(a) Fabrication steps of the FDBC actuator, (b) actuation performance of a FDBC actuator compared with a pure BC actuator, (c) harmonic response of FDBC actuators using EMIM-BF4 (Kim et al. 2013. © 2013 IOP Publishing Ltd. Reproduced by permission of IOP Publishing. All rights reserved.)
2.4 Nanocarbon-Composite Membrane Materials
Reinforcement by the incorporation of electrically conductive 0D/1D/2D/3D carbon nanoparticles into polymer matrix is a promising approach to achieve improved electro-chemo-mechanical properties including enhanced ionic transport properties and tunable mechanical stiffness over pristine polymers. Therefore, conductive carbon nanoparticles such as 0D (fullerene, polyhydroxylated fullerene (PHF)), 1D (SWNT, MWNT, CNF), 2D (Graphene), and 3D (hierarchical G-CNT-Fe/G-CNT-Pd carbon nanostructure) have been reinforced into ionic polymer membranes to enhance their physicochemical properties and actuation performance. Several nanocomposite actuators have been investigated, i.e., (i) 0D carbon nanostructures: C60-reinforced Nafion (Jung et al. 2010; Oh et al. 2010), fullerenol-cellulose (Li et al. 2011), and fullerenol-SPEI (Rajagopalan and Oh 2011), (ii) 1D carbon nanostructures : CNF-reinforced SSEBS (Wang et al. 2009), (iii) 2D carbon nanostructures : Graphene-Nafion (Jung et al. 2011), Graphene oxide-chitosan (Jeon et al. 2013), and Ionic polymer-graphene composite (IPGC, Kim et al. 2014b), (iv) 3D carbon nanostructures : 3D G-Fe-reinforced polyurethane (Lee et al. 2014), and 3D G-Pd-reinforced conducting polymer (Kim et al. 2014a).
Especially graphene is an intriguing 2D flat material of monolayer carbon atoms whose distinct properties make it very promising in actuator applications. Recently, a graphene-reinforced Nafion nanocomposite actuator was developed as shown in Fig. 12a (Jung et al. 2011). The layered structure of graphene in the graphene-Nafion composites showed that graphene layers are aligned parallel to the surface of membrane and can provide tortuous pathway for ion transport (Fig. 12b, c). Although higher graphene concentration showed a decrease in water adsorption caused by the reduction of degree of ion clustering due to a decrease in the cluster size and the exchange sites per cluster, a marginal increase in IEC with increasing graphene was observed, thereby leading to enhanced actuation performance. The tip displacement of the 1.0 wt% graphene-reinforced actuator is almost two times that of the recast Nafion-based IPMC actuator (Fig. 12d). These results demonstrate that the electro-chemo-mechanical properties and actuation performances were significantly improved due to a minute loading of graphene, resulting from the great interaction between graphene and Nafion.
Graphene-Nafion polymer actuator ; (a) cross-sectional SEM image, (b) magnified SEM image, (c) schematic representation of ionic conductivity in hydrated graphene-Nafion composites, (d) harmonic response (Jung et al. 2011. © 2010 Elsevier Ltd. Reproduced by permission of Elsevier BV. All rights reserved.)
In order to develop an economically viable, highly durable, and bio-friendly ionic polymer actuator with superior electro-chemo-mechanical properties, a simple route for a high-performance ionic bio-nanocomposite based on pendent sulfonated chitosan (PSC) and functionalized graphene oxide (GO) have been investigated (Jeon et al. 2013). The amine groups in the chitosan were actively used to tune the degree of sulfonation (DOS) of PSC by controlled reaction with 1,3-propyl sultone and with GO. Thus, PSC can act as a higher ionic-exchangeable membrane due to the availability of propyl sulfonic acid groups that can strongly binding with free amines and with ionic liquid. Furthermore, electro-chemo-mechanical activities of PSC can be maximized by simply reinforcing with GO, resulting in improving in-plane mechanical stiffness and electro-chemo-mechanical properties through strong ionic interactions and bonding with free amines and sulfonic acid groups (Fig. 13a). Minute loading of graphene oxide (1.0 wt%) to PSC greatly improves step responses without the back-relaxation phenomenon (Fig. 13b, c). This improvement can be attributed to relatively higher ionic conductivity and large capacitance, and effective delay of ionic liquid migration in the reduced ionic pores homogeneously distributed in GO(1.0 wt%)-PSC-IL matrix.
(a) Synthesis of GO-PSC-IL bio-nanocomposite membrane, (b) electro-mechanically deformed shapes of GO(1.0 wt%)-PSC-IL actuator under a step input, (c) step responses of GO(1.0 wt%)-PSC-IL actuator according to driving voltages (Jeon et al. 2013. © 2013 WILEY-VCH Verlag GmbH & Co. Reproduced by permission of John Wiley & Sons, Inc. All rights reserved.)
