Abstract
Teeth can be lost due to many reasons including periodontitis, trauma and/or profound caries. Though current treatment modalities such as implantology or prosthesis may provide esthetical and functional restorations of missing teeth, new strategies are required to overcome limitations of traditional treatments towards more biologic treatment methods. Research aiming bio-engineered tooth (bio-tooth) has largely focused on developing functional tooth and attachment apparatus which integrates into bone through a natural periodontal ligament (PDL) employing stem cells. There are several issues, including appropriate scaffold to create tooth structure, proper cell types and optimum growth, and differentiation conditions for cells need to be clarified before realizing stem cell use in clinics. The selection of appropriate stem cells and biomaterials controlling cell functions are highly critical for bio-engineers to construct a whole root/tooth. This chapter will focus on stem cells (dental/oral tissues derived or others), induced pluripotent stem cells and embryonic stem cells which might be used for cell/scaffold based teeth regeneration, and future prospective of stem cells in dentistry.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
9.1 Introduction
Regeneration of tissues and organs requires highly specific orchestrations on cell/extracellular matrix (ECM)/scaffold interactions depending on the tissue characteristics. Bioengineering of tooth and surrounding periodontal tissues is challenging due to their structural complexity. Tooth development requires epithelial-mesenchymal interactions, and signaling pathways of these interactions are still unclear. To overcome these difficulties, various cells such as periodontal ligament (PDL) fibroblasts, osteoblasts, cementoblasts, odontoblasts and ameloblasts have been tried to induce new PDL, bone and cementum for new periodontal attachment apparatus and dentin, and enamel for new crown development. In the last decade, mesenchymal stem cells (MSCs) are also studied in periodontal tissue regeneration approaches. Currently, cell-based therapies using MSCs are very popular to regenerate dental tissues [1, 2]. In this regard, the accessibility and quality of the stem cells are very critical for cell-based dental tissue engineering.
9.2 Stem Cells in Dentistry
In the last decade, different sources including dental follicles, apical papilla, exfoliated deciduous teeth pulp, permanent teeth (premolar, molar) pulp and PDL have been investigated for MSCs isolation [3–7]. Recent studies demonstrated new and more accessible sources for stem cell-like populations including gingiva, palatal connective tissue and oral mucosa [8–10]. Comparison of MSCs originated from oral or dental tissues with bone marrow MSCs (BMMSCs) demonstrated that these cells possess similar characteristics for their differentiation capacities [5–12].
9.2.1 Stem Cells Studies in Reconstruction of Cranio-Facial Tissues
Bone regeneration therapies are frequently required because of trauma, infection, congenital conditions and cancer. Dental implant therapies are needed to rehabilitate patients for functional, phonation and esthetic reasons. Dental implant-supported therapies may have some difficulties if the bone tissue is insufficient at the treatment area. Cell-based therapies combined with appropriate scaffolds may help to overcome the limitations of currently used biomaterials including xenografts, autografts, allografts, and alloplastic materials in these challenging situations [13, 14]. Stem cell therapy can be beneficial on treatments of craniofacial bone defects, i.e. sinus lift, extraction socket preservation, and bone augmentation procedures to prepare bone for implant insertion [15]. Although there are several animal studies in goat [16], canine [17, 18] and sheep [19], supporting MSC-based therapies for the purpose of bone regeneration, only case and/or case series were published in the literature as human studies [20–23].
Recently, randomized clinical trials for stem cell-based therapies have been mostly started in dentistry. To reconstruct localized craniofacial bone defects, Kaigler et al. [24] planned a randomized and controlled clinical trial with mixed stem and progenitor cell population enriched in CD14 and CD90 positive cells isolated from bone marrow (tissue repair cells, TRC) for socket preservation after tooth extraction. Guided bone regeneration (GBR) as control group or TRC transplantation as test group were applied to the participants. No adverse affect was reported after 1-year following the therapy. The clinical, histological and radiographic evaluations of the study demonstrated that TRC therapy increased alveolar bone regeneration compared to GBR therapy. Test group needed less secondary bone grafting during implant insertion. Bony dehiscence exposure on the implants was noted 5-fold longer in the control group compared to the test group [24]. In a very recent study, same group from University of Michigan investigated transplantation of autologous cells enriched for CD90+ stem cells and CD14+ monocytes in the reconstruction of bone deficiencies of the maxillary sinus in a randomized and controlled clinical trial. Patients with 50–80 % maxillary sinus deficiency were randomly allocated to two groups: (i) stem cells combined with β-tricalcium phosphate scaffold group and (ii) control group (scaffold alone). While radiographic analysis showed no difference in the total bone volume gained between test and control groups, 4 months after treatment, bone density in test group was found to be higher. Bone core biopsies of the test group showed better bone quality than control group [25]. In addition, the authors reported no adverse effects after the 1-year follow-up, suggesting that cell-based therapy is safe for maxillary sinus reconstruction and may be an alternative for other maxillofacial bone defects.
9.2.2 Stem Cell Studies in Periodontal and Peri-Implantal Regeneration
BMMSCs were used for periodontal regeneration to promote new cementum, PDL and alveolar bone [26, 27]. BMMSCs seeded biodegradable scaffolds were used for the extraction socket preservation and additional benefit for the preservation of alveolar bone walls was observed in the cell seeded group when compared to control groups [28]. Stem cells isolated from pulp (DPSCs) and PDL (PDLSCs) have been used in several animal and human studies for regenerative periodontal and peri-implantal treatment [29–33]. In canine peri-implant defect models, PDLSCs and BMMSCs were compared for their alveolar bone regeneration capacities [30], and it was found that BMMSC group provided highest new bone formation rate. Transplantation of progenitor cells were thought as an effective and safe alternative in the treatment of human periodontitis; therefore, autologous PDLSCs were applied to the periodontal defects [33]. Upon the well-documented satisfactory results in animal studies [34], further randomized clinical trials with these stem cells are warranted to determine additional benefits of dental/oral stem cell-based therapies [35].
9.2.3 Stem Cells Studies in Pulp Regeneration
Aim of the regenerative endodontics is to convert the non-vital tooth into vital substitute to pathological pulp with functional healthy pulp tissue [36]. For this purpose, DPSCs or other MSCs from different sources have been investigated for revitalization/revascularization procedures in dentistry. Recent studies reported the presence of MSCs in human inflamed pulps [37] and inflamed periapical tissues [38]. Therefore, even infected pulp tissue can be used to obtain autologous MSCs in pulp regeneration treatment. Ravindran et al. investigated differentiation ability of human PDLSCs and BMMSCs into odontogenic lineage [39]. Histological and immunohistochemical analysis revealed that a vascularized pulp-like tissue could be formed by BMMSCs, PDLSCs and DPSCs. They concluded that the biomimetic scaffolds may promote odontogenic differentiation of BMMSCs, PDLSCs and DPSCs. To regenerate pulp, stem cells and biomimetic extracellular matrix combination provides new perspective toward possible therapeutic application in endodontics. Recently, a combination of CD31− and CD105+ DPSC-seeded scaffolds was used for dental pulp regeneration in a canine pulpectomy model [40]. The potential of DPSCs in regenerating pulp-like tissue was proved in immature canine teeth [41]. From a clinical perspective, although these studies give promise, further studies are strictly needed to establish new methods and proper parameters to provide functional pulp regeneration; i.e. appropriate cells, scaffolds, growth factors and clinical application procedures.
9.2.4 Stem Cells in Tooth Development, Bio-tooth or Bio-root
As regeneration of a single tissue compartment of tooth and periodontium, namely bone, PDL or pulp, is even complicated matter, creating a functional whole tooth and appropriate interaction of every single tissue of this organ become a real challenge. The knowledge about tooth development was obtained from the laboratory mice. The regulation of the signals on the tooth initiation and morphogenesis is not still enough clear. In human body, biologically replacement of congenitally missing or lost teeth still remains as a dream.
Two possible ways have been proposed to obtain biological tooth:
-
(i)
Using cells with tooth forming ability, and transplantation to the jaw bone [42, 43].
-
(ii)
Using cells to create the every single compartment of tooth including PDL, pulp and cementum, and seeding these cells to the bio-printed tooth scaffold or decellularized natural tooth [44, 45].
A number of animal studies were performed for whole-tooth bioengineering [46, 47]. Most realistic thought seems to use cells with tooth-forming capacity, transplantation of tooth germ to the jaw, and allowing the formation of a physiological root [1]. Obtaining a biologically mimicked and fully-functional tooth is the main objective for missing teeth due to trauma or periodontal and pulpal disease [44]. Researchers actively follows recent developments in stem cell-mediated tissue regeneration in dentistry [48–50]. In order to regenerate functional pulp and PDL, researchers have explored the characteristics of MSCs isolated from dental tissues [51–53]. In this sense, differentiation ability differences of various cells have been investigated; i.e. DPSCs have found prone to dentinogenesis [52] and PDLSCs to cementogenesis [54]. Cells should be used according to their potentials (proliferation, differentiation and immuno-regulatory), and the targeted tissue/organ in cell-based regenerative therapies [55, 56].
