Abstract
Hemostasis occurs through a complex process that involves numerous different blood clotting proteins. These blood clotting proteins interact in the initiation, amplification, and propagation phases of the coagulation cascade. Platelets also function to stop bleeding by creating platelet plugs at the site of injury. Furthermore, von Willebrand factor (VWF) binds to factor VIII, platelet surface glycoproteins, and connective tissue to assist in hemostasis. When the coagulation cascade is initiated, fibrinolysis will also occur simultaneously. Fibrinolysis is the physiological mechanism that dissolves clots and maintains vessel patency. Hemorrhage occurs when the clotting cascade is interrupted through a deficiency or malfunction in one or more of these clotting proteins or when there is a disorder of fibrinolysis. In addition, a qualitative or quantitative defect in platelets or VWF can also lead to a bleeding event. The etiology of bleeding events in patients may be multifaceted, and managing these bleeding patients can be complex and difficult at times. Treatment is generally aimed at replacing the missing or defective blood clotting protein or platelets. This chapter highlights various medications, both recombinant and human derived, that are utilized in the management of bleeding patients. Medication uses listed in this chapter are both based on manufacturer-approved indications and also on unlabeled indications, which have been reported in case reports, case series, and more rigorous studies.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Bleeding
- Hemorrhage
- Management
- Adult
- Pediatric
- Tranexamic acid
- Aminocaproic acid
- Fibrinogen concentrate
- Recombinant factor VIIa
- Desmopressin
- Antihemophilic factor/von Willebrand factor complex (human)
- Phytonadione
- Four-factor prothrombin complex concentrate
- Thrombin powder
- Protamine
- Anti-inhibitor coagulant complex
Hemostatic Agents Used to Stop Bleeding
Tranexamic Acid
Brand Names (USA): Lysteda™, Cyklokapron™
Description: Tranexamic acid is a synthetic amino acid that is classified as antifibrinolytic. It is a competitive inhibitor of plasminogen activation, which becomes a noncompetitive inhibitor at higher concentrations. Tranexamic acid displaces plasminogen from fibrin and resu lts in the inhibition of fibrinolysis. Tranexamic acid is approximately ten times more potent than aminocaproic acid, and its elimination half-life is 2–11 h [1, 2]. Tranexamic acid is available in an intravenous solution and an oral tablet. In addition, an oral solution may be compounded. If 10 mL of the compounded oral 5 % solution is swallowed, this provides 500 mg of tranexamic acid orally [3]. However, this oral solution is mostly studied for topical use.
Adult Use: Tranexamic acid can be used for menorrhagia [2], blood loss reduction during elective cesarean section [4], blood loss reduction during hip fracture surgery [5], blood loss reduction in orthognathic surgery [6], blood loss reduction in dental procedure patients on oral anticoagulant therapy [7], prevention of perioperative bleeding associated with cardiac and spinal surgery [8–11], blood loss reduction during total hip and total knee replacement surgery [12–18], and trauma-associated hemorrhage [19].
Pediatric Use: In the pediatric setting, tranexamic acid can be used for the prevention of bleeding associated with extracorporeal membrane oxygenation (ECMO) during surgery for congenital diaphragmatic hernia (CDH) repair [20, 21], prevention of perioperative bleeding associated with cardiac surgery [22–24], menorrhagia [2], blood loss reduction in hemophilia patients undergoing tooth extraction [25], treatment of hemoptysis in cystic fibrosis patients [26–28], and prevention of perioperative bleeding associated with spinal surgery and craniosynostosis surgery [29–32].
Adverse Effects and Monitoring Parameters: Orally administered tranexamic acid can cause gastrointestinal upset, headache, abdominal pain, muscle pain, and thrombosis (Table 34.1). Visual defects may occur; thus, patients should undergo routine ophthalmologist examinations. Intravenous tranexamic acid can also cause hypotension with rapid administration [33]. The dose and frequency of tranexamic acid should be adjusted in patients with renal dysfunction (see Table 34.2). Importantly, tranexamic acid should not be used when there is evidence of active intravascular thrombosis [1, 2]. Caution is advised if antifibrinolytics are used together with prothrombin complex concentrates (PCC) or activated prothrombin complex concentrates (APCC) because of the risk of thrombosis. If treatment with both agents is deemed necessary, it is recommended to wait 4–6 h after the last dose of PCC or APCC before administering antifibrinolytic s [34].
Aminocaproic Acid
Brand Name (USA): Amicar™
Description: Aminocaproic acid is an antifibrinolytic that binds competitively to plasminogen, which reduces the conversion of plasminogen to plasmin, resulting in the inhibition of fibrin degradation. The main difference between aminocaproic acid and tranexamic acid is that tranexamic binds more strongly to plasminogen; thus, aminocaproic acid is less potent than tranexamic acid. Aminocaproic acid has an elimination half-life of 2 h and may accumulate in patients with renal dysfunction. Thus, a reduced dose may be necessary in anephric patients or those with renal dysfunction. Aminocaproic acid is available as an intravenous solution, oral solution, and oral tablet [35, 36].
Adult Use: Aminocaproic acid can be used to enhance hemostasis when fibrinolysis contributes to bleeding [35], control of acute bleeding [35], control of bleeding with severe thrombocytopenia [37, 38], control of bleeding in congenital and acquired coagulation disorder [39], blood loss reduction in patients on oral anticoagulant therapy undergoing dental procedures [40], and prevention of perioperative bleeding associated with cardiac surgery [41, 42].
Pediatric Use: Aminocaproic acid can be used in children for the prevention of perioperative bleeding associated with cardiac and spinal surgery [42–44] and prevention of bleeding associated with ECMO [45–47].
Adverse Effects and Monitoring Parameters: The most common adverse effect of aminocaproic acid is gastrointestinal upset (Table 34.3). Other adverse effects include thrombosis and an increase in blood urea nitrogen (BUN) and skeletal muscle weakness. Aminocaproic acid can also cause skeletal muscle weakness; therefore, creatine phosphokinase (CPK) should be monitored, and treatment should be discontinued with a significant rise in CPK. Other monitoring parameters include fibrinogen, BUN, and creatinine. Importantly, aminocaproic acid should not be used when there is evidence of active intravascular thrombosis [ 35].
Fibrinogen Concentrate (Human)
Brand Name (USA): RiaSTAP™
Description: Fibrinogen concentrate (coagulation factor I) is generated from pooled human plasma and is a physiological substrate of thrombin, factor XIIIa, and plasmin. Soluble fibrinogen is converte d to insoluble fibrin. Fibrin is stabilized by factor XIIIa, which induces cross-linking of fibrin polymers to provide strength and stability to the blood clot. The cross-linked fibrin is the end result of the coagulation cascade. Human fibrinogen concentration has a fairly long elimination half-life of 61–97 h; however, this half-life may be decreased in children and adolescents. Fibrinogen concentrate is available as intravenous powder, for reconstitution [48].
Adult Use: Fibrinogen concentrate can be used in the treatment of acute bleeding episodes in patients with congenital fibrinogen deficiency (afibrinogenemia and hypofibrinogenemia) [48], supportive therapy in trauma patients who are bleeding [49–53], blood loss reduction in cardiovascular surgery [49, 54, 55], blood loss reduction in postpartum hemorrhage [56], and improvement in clot firmness after orthopedic surgery [57].
Pediatric Use: Fibrinogen concentrate can be used in children for the treatment of congenital fibrinogen deficiency [58, 59] and reduction of blood loss after surgical craniosynostosis repair [60].
Adverse Effects and Monitoring Parameters: Fibrinogen concentrate may cause hypersensitivity reactions, thrombosis, and headache (Table 34.4). Similar to all plasma-derived factor products, fibrinogen concentrate may also transmit disease, since the product is derived from human plasma. Monitoring parameters include fibrinogen levels and signs/symptoms of thrombosis and hypersensitivity. A target fibrinogen level of 100 mg/dL should be maintained until hemostasis occurs and wound healing is complete. The reference range for normal fibrinogen is 200–450 m g/dL [48].
