Abstract
Hypophysitis, a chronic inflammation of the pituitary gland, is a rare condition which can be classified as secondary or primary. Secondary hypophysitis includes the cases where an etiological agent is identified, whereas primary hypophysitis refers to those that do not currently have identifiable causes. Primary hypophysitis is the most common form of hypophysitis and comprises five histologic forms: lymphocytic, granulomatous, xanthomatous, necrotizing, and immunoglobulin G4 (IgG4) plasmacytic. The two main types are lymphocytic and granulomatous hypophysitis. IgG4-related hypophysitis is a recently reported new variant. Although lymphocytic hypophysitis is the most common form of primary hypophysitis, it is rare, representing less than 1 % of pituitary masses and estimated to be the cause of hypopituitarism in 0.5 % of cases. Lymphocytic hypophysitis predominantly affects women, particularly during late pregnancy or in the early postpartum period. However, cases in men, menopausal women, and children have been described. Available data strongly suggest an autoimmune pathogenesis: association with autoimmune conditions affecting other organs in 50 % of cases (thyroid, parathyroid, and adrenal glands), histopathological findings comprising fibrosis and lymphocytic infiltration, with antipituitary antibodies described in a few cases. Granulomatous hypophysitis represents less than 1 % of all operated cases of hypophysitis. It is usually diagnosed in middle- to old-age patients without gender predominance. Primary granulomatous hypophysitis is rare and its pathogenesis is still not understood. The secondary form of granulomatous hypophysitis is more common, associated with tuberculosis, sarcoidosis, syphilis, Langerhans cell histiocytosis, or Rathke cleft cyst (RCC) rupture. Pathology demonstrates the formation of granulomas, aggregates of lymphocytes, and epithelioid histiocytes with multinucleated giant cells. It usually involves the anterior lobe but may extend to involve the posterior lobe, the stalk, and even the hypothalamus. Xanthomatous hypophysitis predominantly affects young females. Its cause remains unknown, although autoimmune, infectious, and localized endothelial dysfunction have been suggested. It is pathologically characterized by mixed inflammatory infiltrate of foamy histiocytes called xanthoma cells and mature lymphocytes with cyst-like areas typically confined to the anterior pituitary. The basic structure of anterior pituitary is usually preserved without alteration of the pituitary stalk. Necrotizing hypophysitis is pathologically characterized by a marked mononuclear infiltrate showing significant necrosis. It involves the adenohypophysis, pituitary stalk, infundibuloneurohypophysitis, and hypothalamus. The pathogenesis of this rare hypophysitis variant remains unknown. IgG4-related hypophysitis is part of a multifocal systemic disease of unknown etiology called “IgG4-related autoimmune disease.” This disease, which mainly affects middle-aged to elderly males, is characterized by elevated serum IgG4 concentration and tissue infiltration by IgG4+ plasma cells with fibrosis and sclerosis. Diagnostic criteria include involvement of various organs such as the CNS, salivary glands, lacrimal glands, thyroid gland, lungs, pancreas, bile ducts, kidneys, prostate, lymph nodes, retroperitoneum, mesentery, gastrointestinal tract, skin, breast, and arteries. Adenohypophysitis, infundibuloneurohypophysitis, or panhypophysitis may be distinguished according to the anatomic involvement of the gland. Classical manifestations of adenohypophysitis may be tumoral or endocrine symptoms. Tumoral syndrome consists of headaches and visual impairment. The sudden onset of these signs is related to the rapid pituitary volume increase. Oculomotor disorders caused by cavernous sinus involvement are seldom seen. Endocrine symptoms consist of partial or total anterior hypopituitarism. ACTH secretion is most frequently impaired, followed by TSH and LH/FSH secretion. Hyperprolactinemia caused by pituitary stalk compression can be seen. Hypogonadism is typically encountered in xanthomatous hypophysitis. A few cases of aseptic meningitis with meningeal