Abstract
The retina is supplied by a double circulatory system: the outer layers of the retina (the photoreceptors and the pigment epithelium) are avascular and supplied by the choriocapillary network via diffusion. The inner layers of the retina are supplied by the central retinal artery. An anastomosis between the two circulatory systems is rare. The submacular and the peripapillary regions are supplied by the short posterior ciliary arteries. Both circulatory systems belong to the system of the ophthalmic artery. The blood from the choroidea is drained through the vortex veins. The vortex veins open into the inferior and the superior ophthalmic veins. The inferior ophthalmic vein ends in the pterygoid venous plexus, whereas the superior ophthalmic vein opens into the cavernous sinus. There is collateral circulation between the inferior and superior ophthalmic veins. The central retinal vein drains blood from the retina and the prelaminar part of the optic nerve, and opens into the cavernous sinus. Therefore, on the venous side, there is communication between the retinal and the choroidal circulation.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Optic Disc
- Cystoid Macular Edema
- Lamina Cribrosa
- Anterior Ischemic Optic Neuropathy
- Superior Ophthalmic Vein
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
The retina is supplied by a double circulatory system: the outer layers of the retina (the photoreceptors and the pigment epithelium) are avascular and supplied by the choriocapillary network via diffusion. The inner layers of the retina are supplied by the central retinal artery. An anastomosis between the two circulatory systems is rare. The submacular and the peripapillary regions are supplied by the short posterior ciliary arteries. Both circulatory systems belong to the system of the ophthalmic artery. The blood from the choroidea is drained through the vortex veins. The vortex veins open into the inferior and the superior ophthalmic veins. The inferior ophthalmic vein ends in the pterygoid venous plexus, whereas the superior ophthalmic vein opens into the cavernous sinus. There is collateral circulation between the inferior and superior ophthalmic veins. The central retinal vein drains blood from the retina and the prelaminar part of the optic nerve, and opens into the cavernous sinus. Therefore, on the venous side, there is communication between the retinal and the choroidal circulation.
Occlusion of the retinal vessels selectively affects the inner retina. The occlusion is central if it is developed within the optic disc, and therefore, the occlusion site itself cannot be visualized with the ophthalmoscope. A branch occlusion is an occlusion distal to the lamina cribrosa. A circulatory disorder in the short posterior ciliary arteries leads to ischemic optic neuropathy.
Trunk and Branch Occlusion of the Central Retinal Artery
The pathomechanism of the arterial occlusion may be either thrombosis or embolism. Embolism is the cause in more than twothirds of branch occlusion cases, and in only one-third of trunk occlusions. The most widely accepted view is that trunk occlusions are most often caused by a thrombus developed in the area of the lamina cribrosa or immediately behind it, in the proximal direction. Thrombus formation is usually due to atherosclerosis, but it may also be caused by malformation, inflammation, trauma or coagulopathy. Sixty percent of patients with trunk occlusion have hypertension, and 25 % of them have diabetes mellitus. A potential source of embolism is detected in only 40 % of the cases (Table 19.1).
In case of arterial trunk occlusion, the patient reports pain-free, sudden loss of vision, the fundus shows signs of diffuse ischemia, and the macula is cherry-colored. The clinical picture is usually unambiguous. In uncertain cases, fluorescein angiography may be of help. It is typical that the ‘occluded’ vessels almost always show blood flow, and complete occlusion is very rare. The arm-to-retina time is prolonged, and the arterial filling is considerably slower than normal. The flow of the dye, the advancing peak is characteristic. The arteriovenous transit is also very slow. The late-phase images often show a stained optic disc. The optical coherence tomography supports the observation that in case of arterial occlusion, it is primarily the inner retina that is damaged, intracellular edema occurs because of the ischemia.