Recent attention to the correlation of morphological structure (microphase-separated morphology, well-dispersed porous structures and self-assembled nanostructures, etc.) with ion migration mechanisms to enable more efficient ion transport has led to new ways to make high-performance IPMC actuators. There are several research approaches to easily controlling hydrophobic and hydrophilic composition, size of ionic nanochannels, and their stiffness of ionic polymer membranes as well as modulating significant factors such as their ionic-exchange capacity, degree of sulfonation, capacitance, ionic conductivity, liquid electrolyte uptake, and mechanical properties. As one of the recent advances in ionic polymer membranes, herein we briefly introduced promising alternative ionic polymer membranes such as self-assembled sulfonated polyimide block copolymers, functional cellulose-based biopolymers, and graphene-reinforced nanocomposites, and technical considerations including freeze drying method, all-solution process, electrospinning technique, and reinforcement of conductive nanoparticles. Especially, the sulfonated 3D-networked porous structure membranes based on sulfonated polyimide block copolymer and pendent sulfonated chitosan biopolymers can be promising candidates for high-performance ionic polymer membrane materials. Furthermore, electrically conductive and hierarchical nanoparticle additives like 3D carbon nanostructures can greatly increase the output tip displacement, generated blocking force and energy efficiency even more. However, there is still need for more challenges and research studies to realize full potential of the ionic polymer-based high-performance IPMCs and overcome their insufficient controllable structure, material properties, and low repeatability and robustness of the actuation performance.
3 Conclusions
In this chapter, recent developments in electrode materials and ionic polymer membranes used for manufacturing IPMCs were reviewed. Although noble metals such as platinum and gold are commonly used for electrodes in water-based systems and applications for their excellent electrochemical properties, also various nonmetallic conductive carbon derivatives are considered as promising alternatives for fabricating “dry-type” IPMC actuators. These carbon derivatives include nanotubes and nanoporous-activated and carbide-derived carbons. While their electric conductivity is generally less than that of noble metals, the mentioned carbon materials offer some important qualities such as high specific surface area and lower cost.
In spite of wide popularity of Nafion membranes, other less expensive and better-performing ionomers have been explored for IPMCs. One of the alternatives is sulfonated hydrocarbon polymers that offer cost-effectiveness, easy fabrication, tunable stiffness, and high ion transport properties, resulting from their controllable monomer composition. Also, naturally abundant functional biopolymers, such as cellulose-derivatives and chitosan with high ionic conductivity, environmental friendliness, and low cost, have been used for IPMC membrane. A promising approach is also reinforcement by incorporating functional carbon nanoparticles, such as fullerenes, nanotubes, and graphene, in a polymer matrix, resulting in a high-performance nanocomposite membrane.
References
Akle BJ, Leo DJ (2008) Single-walled carbon nanotubes – ionic polymer electroactive hybrid transducers. J Intell Mater Syst Struct 19:905–915
Akle BJ, Bennett MD, Leo DJ (2006) High-strain ionomeric-ionic liquid electroactive actuators. Sensors Actuators A Phys 126(1):173–181
Akle BJ, Bennett MD, Leo DJ et al (2007a) Direct assembly process: a novel fabrication technique for large strain ionic polymer transducers. J Mater Sci 42(16):7031–7041
Akle B, Nawshin S, Leo D (2007b) Reliability of high strain ionomeric polymer transducers fabricated using the direct assembly process. Smart Mater Struct 16(2):S256–S261
Bennett MD, Leo DJ (2004) Ionic liquids as stable solvents for ionic polymer transducers. Sensors Actuators A Phys 115:79–90
Cheedarala RV, Jeon JH, Kee CD et al (2014) Bio-inspired all-organic soft actuator based on π-π stacked 3D ionic network membrane and Ultra-Fast Solution Processing. Adv Funct Mater (In press)