syndrome have been reported. Infundibuloneurohypophysitis occurs in older patients without gender predilection, and typically presents with diabetes insipidus. Young age at onset (<30 years), vasopressin-cell antibodies, and association with other autoimmune diseases support the autoimmune hypothesis for pathogenesis. Diabetes insipidus is seldom encountered in xanthomatous hypophysitis. The role of MRI in primary hypophysitis is to eliminate a pituitary adenoma. Diffuse and symmetric enlargement of the pituitary gland is seen in adenohypophysitis, with frequent upward tongue-like extension. Optic chiasm compression is inconstant. This tumoral syndrome contrasts with a normal-sized pituitary fossa. Signal intensity is usually hypointense on T1WI and more or less hyperintense on T2WI. Postgadolinium enhancement is usually homogeneous and intense but can be moderate and heterogeneous (Fig. 40.1). The pseudocapsule formed by compressed normal pituitary gland is sometimes not seen after gadolinium administration. A nonspecific perisellar dural enhancement is frequent (Fig. 40.2). This feature, classically described in hypophysitis and meningiomas, can also be encountered in pituitary macroadenomas. A noticeable delay in pituitary enhancement compared with the normal gland (<60 s) has been described on dynamic CE studies. In contrast, we have observed during dynamic MRI an intense and early enhancement of some foci presenting a pronounced hyperintensity on T2WI and probably corresponding to a localized inflammatory process (Fig. 40.3). Enhancement of the diaphragm has been reported in a few cases. A peripheral enhancement pattern seems more common in xanthomatous hypophysitis than in other types of hypophysitis. Restitutio ad integrum is rare (Fig. 40.4). Recurrence can be observed after treatment withdrawal (Fig. 40.5). Anterior pituitary atrophy is frequently the final outcome of adenohypophysitis. Another more specific but later sign has been described by Nakata in lymphocytic hypophysitis, appearing 2–20 months after the initial MR examination. It consists of a dark fibrotic rim encircling the pituitary gland on T2WI (Fig. 41.4). Infundibuloneurohypophysitis is characterized by a thickened pituitary stalk and a loss of the posterior pituitary bright spot as seen on axial noncontrast T1WI. Pituitary stalk thickening usually resolves with time, sometimes very quickly. In the end, atrophy takes place with a threadlike pituitary stalk and a decrease in size of the anterior lobe (Fig. 40.6). However, recurrence of the stalk thickening can be seen. Loss of the posterior lobe bright spot is usually permanent. In cases of panhypophysitis, signs of both entities will be seen. The main differential diagnosis is pituitary adenoma. Other nonadenomatous pituitary masses and secondary hypophysitis can be difficult to distinguish from primary hypophysitis. Approximately 40 % of patients are misdiagnosed as having pituitary macroadenoma and undergo unnecessary surgery. Hence, distinguishing primary hypophysitis from pituitary adenoma is very important. On MR scans, pituitary adenoma can easily be distinguished from normal pituitary parenchyma. The normal pituitary gland is most often located at the upper part of the sella, anteriorly or lateral to the adenoma. It is best seen as a pseudocapsule on coronal T1WI after contrast administration. Another distinctive feature is the absence of thickening of the pituitary stalk. Diabetes insipidus is seldom encountered in adenomas and the posterior bright spot is present, although often ectopic. The other nonadenomatous pituitary masses are germinoma, choristoma, and pituitary metastases. Another differential diagnosis to consider is pituitary hyperplasia secondary to hypothyroidism. In this case, the posterior bright spot is present, the pituitary stalk is normal, and there is no delayed enhancement on dynamic postcontrast scans. Distinguishing primary hypophysitis from secondary hypophysitis is a diagnosis of exclusion (Fig. 40.7). Investigations are required to rule out numerous etiologies; administration of immunomodulatory drugs such as CTLA-4 blocking antibody (ipilimumab) or interferon-α, Wegener