9.3 Oral/Dental Tissue-Derived MSCs
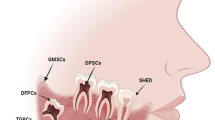
Oral/dental MSCs have become more popular due to their similarities with other MSCs based on their characteristics, relative ease of obtaining and propagating. Examination of differentiation and proliferation capacities of these oral/dental tissue-derived MSCs has been previously carried out in detailed in vivo and in vitro studies. As the first report on this topic, Gronthos et al. have revealed that stem cells derived from the wisdom teeth’s dental pulp has formed dentin/pulp-like structures in vivo and in vitro [57]. The same study group has subsequently accomplished to produce ectomesenchymal stem cells from exfoliated deciduous teeth (SHEDs) [58]. Later on, cells with MSC characteristic were successfully isolated from pulp tissue of supernumerary [59], natal tooth [60] and human third molar germs of young adults [61].
9.3.1 Oral Tissue-Derived MSCs
9.3.1.1 Gingiva-Derived MSCs
Gingival tissue is a part of the unique soft tissue that surrounds teeth; covering the alveolar ridges, palatal and retromolar regions [62, 63]. In addition, because gingival tissue is a distinctive component of the oral mucosal immunity, it plays a significant role in periodontal protection and wound healing. Therefore, gingival tissue participates in the mucosal barrier to stand against bacterial infection, sudden thermal and chemical changes. Another important feature of the gingival tissue is its unique scar-free healing process after the damage occurring in oral tissue [62, 64, 65]. Thus, gingival tissue derived-cells are admitted as potential MSC source because of unique characteristics such as regeneration ability, wound closure, clonogenicity, immunomodulatory properties and multipotent differentiation capacity like other MSCs [65, 66]. A new area of research on stem cell types obtained from periodontal connective tissues where gingival tissue was firstly used for the isolation of progenitor/stromal cell population by Zhang et al. has emerged. MSCs derived from gingival tissue (GMSCs), which are clonogenic colonies, can exhibit stem cell properties and express typical MSC surface markers. They have the capacity of differentiation toward multiple mesodermal lineages in vitro, and have stable phenotype and telomerase activity in long-term cultures [66]. Recent studies have shown that GMSCs are not prone to tumor formation whether they are obtained from healthy or inflamed\hyperplastic gingival tissue, indicating a tremendous potential for therapeutic applications [67]. GMSCs are considered as an accessible cell population because gingival tissues can be obtained from general dental procedures and treated as biomedical waste [68]. Indeed, gingival tissues can be obtained during tooth extraction, dental implantation or periodontal surgery [69]. Thus, GMSCs can be easily isolated from the patient with minimum disturbance. Human gingival tissue is a potential MSC source for the future clinical use for regeneration and repair considering its accessibility and availability.
9.3.1.2 Oral Mucosa-Derived MSCs
Oral cavity is covered by the oral mucosa (OM) as known. It has been showed that characteristics of OM-derived fibroblasts and fetal-derived fibroblasts are similar in some respects [70, 71]. The human OM has been suggested as a novel source for therapeutic adult stem cells after Marynka’s study in 2008 providing first evidence that the oral mucosa lamina propria (OMLP) gives rise to a robust multipotent stem cell population [72]. The same group also reported that the adult human OMLP can generate trillions of stem cells and 95 % of them can express MSC markers and so are referred as human oral mucosa stem cells (hOM-MSCs) . hOM-MSCs have the capacity to differentiate in vitro into lineages of the three germ layers. Their implantation in vivo, after stimulation with dexamethasone, resulted in the formation of lineage mixed tumors consisting of tissues that develop from cranial neural crest cells during embryogenesis [73]. A high percentage of these cells (60–80 %) expressed fundamental neural and neural crest stem cell markers, and were positive for Oct4, Sox2, and Nanog. hOM-MSCs were differentiated into mesodermal (osteogenic, chondrogenic and adipogenic), definitive endoderm and ectodermal (neuronal) lineages in culture conditions, and they also shared all known MSC markers. Therefore, hOM-MSCs might be an alternative source to provide human MSCs. hOM-MSCs could possibly be clinically used for oral diseases and tissue regeneration in the future due to their promising differentiation capacity and easy isolation property.
9.3.1.3 Palatal Connective Tissue-Derived MSCs
The palatal-derived cells were previously isolated by Roman et al. for the first time in 2012, and these cells were named as progenitor-like cells but their characteristics were not completely studied [74]. Later, the characteristics of the cells isolated from the palate tissues were investigated by the same research group [9]. This study demonstrated that the basic characteristics to define cells as MSCs were met by the cells from palatal connective tissue. Palatal connective-derived MSCs are a type of adult stem cells which are easy to isolate, culture and manipulate under in vitro conditions [10]. These cells are characterized by high plasticity and can become important cell sources for regenerative therapy.
9.3.1.4 Palatal Adipose Tissue-Derived MSCs
Autologous MSCs isolated from palatal adipose tissue might have potential clinical use in regenerative alveolar bone/cranio-facial bone and periodontal therapy, and gingival recession treatments [10, 75]. More recently, our group has designed a study in order to make a comparative analysis between MSCs obtained from adipose tissue-derived lipoaspirate (LAT) and palatal adipose tissue (PAT) based on their immunophenotypic and immunogenetic properties, proliferation and differentiation potential [unpublished data]. The results demonstrated that the cell surface marker expression profile of the PAT- and LAT-MSCs showed similarities, and they expressed all MSC markers, except CD11b, CD34, CD45, CD106, CD117 and HLA-DR. PAT-MSCs showed differentiation potential into adipocytes, osteocytes and neuro-glial like cells under proper conditions like LAT-MSCs. The level of Alkaline Phosphatase (ALP) activity of PAT-MSCs was found to be higher than the LAT-MSCs after the osteogenic differentiation in culture [unpublished data]. Results of this study pointed that PAT-MSCs are likely to have more osteogenic potential when compared to LAT-MSCs.
9.3.2 Dental-Derived MSCs
9.3.2.1 Dental Follicle-Derived MSCs (DFSCs)
The dental follicle (DF) has a loose connective tissue structure. It is thought that the dental follicle derived from third molar and wisdom tooth contains progenitor cells which are originated from cementoblasts, PDL cells and osteoblasts [6, 76, 77]. Like the other dental stem cells, DFSCs express similar cell surface antigens, and have the capability to form hard tissue in vitro and in vivo along with displaying extensive proliferative ability [78]. On the other hand, they can form the tissues of the periodontium including alveolar bone, PDL and cementum while they express the putative stem cell markers including Notch‐1 and Nestin [77]. Recent studies show that DPSCs and DFSCs derived from the same tooth and donor have the ability to form colonies, and although they show similar immunophenotypic characteristics they had different levels of gene expressions.
When DFSCs and DPSCs are compared, DFSCs seemed to proliferate faster and contained cells larger in diameter. DFSCs also exhibited a higher potential to form adipocytes and a lower potential to form chondrocytes and osteoblasts with respect to DPSCs. Unlike DFSCs, DPSCs were able to produce the transforming growth factor (TGF)-β and suppressed the proliferation of peripheral blood mononuclear cells, which could be neutralized with anti-TGF-β antibody [78].
9.3.2.2 Apical Papilla-Derived MSCs
Recent studies have described the physical and histological properties of the dental papilla located at the apex of developing human permanent teeth, and this tissue is named as the “apical papilla ”. Because this tissue is loosely attached to the apex of the developing root, it can easily be detached from it [79]. Discovery of human apical papilla MSCs have been accomplished by Sonoyama et al. in 2006, and they called these cells as “stem cells from the apical papilla (SCAPs) ”. In this study, it has been demonstrated that SCAPs are a promising cell source for regeneration of bio-roots for future clinical applications by utilizing them to engineer bio-roots using swine as an animal model [44]. Afterwards, the same group have shown that apical papilla comprises less cellular and vascular components in comparison with the pulp tissue. SCAPs have displayed two to three times greater proliferation rate in comparison with DPSCs. Both SCAPs and DPSCs showed weak adipogenic differentiation potentials although they were as potent as BMMSCs in terms of osteo/dentinogenic differentiation potential. Furthermore, it has been found that the immunophenotypic properties of SCAPs and DPSCs show similarities in osteogenic and dentinogenic gene profiles of growth factor receptors. A broad variety of neurogenic markers such as nestin and neurofilament are also expressed by SCAPs [80]. Besides playing a fundamental role in pulp healing and regeneration, SCAPs also contribute to the formation of developing odontoblasts which are responsible for dentinogenesis and radicular pulp formation [80, 81]. The importance of SCAPs for the apexogenesis of developing roots and constant root maturation in teenagers with endodontic diseases have been reported in recent clinical studies [82, 83]. In addition, SCAPs are also candidates to be used for dental tissue regeneration due to their remarkable regeneration capability. In vivo recombination of SCAPs and biological scaffolds resulted in generation of dentin-pulp-like tissues in the empty root canal space and bioengineered roots that can support a porcelain crown [6, 84]. It has been hypothesized that the insulin growth factor 1 (IGF-1) has a very important role in the differentiation and proliferation of SCAPs. SCAPs were isolated from juvenile third human molar apex and treated with exogenous IGF-1 for this rationale. Afterwards, in vitro and in vivo studies were conducted for the evaluation of the effects of IGF-1 on SCAPs . The increase of osteogenesis and osteogenic differentiation potential and decrease of dentinogenesis and odontogenic differentiation potential of SCAPs by IGF-1 treatment was also reported in the study of Wang et al., indicating that SCAPs treated with IGF-1 may be used as a potential candidate for bone tissue engineering [85].