Factor VIIa (Recombinant)
Brand Name (USA): NovoSeven™ RT
Description: Recombinant factor VIIa is a vitamin K-dependent glycoprotein that promotes hemostasis by activating the extrinsic pathway of the coagulation cascade. Factor VII complexes with tissue factor and activates coagulation factors IX and X. When complexed with other factors, coagulation factor Xa converts prothrombin to thrombin, a key step in the formatio n of a fibrin-platelet hemostatic plug. Recombinant factor VIIa has a short terminal half-life of 2.6–3.1 h, thus a need for frequent dosing. Recombinant factor VIIa is available as an intravenous solution [61].
Adult Use: Recombinant factor VIIa is indicated for use in patients with hemophilia A or B with inhibitors [61], congenital factor VII deficiency [61], acquired hemophilia [61], and Glanzmann’s thrombasthenia [61]. There have been numerous studies and reports on the unlabeled use of recombinant factor VIIa in bleeding patients. The paragraph below is not an exhaustive list but rather a general summary of the use of this product in different patient populations. Recombinant factor VIIa has been reported to be used in patients with warfarin-related intracerebral hemorrhage (ICH) [62, 63], refractory bleeding after cardiac or liver surgery in nonhemophilic patients [63–67], anticoagulation reversal [68–70], blood loss reduction after cardiac surgery [71], coagulopathy reversal in isolated traumatic brain injury (TBI) [72], diffuse alveolar hemorrhage in bone marrow transplant (BMT) patients [73], bridge to transplant in end-stage liver disease patients [73], trauma-related coagulopathy [73], refractory perioperative bleeding in noncardiac patients [73], life-threatening refractory hemorrhage of any cause in severely coagulopathic patients [63, 73], blood loss reduction in abdominal trauma patients [74], and esophageal varices [63].
Pediatric Use: Recombinant factor VIIa can also be utilized in the pediatric population for reduction in blood loss and requirement for blood products after cardiac surgery [75, 76], coagulopathies [77], treatment of severe bleeding associated with dengue hemorrhagic fever [78], liver impairment [79, 80], and nonhemophilic hemorrhages [81].
Adverse Effects and Monitoring Parameters: Recombinant factor VIIa may cause antibody formation, hypersensitive reactions, thromboembolic events, and hyper- or hypotension (Table 34.5). Monitoring parameters include evidence of hemostasis. The prothrombin time (PT), international normalized ratio (INR), activated partial thromboplastin time (PTT), and factor VII may also be useful as adjunct tests to evaluate efficacy [61].
Desmopressin
Brand Names (USA): DDAVP™, Stimate™
Description: Desmopressin is a synthetic analogue of vasopressin, but the molecular structure is modified from vasopressin to reduce its vasoactive actions; vasopressin activates both V1 and V2 receptors where desmopressin only stimulates V2 receptors. Desmopressin incr eases plasma levels of von Willebrand factor (VWF), factor VIII (FVIII), and tissue plasminogen activator (t-PA) contributing to a shortened PTT and bleeding time. These effects are likely due to stimulating the release of VWF from endothelial storage sites; however, this mechanism is not fully understood, and several hypotheses exist [82–86]. The secreted t-PA is inactivated by plasminogen activator inhibitor and thus does not seem to promote fibrinolysis or bleeding. Most patients with type 1 von Willebrand disease (VWD) and FVIII/VWF levels greater than 10 units/mL will respond to desmopressin, but patients with type 2 VWD have a more variable response. Prior to utilizing desmopressin for therapy, a therapeutic trial should be conducted to determine a patient’s response. To test responsiveness, blood samples are taken 30–60 min and 4 h after an intravenous injection of desmopressin to obtain a reliable figure on recovery and clearance of FVIII and VWF [87]. Desmopressin has an elimination half-life of 2–4 h; however, the half-life is prolonged to 9 h in patients with renal impairment (See Table 34.7) [35].
Formulations: Desmopressin is available as a 4 mcg/mL injection and a 1.5 mg/mL nasal solution. The nasal formulation is ~2.75 times less potent than the injection formulation. Therefore, the nasal solution is often used for minor bleeding, while the intravenous injection is preferred for surgical bleeding prophylaxis and major bleeding. Desmopressin is also available as an oral tablet and a rhinal tube [83].
Adult Use: Desmopressin is utilized in the treatment and prevention of bleeding episodes in mild-to-moderate hemophilia A and mild-to-moderate VWD type 1 patients who respond to a desmopressin challenge [34, 88, 89], uremia associated with acute or chronic renal failure [90], prevention of surgical bleeding in patients with uremia [91], stabilization of platelet function in intracranial hemorrhage [92], blood loss reduction after cardiac surgery [93, 94], blood loss reduction in dental procedures [83], and blood loss reduction in patients with liver cirrhosis [95–98]. It is recommended to utilize VWF in addition to desmopressin if the postsurgical treatment is necessary for more than three days [83].
Pediatric Use: In the pediatric setting, desmopressin is utilized for heavy menstrual bleeding in adolescent females [99, 100], congenital VWD in patients who respond to a desmopressin challenge [82], congenital platelet defect disorders in patients who respond to a desmopressin challenge [82], circumcision in combined factor V and VIII deficiency [101], tonsillectomy and adenoidectomy [83], and otologic surgery [83]. Studies have shown that desmopressin administered as a one-time dose after cardiopulmonary bypass failed to reduce blood loss after cardiovascular surgery in pediatrics [102–104]. Of note, children under two years of age tend to have a lower response to desmopressin in comparison to older children [83].
Adverse Effects and Monitoring Parameters: Desmopressin may cause flushing, hypo- or hypertension, headache, fatigue, hyponatremia, abdominal pain, abnormal lacrimation (intranasal formulation), conjunctivitis (intranasal formulation), and ocular edema (intranasal formulation) (Tables 34.6 and 34.7). Monitoring parameters include fluid intake, urine volume, and signs/symptoms of hyponatremia [83]. Young children may be at an increased risk for hyponatremia-induced seizures when the intravenous formulation is utilized; fluid restriction and careful monitoring of serum sodium levels and urine output are warranted [105–109]. Due to the risk of hyponatremia, some institutions adopt sodium limits associated with desmopressin administration, i.e., avoid desmopressin if sodium is less than 130 meq/L. Tachyphylaxis can occur with consecutive dosing of desmopressin; thus, it is recommended to use specific factor concentrates or platelet transfusions, depending on the underlying disease, if hemostasis after major trauma or surgery is desired [110].
Antihemophilic Factor/von Willebrand Factor Complex (Human)
Brand Name (USA): Humate-P™
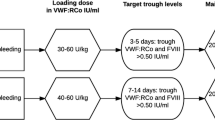
Description: Humate-P™ is a factor product that is derived from human plasma and contains FVIII, VWF, and small amounts of fibrinogen and albumin [111]. Its use is primarily to replace endogenous factor VIII and VWF in patients with hemophilia A or VWD. Factor VIII in conjunction with activated factor IX activates factor X which converts prothrombin to thrombin and fibrinogen to fibrin. VWF promotes platelet aggregation and adhesion to damaged vasculature and acts as a carrier protein for factor VIII. Circulating levels of functional VWF are measured as ristocetin cofactor (VWF:RCo) activity. The average ratio of VWF:RCo to FVIII in Humate-P™ is 2.4:1, which is more similar to the ratio in normal human plasma in comparison to other VWF/FVIII products. The elimination hale-life of VWF:RCo in Humate-P™ has a wide range of 3–34 h in patients with VWD. Humate-p™ is available as an intravenous powder for reconstitution [112].
Adult Use: Humate-P™ is utilized in adult patients for the prevention and treatment of bleeding episodes in patients with hemophilia A [111]); treatment of spontaneous or trauma-induced bleeding and prevention of excessive bleeding during and after surgery in patients with severe VWD [111, 113, 114], including mild or moderate disease where use of desmopressin is known or suspected to be inadequate [111]); reduction of postpartum blood loss in VWD type 3 patients [115]; and development of acquired von Willebrand disease after ventricular assist device implantation [112]. When used for surgical prophylaxis, target levels of VWF:RCo should be approximately 100 units/dL and, at least for the first 3 days of treatment, a nadir of 50 units/dL VWF:RCo, as well as similar targets for FVIII [83]. Humate-P™ administered as a continuous infusion has also been reported to be successful for surgical prophylaxis [83].