granulomatosis, tuberculosis, sarcoidosis, syphilis, tuberculosis, and Langerhans and non-Langerhans cell histiocytosis (see Chap. 41). Other etiologies may induce a secondary hypophysitis such as ruptured RCC, pituitary abscess, and Takayasu disease. The T2 dark fibrotic rim around the pituitary gland and along the dura mater, seen in the chronic stage of lymphocytic and IgG4-related hypophysitis (Figs. 40.8 and 40.9) is not a distinctive sign for secondary hypophysitis and can be observed in granulomatosis, sarcoidosis, or tuberculosis. Primary hypophysitis management remains controversial. Surgery may be indicated when the tumoral syndrome is important. Otherwise, when the diagnosis of primary hypophysitis is the most likely, medical treatment is initiated. Medical treatment consists of corticosteroid therapy and hormonal replacement therapy. This treatment should be carried out under clinical, biological, and neuroradiological supervision. Different outcomes have been reported. The disease may be self-limited, show a relapsing and remitting course, or progress to permanent hypopituitarism. In cases where the tumoral syndrome persists, surgery is indicated to avoid complications.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Hypophysitis, a chronic inflammation of the pituitary gland, is a rare condition which can be classified as secondary or primary. Secondary hypophysitis includes the cases where an etiological agent is identified, whereas primary hypophysitis refers to those that do not currently have identifiable causes. Primary hypophysitis is the most common form of hypophysitis and comprises five histologic forms: lymphocytic, granulomatous, xanthomatous, necrotizing, and immunoglobulin G4 (IgG4) plasmacytic. The two main types are lymphocytic and granulomatous hypophysitis. IgG4-related hypophysitis is a recently reported new variant. Although lymphocytic hypophysitis is the most common form of primary hypophysitis, it is rare, representing less than 1 % of pituitary masses and estimated to be the cause of hypopituitarism in 0.5 % of cases. Lymphocytic hypophysitis predominantly affects women, particularly during late pregnancy or in the early postpartum period. However, cases in men, menopausal women, and children have been described. Available data strongly suggest an autoimmune pathogenesis: association with autoimmune conditions affecting other organs in 50 % of cases (thyroid, parathyroid, and adrenal glands), histopathological findings comprising fibrosis and lymphocytic infiltration, with antipituitary antibodies described in a few cases. Granulomatous hypophysitis represents less than 1 % of all operated cases of hypophysitis. It is usually diagnosed in middle- to old-age patients without gender predominance. Primary granulomatous hypophysitis is rare and its pathogenesis is still not understood. The secondary form of granulomatous hypophysitis is more common, associated with tuberculosis, sarcoidosis, syphilis, Langerhans cell histiocytosis, or Rathke cleft cyst (RCC) rupture. Pathology demonstrates the formation of granulomas, aggregates of lymphocytes, and epithelioid histiocytes with multinucleated giant cells. It usually involves the anterior lobe but may extend to involve the posterior lobe, the stalk, and even the hypothalamus. Xanthomatous hypophysitis predominantly affects young females. Its cause remains unknown, although autoimmune, infectious, and localized endothelial dysfunction have been suggested. It is pathologically characterized by mixed inflammatory infiltrate of foamy histiocytes called xanthoma cells and mature lymphocytes with cyst-like areas typically confined to the anterior pituitary. The basic structure of anterior pituitary is usually preserved without alteration of the pituitary stalk. Necrotizing hypophysitis is pathologically characterized by a marked mononuclear infiltrate showing significant necrosis. It involves the adenohypophysis, pituitary stalk, infundibuloneurohypophysitis, and hypothalamus. The pathogenesis of this rare hypophysitis variant remains unknown. IgG4-related hypophysitis is part of a multifocal systemic disease of unknown etiology called “IgG4-related autoimmune disease.” This disease, which mainly