The retina is thickened. Since the edema is intracellular, there are no low-reflectivity cystoid spaces on the topogram. The layers of the inner retina, supposedly because of coagulation necrosis, are strongly reflective, and cast a shadow over the photoreceptor layers and the pigment epithelium–choriocapillary complex. Since the area of the fovea lacks the inner retinal layers, there is no shadowing effect here, and the base of the choriocapillary–pigment epithelium complex shows higher reflectivity than its surroundings (Fig. 19.1). During the follow-up of the patient, thinning of the retina is observed in case of chronic vessel occlusion. The macular volume decreases, and in case of trunk occlusion, the peripapillary nerve fiber layer also becomes thinner. A useful indicator for the follow-up of the patient is the change in macular volume, foveal thickness and peripapillary nerve fiber layer (RNFL) measured with OCT.
Central retinal artery occlusion with a small flame-shaped hemorrhage on the optic disc. The inner retinal layers are thickened and hyperreflective and cast a shadow over the photoreceptors and the pigment epithelium–choriocapillary complex. The FLAG images show the slow arterial filling, and the advancing peak of the dye can be followed well
The emboli causing branch occlusion can be divided into three main groups: cholesterol (Hollenhorst plaque), platelet fibrin and calcific emboli. Less frequent forms are emboli that contain tumor cells, emboli from a septic source, and fat emboli formed in case of large-bone fractures. Similarly to trunk occlusions, branch occlusions may also be caused by local, ophthalmological lesions. Systemic hematological or coagulation disorders may even lead to recurrent occlusions. The patient reports a painfree, sudden-onset loss of vision in the visual field segment that corresponds to the affected branch, and central vision is intact in most of the cases. The milk-white retinal area on the fundus corresponds to the ischemic area supplied by the occluded artery. The edge of the lesion is determined by the drainage area of the neighboring vein branch. The FLAG shows a circulatory block and a considerably decreased flow of the dye in the affected artery branch, and as a consequence, the circulation in the neighboring vein branches is also slower. On the OCT images, the inner retinal layers are considerably and diffusely thickened and hyperreflective in the area that corresponds to the occluded artery branch, there is a consequential shadow over the photoreceptors and the pigment epithelium–choriocapillary complex, and the cystoid spaces characteristic to extracellular edema are absent.
Trunk and Branch Occlusion of the Central Retinal Vein
Venous occlusions can be sorted into three groups based on location. Trunk (central), hemicentral and branch occlusions are distinguished. In case of a trunk occlusion, the entire central retinal vein is occluded. Hemicentral occlusion means the occlusion of one trunk of the congenitally dual-trunk central retinal vein. About 20 % of the population have a dual-trunk central retinal vein. Branch occlusion is most commonly seen in the superior temporal vein branch. Retinal venous circulatory disorders are relatively common. The incidence of venous trunk occlusion is second to that of diabetic retinopathy. The most common predisposing conditions are diabetes mellitus, arterial hypertension and atherosclerotic cardiovascular lesions. It is known that it is five times more common in patients with open-angle glaucoma, presumably due to the damage to the structure of the lamina cribrosa caused by the elevated intraocular pressure. Acute closed-angle glaucoma may also provoke venous trunk occlusion.
The pathomechanism of venous trunk occlusion is not fully clarified. There is a theory according to which the occlusion is caused by a thrombus formed in the area of the lamina cribrosa or directly behind it. The adjacent artery has a role in the formation of the thrombus because its wall is thickened due to arteriosclerosis and, by exerting pressure on the wall of the vein, it leads to turbulent flow and, consequentially, endothelial cell damage in it. Endothelial cell proliferation is also assumed to occur in the process of thrombus formation. According to another theory, the thrombosis of the vein trunk is an end stage caused by numerous possible primary factors, e.g., compression or inflammation of the optic nerve, conditions of the orbit, structure abnormalities of the lamina cribrosa, or hemodynamic factors. Since the already slow blood flow in the veins is against a relatively high resistance, the venous circulation is especially sensitive to changes in hematological factors (increased erythrocyte sedimentation rate, increased blood viscosity, elevated hematocrit, antithrombin III, fibrinogen and homocysteine levels, presence of antiphospholipid antibodies or lupus anticoagulant, activated protein C deficiency). In case of venous circulatory disorders developed by young patients, the hematological assessment may find antiphospholipid syndrome or Leiden point mutation.
Some opine that the two forms of venous trunk occlusion, the ischemic type and the non-ischemic type are manifestations of different severity of the same disease. Others think that the two types differ in their pathogenesis. In the ischemic form, there is also a severe arterial circulatory disorder. In the non-ischemic form, the thrombus is located behind the lamina cribrosa, in a more distal position.