Pediatric Use: Humate-P™ is utilized in pediatric patients with VWD undergoing surgery, bleeding events in VWD [116, 117], prophylaxis in VWD [116], and valproate-associated von Willebrand syndrome [117].
Adverse Effects and Monitoring Parameters: Adverse effects of Humate-P™ include antibody formation, hypersensitivity, thrombotic events, rash, dizziness, headache, and nausea/vomiting (Table 34.8). Monitoring parameters include heart rate, blood pressure, AHF levels prior to and during treatment, inhibitor development, hematocrit, signs/symptoms of intravascular hemolysis, bleeding, and VWF activity. In surgical patients, monitor VWF:RCo at baseline and after surgery and trough VWF:RCo and FVIII:C daily. Humate-P™ can also transmit infections since it’s derived from human plasma [ 111].
Antihemophilic Factor/von Willebrand Factor Complex (Human)
Brand Name (USA): Alphanate™
Description: Alphanate™ is derived from human plasma and contains FVIII, VWF, and other plasma proteins. Alphanate™ is used to replace endogenous factor VIII and VWF. The average ratio of VWF:RCo to FVIII in Alphanate™is not provided by the manufacturer but is approximately 1:1. Of note, Alphanate™ has less VWF per unit when compared with Humate-P™. The elimination half-life range for Alphanate™ is the same as Humate-P™ (3–34 h). Alphanate™ is available as an intravenous powder for reconstitution [83].
Adult Use: Alphanate™ is utilized for the prevention and treatment of hemorrhagic episodes in patients with hemophilia A [118] and prophylaxis with surgical and/or invasive procedures in patients with VWD when desmopressin is either ineffective or contraindicated [118, 119]. Alphanate™ is not indicated for surgical prophylaxis in patients with severe VWD, type 3 [83, 118].
Pediatric Use: Alphanate™ is used in pediatric patients with VWD for the treatment of bleeding episodes [120] and prophylaxis prior to surgery [118, 120].
Adverse Effects and Monitoring Parameters: Alphanate™ can cause antibody formation, hypersensitivity, thrombotic evens, rash, face edema, headache, dizziness, and nausea (Table 34.9). Alphanate™ can also cause transmission of infections since it is derived from human plasma. Monitoring parameters are the same as those listed for Humate-P™.
Phytonadione (Vitamin K)
Brand Name (USA): Mephyton™
Description: Phytonadione is a vitamin that is necessary for the liver to synthesize factors II, VII, IX, and X. These vitamin-K dependent coagulation factors are γ-carboxylated by the action of vitamin K and glutamyl carboxylase. Phytonadione is available as an intravenous aqueous colloidal solution and an oral tablet [121].
Adult Use: Phytonadione is used in the prevention and treatment of hypoprothrombinemia caused by vitamin K antagonist (VKA)-induced or other drug-induced vitamin K deficiency [121], hypoprothrombinemia due to drugs or factors limiting absorption or synthesis [121], vitamin K deficiency secondary to VKA [122–124], and preprocedural/surgical INR normalization in patients receiving warfarin [122, 125].
Pediatric Use: Phytonadione is used in pediatric patients to treat vitamin K deficiency secondary to vitamin K antagonist administration [121] and bleeding in patients with chronic cholestasis [126].
Adverse Effects and Monitoring Parameters: Phytonadione can cause hypersensitivity reactions, flushing, dizziness, and abnormal taste (Table 34.10). PT, INR, and hypersensitive reactions should be monitored following phytonadione administration [ 121].
Four-Factor Prothrombin Complex Concentrate (Human)
Brand Name (USA): Kcentra™
Description: Kcentra™ is derived from human plasma and contains factors II, VII, IX, and X proteins C and S. Coagulation factors II, IX, and X are part of the intrinsic coagulation pathway, while factor VII is part of the extrinsic coagulation pathway. Ultimately, these factors facilitate the activation of prothrombin into thrombin which converts fibrinogen into fibrin resulting in clot formation. Proteins C and S are vitamin K-dependent inhibiting enzymes involved in regulating the coagulation process. Protein S serves as a cofactor for protein C, which is converted to activated protein C (APC). APC is a serine protease that inactivates factors Va and VIIIa, limiting thrombotic formation. The elimination half-life of this product is dependent on the half-life of its individual components: factor II, 48–60 h; factor VII, 1.5–6 h; factor IX, 20–24 h; factor X, 24–48 h; protein C, 1.5–6 h; and protein S, 24–48 h. Kcentra™ is available as an intravenous powder for reconstitution [127]. Three-factor prothrombin complex concentrates (Bebulin™ and Profilnine™) differ from Kcentra in that they do not contain factor VII but only contain factors II, IX, and X (see Tables 34.12 and 34.13) [128–130].
Adult Use: Kcentra™ is indicated for VKA reversal in patients with acute major bleeding or need for an urgent surgery/invasive procedure [127]. Reports have also shown Kcentra™ to be effective in the reversal of direct factor Xa anticoagulants [131, 132], an alternative agent to fresh frozen plasma (FFP) in patients with serious/life-threatening bleeding related to vitamin K antagonist therapy [133], and in acquired, non-warfarin-related coagulopathy in major trauma and surgery [134–137].
Pediatric Use: Data supporting four-factor prothrombin complex concentrate use in pediatric patients is limited to case reports or case series. These data show prothrombin complex concentrate can be used for prophylaxis in patients with severe congenital factor X deficiency [138, 139] and in dilutional coagulopathy [140]. Prothrombin complex concentrate may also be useful in pediatric patients with limited total blood volume and high risk of volume overload; however, there have been no formal studies validating this.
Adverse Effects and Monitoring Parameters: Kcentra™ can cause hypersensitivity reactions, hypercoagulopathy, hypo- or hypertension, tachycardia, headache, and nausea/vomiting (Tables 34.11, 34.12, and 34.13). Infection can also be transmitted since Kcentra™ is derived from human plasma. The INR should be monitored at baseline and at 30 min post dose, and a patient’s clinical response should be monitored during and after treatment [127].
Thrombin Powder
Brand Name (USA): Recothrom™
Description: Thrombin is a to pical product that is made through recombinant DNA technology. Thrombin activates platelets and catalyzes the conversion of fibrinogen to fibrin to promote hemostasis. Thrombin is available as a topical powder for recon struction, topical pad, topical solution, and topical sponge [141].
Adult Use: Thrombin is utilized for hemostasis [141]; control of localized, accessible bleeding from lacerated tissues [34]; control of bleeding after dental extractions or at surgical sites [34]; and reduction of blood loss in total knee arthroplasty [142].
Pediatric Use: Thrombin powder has been studied in pediatric patients and is approved to aid in hemostasis, specifically in burn patients [141].
Adverse Effects and Monitoring Parameters: Patients who receive thrombin powder should be monitored for abnormal hemostasis (Table 34.14). Thrombin powder may also cause pruritus. Of note, this product is for topical use only [141].
Protamine Sulfate
Brand Name (USA): Not applicable
Description: Protamine is a strongly alkaline substance and is derived from the sperm of salmon and other fish species. When protamine is administered alone, it has anticoagulant effects. However, when protamine is administered in the presence of heparin, a strong acidic medication, a stable salt is formed, and the anticoagulant activity of both medications is lost. Protamine has a very rapid onset of action (5 min), and the elimination half-life is approximately 7 min. However, when protamine is administered, it neutralizes the heparin; therefore, subsequent doses are not usually required. Protamine is available as an intravenous solution [143].
Adult Use: Protamine is utilized in adults for the reversal of heparin and low molecular weight heparins [143, 144]. When heparin is given as a continuous IV infusion, only heparin given in the preceding several hours should be considered when administering protamine [145]. Protamine can also be utilized for low molecular weight heparin (LMWH) overdose, but the anti-Xa activity is never completely neutralized [146–148]. Protamine is also used to neutralize heparin in patients previously on cardiopulmonary bypass, the most effective dosing being individualized management [149], and to reduce bleeding complications after carotid endarterectomy [150].
