Abstract
Patent ductus arteriosus (PDA) continues to be a frequent complication of extremely premature birth, despite the more generalized use of antenatal steroids, postnatal surfactant administration, and the improvement of noninvasive ventilatory strategies. Its incidence is inversely related to gestational age, such that it affects almost 60% of infants less than 28 weeks’ gestation. Spontaneous closure of the ductus occurs in 30% of infants with very low birth weight (<1500 g). When the PDA is hampering the neonate’s well-being, judicious evaluation and tailoring of the best therapeutic strategy should be performed. Ibuprofen actually seems to be the preferred drug for pharmacological treatment because of its favorable risk/benefit ratio; the therapeutic response of the most immature infants however is limited. Surgical ligation of the duct should be considered as a backup treatment due to the risks of serious complications during and after the procedure.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
1 Salient Points
-
PDA continues to be a frequent complication of extremely premature birth (60% of infants less than 28 weeks’ gestation), despite the more generalized use of antenatal steroids, postnatal surfactant administration, and the improvement of noninvasive ventilatory strategies.
-
A large left to right shunt may cause profound hemodynamic disturbances to local organs and the brain.
-
Ibuprofen actually seems to be the preferred drug for pharmacological treatment because of its favorable risk/benefit ratio; the therapeutic response of the most immature infants however is limited.
-
Surgical ligation of the duct should be considered as a backup treatment due to the risks of serious complications during and after the procedure.
2 Epidemiology
Patent ductus arteriosus (PDA) is the most common cardiac abnormality of the preterm infant. Its incidence is inversely related to gestational age, such that it affects almost 60% of infants less than 28 weeks’ gestation. Data published in 2007 demonstrated that spontaneous closure of the ductus occurs in 30% of infants with birthweights below 1500 g (very low birth weight [VLBW]) (Fanaroff et al. 2007). It is obvious that percentages are decreasing for each lower birthweight category (Table 1), but also that a markedly wide variation exists among centers. This may reflect the influence of varying factors on the closure of the ductus.
Higher closing rates, and as a consequence lower incidences of PDA, have been reported in several papers for European infants. A randomized comparative multicenter trial that compared early (day 3) versus late (day 7) indomethacin treatment for PDA in preterm infants with gestational ages between 26 and 31 weeks and suffering from respiratory distress syndrome (RDS), revealed that 281 of 380 (74%) spontaneously closed their duct by the age of 7 days (Overmeire et al. 2001a). In 210 infants who received placebo in a double blind randomized trial of ibuprofen prophylaxis, high spontaneous closing rates on the 3rd to 4th day of life were confirmed by echocardiographic assessment (Overmeire et al. 2004a). Data are displayed for five birthweight and gestational age categories (Table 2).
Data from the neonatal registry of the Belgian college of physicians, demonstrate a closed or non-symptomatic PDA in 46.7% of 2635 VLBW infants for the years 2007–2009 (College of Physicians for the Mother and Newborn, Section Neonatology). As compared to these numbers, the closure rates described by Koch et al (2006) in the United States seem lower, e.g., 34% on the third day of life in a group of 122 infants with birth weights below 1000 g. Other US data report 31% closure below 1000 g and 67% in a group of 65 VLBW infants (Nemerofsky et al. 2008). It is clear that a wide variation exists in spontaneous ductal closure in preterm infants. In addition to immaturity, which is the factor that contributes most to PDA, various cofactors affect the occurrence of PDA. For many years, the presence of severe RDS has been recognized as a delaying factor for closure (Bancalari et al. 2005), as is the association with sepsis (Gonzalez et al. 1996). Intrauterine growth restriction also affects the behavior of the ductus (Robel-Tillig et al. 2003). The group of Rakza demonstrated convincingly that hypotrophic infants had significantly larger left to right shunts appearing earlier after birth and needing earlier treatment than eutrophic controls (Rakza et al. 2007). The authors explain that chronic hypoxia in utero may have caused a faster decrease of the pulmonary vascular resistance through the production of higher noradrenaline levels. Additionally, major anomalies of both the intima and media of the ductal vessal wall have been described (Ibara et al. 1994). It is a common belief that excessive fluid administration during the first days of life is associated with the occurrence of PDA. Unfortunately, poor evidence exists to support this view. A systematic meta-analysis on this topic was last updated in 2008. Four of the five included trials date from the era before antenatal steroids and exogenous surfactant therapy and the one from 1999 shows very weak evidence. Nevertheless, the authors conclude that the practice of restricting water intake to physiological needs in preterm infants might be expected to decrease the risk of PDA (Bell and Acarregui 2008). The role of cortisol was highlighted by the studies of Watterberg demonstrating an inverse relationship between serum cortisol levels on postnatal days 3–4 and 5–6 and the occurrence of PDA (Watterberg et al. 2000). It is known that both antenatal (Abbasi et al. 2000; Chorne et al. 2007a) and postnatal steroid administration are associated with lower incidences of PDA (Halliday et al. 2001; Doyle et al. 2010). The prenatal use of NSAIDs as tocolytics has an impact on the postnatal reactivity of the ductus. Indomethacin and ibuprofen freely cross the placenta (Hammerman et al. 1998; Norton et al. 1993). During pregnancy they have a vasoconstrictive effect on the ductus and fetal pulmonary circulation which increases with advancing gestational age. Antenatally induced changes in the fetal ductal wall lead to a decreased reactivity of the ductus to postnatal NSAID treatment with an increased rate of treatment failure (Reese et al. 2009; Soraisham et al. 2010). The use of magnesium sulfate in pregnant women has been associated with a higher risk of developing PDA in extremely low birth weight infants (Moral et al. 2007) and with a reduced responsiveness to indomethacin prophylaxis (Katayama et al. 2010). Other large studies did not confirm these observations (Rouse et al. 2008).
Many pharmacologic agents that are commonly used in the early neonatal period may influence the behavior of the ductus. Although caffeine has no direct effect on preterm sheep ductus’ contractility (Clyman and Roman 2007), markedly less treatment for PDA was observed in the group of infants who received caffeine in a large multicenter clinical trial including 2,006 subjects (Schmidt et al. 2006a). In contrast to what was suggested years ago, there actually seems less evidence for a reducing effect of the postnatal administration of thyroid hormone (Osborn and Hunt 2007) and the shielding of the thorax during phototherapy (Travadi et al. 2006) on the occurrence of PDA. The administration of furosemide, by increasing circulating prostaglandin E2 levels, has been associated with an increased incidence of PDA in a study of 66 infants with RDS (Green et al. 1983). Six of seven studies that investigated the use of furosemide in preterm infants with RDS of less than 5 days of age were performed before the systematic application of prenatal steroids, surfactant or fluid restriction. However, a systematic review combining the trials indicated an increased risk of PDA and hypovolemia (Brion and Soll 2008). More recent data on the concomittant use of furosemide during NSAID treatment for PDA underscore the risk of inducing transient renal failure (Toyoshima et al. 2010) but do not confirm the clinical effect of delaying the closure of the ductus (Andriessen et al. 2009; Lee et al. 2009).