9.3.2.3 PDLSCs
PDL is a gap interlaying the cementum and alveolar bone functioning as a replacement of the follicle region, which encloses the developing tooth during the cap and bud stages. Follicle (Sharpey’s fibers) or cementoblast (in cellular intrinsic fiber cementum) originated fibers may be used to insert into the cementum layer. As PDL matures during the tooth eruption, it prepares to support the functional tooth for the occlusal forces [86, 87]. Major collagen bundles (principal fibers) occupy whole mature PDL by embedding in both cementum and alveolar bone. The maximization of the forces to be placed on the tooth during mastication is caused by the arrangement of fibers in specific orientations [4, 87]. Previous studies indicate that cementoblast-like cells, adipocytes and connective tissue with rich collagen structure can be produced from cell populations found in PDL which can differentiate into mesenchymal cell lineages [88, 89]. The study of Ponnaiyan et al. proved that embryonic stem cell markers Oct4 and Nanog (weak for PDLSCs) and the mesodermal marker vimentin are expressed both in DPSCs and PDLSCs. Strong expression of MSC markers (CD73 and CD90) in DPSCs and PDSCs were also shown by immunophenotyping experiments. These results indicated that MSC markers were expressed in both stem cells at different levels, suggesting that DPSCs are more primitive stem cell type with respect to PDLSCs [90]. Functional and cellular characteristics of MSCs derived from pulp and PDL from identical donors have been compared in a recent study [91]. The results of this study proved that DPSCs and PDLSCs differed from each other in differentiation potentials and as well as expression levels of mesenchymal (CD105) and pluripotent/multipotent stem cell–associated cell surface antigens (SSEA4, CD117, CD123, andCD29). DPSCs, and PDLSCs also had different response patterns when exposed to pro-inflammatory cytokines [91].
In one of our group’s recent study , different cell behaviors were seen in MSCs isolated from pulp and PDL tissues [5]. In this study, PDLSCs expressed higher levels of HLA-G, which is a major histocompatibility complex (MHC) class I molecule that functions as an immune modulatory molecule, when compared to DPSCs based on the immunohistochemical data. HLA-G inhibits cytolytic function of natural killer (NK) and cytotoxic T cells, the alloproliferative response of CD4+ T cells, the proliferation of NK and T cells, and the maturation and function of dendritic cells which have the ability to protect tissues from the immune system attacks [92]. PDLSCs had much higher levels of IL-6 and IL-10 expressions than DPSCs. While these cytokines play an important role in immune regulation, it was also demonstrated that both IL-10 and HLA-G are essential for the full immunosuppression mediated by MSCs [93]. IL-6, a pro-inflammatory cytokine, can also mediate immunosuppressive functions that might involve in the induction of IL-10 which is an anti-inflammatory cytokine.
Immunomodulatory characteristics of PDLSCs were examined by Wada et al. as a candidate sources for new allogeneic stem cell-based therapies. It is confirmed by this research group that PDLSCs, DPSCs and BMMSCs can inhibit proliferation of peripheral blood mononuclear cells (PBMNC) via stimulation of mitogen or an allogeneic-mixed lymphocyte reaction (MLR). The results of their study stated that soluble factors produced by activated PBMNCs mediated the immunosuppressive effect of PDLSCs, BMMSCs and DPSCs [94]. Similar to Wada et al., our study showed the expression of IL-6, IL-10 and HLA-G with respect to their immunoregulatory relationship. In addition, our study also demonstrated that although PDLSCs had higher IL-6 and IL-10 mRNA expression levels, DPSCs seemed to have more stemness characteristics, and higher BMP-2 and BMP-6 mRNA expression levels, indicating that PDLSCs are more likely to be preferred in clinical trials compared to DPSCs due to their superior immunomodulatory properties [5].
Lei et al. has reported that MSC characteristics of DPSCs and PDLSCs can be sustained after in vivo implantation but when compared with PDLSCs, DPSCs seems to be more stable under in vivo conditions [95]. This study also suggested that further studies need to be done to understand the mechanisms lying beneath the determination of the reduction of lineage-specific differentiation of PDLSCs. Comparison of DPSCs and PDLSCs in our study has shown that DPSCs had higher proliferation and telomerase activity [5]. The reduction of lineage-specific differentiation of PDLSCs may explain the reason of the low proliferation and telomerase activity of PDLSCs. PDLSCs, BMMSCs and DPSCs seem to share similarities in their differentiation potentials, and cell surface marker characteristics [86]. Cementum/PDL-like structures were formed when PDLSCs were transplanted into immune compromised mice. Bone generation was observed when human PDLSCs were expanded ex vivo and seeded into 3D scaffolds (fibrin sponge, bovine-derived substitutes) [96]. These PDLSCs also seemed to preserve their stem cell properties and tissue regeneration potentials. In conclusion, overall data proposes that the PDLSC population might be used for creating a biological root to be used like a metal implant by capping with an artificial dental crown.
9.3.2.4 DPSCs (Natal, Deciduous and Adult)
Stem cells residing in the dental pulp showing similar characteristics with BMMSCs and generating the mineralized matrix of dentin due to their ability to differentiate into odontoblasts were first reported by Gronthos and co-workers [57]. Based on the surface marker expression of both cell types, they proposed that they can both adhere to plastic, form colonies, and display similar phenotypes with each other. Two different types of DPSCs were identified, which are similar to stem and stem-like cells in subsequent studies. While hematopoietic markers (CD34/CD45/CD14) are not expressed by the first type, MSC specific markers (STRO1/CD29/CD44/CD13) are strongly expressed [58, 97, 98]. The second type of DPSCs are constituted by C-kit+/CD34+/CD45− cells which have osteogenic differentiation potential both in vivo and in vitro [99, 100]. Then after, cells displaying stem cell characteristics were isolated from pulp tissue of deciduous and wisdom tooth [97, 99, 100], supernumerary natal tooth [4] and human third molar germs of young adults [5]. Recent studies indicate that DPSCs have the ability to differentiate into a broad range of cell lineages, including odontoblasts that can produce dentin, osteoblasts, adipocytes, skeletal and smooth muscle cells, elastic cartilage cells, endothelial and neural cells both in vivo and in vitro conditions [97–105]. Even though adult stem cells share very similar behaviors both in vivo and in vitro, they carry some specific characteristics of the tissue that they were derived from. The impact of these differences on biological and clinical processes, their origin and the generation mechanism that lies beneath are still unclear. Comparing the differences or similarities between stem cell types is one way to understand these mechanisms. Such comparisons should be focused on aspects of biological marker discovery , characterization of their proliferation capacity and differentiation potential along with other characteristics. In this sense, our research group isolated putative stem cells derived from human impacted third molar dental pulp (hDP), broadly characterized and compared with human BMMSCs [106]. We found out that in contrast to hBMMSCs, cytokeratin (CK) -18 and -19, which may be involved in the dentine repair and odontoblast differentiation, are strongly expressed in hDPSCs [106]. By showing the expression of numerous specific proteins of neural stem cells (NSCs) and neurons, the essential neuro-glia characteristics of hDPSCs were demonstrated. While these cells can differentiate into chondrogenic, osteogenic and adipogenic lineages, they also share some specific characteristics like expressing some NSC- and epithelial-related markers. hDPSCs have the ability to differentiate into both vascular endothelial and neural cells under distinct conditions in vitro. hDPSCs are located in the perivascular niche of dental pulp, and because hDPSCs are originated from migrating cranial neural crest cells, their neurogenicity is more potent with respect to hBMMSCs. Neural crest cells differentiate into a wide range of cell types including cells of dental papilla, dental follicle and neurons of the peripheral nervous system during embryonic development. Considering this, it has been shown that transplanted DPSCs can stay alive for a long time [107] and may induce neuroplasticity [108] in the central nervous system of experimental animal models. Considerable recovery from neurological dysfunction has been reported in studies mentioning DPSCs injection into the right dorsolateral striatum of animals subjected to middle cerebral arteryocclusion (MCAO) [109].
After rats with induced cortical lesions were injected into their cerebrospinal fluid with DPSCs which are pre-differentiated into neurons, these cells integrated into the host brain and exhibited some neuronal properties, indicating that they may be used as valuable sources for neuro‐ and glio-genesis in vivo [110]. The neuroregenerative effects of DPSCs in rodent spinal cord injury (SCI) models have also been reported in a recent study. High levels of trophic‐factor expression in the tissue, better tissue organization and the existence of many axons or oligodendrocytes and neurons with synapses in DPSCs transplanted mouse models of compressive SCI suggested that DPSCs may be possible candidates for therapeutic intervention for the treatments of SCI and central nervous system disorder in humans [111].