affects middle-aged to elderly males, is characterized by elevated serum IgG4 concentration and tissue infiltration by IgG4+ plasma cells with fibrosis and sclerosis. Diagnostic criteria include involvement of various organs such as the CNS, salivary glands, lacrimal glands, thyroid gland, lungs, pancreas, bile ducts, kidneys, prostate, lymph nodes, retroperitoneum, mesentery, gastrointestinal tract, skin, breast, and arteries. Adenohypophysitis, infundibuloneurohypophysitis, or panhypophysitis may be distinguished according to the anatomic involvement of the gland. Classical manifestations of adenohypophysitis may be tumoral or endocrine symptoms. Tumoral syndrome consists of headaches and visual impairment. The sudden onset of these signs is related to the rapid pituitary volume increase. Oculomotor disorders caused by cavernous sinus involvement are seldom seen. Endocrine symptoms consist of partial or total anterior hypopituitarism. ACTH secretion is most frequently impaired, followed by TSH and LH/FSH secretion. Hyperprolactinemia caused by pituitary stalk compression can be seen. Hypogonadism is typically encountered in xanthomatous hypophysitis. A few cases of aseptic meningitis with meningeal syndrome have been reported. Infundibuloneurohypophysitis occurs in older patients without gender predilection, and typically presents with diabetes insipidus. Young age at onset (<30 years), vasopressin-cell antibodies, and association with other autoimmune diseases support the autoimmune hypothesis for pathogenesis. Diabetes insipidus is seldom encountered in xanthomatous hypophysitis. The role of MRI in primary hypophysitis is to eliminate a pituitary adenoma. Diffuse and symmetric enlargement of the pituitary gland is seen in adenohypophysitis, with frequent upward tongue-like extension. Optic chiasm compression is inconstant. This tumoral syndrome contrasts with a normal-sized pituitary fossa. Signal intensity is usually hypointense on T1WI and more or less hyperintense on T2WI. Postgadolinium enhancement is usually homogeneous and intense but can be moderate and heterogeneous (Fig. 40.1). The pseudocapsule formed by compressed normal pituitary gland is sometimes not seen after gadolinium administration. A nonspecific perisellar dural enhancement is frequent (Fig. 40.2). This feature, classically described in hypophysitis and meningiomas, can also be encountered in pituitary macroadenomas. A noticeable delay in pituitary enhancement compared with the normal gland (<60 s) has been described on dynamic CE studies. In contrast, we have observed during dynamic MRI an intense and early enhancement of some foci presenting a pronounced hyperintensity on T2WI and probably corresponding to a localized inflammatory process (Fig. 40.3). Enhancement of the diaphragm has been reported in a few cases. A peripheral enhancement pattern seems more common in xanthomatous hypophysitis than in other types of hypophysitis. Restitutio ad integrum is rare (Fig. 40.4). Recurrence can be observed after treatment withdrawal (Fig. 40.5). Anterior pituitary atrophy is frequently the final outcome of adenohypophysitis. Another more specific but later sign has been described by Nakata in lymphocytic hypophysitis, appearing 2–20 months after the initial MR examination. It consists of a dark fibrotic rim encircling the pituitary gland on T2WI (Fig. 41.4). Infundibuloneurohypophysitis is characterized by a thickened pituitary stalk and a loss of the posterior pituitary bright spot as seen on axial noncontrast T1WI. Pituitary stalk thickening usually resolves with time, sometimes very quickly. In the end, atrophy takes place with a threadlike pituitary stalk and a decrease in size of the anterior lobe (Fig. 40.6). However, recurrence of the stalk thickening can be seen. Loss of the posterior lobe bright spot is usually permanent. In cases of panhypophysitis, signs of both entities will be seen. The main differential diagnosis is pituitary adenoma. Other nonadenomatous pituitary masses and secondary hypophysitis can be difficult to distinguish from primary hypophysitis. Approximately 40 % of patients are misdiagnosed as having pituitary macroadenoma and undergo unnecessary