Abstract
Now a day, nanomaterial plays important role in every field due to their unique properties which are completely different from the bulk materials. Material Properties are dynamically changed with reduction in the crystallite size. Transparent conducting oxides (TCOs) are optically transparent to visible light, but electronically conductive. Owing to these properties, they have broad industrial applications such as optoelectronic devices and photovoltaics . Doped Tin Oxide is an oxygen deficient material which could be beneficial for transparent conducting oxide. The SnO2 was doped with Cu as a dopant element at different concentration. Mede-A software for material design was used to obtain the theoretical data which is to be compared with the practical results. Co-precipitation method was used to prepare Cu-doped SnO2 . Studies on structural properties of undoped and doped SnO2 were done by X-ray diffraction. The XRD results have shown that the size of the nanoparticles decreases down to 51 nm with increase in dopant concentration. Optical Properties were studied by UV-visible spectroscopy. Band gap was found to decreases down to 2.25 eV with increase of dopant content in samples.
Access provided by CONRICYT-eBooks. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
In the recent time nanoparticle plays important role in every field, due to their unique mechanical, chemical and electrical properties which are completely different from the bulk material. Semiconductor materials are widely used in electronic devices but in the field of optoelectronic these materials have some limitations. To overcome these limitations metal oxides are used. Transparent conducting oxides (TCOs) are electronically conductive and optically transparent to visible light. Due to these properties, they are extensively used in industrial applications such as optoelectronic devices and photovoltaics [1]. Transparent conductive oxide thin film of ITO, SnO2 and ZnO are widely used in the field of optoelectronic such as transparent electrode in touch panel, flat panel displays and OLED (Organic light emitting diode). Successful performance of these applications depends on a combination of good transparency and low resistivity of TCOs, mostly achieved by controlling chemical compositions of wide band-gap semiconducting materials with various doping agents [2]. Now a days ITO is most widely used material for TCO. SnO2 is used in gas sensor, solar cell , display devices and other optoelectronic application. Indium is replaced by low cost and easily available material such as Cu. The electrical conductivity of SnO2 is increased with introducing the foreign doping element [3]. SnO2 has a rutile crystal structure in which Sn atoms are present at each corner and are surrounded by oxygen atoms. Doping can cause the impurity atoms to either replace Tin atoms or occupy the void space. The SnO2 nanoparticle are prepared by using different method such as CVD , sol-gel, co-precipitation and thermal evaporation method [4]. Among the different methods for the doped and un-doped SnO2 (multi-component TCO) production the co-precipitation method is the most useful method for mass production [5]. In the present work we have investigated the optical and structural properties of Cu doped SnO2 and results are compared with the theoretical data obtained from the material design software Mede-A. For SnO2 structural conformation and particle size calculation X-ray diffraction (XRD ) is used. Doping of Cu in SnO2 at different concentration is done to analyze the changes in band gap, conductivity and optical transmittance.
2 Experimental Details
Modelling
VASP 5.3 module of Mede-A was used to observe the structural and optical properties of undoped and doped SnO2 .
Figure 1 represent the designed model of undoped SnO2 by material design software Mede-A in which there are 2 atoms of Sn are present and 4 atoms of oxygen are there and Fig. 2. Represent the designed model of Cu-doped SnO2 using Mede-A in which 1 Cu atom is present, 15 Sn atoms and 32 atoms of oxygen are present.
Materials Used
The material used to synthesize Undoped and doped SnO2 nanoparticles are Stannous Chloride Dihydrates (SnCl2.2H2O), Sodium Hydroxide (NaOH) and Cupric Chloride Dihydrate (CuCl2.2H2O). SnCl2.2H2O was used as precursor to synthesized SnO2 nanoparticles and NaOH was used as reducing agent. The CuCl2.2H2O and FeCl3 are the dopant materials. All the chemicals were used of Loba Chemie with high purity.
Synthesis Procedure
See Fig. 3.
3 Results and Discussion
Structural Properties
Structural properties are determined by the XRD pattern of all the prepared SnO2 nanoparticles. XRD Results obtained from Mede-A are compared with the practical data of sample.