Partial locomotor function recovery has been reported in completely transected rat spinal cord after hDPSC transplantation [112]. However, relatively less recovery of locomotor functions was detected after transplantation of human BMMSCs or skin-derived fibroblasts. It has been stated by the same research group that hDPSCs present three major neuroregenerative activities ; (i) SCI-induced apoptosis of neurons, oligodendrocytes and astrocytes are inhibited by DPSCs that improves the preservation of myelin sheaths and neuronal filaments, (ii) they directly inhibit various axon growth inhibitors including chondroitin sulfate proteoglycan and myelin-associated glycoprotein via paracrine mechanisms, and (iii) they replace the lost cells by differentiating into mature oligodendrocytes under severe conditions of SCI. In line with these findings, results of our study state that due to their cell-autonomous and paracrine neuroregenerative activities, tooth-derived stem cells may offer therapeutic benefits for treating SCI [112]. Another finding of our study is that preclinical animal disease models, including myocardial infarction, colitis and systemic lupus erythematosus (SLE) may be treated via using the significant therapeutic benefits provided by the array of trophic factors produced by engrafted DPSCs [113, 114]. In correlation, DPSCs are found to be highly proliferative, self-renewing and multipotent cell population that can actively secrete a broad range of trophic, immuno-modulatory and anti-inflammatory factors. It has also been suggested by preliminary studies that other than exhibiting self‐renewal and multi-differentiation potential, dental tissue-derived MSCs also have immunomodulatory functions and potent tissue regenerative properties [115–117]. We have also shown the regulation of T-cell functions via expression and secretion of soluble factors/cytokines such as HLA-G, HGF-β1, IL-6, IL-10, TGF-β1, ICAM-1 and VCAM-1 by hDPSCs in both direct and indirect co-culture systems [117].
SHEDs can proliferate faster than DPSCs and BMMSCs (SHEDs > DPSCs > BM-MSCs). If they are cultured in neurogenic differentiation medium, SHEDs can form sphere-like clusters due to their high proliferation rate, which either adhere to the culture dish or float freely in the culture medium aggregating in clusters. Dissociation of these sphere-like clusters can be done via passaging through needles and grown on dishes coated with 0.1 % gelatin as individual fibroblastic cells afterwards, demonstrating that the process can bring about a remarkable proliferative capacity analogous to that of NSCs [58]. SHEDs have also been isolated and termed as “immature DPSCs (iDPSCs) ” by another research group [118]. As well as correlating with the results of studies described above, they also found out that iDPSCs express embryonic stem cell markers, including Oct4, Nanog, tumor recognition antigens (TRA-1-60 and TRA-1-81) and stage specific embryonic antigens (SSEA-3, SSEA-4).
Successful isolation and characterization of MSCs derived from human natal dental pulp (hNDP) were first declared by our research group [119] and these hNDP-MSCs were directionally differentiated to osteogenic, chondrogenic, adipogenic, myogenic and neurogenic lineages. Unlike CD3, CD8, CD11b, CD14, CD15, CD19, CD33, CD34, CD45, CD117, and HLA-DR, hNDP-MSCs expressed CD13, CD44, CD90, CD146 and CD166. hNDP-SCs seemed more developed and metabolically active cells based on their ultrastructural characteristics. Under basal conditions and without any stimulation towards differentiation, hNDP-SCs were able to express particular adipogenic (leptin, adipophilin and PPARγ), neurogenic (γ-enolase, MAP2a,b, c-fos, nestin, NF-H, NF-L, GFAP and betaIII tubulin), myogenic (desmin, myogenin, myosin-IIa, and α-SMA), osteogenic (osteonectin, osteocalcin, osteopontin, Runx-2, and type I collagen) and chondrogenic (type II collagen, SOX9) markers along with embryonic stem cell markers including Oct4, Rex-1, FoxD-3, Sox2, and Nanog. Adipogenic, osteogenic, chondrogenic, myogenic and neurogenic differentiation potentials of hNDP-SCs have also been demonstrated [119].
In one of our recent studies, phenotypic and proteomic characteristics of hDPSCs derived from a natal, an exfoliated deciduous and an impacted third molar tooth were comparatively analyzed [120]. All three stem cells displayed similar features on morphology, proliferation rates, expression of various cell surface markers, and differentiation potentials into adipocytes, osteocytes and chondrocytes. Furthermore, using 2DE approach coupled with MALDI-TOF/TOF, we have generated a common 2DE profile for all three stem cells. We found that 62.3 ± 7 % of the protein spots were conserved among the three MSC lines. Sixty-one of these conserved spots were identified by MALDI-TOF/TOF analysis . Classification of the identified proteins based on biological function revealed that proteins that are involved in protein folding machinery along with many structurally important proteins are predominantly expressed by all three stem cell lines. Some of these proteins may hold importance in understanding specific characteristics of hDPSCs [120].
To sum up, ongoing researches on DSCs is growing at an exceptional rate. Teeth- derived stem cells can be obtained in a convenient and minimally invasive way, and are easily accessible. Based on the discussion above, these new stem cell sources could be exploited for cellular therapies and ultimately for the development of regenerative treatment methods. Although these cells guarantee a donor match (autologous transplant) for life, they can also be used partly for close relatives.
9.4 Induced Pluripotent Stem (iPS) Cells in Dentistry
The discovery of iPS cells by Dr. Shinya Yamanaka is a milestone in stem cell research, and created a new approach in regenerative medicine [121–123]. iPS cells are obtained by reprogramming of somatic cells with gene transfer of transcription factors (Oct4, Sox2, Klf4, and c-Myc) which are highly expressed in embryonic stem cells. In the dental field, researchers are actively working with iPS cells for tooth regeneration [124]. Tooth development requires epithelial-mesenchymal interactions during the early stages of morphogenesis, and these cells come from different embryonic layer. To form tooth/root, iPS cells should be differentiated into both epithelial and mesenchymal lineages. If iPS cells can be induced separately to epithelial cells which express ameloblasts-specific proteins (i.e., cytokeratin, ameloblastin, amelogenin, enamelin) and mesenchymal cells which display odontogenic potential, and if the interactions of these two lineages could be provided later, functional tooth regeneration seems possible. Although iPS cells are promising for tooth/root regeneration and tooth like-structures obtained in mouse models, tooth regeneration process using iPS cells in humans, however, cannot be that much easy. While in mouse, the success rate of tooth regeneration using mesenchyme and epithelium derived from iPS cells can be 100 %, success rate of obtaining the tooth-like structures can be only 30 % in human studies. The difference of success rates between two groups can be explained with lack of uniformity in the epithelium derived from human iPS cells, and lack of the capacity to secrete extracellular matrix required for tooth regeneration [125, 126]. Furthermore, these variations can be due to differences in species and signaling at the stages of tooth formation in human vs. mouse. Therefore, some challenges still remain in creating root/tooth formation from human iPS cells as follows;
-
(i)
Immunogenicity of iPS cells
-
(ii)
No established feasible reprogramming method
-
(iii)
Lack of reproducible method due to significant differences between species
-
(iv)
Lack of information to accelerate human tooth development in vitro or in vivo
-
(v)
Lack of information in regulatory mechanism for iPS cells
-
(vi)
Insufficiency in regulation of the shape and size of the tooth
iPS cells can be differentiated into both epithelial and mesenchymal cells, and expanded and maintained for tooth bioengineering [127]. Liu et al. claimed that iPS cells had more potential in tooth regeneration when compared to other stem cells due to having better proliferation and differentiation capacities [128]. In addition to form epithelium and/or mesenchyme layer of tooth germ with iPS cells, these cells can also be used to obtain functional adult MSCs [129, 130]. For the first time, Hynes and co-workers investigated the pre-clinical utility of iPS cell-derived MSC-like cells for the treatment of periodontal fenestration defect model in rats [131]. Their results demonstrated that iPSC-MSC-like cells increased regeneration efficiency of periodontal tissues. Yang et al. used iPS cells-derived MSCs in the treatment of experimentally induced periodontitis model in rat as well, and they observed significantly decreased inflammatory infiltrates in periodontal tissues after systemic and local application of iPSC-MSCs treatment [132]. These two studies mentioned above concluded that iPSC-MSCs might provide a promising approach for the treatment of periodontal defects, and can be used as a source for not only for periodontal tissue engineering but also orthopedic applications and dental tissue engineering [133]. Ozeki et al. reported a method to differentiate mouse iPS cells into odontoblast-like cells expressing mature odontoblast markers, dentin sialophosphoprotein, and dentin matrix protein 1, and displaying physiologic and functional characteristics of odontoblasts in vitro. The generation of odontoblast cells from iPS cells may provide new clinical application area for the treatment of dental pulp regeneration [134] Since MSC-like cells derived from different iPS cell lines might demonstrate variability in their differentiation potential, detailed characterization studies regarding iPS cell-derived MSC-like cells is critical. Furthermore, safety, efficacy and economical concerns should be taken in to consideration as well [128, 133].
9.5 Current Approach in the Treatment of Missing Teeth
There are various alternative methods for the management of oral conditions due to periodontal disease, profound caries, congenital missing teeth, failure in endodontic treatment, tumor, and trauma, which may result in partial or full edentulism [135]. While the only choices for patients were conventional prosthesis including fixed prosthesis and full/partial dentures until dental implants were discovered, dental implant supported fixed and removable prosthesis have currently been offered to patients as a promising option (Figs. 9.1, 9.2, 9.3, 9.4, and 9.5) . As patient’s expectations and life standards increase, more options are being presented to the patients. However, economic condition can limit the alternative interventions, and is important for decision-making for the management of tooth loss.
