Abstract
The present work reports the synthesis of nanocomposites (NCs) prepared by amine functionalization of nanographite platlets (NGPs) coupled with diglycidyl ether of bisphenol A (DGEBA) epoxy resin. NGPs were treated with 3:1 mixture of concentrated H2SO4/HNO3, and then grafted with triethylene-tetramine (TETA) that contributes uniform dispersion of NGPs within the epoxy matrix. In particular, the amine functionalization of NGPs with triethylene-tetramine (TETA) purposed to attain better dispersion and strong interfacial interaction between the filler and the matrix. The TETA functionalized NGPs/epoxy nanocomposites (DGEBA/TETA-NGPs) were produced by molding curing method. The synthesized nanocomposites were characterized by FTIR and SEM techniques. The electrical and thermal properties of the nanocomposites at various TETA-NGP loadings were investigated and found to attain an increase in the conductivity and thermal stability compared with that of neat epoxy resin.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
Carbon fillers have gained high importance for their exceptional electrical, mechanical and thermal properties. Polymer nanocomposites reinforced with carbon fillers have been investigated for numerous potential applications [1]. In particular, the epoxy composites are extensively used for aerospace application, electronic materials and many other industrial applications [2]. Moreover, the synergistic effect between the epoxy matrix and the carbon filler can generates a superior nanocomposite material which hold the promise of providing high mechanical strength, lightweight, flexibility and multifunctional properties. However, the challenges faced while incorporating the carbon filler into the epoxy matrix are phase separation, aggregation, lack of interfacial bonding, poor dispersion and adhesion within the matrix. These problems can be overcome by functionalization of the reinforced carbon filler, which provides multiple bonding sites without affecting the desired properties to make them simply dispersible in the polymer matrix [3]. So far, various physical and chemical methods of carbon filler functionalization have been reported. Among them, the chemical modification by reactive functional group mainly the amine-functionalization of the carbon filler are much researched because of the high reactivity of the amine group to enhance the interfacial adhesion and homogeneous dispersion of the filler into the matrix [4, 5]. The chemical functionalization was proposed to provide the stable covalent bond between the filler and the matrix as compared to the weak Vander wall interaction acquired by non-functionalized filler. Moreover, after amine functionalization the hydrophobic carbon filler become more hydrophilic in nature due to presence the amine functional groups and can easily react with the functional groups present in the epoxy matrix [6].
In the present study efforts have been made to synthesize epoxy nanocomposite using nanographite platlets (NGPs) as filler and Bisphenol A diglycidyl ether (DGEBA) epoxy as matrix. The NGPs after the acid treatment were functionalized by the amine groups using triethlyenetetramine (TETA). Epoxy based nanocomposites were prepared by molding curing method. The main objective of the paper is to study the influence of the TETA functionalized NGPs on the covalent interaction and their effect on the electrical and thermal properties of the composite (DGEBA/TETA-NGPs) at various TETA-NGPs loadings. The morphology of the composites was analyzed. The development of such material has significant potential for various engineering application.
2 Experimental Details
2.1 Materials and Characterization
The natural graphite was purchased from (Asbury Carbon Inc) to prepare NGPs. Bisphenol A diglycidyl ether (DGEBA, Sigma-Aldrich), triethyltetramine (TETA, Sigma-Aldrich, 99 %), thionyl chloride (SOCl2, Sigma-Aldrich, 97 %), sulphuric acid (H2SO4, Merck), Nitric acid (HNO3, Merck) were used as received.
FTIR spectra were recorded using KBr pellet on a Nicolet 5700 FTIR spectrophotometer from 400 to 4000 cm−1 wave number at room temperature. The spectra were collected with a resolution of 4 cm−1 performing 32 scans. The morphology of the NGPs and epoxy nanocomposite samples were determined by scanning electron microscopy (SEM) [SEM, Zeiss (MA EVO-18 Special Edition)]. The sample preparation was executed by dispersion of the material in ethanol using ultra-sonication. The samples were gold coated after placing the small drop of suspended material on the silicon wafers. The thermal stability of the samples were measured by thermogravimetric analyzer (Mettler Toledo TGA/SDTA 851e) at temperature range of 25–700 °C under an inert atmosphere (flowing N2 gas). The standard two-probe technique (Keithley programmable current source-model 6517 B) was used to measure the conductivity of the films at room temperature. The rectangular films of (2 cm × 2 cm) were prepared for electrical measurement and the ohmic contacts were made on the films by using the silver paste.
2.2 Acid Treatment of NGPs
NGPs were prepared by the modified Hummer’s method as reported earlier by our group [7]. In a round bottom flask the pristine NGPs flakes were first treated with nitric acid (20 ml, 50 wt%). After 40 h reflux, the mixture was cooled at room temperature. Enormous amount of de-ionized water was diluted in the resulting mixture and then filtered using vacuum pump. The mixture was washed numerous times using de-ionized water until the pH reached 7, and then dried at 110 °C for 2 days under vacuum. The acid modified NGPs were obtained by 5 h ultrasonication of unmodified NGPs (U-NGPs) with H2SO4/HNO3 with (3:1) volume ratio at room temperature. The resulting solution was filtered and washed several times with deionized water till the solution pH reached neutral. Then the mixture was dried at 60 °C in vacuum and the A-NGPs were obtained.