Pediatric Use: Protamine is utilized in the pediatric patient to reverse heparin and low molecular weight heparin, to neutralize heparin from combined estimated blood volume of the patient and cardiopulmonary bypass circuit [151, 152], and to treat severe post-reperfusion coagulopathy in liver transplant patients [153].
Adverse Effects and Monitoring Parameters: Severe hypotension can occur with rapid administration of protamine; thus, protamine should be administered over at least a 10-min period (Table 34.15). Transient hypotension can still be expected within 3–4 min after administration [154]. There is also a risk for anaphylaxis with protamine administration secondary to histamine release, which has been reported mainly during cardiac surgeries [155]. Since protamine has weak anticoagulant activity, due to an interaction with platelets and proteins including fibrinogen, protamine overdose can cause bleeding. This effect should be distinguished from the rebound anticoagulation that may occur 30 min to 18 h following the reversal of heparin with protamine [143].
Anti-inhibitor Coagulant Complex (Human)
Brand Name (USA): Feiba NF™
Description: Anti-inhibitor coagulant complex is a human plasma-derived factor product and contains nonactivated factors II, IX, and X and activated factor VII. Anti-inhibitor coagulant complex also contains factor VIII bypassing activity at approximately equal unitages to the other factors and 1–6 units of factor VIII coagulant antigen per milliliter. Anti-inhibitor coagulant complex shortens the activated partial thromboplastin time of plasma containing factor VIII inhibitor. Strengths are expressed in terms of factor VIII inhibitor bypassing activity, and one unit of activity is defined as the amount of anti-inhibitor coagulant complex that shortens the PTT of a high-titer factor VIII inhibitor reference plasma to 50 % of the blank value. The elimination half-life of anti-inhibitor coagulant complex is approximately 4–7 h. Anti-inhibitor coagulant complex is available as an intravenous powder for reconstitution [156].
Adult Use: Anti-inhibitor coagulant complex is utilized in adults for control and prevention of bleeding episodes in hemophilia patients with inhibitors [156] and moderate to severe bleeding in patients with acquired hemophilia [157, 158]. Anti-inhibitor coagulant complex is also used for perioperative management in hemophilia patients with inhibitors, life-threatening bleeding associated with dabigatran use [159–165], and life-threatening bleeding associated with rivaroxaban use [166]; reversal of warfarin-related bleeding [167]; and management of refractory bleeding in cardiac surgery [168].
Pediatric Use: Anti-inhibitor coagulant complex is utilized in pediatrics for control and prevention of bleeding episodes in hemophilia patients with inhibitors [156], prevention of bleeding episodes in factor X deficiency [169], and treatment of hemothorax in children with congenital coagulopathy [170].
Adverse Effects and Monitoring Parameters: Thrombotic and thromboembolic events can occur following anti-inhibitor coagulant complex use, especially with doses ≥ 100 units/kg (Table 34.16). Therefore, caution is advised in patients with atherosclerotic disease, crush injury, septicemia, or concomitant treatment with factor VIIa or antifibrinolytics due to increased risk of developing thrombotic events from circulating tissue factor or predisposing coagulopathy. Infection can also be transmitted since anti-inhibitor coagulant complex is derived from human plasma. Monitoring parameters include signs of symptoms of DIC, hemoglobin, and hematocrit. Of note, PTT and thromboelastography (TEG) should not be utilized to monitor response; DIC can occur when practitioners attempt to normalize these values with anti-inhibitor coagulant complex [156].
References
Cyklokapron [package insert]. Pharmacia & Upjohn Co., New York, NY; 2013
Lysteda [package insert]. Ferring Pharmaceutical Inc., Parsippany, NJ; October 2013
Pasi KJ, Collins PW, Keeling DM, Brown SA, Cumming AM, Dolan GC, et al. Management of von Willebrand disease: a guideline from the UK Haemophilia Centre Doctors' Organization. Haemophilia. 2004;10(3):218–31.
Gungorduk K, Yildirim G, Asicioglu O, Gungorduk OC, Sudolmus S, Ark C. Efficacy of intravenous tranexamic acid in reducing blood loss after elective cesarean section: a prospective, randomized, double-blind, placebo-controlled study. Am J Perinatol. 2011;28(3):233–40.
Zufferey PJ, Miquet M, Quenet S, Martin P, Adam P, Albaladejo P, et al. Tranexamic acid in hip fracture surgery: a randomized controlled trial. Br J Anaesth. 2010;104(1):23–30.
Choi WS, Irwin MG, Samman N. The effect of tranexamic acid on blood loss during orthognathic surgery: a randomized controlled trial. J Oral Maxillofac Surg. 2009;67(1):125–33.
Carter G, Goss A. Tranexamic acid mouthwash--a prospective randomized study of a 2-day regimen vs 5-day regimen to prevent postoperative bleeding in anticoagulated patients requiring dental extractions. Int J Oral Maxillofac Surg. 2003;32(5):504–7.
Fergusson DA, Hebert PC, Mazer CD, Fremes S, MacAdams C, Murkin JM, et al. A comparison of aprotinin and lysine analogues in high-risk cardiac surgery. N Engl J Med. 2008;358(22):2319–31.
Nuttall GA, Gutierrez MC, Dewey JD, Johnson ME, Oyen LJ, Hanson AC, et al. A preliminary study of a new tranexamic acid dosing schedule for cardiac surgery. J Cardiothorac Vasc Anesth. 2008;22(2):230–5.
Elwatidy S, Jamjoom Z, Elgamal E, Zakaria A, Turkistani A, El-Dawlatly A. Efficacy and safety of prophylactic large dose of tranexamic acid in spine surgery: a prospective, randomized, double-blind, placebo-controlled study. Spine. 2008;33(24):2577–80.
Wong J, El Beheiry H, Rampersaud YR, Lewis S, Ahn H, De Silva Y, et al. Tranexamic Acid reduces perioperative blood loss in adult patients having spinal fusion surgery. Anesth Analg. 2008;107(5):1479–86.
Gandhi R, Evans HM, Mahomed SR, Mahomed NN. Tranexamic acid and the reduction of blood loss in total knee and hip arthroplasty: a meta-analysis. BMC Res Notes. 2013;6:184.
Oremus K, Sostaric S, Trkulja V, Haspl M. Influence of tranexamic acid on postoperative autologous blood retransfusion in primary total hip and knee arthroplasty: a randomized controlled trial. Transfusion. 2014;54(1):31–41.
Dhillon MS, Bali K, Prabhakar S. Tranexamic acid for control of blood loss in bilateral total knee replacement in a single stage. Indian J Orthop. 2011;45(2):148–52.
Kelley TC, Tucker KK, Adams MJ, Dalury DF. Use of tranexamic acid results in decreased blood loss and decreased transfusions in patients undergoing staged bilateral total knee arthroplasty. Transfusion. 2014;54(1):26–30.
Camarasa MA, Olle G, Serra-Prat M, Martin A, Sanchez M, Ricos P, et al. Efficacy of aminocaproic, tranexamic acids in the control of bleeding during total knee replacement: a randomized clinical trial. Br J Anaesth. 2006;96(5):576–82.
Lozano M, Basora M, Peidro L, Merino I, Segur JM, Pereira A, et al. Effectiveness and safety of tranexamic acid administration during total knee arthroplasty. Vox Sang. 2008;95(1):39–44.
Alvarez JC, Santiveri FX, Ramos I, Vela E, Puig L, Escolano F. Tranexamic acid reduces blood transfusion in total knee arthroplasty even when a blood conservation program is applied. Transfusion. 2008;48(3):519–25.
Shakur H, Roberts I, Bautista R, Caballero J, Coats T, Dewan Y, et al. Effects of tranexamic acid on death, vascular occlusive events, and blood transfusion in trauma patients with significant haemorrhage (CRASH-2): a randomised, placebo-controlled trial. Lancet. 2010;376(9734):23–32.