Various other frequently used drugs in the NICU may have unexpected vasodilatory actions on the ductus. To date, no clinically relevant effect on the behavior of the ductus has been described by the inhalation of nitric oxide (Schreiber et al. 2003; Askie et al. 2010; Mercier et al. 2010). Aminoglycosides, cimetidine, ranitidine and heparin are other possible candidates with dilating effects on the ductus meriting further prospective evaluation (Reese et al. 2010).
3 Physiology and Pathogenesis
During fetal life the patency of the ductus is essential. It allows 90% of the right ventricular output to bypass the highresistance pulmonary vascular bed. Fetal patency of the ductus before birth and the closure after birth is the result of a balanced interaction of locally produced and circulating mediators, low oxygen tension and the unique structure of the vessel wall. Prostaglandins, converted from arachidonic acid by COX enzymes, play a major role in maintaining ductal patency in utero. There are two separate genes encoding COX proteins, COX-1 and COX-2, and a variant of COX-1 informally termed COX-3 has also been identified (Chandrasekharan et al. 2002). COX-1 is mostly constitutive whereas COX-2 is highly expressed during inflammation. COX-2 has been found to be increasingly expressed in the fetal ductus arteriosus with advancing gestation and is the main contributor to local PGE2 generation in the ductus at term (Smith et al. 2001). Of the five major prostanoids (PGE2, PGF2α, PGD2, PGI2 and TXA2), predominantly PGE2 and PGI2 have high levels in the fetus because of high placental production and low clearance by the fetal lungs. They are the most potent ductal relaxants.
The increase in postnatal oxygen tension along with a decreased sensitivity of the ductus to PGE2 as the fetus approaches term, initiate the closure of the ductus (Schneider and Moore 2006). In the term infant constriction begins within the first few hours after birth and functional closure is usually completed within 24–48 h of age.
After the initial functional constriction, a phase of neointimal thickening and remodeling occurs, leading to anatomical occlusion after several days (Clyman et al. 1998). Recent experiments have shown that platelets play a crucial role by promoting a thrombotic sealing and supporting luminal remodeling for the definite closure of the ductus (Echtler et al. 2010; Clyman and Chemtob 2010).
In the preterm infant the closure usually takes longer. The immature ductus has an increased sensitivity to PGE2 and its constriction after birth is weaker. At comparable O2 concentrations the ductus of the very immature fetus generates far lower tensions than those observed in the near-term fetus precluding definitive closure. NO synthase expression in the vasa vasorum of the vessel media is further increased and impedes the critical degree of contraction which is essential to elicit tissue hypoxia, initate remodeling and ultimately definitive closure of the ductus (Kajino et al. 2001). This carries an increased risk of reopening. A similar mechanism is believed to contribute to the failure of ductal closure of fetus previously exposed to maternal indomethacin (Clyman et al. 2001). In addition, there is evidence that the major vasorelaxant nitric oxide (NO) plays a role in the very immature ductus. Inhibition of NO synthase was found to exert a greater effect in closing the ductus of the more immature fetus whereas COX inhibition exerted a greater effect in the near-term fetus. The combined treatment with NO synthase and COX inhibitors was more effective than simply using COX inhibitors, but severe side effects precluded the introduction of this approach in clinical practice (Seidner et al. 2001).
4 Pathophysiology
Almost all morbidity associated with PDA in preterm infants may be explained by the persistent left to right shunt through the vessel, causing a systemic hypoperfusion and a pulmonary hyperperfusion. In most infants, the heart can cope with the increasing demands by increasing left ventricular stroke volume and heart rate.
4.1 Pulmonary Effects
The lungs receive an increased perfusion which may lead to decreased lung compliance, lung edema, and increased risk of pulmonary hemorrhage (Stefano et al. 1991; McCurnin et al. 2008). Symptoms and signs appear as the limits of compensatory mechanisms are reached. An association between the duration of the left to right shunt and the development of bronchopulmonary dysplasia has been demonstrated (Marshall et al. 1999), although the simultaneous presence of an infection largely increases the risk (Gonzalez et al. 1996). Unfortunately, in studies of prophylactic treatment of PDA the incidence of BPD was not reduced. This may be explained by the study design, as all infants in the control group were given a back-up treatment only 1–3 days after the randomization. Alternatively, indomethacin may promote BPD in itself by inducing moderate renal dysfunction leading to increased lung fluid (Schmidt et al. 2006b). Experiments in baboons confirmed that a negative influence of a moderate left to right shunt increases clearly pulmonary to systemic flow ratio and exerts a negative effect on pulmonary mechanics. The most striking observation was an arrested alveolar growth and a reduced alveolar branching causing a significantly lower alveolar surface area and complexicity in the subjects that were exposed to persisted shunting (McCurnin et al. 2008).
4.2 Systemic Effects
Other organ systems may suffer hypoperfusion, including the gut, kidneys and the brain. A persistent patency of the ductus has been shown to be a risk factor for increased mortality. (Brooks et al. 2005; Noori et al. 2009). Disturbances in renal perfusion have been shown (Romagnoli et al. 2000). The decrease in mesenteric blood flow (Meyers et al. 1991; McCurnin and Clyman 2008) has been associated with increased risk of gut ischemia, necrotizing enterocolitis (Coombs et al. 1990), and causing a prolonged interval to full feeds (Patole et al. 2007). In infants with PDA the superior mesenteric artery flow velocities were significantly lower postprandially as compared to those with closed duct (McCurnin and Clyman 2008).
4.3 Cerebral Effects
Already in the early 1980s hypoperfusion of the neonatal brain as a result of ductal left to right shunt was demonstrated by Doppler flow studies (Perlman et al. 1981) and confirmed repeatedly on many occasions with other imaging techniques (Lundell et al. 1986; Shortland et al. 1990).
More recently, an impressive lowering of the regional oxygen saturation in the brain could be visualized in infants with a moderate ductal shunt by use of near infrared spectroscopy (NIRS), and a prompt amelioration after closure of the ductus (Lemmers et al. 2008). Although PDA has been clearly associated with all above-mentioned morbidities, in many situations, its causal role is not straightforward.
5 Pharmacological Treatment
Because left to right shunt through the ductus is associated with increased morbidity and mortality and because prostaglandins play a major role in patency of the ductus, for decades cyclooxygenase inhibitors have been used to treat PDA (Evans 2003). Successful closure of the duct has been reported to occur in 40–80% of treated infants. The risk of reopening increases to above 20% in the smallest infants. Because the process of intimal cushion formation during closure of the duct is inhibited by blockade of prostaglandins in the most immature infants, COX inhibitors are paradoxically less effective in extremely preterm neonates (Yokoyama et al. 2006; Ivey and Srivastava 2006). Until 2004, indomethacin was the only COX inhibitor approved for treatment of PDA in most countries. Since late 1990s ibuprofen has been studied as an alternative for the treatment and prophylaxis of PDA.