In dentistry, evidence-based approaches indicate that root canal treatment is the most cost-effective treatment options for the treatment of teeth with irreversible pulpitis and coronal lesions. If initial root canal treatment fails, orthograde retreatment can be the most cost-effective way. However, if root canal retreatment is not successful, extraction and/or implant-supported crown would be more cost-effective compared to traditional prosthesis including fixed or removable partial dentures [135].
In molars with furcation involvement, non-surgical periodontal therapy is more effective for Class I types, and the therapy costs less than implant-supported single crowns. However, molars with class II-III need periodontal surgeries, and further bone graft materials and membranes for guided tissue regeneration, meaning that teeth can be saved successfully but it may not always be cost effective. Quality and survival rates of the treatment plan are very important for the replacement of missing single tooth. Implant-supported single crown provides better results in comparison with fixed partial prostheses. However, implant-supported prosthesis especially in partially or totally edentulous cases may cost more but provides superior survival rates when compared to partial or full dentures [135]. Gjengedal et al. compared the dietary intake of edentulous subjects who had conventional mandibular complete dentures or implant-supported overdenture, and recorded food avoidance of the patients [136]. The results of their study demonstrated that while there was no significant difference regarding food choices and nutrient intake between two groups, better chewing ability and greater willingness to eat more of certain food were reported in implant supported overdenture group. The chewing ability and capacity are very important for patients, and complete dentures may present oral disability. Chewing efficiency is critical to maintain quality of life and adequate nutrition. Using dentures or fixed prosthesis supported with dental implants improves life standards and nutritional status [137]. Numerous alternatives have been presented for implant supported dentures including fixed, removable or hybrid type according to bone amount (width/height), bone quality of jaw, oral hygiene, habits, systemic conditions and expectations of the patients. Esthetic, phonetic, functional and financial parameters can also be determinative for decision of the patients and dentist (prosthodontist and periodontist/oral surgeon).
For a successful treatment, osseointegration of the implants with bone should take place. Osseointegration is defined as the direct structural and functional connection between living bone and the surface of implant without intervening soft tissue. In dentistry, the implementation of osseointegration started in the mid-1960s as results of the study performed by Branemark who was anatomist professor [138]. Osseointegration is a dynamic process in which implant characteristics (macro-topography, micro-topography and surface properties) play critical role for cell behavior [139, 140]. However, the survival rate of the dental implants are very high (95–99 %), osseointegration of bone to titanium dental implant provides very rigid connection when compared to natural tooth. Natural tooth has periodontium and periodontal ligament tissue surrounding root surface which is unique tissue in the body allows tooth for the mobility for compensation of occlusal forces or trauma, and provides the maintenance of the periodontium . PDL composes different cell types including fibroblasts, MSCs, nerve cells and extracellular matrix and firm collagen fibers [140]. These cells have the capacity to differentiate into cementoblasts and/or osteoblasts, and have roles in repair/regeneration and immunoregulation of cell within the periodontium [140].
Dental implant-supported rehabilitations have some limitations due to lack of periodontal ligament around implants that maintains periodontium and proprioception during chewing. Dental implant supported prosthesis cannot mimic biologically active system like in tooth surrounding periodontal ligament and alveolar bone. There is direct integration and rigid connection with bone and titanium implants. Rehabilitation of mouth with denture (with/without implant) may present some complications like denture induced stomatitis and traumatic ulcers . Furthermore, dental implant-supported therapies may have some early and delayed complications (failure of implants, broken implants, peri-implantitis, decementation of prosthesis) [141]. To overcome these limitations, stem cell-based tooth regeneration has been considered as a fantastic option, combining tissue engineering techniques and stem cells [142].
9.6 Future Prospective of Stem Cells in Dentistry
9.6.1 Utility of Stem Cells to Create Whole Tooth Organ .
During the last decade, stem cell-based tooth regeneration studies presented attractive approaches for lost teeth. For this purpose, embryonic, adult stem cells and recently iPS cells have been investigated as potential cell sources for tooth regeneration. Since using embryonic stem cells leads to ethical concerns, iPS and adult stem cells seem more promising approaches for regenerative dentistry.
9.6.2 What Is Bio-tooth? Is It Possible?
Regeneration of a living tooth is the final aim of dentistry for the replacement of a lost tooth. There are two different approaches to create bio-tooth; only cell or cell-scaffold based approaches.
9.6.2.1 Cell-Based Tooth Regeneration
The cell-based regeneration process can be simply defined as obtaining of tooth germ using the epithelial and mesenchymal cells (derived from embryo or iPS cells). These cells can be derived from either dental or non-dental sources.
9.6.2.2 Using Epithelial and Mesenchymal Cells Derived from Dental Source
Oshima et al. described a protocol for three-dimensional bioengineered tooth germ reconstitution using tooth germ-derived epithelial and mesenchymal cells [43, 46]. They also described methods for analysis utilized for in vitro and in vivo studies of tooth development. Oshima’s group ectopically produced a bioengineered tooth containing periodontal ligament and alveolar bone, and they engrafted this bioengineered tooth into a jaw bone through bone integration. This bioengineered tooth could perform normal physiological tooth functions, including masticatory and perceptive potential, in mouse.
9.6.2.3 Using One of Epithelial or Mesenchymal Cells from Non-dental Sources
Mesenchymal cells derived from bone marrow stroma as a non-dental source were used for tooth formation firstly by Ohazama et al. in 2004 [143]. Their study demonstrated that adult BMMSCs with the embryonic inductive tooth epithelium cells could induce tooth formation in an adult body. Later, Angelova Volponi et al. showed that adult human epithelial cells (non-dental source) combined with mouse embryonic mesenchymal cells could also produce tooth-like structure in renal capsule of the mouse [144]. Micro-CT analysis of the transferred tissues revealed obvious tooth-like structures. Histological sections confirmed the presence of obvious teeth structure with dentin, enamel spaces, and well-vascularized pulp containing odontoblast-like cells expressing dentin sialophosphoprotein and lining the dentin surface. They claimed that these epithelial cells obtained from human gingival are a realistic source to be used in human bio-tooth generation [144]. They concluded that using non-embryonic sources for epithelial or mesenchymal cells is clinically feasible and needs further research to provide sufficient cell numbers for successful tooth formation.
9.6.2.4 Scaffold and Cell-Based Tooth Regeneration (Fig. 9.6)
The main aim of the process is to obtain different compartment of tooth (periodontal ligament and pulp) from MSCs (derived from adult or iPS cells) separately, and put them together into the tooth-like bio-printed scaffold mimicking calcified tooth structure.
Sonoyama et al. aimed to establish a bio-root model to reconstruct a functional tooth in miniature pigs (minipigs) using postnatal stem cells including SCAPs and PDLSCs [44]. Their results demonstrated that this hybrid strategy using autologous DSCs may provide predictable applications. Wei et al. also performed an animal experiment to regenerate bio-root by employing similar hybrid strategy with different cell types (allogeneic DSCs) [45]. Hydroxyapatite tricalcium phosphate root shaped scaffold containing DPSCs covered by PDLSC sheet exhibited normal tooth characteristics after 6 months. In addition, dentinal tubule-like and functional periodontal ligament-like structures without any immunological response were reported. In another study, hydroxyapatite-coated dental implant was covered with embryonic dental follicle tissue, and transplanted into jaw bone of a murine tooth-loss model [145]. Using hydroxyapatite-coated dental implant and DFSCs, fibrous connection was established around the implants, a bio-hybrid organ. This bio-hybrid implant provided function, bone remodeling, and periodontal tissue regeneration including periodontal ligament and cementum. The bio-hybrid implant was claimed to be a promising approach to be used for future tooth replacement therapies. However, to create tooth-like structures, numerous concerns should be elucidated before conducting clinical studies;
-
Which cell combinations are better for human approaches?
-
Heterogeneity of the cells among the patient,
-
Appropriate reciprocal interaction among the cells,
-
The predictability of shape of the growing tooth,
-
Tumorigenicity and immunogenicity of the cells (since one of cell layer is embryonic and obtained from iPS)
9.7 Biomaterials
Developing three dimensional bioengineered tooth for future replacement therapy have been investigated, and in this line, mechanically resistant to the occlusal force and biocompatible biomaterials have been tested. Biomaterials including micro and nano-sized (or their combinations) have been used for this purpose. Biomaterials should provide appropriate micro-environment for cells to form the final structured organ. The materials used for bio-tooth applications should be resistant to chemical and physical abrasions, and provide required mechanical strength and intraoral maintenance with desired function and esthetic. For this purpose, poly(lactide-co-glycolide) (PLGA) (70/30, mol/mol) scaffolds, three types of calcium phosphate contained composites scaffolds that were composed of 50 % of PLGA and 50 % of hydroxyapatite, tricalcium phosphate (TCP) and calcium carbonate hydroxyapatite (CDHA) were evaluated [146]. The results showed that while the calcium phosphate contained compound supported tooth regeneration effectively, the PLGA/TCP scaffold would be more appropriate for the proliferation and differentiation of DPSCs. Furthermore, seeding of rat tooth bud cells on the PLGA/TCP scaffold generated dentin- and pulp-like tissues, indicating that PLGA/TCP scaffold is superior to the other three scaffolds for tooth-tissue regeneration approaches, particularly for dentin formation.