Keijzer R, Wilschut DE, Houmes RJ, van de Ven KP, van den Hout L, Sluijter I, et al. Congenital diaphragmatic hernia: to repair on or off extracorporeal membrane oxygenation? J Pediatr Surg. 2012;47(4):631–6.
van der Staak FH, de Haan AF, Geven WB, Festen C. Surgical repair of congenital diaphragmatic hernia during extracorporeal membrane oxygenation: hemorrhagic complications and the effect of tranexamic acid. J Pediatr Surg. 1997;32(4):594–9.
Graham EM, Atz AM, Gillis J, Desantis SM, Haney AL, Deardorff RL, et al. Differential effects of aprotinin and tranexamic acid on outcomes and cytokine profiles in neonates undergoing cardiac surgery. J Thorac Cardiovasc Surg. 2012;143(5):1069–76.
Schindler E, Photiadis J, Sinzobahamvya N, Dores A, Asfour B, Hraska V. Tranexamic acid: an alternative to aprotinin as antifibrinolytic therapy in pediatric congenital heart surgery. Eur J Cardiothorac Surg. 2011;39(4):495–9.
Grassin-Delyle S, Couturier R, Abe E, Alvarez JC, Devillier P, Urien S. A practical tranexamic acid dosing scheme based on population pharmacokinetics in children undergoing cardiac surgery. Anesthesiology. 2013;118(4):853–62.
Peisker A, Raschke GF, Schultze-Mosgau S. Management of dental extraction in patients with Haemophilia A and B: a report of 58 extractions. Med Oral Patol Oral Cir Bucal. 2014;19(1):e55–60.
Wong LT, Lillquist YP, Culham G, DeJong BP, Davidson AG. Treatment of recurrent hemoptysis in a child with cystic fibrosis by repeated bronchial artery embolizations and long-term tranexamic acid. Pediatr Pulmonol. 1996;22(4):275–9.
Chang AB, Ditchfield M, Robinson PJ, Robertson CF. Major hemoptysis in a child with cystic fibrosis from multiple aberrant bronchial arteries treated with tranexamic acid. Pediatr Pulmonol. 1996;22(6):416–20.
Hurley M, Bhatt J, Smyth A. Treatment massive haemoptysis in cystic fibrosis with tranexamic acid. J R Soc Med. 2011;104 Suppl 1:S49–52.
Grant JA, Howard J, Luntley J, Harder J, Aleissa S, Parsons D. Perioperative blood transfusion requirements in pediatric scoliosis surgery: the efficacy of tranexamic acid. J Pediatr Orthop. 2009;29(3):300–4.
Neilipovitz DT, Murto K, Hall L, Barrowman NJ, Splinter WM. A randomized trial of tranexamic acid to reduce blood transfusion for scoliosis surgery. Anesth Analg. 2001;93(1):82–7.
Dadure C, Sauter M, Bringuier S, Bigorre M, Raux O, Rochette A, et al. Intraoperative tranexamic acid reduces blood transfusion in children undergoing craniosynostosis surgery: a randomized double-blind study. Anesthesiology. 2011;114(4):856–61.
Goobie SM, Meier PM, Pereira LM, McGowan FX, Prescilla RP, Scharp LA, et al. Efficacy of tranexamic acid in pediatric craniosynostosis surgery: a double-blind, placebo-controlled trial. Anesthesiology. 2011;114(4):862–71.
Tranexamic acid [package insert]. Pfizer, New York, NY; December 2014
Poon MC, Card R. Hemophilia management in transfusion medicine. Transfus Apher Sci. 2012;46(3):299–307.
Aminocaproic acid [package insert]. Regent, Inc., Shirley, NY; November 2005
Gravlee GPSB. Pharmacologic prophylaxis for post-cardiopulmonary bypass bleeding. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2008.
Bartholomew JR, Salgia R, Bell WR. Control of bleeding in patients with immune and nonimmune thrombocytopenia with aminocaproic acid. Arch Intern Med. 1989;149(9):1959–61.
Gardner FH, Helmer 3rd RE. Aminocaproic acid. Use in control of hemorrhage in patients with amegakaryocytic thrombocytopenia. J Am Med Assoc. 1980;243(1):35–7.
Mannucci PM. Hemostatic drugs. N Engl J Med. 1998;339(4):245–53.
Souto JC, Oliver A, Zuazu-Jausoro I, Vives A, Fontcuberta J. Oral surgery in anticoagulated patients without reducing the dose of oral anticoagulant: a prospective randomized study. J Oral Maxillofac Surg. 1996;54(1):27–32.
Vander Salm TJ, Kaur S, Lancey RA, Okike ON, Pezzella AT, Stahl RF, et al. Reduction of bleeding after heart operations through the prophylactic use of epsilon-aminocaproic acid. J Thorac Cardiovasc Surg. 1996;112(4):1098–107.
Florentino-Pineda I, Blakemore LC, Thompson GH, Poe-Kochert C, Adler P, Tripi P. The effect of epsilon-aminocaproic acid on perioperative blood loss in patients with idiopathic scoliosis undergoing posterior spinal fusion: a preliminary prospective study. Spine. 2001;26(10):1147–51.
Chauhan S, Das SN, Bisoi A, Kale S, Kiran U. Comparison of epsilon aminocaproic acid and tranexamic acid in pediatric cardiac surgery. J Cardiothorac Vasc Anesth. 2004;18(2):141–3.
Florentino-Pineda I, Thompson GH, Poe-Kochert C, Huang RP, Haber LL, Blakemore LC. The effect of amicar on perioperative blood loss in idiopathic scoliosis: the results of a prospective, randomized double-blind study. Spine. 2004;29(3):233–8.
Downard CD, Betit P, Chang RW, Garza JJ, Arnold JH, Wilson JM. Impact of AMICAR on hemorrhagic complications of ECMO: a ten-year review. J Pediatr Surg. 2003;38(8):1212–6.
Horwitz JR, Cofer BR, Warner BW, Cheu HW, Lally KP. A multicenter trial of 6-aminocaproic acid (Amicar) in the prevention of bleeding in infants on ECMO. J Pediatr Surg. 1998;33(11):1610–3.
Wilson JM, Bower LK, Fackler JC, Beals DA, Bergus BO, Kevy SV. Aminocaproic acid decreases the incidence of intracranial hemorrhage and other hemorrhagic complications of ECMO. J Pediatr Surg. 1993;28(4):536–40.
RiaSTAP [package insert]. CSL Behring, Kankakee, IL; January 2009
Levy JH, Goodnough LT. How I use fibrinogen replacement therapy in acquired bleeding. Blood. 2015;125(9):1387–93.
Brenni M, Worn M, Bruesch M, Spahn DR, Ganter MT. Successful rotational thromboelastometry-guided treatment of traumatic haemorrhage, hyperfibrinolysis and coagulopathy. Acta Anaesthesiol Scand Suppl. 2010;54(1):111–7.
Schochl H, Forster L, Woidke R, Solomon C, Voelckel W. Use of rotation thromboelastometry (ROTEM) to achieve successful treatment of polytrauma with fibrinogen concentrate and prothrombin complex concentrate. Anaesthesia. 2010;65(2):199–203.
Wafaisade A, Lefering R, Maegele M, Brockamp T, Mutschler M, Lendemans S, et al. Administration of fibrinogen concentrate in exsanguinating trauma patients is associated with improved survival at 6 hours but not at discharge. J Trauma Acute Care Surg. 2013;74(2):387–93.
Schochl H, Nienaber U, Maegele M, Hochleitner G, Primavesi F, Steitz B, et al. Transfusion in trauma: thromboelastometry-guided coagulation factor concentrate-based therapy versus standard fresh frozen plasma-based therapy. Crit Care. 2011;15(2):R83.
Rahe-Meyer N, Solomon C, Hanke A, Schmidt DS, Knoerzer D, Hochleitner G, et al. Effects of fibrinogen concentrate as first-line therapy during major aortic replacement surgery: a randomized, placebo-controlled trial. Anesthesiology. 2013;118(1):40–50.
Karlsson M, Ternstrom L, Hyllner M, Baghaei F, Skrtic S, Jeppsson A. Prophylactic fibrinogen infusion in cardiac surgery patients: effects on biomarkers of coagulation, fibrinolysis, and platelet function. Clin Appl Thromb Hemost. 2011;17(4):396–404.