The decision to start with pharmacological treatment in an individual infant depends on many factors. It is obvious that if factors in favor of a spontaneous closure of the duct are present, many clinicians will postpone pharmacological treatment by a few days. The appearance of signs and symptoms in the lungs or other organ systems should motivate to the initiation of more prompt treatment.
Schematically, three approaches are possible. Firstly, a conservative management with restriction of fluids combined with optimized respiratory support including positive end expiratory pressure; secondly, the pharmacological treatment with either ibuprofen or indomethacin; and finally surgical ligation of the ductus. Benefits and risks of each approach should be balanced, taken into account the specific characteristics of the infant such as birth weight, postnatal age, antenatal exposure to corticosteroids or NSAIDs, and the presence of comorbidities or infection.
5.1 Hemodynamic Significance of the PDA-Shunt
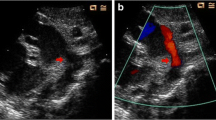
Because invasive catheterization is not possible in small preterm infants, echocardiographic assessment is the method of choice to evaluate ductal patency and magnitude of PDA shunting (Kluckow et al. 2008). The technique allows monitoring the effects of intervention and has been used to guide both doses and the duration of pharmacological treatment (Sperandio et al. 2005; Carmo et al. 2009; Waal and Kluckow 2010). It is generally accepted that a left atrium-to-aortic-root diameter ratio of ≥1.4 in the parasternal long axis view, a ductal diameter of 1.4 mm/kg body weight, and flow reversal in diastole in the descending aorta, indicate a hemodynamically significant left to right shunt (El Hajjar et al. 2005). When the mean end diastolic flow velocity in the left pulmonary artery exceeds 0,2 m/s, there needs to be an additional high index of suspicion of significant left to right shunt (Sehgal and McNamara 2009).
Because frequent echocardiographic studies may destabilize the infant and in some NICUs it is not easy to obtain a bedside cardiac ultrasound, biomarkers such as B-type natriuretic peptide (BNP), NT-pro-BNP and cardiac troponin T (cTNT) are now more frequently used to detect “symptomatic” PDA and to guide treatment (Holmström et al. 2001; Choi et al. 2005; Sanjeev et al. 2005; Attridge et al. 2009). BNP is a vasoregulatory peptide that is released by the ventricles in response to increases in cardiac volume and pressure (El-Khuffash and Molloy 2008). BNP levels did correlate very well with the magnitude of the left to right shunt as examined by echocardiography and reflected the moment of closure. Nevertheless, the high variability in the BNP measurements preclude their use as monitoring for changes in shunt magnitude (Chen et al. 2010). The combination of measurement of biomarkers as a screening tool in those centers where echocardiography is not readily available, a high clinical suspicion and a comprehensive cardiac ultrasound and Doppler assessment, is probably an optimal approach to guide timely treatment of PDA (Chiruvolu et al. 2009).
5.2 Prophylaxis
A vast amount of studies have investigated the timing of pharmacological treatment of PDA. Prophylactic, early pre-symptomatic and symptomatic treatment strategies have been compared (Clyman 1996). More than 30 studies and reviews addressed prophylaxis with non-steroidal anti-inflammatory drugs (NSAIDs) in preterm infants. The last updated meta-analyses available demonstrate that both prophylactic indomethacin and ibuprofen reduce significantly the risk of developing a symptomatic PDA and the need for ductal ligation (Ohlsson and Shah 2011; Fowlie et al. 2010). Nineteen prophylactic indomethacin trials were eligible in which 2,872 infants were included. The incidence of symptomatic PDA was very significantly reduced: typical relative risk (RR) 0.44; 95% confidence interval (CI) 0.38–0.50. In the studies eligible for the ibuprofen review the ductus had closed spontaneously in 58% of the infants of the control group by the third day of life. Based on available data the prophylactic administration of ibuprofen cannot be recommended. Indomethacin offers the additional effect of reducing the occurrence of intraventricular hemorrhage (IVH) (typical RR 0.66 95% CI 0.53–0.82). No positive effect on any other outcome parameter could be demonstrated. In particular, no decrease in the rates of bronchopulmonary dysplasia, death, necrotizing enterocolitis, or white matter disease. Notwithstanding the decrease of IVH, no improved neurodevelopmental outcome has been demonstrated (Fowlie et al. 2010; Schmidt et al. 2001).
5.3 Treatment
Indomethacin and ibuprofen have also been extensively studied for the treatment of PDA. Ibuprofen disturbs significantly less regional circulations, which may offer less dysfunction of kidney, intestines, and brain (Overmeire et al. 1997; Patel et al. 2000; Pezzati et al. 1999). Urine production was less affected in preterm infants (n = 148) that were treated on day 2–3 of life with ibuprofen than in those receiving indomethacin (Overmeire et al. 2000). This observation resulted in ibuprofen being introduced for clinical use in preterm infants. Necrotizing enterocolitis was diagnosed twice as often in the indomethacin group (8 versus 4; p = 0,37) (Overmeire et al. 2000). Cerebral oxygen availability has repeatedly been shown to be less affected with ibuprofen (Patel et al. 2000; Mosca et al. 1997). Systematic reviews of the trials that evaluated indomethacin or ibuprofen for early treatment of a non-symptomatic or symptomatic PDA confirmed a comparable efficacy of both drugs (Thomas et al. 2005). The most recently updated systematic meta-analysis included 20 studies (Ohlsson et al. 2010). There was no statistically significant difference between indomethacin and ibuprofen efficacy or in failure to close the ductus in 19 comparative studies including 956 infants with a typical relative risk (RR) of 0.94 (95% CI 0.76–1.17). Infants receiving ibuprofen treatment have less evidence of transient renal failure and less risk of developing necrotizing enterocolitis: 15 studies including 865 infants; typical RR 0.68 (95% CI 0.47–0.99). No other differences were noted, e.g., mortality, reopening of the ductus, need for ligation, duration of ventilatory support, duration of supplementary oxygen and development of BPD.
The conventional doses of indomethacin and ibuprofen are different, based on their respective pharmacokinetic parameters. Adapted dosing schemes have been tried to augment efficacy/side effect ratio of indomethacin, however, with variable success (Herrera et al. 2007) (see also section “NSAID Dosing Schemes”).