Selection of optimal scaffold for future clinical application remains a questionable, and further research is required to improve the features of the materials for tooth regeneration applications. In particular, recent developments including composites, biomaterials (nanofibrous scaffolds, hydrogel systems, laser-fabricated nanostructures) and cell-based bio-printing methods seem promising to produce proper scaffolds for dental tissue engineering.
9.7.1 Bio-implant vs. Bio-tooth?
Gault et al. evaluated PDLSC-seeded titanium implant to create bio-implant (Fig. 9.7), and named the structure as ‘ ligaplant ’ [147]. They placed titanium dental implant to the extraction socket and reported new bone and PDL tissue development around the implants at the end of treatment. They claimed that biological mimicking of tooth with dental implant can be applicable in clinical dentistry. Their investigation demonstrated the application of ligament-anchored implants, which have advantages over osseointegrated oral implants since they don’t have rigid fixation. In addition, they concluded that ligaplant induced the formation of new bone and new PDL in the vicinity due to their remarkable potential in periodontal tissue regeneration [147, 148]. On the other hand, as there is no cementum on the titanium surface, and collagen fibers cannot be placed around of the titanium implants like in natural tooth environment, bio-implant cannot exactly mimic natural structure around the tooth. Without cementum layer, cell-seeded titanium implant cannot provide biological expectations. Many questions remain with the ligaplant to be solved with long-term clinical findings [148]. However, this bio-hybrid (cells and titanium material combination) technology for tooth replacement can find a place in both periodontology/oral implantology, bio-tooth philosophy looks more applicable and more biological thought. But further pre-clinical studies in large animal models or human clinical trials using patient tissue-derived cells are needed to realize future human clinical applications [149]. Making a functional bio-tooth using stem cells may be much more complicated than expected. Several issues including identification and stemness of stem cells, dental morphogenesis, determination tooth type, odontogenic signals, controllable bio-tooth growth and eruption, and host-graft immunorejection in the jaws must be solved [35, 124, 149].
9.8 Conclusion and Future Trends or Directions
The final aim of the regenerative dentistry is to create functional whole tooth organ, mimicking dental hard and soft tissues. Various stem cells have been used in tooth bioengineering studies to evaluate their potential. Technologies using MSCs and iPS cells might be the new era of personalized dentistry but due to heterogeneity among the patients, studies should be focused an individually-targeted approach . Functional cell-based tooth replacement therapy requires collaborative studies conducted by bio-engineers, biologists, chemists and dentists. Mechanically and topographically appropriate biomaterials should be investigated for functional tooth organ studies. For successful tooth regeneration, more information is needed on genetic and cellular mechanisms regulating growth of the tooth crown and root, guiding tooth development, to understand the specification of important cell lineages including ameloblasts, odontoblasts, and cementoblasts.
References
Jussila M, Juuri E, Thesleff I (2013) Tooth morphogenesis and renewal. In: Huang GTJ, Thesleff I (eds) Stem cells in craniofacial development and regeneration. Wiley, New York, pp 109–134
Foster BL, Nociti FH, Somerman MJ (2013) Tooth root development. In: Huang GTJ, Thesleff I (eds) Stem cells in craniofacial development and regeneration. Wiley, New York, pp 153–178
Akiyama K, Chen C, Gronthos S, Shi S (2012) Lineage differentiation of mesenchymal stem cells from dental pulp, apical papilla, and periodontal ligament. Methods Mol Biol 887:111–121
Hakki SS, Bozkurt SB, Hakki EE, Turac G, Yilmaz I, Karaoz E (2014) BMP-2,-6 and BMP-7 differently regulate osteogenic differentiation of human periodontal ligament stem cells (hPDLSCs). J Biomed Mater Res B 02(1):119–130
Hakki SS, Kayis SA, Hakki EE, Bozkurt SB, Duruksu G, Unal ZS, Turaç G, Karaoz E (2015) Comparison of mesenchymal stem cells isolated from pulp and periodontal ligament. J Periodontol 86(2):283–291
Huang GT, Gronthos S, Shi S (2009) Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine. J Dent Res 88(9):792–806
Gosau M, Götz W, Felthaus O, Ettl T, Jäger A, Morsczeck C (2013) Comparison of the differentiation potential of neural crest derived progenitor cells from apical papilla (dNC-PCs) and stem cells from exfoliated deciduous teeth (SHED) into mineralising cells. Arc Oral Biol 58(6):699–706
Yang H, Gao LN, An Y et al (2013) Comparison of mesenchymal stem cells derived from gingival tissue and periodontal ligament in different incubation conditions. Biomaterials 34(29):7033–7047
Roman A, Soancă A, Florea A, Páll E (2013) In vitro characterization of multipotent mesenchymal stromal cells isolated from palatal subepithelial tissue grafts. Microsc Microanal 19(2):370–380
Grimm WD, Dannan A, Giesenhagen B, Schau I, Varga G, Vukovic MA, Sirak SV (2014) Translational research: palatal-derived ecto-mesenchymal stem cells from human palate: a new hope for alveolar bone and cranio-facial bone reconstruction. Int J Stem Cells 7(1):23–29
Hynes K, Menicanin D, Gronthos S, Bartold PM (2012) Clinical utility of stem cells for periodontal regeneration. Periodontol 59(1):203–227
Xiao L, Nasu M (2014) From regenerative dentistry to regenerative medicine: progress, challenges, and potential applications of oral stem cells. Stem Cells Cloning 7:89–99
Padial-Molina M, O’Valle F, Lanis A, Mesa F, Ehrenfest DM, Wang HL, Galindo-Moreno P (2015) Clinical application of mesenchymal stem cells and novel supportive therapies for oral bone regeneration. Biomed Res Int 2015:341327
Farré-Guasch E, Wolff J, Helder MN, Schulten EA, Forouzanfar T, Klein-Nulend J (2015) Application of additive manufacturing in oral and maxillofacial surgery. J Oral Maxillofac Surg. doi:10.1016/j.joms.2015.04.019
Pagni G, Kaigler D, Rasperini G, Avila-Ortiz G, Bartel R, Giannobile WV (2012) Bone repair cells for craniofacial regeneration. Adv Drug Deliv Rev 64(12):1310–1319
Zhao W, Lu JY, Hao YM, Cao CH, Zou DR (2014) Maxillary sinus floor elevation with a tissue-engineered bone composite of deciduous tooth stem cells and calcium phosphate cement in goats. J Tissue Eng Regen Med. doi:10.1002/term.1867
Yu BH, Zhou Q, Wang ZL (2014) Comparison of tissue-engineered bone from different stem cell sources for maxillary sinus floor augmentation: a study in a canine model. J Oral Maxillofac Surg 72(6):1084–1092
Zhang W, Zhang X, Wang S, Xu L, Zhang M, Wang G, Jin Y, Zhang X, Jiang X (2013) Comparison of the use of adipose tissue-derived and bone marrow-derived stem cells for rapid bone regeneration. J Dent Res 92(12):1136–1141
Oshima T, Duttenhoefer F, Xavier S, Nelson K, Sauerbier S (2014) Can mesenchymal stem cells and novel gabapentin-lactam enhance maxillary bone formation? J Oral Maxillofac 72(3):485–495
Yamada Y, Nakamura S, Ueda M, Ito K (2013) Osteotome technique with injectable tissue-engineered bone and simultaneous implant placement by cell therapy. Clin Oral Implants Res 24(4):468–474
Yamada Y, Nakamura S, Klein OD, Ito K (2014) Current trends in stem cell therapy for improvement of bone quality. Histol Histopathol 29(6):691–697
Yamada Y, Hara K, Nakamura S, Ueda M, Ito K, Nagasaka T (2013) Minimally invasive approach with tissue engineering for severe alveolar bone atrophy case. Int J Oral Maxillofac Surg 42(2):260–263
Rickert D, Vissink A, Slot WJ, Sauerbier S, Meijer HJ, Raghoebar GM (2014) Maxillary sinus floor elevation surgery with BioOss® mixed with a bone marrow concentrate or autogenous bone: test of principle on implant survival and clinical performance. Int J Oral Maxillofac Surg 43(2):243–247
Kaigler D, Pagni G, Park CH, Braun TM, Holman LA, Yi E, Tarle SA, Bartel RL, Giannobile WV (2013) Stem cell therapy for craniofacial bone regeneration: a randomized, controlled feasibility trial. Cell Transplant 22(5):767–777