The clinical presentation of the ischemic and non-ischemic venous trunk occlusion is similar. There are dilated veins of deformed course on the fundus and dot- or puddle-like hemorrhages in all four quadrants of the retina, cotton wool spots characteristic to infarcts may appear in the nerve fiber layer, the macula is edematous, the capillaries around the optic disc are markedly dilated and the optic disc also shows edema. Making a distinction between the two forms is important. In the non-ischemic form, the prognosis is more favorable, there is a higher chance of vision improvement, and neoangiogenesis is less common. It is known, however, that in one-third of the cases, the non-ischemic form may transform into ischemic form over the first 3 years. In one tenth of these patients, the transformation into the ischemic form occurs in the first 4 months.
It is not easy to tell the two forms apart in the acute phase. Examination of the pupils is considered to be very important. In the non-ischemic type, there is usually no or only a very mild afferent pupillary defect. Less bleeding can be seen on the fundus. If there are cotton wool spots, they are low in number and mainly located around the optic disc. On the fluorescein angiogram, staining can be observed along the dilated retinal veins that have deformed course, there is a slow, continuous leakage of dye from the dilated capillaries around the optic disc, and microaneurysms can be seen. The retinal capillary bed is intact. In the long term, vision is determined by the cystoids macular edema caused by the chronic circulatory disorder in the capillaries. The process resolves within 6–12 months, leaving a pigment disorder in the area of the macula behind, but an epiretinal membrane or subretinal fibrosis may also occur at the posterior pole. All of this can be followed well with OCT.
In the ischemic form, there is marked vision loss, and the number and extension of bleedings and cotton wool spots is increased. A considerable cystoid edema can be observed in the macula, although covered with hemorrhages. In 60 % of the cases, neoangiogenesis occurs in the anterior segment (iris, chamber angle) in the first 9 weeks, and neovascular glaucoma is developed within 3 months. (In case of venous trunk occlusion, neoangiogenesis is possible but not typical in the posterior segment, on the optic disc and in other areas.) The fluorescein angiogram is hard to evaluate because the extensive hemorrhages block the macular edema and cover the non-perfused areas. There are studies according to which in case of a non-perfused area the size of 10 or more optic discs, the risk of neoangiogenesis in the anterior segment is very high and therefore such forms should be considered as ischemic. Other studies are more permissive and consider a visual acuity of lower than 0.1 or a non-perfused retinal area the size of 30 or more optic discs to be the threshold. Despite the bleedings, OCT is suitable for the early detection of macular edema. Both fluorescein angiography and optical coherence tomography have a role not primarily in making the diagnosis or distinguishing between the ischemic and the non-ischemic form but rather in monitoring the course of the disease. Non-perfused areas are easier to detect with fluorescein angiography once the hemorrhages are resorbed. (It is worth taking photos not only of the posterior central areas but, with directed view, also of the periphery.) Neoangiogenesis is easier to detect or, if suspected based on the ophthalmoscopic picture, confirm. The condition of the macula can be followed well with OCT (Figs. 19.2 and 19.3). In case of venous occlusions, OCT provides information also about the optic disc. In the acute phase, sector-like peripapillary nerve fiber layer (RNFL) thickening may occur in case of branch occlusion, whereas concentric RNFL thickening can be measured around the optic disc in case of trunk occlusions. During follow-up, the edema of the nerve fiber layer decreases in an average of 2 months, and atrophy of the RNFL and its decrease below the normal value can be observed 6–8 months after the occlusion.