5.4 Risks of Treatment
Indomethacin and ibuprofen are not devoid of adverse drug reactions. Due to their vasoconstrictive effects, both drugs affect the perfusion of various organ systems to a certain extent, e.g., the gut, kidney and brains. Although ibuprofen initially seemed to have a more favorable safety profile with less disturbance of regional circulations (Overmeire et al. 1997, 2000; Patel et al. 2000; Pezzati et al. 1999; Mosca et al. 1997), the occurrence of unexpected hypoxemia caused a lot of concern. Acute pulmonary hypertension developed in three preterm infants immediately after the infusion of ibuprofen THAM-buffered solution (Gournay et al. 2002). A clear explanation for this phenomenon is lacking. One infant of 169 treated with ibuprofen-lysine presented the same adverse effect, but no causal relationship could be found between PPHN and ibuprofen-lysine in another 229 treated infants (Overmeire et al. 2004a; Mosca et al. 2002; Aranda et al. 2009). Additional studies could not confirm a clear association between the use of ibuprofen-lysine and severe hypoxemia. Although the trial that first reported this serious adverse effect was halted by the national authorities (Gournay et al. 2004), the ibuprofen formulation under investigation became registered for clinical use in Europe in 2004. Ibuprofen-lysine was registered for treatment and prophylaxis of PDA in preterm infants in the US in 2006. Ibuprofen is insoluble in water and more than 90% bound to serum albumine (Aranda et al. 1997). An in vitro model demonstrated that at higher ibuprofen concentrations (750 μmol/L or 150 mg/L) in infant serum containing bilirubin, the fraction of unbound bilirubin increases fourfold (Ahlfors 2004). This displacement of bilirubin from albumin binding sites may be clinically relevant in jaundiced preterm infants, as high levels of free bilirubin are associated with brain damage, hearing loss and kernicterus (Ahlfors 2004; Ahlfors et al. 2006). When ibuprofen is administered in the recommended dosages of 10, 5 and 5 mg/kg at 24 hourly intervals to neonatal infants, ibuprofen serum peak levels of 20–40 mg/L are reached (Overmeire et al. 2001b). According to the in vitro model, an increase of 10% of the free fraction of bilirubin can be expected at such ibuprofen levels. Three smaller studies exploring the rise of unbound bilirubin were rather reassuring (Overmeire et al. 2004b; Amin and Miravalle 2011; Diot et al. 2010).
Additional risks of NSAID use for closing PDA in preterm infants are the occurrence of an isolated ileal perforation, particularly when there is concomitant use of steroids (Watterberg et al. 2004; Paquette et al. 2006). Although predominantly reported for indomethacin, the risk may be similar for ibuprofen as its mechanism is related to microvascular changes and not to disturbances of the regional perfusion of the gut (Tatli et al. 2004). Increased bleeding tendency has been described after indomethacin use (Corazza et al. 1984), but except for some increased occult blood appearing in stools this does not seem to cause clinically relevant problems. Some neonatal intensive care units are reluctant to continue or introduce enteral feedings during NSAID treatment. Eighty percent of the infants that participated in the European trials of prophylactic and early therapeutic NSAID for PDA were receiving trophic feedings without any apparent untoward effect (Overmeire et al. 2000, 2001a, 2004a; Gournay et al. 2004). The most recent meta-analysis showed that necrotizing enterocolitis is less likely to occur post ibuprofen than post indomethacin treatment (Ohlsson et al. 2010).
5.5 NSAID Dosing Schemes
The pharmacokinetic parameters vary widely in preterm infants due to physiological changes that occur after birth. Studies have shown large prolonged half-life for indomethacin (11–36 h) and reduced clearance rate in the preterm infant, both of which reveal marked interindividual variations (Thalji et al. 1980; Shaffer et al. 2002). The volume of distribution also varies and is influenced by the patency of the ductus (Gal et al. 1991). Accordingly, optimal therapeutic dosing is difficult to establish (Guimarães et al. 2009). The most widely used doses for indomethacin are three times 0.1–0.25 mg/kg administered every 12–24 h. The lower dose with longer interval is recommended when treatment is initiated on the first day. The registered dosing scheme for ibuprofen consists of a first dose of 10 mg/kg followed by a second and third dose of 5 mg/kg at 24 hourly intervals.
In order to improve efficacy/side effect ratio of NSAID during treatment for PDA, a variety of adapted dosing schemes have been studied. As it has been observed that PGE2 production resurges within 5 days of indomethacin treatment (Seyberth 1983), a prolonged course consisting of additional indomethacin doses has been tried (Herrera et al. 2007). Unfortunately, no significant benefit for closure rates, not less reopenings or ligations, and no improvement for BPD, IVH or mortality rates has been obtained. A lower proportion of infants with diminished urine output was observed (typical RR 0.27; 95% CI 0.13–0.6) but at the expense of an increased risk of NEC (RR 1.87; 95% CI 1.07–3.27) (Herrera et al. 2007).
The continuous infusion of indomethacin has been advocated to avoid disturbances in cerebral perfusion (Christmann et al. 2002), but was reported later to be less effective in extremely low birthweight infants (Vries et al. 2005). Interestingly, by applying a stepwise increasing dosage of indomethacin based on echographic evaluation, the group of Sperandio et al (Sperandio et al. 2005) obtained ductal closing rates up to 80% without apparently increasing side effects. After an initial standard treatment, subsequent doses were given if the ductus persisted as assessed by echocardio- Doppler. Cumulative indomethacin doses were as high as 6 mg/kg (Sperandio et al. 2005). As the efficacy of ibuprofen at extremely low gestational ages is comparably reduced to indomethacin (Su et al. 2008), the administration of additional doses has been investigated. In a group of 25 VLBW infants that received a second course of three doses of ibuprofen the additional closure was 48% (Lago et al. 2002). Su et al (Su et al. 2008) obtained an additional closure rate of 50% by administrating up to six doses of ibuprofen. Similar efficacy was demonstrated after the first and second course in a study population of 160 infants with birthweight below 1000 g with a cumulative closure rate of 65% (Richards et al. 2009). In order to optimize the efficacy of ibuprofen, an adapted dosing scheme based on postnatal age has been proposed (Hirt et al. 2008). Further studies are warranted to investigate whether an individualized ibuprofen dosing scheme would result in an increased benefit/risk ratio.
6 Surgical Treatment
Since the introduction of indomethacin in the 1980s, surgical ligation has been reserved for those infants for whom treatment with NSAIDs fails or is contraindicated (Gersony et al. 1983). Beyond the third to fourth week of life, efficacy of COX-inhibitors rapidly declines as the patency of the ductus is less regulated by prostaglandins. Ligation then becomes an option, however it remains nonetheless associated with a number of complications such as pneumothorax, bleeding, IVH, chylothorax as a result of thoracic duct injury, vocal cord paralysis, wound infection, hypotension, and left ventricular dysfunction in the days after the procedure (Little et al. 2003; Sørensen et al. 2010; McNamara et al. 2010; Seghaye et al. 1997; Moin et al. 2003). The morbidity rate ranges from 1 to 16% and mortality from 0% to 10%. In a large retrospective study, surgical ligation and indomethacin treatment for significant PDA were associated with a comparable risk for NEC or NEC-related gastrointestinal complications (O’Donovan et al. 2003) but mechanics of breathing significantly improved after ligation (Szymankiewicz et al. 2004). However, there are no recent controlled comparisons between pharmacological, conservative and surgical methods of PDA closure. Only one trial in 1983 enrolling 154 infants, compared initial surgical ligation versus medical treatment (Gersony et al. 1983). No statistically significant difference was found between the groups for mortality, chronic lung disease, NEC or IVH. An increase in pneumothorax (RR = 2.68; CI 1.45–4.03) and retinopathy of prematurity (RR = 3.80; CI 1.12–12.93) was observed in the ligated group. The use of prophylactic ligation cannot be recommended based on the limited current evidence (Mosalli and Alfaleh 2008). Additional data from the TIPP study indicated that ligation of the ductus was associated with a higher risk of BPD, severe retinopathy of prematurity and neurosensory impairment (Kabra et al. 2007). A retrospective analysis of 446 infants showed that ligation was significantly associated with chronic lung disease (Chorne et al. 2007b). In a group of 20 infants, a further decrease of oxygenation of the brain was observed by use of near infra red spectroscopy (Lemmers et al. 2010). No increased brain injury, however, was noted in a preterm baboon model (Loeliger et al. 2009). Because of the associated risks of ductal ligation in preterm infants in recent papers and the absence of recent comparative clinical trials, the primary surgical closure of the ductus does not seem advisable (Malviya et al. 2008).