Kaigler D, Avila-Ortiz G, Travan S, Taut AD, Padial-Molina M, Rudek I, Wang F, Lanis A, Giannobile WV (2015) Bone engineering of maxillary sinus bone deficiencies using enriched CD90+ stem cell therapy: a randomized clinical trial. J Bone Miner Res. doi:10.1002/jbmr.2464
Hasegawa N, Kawaguchi H, Hirachi A, Takeda K, Mizuno N, Nishimura M, Koike C, Tsuji K, Iba H, Kato Y, Kurihara H (2006) Behavior of transplanted bone marrow-derived mesenchymal stem cells in periodontal defects. J Periodontol 77:1003–1007
Kawaguchi H, Hirachi A, Hasegawa N, Iwata T, Hamaguchi H, Shiba H, Takata T, Kato Y, Kurihara H (2004) Enhancement of periodontal tissue regeneration by transplantation of bone marrow mesenchymal stem cells. J Periodontol 75:1281–1287
Marei MK, Nouh SR, Saad MM, Ismail NS (2005) Preservation and regeneration of alveolar bone by tissue-engineered implants. Tissue Eng 11:751–767
Ito K, Yamada Y, Nakamura S, Ueda M (2011) Osteogenic potential of effective bone engineering using dental pulp stem cells, bone marrow stem cells, and periosteal cells for osseointegration of dental implants. Int J Oral Maxillofac Implants 26(5):947–954
Kim SH, Kim KH, Seo BM et al (2009) Alveolar bone regeneration by transplantation of periodontal ligament stem cells and bone marrow stem cells in a canine peri-implant defect model: a pilot study. J Periodontol 80(11):1815–1823
Liu Y, Zheng Y, Ding G et al (2008) Periodontal ligament stem cell-mediated treatment for periodontitis in miniature swine. Stem Cells 26(4):1065–1073
Khorsand A, Eslaminejad MB, Arabsolghar M et al (2013) Autologous dental pulp stem cells in regeneration of defect created in canine periodontal tissue. J Oral Implantol 39(4):433–443. doi:10.1563/AAID-JOI-D-12-00027
Feng F, Akiyama K, Liu Y et al (2010) Utility of PDL progenitors for in vivo tissue regeneration: a report of 3 cases. Oral Dis 16(1):20–28
Fawzy El-Sayed KM, Mekhemar MK, Beck-Broichsitter BE, Bähr T, Hegab M, Receveur J, Heneweer C, Becker ST, Wiltfang J, Dörfer CE (2015) Periodontal regeneration employing gingival margin-derived stem/progenitor cells in conjunction with IL-1ra-hydrogel synthetic extracellular matrix. J Clin Periodontol 42(5):448–457
Liu J, Yu F, Sun Y, Jiang B, Zhang W, Yang J, Xu GT, Liang A, Liu S (2015) Concise reviews: characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells 33(3):627–638
Bansal R, Jain A, Mittal S (2015) Current overview on challenges in regenerative endodontics. J Conserv Dent 18(1):1–6
Alongi DJ, Yamaza T, Song Y et al (2010) Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen Med 5:617–631
Liao J, Al Shahrani M, Al-Habib M, Tanaka T, Huang GT (2011) Cells isolated from inflamed periapical tissue express mesenchymal stem cell markers and are highly osteogenic. J Endod 37:1217–1224
Ravindran S, Huang CC, George A (2014) Extracellular matrix of dental pulp stem cells: applications in pulp tissue engineering using somatic MSCs. Front Physiol 4:395
Ishizaka R, Iohara K, Murakami M, Fukuta O, Nakashima M (2012) Regeneration of dental pulp following pulpectomy by fractionated stem/progenitor cells from bone marrow and adipose tissue. Biomaterials 33:2109–2118
Wang Y, Zhao Y, Jia W, Yang J, Ge L (2013) Preliminary study on dental pulp stem cell-mediated pulp regeneration in canine immature permanent teeth. J Endod 39:195–201
Nakao K, Morita R, Saji Y, Ishida K, Tomita Y, Ogawa M, Saitoh M, Tomooka Y, Tsuji T (2007) The development of a bioengineered organ germ method. Nat Methods 4(3):227–230
Oshima M, Mizuno M, Imamura A, Ogawa M, Yasukawa M, Yamazaki H, Morita R, Ikeda E, Nakao K, Takano-Yamamoto T, Kasugai S, Saito M, Tsuji T (2011) Functional tooth regeneration using a bioengineered tooth unit as a mature organ replacement regenerative therapy. PLoS One 6(7):e21531
Sonoyama W, Liu Y, Fang D et al (2006) Mesenchymal stem cell-mediated functional tooth regeneration in swine. Plos One 20:1
Wei F, Song T, Ding G, Xu J, Liu Y, Liu D, Fan Z, Zhang C, Shi S, Wang S (2013) Functional tooth restoration by allogeneic mesenchymal stem cell-based bio-root regeneration in swine. Stem Cells Dev 22(12):1752–1762
Oshima M, Ogawa M, Yasukawa M, Tsuji T (2012) Generation of a bioengineered tooth by using a three-dimensional cell manipulation method (organ germ method). Methods Mol Biol 887:149–165
Takahashi C, Yoshida H, Komine A, Nakao K, Tsuji T, Tomooka Y (2010) Newly established cell lines from mouse oral epithelium regenerate teeth when combined with dental mesenchyme. In Vitro Cell Dev Biol Anim 46:457–468
Egusa H, Sonoyama W, Nishimura M, Atsuta I, Akiyama K (2012) Stem cells in dentistry-Part II: clinical applications. J Prosthodont Res 56(4):229–248
Lin NH, Gronthos S, Marrtold P (2009) Stem cells and future periodontal regeneration. Periodontol 51:239–251
Steindorff MM, Lehl H, Winkel A, Stiesch M (2014) Innovative approaches to regenerate teeth by tissue engineering. Arch Oral Biol 59(2):158–166
Menicanin D, Mrozik KM, Wada N et al (2014) Periodontal ligament derived stem cells exhibit the capacity for long-term survival, self-renewal and regeneration of multiple tissue types in vivo. Stem Cells Dev 23:1001–1011
Rosa V, Zhang Z, Grande RH, Nör JE (2013) Dental pulp tissue engineering in full-length human root canals. J Dent Res 92(11):970–975
Syed-Picard FN, Ray HL Jr, Kumta PN, Sfeir C (2014) Scaffoldless tissue-engineered dental pulp cell constructs for endodontic therapy. J Dent Res 93:250–255
Torii D, Konishi K, Watanabe N, Goto S, Tsutsui T (2014) Cementogenic potential of multipotential mesenchymal stem cells purified from the human periodontal ligament. Odontology. doi:10.1007/s10266-013-0145-y
Otsu K, Kumakami-Sakano M, Fujiwara N, Kikuchi K, Keller L, Lesot H, Harada H (2014) Stem cell sources for tooth regeneration: current status and future prospects. Front Physiol 5:36. doi:10.3389/fphys.2014.00036
Yan M, Yu Y, Zhang G, Tang C, Yu J (2011) A journey from dental pulp stem cells to a bio-tooth. Stem Cell Rev 7(1):161–171
Gronthos S, Mankani M, Brahim J, Robey PG, Shi S (2000) Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 97:13625–13630
Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, Shi S (2003) SHED: stem cells from human exfoliated deciduous teeth. Proc Natl Acad Sci U S A 100:5807–5812
Huang AH, Chen YK, Lin LM, Shieh TY, Chan AW (2008) Isolation and characterization of dental pulp stem cells from a supernumerary tooth. J Oral Pathol Med 37:571–574
Karaoz E, Okcu A, Gacar G, Saglam O, Yuruker S, Kenar H (2010) A comprehensive characterization study of human bone marrow MSCs with an emphasis on molecular and ultrastructural properties. J Cell Physiol 226:1367–1382
Yalvac ME, Ramazanoglu M, Tekguc M, Bayrak OF, Shafigullina AK, Salafutdinov II, Blatt NL, Kiyasov AP, Sahin F, Palotás A, Rizvanov AA (2010) Human tooth germ stem cells preserve neuro-protective effects after long-term cryo-preservation. Curr Neurovasc Res 7:49–58
Palmer R, Lubbock M (1995) The soft connective tissues of the gingiva and periodontal ligament: are they unique? Oral Dis 1:230–237
Bath-Balogh M, Fehrenbach MJ (2011) Illustrated dental embryology, histology, and anatomy. Elsevier, St Louis, p 123
Schor SL, Ellis I, Irwin CR (1996) Sub- populations of fetal-like gingival fibroblasts: characterization and potential significance for wound healing and the progression of periodontal disease. Oral Dis 2(2):155e66
Häkkinen L, Uitto VJ, Larjava H (2000) Cell biology of gingival wound healing. Periodontol 24:127e52
Zhang Q, Shi S, Liu Y et al (2009) Mesenchymal stem cells derived from human gingiva are capable of immunomodulatory functions and ameliorate inflammation-related tissue destruction in experimental colitis. J Immunol 183(12):7787e98
Tang L, Li N, Xie H, Jin Y (2011) Characterization of mesenchymal stem cells from human normal and hyperplastic gingiva. J Cell Physiol 226(3):832e42
Ge S, Mrozik KM, Menicanin D, Gronthos S, Bartold PM (2012) Isolation and characterization of mesenchymal stem cell-like cells from healthy and inflamed gingival tissue: potential use for clinical therapy. Regen Med 7(6):819e32
Egusa H, Okita K, Kayashima H et al (2010) Gingival fibroblasts as a promising source of induced pluripotent stem cells. PLoS One 5(9):e12743
Schor SL, Ellis I, Irwin CR et al (1996) Subpopulations of fetal-like gingival fibroblasts: characterisation and potential significance for wound healing and the progression of periodontal disease. Oral Dis 2:155–166