surgery. Hence, distinguishing primary hypophysitis from pituitary adenoma is very important. On MR scans, pituitary adenoma can easily be distinguished from normal pituitary parenchyma. The normal pituitary gland is most often located at the upper part of the sella, anteriorly or lateral to the adenoma. It is best seen as a pseudocapsule on coronal T1WI after contrast administration. Another distinctive feature is the absence of thickening of the pituitary stalk. Diabetes insipidus is seldom encountered in adenomas and the posterior bright spot is present, although often ectopic. The other nonadenomatous pituitary masses are germinoma, choristoma, and pituitary metastases. Another differential diagnosis to consider is pituitary hyperplasia secondary to hypothyroidism. In this case, the posterior bright spot is present, the pituitary stalk is normal, and there is no delayed enhancement on dynamic postcontrast scans. Distinguishing primary hypophysitis from secondary hypophysitis is a diagnosis of exclusion (Fig. 40.7). Investigations are required to rule out numerous etiologies; administration of immunomodulatory drugs such as CTLA-4 blocking antibody (ipilimumab) or interferon-α, Wegener granulomatosis, tuberculosis, sarcoidosis, syphilis, tuberculosis, and Langerhans and non-Langerhans cell histiocytosis (see Chap. 41). Other etiologies may induce a secondary hypophysitis such as ruptured RCC, pituitary abscess, and Takayasu disease. The T2 dark fibrotic rim around the pituitary gland and along the dura mater, seen in the chronic stage of lymphocytic and IgG4-related hypophysitis (Figs. 40.8 and 40.9) is not a distinctive sign for secondary hypophysitis and can be observed in granulomatosis, sarcoidosis, or tuberculosis. Primary hypophysitis management remains controversial. Surgery may be indicated when the tumoral syndrome is important. Otherwise, when the diagnosis of primary hypophysitis is the most likely, medical treatment is initiated. Medical treatment consists of corticosteroid therapy and hormonal replacement therapy. This treatment should be carried out under clinical, biological, and neuroradiological supervision. Different outcomes have been reported. The disease may be self-limited, show a relapsing and remitting course, or progress to permanent hypopituitarism. In cases where the tumoral syndrome persists, surgery is indicated to avoid complications.
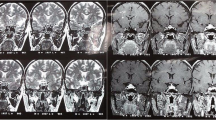
A 43-year-old woman with headache, corticotropic insufficiency, and hyperprolactinemia. (a, b) Coronal T1 and T2 WIs. Symmetric intra- and suprasellar mass, hypointense on T1, hyperintense on T2, with compression of the optic chiasm (curved arrow). (c, d) Coronal and sagittal CE T1WIs. Intense, diffuse, and homogeneous enhancement of the mass. Presellar and retrosellar dural enhancement along the clivus (straight arrows). A pituitary adenoma was initially suspected and the patient underwent surgery. Histopathological examination revealed a lymphocytic hypophysitis
A 46-year-old-woman with headache, corticotropic insufficiency, central hypothyroidism, and moderate hyperprolactinemia. Diagnosis of Hashimoto’s thyroiditis was made by detection of high levels of antithyroid peroxidase antibodies. (a) Sagittal T1WI. Enlarged pituitary gland in a normal-sized pituitary fossa. (b, c) Coronal T1 and T2 WIs. Symmetric enlarged pituitary gland with suprasellar extension. The mass is slightly hyperintense on T2 and relatively hypointense to temporal white matter on T1WI. (d, e) Sagittal and coronal CE T1WIs. Homogeneous enhancement. Presellar and retrosellar dural enhancement (arrows)
Presumed lymphocytic hypophysitis in a 27-year-old woman presenting with severe unusual retro-orbital headache at the fifth month of a first pregnancy. No pituitary deficit. No visual field defect. (a, b) Sagittal T1 and CE T1 WIs. Pituitary mass with suprasellar tongue-like extension (curved arrows) and strong enhancement after gadolinium perfusion. (c, d) Coronal T1 and CE T1 WIs. Symmetrical enlargement of the lesion and compression of the optic chiasm. (e) Coronal T2WI detects two areas of hyperintensity (straight arrows) exactly corresponding to foci of marked enhancement during dynamic MRI (straight arrows in f)
Same patient as in Fig. 40.3. (a–c) Coronal T1WI at diagnosis, 2 and 6 months later with conservative management. Normalization of the sellar content. The patient is asymptomatic after a normal delivery
Relapsing panhypophysitis in a 48-year-old woman with anterior pituitary deficit and diabetes insipidus. (a, b) Coronal and sagittal T1WIs. Enlarged and hypointense pituitary gland (asterisk). (c) Coronal T2WI. Heterogeneous hyperintensity of the mass. (d) Sagittal CE T1WI. Intense enhancement after contrast administration. (e, f) Coronal and sagittal T1WIs 1 month after initiation of medical therapy (a new MRI was performed for immediate preoperative evaluation of what was diagnosed as a pituitary macroadenoma). Rapid shrinkage of the pituitary content; atrophy of the pituitary stalk; ectopic ADH storage (small arrow). (g, h) Coronal and sagittal T1WIs at 3 months. Clinical relapse with recurrence of pituitary gland enlargement. (i, j) Coronal and sagittal T1WIs. MR follow-up 6 years later showing an empty sella (curved arrow) and ptosis of the optic chiasm (thick arrow)
Infundibuloneurohypophysitis in a 3-year-old boy presenting with diabetes insipidus. Incidental intrasellar RCC. (a, b) Sagittal CE T1WI and axial noncontrast T1WI at diagnosis. Enlarged pituitary stalk (curved arrow). The posterior lobe (thick arrow) is not T1 hyperintense but clearly enlarged and outlined by a compressed arch-like RCC (thin arrow). (c, d) Same sequences 1 month later. Shrinkage of the pituitary stalk and posterior lobe (thick arrow). The small RCC is represented by a thin linear nonenhanced area (straight arrow in c). (e, f) Sagittal and axial noncontrast T1WIs 3 years later. Further atrophy of the pituitary stalk (curved arrow) and posterior lobe (thick arrow). Enlarged T1-hyperintense RCC (thin arrow)
Secondary hypophysitis. Presumed autoimmune hypophysitis after treatment with ipilimumab in a patient with metastatic melanoma. (a, b) Coronal T1 and T2 WIs. (c) Sagittal CE T1WI. Thickened pituitary stalk, T1 hypointense, T2 hyperintense: the stalk is quite transparent, but strongly enhanced after gadolinium administration (curved arrows), as is the posterior pituitary (short arrow). Size and signal of the anterior pituitary are normal (straight arrows) (Courtesy of G. Raverot, MD, PhD)
An 80-year-old man with hypopituitarism due to IgG4 hypophysitis. Past history of infiltrative lesions in the lungs and kidneys whose biopsy led to the diagnosis of IgG4 disease. (a) Sagittal and (b) coronal T2WIs demonstrate a perisellar hypointense infiltrate gliding along the clivus (curved arrow) and encircling the pituitary gland itself (arrows). This inflammatory lesions enhance markedly after gadolinium administration, as seen on (c) sagittal and (d) coronal CE T1WIs
A 30-year-old man with history of pulmonary and renal lesions related to IgG4-related disease. Four years later, occurrence of an anterior pituitary deficit and diabetes insipidus. (a, b) Coronal T2WI. (c) Sagittal heavily T2WI. (d, e) Coronal and sagittal CE T1WIs. Marked peripheral T2 hypointensity encircling the pituitary gland and the cavernous sinuses (long arrows), extending to the clivus (asterisk) and the tentorium cerebelli (short arrows) due to fibrous pachymeningitis (asterisk). Peripheral enhancement (thin arrows) sparing the center (asterisk)
Further Reading
Khare S, Jaktab VS, Budyal SR et al (2015) Primary (autoimmune) hypophysitis: a single centre experience. Pituitary 18:16–22
Nagi S, Megdiche H, Nouira K et al (2002) Hypophysite granulomateuse idiopathique: aspects cliniques et radiologiques. J Neuroradiol 29:43–48
Nakata Y, Sato N, Masumoto T et al (2010) Parasellar T2 dark sign on MR imaging in patients with lymphocytic hypophysitis. Am J Neuroradiol 31:1944–1950
Author information
Authors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Nagi, S. (2016). Primary Hypophysitis. In: MRI of the Pituitary Gland. Springer, Cham. https://doi.org/10.1007/978-3-319-29043-0_40
Download citation
DOI: https://doi.org/10.1007/978-3-319-29043-0_40
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-29041-6
Online ISBN: 978-3-319-29043-0
eBook Packages: MedicineMedicine (R0)