Abstract
Insomnia is a common disorder characterized by difficulties initiating or maintaining sleep and is associated with lower quality of life, neuropsychiatric disorders, and increased risk of mortality. Cognitive behavioral therapy for insomnia (e.g., CBT-I) is the most common and widely researched nonpharmacological treatment available. However, a significant proportion of individuals do not respond to CBT-I or experience incomplete symptom reduction. Theoretical considerations and preliminary empirical evidence indicate that mindfulness-based cognitive therapy (MBCT) is a promising intervention for insomnia. Skills in experiential awareness, attentional control, and acceptance acquired through MBCT training can be used to target key cognitive vulnerabilities associated with chronic insomnia. Evidence from several single arm studies and one randomized controlled trial indicates that MBCT is effective for improving sleep, particularly in individuals with symptoms of depression and a history of major depressive disorder.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Insomnia
- Sleep disorders
- Mindfulness-based cognitive therapy
- MBCT
- Mindfulness-based interventions for insomnia
Clinical Case Study
Patient Presentation
Manu is a 51-year-old married man with multiple lifetime depressive episodes seeking mindfulness-based treatment for depression relapse prevention. At intake his depressive symptoms reflected partial remission from depression (Beck Depression Inventory score of 18). Manu reported being in good health overall with no chronic medical conditions apart from depression. Manu's primary goal for treatment was to "learn ways to prevent another [depressive] episode"; however, initial interviews revealed that sleep difficulties were a source of considerable distress and were inter-related with mood issues (e.g., patient reported low mood on days following poor sleep which resulted in heightened anxiety about getting enough sleep in order to avoid depressed mood).
Manu reported difficulty with sleep initiation (average sleep onset latency of 90 min) and sleep maintenance (woke 2–3 times each night). Average total sleep time was roughly 5 h but average time in bed was 9 h (consistent sleep schedule of 9:30 pm to 6:30 am). His sleep efficiency was 56 %. Manu denied use of nicotine, caffeine, and alcohol. Manu indicated that over the past several months he had reduced his overall activity levels (socializing with friends, engaging in activities with wife and children) in favor of "resting" in his bed as a response to daytime fatigue and to make up for the sleep he was not getting at night.
Treatment Course
Manu participated in the 8-week mindfulness-based cognitive therapy (MBCT) group intervention for depression relapse prevention [1]. Weekly 2.5 h sessions focused on mindfulness skills (increasing awareness of thoughts, emotions, and physical sensations, and the tendency to react with attachment or aversion to these internal experiences; and developing a nonjudgmental and accepting stance toward internal experiences) and psychoeducation about depression and relapse (e.g., the cognitive model of depression, links between sad mood, negative automatic thoughts, and maintenance of low mood). A detailed description of session content and homework assignments is available in the MBCT manual [1].
Manu had expressed an interest in mindfulness and remained engaged throughout treatment. After the first several sessions, he noted a tendency to frequently scan for signs of low mood and would engage in maladaptive secondary appraisals if any emerged—"why am I feeling down again—I’m going to be depressed—I will never get better". Manu found that these thoughts were typically associated with increased autonomic arousal—"tightness and fluttering" in his chest and stomach and noted that, at bedtime, he experienced many negative thoughts and beliefs (“I have to get 8 h to make up for last night” “I can’t function on less than 8 h”) and feelings (frustration, anxiety, hopelessness).
Manu struggled with the concept of letting go of the effort to control thoughts rather than the thoughts themselves. Like many other individuals new to mindfulness meditation , Manu held the idea that he must rid his mind of distressing thoughts and described attempts to "just cut it off". In session 6 however, Manu reported sitting with his worry thoughts and his physical sensations of anxiety and using his breath to bring his attention back whenever he "ended up downstream". Manu noted that he did not fall asleep but felt less tense and overwhelmed by needing to get to sleep. During one inquiry session, Manu expressed frustration with not falling asleep despite "doing all the right things" like abstaining from coffee and alcohol and giving himself plenty of time to sleep. The facilitator inquired about Manu's experience of sleepiness and noted that he may be confusing fatigue with sleepiness. Manu was encouraged to observe the difference between these sensations.
Treatment Outcome
Post-MBCT training, Manu reported an average total sleep time of 6.5 h with sleep efficiency >90 %. He had adjusted his time in bed to approximately 7 h after realizing that he did not need as much sleep as he had previously believed. He reported satisfaction with the amount and quality of sleep and ability to function effectively in the daytime. His BDI score was reduced to a score of 7.
Pathway for Clinical Improvement
Manu's skill development in the three key domains influenced by MBCT likely contributed to resolution of sleep difficulties. First, Manu's awareness of ruminative thought cycles led him to practice attentional control thereby supporting “decentering” from worry thoughts and distorted beliefs and resulting in reductions in negative primary and secondary (metacognitive) appraisals and physiological arousal. Practicing mindful awareness also decreased time spent on sleep-interfering behaviors like calculating time left to sleep. Finally, increased acceptance of difficult thoughts, emotions, and physical sensations allowed Manu to let go of his desire (and desperate behaviors) to make sleep happen. Each of these skills likely contributed to Manu’s overall diminished worry about the consequences of poor sleep and increased confidence in his ability to function well in the daytime with fewer hours of sleep than previously believed.
The Problem of Insomnia
Insomnia is a complex disorder of varied etiology that is characterized by the presence of an individual's report of difficulty with sleep [2]. The complexity of insomnia is due, in part, to a lack of universally defined diagnostic criteria. Hallmark symptoms common to the three different classification systems used to diagnose insomnia include difficulties in initiating sleep or in maintaining sleep [3–5]. The term “insomnia” has different meanings based on an individual’s clinical presentation. Ohayon and Reynolds [6] compare “insomnia” to “pain” whereby it can be considered a complaint (related to sleep duration or quality), a symptom (part of a sleep or other organic disorder), or a diagnosis (e.g., primary insomnia or secondary insomnia, a consequence of a primary diagnosis such as substance abuse or medical or mental illness). Prevalence rates of insomnia vary based on the degree of distinction between these domains and also the diagnostic classification system used. Results from a recent epidemiological study that differentiated the various presentations of insomnia indicate that 37 % of individuals reported sleep complaints (e.g., light or short sleep and sleep dissatisfaction), 35 % reported at least one symptom of insomnia (difficulty initiating or maintaining sleep and nonrestorative sleep at least 3 nights per week), and 7 % met diagnostic criteria for insomnia based on the DSM-IV classification [6].
Insomnia is associated with a range of debilitating outcomes including increased psychosocial and physical morbidities, financial consequences, and mortality. Decades of population-based studies demonstrate that individuals with insomnia experience significantly impaired quality of life on nearly all domains including mental, emotional, physical, and social functioning (for review, see [7]). Individuals with sleep disturbance also experience significant daytime impairment, which frequently leads to poor work performance, higher rates of absenteeism, and economic loss [8]. Finally, insomnia is causally linked with higher mortality including increased suicide [9] and preventable accidental deaths (e.g., motor vehicle accidents) [10].
Identification of the causal determinants of insomnia is difficult not only because of nonstandard diagnostic and classification systems but also because the symptom profile of insomnia overlaps considerably with other psychological and physical health conditions . Indeed, insomnia is more frequently associated with psychiatric disorders, most commonly depression, than any other disorder [11]. Consensus regarding whether insomnia is antecedent to or a result of psychiatric disorders has not been reached; however, at least one large-scale epidemiological study demonstrated that insomnia more often preceded rather than followed incident cases of a mood disorder [12]. Still, it is widely agreed that there may be bidirectional relationships between insomnia and psychiatric disorders and that these conditions share pathophysiological pathways that make individuals vulnerable to both conditions [2].
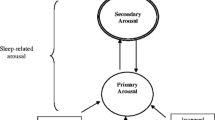
Several models have been proposed to explain the pathophysiology of insomnia (Psychobiological Inhibition Model [13]; Hyperarousal Model [14]; Cognitive Model [15]; Neurocognitive Model [16]). Common to each of these is that cognitive/affective processes play a primary role in activating the clinical complaint of insomnia [13] and that mental hyperarousal [15] or deficits in achieving mental de-arousal [13] contribute to the maintenance and chronicity of this disorder. In Fig. 3.1, we present a model of insomnia that integrates core cognitive and behavioral processes from several extant theoretical frameworks (e.g., [17, 18]). In general, insomnia is thought to be initiated and perpetuated by the following sequential cognitive and behavioral processes: (1) Excessive daytime and nighttime rumination [19]; (2) Primary arousal (i.e., initial negative appraisal about daytime consequences of poor sleep that results in distress and physiological activation)—“If I don’t sleep tonight, I’m going to fail at my job tomorrow and be fired” [15]; (3) Secondary arousal (i.e., the negative secondary or metacognitive evaluation or judgment of initial (primary) arousal, which leads to continuing distress and physiological activation)—“I hate how I’m feeling and shouldn’t be feeling this way.” [18]; (4) Excessive monitoring of and selective attention to internal (e.g., bodily sensations) and/or external (e.g., clock) sleep cues that are either consistent or inconsistent with falling asleep [15]. Hand in hand with selective attention is a dysfunctional perceived need for control and engagement in sleep effort (e.g., actively trying to sleep or increasing sleep opportunity) [20]; (5) Distorted perceptions about sleep impairment (i.e., regularly overestimating sleep loss). Misperceptions of sleep deficit frequently lead to excessive negative cognitions about sleep thus fortifying a vicious cycle of chronic insomnia.
On the basis of this integrative cognitive model of insomnia, the most successful interventions for sleep disturbance are those that target each of the key predisposing vulnerabilities, namely negative cognitions, dysfunctional cognitive and affective and behavioral regulatory strategies (e.g., suppression, selective attention, monitoring, sleep effort) and distorted perceptions about sleep.
Theoretical Rationale of MBCT for Insomnia
Cognitive behavior therapy for insomnia (CBT-I) is one of the most widely researched psychobehavioral interventions for insomnia. CBT-I has evolved as a multicomponent treatment approach and combines: (1) cognitive strategies such as thought restructuring to change dysfunctional beliefs and attitudes about sleep; and (2) behavioral techniques such as sleep restriction and stimulus control to promote healthy sleep habits [21]. Although CBT-I is considered to be an effective and first-line treatment (for review and meta-analysis, see [22, 23]), a significant proportion (19–26 %) of individuals do not benefit from CBT-I, and the average overall improvement among those who do respond is only 50–60 % [24]. This change, while statistically significant, does not represent enough clinical improvement to classify those who do respond to CBT-I as “good sleepers” [15]. Thus, additional and/or complementary treatment approaches are needed for the substantial number of CBT-I “nonresponders” and for those who have experienced only partial symptomatic relief from CBT-I.
One particularly promising treatment approach for insomnia is MBCT. MBCT was developed as a treatment to prevent depression relapse in individuals with recurrent major depressive disorder. It is based on the Mindfulness-Based Stress Reduction (MBSR) program and combines mindfulness meditation practices with cognitive therapy to help reduce depressogenic and ruminative thinking, common antecedents to major depression relapse. Several RCTs have demonstrated MBCT is effective for reducing depression relapse and depressive symptoms [25–27]. Three theoretical considerations indicate that MBCT may be a promising treatment for insomnia.
First, MBCT is believed to target experiential awareness, attentional control, and acceptance; deficits in each of these domains have been linked to poor sleep [18, 28, 29]. For instance, mindfulness-based practices , such as breath-focused meditation, body scanning, and mindful stretching, promote experiential awareness of a range of experiences including internal (e.g., thoughts, emotions, physiological sensations) and external stimuli (e.g., sights, sounds) [30]. During these exercises, participants acquire skills in attentional control by focusing attention on the breath (sustained attention) and redirecting attention to this anchor whenever one’s thoughts wander (attention inhibition) [31]. Finally, participants learn to change their relationship to their experiences by learning to accept, rather than avoid or control negatively perceived thoughts, emotions, and physical sensations. Skills in acceptance are learned by nonjudgmentally observing one’s thoughts, feelings, and physical sensations and by viewing such experiences as passing mental events, rather than facts [32].
Experiential awareness, attentional control, and acceptance, the three domains putatively improved by MBCT, collectively target each of the cognitive and behavioral vulnerabilities associated with poor sleep (see Fig. 3.1). First, increased awareness of internal and external experiences (e.g., thoughts and behaviors) should target each of the processes that contribute to the maintenance of insomnia: (1) rumination; (2) primary arousal; (3) secondary arousal; (4) sleep monitoring/selective attention and effort; and (5) distorted perceptions. Second, attentional control should enable individuals to disengage from negative thoughts and/or beliefs about sleeplessness by disrupting selective attention toward internal/external sleep-related threat cues. Thus, skills in attentional control should target processes (1)–(4) above. Finally, skills in acceptance should foster a less contentious and more flexible relationship to one’s thoughts, emotions, and sensations by promoting the ability to approach, rather than avoid, and to engage with such experiences with equanimity instead of with judgment. Therefore, acceptance should target the following processes: (3) secondary arousal; (4) sleep monitoring/selective attention and effort; and (5) distorted perceptions.
A second consideration that indicates that MBCT may be a helpful treatment for insomnia is that insomnia is frequently comorbid with mood disorders like depression [33], and extensive studies have shown MBCT is effective for preventing depression and reducing depressive symptoms [26, 27, 34]. Thus, although the relationship between depression and insomnia is likely bidirectional [35], one would expect that MBCT-related reductions in depression relapse and depressive symptoms may also lead to improvements in sleep.
Finally, the mindfulness skills imparted through MBCT have broad implications for well-being and may additionally have long-term effects that are sustained over time. For example, mindfulness can be applied to a range of daily psychological and behavioral phenomena (e.g., healthy decision making) that may either directly (i.e., via processes discussed above) or indirectly (e.g., via improvements in chronic disease symptomology) reduce insomnia. Relatedly, MBCT may have cumulative benefits that are sustained over the long term [36]. Collectively, these features of MBCT suggest that it may be a particularly promising intervention for individuals who suffer from insomnia.
Evidence of MBCT for Insomnia and Mechanisms of Change
The majority of the evidence for mindfulness-based interventions (MBIs) for insomnia comes from studies that have tested the MBSR intervention or a tailored derivative of this protocol for insomnia (e.g., mindfulness-based therapy for insomnia or MBTI: [27, 37]). A thorough review of such studies is beyond the scope of this chapter. However, compelling evidence from three recent studies demonstrates especially promising effects of MBTI on objective and subjective assessments of insomnia. MBTI combines mindfulness meditation exercises modeled after the MBSR protocol with CBT-I. In a single-arm study with participants who met diagnostic criteria for psychophysiological insomnia (a subtype of primary insomnia characterized by heightened arousal in bed) [38], MBTI was associated with improvements in self-reported total wake time (TWT), number of awakenings, time in bed, sleep efficiency, insomnia symptom severity, and pre-sleep somatic and cognitive arousal [37]. These improvements were maintained over a 12-month follow-up period [39]. Building on the limitations of single-arm trial, Ong and colleagues [40] conducted a three-arm RCT comparing MBTI versus MBSR versus a self-monitoring control condition (SM). Compared to SM, MBTI and MBSR demonstrated superior effects on self-reported TWT, pre-sleep arousal, insomnia severity, and objective measures of TWT and total sleep time (TST) using wrist actigraphy. Effects of MBTI and MBSR were maintained at a 6-month follow-up and the highest rates of improvement on the Insomnia Severity Index (ISI :[41]) and insomnia remission were found in the MBTI group.
Results from these studies demonstrate that features of MBSR can be combined with elements from the CBT-I protocol to yield a viable treatment for chronic insomnia. Effect sizes for MBTI are comparable to those found for other behavioral interventions for insomnia [42, 43], and MBTI appears to be superior to MBSR for reducing self-reported severity of insomnia symptoms over the long term. One advantage to adding a mindfulness component to the CBT-I intervention may be that MBTI presents an additional treatment option for patients who relate more to acceptance-based approaches than to cognitive restructuring. Also, the benefits of mindfulness may generalize across greater domains of healthy functioning (e.g., reductions in somatic complaints associated with other comorbid conditions) that may directly or indirectly improve sleep. Given the especially high comorbidity between insomnia and certain chronic diseases and mood disorders, developing and testing MBIs that target multiple risk factors for insomnia is critical. For example, MBCT’s effects on emotion regulation [44, 45] and reductions in rumination and mood disorder symptoms [46], which are each linked to sleep disturbance [19, 47], suggest it may be an additional treatment option for insomnia.
To date, five studies have examined the effects of the standard MBCT intervention or a modified version of the protocol for insomnia (for review, see [48]). Initial pilot testing of MBCT’s effects on reducing symptoms of primary insomnia was conducted by Heidenreich and colleagues [49]. The standardized 8-week MBCT protocol was modified with “cognitive elements tailored to patients with primary insomnia rather than a depressive disorder.” Results indicated pre–post improvements in self-reported total sleep time and several domains of thought control as well as reductions in sleep-related monitoring and worry. Ree and colleagues [50] conducted an additional single arm study in a heterogeneous group of adult outpatients with psychiatric presentations. Immediately following the MBCT intervention, self-reported insomnia severity symptom scores decreased from subthreshold insomnia to values within the normal range. Post-treatment improvements were maintained at 3-month follow-up with a large effect size. A more recent study that similarly employed a pre–post design examined the effects of MBCT on self-reported sleep disturbance among older adults with history of depression or anxiety in partial or full remission [51]. The MBCT protocol was modified slightly for the aging sample (e.g., length of seated meditations and duration of each weekly session were slightly shorter to accommodate the aging sample). Results indicated that participants experienced a 14.5 % improvement in self-reported sleep problems.
Although promising, conclusions about the efficacy of MBCT for insomnia are difficult to draw based on these studies due to the lack of a control group and objective and comprehensive subjective (e.g., sleep diaries) measures of sleep and limited reported information regarding modifications made to the MBCT protocol (i.e., [49]).
The strongest evidence for the effects of MBCT for insomnia comes from two sets of results from a randomized controlled trial conducted by Britton and colleagues [52, 53]. Individuals with recurrent depression in full or partial remission and with residual sleep complaints were randomized to either MBCT or a waitlist control condition (WLC) . Objective assessments of sleep were measured via polysomnography (PSG) , and comprehensive assessments of self-reported sleep were measured with sleep diaries at baseline and over the course of the intervention.
Their first investigation included a medication-free sample [52]; the second included individuals who were taking ADM [53]. Polysomnography data from the initial study indicated that, contrary to hypotheses, MBCT participants experienced significantly more awakenings during the initial or “light” sleep stage (Stage 1) and decreased “deep sleep” or slow wave sleep (Stages 3 and 4) relative to controls. Subjective reports from sleep diaries indicated that sleep was improved in the MBCT group but not over and above improvements in the control condition. Participants in the MBCT group experienced greater improvements in depressive symptoms compared to the control condition and these reductions were associated with improvements in both objective and subjective assessments of sleep.
Results from the second study in individuals currently taking ADM indicated that MBCT participants spent less time awake in bed (TWT: total wake time) and had higher sleep efficiency relative to controls, according to both objective (PSG) and subjective measures (sleep diaries). However, when “wake after sleep onset” (WASO) and “sleep onset latency” (SOL) were analyzed separately (the two indices of TWT), no differences were found using objective PSG and only SOL approached statistical significance using subjective sleep diaries. Also, no changes were found in the total amount of sleep or the amount of “deep” (Stages 3 and 4) or “light” (Stage 1) sleep as a result of MBCT. Finally, improvements in self-reported sleep continuity (e.g., sleep efficiency and WASO), but not objective measures of sleep were correlated with reductions in depression symptoms.
What can be concluded about the effects of MBCT on insomnia from these studies? First, both studies suggest that MBCT is effective for improving self-reported sleep. Further, the magnitude of the effect of MBCT on TWT is comparable to MBTI [40]. Second, objective improvements in sleep as a result of MBCT were found only in the sample taking ADM. The medication-free sample experienced increased sleep disturbance according to objective assessments. The beneficial effect in individuals currently taking ADM may represent an additive advantage of MBCT plus medication on objective sleep outcomes. Also, complete depression remission rates in the MBCT group among the medication-free sample were lower (35 %) than the sample taking ADM (50 %), which may indicate that individuals in full remission are more likely to experience objective sleep benefits from MBCT. This interpretation is consistent with theoretical considerations that endorse the use of MBCT as a depression prevention intervention that may be most effective for individuals with minimal depressive symptoms [54]. Further work is needed to identify which types of patients with insomnia complaints are most likely to benefit from MBCT.
Two notable limitations of these investigations should guide future scientific inquiry into the benefits of MBCT for insomnia. First, the long-term effects of MBCT for sleep complaints are largely unknown. Among the five MBCT studies to date, only Ree and colleagues [50] examined the effects of MBCT beyond an immediate post-intervention assessment. Second, the clinical profile of participants across these studies was heterogeneous, which limits conclusions about generalizability. For example, only three MBCT studies included patients who met specified insomnia symptom criteria [49, 52, 53]. Also, with the exception of the study conducted by Heidenrich and colleagues [49], each study examined the effects of MBCT for comorbid mood disorders and insomnia symptoms. Therefore, minimally, results may generalize only to the 40 % of individuals with comorbid psychopathology and insomnia.
Mechanisms
The authors are unaware of any study that has formally tested mechanisms underlying the effects of MBCT for insomnia. Two studies demonstrated the effects of MBCT for thought control and rumination. It is unclear, however, whether these improvements correlated with changes insomnia [49, 51]. In the two RCTs of MBCT for insomnia, reductions in depressive symptoms were related to improvements in sleep disturbance, which suggests that improvements in mood may be one possible mechanism underlying the effects of MBCT for sleep disturbance [52, 53]. One additional hypothesis put forward by Britton and colleagues is that, similar to ADM, MBCT may increase neurotransmitters (e.g., norepinephrine, serotonin, and dopamine) [52]. This idea may explain their paradoxical finding whereby improved mood and subjective sleep quality was experienced with a concomitant increase in objectively measured sleep arousal [52].
The wider literature on MBIs for insomnia offers additional insight into candidate mechanisms. Evidence for MBTI-related reductions in pre-sleep arousal [37, 39, 40] suggests this may be a mechanism of MBIs that is consistent with conceptual models [15, 18] and preliminary evidence which indicated that cognitive and somatic arousal predict sleep disturbance [55]. Given the bidirectional link between mood disorders and sleep, additional mechanisms worthy of consideration are those that explain MBCT’s effects on depressive disorder outcomes (e.g., self-compassion, improved attentional control, emotion regulation) (for a review, see [56]).
The collective literature on MBIs for insomnia and MBCT for depression provides insight into several plausible mechanisms by which MBCT reduces insomnia. Future investigations are needed to understand the specificity of the effects of MBIs for insomnia (e.g., benefits due to mindfulness rather than nonspecific therapeutic elements such as alliance with instructor or group members) as well as the mechanisms underlying MBIs. A definitive test of each of these would require several assessments of the hypothesized mediator (i.e., pre-intervention, during, and post-intervention) in order to detect the time-course of intervention-related changes, and a demonstration that the mechanisms of MBCT differ from the mechanisms underlying the effects of attention control conditions [27]. Prior to this, however, additional work may be required to first develop a clear conceptual model to guide the testing of treatment mechanisms [18]. Ong and colleagues’ “two-level model of sleep-related arousal” [18] has laid the necessary initial groundwork by integrating extant theory from cognitive and behavioral models with mindfulness-based concepts and processes (e.g., metacognition and secondary arousal) that are associated with sleep disturbance and that are targeted by MBIs .
Modifications of MBCT for Insomnia
The strongest evidence for the effects of MBCT is for individuals with comorbid insomnia complaints and elevated depressive or anxiety symptoms or recurrent major depressive disorder [50–53]. It appears that MBCT may be effective for this patient population, in part, because it targets cognitive and physiological vulnerabilities that underlie both disorders. We provide a few additional considerations for the delivery of MBCT to this population. We recommend that providers be familiar with the diagnosis of insomnia and be able to rule out other sleep disorder s (e.g., circadian rhythm sleep–wake disorders, obstructive sleep apnea hypopnea, restless legs syndrome, nightmare disorder) that may contribute to, or complicate, patients’ symptoms, and that may necessitate a referral to a sleep specialist. We also recommend that providers screen for substance use, including caffeine, nicotine, and alcohol, as these can have deleterious effects on sleep and mood, and because patients may need additional information and/or substance use treatment prior to engaging in MBCT.
With regard to modification of group session content, we recommend the dissemination of basic information on sleep processes. For example, information on sleep drive and guided discussion to help participants distinguish sleepiness from fatigue may support important behavioral changes (e.g., reducing sleep effort) [18, 57]. Also, to prevent the use of mindfulness practice as a form of sleep effort, providers may want to discourage the use of formal mindfulness practice at bedtime for the first several sessions [37]. Finally, daytime sleepiness and fatigue may affect patients’ ability to learn and retain session information. Thus, providers will want to consider timing of group sessions and may schedule session times only after a discussion with participants about when they experience the lowest levels of sleepiness and/or fatigue over the course of the day.
Summary and Conclusions
Nearly 40 % of individuals suffer from symptoms of insomnia. Despite being common and a risk factor for a range of morbidities and mortality, a significant portion of individuals with insomnia go untreated [58] or do not experience benefit from prevailing nonpharmacological interventions [59]. Theoretical considerations from widely accepted models of insomnia and from conceptual frameworks of the cognitive processes targeted by mindfulness-based practices suggest that MBCT may be a promising intervention for insomnia. Another reason MBCT may be a favorable treatment for insomnia is because it reduces depression, which is experienced by over 1 in 3 individuals with insomnia. Because these two disorders are thought to share common pathophysiological pathways, improvements in depression should also reduce symptoms of insomnia. Indeed, compelling preliminary empirical evidence indicates that MBCT reduces symptoms of insomnia in individuals with depressive symptoms and a history of recurrent major depression [52, 53]. Further, the magnitude of MBCT-related improvements in self-reported sleep is similar to those observed in another trial of mindfulness-based therapy for insomnia (MBTI) , a newly developed intervention that combines mindfulness exercises from MBSR with CBT-I [40]. Collectively, these studies demonstrate that interventions featuring mindfulness meditation have positive patient-reported benefits and may be viable options for individuals with insomnia seeking treatments that are not based strictly on cognitive behavioral techniques. Additional research is needed to determine the varying degrees of efficacy among mindfulness-based approaches for insomnia (e.g., MBSR, MBCT, and MBTI) and how each of these compares to standard psychobehavioral treatments such as CBT-I.
Also, further work is needed to understand the mechanisms underlying each of these interventions and also which treatments will confer the greatest benefit for which types of patients. Based on the strongest evidence to date, it appears that MBCT may be ideally suited to treat insomnia in individuals with comorbid depression symptoms. Some early evidence suggests that a tailored MBCT protocol that includes information on sleep education may be effective for improving self-reported sleep in individuals with primary insomnia [49]. Treatment development studies and RCTs are needed to test whether the effects of such modifications are stronger than those for extant mindfulness-based treatments that reduce symptoms of insomnia (e.g., MBTI and the standardized MBCT and MBSR protocols).
In sum, MBCT appears to be a promising intervention for reducing symptoms of insomnia, particularly individuals with depressive symptoms and a history of depression.
References
Segal Z, Williams MG, Teasdale JD. Mindfulness-based cognitive therapy for depression. New York, NY: The Guilford Press; 2013.
Roth T. Insomnia: definition, prevalence, etiology, and consequences. J Clin Sleep Med. 2007;3(5 Suppl):S7–10.
American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5 2013. Available from: http://dsm.psychiatryonline.org/book.aspx?bookid=556.
World Health Organization. International classification of diseases 10th Revision 2015. Accessed 24 July 2015. Available from: http://www.who.int/classifications/icd/en/.
American Academy of Sleep Medicine. International classification of sleep disorders: diagnostic and coding manual 2. Westchester; 2005.
Ohayon MM, Reynolds 3rd CF. Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the International Classification of Sleep Disorders (ICSD). Sleep Med. 2009;10(9):952–60. doi:10.1016/j.sleep.2009.07.008.
Ishak WW, Bagot K, Thomas S, Magakian N, Bedwani D, Larson D, et al. Quality of life in patients suffering from insomnia. Innov Clin Neurosci. 2012;9(10):13–26.
Kessler RC, Berglund PA, Coulouvrat C, Hajak G, Roth T, Shahly V, et al. Insomnia and the performance of US workers: results from the America insomnia survey. Sleep. 2011;34(9):1161–71. doi:10.5665/sleep.1230.
Turvey CL, Conwell Y, Jones MP, Phillips C, Simonsick E, Pearson JL, et al. Risk factors for late-life suicide: a prospective, community-based study. Am J Geriatr Psychiatry. 2002;10(4):398–406.
Leger D, Bayon V, Ohayon MM, Philip P, Ement P, Metlaine A, et al. Insomnia and accidents: cross-sectional study (EQUINOX) on sleep-related home, work and car accidents in 5293 subjects with insomnia from 10 countries. J Sleep Res. 2014;23(2):143–52. doi:10.1111/jsr.12104.
Benca RM. Consequences of insomnia and its therapies. J Clin Psychiatry. 2001;62 Suppl 10:33–8.
Ohayon MM, Roth T. Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res. 2003;37(1):9–15.
Espie CA. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu Rev Psychol. 2002;53:215–43. doi:10.1146/annurev.psych.53.100901.135243.
Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, et al. The hyperarousal model of insomnia: a review of the concept and its evidence. Sleep Med Rev. 2010;14(1):19–31. doi:10.1016/j.smrv.2009.04.002.
Harvey AG. A cognitive model of insomnia. Behav Res Ther. 2002;40(8):869–93.
Perlis ML, Giles DE, Mendelson WB, Bootzin RR, Wyatt JK. Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res. 1997;6(3):179–88.
Harvey AG, Tang NK. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psychol Bull. 2012;138(1):77–101. doi:10.1037/a0025730.
Ong JC, Ulmer CS, Manber R. Improving sleep with mindfulness and acceptance: a metacognitive model of insomnia. Behav Res Ther. 2012;50(11):651–60. doi:10.1016/j.brat.2012.08.001.
Carney CE, Harris AL, Moss TG, Edinger JD. Distinguishing rumination from worry in clinical insomnia. Behav Res Ther. 2010;48(6):540–6. doi:10.1016/j.brat.2010.03.004.
Espie CA, Broomfield NM, MacMahon KM, Macphee LM, Taylor LM. The attention-intention-effort pathway in the development of psychophysiologic insomnia: a theoretical review. Sleep Med Rev. 2006;10(4):215–45. doi:10.1016/j.smrv.2006.03.002.
Morin CM. Cognitive-behavioral therapy of insomnia. Sleep Med Clin. 2006;1(3):375–86. doi:10.1016/j.jsmc.2006.06.008.
Trauer JM, Qian MY, Doyle JS, Rajaratnam SM, Cunnington D. Cognitive behavioral therapy for chronic insomnia: a systematic review and meta-analysis. Ann Intern Med. 2015;163(3):191–204. doi:10.7326/m14-2841.
Taylor DJ, Pruiksma KE. Cognitive and behavioural therapy for insomnia (CBT-I) in psychiatric populations: a systematic review. Int Rev Psychiatry. 2014;26(2):205–13. doi:10.3109/09540261.2014.902808.
Morin CM, Culbert JP, Schwartz SM. Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry. 1994;151(8):1172–80.
Kuyken W, Hayes R, Barrett B, Byng R, Dalgleish T, Kessler D, et al. Effectiveness and cost-effectiveness of mindfulness-based cognitive therapy compared with maintenance antidepressant treatment in the prevention of depressive relapse or recurrence (PREVENT): a randomised controlled trial. Lancet. 2015;386(9988):63–73. doi:10.1016/s0140-6736(14)62222-4.
Williams JM, Crane C, Barnhofer T, Brennan K, Duggan DS, Fennell MJ, et al. Mindfulness-based cognitive therapy for preventing relapse in recurrent depression: a randomized dismantling trial. J Consult Clin Psychol. 2014;82(2):275–86. doi:10.1037/a0035036.
Shallcross AJ, Gross JJ, Visvanathan PD, Kumar N, Palfrey A, Ford BQ, et al. Relapse prevention in major depressive disorder: mindfulness-based cognitive therapy versus an active control condition. J Consult Clin Psychol. 2015;83(5):964–75. doi:10.1037/ccp0000050.
Woods H, Marchetti LM, Biello SM, Espie CA. The clock as a focus of selective attention in those with primary insomnia: an experimental study using a modified Posner paradigm. Behav Res Ther. 2009;47(3):231–6. doi:10.1016/j.brat.2008.12.009.
Harvey A. The attempted suppression of presleep cognitive activity in insomnia. Cogn Ther Res. 2003;27(6):593–602. doi:10.1023/A:1026322310019.
Jha AP, Krompinger J, Baime MJ. Mindfulness training modifies subsystems of attention. Cogn Affect Behav Neurosci. 2007;7(2):109–19.
Tang YY, Ma Y, Wang J, Fan Y, Feng S, Lu Q, et al. Short-term meditation training improves attention and self-regulation. Proc Natl Acad Sci U S A. 2007;104(43):17152–6. doi:10.1073/pnas.0707678104.
Shallcross AJ, Troy A, Mauss IB. Regulation of emotions under stress. Emerging trends in the social and behavioral sciences. John Wiley & Sons, Inc.; Hoboken, New Jersey. 2015.
Staner L. Comorbidity of insomnia and depression. Sleep Med Rev. 2010;14(1):35–46. doi:10.1016/j.smrv.2009.09.003.
Ma SH, Teasdale JD. Mindfulness-based cognitive therapy for depression: replication and exploration of differential relapse prevention effects. J Consult Clin Psychol. 2004;72(1):31–40. doi:10.1037/0022-006x.72.1.31.
Talbot LS, Stone S, Gruber J, Hairston IS, Eidelman P, Harvey AG. A test of the bidirectional association between sleep and mood in bipolar disorder and insomnia. J Abnorm Psychol. 2012;121(1):39–50. doi:10.1037/a0024946.
Mathew KL, Whitford HS, Kenny MA, Denson LA. The long-term effects of mindfulness-based cognitive therapy as a relapse prevention treatment for major depressive disorder. Behav Cogn Psychother. 2010;38(5):561–76. doi:10.1017/s135246581000010x.
Ong JC, Shapiro SL, Manber R. Combining mindfulness meditation with cognitive-behavior therapy for insomnia: a treatment-development study. Behav Ther. 2008;39(2):171–82. doi:10.1016/j.beth.2007.07.002.
Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27(8):1567–96.
Ong JC, Shapiro SL, Manber R. Mindfulness meditation and cognitive behavioral therapy for insomnia: a naturalistic 12-month follow-up. Explore (NY). 2009;5(1):30–6. doi:10.1016/j.explore.2008.10.004.
Ong JC, Manber R, Segal Z, Xia Y, Shapiro S, Wyatt JK. A randomized controlled trial of mindfulness meditation for chronic insomnia. Sleep. 2014;37(9):1553–63. doi:10.5665/sleep.4010.
Morin CM, Belleville G, Bélanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–8.
Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. 2001;285(14):1856–64.
Buysse DJ, Germain A, Moul DE, Franzen PL, Brar LK, Fletcher ME, et al. Efficacy of brief behavioral treatment for chronic insomnia in older adults. Arch Intern Med. 2011;171(10):887–95. doi:10.1001/archinternmed.2010.535.
Britton WB, Shahar B, Szepsenwol O, Jacobs WJ. Mindfulness-based cognitive therapy improves emotional reactivity to social stress: results from a randomized controlled trial. Behav Ther. 2012;43(2):365–80. doi:10.1016/j.beth.2011.08.006.
Troy A, Shallcross AJ, Davis TS, Mauss IB. History of mindfulness-based cognitive therapy is associated with increased cognitive reappraisal ability. Mindfulness. 2013;4(3):213–22. doi:10.1007/s12671-012-0114-5.
Keune PM, Bostanov V, Hautzinger M, Kotchoubey B. Mindfulness-based cognitive therapy (MBCT), cognitive style, and the temporal dynamics of frontal EEG alpha asymmetry in recurrently depressed patients. Biol Psychol. 2011;88(2–3):243–52. doi:10.1016/j.biopsycho.2011.08.008.
Baglioni C, Spiegelhalder K, Lombardo C, Riemann D. Sleep and emotions: a focus on insomnia. Sleep Med Rev. 2010;14(4):227–38. doi:10.1016/j.smrv.2009.10.007.
Larouche M, Cote G, Belisle D, Lorrain D. Kind attention and non-judgment in mindfulness-based cognitive therapy applied to the treatment of insomnia: state of knowledge. Pathol Biol (Paris). 2014;62(5):284–91. doi:10.1016/j.patbio.2014.07.002.
Heidenreich T, Tuin I, Pflug B, Michal M, Michalak J. Mindfulness-based cognitive therapy for persistent insomnia: a pilot study. Psychother Psychosom. 2006;75(3):188–9. doi:10.1159/000091778.
Ree MJ, Craigie MA. Outcomes following mindfulness-based cognitive therapy in a heterogeneous sample of adult outpatients. Behav Chang. 2007;24(02):70–86. doi:10.1375/bech.24.2.70.
Foulk MA, Ingersoll-Dayton B, Kavanagh J, Robinson E, Kales HC. Mindfulness-based cognitive therapy with older adults: an exploratory study. J Gerontol Soc Work. 2013;57(5):498–520. doi:10.1080/01634372.2013.869787.
Britton WB, Haynes PL, Fridel KW, Bootzin RR. Polysomnographic and subjective profiles of sleep continuity before and after mindfulness-based cognitive therapy in partially remitted depression. Psychosom Med. 2010;72(6):539–48. doi:10.1097/PSY.0b013e3181dc1bad.
Britton WB, Haynes PL, Fridel KW, Bootzin RR. Mindfulness-based cognitive therapy improves polysomnographic and subjective sleep profiles in antidepressant users with sleep complaints. Psychother Psychosom. 2012;81(5):296–304. doi:10.1159/000332755000332755.
Segal ZV, Teasdale JD, Williams JMG. Mindfulness-based cognitive therapy: theoretical rationale and empirical status. In: Hayes SC, Follette VM, Linehan MM, editors. Mindfulness and acceptance: expanding the cognitive-behavioral tradition. New York: Guilford Press; 2004. p. 45–65.
Wicklow A, Espie CA. Intrusive thoughts and their relationship to actigraphic measurement of sleep: towards a cognitive model of insomnia. Behav Res Ther. 2000;38(7):679–93.
van der Velden AM, Kuyken W, Wattar U, Crane C, Pallesen KJ, Dahlgaard J, et al. A systematic review of mechanisms of change in mindfulness-based cognitive therapy in the treatment of recurrent major depressive disorder. Clin Psychol Rev. 2015;37:26–39. doi:10.1016/j.cpr.2015.02.001.
Ong J, Sholtes D. A mindfulness-based approach to the treatment of insomnia. J Clin Psychol. 2010;66(11):1175–84. doi:10.1002/jclp.20736.
Ozminkowski RJ, Wang S, Walsh JK. The direct and indirect costs of untreated insomnia in adults in the United States. Sleep. 2007;30(3):263–73.
Murtagh DR, Greenwood KM. Identifying effective psychological treatments for insomnia: a meta-analysis. J Consult Clin Psychol. 1995;63(1):79–89.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Shallcross, A.J., Visvanathan, P.D. (2016). Mindfulness-Based Cognitive Therapy for Insomnia. In: Eisendrath, S. (eds) Mindfulness-Based Cognitive Therapy. Springer, Cham. https://doi.org/10.1007/978-3-319-29866-5_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-29866-5_3
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-29864-1
Online ISBN: 978-3-319-29866-5
eBook Packages: MedicineMedicine (R0)