7 Conclusions
PDA continues to be a frequent complication of extremely premature birth, despite the more generalized use of antenatal steroids, postnatal surfactant administration and the improvement of non-invasive ventilatory strategies. Many perinatal factors influence the development of a symptomatic PDA. A large left to right shunt may cause profound hemodynamic disturbances to local organs and the brain. From the NSAIDs studied, ibuprofen actually seems to be the preferred drug for pharmacological treatment because of its more favorable benefit/risk ratio. The therapeutic response of the most immature infants, however, is limited for all investigated COX-inhibitors. Surgical ligation of the duct should be considered as a backup treatment, because it carries the risk of serious complications and destabilization of the infant. Unfortunately, in spite of many studies, investigations and trials, there is no generally accepted and conclusive evidence to decide at which moment and to which infants the treatment of the ductus should be pursued in order to improve their long-term outcome.
References
Abbasi S, Hirsch D, Davis J et al (2000) Effect of single versus multiple courses of antenatal corticosteroids on maternal and neonatal outcome. Am J Obstet Gynecol 182:1243–1249
Ahlfors CE (2004) Effect of ibuprofen on bilirubin-albumin binding. J Pediatr 144:386–388
Ahlfors CE, Marshall GD, Wolcott DK et al (2006) Measurement of unbound bilirubin by the peroxidase test using zone fluidics. Clin Chim Acta 365:78–85
Amin SB, Miravalle N (2011) Effect of ibuprofen on bilirubin-albumin binding affinity in premature infants. J Perinat Med 39:55–58
Andriessen P, Struis NC, Niemarkt H et al (2009) Furosemide in preterm infants treated with indomethacin for patent ductus arteriosus. Acta Paediatr 98:797–803
Aranda JV, Varvarigou A, Beharry K et al (1997) Pharmacokinetics and protein binding of intravenous ibuprofen in the premature newborn infant. Acta Paediatr 86:289–293
Aranda JV, Clyman R, Cox B et al (2009) A randomized, doubleblind, placebo-controlled trial on intravenous ibuprofen l-lysine for the early closure of nonsymptomatic patent ductus arteriosus within 72 hours of birth in extremely low-birth-weight infants. Am J Perinatol 26:235–245
Askie LM, Ballard RA, Cutter G et al (2010) Inhaled nitric oxide in preterm infants: a systematic review and individual patient data meta-analysis. BMC Pediatr 10:15
Attridge JT, Kaufman D, Lim DS (2009) B-type natriuretic peptide to guide therapy of patent ductus arteriosus. Arch Dis Child Fetal Neonatal Ed 94:F178–F182
Bancalari E, Claure N, Gonzalez A (2005) Patent ductus arteriosus and respiratory outcome in premature infants. Biol Neonate 88:192–201
Bell EF, Acarregui MJ (2008) Restricted versus liberal water intake for preventing morbidity and mortality in preterm infants. Cochrane Database Syst Rev (1):CD000503
Brion LP, Soll RF (2008) Diuretics for respiratory distress syndrome in preterm infants. Cochrane Database Syst Rev (1):CD001454
Brooks JM, Travadi JN, Patole SK et al (2005) Is surgical ligation of patent ductus arteriosus necessary? The Western Australian experience of conservative management. Arch Dis Child 90:F235–F239
Carmo KB, Evans N, Paradisis M (2009) Duration of indomethacin treatment of the preterm patent ductus arteriosus as directed by echocardiography. J Pediatr 155:819–822
Chandrasekharan NV, Dai H, Roos KL et al (2002) COX-3, a cyclooxygenase-1 variant inhibited by acetaminophen and other analgesic/antipyretic drugs: cloning, structure, and expression. Proc Natl Acad Sci 99:13926–13931
Chen S, Tacy T, Clyman R (2010) How useful are B-type natriuretic peptide measurements for monitoring changes in patent ductus arteriosus shunt magnitude? J Perinatol 30:780–785
Chiruvolu A, Punjwani P, Ramaciotti C (2009) Clinical and echocardiographic diagnosis of patent ductus arteriosus in premature neonates. Early Hum Dev 85:147–149
Choi BM, Lee KH, Eun BL et al (2005) Utility of rapid B-type natriuretic peptide assay for diagnosis of symptomatic patent ductus arteriosus in preterm infants. Pediatrics 115:e255–e261
Chorne N, Jegatheesan P, Lin E et al (2007a) Risk factors for persistent ductus arteriosus patency during indomethacin treatment. J Pediatr 151:629–634
Chorne N, Leonard C, Piecuch R, Clyman RI (2007b) Patent ductus arteriosus and its treatment as risk factors for neonatal and neurodevelopmental morbidity. Pediatrics 119:1165–1174
Christmann V, Liem KD, Semmekrot BA, van de Bor M (2002) Changes in cerebral, renal and mesenteric blood flow velocity during continuous and bolus infusion of indomethacin. Acta Paediatr 91:440–446
Clyman RI (1996) Recommendations for the postnatal use of indomethacin: an analysis of four separate treatment strategies. J Pediatr 128:601–607
Clyman R, Chemtob S (2010) Vessel remodeling in the newborn: platelets fill the gap. Nat Med 16:33–35
Clyman RI, Roman C (2007) The effects of caffeine on the preterm sheep ductus arteriosus. Pediatr Res 62:167–169
Clyman RI, Waleh N, Black SM et al (1998) Regulation of ductus arteriosus patency by nitric oxide in fetal lambs: the role of gestation oxygen tension and vasa vasorum. Pediatr Res 43:633–644
Clyman RI, Chen YQ, Chemtob S et al (2001) In utero remodeling of the fetal lamb ductus arteriosus: the role of antenatal indomethacin and avascular zone thickness on vasa vasorum proliferation, neointima formation, and cell death. Circulation 103:1806–1812
College of Physicians for the Mother and Newborn, Section Neonatology. Federal Public Health Services, Belgium
Coombs RC, Morgan ME, Durbin GM et al (1990) Gut blood flow velocities in the newborn: effects of patent ductus arteriosus and parenteral indomethacin. Arch Dis Child 65:1067–1071
Cooper-Peel C, Brodersen R, Robertson A (1996) Does ibuprofen affect bilirubin-albumin binding in newborn infant serum? Pharmacol Toxicol 79:297–299
Corazza MS, Davis RF, Merrit TA et al (1984) Prolonged bleeding time in preterm infants receiving indomethacin for patent ductus arteriosus. J Pediatr 105:292–296
de Vries NK, Jagroep FK, Jaarsma AS et al (2005) Continuous indomethacin infusion may be less effective than bolus infusions for ductal closure in very low birth weight infants. Am J Perinatol 2:71–75