Chung CK, Fung PK, Hong YZ, Ju MS, Lin CCK, Wu TC (2006) A novel fabrication of ionic polymer-metal composites (IPMC) actuator with silver nano-powders. Sensors Actuators B Chem 117(2):357–375
Fujiwara N, Asaka K, Nishimura Y et al (2000) Preparation of gold − solid polymer electrolyte composites as electric stimuli-responsive materials. Chem Mater 12(6):1750–1754
Fukushima T, Asaka K, Kosaka A et al (2005) Fully plastic actuator through layer-by-layer casting with ionic-liquid-based bucky gel. Angew Chem Int Ed 44:2410–2413
Gao R, Wang D, Heflin JR et al (2012) Imidazolium sulfonate-containing pentablock copolymer–ionic liquid membranes for electroactive actuators. J Mater Chem 22:13473–13476
Gogotsi Y, Nikitin A, Ye H et al (2003) Nanoporous carbide-derived carbon with tunable pore size. Nat Mater 2(9):591–594
Hong CH, Ki SJ, Jeon JH et al (2013) Electroactive bio-composite actuators based on cellulose acetate nanofibers with specially chopped polyaniline nanoparticles through electrospinning. Compos Sci Technol 87:135–141
Imaizumi S, Kokubo H, Watanabe M (2012) Polymer actuators using ion-gel electrolytes prepared by self-assembly of ABA-triblock copolymers. Macromolecules 45(1):401–409
Imaizumi S, Ohtsuki Y, Yasuda T et al (2013) Printable polymer actuators from ionic liquid, soluble polyimide, and ubiquitous carbon materials. ACS Appl Mater Inter 5(13):6307–6315
Jeon JH, Kang SP, Oh IK (2009) Novel biomimetic actuator based on SPEEK and PVDF. Sensor Actuators B 143(1):357–364
Jeon JH, Kumar R, Kee CD et al (2013) Dry-type artificial muscles based on pendent sulfonated chitosan and functionalized graphene oxide for greatly enhanced ionic interactions and mechanical stiffness. Adv Funct Mater 23(48):6007–6018
Johanson U, Mäeorg U, Sammelselg V, Brandell D, Punning A, Kruusmaa M, Aabloo A (2008) Electrode reactions in Cu-Pt coated ionic polymer actuators. Sensor Actuators B 31:340–346
Jo CH, Pugal D, Oh IK et al (2013) Recent advances in ionic polymer-metal composite actuators and their modeling and applications. Prog Polym Sci 38(7):1037–1066
Jung JH, Vadahanambi S, Oh IK (2010) Electro-active nano-composite actuator based on fullerene-reinforced Nafion. Compos Sci Technol 70(4):584–592
Jung JH, Jeon JH, Sridhar V et al (2011) Electro-active graphene–Nafion actuators. Carbon 49(4):1279–1289
Kim SM, Kim KJ (2008) Palladium buffer-layered high performance ionic polymer-metal composites. Smart Mater Struct 17(3):035011
Kim KJ, Shahinpoor M (2003) Ionic polymer-metal composites: II. Manufacturing techniques. Smart Mater Struct 12(1):65–79
Kim SS, Jeon JH, Kee CD et al (2013) Electro-active hybrid actuators based on freeze-dried bacterial cellulose and PEDOT:PSS. Smart Mater Struct 22(8):085026
Kim HJ, Randriamahazaka H, Oh IK (2014a) Highly conductive, capacitive, flexible and soft electrodes based on 3D graphene-nanotube-palladium hybrid and conducting polymer. Small 10(24):5023–5029
Kim J, Jeon JH, Kim HJ et al (2014b) Durable and water-floatable ionic polymer actuator with hydrophobic and asymmetrically laser-scribed reduced graphene oxide paper electrodes. ACS Nano 8(3):2986–2997
Lee DY, Park IS, Lee MH et al (2007) Ionic polymer–metal composite bending actuator loaded with multi-walled carbon nanotubes. Sensors Actuators A Phys 133(1):117–127
Lee SH, Jung JH, Oh IK (2014) 3D networked graphene-ferromagnetic hybrids for fast shape memory polymers with enhanced mechanical stiffness and thermal conductivity. Small 10(19):3880–3886
Li J, Vadahanambi S, Kee CD et al (2011) Electrospun fullerenol-cellulose biocompatible actuators. Biomacromolecules 12(6):2048–2054
Lu J, Kim SG, Lee S et al (2008a) A biomimetic actuator based on an ionic networking membrane of poly(styrene-alt-maleimide)-incorporated poly(vinylidene fluoride). Adv Funct Mater 18(8):1290–1298
Lu J, Kim SG, Lee S et al (2008b) Fabrication and actuation of electro-active polymer actuator based on PSMI-incorporated PVDF. Smart Mater Struct 17(4):045002
Lu W, Hartman R, Qu L et al (2011) Nanocomposite electrodes for high-performance supercapacitors. J Phys Chem Lett 2(6):655–660
Nemat-Nasser S (2002) Micromechanics of actuation of ionic polymer-metal composites. J Appl Phys 92:2899–2915