Central retinal vein occlusion. Upper images: In the acute phase, dilated veins of deformed course, hemorrhages and numerous cotton wool spots around the optic disc can be seen. OCT shows macular edema, and thickening, increased reflectivity and shadowing effect of the inner retinal layers. On the fluorescein angiograms, staining along the dilated vein branches of deformed course and mild dye leakage along the superior temporal venous branch can be observed. Lower images: 9 months later, the hemorrhages are almost completely absorbed, and there is cystoid edema in the macula. Circulation in the large vessels is almost completely restored but the superior temporal vein branch is still thickened and of deformed course, and there is late filling in it
Hemispheric occlusion in the inferior central vein branch. The upper images show the acute phase and the lower images the status 9 months later. Despite the hemorrhages, the OCT shows the cystoid edema of the macula already in the acute phase. The FLAG makes it clear that filling is late both in the inferior nasal and the inferior temporal vein branches. Nine months later, the cystoid macular edema, although decreased, is not yet resolved. Edema can be observed beneath the papillomacular zone. The foveal avascular zone is broadened. There are dilated collaterals between the inferior and superior temporal vessel arches. Temporal to the macula, and nasally in the mid-periphery, severe damage to the capillary network can be seen. Especially in the nasal periphery, circumscribed neoangiogenesis is possible
Venous branch occlusion is three times as common as trunk occlusion. It almost always occurs in the arteriovenous crossing, where the artery and vein run in a common sheath. The artery is on the top, in the direction of the vitreous body. The artery, which is rigid because of arteriosclerosis, compresses the wall of the vein. Therefore, turbulent flow and, consequentially, endothelial cell damage and thrombus formation start within the vein. The majority of branch occlusions are superotemporal. The likely reason for this is that the highest number of arteriovenous crossings is found here. Rarely, branch occlusion is caused by a local eye condition, e.g., inflammation such as toxoplasmosis, or Eales disease. It may accompany Coats’ disease, macroaneurysm, papillary drusen, retinal capillary drusen and other systemic diseases (sarcoidosis, Behçet’s syndrome). Glaucoma is a known risk factor.
The clinical picture is characteristic. Patients report sudden-onset blurred vision and a defect in the visual field that corresponds to the occluded vessel. In the acute phase, flame-like hemorrhages can be observed along the occluded vein branch on the fundus. The number of hemorrhages reflects the degree of the occlusion. FLAG is an efficient tool in the confirmation of the diagnosis and planning the therapy. Arterial filling is generally normal, and the capillaries are dilated and show a deformed course. The circulation in the occluded vein branch is considerably slower and late. Hypofluorescence can be seen because of the hemorrhages and the nonperfused areas. As the hemorrhages are resorbed, the extension of the damage to the retinal capillary bed is more and more visible. Collateral circulation is formed between the superior and inferior vessel branches, and it crosses the horizontal boundary. In case of any doubt, fluorescein angiography helps safely distinguish between collateral vessels and neoangiogenesis on the optic disc or other places of the retina. Optic coherence tomography is good for the detection of cystoid macular edema, as well as the monitoring of its slow absorption (taking 6–12 months) and the development of any complications (epiretinal, subretinal fibrosis) during follow-up.
Anterior Ischemic Optic Neuropathy (AION)
Ischemia of the optic nerve, owing to its structure, most commonly occurs in the area of the optic disc. As a result of the impaired blood supply of the optic disc, the perfusion in the tightly packed nerve fibers may drop below a critical level. The most common manifestation form of optic nerve ischemia is anterior ischemic optic neuropathy but a similar picture can be seen in diabetic papillopathy, hypertensive papillopathy and optic neuropathy associated with migraine, which are also likely to be of ischemic origin. Ischemia of the intraorbital segment of the optic nerve (posterior ischemic optic neuropathy, PION) is a rare condition.
Anterior ischemic optic neuropathy has two forms. In giant cell arteritis, arteritic AION is caused by the vasculitis in the short posterior ciliary arteries. The medical history (‘temporal arteritis’: headache, sensitivity of the scalp, masticatory pain) and the significantly increased erythrocyte sedimentation rate (70–120 mm/h) are characteristic. In non-arteritic AION, the circulatory disorder affects the branches that directly supply the optic disc and that are distal to the short posterior ciliary arteries. Risk factors include hypertension, diabetes, ischemic heart disease, and hypercholesterolemia, as well as increased bleeding tendency and perfusion impairment due to elevated intraocular pressure.
The clinical picture is dominated by the deterioration of vision. It is characterized by a relative afferent pupillary defect and a curved scotoma most often developed in the inferior half of the visual field, which is connected with the center. On the fundus, initially there is an edematous optic disc with blurred edges and striped hemorrhages around it, and then sectors and, finally, the whole area of the optic disc becomes atrophic.