de Waal K, Kluckow M (2010) Functional echocardiography; from physiology to treatment. Early Hum Dev 86:149–154
del Moral T, Gonzalez-Quintero VH, Claure N et al (2007) Antenatal exposure to magnesium sulfate and the incidence of patent ductus arteriosus in extremely low birth weight infants. J Perinatol 27:154–157
Diot C, Kibleur Y, Desfrere L (2010) Effect of ibuprofen on bilirubin-albumin binding in vitro at concentrations observed during treatment of patent ductus arteriosus. Early Hum Dev 86:315–317
Doyle LW, Ehrenkranz RA, Halliday HL (2010) dexamethasone treatment in the first week of life for preventing bronchopulmonary dysplasia in preterm infants: a systematic review. Neonatology 98:217–224
Echtler K, Stark K, Lorenz M et al (2010) Platelets contribute to postnatal occlusion of the ductus arteriosus. Nat Med 16:75–82
El Hajjar M, Vaksmann G, Rakza T et al (2005) Severity of the ductal shunt: a comparison of different markers. Arch Dis Child Fetal Neonatal Ed 90:F419–F422
El-Khuffash AF, Molloy EJ (2008) Influence of a patent ductus arteriosus on cardiac troponin t levels in preterm infants. J Pediatr 153:350–353
Evans N (2003) Current controversies in the diagnosis and treatment of patent ductus arteriosus in preterm infants. Adv Neonatal Care 3:168–177
Fanaroff AA, Stoll BJ, Wright LL et al (2007) Trends in neonatal morbidity and mortality for very low birthweight infants. Am J Obstet Gynecol 196(147):e1–e8
Fowlie PW, Davis PG, McGuire W (2010) Prophylactic intravenous indomethacin for preventing mortality and morbidity in preterm infants. Cochrane Database Syst Rev (7):CD000174
Gal P, Ransom JL, Weaver RL et al (1991) Indomethacin pharmacokinetics in neonates: the value of volume of distribution as a marker of permanent patent ductus arteriosus closure. Ther Drug Monit 13:42–45
Gersony WM, Peckham GJ, Ellison RC et al (1983) Effects of indomethacin in premature infants with patent ductus arteriosus: results of a national collaborative study. J Pediatr 102:895–906
Gonzalez A, Sosenko IR, Chandar J et al (1996) Influence of infection on patent ductus arteriosus and chronic lung disease in premature infants weighing 1000 grams or less. J Pediatr 128:470–478
Gournay V, Savagner C, Thiriez G et al (2002) Pulmonary hypertension after ibuprofen prophylaxis in very preterm infants. Lancet 359:1486–1488
Gournay V, Roze JC, Kuster A et al (2004) Prophylactic ibuprofen versus placebo in very premature infants: a randomised, doubleblind, placebo-controlled trial. Lancet 364:1939–1944
Green TP, Thompson TR, Johnson DE, Lock JE (1983) Furosemide promotes patent ductus arteriosus in premature infants with the respiratory-distress syndrome. N Engl J Med 308:743–748
Guimarães H, Rocha G, Tomé T et al (2009) Non-steroid anti-inflammatory drugs in the treatment of patent ductus arteriosus in European newborns. J Matern Fetal Neonatal Med 22(Suppl 3):77–80
Halliday HL, Patterson CC, Halahakoon CW, Behalf of the European Multicenter Steroid Study Group (2001) A multicenter, randomized open study of early corticosteroid treatment (OSECT) in preterm infants with respiratory illness: comparison of early and late treatment and of dexamethasone and inhaled budesonide. Pediatrics 107:232–240
Hammerman C, Glaser J, Kaplan M et al (1998) Indomethacin tocolysis increases postnatal patent ductus arteriosus severity. Pediatrics 102:E56
Herrera C, Holberton J, Davis P (2007) Prolonged versus short course of indomethacin for the treatment of patent ductus arteriosus in preterm infants. Cochrane Database Syst Rev (2):CD003480
Hirt D, Van Overmeire B, Treluyer JM et al (2008) An optimized ibuprofen dosing scheme for preterm neonates with patent ductus arteriosus, based on a population pharmacokinetic and pharmacodynamics study. Br J Clin Pharmacol 65:629–636
Holmström H, Hall C, Thaulow E (2001) Plasma levels of natriuretic peptides and hemodynamic assessment of patent ductus arteriosus in preterm infants. Acta Paediatr 90:184–191
Ibara S, Tokunaga M, Ikenoue T et al (1994) Histologic observation of the ductus arteriosus in premature infants with intrauterine growth retardation. J Perinatol 14:411–416
Ivey KN, Srivastava D (2006) The paradoxical patent ductus arteriosus. J Clin Invest 116:2863–2865
Kabra NS, Schmidt B, Roberts RS et al (2007) Neurosensory impairment after surgical closure of patent ductus arteriosus in extremely low birth weight infants: results from the Trial of Indomethacin Prophylaxis in Preterms. J Pediatr 150:229–234
Kajino H, Chen YQ, Seidner SR et al (2001) Factors that increase the contractile tone of the ductus arteriosus also regulate its anatomic remodeling. Am J Phys Regul Integr Comp Phys 281:R291–R301
Katayama Y, Minami H, Enomoto M et al (2010) Antenatal magnesium sulfate and the postnatal response of the ductus arteriosus to indomethacin in extremely preterm neonates. J Perinatol 31:21–24
Kluckow M, Seri I, Evans N (2008) Echocardiography and the neonatologist. Pediatr Cardiol 29:1043–1047
Koch J, Hensley G, Roy L et al (2006) Prevalence of spontaneous closure of the ductus arteriosus in neonates at a birth weight of 1000 grams or less. Pediatrics 117:1113–1121
Lago P, Bettiol T, Salvadori S et al (2002) Safety and efficacy of ibuprofen versus indomethacin in preterm infants treated for patent ductus arteriosus: a randomised controlled trial. Eur J Pediatr 161:202–207
Lee BS, Byun SY, Chung ML et al (2009) Effect of furosemide on ductal closure and renal function in indomethacin-treated preterm infants during the early neonatal period. Acta Paediatr 98:797–803
Lemmers PMA, Toet MC, van Bel F (2008) Impact of patent ductus arteriosus and subsequent therapy with indomethacin on cerebral oxygenation in preterm infants. Pediatrics 121:142–147
Lemmers PM, Molenschot MC, Evens J et al (2010) Is cerebral oxygen supply compromised in preterm infants undergoing surgical closure for patent ductus arteriosus? Arch Dis Child Fetal Neonatal Ed 95:F429–F434
Little DC, Pratt TC, Blalock SE et al (2003) Patent ductus arteriosus in micropreemies and full-term infants: the relative merits of surgical ligation versus indomethacin treatment. J Pediatr Surg 38:492–496
Loeliger M, Inder TE, Dalitz PA et al (2009) Developmental and neuropathological consequences of ductal ligation in the preterm baboon. Pediatr Res 65:209–214
Lundell BP, Sonesson SE, Cotton RB (1986) Ductus closure in preterm infants. Effects on cerebral hemodynamics. Acta Paediatr Scand Suppl 329:140–147
Malviya M, Ohlsson A, Shah S (2008) Surgical versus medical treatment with cyclooxygenase inhibitors for symptomatic patent ductus arteriosus in preterm infants. Cochrane Database Syst Rev (1): CD003951
Marshall DD, Kotelchuck M, Young TE et al (1999) Risk factors for chronic lung disease in the surfactant era: a North Carolina population-based study of very low birth weight infants. North Carolina Neonatologists Association. Pediatrics 104:1345–1350
McCurnin D, Clyman RI (2008) Effects of a patent ductus arteriosus on postprandial mesenteric perfusion in premature baboons. Pediatrics 122:e1262–e1267
McCurnin D, Seidner S, Chang LY et al (2008) Ibuprofen-induced patent ductus arteriosus closure: physiologic, histologic, and biochemical effects on the premature lung. Pediatrics 121:945–956
McNamara PJ, Stewart L, Shivananda SP et al (2010) Patent ductus arteriosus ligation is associated with impaired left ventricular systolic performance in premature infants weighing less than 1000 g. J Thorac Cardiovasc Surg 140:150–157
Mercier JC, Hummler H, Durrmeyer X et al (2010) Inhaled nitric oxide for the prevention of bronchopulmonary dysplasia in premature babies (EUNO): a randomised controlled trial. Lancet 376:346–354
Meyers RL, Alpan G, Lin E, Clyman RI (1991) Patent ductus arteriosus, indomethacin, and intestinal distension: effects on intestinal blood flow and oxygen consumption. Pediatr Res 29:569–574
Moin F, Kennedy KA, Moya FR (2003) Risk factors predicting vasopressor use after patent ductus arteriosus ligation. Am J Perinatol 20:313–320
Mosalli R, Alfaleh K (2008) Prophylactic surgical ligation of patent ductus arteriosus for prevention of mortality and morbidity in extremely low birth weight infants. Cochrane Database Syst Rev (1):CD006181
Mosca F, Bray M, Lattanzio M et al (1997) Comparative evaluation of the effects of indomethacin and ibuprofen on cerebral perfusion and oxygenation in preterm infants with patent ductus arteriosus. J Pediatr 131:549–554
Mosca F, Bray M, Stucchi I, Fumagalli M (2002) Pulmonary hypertension after ibuprofen prophylaxis in very preterm infants. Lancet 360:1023–1024
Nemerofsky SL, Parravicini E, Bateman D et al (2008) The ductus arteriosus rarely requires treatment in infants 1000 grams. Am J Perinatol 25:661–666
Noori S, McCoy M, Friedlich P et al (2009) Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics 123:e138–e144
Norton ME, Merrill J, Cooper BA et al (1993) Neonatal complications after the administration of indomethacin for preterm labor. N Engl J Med 329:1602–1607
O’Donovan DJ, Baetiong A, Adams K et al (2003) Necrotizing enterocolitis and gastrointestinal complications after indomethacin therapy and surgical ligation in premature infants with patent ductus arteriosus. J Perinatol 23:286–290
Ohlsson A, Shah S (2011) Ibuprofen for the prevention of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev (7):CD004213
Ohlsson A, Walia R, Shah S (2010) Ibuprofen for the treatment of patent ductus arteriosus in preterm and/or low birth weight infants. Cochrane Database Syst Rev (4):CD003481
Osborn DA, Hunt RW (2007) Prophylactic postnatal thyroid hormones for prevention of morbidity and mortality in preterm infants. Cochrane Database Syst Rev (1):CD005948
Paquette L, Friedlich P, Ramanathan R, Seri I (2006) Concurrent use of indomethacin and dexamethasone increases the risk of spontaneous intestinal perforation in very low birth weight neonates. J Perinatol 26:486–492
Patel J, Roberts I, Azzopardi D et al (2000) Randomized doubleblind controlled trial comparing the effects of ibuprofen with indomethacin on cerebral hemodynamics in preterm infants with patent ductus arteriosus. Pediatr Res 47:36–42
Patole SK, Kumaran V, Travadi JN et al (2007) Does patent ductus arteriosus affect feed tolerance in preterm neonates? Arch Dis Child Fetal Neonatal Ed 92:F53–F55
Perlman JM, Hill A, Volpe JJ (1981) The effect of patent ductus arteriosus on flow velocity in the anterior cerebral arteries: ductal steal in the premature newborn infant. J Pediatr 99:767–771
Pezzati M, Vangi V, Biagiotti R et al (1999) Effects of indomethacin and ibuprofen on mesenteric and renal blood flow in preterm infants with patent ductus arteriosus. J Pediatr 135:733–738
Rakza T, Magnenant E, Klosowski S et al (2007) Early hemodynamic consequences of patent ductus arteriosus in preterm infants with intrauterine growth restriction. J Pediatr 151:624–628
Reese J, Waleh N, Poole SD et al (2009) Chronic in utero cyclooxygenase inhibition alters PGE2-regulated ductus arteriosus contractile pathways and prevents postnatal closure. Pediatr Res 66:155–161
Reese J, Veldman A, Shah L et al (2010) Inadvertent relaxation of the ductus arteriosus by pharmacologic agents that are commonly used in the neonatal period. Semin Perinatol 34:222–230
Richards J, Johnson A, Fox G, Campbell M (2009) A second course of ibuprofen is effective in the closure of a clinically significant PDA in ELBW infants. Pediatrics 124:e287–e292
Robel-Tillig E, Knüpfer M, Vogtmann C (2003) Cardiac adaptation in small for gestational age neonates after prenatal hemodynamic disturbances. Early Hum Dev 72:123–129
Romagnoli C, De Carolis MP, Papacci P et al (2000) Effects of prophylactic ibuprofen on cerebral and renal hemodynamics in very preterm neonates. Clin Pharmacol Ther 67:676–683
Rouse DJ, Hirtz DG, Thom E et al (2008) A randomized, controlled trial of magnesium sulfate for the prevention of cerebral palsy. N Engl J Med 359:895–905
Sanjeev S, Pettersen M, Lua J et al (2005) Role of plasma B-type natriuretic peptide in screening for hemodynamically significant patent ductus arteriosus in preterm neonates. J Perinatol 25:709–713
Schmidt B, Davis P, Moddemann D et al (2001) Long-term effects of indomethacin prophylaxis in extremely-low-birth-weight infants. N Engl J Med 344:1966–1972
Schmidt B, Roberts RS, Davis P et al (2006a) Caffeine therapy for apnea of prematurity. N Engl J Med 354:2112–2121
Schmidt B, Roberts RS, Fanaroff A et al (2006b) Indomethacin prophylaxis, patent ductus arteriosus, and the risk of bronchopulmonary dysplasia: further analyses from the Trial of Indomethacin Prophylaxis in Preterms (TIPP). J Pediatr 148:713–714
Schneider DJ, Moore JW (2006) Patent ductus arteriosus. Circulation 114:1873–1882
Schreiber MD, Gin-Mestan K, Marks JD et al (2003) Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med 349:2099–2107
Seghaye MC, Grabitz R, Alzen G et al (1997) Thoracic sequelae after surgical closure of the patent ductus arteriosus in premature infants. Acta Paediatr 86:213–216
Sehgal A, McNamara PJ (2009) Does echocardiography facilitate determination of hemodynamic significance attributable to the ductus arteriosus? Eur J Pediatr 168:907–914
Seidner SR, Chen YQ, Oprysko PR et al (2001) Combined prostaglandin and nitric oxide inhibition produces anatomic remodeling and closure of the ductus arteriosus in the premature newborn baboon. Pediatr Res 50:365–373
Seyberth HW (1983) Effect of prolonged indomethacin therapy on renal function and selected vasoactive hormones in VLBW infants with symptomatic patent ductus arteriosus. J Pediatr 103:979–984
Shaffer CL, Gal P, Ransom JL et al (2002) Effect of age and birth weight on indomethacin pharmacodynamics in neonates treated for patent ductus arteriosus. Crit Care Med 30:343–348
Shortland DB, Gibson NA, Levene MI et al (1990) Patent ductus arteriosus and cerebral circulation in preterm infants. Dev Med Child Neurol 32:386–393
Smith GC, Wu WX, Nijland MJ et al (2001) Effect of gestational age, corticosteroids, and birth on expression of prostanoid EP receptor genes in lamb and baboon ductus arteriosus. J Cardiovasc Pharmacol 37:697–704
Soraisham AS, Dalgleish S, Singhal N (2010) Antenatal indomethacin tocolysis is associated with an increased need for surgical ligation of patent ductus arteriosus in preterm infants. J Obstet Gynaecol Can 32:435–442
Sørensen CM, Steensberg JN, Greisen G (2010) Surgical ligation of patent ductus arteriosus in premature infants. Dan Med Bull 57:A4160
Sperandio M, Beedgen B, Feneberg R et al (2005) Effectiveness and side effects of an escalating, stepwise approach to indomethacin treatment for symptomatic patent ductus arteriosus in premature infants below 33 weeks of gestation. Pediatrics 116:1361–1366
Stefano JL, Abbasi S, Pearlman SA et al (1991) Closure of the ductus arteriosus with indomethacin in ventilated neonates with respiratory distress syndrome; effects on pulmonary compliance and ventilation. Am Rev Respir Dis 143:236–239
Su BH, Lin HC, Chiun HY et al (2008) Comparison of ibuprofen and indomethacin for early-targeted treatment of patent ductus arteriosus in extremely premature infants: a randomized controlled trial. Arch Dis Child 93:F94–F99
Szymankiewicz M, Hodgman JE, Siassi B, Gadzinowski J (2004) Mechanics of breathing after surgical ligation of patent ductus arteriosus in newborns with respiratory distress syndrome. Biol Neonate 85:32–36
Tatli MM, Kumral A, Duman N et al (2004) Spontaneous intestinal perforation after oral ibuprofen treatment of patent ductus arteriosus in two very-low-birthweight infants. Acta Paediatr 93:999–1001
Thalji AA, Carr I, Yeh TF et al (1980) Pharmacokinetics of intravenously administered indomethacin in premature infants. J Pediatr 97:995–1000
Thomas RL, Parker GC, Van Overmeire B, Aranda JV (2005) A meta-analysis of ibuprofen versus indomethacin for closure of patent ductus arteriosus. Eur J Pediatr 164:135–140
Toyoshima K, Momma K, Nakanishi T (2010) In vivo dilatation of the ductus arteriosus induced by furosemide in the rat. Pediatr Res 67:173–176
Travadi J, Simmer K, Ramsay J et al (2006) Patent ductus arteriosus in extremely preterm infants receiving phototherapy: does shielding the chest make a difference? A randomized, controlled trial. Acta Paediatr 95:1418–1423
Van Overmeire B, Follens I, Hartmann S et al (1997) Treatment of patent ductus arteriosus with ibuprofen. Arch Dis Child 76:F179–F184
Van Overmeire B, Smets K, Lecoutere D et al (2000) A comparison of ibuprofen and indomethacin for closure of patent ductus arteriosus. N Engl J Med 343:674–681
Van Overmeire B, Van de Broek H, Van Laer P et al (2001a) Early versus late indomethacin treatment for patent ductus arteriosus in premature infants with respiratory distress syndrome. J Pediatr 138:205–211
Van Overmeire B, Touw D, Schepens PJC et al (2001b) Ibuprofen pharmacokinetics in preterm infants with patent ductus arteriosus. Clin Pharmacol Ther 70:336–343
Van Overmeire B, Allegaert K, Casaer A et al (2004a) Prophylactic ibuprofen in premature infants: a multicentre, randomised, doubleblind, placebo-controlled trial. Lancet 364:1945–1954
Van Overmeire B, Vanhagendoren S, Schepens PJ, Ahlfors CE (2004b) The influence of ibuprofen-lysine on unbound bilirubin plasma levels in preterm neonates. Pediatr Res 55:474A
Watterberg KL, Scott SM, Backstrom C et al (2000) links between early adrenal function and respiratory outcome in preterm infants: airway inflammation and patent ductus arteriosus. Pediatrics 105:320–324
Watterberg KL, Gerdes JS, Cole CH et al (2004) Prophylaxis of early adrenal insufficiency to prevent bronchopulmonary dysplasia: a multicenter trial. Pediatrics 114:1649–1657
Yokoyama U, Minamisawa S, Quan H et al (2006) Chronic activation of the prostaglandin receptor EP4 promotes hyaluronan-mediated neointimal formation in the ductus arteriosus. J Clin Invest 116:3026–3034
Author information
Authors and Affiliations
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2018 Springer International Publishing AG, part of Springer Nature
About this entry
Cite this entry
Van Overmeire, B. (2018). Patent Ductus Arteriosus. In: Buonocore, G., Bracci, R., Weindling, M. (eds) Neonatology. Springer, Cham. https://doi.org/10.1007/978-3-319-29489-6_219
Download citation
DOI: https://doi.org/10.1007/978-3-319-29489-6_219
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-29487-2
Online ISBN: 978-3-319-29489-6
eBook Packages: MedicineReference Module Medicine