2.3 TETA Modification of NGPs
The acid treated NGPS (A-NGPs) were functionalized with thionyl chloride for acylation. The reaction was performed with the treatment of thionyl chloride along with the DMF (N, N dimethyl formamide) solvent, and the mixture was allowed to stir at 70 °C and refluxed for 24 h. After the acyl functionalization, the solution was washed and extracted with anhydrous THF. The material was then dried and the solvent was evaporated in vacuum at 60 °C. The resulting acyl-chlorinated NGPs (AC-NGPs) were obtained. The surface acylated NGPs (AC-NGPs) were further treated with TETA at 100 °C for 96 h for amine functionalization. The mixture attained was washed by anhydrous ethanol to remove excessive TETA within the amine functionalized NGPs, and then filtered and dried in vacuum for 24 h. The TETA functionalized NGPs were then obtained.
2.4 Preparation of TETA-NGPs/DGEBA Composites
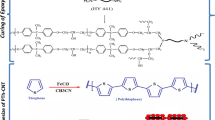
The composite of functionalized NGPs and epoxy matrix was prepared by molding curing method. In a typical experiment a 100 ml flask charged with proper amount of TETA-NGPs was dispersed in acetone. After 40 min sonication at room temperature, epoxy resin (DGEBA) was added to the black suspension. The reaction mixture was ultrasonicated at 50 °C until the acetone was removed completely. After stirring for 1 h, 10 % TETA curing agent was added and further stirring was performed in a magnetic stirrer for 6 h. The reaction mixture was degassed for 0.5 h to remove bubbles in a vacuum oven. After degasification, the reaction mixture was quickly poured into the stainless steel molds which were coated with the mold releasing agent. The curing time of 2 h at 50 °C and 2 h at 100 °C was obtained by the mixture in an oven. The epoxy nanocomposite was prepared at various compositions by varying the TETA-NGPs loadings (1, 2, 3, 4 and 5 %) in the epoxy matrix (DGEBA). Thus the modified TETA-NGPs/DGEBA were obtained at various TETA-NGPs loadings. The process of synthesis for preparing TETA-NGPs/DGEBA was depicted in Scheme 1.
3 Results and Discussion
3.1 Fourier Transform Infra-red Spectroscopy (FTIR)
In Fig. 1 the NGP shows characteristic absorption bands at 3430, 1635 and 1500 cm−1 which is attributed to hydroxyl group, C=C and deformed C–C bond, receptively. The TETA modified NGPs shows the band at 1610 cm−1 corresponds to the absorption of –NH bending and stretching. Whereas the –C=O stretching was obtained at 1650 cm−1 [8]. In addition, a strong peak at 1130 cm−1 is attributed to the existence of—CN stretching in TETA functionalization. Moreover, a distinct peak at 3100 cm−1 reveals the stretching of amide groups (–NH2) [9]. However, in the case of DGEBA/TETA-NGPs, there are characteristic peaks of DGEBA at 2935, 2846, 1432 and 1385 cm−1 which is due to the symmetric and asymmetric vibration of methylene and the absorption of benzene ring of DGEBA molecule. A prominent peak at 1715 cm−1 confirms the existence of C=O bond of ester, indicating the presence of covalent ester bond after grafting with functionalized TETA-NGPs. The results of FTIR indicate the successful synthesis of DGEBA/TETA-NGPs through strong covalent interaction between the amine functionalized NGP and DGEBA molecules.
3.2 Scanning Electron Microscopy (SEM)
The morphology of the NGPs and the TETA-NGPs/DGEBA within the epoxy matrix were observed by using SEM analysis. Figure 2a shows the SEM micrograph of the pristine NGPs depicting bare flat and flaky structure of the layered graphene sheets. A strong interconnected structure due to the dispersion of TETA functionalized NGPs within the DGEBA matrix was observed for TETA-NGPs/DGEBA in Fig. 2b.
3.3 Electrical Conductivity
The conductivity of the TETA-NGPs/DGEBA films at various TETA-NGPs loadings was measured at room temperature with a potential window from −10 to 10 V. The variation of the electrical conductivity of the epoxy nanocomposites as a function of NGP wt% at room temperature is shown in Fig. 3. The conductivity of DGEBA/TETA-NGPs increased from 5.2 × 10−15 S/cm for neat epoxy to 1.4 × 10−3 S/cm for 5 wt% TETA-NGPs loadings. The conductivities of the DGEBA/TETA-NGPs nanocomposites increases with the increase in the TETA-NGPs content in wt% and then levels off at 5 wt% of NGPs loadings. Consequently adding more nanofiller did significantly alter the resistance. This shows that the amine functionalization of NGPs facilitates the homogeneous dispersion within the DGEBA matrix which resulting into the formation of conductive networks and hence increase in the electrical conductivity of the composite [10]. Moreover on increasing the filler concentration, more charge carriers may be able to “hop” by tunneling, resulting in increase in electrical conductivity. The critical nanofiller content above which sharp increase in electrical conductivity occurs is known as “percolation threshold”. According to the percolation theory, the percolation threshold is the certain critical concentration of nanofiller at which a conductive path is formed within the composite which exhibits the transition of the material from non-ohmic to ohmic conduction. TETA-NGPs/DGEBA exhibits electrical percolation around 3 wt% of TETA-NGPs loadings after which electrical conductivity increases sharply.