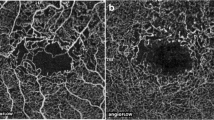
With the localization of the circulatory disorder, FLAG may help in the distinction of the two types. In case of arteritic AION, filling is considerably late in the optic disc and the neighboring choroidea (30–70 s, Fig. 19.4). In case of non-arteritic AION, there is late filling in the optic disc but the dye appears earlier than in AION of vasculitic origin. In non-arteritic AION, filling in the peripapillary choroidea is not late, and the flow is not or only slightly different from that seen in healthy control subjects of the same age. The change in the thickness of the retinal nerve fiber layer can be monitored with optic coherence tomography (Fig. 19.5). Thickening of the nerve fiber layer can be detected already in the early phase, and it increases further when an infarct occurs in the optic disc. Simultaneously with the atrophy of the optic nerve, the nerve fiber layer gets thinner and the cup/disc (C/D) ratio increases.
References
Arnold AC, Hepler RS. Fluorescein angiography in acute anterior ischemic optic neuropathy. Am J Ophthalmol. 1994;117:222–30.
Asefzadeh B, Ninyo K. Longitudinal analysis of retinal changes after branch retinal artery occlusion using optical coherence tomography. Optometry. 2008;79:85–9.
BCSC Retina and Vitreous. 2004, Section 12. P. 136–45.
Contreras I, Rebolleda G, Noval S. Optic disc evaluation by optical coherence tomography in nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Visual Sci. 2007;48:4087–92.
The Central Retinal Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol. 1997;115:486–91.
DeLeon-Ortega J, Carroll KE, Arthur SN. Correlations between retinal nerve fiber layer and visual field in eyes with nonarteritic anterior ischemic optic neuropathy. Am J Ophthalmol. 2007;143:288–94.
Duker JS, Sivalingam A, Brown GC, Reber R. A prospective study of acute central retinal artery obstruction. Arch Ophthalmol. 1991;109:339–42.
Hayreh SS, Zimmermann MB, Podhajsky P. Incidence of various types retinal vein occlusion and their recurrence in demographic characteristics. Am J Ophthalmol. 1994;117:429–41.
Hayreh SS. Ischemic optic neuropathy. Progr Retinal Eye Res. 2009;28:34–62.
Hayreh SS. HCRVO: pathogenesis, clinical features, and natural history. Arch Ophthalmol. 1980;98:1600–8.
Hayreh SS, Jonas JB. Optic disc morphology after arteritic anterior ischemic optic neuropathy. Ophthalmology. 2001;108:1586–94.
Johnson TM. El-defrawy: prevalence of factor V Leiden and activated protein C resistance in central retinal vein occlusion. Retina. 2001;21:161–6.
Jonas JB, Xu L. Optic disc morphology in eyes after nonarteritic anterior ischemic optic neuropathy. Invest Ophthalmol Visual Sci. 1993;34:2260–5.
Leung CK, Tham CC, Mohammed S, Li EY, Leung KS, Chan WM, Lam DS. In vivo measurements of macular and nerve fibre layer thickness in retinal arterial occlusion. Eye (Lond). 2007;12:1464–8.
Siatkowski RM, Gass JDM, Glaser JS. Fluorescein angiography in the diagnosis of giant cell ateritis. Am J Ophthalmol. 1993;115:57–63.
Schuman JS, Puliafito CA, Fujimoto JG. Optical tomography of ocular diseases. Thorofare: Slack Inc.; 2004.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Récsán, Z., Szepessy, Z. (2016). The Role of Fluorescein Angiography and Optical Coherence Tomography in the Examination of Circulatory Disorders of the Optic Disc. In: Somlai, J., Kovács, T. (eds) Neuro-Ophthalmology. Springer, Cham. https://doi.org/10.1007/978-3-319-28956-4_19
Download citation
DOI: https://doi.org/10.1007/978-3-319-28956-4_19
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28954-0
Online ISBN: 978-3-319-28956-4
eBook Packages: MedicineMedicine (R0)