Oh IK, Jung JH, Jeon JH et al (2010) Electro-chemo-mechanical characteristics of fullerene-reinforced ionic polymer-metal composite transducers. Smart Mater Struct 19(7):075009
Palmre V, Brandell D, Maeorg U et al (2009) Nanoporous carbon-based electrodes for high strain ionomeric bending actuators. Smart Mater Struct 18(9):095028
Palmre V, Lust E, Jänes A et al (2011) Electroactive polymer actuators with carbon aerogel electrodes. J Mater 21:2577–2583
Palmre V, Torop J, Arulepp M et al (2012) Impact of carbon nanotube additives on carbide-derived carbon-based electroactive polymer actuators. Carbon 50(12):4351–4358
Palmre V, Kim SJ, Pugal D, Kim K (2014) Improving electromechanical output of IPMC by high surface area Pd-Pt electrodes and tailored ionomer membrane thickness. Int J Smart Nano Mater 5(2):99–113
Presser V, Heon M, Gogotsi Y (2011) Carbide-derived carbons – from porous networks to nanotubes and graphene. Adv Funct Mater 21:810–833
Pugal D, Kim KJ, Aabloo A (2011) An explicit physics-based model of ionic polymer-metal composite actuators. J Appl Phys 110:084904–084909
Punning A, Kruusmaa A, Aabloo A (2007) Surface resistance experiments with IPMC sensors and actuators. Sensors Actuators A Phys 133(1):200–209
Rajagopalan M, Oh IK (2011) Fullerenol-based electroactive artificial muscles utilizing biocompatible polyetherimide. ACS Nano 5(3):2248–2256
Rajagopalan M, Jeon JH, Oh IK (2010) Electric-stimuli-responsive bending actuator based on sulfonated polyetherimide. Sensor Actuators B 151(1):57–64
Shahinpoor M, Kim KJ (2001) Ionic polymer-metal composites: I. Fundamentals. Smart Mater Struct 10(4):819–833
Siripong M, Fredholm S, Nguyen QA, Shih B, Itescu J, Stolk J (2006) Symposium W – electroresponsive polymers and their applications, MRS proceedings. Mater Res Soc 889.
Song J, Jeon JH, Oh IK et al (2011) Electro-active polymer actuator based on sulfonated polyimide with highly conductive silver electrodes via self-metallization. Macromol Rapid Commun 32(19):1583–1587
Sugino T, Kiyohara K, Takeuchi I et al (2011) Improving the actuating response of carbon nanotube/ionic liquid composites by the addition of conductive nanoparticles. Carbon 49:3560–3570
Torop J, Palmre V, Arulepp M, Sugio T, Asaka K, Aabloo A (2011) Flexible supercapacitor-like actuator with carbide-derived carbon electrodes. Carbon 49:3113–3119
Vargantwar PH, Roskov KE, Ghosh TK et al (2012) Enhanced biomimetic performance of ionic polymer–metal composite actuators prepared with nanostructured block ionomers. Macromol Rapid Commun 33(1):61–68
Wallmersperger T, Akle BJ, Leo DJ et al (2008) Electrochemical response in ionic polymer transducers: an experimental and theoretical study. Compos Sci Technol 68:1173–1180
Wang XL, Oh IK, Lu J et al (2007) Biomimetic electro-active polymer based on sulfonated poly (styrene-b-ethylene-co-butylene-b-styrene). Mater Lett 61(29):5117–5120
Wang XL, Oh IK, Kim JB (2009) Enhanced electromechanical performance of carbon nano-fiber reinforced sulfonated poly(styrene-b-[ethylene/butylene]-b-styrene) actuator. Compos Sci Technol 69(13):2098–2101
Wang XL, Oh IK, Chen TH (2010a) Electro-active polymer actuators employing sulfonated poly(styrene-ran-ethylene) as ionic membranes. Polym Int 59(3):305–312
Wang XL, Oh IK, Lee S (2010b) Electroactive artificial muscle based on crosslinked PVA/SPTES. Sensor Actuators B 150(1):57–64
Wang XL, Oh IK, Xu L (2010c) Electro-active artificial muscle based on irradiation-crosslinked sulfonated poly(styrene-ran-ethylene). Sensor Actuators B 145(2):635–642
Acknowledgments
KJK acknowledges the financial support from various agencies who supported IPMC research including the US Office of Naval Research (ONR), US National Science Foundation (NSF), and National Aeronautics and Space Administration (NASA).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this entry
Cite this entry
Kim, K., Palmre, V., Jeon, JH., Oh, IK. (2016). IPMCs as EAPs: Materials. In: Carpi, F. (eds) Electromechanically Active Polymers. Polymers and Polymeric Composites: A Reference Series. Springer, Cham. https://doi.org/10.1007/978-3-319-31530-0_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-31530-0_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-31528-7
Online ISBN: 978-3-319-31530-0
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics