Abstract
The rupestrian grasslands in Minas Gerais State comprise headwaters of important watersheds that drainages for millions citizens in over 400 cities in São Francisco and Doce river basins. The human activities in the rupestrian grasslands include domestic supply, agriculture, forestry, cattle raising, industry, and mineral extraction. This chapter addresses the ecological conditions of streams in terms of water quality (physical and chemical characteristics, nutrient availability), habitat quality and structure (diversity of benthic macroinvertebrates, structure of the riparian vegetation, riparian food webs, invertebrate drift), and ecosystem functioning (allochthonous and autochthonous production, dynamics of coarse and fine particulate organic matter, leaf litter breakdown of native and alien species). A synthesis of 20 years of ongoing research on the headwaters in the rupestrian grasslands is included, together with perspectives for future conservation and management of water resources.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 The Headwater Streams of the Espinhaço Mountains
Freshwaters harbor a rich diversity of species and habitats. Although they cover only 0.8 % of the Earth’s surface, 10 % of all animal species live in freshwaters (at least 126,000 plant and animal species), including 35 % of all vertebrate species. These species combine to provide a wide range of critical services for humans, such as flood protection, food, water filtration and carbon sequestration (Collen et al. 2013). Despite the high number of species described in freshwaters and their importance for biodiversity, anthropogenic disturbances have been increasing dramatically since the last Century. As a result, the decline in freshwater biodiversity is outpacing that of other ecosystems (Dudgeon et al. 2006). Freshwaters are hotspots of biodiversity that are being threatened by fragmentation and habitat destruction, introduction of invasive species, pollution, human population growth, as well as overharvesting (Stendera et al. 2012; Macedo et al. 2014).
The Espinhaço Mountain Range shows headwaters for a large number of watercourses which house a high number of aquatic and semi-aquatic plant and animal species. The water quality in the headwaters in this region is excellent and achieves the highest level of the Brazilian Environmental Legislation (“special class” according to CONAMA 357/2005). These headwaters form three important river basins in the Cerrado biome: São Francisco, Doce and Jequitinhonha. The freshwater biodiversity in the Espinhaço Mountain Range includes 27 endemic fish species, 162 fish species that inhabit small creeks and low-order streams , and 12 fish species threatened of extinction (Alves et al. 2008); 105 anuran species, of which 28 are endemics species (Leite et al. 2008).
Human activities in the Espinhaço Mountain Range, including agriculture, mining, and dam construction, have increased the environmental impacts on the freshwaters , which change physical habitats, chemical water quality and negatively influence native plant and animal species. The anthropogenic disturbances by industrial, urban, agro-cattle and mining are responsible not only for local changes that threaten aquatic biodiversity but also influence basin water quality due to the bioaccumulation and biomagnification of heavy metals, Polycyclic Aromatic Hydrocarbons (PAHs), insecticides, herbicides, fungicides and other chemical compounds along the food webs. Experiments in Parque Nacional da Serra do Cipó streams have been conducted to assess the habitat-species composition associations and to test global hypotheses in international collaboration networks. In this chapter, we describe the features of the land and the scientific experience of the last 20 years of studies conducted in Espinhaço Mountain Range region.
2 River Basin Beta Diversity and Trends in the Distribution of Aquatic Invertebrates in the Headwater Streams of the Parque Nacional da Serra do Cipó
2.1 Beta Diversity in River Basins
Headwaters comprise the majority (c. 70 %) of the total length of any river basin (Benda et al. 2005). Due to the longitudinal linkage, the water quality upstream affects the water quality downstream . Headwater streams are also important for the maintenance of the biodiversity of the whole river basin. Many invertebrates and vertebrates reproduce in headwaters, from which they can potentially colonize downstream reaches (Clarke et al. 2008).
Whittaker (1960) coined the term “alpha diversity” (α) to account to the variety of species found locally and the term “beta diversity” (β) to account to the composition dissimilarity between local sites. Because headwaters generally have narrow and shallow channels, each stream reach usually harbor less species than medium size river reaches do. Authors argue that medium-size river reaches are likely to present the highest taxonomic diversity of the river network, small streams and large rivers presenting lower diversity (Allan and Castillo 2007). Thus, headwater streams usually present lower alpha diversity when compared to wider and deeper medium size downstream reaches. On the other hand, because headwater reaches are usually highly isolated from each other, they typically present higher beta diversity , especially when landscape features like hilly terrain and dense vegetation hamper aerial dispersion (Finn and Poff 2005). Consequently, the total species diversity found in all headwater reaches accounts for the majority of the total species diversity found in the whole basin. Whittaker named this regional diversity as “gamma diversity” (γ).
For a long time, stream ecologists have focused on the factors driving the diversity of species at the site scale (i.e., α diversity). More recently, a large number of studies started to investigate the dissimilarity of species composition among sites (i.e., β diversity) (Legendre et al. 2005; Tuomisto and Ruokolainen 2006; Melo et al. 2011). Both aspects of diversity must be considered because it is the interaction between alpha and beta diversities that defines the total diversity (gamma) found in any region.
2.2 Assessing Key Spatial Scales
Stream ecosystems can be investigated at many spatial scales, from centimeters to kilometers. Frissel et al. (1986) recognized several discrete spatial levels , ranging from microhabitats to river basins (Fig. 5.1). The biological assemblages at any site are structured by environmental factors acting at all those spatial levels (Allan 2004). For aquatic invertebrates, at the microhabitat level assemblages are constrained by the type of bottom substrate, microflow regimes near the streambed and, possibly, by interactions among species. At the stream reach level, riparian zone characteristics can play a stronger role; while at the basin level, assemblages are affected by the vegetation type and human land uses. Identifying the spatial level that presents the highest beta diversity of biological assemblages is critical to determine which spatial scale should be mostly considered in conservation and ecosystem management actions. For instance, if we find that most of the assemblage dissimilarity is found between stream reaches, it is not worthwhile to apply all the available resources for conserving a single or few stream reaches. In this scenario, even if one stream reach is superbly well protected, much of the regional biodiversity can be lost because other stream reaches were not considered.
Hierarchical approaches in stream ecology, after Frissel et al. (1986)
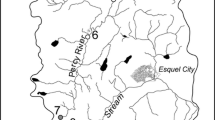
A long term study was performed in the Parque Nacional da Serra do Cipó to identify the “key” spatial scales responsible for the highest β diversity of aquatic invertebrates (Ligeiro et al. 2010a). We used a hierarchical nested sampling design across multiple spatial levels at the Mascates River basin. The Mascates basin belongs to the São Francisco River Basin, one of the most important basins for the Brazilian economy. We selected three headwater streams in this basin, representing the “stream segment” spatial level of Frissel et al. (1986): the Pedras stream, the Farofa stream, and the Taioba stream (Fig. 5.2a–c). They are all narrow (~4 meters wide) and shallow (~0.5 meters deep) streams, with sandy/rocky substrate, presenting riffle/pool sequences and ecological preservation but without dense riparian vegetation. In all aspects, they well represent the typology of the headwater streams found in the Serra do Cipó region. In each stream segment, we sampled in two stream reaches, longitudinal stretches of the streams of approximately 50 meters each. In each reach, we sampled in three riffles, representing the “habitat” spatial level of Frissel et al. (1986). Finally, in each riffle we took three samples of the stream benthos, the “microhabitat” spatial level of Frissel et al. (1986). We performed this sampling design for each of the three types of substrates, representing the heterogeneity at the microhabitat level: stones, gravel, and leaf litter. In total, 162 samples were taken from the three types of substrates in the Mascates River basin (Fig. 5.3). The detailed procedures can be found in Ligeiro et al. (2010a).
Sampling design for assessing the beta diversity of macroinvertebrate assemblages across multiple spatial scales in a small river basin in Parque Nacional da Serra do Cipó (Brazil). Adapted from Ligeiro et al. (2010a)
For assessing the spatial levels of the greater aquatic invertebrate beta diversity (“key spatial scales”), we used an additive partitioning diversity approach (Veech et al. 2002). First, the average species richness is calculated for the sampling units of each spatial level. Then, the β diversity of each spatial level (L) is defined by subtracting the average species richness of that level from the average species richness of the next higher spatial level (L + 1). For instance, the β diversity at the stream reach level is equal to the average species richness of the stream segment level minus the average species richness of the stream reach level, and so on for all the spatial levels considered. Consequently, the γ diversity coincides with the sum of the α diversity (the average species richness found in the smallest spatial level) with the beta diversity calculated for each spatial level considered (γ = α + β 1 + β 2 + β 3 + β 4).
For the three substrate types selected we determined that most of the variation in aquatic invertebrates composition was found at the stream segment spatial level, indicating that the biological dissimilarity between the three headwater streams studied (β 4) was much greater than the dissimilarities found between stream reaches (β 3), habitats (β 2), and microhabitats (β 1) (Fig. 5.4). This high similarity found within each headwater stream can be partially explained by the unidirectional water flow from upstream to downstream, which promotes the passive and active dispersion of invertebrate adults and juveniles through water drift, homogenizing the many sites along the same stream (Allan and Castillo 2007).
Distribution of macroinvertebrate diversity in Parque Nacional da Serra do Cipó (Brazil) across multiple spatial scales according to the additive diversity partitioning approach (more details in the text), for each of the main substrate types found in the streambeds. a Leaf litter. b Stones. c Gravel
In parallel to the partitioning of the aquatic invertebrate diversity across multiple spatial levels , we found great dissimilarities between the samples of the three substrate types considered, mainly between the organic substrate (leaf litter) and the inorganic substrates (stones and gravel) (Ligeiro et al. 2010a). The substrate types are very important for aquatic invertebrates, defining the availability of shelter from predators and natural disturbances (e.g., flashfloods), and also the availability and quality of food resources (e.g., particulate organic matter, prey).
2.3 Implications of β Diversity for Ecosystem Management and Conservation
The fact that we identified the stream segment (represented in our study by the three headwater streams sampled) as the key spatial level for the biodiversity of aquatic invertebrates has important implications for management and conservation thinking of Serra do Cipó streams and Cerrado streams in general. First, this result indicates that sampling many headwater streams is essential to properly represent the biodiversity of headwater basins. As resources (money, time, qualified personal) are often limited for any scientific research or conservation program (Hughes and Peck 2008), it is ineffective to concentrate many samples in one single stream segment, rather the sampling effort should be spread through the highest possible number of headwater streams . The substrate type was an important factor differentiating invertebrate assemblages; therefore, it is also important to consider microhabitat heterogeneity when conducing biodiversity surveys.
Because most of the diversity of the aquatic invertebrates of Parque Nacional da Serra do Cipó was generated by the dissimilarities between the headwater streams, conservation and management actions should invest in protecting the highest number possible of headwater streams in the region. Each stream impaired by human activities can mean the loss of aquatic species in the whole region. Because each stream is relatively internally homogeneous, occasional alterations conducted in some parts of the streams are likely not to greatly impair the biodiversity at a larger spatial scale. This scenario suggests that the best management approach is not to isolate a few streams from human contact, but to wisely promote sustainable human use (recreational, human and familiar agriculture supply) of the existing streams, in this way allowing each one to harbor and conserve its unique diversity.
3 Leaf Breakdown and Organic Matter Dynamics
In low order streams, tree canopy cover commonly limits light availability to streambed. Thus, the reduced stream’s primary production and allochthonous organic matter inputs are the main energy source for the aquatic ecosystems. The Espinhaço Range is rich in headwater streams, particularly between the altitudes of 500 and 2000 meters a.s.l. In this region different habitats of the rupestrian grasslands co-occur, including rock outcrops, open grasslands and forests (Gonçalves et al. 2006a, b, c; Chaps. 1, 6 and 7). Headwaters at higher altitudes (1st and 2nd order streams) are bordered by woody grasslands, while at the lower sections rivers flow through forest habitats.
The energetic base is maintained by the production of algae and bacteria (Callisto et al. 2004). Bacteria use the dissolved organic carbon, which can be observed by the tea color of the water (Janzen 1974). The waters are generally nutrient-poor due to the low nutrient content in the soil (Chap. 3), which also limits the aquatic productivity. We hypothesize that this could lead to the dependency of the productivity on the biofilm (e.g., algae and periphyton) that colonizes rocks, gravel, and trunks in the streambed. Along the longitudinal gradient, larger streams also have low productivity due to lack of light (due to water turbidity), despite possessing higher concentration of nutrients in the water. In those segments of the watercourse, energy depends on the organic matter derived from the plants on its banks (Coarse Particulate Organic Matter, CPOM, and Fine Particulate Organic Matter, FPOM), while their productivity depends on the dynamics of the allochthonous organic matter. On the other hand, studies on the primary production in these ecosystems are still rare. Furthermore, little is known about the abundance and dynamics of dissolved organic matter (DOM) and fine particulate organic matter (FPOM).
In general, the abundance of gathering-collectors is negatively related with the FPOM; where the FPOM concentration is low, gathering-collectors drift away (Castro et al. 2013a). When the FPOM is available in high concentration, the filtering collectors are abundant. This suggests that invertebrates’ drift is related to food availability while high drift movement by the gathering-collectors is related to their searching for deposited FPOM as food. During the rainy seasons and flashfloods, bottom instability may lead to FPOM drift, the main food resource in tropical headwater streams (Castro et al. 2013b). For instance, the drift of invertebrate assemblages along a longitudinal gradient in a headwater stream were mostly composed by aquatic insects. Among the 91 taxa, Chironomidae (Diptera: 33 genera), Trichoptera (18 genera), and Ephemeroptera (13 genera) showed the highest taxonomic richness values (Callisto and Goulart 2005).
We conducted three studies on the dynamics of organic matter in the Espinhaço Range (Gonçalves et al. 2006a; França et al. 2009; Gonçalves and Callisto 2013) (Fig. 5.5). The diversity of plant species in the riparian zone ranged from 15 (stream reaches surrounded by rocky outcrops) to 192 in a reach 1300 m a.s.l. (along with 128 species contributed for inputs of CPOM). In the three sites, 13 species were common to at least two sites, and the genus Ocotea was common in all sites. The CPOM productivity from the riparian vegetation (measured by vertical and terrestrial inputs) ranged from 1.8–4.6 t ha−1 year−1. This productivity was seasonal being higher during the transition between periods of drought and rain, with maximum values occurring in October. Most of the CPOM falling in the soil from the riparian zone goes to the stream (between 82 and 100 % of the total CPOM that reaches the ground). This occurs because headwater streams in the Espinhaço Range have a steep slope and narrow channel morphology. Thus, the decomposition of CPOM (especially leaves, representing more than 60 % of the total CPOM) is fundamental to understand the functioning of the stream ecosystems in the Espinhaço Range. We found 9 studies on this topic in the rupestrian grasslands of the Espinhaço Range (Fig. 5.5), where nine native species were studied (Myrcia guyanensis, Miconia chartacea, Protium brasiliense, Protium heptaphyllum, Coccoloba cereifera, Ocotea sp., Baccharis platypoda, Baccharis concinna, Baccharis dracunculifolia), two exotic species (Eucalyptus grandis and Alnus glutinosa) and two studies of “mixed leaves” (Gonçalves et al. 2006b; Moretti et al. 2007a, b). Most studies were conducted between April and September during the years 2001–2009.
The leaves of the native plant species had low nitrogen (1.15 %) and phosphorus (0.0914 %), and high levels of lignin (31 %) and polyphenols (15 %). The leaf breakdown rate of the native species was −0.0039 day−1, mixed leaves −0.0111 day−1, Eucalyptus grandis −0.005 day−1 and Alnus glutinosa −0.014 day−1. The resulting k values indicate that the native leaves have slow decomposition, while the mixed and exotic species are characterized by intermediate decomposition (Ligeiro et al. 2010b; Gonçalves et al. 2014). There are two factors that could influence these results: the low quality of the leaf litter of the rupestrian grassland plants and the low concentration of the nutrients in the headwater streams in the Espinhaço Range, as demonstrated by Medeiros et al. (2015).
Rupestrian grassland leaves decomposing in streams had low fungal biomass, measured as ergosterol concentration. Here, ergosterol values ranged from 62 to 278 μg g−1. On the other hand, for the small leaves of Baccharis dracunculifolia and B. concinna (Alvim et al. 2015), the ergosterol values were high (908 μg g−1 in both) compared to the concentrations in the leaves of an exotic species widely used in decomposition experiments (A. glutinosa). This last species was also studied in the headwater streams at Espinhaço Range region, and presented ergosterol max value of 573 μg g−1 (Gonçalves et al. 2006c).
The Total Microbial Biomass estimations (fungi, bacteria and other microorganisms through the concentration of ATP) also indicate low participation of the microbial decomposers (concentration in rupestrian grassland leaves between 42 and 194 nmoles ATP g−1, 4278−4023 nmoles ATP g−1 B. dracunculifolia and B. concinna, respectively, and 531 nmoles ATP g−1 in A. glutinosa). These results indicate that, besides fungi, other microorganisms, such as bacteria, proportionally may play a major role in litter decomposition. We believe that the lack of nutrients in leaf litter and in the waters negatively affect fungal activity. Before that, bacteria could be more effective in the decomposition of leaf detritus than fungi, especially in the first phase of the decomposition process (sensu Degradative Ecological Succession, Begon et al. 2007; Gonçalves et al. 2006b, c, 2007).
Detritivorous invertebrates are largely represented by the larvae of Chironomidae (Diptera) (over 60 % of all assemblages associated with organic detritus, Callisto et al. 2007). Among the trophic guilds, we found the gathering-collectors (using fine particles of particulate organic matter—FPOM) were dominant, representing an average of 62 % of the individuals (mainly chironomids), while the shredders (which feed directly from particles greater than 1 mm—CPOM) represented 0–20 % of invertebrates. These results indicate that the influence of invertebrates in leaf fragmentation is low, which is likely due to the low nutritional value of the leaves associated with the reduced fungal biomass. However, the aquatic invertebrates preferentially use FPOM with a high biomass of bacteria and easy digestibility (Callisto and Graça 2013).
Due to the low quality of the leaves from the riparian zone (e.g., low nitrogen and phosphorus, high cellulose and ligninins), the decomposition is slow, leading to the accumulation of CPOM on the streambeds. We postulate that this CPOM is basically processed by physical fragmentation because the biological effect is reduced. Thus, the energy could be dependent on the FPOM (originated by aquatic or terrestrial origin by physical and biological factors) when the processing of the CPOM is inefficient. On the other hand, the FPOM can have a proportionally greater role as energy and nutrient sources to the trophic web, despite its biomass being lower when compared to the fraction of the CPOM (Callisto and Graça 2013). These ecosystems have complex food webs, which are still poorly known. Another factor to be considered in ecological studies could be the low redundancy in the components of the trophic web and a dependency on allochthonous organic matter (CPOM and FPOM) indicating a high ecological fragility of these ecosystems. This is due to the dependence of the dynamic and complex processes that are connected by many environmental variables (vegetation composition, climate, roughness, slope the banks and the stream channel, flow, etc.), which may lead to the decreased resilience of these ecosystems to anthropic activities or global climate change.
Due to the ecological fragility of the aquatic ecosystems of the Espinhaço Range, the need for a biodiversity conservation policy is urgently needed. This could be implemented through policies from the Federal Government that are extended for Minas Gerais and Bahia states and their watersheds , which involve many more states. Another important aspect is a network of systematic studies to raise the level of basic information, allowing the development of an ecological model for sustainable growth of the entire mountain range complex. From this, the ecological and economic zoning of the region should be established, considering the use and occupation of the soil and water resources.
4 The Conservation of Benthic Invertebrates and Their Use as Bioindicators in a Global Perspective
In addition to its importance as a potential area for water supply and aquatic resources, Espinhaço Range headwater streams have been used for agriculture and mine companies, and also represent important aquatic resources for other uses, such as recreation and wildlife maintenance. The stream ecology studies in this region have been developed during the last 20 years. The first years (1995–2000) were dedicated to surveys of the distribution and structure of the aquatic communities, assessments of the existing habitats/micro-habitats, the availability of trophic resources, and of the distribution patterns of the aquatic communities (Galdean et al. 1999). These studies adopted the utilization of higher taxonomic levels and/or functional groups of organisms, together with the basic ecological features of the environments (Barbosa and Galdean 1997). The basic information provided the basis for defining conservation priorities and sustainable uses for the area in the Parque Nacional da Serra do Cipó. Some regions located within the Parque Nacional da Serra do Cipó are in “nearly” pristine conditions, while some other regions have been affected by human activities to varying degrees (Galdean et al. 2000). The most important impacts are related to past deforestation, the disposal of untreated organic sewage from the riverine human populations, cattle ranching, and agriculture. In recent years, some parts of the Espinhaço Range have been severely degraded by iron mining, which caused heavy siltation of the headwaters and deforestation of the riparian zone , resulting in the local extinctions of native aquatic species.
In general, the waters are dark in color, relatively well oxygenated (>80 % saturation), nutrient poor (soluble reactive phosphorus <20 μg L−1, total phosphorus <50 μg L−1) and of low conductivity (<20 uS cm−1), with a pH ranging between 5 and 6 (Galdean et al. 1999, 2000; Fernandes et al. 2014). The benthic macroinvertebrate communities are dominated by larvae of the aquatic insects Ephemeroptera, Plecoptera, Trichoptera, and Diptera-Chironomidae. Crustacea, Bivalvia and Oligochaeta may also be found in low densities in some areas (Galdean et al. 1999). These taxa colonize riverbed rocks, clay and stony substrates, aquatic macrophytes and riparian vegetation. Filamentous algae, mosses and lichens, and coarse and fine organic detritus, on or in between these habitats, form the major microhabitats in the headwater streams . These results have been used as an instrument to assess the present biodiversity, and thus represent an aid in conserving the existing rivers’ benthic fauna.
A global survey of the stream fauna (146 sites from 16 areas located in six continents) included data from the streams at the Espinhaço Range to test the hypothesis on the scarcity of shredders in tropical sites. Both the evolutionary adaptation of shredders to cool waters, which might cause their scarcity in the tropics for physiological reasons, as well as more plant defenses against herbivores in tropical areas than those in the temperate zone, were assessed by Boyero et al. (2011a, b). In fact, tropical streams possess few shredders compared to temperate streams, but their distribution varies at multiple spatial scales and the great majority of the variation occurred among areas within zones, particularly when the richness was quantified as the number of species per site. However, shredder assemblage composition differs between the temperate and tropical zones, which suggest that the distribution of several taxa strongly depends on temperature (Boyero et al. 2012). The hypothesis of plant defenses against herbivores was not corroborated, because leaf toughness was not related to shredder densities or species richness. Perhaps, this finding is supported by the fact that the richness of litter entering into and retained in streams is greater in the tropics. Thus, shredders have a lower chance of re-encountering a leaf of any particular species, which makes it difficult to find palatable leaves and/or develop anti-herbivore strategies (as proposed by Wantzen et al. 2002). Additional global biodiversity aspects were discussed in a series of articles by Boyero et al. (2011a, b, 2012, 2015a, b).
5 Anthropogenic Disturbances, Threats to Headwaters, Future Perspectives and Global Climate Change
Freshwater species are consistently under a greater level of threat than those resident in terrestrial ecosystems (Collen and Boehm 2012; Collen et al. 2013). These patterns of threat are mediated by the high rates of habitat loss and degradation, pollution and overexploitation, which are particularly problematic for species inhabiting flowing waters. Land-use change driving habitat loss and degradation affects the majority of threatened freshwater species. As stated by Kominoski and Rosemond (2012), fluxes and pools of organic matter are critical food and habitat resources at relatively small scales. On larger scales, alterations in the availability, retention, and processing rates of detritus can affect the delivery of organic matter to downstream organisms and fluxes of C to the atmosphere and oceans.
The Espinhaço Range headwater streams are impacted by different human uses. Climate change is expected to affect aquatic ecosystems strongly by altering the quantity, characteristics, processing, and retention of inputs of terrestrial detritus and sediments (www.ipcc.ch). The principal climate drivers affecting detrital dynamics in aquatic ecosystems are increases in atmospheric CO2 and its effects on terrestrial organic matter, changes in precipitation and associated hydrologic impacts, and increases in temperature and siltation.
Altered patterns of precipitation will make extreme events (e.g., droughts and floods) part of flow regimes, which directly influence water quantity and indirectly influence the availability and biological processing of detrital resources and sediment deposits. Drought reduces physical breakdown rates and slows biological processing. Higher discharge will accelerate the transport of sediments and physical breakdown of organic matter. High-flow events can scour detritus from stream reaches.
According to our results the future research projects on headwater streams at the Espinhaço Range should focus on strategic issues and perspectives for future conservation and the management of water resources, including:
-
(i)
Incorporate measures of organic matter processing as indices of stream ecosystem function to complement structural measures (water quality, taxonomic composition) in stream health monitoring (Piggott et al. 2015);
-
(ii)
Investigate how deposited fine sediments and elevated nutrient concentrations interact to affect stream water quality and freshwater biodiversity in a climate-change future scenario.
The resulting information will allow the testing of individual stressor hypotheses, such as: (i) nutrient enrichment generally increase the measures of decomposition rate; (ii) the positive effect of nutrient enrichment are weaker or suppressed when fine sediment is also present.
References
Allan JD (2004) Landscape and riverscapes: The influence of land use on river ecosystems. Ann Rev Ecol Evol Syst 35:257–284
Allan JD, Castillo MM (2007) Stream ecology: Structure and function of running waters. Springer, Netherlands
Alves CBM, Lean CG, Brito MFG, Santos ACA (2008) Biodiversidade e conservação de peixes do complexo do Espinhaço. Megadiv 4:145–164
Alvim EACC, Medeiros AO, Rezende RS, Gonçalves JF (2015) Small leaf breakdown in a Savannah headwater stream. Limnol 51:131–138
Barbosa FAR, Galdean N (1997) Ecological taxonomy: A basic tool for biodiversity conservation. Tren Ecol Evol 12:359–360
Begon M, Townsend CR, Harper JL (2007) Ecologia: de indivíduos a ecossistemas. Artmed, São Paulo
Benda L, Hassan MA, Church M, May CL (2005) Geomorphology of steepland headwaters: The transition from hill slopes to channels. J Am Water Res Assoc 41:835–851
Boyero L, Pearson R, Dudgeon D, Graça MAS, Gessner MO, Albarino R, Ferreira V, Mathuriau C, Boulton A, Arunachalam M, Callisto M, Chauvet E, Ramirez A, Chara J, Moretti MS, Gonçalves JFJr (2011a) Global distribution of a key trophic guild contrasts with common latitudinal diversity patterns. Ecol 92:1839–1848
Boyero L, Pearson RG, Gessner MO, Barmuta LA, Ferreira V, Graça MAS, Dudgeon D, Boulton AJ, Callisto M, Chauvet E, Helson JE, Bruder A, Albariño RJ, Yule CM, Arunachalam M, Davies JN, Figueroa R, Flecker AS, Ramírez A, Death RG, Iwata T, Mathooko JM, Mathuriau C, Gonçalves, JF, Moretti MS, Jinggut T, Lamothe S, M Erimba C, Ratnarajah L, Schindler MH, Castela J, Buria LM, Cornejo A, Villanueva V, West DC (2011b) A global experiment suggests climate warming will not accelerate litter decomposition in streams but might reduce carbon sequestration. Ecol Lett 14:289–294
Boyero L, Pearson RG, Dudgeon D, Ferreira V, Graça MAS, Gessner MO, Boulton AJ, Chauvet E, Yule CM, Albariño RJ, Ramírez A, Helson JE, Callisto M, Arunachalam M., Chará J, Figueroa R, Mathooko JM, Gonçalves JF, Moretti MS, Chará-Serna AM, Davies JN, Encalada A, Lamothe S, Buria LM, Castela J, Cornejo A, Li AOY, M’Erimba C, Villanueva VD, Zúñiga C, Swan CM, Barmuta LA (2012) Global patterns of stream detritivore distribution: Implications for biodiversity loss in changing climates. Global Ecol Biogeog 21:134–141
Boyero L, Pearson R, Gessner MO, Dudgeon D, Ramirez A, Yule C, Callisto M, Pringle C, Encalada A, Arunachalam M, Mathooko J, Helson J, Rincon J, Bruder A, Cornejo A, Flecker AS, Mathuriau C, Merimba C, Moretti MS, Gonçalves JF, Jinggut T (2015a) Leaf-litter breakdown in tropical streams: Is variability the norm? Freshwat Sci. doi:10.1086/681093
Boyero L, Pearson R, Swan CM, Hui C, Albarino R, Arunachalan R, Callisto M, Chara J, Chara-Serna AM, Chauvet E, Cornejo A, Dudgeon D, Encalada A, Ferreira V, Gessner MO, Gonçalves JF, Graça MAS, Helson J, MathookoJ, McKie B, Moretti MS, Yule C (2015b) Latitudinal gradient of nestedness and its potential drivers in stream detritivores. Ecography 38:1–7
Callisto M, Goulart MD (2005) Invertebrate drift along a longitudinal gradient in a Neotropical stream in Serra do Cipó National Park, Brazil. Hydrobiol 539:47–56
Callisto M, Graça MAS (2013) The quality and availability of fine particulate organic matter for collector species in headwater streams. Int Rev Hydrobiol. doi:10.1002/iroh.201301524
Callisto M, Goulart M, Medeiros AO, Moreno P, Rosa CA (2004) Diversity assessment of benthic macroinvertebrates, yeasts, and microbiological indicators along a longitudinal gradient in Serra do Cipó, Brazil. Braz J Biol 64:743–755
Callisto M, Gonçalves JF, Graca MAS (2007) Leaf litter as a possible food source for chironomids in headwater streams. Rev Brasil Zool 24:442–448
Castro DMP, Hughes RM, Callisto M (2013a) Effects of flow fluctuations on the daily and seasonal drift of invertebrates in a tropical river. Ann Limnol Int J Limnol. doi:10.1051/limn/2013051
Castro DMP, Hughes RM, Callisto M (2013b) Influence of peak flow changes on the macroinvertebrate drift downstream of a Brazilian hydroelectric dam. Braz J Biol 73:775–782
Clarke A, MacNally R, Bond N, Lake PS (2008) Macroinvertebrate diversity in headwater streams: A review. Freshwat Biol 53:1707–1721
Collen B, Boehm M (2012) The growing availability of invertebrate extinction risk assessments—A response to Cardoso et al. (October 2011): Adapting the IUCN red list criteria for invertebrates. Biol Conserv 149:145–146
Collen B, Whitton F, Dyer EE, Baillie JEM, Cumberlidge N, Darwall WRT, Richman NI, Pollock C, Soulsby A-M, Bohm M (2013) Global patterns of freshwater species diversity, threat and endemism. Global Ecol Biogeog. doi:10.1111/geb.12096
Dudgeon D, Arthington AH, Gessner MO, Kawabata Z-I, Knowler DJ, Lévêque C, Naiman RJ, Prieur-Richard A-H, Soto D, Stiassny MLJ, Sullivan CA (2006) Freshwater biodiversity: Importance, threats, status and conservation challenges. Biol Rev 81:163–182
Fernandes GW, Barbosa NPU, Negreiros D, Paglia AP (2014) Challenges for the conservation of vanishing megadiverse rupestrian grasslands. Nat Cons 12:162–165
Finn DS, Poff NL (2005) Variability and convergence in benthic communities along the longitudinal gradients of four physically similar rocky mountain streams. Freshwat Biol 50:243–261
França JS, Gregório RS, D’Arc JD, Gonçalves JF, Ferreira FA, Callisto M (2009) Composition and dynamics of allochthonous organic matter inputs and benthic stock in a Brazilian stream. Mar Fresh Res 60:990–998
Frissel CA, Liss WJ, Warren CE, Hurley MD (1986) A hierarchical framework for stream habitat classification—viewing streams in a watershed context. Environ Manag 10:199–214
Galdean N, Barbosa FAR, Callisto M, Rocha LA, Margarida MGSMM (1999) A proposed typology for the rivers of Serra do Cipó (Minas Gerais, Brazil) based on the diversity of benthic macroinvertebrates and the existing habitats. Trav Mus Natl Hist Nat Grigore Antipa 41:445–453
Galdean N, Callisto M, Barbosa FAR (2000) Lotic ecosystems of Serra do Cipó, Southeast Brazil: Water quality and a tentative classification based on the benthic macroinvertebrate community. Aq Ecosyst Heal Manag 3:545–552
Gonçalves JF, Callisto M (2013) Organic-matter dynamics in the riparian zone of a tropical headwater stream in Southern Brazil. Aquat Bot 109:8–13
Gonçalves JF, França JS, Callisto M (2006a) Dynamics of allochthonous organic matter in a tropical Brazilian headstream. Braz Arch Biol Technol 49:967–973
Gonçalves JF, França JS, Medeiros AO, Rosa CA, Callisto M (2006b) Leaf breakdown in a tropical stream. Int Rev Hydrobiol 91:164–177
Gonçalves JF, Graça MAS, Callisto M (2006c) Leaf-litter breakdown in 3 streams in temperate, mediterranean, and tropical Cerrado climates. J N Am Benthol Soc 25:344–355
Gonçalves JF, Graça MAS, Callisto M (2007) Litter decomposition in a cerrado savannah stream is retarded by leaf toughness, low dissolved nutrients and a low density of shredders. Freshwat Biol 52:1440–1451
Gonçalves JF, Martins RT, Ottoni BMP, Couceiro SRM (2014) Uma visão sobre a decomposição foliar em sistemas aquáticos brasileiros. In: Hamada N, Nessimian J.L, Querino R.B (eds). Insetos aquáticos na Amazônia brasileira: taxonomia, biologia e ecologia. 1ed. Manaus: Editora do INPA, vol. I, pp. 89–116
Hughes RM, Peck DV (2008) Acquiring data for large aquatic resource surveys: The art of compromise among science, logistics, and reality. J N Am Benthol Soc 27:837–859
Janzen DH (1974) Tropical blackwater rivers, animals, and mast fruiting by the dipterocarpaceae. Biotropica 6:69–103
Kominoski JS, Rosemond AD (2012) Conservation from the bottom up: Forecasting effects of global change on dynamics of organic matter and management needs for river networks. Freshwat Sci 31:51–68
Legendre P, Borcard D, Peres-Neto PR (2005) Analyzing beta diversity: Partitioning the spatial variation of community composition data. Ecol Monogr 75:435–450
Leite FSF, Juncá FA, Eterovick PC (2008) Status do conhecimento, endemismo e conservação de anfíbios anuros da Cadeia do Espinhaço, Brasil. Megadiv 4:182–200
Ligeiro R, Melo AS, Callisto M (2010a) Spatial scale and the diversity of macroinvertebrates in a neotropical catchment. Freshw Biol 55:424–435
Ligeiro R, Moretti MS, Gonçalves JF, Callisto M (2010b) What is more important for invertebrate colonization in a stream with low-quality litter inputs: Exposure time or leaf species? Hydrobiol 235:1–2
Macedo DR, Hughes RM, Ligeiro R, Ferreira W, Castro MA, Junqueira NT, Oliveira DR, Firmiano KR, Kaufmann PR, Pompeu PS, Callisto M (2014) The relative influence of catchment and site variables on fish and macroinvertebrate richness in cerrado biome streams. Landsc Ecol 29:1001–1016
Medeiros AO, Callisto M, Graça MAS, Ferreira V, Rosa CA, França JS, Eller AP, Rezende RS, Gonçalves JF (2015) Microbial colonization and litter decomposition in a cerrado stream is limited by low dissolved nutrient concentration: Evidence from a manipulative experiment. Limnetica (in press) 34:283–292
Melo AS, Shneck F, Hepp LU, Simões NR, Siqueira T, Bini LM (2011) Focusing on variation: Methods and applications of the concept of beta diversity in aquatic ecosystems. Acta Limnol Bras 23:318–331
Moretti M, Gonçalves JF, Callisto M (2007a) Leaf breakdown in two tropical streams: Differences between single and mixed species packs. Limnol 37:250–258
Moretti MS, Gonçalves JF, Ligeiro R, Callisto M (2007b) Invertebrates colonization on native tree leaves in a neotropical stream (Brazil). Int Rev Hydrobiol 92:199–210
Piggott JJ, Nyougi DK, Townsend CR, Mathaei CD (2015) Multiple stressors and stream ecosystem functioning: Climate warming and agricultural stressors interact to affect processing of organic matter. J Appl Ecol. doi:10.1111/1365-2664.12480
Stendera S, Adrian R, Bonada B, Cañedo-Argüelles M, Hungueny B, Januschke K, Pletterbauer F, Hering D (2012) Drivers and stressors of freshwater biodiversity patterns across different ecosystems and scales: A review. Hydrobiol 696:1–28
Tuomisto H, Ruokolainen K (2006) Analyzing or explaining beta diversity? Understanding the targets of different methods of analysis. Ecol 87:2697–2708
Veech JA, Summerville KS, Crist TO, Gering JC (2002) The additive partitioning of species diversity: recent revival of an old idea. Oikos 99:3–9
Wantzen KM, Wagner R, Suetfeld R, Junk WJ (2002) How do plant–herbivore interactions of trees influence coarse detritus processing by shredders in aquatic ecosystems of different latitudes? Verh Int Ver Theor Angew Limnol 28:815–821
Whittaker RH (1960) Vegetation of the Siskiyou mountains, Oregon and California. Ecol Monog 26:1–80
Acknowledgments
The authors are grateful to Geraldo W. Fernandes for the invitation to contribute with this chapter and for his constructive comments on early versions. Manuel Graça and Irineu Bianchini offered carefull reading and insights to previous version of this chapter. Our studies have been supported by Conselho Nacional de Pesquisa e Desenvolvimento (CNPq), Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Fundação Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), P&D ANEEL (GT-487) and CEMIG-Programa Peixe Vivo. MC was awarded research productivity grants CNPq (No. 303380/2015-2) and CNPq research grant (446155/2014-4) and Minas Gerais researcher grant FAPEMIG PPM-77/13 and PPM IX-02/2015. JFG-Jr was supported by CNPq (Proc. 472.328/01–8), FAPEMIG (Proc. 1085/03, APQ-2051-5.03/07, and FAPEMIG/PRONEX 20/2006, 465/07) and PADI-Project AWARE Foundation. The authors are grateful for the logistical support provided by IBAMA, IEF-MG and the US Fish and Wildlife Service.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Callisto, M., Gonçalves, J.F., Ligeiro, R. (2016). Water Resources in the Rupestrian Grasslands of the Espinhaço Mountains. In: Fernandes, G. (eds) Ecology and Conservation of Mountaintop grasslands in Brazil. Springer, Cham. https://doi.org/10.1007/978-3-319-29808-5_5
Download citation
DOI: https://doi.org/10.1007/978-3-319-29808-5_5
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-29807-8
Online ISBN: 978-3-319-29808-5
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)