Wikkelsoe AJ, Afshari A, Stensballe J, Langhoff-Roos J, Albrechtsen C, Ekelund K, et al. The FIB-PPH trial: fibrinogen concentrate as initial treatment for postpartum haemorrhage: study protocol for a randomised controlled trial. Trials. 2012;13:110.
Mittermayr M, Streif W, Haas T, Fries D, Velik-Salchner C, Klingler A, et al. Hemostatic changes after crystalloid or colloid fluid administration during major orthopedic surgery: the role of fibrinogen administration. Anesth Analg. 2007;105(4):905–17.
Acharya SS, Dimichele DM. Rare inherited disorders of fibrinogen. Haemophilia. 2008;14(6):1151–8.
Kreuz W, Meili E, Peter-Salonen K, Haertel S, Devay J, Krzensk U, et al. Efficacy and tolerability of a pasteurised human fibrinogen concentrate in patients with congenital fibrinogen deficiency. Transfus Apher Sci. 2005;32(3):247–53.
Haas T, Fries D, Velik-Salchner C, Oswald E, Innerhofer P. Fibrinogen in craniosynostosis surgery. Anesth Analg. 2008;106(3):725–31.
NovoSeven RT [package insert]. Novo Nordisk, Plainsboro, NJ; July 2014
Morgenstern LB, Hemphill 3rd JC, Anderson C, Becker K, Broderick JP, Connolly Jr ES, et al. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2010;41(9):2108–29.
Mallarkey G, Brighton T, Thomson A, Kaye K, Seale P, Gazarian M. An evaluation of eptacog alfa in nonhaemophiliac conditions. Drugs. 2008;68(12):1665–89.
Chapman AJ, Blount AL, Davis AT, Hooker RL. Recombinant factor VIIa (NovoSeven RT) use in high risk cardiac surgery. Eur J Cardiothorac Surg. 2011;40(6):1314–8.
Ferraris VA, Brown JR, Despotis GJ, Hammon JW, Reece TB, Saha SP, et al. 2011 update to the Society of Thoracic Surgeons and the Society of Cardiovascular Anesthesiologists blood conservation clinical practice guidelines. Ann Thorac Surg. 2011;91(3):944–82.
Karkouti K, Beattie WS, Crowther MA, Callum JL, Chun R, Fremes SE, et al. The role of recombinant factor VIIa in on-pump cardiac surgery: proceedings of the Canadian Consensus Conference. Can J Anaesth. 2007;54(7):573–82.
Bruckner BA, DiBardino DJ, Ning Q, Adeboygeun A, Mahmoud K, Valdes J, et al. High incidence of thromboembolic events in left ventricular assist device patients treated with recombinant activated factor VII. J Heart Lung Transplant. 2009;28(8):785–90.
Ilyas C, Beyer GM, Dutton RP, Scalea TM, Hess JR. Recombinant factor VIIa for warfarin-associated intracranial bleeding. J Clin Anesth. 2008;20(4):276–9.
Rowe AS, Turner RM. Coagulation factor VIIa (recombinant) for warfarin-induced intracranial hemorrhage. Am J Health Syst Pharm. 2010;67(5):361–5.
Pinner NA, Hurdle AC, Oliphant C, Reaves A, Lobo B, Sills A. Treatment of warfarin-related intracranial hemorrhage: a comparison of prothrombin complex concentrate and recombinant activated factor VII. World Neurosurg. 2010;74(6):631–5.
Payani N, Foroughi M, Dabbagh A. The effect of intravenous administration of active recombinant factor VII on postoperative bleeding in cardiac valve reoperations; a randomized clinical trial. Anesth Pain Med. 2015;5(1), e22846.
Yuan Q, Wu X, Du ZY, Sun YR, Yu J, Li ZQ, et al. Low-dose recombinant factor VIIa for reversing coagulopathy in patients with isolated traumatic brain injury. J Crit Care. 2015;30(1):116–20.
Bain J, Lewis D, Bernard A, Hatton K, Reda H, Flynn J. Implementation of an off-label recombinant factor VIIa protocol for patients with critical bleeding at an academic medical center. J Thromb Thrombolysis. 2014;38(4):447–52.
Yao D, Li Y, Wang J, Yu W, Li N, Li J. Effects of recombinant activated factor VIIa on abdominal trauma patients. Blood Coagul Fibrinolysis. 2014;25(1):33–8.
McQuilten ZK, Barnes C, Zatta A, Phillips LE. Off-label use of recombinant factor VIIa in pediatric patients. Pediatrics. 2012;129(6):e1533–40.
Ekert H, Brizard C, Eyers R, Cochrane A, Henning R. Elective administration in infants of low-dose recombinant activated factor VII (rFVIIa) in cardiopulmonary bypass surgery for congenital heart disease does not shorten time to chest closure or reduce blood loss and need for transfusions: a randomized, double-blind, parallel group, placebo-controlled study of rFVIIa and standard haemostatic replacement therapy versus standard haemostatic replacement therapy. Blood Coagul Fibrinolysis. 2006;17(5):389–95.
Greisen G, Andreasen RB. Recombinant factor VIIa in preterm neonates with prolonged prothrombin time. Blood Coagul Fibrinolysis. 2003;14(1):117–20.
Corrales-Medina VFSW. Viral and rickettsial infections. In: McPhee SJPM, editor. Current medical diagnosis and treatment. 48th ed. New York: McGraw-Hill; 2009.
Pettersson M, Fischler B, Petrini P, Schulman S, Nemeth A. Recombinant FVIIa in children with liver disease. Thromb Res. 2005;116(3):185–97.
Brown JB, Emerick KM, Brown DL, Whitington PF, Alonso EM. Recombinant factor VIIa improves coagulopathy caused by liver failure. J Pediatr Gastroenterol Nutr. 2003;37(3):268–72.
Jen H, Shew S. Recombinant activated factor VII use in critically ill infants with active hemorrhage. J Pediatr Surg. 2008;43(12):2235–8.
Hanebutt FL, Rolf N, Loesel A, Kuhlisch E, Siegert G, Knoefler R. Evaluation of desmopressin effects on haemostasis in children with congenital bleeding disorders. Haemophilia. 2008;14(3):524–30.
Nichols WL, Hultin MB, James AH, Manco-Johnson MJ, Montgomery RR, Ortel TL, et al. von Willebrand disease (VWD): evidence-based diagnosis and management guidelines, the National Heart, Lung, and Blood Institute (NHLBI) Expert Panel report (USA). Haemophilia. 2008;14(2):171–232.
Franchini M, Lippi G. The use of desmopressin in acquired haemophilia A: a systematic review. Blood Transfus. 2011;9(4):377–82.
Mannucci PM. Desmopressin (DDAVP) in the treatment of bleeding disorders: the first 20 years. Blood. 1997;90(7):2515–21.
Kaufmann JE, Vischer UM. Cellular mechanisms of the hemostatic effects of desmopressin (DDAVP). J Thromb Haemost. 2003;1(4):682–9.
Svensson PJ, Bergqvist PB, Juul KV, Berntorp E. Desmopressin in treatment of haematological disorders and in prevention of surgical bleeding. Blood Rev. 2014;28(3):95–102.
Desmopressin [package insert]. Ferring Pharmaceuticals Inc., Parsippany, NJ; April 2008
Stimate [package insert]. CSL Behring, King of Prussia, PA; December 2010
Watson AJ, Keogh JA. 1-Deamino-8-d-arginine vasopressin (DDAVP): a potential new treatment for the bleeding diathesis of acute renal failure. Pharmatherapeutica. 1984;3(9):618–22.
Mannucci PM, Remuzzi G, Pusineri F, Lombardi R, Valsecchi C, Mecca G, et al. Deamino-8-d-arginine vasopressin shortens the bleeding time in uremia. N Engl J Med. 1983;308(1):8–12.
Kapapa T, Rohrer S, Struve S, Petscher M, Konig R, Wirtz CR, et al. Desmopressin acetate in intracranial haemorrhage. Neurol Res Int. 2014;2014:298767.
Besser MW, Ortmann E, Klein AA. Haemostatic management of cardiac surgical haemorrhage. Anaesthesia. 2015;70 Suppl 1:87–95. e29–e31.