3.4 Thermogravimetric Analysis (TGA)
Thermogravimetric analysis (TGA) under N2 atmosphere was used to investigate the thermal stability of the TETA-NGPs/DGEBA composite. Figure 4 shows the TGA spectra of NGPs, pure DGEBA and TETA-NGPs/DGEBA. NGP shows initial decomposition at 100 °C and becomes rapid at 150 °C then the major mass loss occurs at 460 °C where it decomposes completely which is presumably due to the pyrolysis of labile oxygen-containing groups such as –OH, COOH etc. Pure DGEBA epoxy decomposes at 280 °C and begins degradation at 460 °C, which is ascribed to the main-chain pyrolysis. The TETA-NGP/DGEBA begin to decompose at higher temperature (360 °C) as compared to that of pure DGEBA at (280 °C). This remarkable improvement in the thermal stability of the TETA-NGPs/DGEBA composite is due to the strong covalent interaction between the TETA functionalized NGPs and DGEBA. The amine functionalized NGPs in the composite act as a physical barrier which delays the degradation. Moreover, the ultrahigh aspect ratio of the filler could create a tortuous path for the volatile degradation products. The slow degradation of the epoxy chains that absorbed at the matrix filler interface may also contribute to the enhanced thermal stability of the TETA-NGP/DGEBA nanocomposites [11].
4 Conclusions
In the work, TETA functionalized NGPs was successfully attached to the epoxy DGEBA with molding curing method. The uniform dispersion of the functionalized NGPs in the DGEBA matrix was demonstrated by SEM morphology. The homogeneous dispersion and the strong interfacial bonding of TETA modified NGPs and DGEBA at different loadings are supportive to improve the electrical properties by forming conducting pathway within the matrix. A remarkable thermal property of TETA-NGPs/DGEBA nanocomposite was obtained with 5 wt% of TETA-NGPs loadings due to the strong covalent interaction of TETA-NGPs and DGEBA matrix which act as a physical barrier to delay the degradation. The proposed reinforcement of TETA functionalized NGP filler into the DGEBA matrix significantly improve the electrical and thermal properties of the composite.
References
T. Ramanathan, A.A. Abdala, S. Stankovich, D.A. Dikin, M. Herrera-Alonso, R.D. Piner, D.H. Adamson, H.C. Schniepp, X. Chen, R.S. Ruoff, S.T. Nguyen, I.A. Aksay, R.K. Prud’Homme, L.C. Brinson, Nat. Nanotechnol. 3, 327–331 (2008)
J. Jia, X. Sun, X. Lin, X. Shen, Y. Mai, J. Kim, ACS Nano 8, 5774–5783 (2014)
S.H. Park, P.R. Bandaru, Polymer 51, 5071–5077 (2010)
H. Ribeiro, W.M. Silva, M.F. Rodrigues, J.C. Neves, R. Paniago, C. Fantini, H.D.R. Calado, L.M. Seara, G. Goulart Silva, J. Mater. Chem. 48, 7883–7892 (2013)
Q. Zehua, W. Guojian, J. Appl. Poly. Sci. 124, 403–411 (2012)
J. Langea, B. Nicolasa, J. Galy, J.-F. Gerar, Polymer 43, 5985–5994 (2002)
P. Mazumdar, S. Rattan, M. Mukherjee, RSC Adv. 5, 69573–69582 (2015)
J.F. Shen, W.S. Huang, L.P. Wu, Mater. Sci. Eng. A 464, 151–156 (2007)
J.Z. Kovacs, B.S. Velagala, K. Schulte, W. Bauhofer, Compos. Sci. Technol. 67, 922–928 (2007)
J. Karippal, H.N. Narasimha Murthy, K.S. Rai, M. Krishna, M. Sre jith, Polym. Bull. 65, 849–861 (2010)
F. Jin, C. Ma, S. Park, Mater. Sci. Eng. A 528, 8517–8522 (2011)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this paper
Cite this paper
Mazumdar, P., Rattan, S. (2017). Improved Electrical and Thermal Properties of TETA Functionalized NGPs/Epoxy Nanocomposites. In: Jain, V., Rattan, S., Verma, A. (eds) Recent Trends in Materials and Devices. Springer Proceedings in Physics, vol 178. Springer, Cham. https://doi.org/10.1007/978-3-319-29096-6_53
Download citation
DOI: https://doi.org/10.1007/978-3-319-29096-6_53
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-29095-9
Online ISBN: 978-3-319-29096-6
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)