Scherrer formula is used to calculate the crystallite size using the following equation,
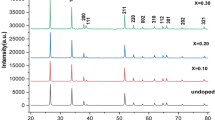
where d is the crystallite size, λ is the wavelength of X-ray used, and θ is the Bragg angle of diffraction peaks. Figure 4 shows the XRD patterns of all the samples and Intense diffraction peaks of the SnO2 were observed at 29.82°, 33.705°, 45.454° and 56.474° which were indexed to the (110), (101), (200) and (220) planes, respectively, up to 10 % of Cu doping. It is evident from XRD patterns that SnO2 has rutile type structure with Sn at octahedral site and O at tetragonal site. The results show that as doping increases the crystalline size decreases.
Optical Properties
The reflection spectra in the UV-VIS range of SnO2 : Cu nanoparticles with 6 % Cu concentrations are presented in Fig. 5 The optical band gap (Eg) for Cu-doped SnO2 nanoparticles can be determined by extrapolation from the absorption edge which is given by the following equation:
where A is a constant and hν is the photon energy. Cu doped SnO2 powders showed optical band gap decrease from 3.17 down to 2.25 eV when the Cu concentration increased up to 10 %. This result was compared with the Mede-A results as shown in Fig. 6.
The density of states (DOS) of a system describes the number of states per interval of energy at each energy level that are available to be occupied. Unlike isolated systems, like atoms or molecules in gas phase, the density distributions are not discrete like a spectral density but continuous. A high DOS at a specific energy level means that there are many states available for occupation. A DOS of zero means that no states can be occupied at that energy level. In general, a DOS is an average over the space and time domains occupied by the system. The graph is plotted between the intensity and energy.
Table 1 shows the Bandgap and crystal size of the pure SnO2 and Cu doped SnO2. The Cu is doped in SnO2 at different concentration. When the doping concentration of Cu is increased the crystal size is decrease from 90 to 51.46 nm. As well as with decreasing the crystal size the bandgap is also reduced from 3.17 to 2.25 eV. At lower doping concentration (2 %) of Cu there was an unexpected increment in the band gap from 3.17 to 4.49 eV. But when the doping concentration of Cu is increased from 2 to 6 and 10 % the band gap is decreased to 2.25 eV that is lower than pure SnO2. It means with increasing the concentration of Cu in SnO2 the conductivity is increased due to decreasing the Bandgap.
4 Conclusions
In this study, Cu-doped SnO2 nanoparticles were prepared by co-precipitation method . From UV-Vis spectroscopy it is found that the band gap of Cu doped SnO2 shows a decrease from 3.17 to 2.25 eV with the Cu concentration increase up to 10 %. The XRD analysis revealed that the crystallites have the expected SnO2 tetragonal phase and crystallite size reduces with Cu doping up to 10 mol% down to 51 nm.
References
A. Bouaine et al., Structural, optical, and magnetic properties of Co-doped SnO2 powders synthesized by the co-precipitation technique. J. Phys. Chem. C 111(7), 2924–2928 (2007)
S. Sohn, H.-M. Kim, Transparent conductive oxide (TCO) films for organic light emissive devices (OLEDs), organic light emitting diode—material, process and devices (2011)
J. Ni, Y. Xiong, Preparation and characterization of transparent conductive zinc doped tin oxide thin films prepared by radio-frequency magnetron sputtering. J. Wuhan Univ. Technol.-Mater Sci. Ed. 26(3), 388–392 (2011)
H. Kim, R.C.Y. Auyeung, A. Piqué, Transparent conducting F-doped SnO2 thin films grown by pulsed laser deposition. Thin Solid Films 516(15), 5052–5056 (2008)
G.E. Patil et al., Preparation and characterization of SnO2 nanoparticles by hydrothermal route. Int. Nano Lett. 2(1), 1–5 (2012)
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this paper
Cite this paper
Singh, G.P., Kaur, N., Abhinav, Kumar, S., Kumar, D. (2017). Effect of Dopant Concentration on Structural and Optical Properties of Cu Doped SnO2 . In: Jain, V., Rattan, S., Verma, A. (eds) Recent Trends in Materials and Devices. Springer Proceedings in Physics, vol 178. Springer, Cham. https://doi.org/10.1007/978-3-319-29096-6_14
Download citation
DOI: https://doi.org/10.1007/978-3-319-29096-6_14
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-29095-9
Online ISBN: 978-3-319-29096-6
eBook Packages: Physics and AstronomyPhysics and Astronomy (R0)