Salzman EW, Weinstein MJ, Weintraub RM, Ware JA, Thurer RL, Robertson L, et al. Treatment with desmopressin acetate to reduce blood loss after cardiac surgery. A double-blind randomized trial. N Engl J Med. 1986;314(22):1402–6.
Mannucci PM, Vicente V, Vianello L, Cattaneo M, Alberca I, Coccato MP, et al. Controlled trial of desmopressin in liver cirrhosis and other conditions associated with a prolonged bleeding time. Blood. 1986;67(4):1148–53.
Agnelli G, Parise P, Levi M, Cosmi B, Nenci GG. Effects of desmopressin on hemostasis in patients with liver cirrhosis. Haemostasis. 1995;25(5):241–7.
Lopez P, Otaso JC, Alvarez D, Rojter S, Podesta A, Albornoz L, et al. Hemostatic and hemodynamic effects of vasopressin analogue DDAVP in patients with cirrhosis. Acta Gastroenterol Lactinoam. 1997;27(2):59–62.
Burroughs AK, Matthews K, Qadiri M, Thomas N, Kernoff P, Tuddenham E, et al. Desmopressin and bleeding time in patients with cirrhosis. Br Med J (Clin Res Ed). 1985;291(6506):1377–81.
Mills HL, Abdel-Baki MS, Teruya J, Dietrich JE, Shah MD, Mahoney Jr D, et al. Platelet function defects in adolescents with heavy menstrual bleeding. Haemophilia. 2014;20(2):249–54.
Amesse LS, Pfaff-Amesse T, Leonardi R, Uddin D, French 2nd JA. Oral contraceptives and DDAVP nasal spray: patterns of use in managing vWD-associated menorrhagia: a single-institution study. J Pediatr Hematol Oncol. 2005;27(7):357–63.
Devecioglu O, Eryilmaz E, Celik D, Unuvar A, Karakas Z, Anak S, et al. Circumcision in a combined factor V and factor VIII deficiency using desmopressin (DDAVP). Turk J Pediatr. 2002;44(2):146–7.
Guay J, Rivard GE. Mediastinal bleeding after cardiopulmonary bypass in pediatric patients. Ann Thorac Surg. 1996;62(6):1955–60.
Seear MD, Wadsworth LD, Rogers PC, Sheps S, Ashmore PG. The effect of desmopressin acetate (DDAVP) on postoperative blood loss after cardiac operations in children. J Thorac Cardiovasc Surg. 1989;98(2):217–9.
Reynolds LM, Nicolson SC, Jobes DR, Steven JM, Norwood WI, McGonigle ME, et al. Desmopressin does not decrease bleeding after cardiac operation in young children. J Thorac Cardiovasc Surg. 1993;106(6):954–8.
Smith TJ, Gill JC, Ambruso DR, Hathaway WE. Hyponatremia and seizures in young children given DDAVP. Am J Hematol. 1989;31(3):199–202.
Molnar Z, Farkas V, Nemes L, Reusz GS, Szabo AJ. Hyponatraemic seizures resulting from inadequate post-operative fluid intake following a single dose of desmopressin. Nephrol Dial Transplant. 2005;20(10):2265–7.
Das P, Carcao M, Hitzler J. DDAVP-induced hyponatremia in young children. J Pediatr Hematol Oncol. 2005;27(6):330–2.
Thumfart J, Roehr CC, Kapelari K, Querfeld U, Eggert P, Muller D. Desmopressin associated symptomatic hyponatremic hypervolemia in children. Are there predictive factors? J Urol. 2005;174(1):294–8.
Weinstein RE, Bona RD, Altman AJ, Quinn JJ, Weisman SJ, Bartolomeo A, et al. Severe hyponatremia after repeated intravenous administration of desmopressin. Am J Hematol. 1989;32(4):258–61.
Ozgonenel B, Rajpurkar M, Lusher JM. How do you treat bleeding disorders with desmopressin? Postgrad Med J. 2007;83(977):159–63.
Humate-P [package insert]. CSL Behring. Kankakee, IL; October 2007
Cushing M, Kawaguchi K, Friedman KD, Mark T. Factor VIII/von Willebrand factor concentrate therapy for ventricular assist device-associated acquired von Willebrand disease. Transfusion. 2012;52(7):1535–41.
Gill JC, Ewenstein BM, Thompson AR, Mueller-Velten G, Schwartz BA. Successful treatment of urgent bleeding in von Willebrand disease with factor VIII/VWF concentrate (Humate-P): use of the ristocetin cofactor assay (VWF:RCo) to measure potency and to guide therapy. Haemophilia. 2003;9(6):688–95.
Dobrkovska A, Krzensk U, Chediak JR. Pharmacokinetics, efficacy and safety of Humate-P in von Willebrand disease. Haemophilia. 1998;4 Suppl 3:33–9.
Mannucci PM. How I treat patients with von Willebrand disease. Blood. 2001;97(7):1915–9.
Lillicrap D, Poon MC, Walker I, Xie F, Schwartz BA. Efficacy and safety of the factor VIII/von Willebrand factor concentrate, haemate-P/humate-P: ristocetin cofactor unit dosing in patients with von Willebrand disease. Thromb Haemost. 2002;87(2):224–30.
Kreuz W, Mentzer D, Becker S, Scharrer I, Kornhuber B. Haemate P in children with von Willebrand's disease. Haemostasis. 1994;24(5):304–10.
Alphanate [package insert]. Grifols Biologicals Inc., Los Angeles, CA; June 2014
Rivard GE, Aledort L. Efficacy of factor VIII/von Willebrand factor concentrate Alphanate in preventing excessive bleeding during surgery in subjects with von Willebrand disease. Haemophilia. 2008;14(2):271–5.
Mannucci PM, Chediak J, Hanna W, Byrnes J, Ledford M, Ewenstein BM, et al. Treatment of von Willebrand disease with a high-purity factor VIII/von Willebrand factor concentrate: a prospective, multicenter study. Blood. 2002;99(2):450–6.
Phytonadione [package insert]. Cardinal Health, Zanesville, OH; November 2013
Patriquin C, Crowther M. Treatment of warfarin-associated coagulopathy with vitamin K. Expert Rev Hematol. 2011;4(6):657–65.
Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuunemann HJ. Executive summary: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):7S–47S.
Hirsh J, Guyatt G, Albers GW, Harrington R, Schunemann HJ. Executive summary: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th edition). Chest. 2008;133(6 Suppl):71S–109S.
Douketis JD, Spyropoulos AC, Spencer FA, Mayr M, Jaffer AK, Eckman MH, et al. Perioperative management of antithrombotic therapy: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e326S–50S.
Glatstein M, Idan-Prusak D, Yahav A, Ovental A, Rimon A, Scolnik D. Emergency use of intravenous phytonadione (vitamin K1) for treatment of severe bleeding in a child with chronic cholestasis. Am J Ther. 2013;20(6):e733–5.
Kcentra [package insert]. CSL Behring, Kankakee, IL; March 2014
Profilnine SD [package insert]. Grifols Biologicals Inc.; Los Angeles, CA; August 2010
Bebulin [package insert]. Baxter; Westlake Village, CA; July 2012
Sorensen B, Spahn DR, Innerhofer P, Spannagl M, Rossaint R. Clinical review: prothrombin complex concentrates—evaluation of safety and thrombogenicity. Crit Care. 2011;15(1):201.
Herzog E, Kaspereit F, Krege W, Doerr B, Mueller-Cohrs J, Pragst I, et al. Effective reversal of edoxaban-associated bleeding with four-factor prothrombin complex concentrate in a rabbit model of acute hemorrhage. Anesthesiology. 2015;122(2):387–98.
Baumann Kreuziger LM, Keenan JC, Morton CT, Dries DJ. Management of the bleeding patient receiving new oral anticoagulants: a role for prothrombin complex concentrates. Biomed Res Int. 2014;2014:583794.
Patanwala AE, Acquisto NM, Erstad BL. Prothrombin complex concentrate for critical bleeding. Ann Pharmacother. 2011;45(7–8):990–9.
Schochl H, Nienaber U, Hofer G, Voelckel W, Jambor C, Scharbert G, et al. Goal-directed coagulation management of major trauma patients using thromboelastometry (ROTEM)-guided administration of fibrinogen concentrate and prothrombin complex concentrate. Crit Care. 2010;14(2):R55.
Gorlinger K, Dirkmann D, Hanke AA, Kamler M, Kottenberg E, Thielmann M, et al. First-line therapy with coagulation factor concentrates combined with point-of-care coagulation testing is associated with decreased allogeneic blood transfusion in cardiovascular surgery: a retrospective, single-center cohort study. Anesthesiology. 2011;115(6):1179–91.
Weber CF, Gorlinger K, Meininger D, Herrmann E, Bingold T, Moritz A, et al. Point-of-care testing: a prospective, randomized clinical trial of efficacy in coagulopathic cardiac surgery patients. Anesthesiology. 2012;117(3):531–47.
Tanaka KA, Mazzeffi M, Durila M. Role of prothrombin complex concentrate in perioperative coagulation therapy. J Intensive Care Med. 2014;2(1):60.
Bowles L, Baker K, Khair K, Mathias M, Liesner R. Prophylaxis with prothrombin complex concentrate in four children with severe congenital factor X deficiency. Haemophilia. 2009;15(1):401–3.
McMahon C, Smith J, Goonan C, Byrne M, Smith OP. The role of primary prophylactic factor replacement therapy in children with severe factor X deficiency. Br J Haematol. 2002;119(3):789–91.
Fuentes-Garcia D, Hernandez-Palazon J, Sansano-Sanchez T, Acosta-Villegas F. Prothrombin complex concentrate in the treatment of multitransfusion dilutional coagulopathy in a paediatric patient. Br J Anaesth. 2011;106(6):912–3.
Thrombin [package insert]. ZymoGenetics, Inc., Seattle, WA; 2008
Suarez JC, Slotkin EM, Alvarez AM, Szubski CR, Barsoum WK, Patel PD. Prospective, randomized trial to evaluate efficacy of a thrombin-based hemostatic agent in total knee arthroplasty. J Arthroplasty. 2014;29(10):1950–5.
Protamine [package insert]. Fresenius Kabi, Lake Zurich, IL; July 2013
Aren C. Heparin and protamine therapy. Semin Thorac Cardiovasc Surg. 1990;2(4):364–72.
Garcia DA, Baglin TP, Weitz JI, Samama MM. Parenteral anticoagulants: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012;141(2 Suppl):e24S–43S.
Enoxaparin [package isnert]. Sanofi-aventis U.S. llC; Bridgewater, NJ; 1993
Dalteparin [package insert]. Pfizer Inc; New York, NY; 1994
Tinzaparin [package insert]. Celgene Corporation; Boulder, CO; April 2008
Vonk AB, Veerhoek D, van den Brom CE, van Barneveld LJ, Boer C. Individualized heparin and protamine management improves rotational thromboelastometric parameters and postoperative hemostasis in valve surgery. J Cardiothorac Vasc Anesth. 2014;28(2):235–41.
Patel RB, Beaulieu P, Homa K, Goodney PP, Stanley AC, Cronenwett JL, et al. Shared quality data are associated with increased protamine use and reduced bleeding complications after carotid endarterectomy in the Vascular Study Group of New England. J Vasc Surg. 2013;58(6):1518–24. e1.
Gautam NK, Schmitz ML, Harrison D, Zabala LM, Killebrew P, Belcher RH, et al. Impact of protamine dose on activated clotting time and thromboelastography in infants and small children undergoing cardiopulmonary bypass. Paediatr Anaesth. 2013;23(3):233–41.
Gruenwald CE, Manlhiot C, Chan AK, Crawford-Lean L, Foreman C, Holtby HM, et al. Randomized, controlled trial of individualized heparin and protamine management in infants undergoing cardiac surgery with cardiopulmonary bypass. J Am Coll Cardiol. 2010;56(22):1794–802.
Gouvea G, Toledo R, Diaz R, Auler L, Enne M, Martinho JM. Protamine sulphate for treatment of severe post-reperfusion coagulopathy in pediatric liver transplantation. Pediatr Transplant. 2009;13(8):1053–7.
Muangmingsuk V, Tremback TF, Muangmingsuk S, Roberson DA, Cipparrone NE. The effect on the hemodynamic stability of varying calcium chloride administration during protamine infusion in pediatric open-heart patients. Anesth Analg. 2001;93(1):92–5.
Suryanarayan D, Schulman S. Potential antidotes for reversal of old and new oral anticoagulants. Thromb Res. 2014;133 Suppl 2:S158–66.
Feiba NH. [package insert]. Baxter Health Corporation. Westlake Village, CA; February 2011
Sallah S. Treatment of acquired haemophilia with factor eight inhibitor bypassing activity. Haemophilia. 2004;10(2):169–73.
Huth-Kuhne A, Baudo F, Collins P, Ingerslev J, Kessler CM, Levesque H, et al. International recommendations on the diagnosis and treatment of patients with acquired hemophilia A. Haematologica. 2009;94(4):566–75.
Dager WE, Gosselin RC, Roberts AJ. Reversing dabigatran in life-threatening bleeding occurring during cardiac ablation with factor eight inhibitor bypassing activity. Crit Care Med. 2013;41(5):e42–6.
Faust AC, Peterson EJ. Management of dabigatran-associated intracerebral and intraventricular hemorrhage: a case report. J Emerg Med. 2014;46(4):525–9.
Kiraly A, Lyden A, Periyanayagam U, Chan J, Pang PS. Management of hemorrhage complicated by novel oral anticoagulants in the emergency department: case report from the northwestern emergency medicine residency. Am J Ther. 2013;20(3):300–6.
Neyens R, Bohm N, Cearley M, Andrews C, Chalela J. Dabigatran-associated subdural hemorrhage: using thromboelastography (TEG((R))) to guide decision-making. J Thromb Thrombolysis. 2014;37(2):80–3.
Schulman S, Ritchie B, Goy JK, Nahirniak S, Almutawa M, Ghanny S. Activated prothrombin complex concentrate for dabigatran-associated bleeding. Br J Haematol. 2014;164(2):308–10.
Heidbuchel H, Verhamme P, Alings M, Antz M, Hacke W, Oldgren J, et al. European Heart Rhythm Association Practical Guide on the use of new oral anticoagulants in patients with non-valvular atrial fibrillation. Europace. 2013;15(5):625–51.
Weitz JI, Quinlan DJ, Eikelboom JW. Periprocedural management and approach to bleeding in patients taking dabigatran. Circulation. 2012;126(20):2428–32.
Maurice-Szamburski A, Graillon T, Bruder N. Favorable outcome after a subdural hematoma treated with feiba in a 77-year-old patient treated by rivaroxaban. J Neurosurg Anesthesiol. 2014;26(2):183.
Stewart WS, Pettit H. Experiences with an activated 4-factor prothrombin complex concentrate (FEIBA) for reversal of warfarin-related bleeding. Am J Emerg Med. 2013;31(8):1251–4.
Balsam LB, Timek TA, Pelletier MP. Factor eight inhibitor bypassing activity (FEIBA) for refractory bleeding in cardiac surgery: review of clinical outcomes. J Cardiac Surg. 2008;23(6):614–21.
Shim YJ, Won DI. Pharmacokinetics and prophylactic use of FEIBA in a child with severe congenital factor X deficiency and recurrent spontaneous intracranial haemorrhage: a case report. Haemophilia. 2013;19(6):e364–7.
Obitko-Pludowska A, Laguna P, Adamowicz-Salach A, Brzewski M, Del Campo KS. Haemothorax in children with congenital coagulopathy. Haemophilia. 2010;16(4):688–91.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Beaty, R.S. (2016). Hemostatic Agents Used to Stop Bleeding. In: Teruya, J. (eds) Management of Bleeding Patients. Springer, Cham. https://doi.org/10.1007/978-3-319-30726-8_34
Download citation
DOI: https://doi.org/10.1007/978-3-319-30726-8_34
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-30724-4
Online ISBN: 978-3-319-30726-8
eBook Packages: MedicineMedicine (R0)