McCulloch CA, Knowles G (1991) Discrimination of two fibroblast progenitor populations in early explant cultures of hamster gingiva. Cell Tissue Res 264:87–94
Marynka K (2008) Human oral mucosa is highly enriched with pluripotent stem cells. Sixth ISSCR Annual Meeting, Philadelphia, PA
Marynka K, Treves S, Yafee M, Rachima H, Gafni Y, Cohen MA, Pitaru S (2010) The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cells. doi:10.1002/stem.425
Roman A, Soanca A, Barbu-Tudoran L, Irimie AI, Pall E (2012) Comparative evaluation of the influence of two resin-based restorative materials on the behavior of progenitor like cells. J Optoelectron Adv Mater 14:491–496
Köseoğlu S, Duran İ, Sağlam M, Bozkurt SB, Kırtıloğlu OS, Hakki SS (2013) Efficacy of collagen membrane seeded with autologous gingival fibroblasts in gingival recession treatment: a randomized, controlled pilot study. J Periodontol 84(10):1416–1424
Hakki SS, Berry JE, Somerman MJ (2001) The effect of enamel matrix protein derivative on follicle cells in vitro. J Periodontol 72(5):679–687
Morsczeck C, Götz W, Schierholz J et al (2005) Isolation of precursor cells (PCs) from human dental follicle of wisdom teeth. Matrix 24:155–165
Honda MJ, Imaizumi M, Tsuchiya S, Morsczeck C (2010) Dental follicle stem cells and tissue engineering. J Oral Sci 52:541–552
Estrela C, Alencar AH, Kitten GT, Vencio EF, Gava E (2011) Mesenchymal stem cells in the dental tissues: perspectives for tissue regeneration. Braz Dent J 22(2):91–98
Sonoyama W, Liu Y, Yamaza T, Tuan RS, Wang S, Shi S, Huang GT-J (2008) Characterization of apical papilla and its residing stem cells from human immature permanent teeth – a pilot study. J Endod 34(2):166–171. doi:10.1016/j.joen.2007.11.021
Huang AH-C, Snyder BR, Cheng P-H, Chan AWS (2008) Putative dental pulp-derived stem/stromal cells promote proliferation and differentiation of endogenous neural cells in the hippocampus of mice. Stem Cells 26(10):2654–2663
Banchs F, Trope M (2004) Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod 30:196–200
Chueh LH, Huang GT (2006) Immature teeth with periradicular periodontitis or abscess undergoing apexogenesis: a paradigm shift. J Endod 32:1205–1213
Huang GT, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, Shi S (2010) Stem/progenitor cell-mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Eng Part A 16(2):605–615
Sainan W, Jinquan M, Zhipeng F, Yan Y, Ming Y, Gang L, Chunbo T, Zilu W, Yangyu Z, Jinhua Y, Guangdong Z (2012) Insulin-like growth factor 1 can promote the osteogenic differentiation and osteogenesis of stem cells from apical papilla. Stem Cell Res 8:346–356
Seo BM, Miura M, Gronthos S et al (2004) Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 364(9429):149e55
Hakki SS, Hakki EE, Nohutcu RM (2009) Regulation of matrix metalloproteinases and tissue inhibitors of matrix metalloproteinases by basic fibroblast growth factor and dexamethasone in periodontal ligament cells. J Periodontal Res 44(6):794–802
McCulloch CA, Bordin S (1991) Role of fibroblast subpopulations in periodontal physiology and pathology. J Periodontal Res 26(3 Pt 1):144–154
Isaka J, Ohazama A, Kobayashi M et al (2001) Participation of periodontal ligament cells with regeneration of alveolar bone. J Periodontol 72:314–323
Ponnaiyan D, Bhat KM, Bhat GS (2012) Comparison of immuno-phenotypes of stem cells from human dental pulp and periodontal ligament. J Oral Implantol 25(1):127–134
Vasandan AB, Shankar SR, Prasad P, Sowmya JV, Bhonde RR, Jyothi PS (2014) Functional differences in mesenchymal stromal cells from human dental pulp and periodontal ligament. J Cell Mol Med 8:344–354
Carosella ED, Favier B, Rouas-Freiss N, Moreau P, Lemaoult J (2008) Beyond the increasing complexity of the immunomodulatory HLA-G molecule. Blood 15:4862–4870
Selmani Z, Naji A, Zidi I (2008) Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4-CD25 high FOXP3+regulatory T cells. Stem Cells 26:212–222
Wada N, Menicanin D, Shi S, Bartold PM, Gronthos S (2009) Immunomodulatory properties of human periodontal ligament stem cells. J Cell Physiol 219(3):667–676
Lei M, Li K, Li B, Gao LN, Chen FM, Jin Y (2014) Mesenchymal stem cell characteristics of dental pulp and periodontal ligament stem cells after in vivo transplantation. Biomaterials 35:6332–6343
Trubiani O, Orsini G, Zini N et al (2008) Regenerative potential of human periodontal ligament derived stem cells on three-dimensional biomaterials: a morphological report. J Biomed Mater Res A 87:986–993
Gronthos S, Brahim J, Li W, Fisher LW, Cherman N, Boyde A, Den-Besten P, Robey PG, Shi S (2002) Stem cell properties of human dental pulp stem cells. J Dent Res 81:531–535
Jo YY, Lee HJ, Kook SY, Choung HW, Park JY, Chung JH, Choung YH, Kim ES, Yang HC, Choung PH (2007) Isolation and characterization of postnatal stem cells from human dental tissues. Tissue Eng 13:767–773
Laino G, d’Aquino R, Graziano A, Lanza V, Carinci F, Naro F, Pirozzi G, Papaccio G (2005) A new population of human adult dental pulp stem cells: a useful source of living autologous Wbrous bone tissue (LAB). J Bone Miner Res 20:1394–1402
d’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, Papaccio G (2007) Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ 14:1162–1171
Gandia C, Arminan A, Garcia-Verdugo JM, Lledo E, Ruiz A, Minana MD, Sanchez-Torrijos J, Paya R, Mirabet V, Carbonell-Uberos F, Llop M, Montero JA, Sepulveda P (2008) Human dental pulp stem cells improve left ventricular function, induce angiogenesis, and reduce infarct size in rats with acute myocardial infarction. Stem Cells 26:638–645
Iohara K, Nakashima M, Ito M, Ishikawa M, Nakasima A, Akamine A (2004) Dentin regeneration by dental pulp stem cell therapy with recombinant human bone morphogenetic protein 2. J Dent Res 83:590–595
Nosrat IV, Widenfalk J, Olson L, Nosrat CA (2001) Dental pulp cells produce neurotrophic factors, interact with trigeminal neurons in vitro, and rescue motoneurons after spinal cord injury. Dev Biol 238:120–132
Otaki S, Ueshima S, Shiraishi K, Sugiyama K, Hamada S, Yorimoto M, Matsuo O (2007) Mesenchymal progenitor cells in adult human dental pulp and their ability to form bone when transplanted into immune compromised mice. Cell Biol Int 31:1191–1197
Yu J, Wang Y, Deng Z, Tang L, Li Y, Shi J, Jin Y (2007) Odontogenic capability: bone marrow stromal stem cells versus dental pulp stem cells. Biol Cell 99:465–474
Karaoz E, Demircan PÇ, Saglam Ö, Aksoy A, Kaymaz F, Duruksu G (2011) Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow derived mesenchymal stem cells. Histochem Cell Biol 136(4):455–473
Arthur A, Rychkov G, Shi S et al (2008) Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells 26:1787–1795
Arthur A, Shi S, Zannettino AC et al (2009) Implanted adult human dental pulp stem cells induce endogenous axon guidance. Stem Cells 27:2229–2237
Yang K‐L, Chen M‐F, Liao C‐H et al (2009) A simple and efficient method for generating Nurr1‐positive neuronal stem cells from hu‐ man wisdom teeth (tNSC) and the potential of tNSC for stroke therapy. Cytotherapy 11:606–617
Király M, Kádár K, Horváthy DB et al (2011) Integration of neuronally pre-differentiated human dental pulp stem cells into rat brain in vivo. Neurochem Int 59:371–381
de Almeida FM, Marques SA, Ramalho Bdos S et al (2011) Human dental pulp cells: a new source of cell therapy in a mouse model of compressive spinal cord injury. J Neurotrauma 28:1939–1949
Sakai K, Yamamoto A, Matsubara K, Nakamura S, Naruse M, Yamagata M, Ueda M (2012) Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest 122(1):80–90
Zhao Y, Wang L, Jin Y, Shi S (2012) Fas ligand regulates the immunomodulatory properties of dental pulp stem cells. J Dent Res 91:948–954
Yamamoto A, Sakai K, Matsubara K, Kano F, Ueda M (2014) Multifaceted neuro-regenerative activities of human dental pulp stem cells for functional recovery after spinal cord injury. Neurosci Res 78:16–20
Pierdomenico L, Bonsi L, Calvitti M et al (2005) Multipotent mesenchymal stem cells with immunosuppressive activity can be easily isolated from dental pulp. Transplantation 80:836–842
Tomic S, Djokic J, Vasilijic S et al (2010) Immunomodulatory properties of mesenchymal stem cells derived from dental pulp and dental follicle are susceptible to activation by toll-like receptor agonists. Stem Cells Dev 20:695–708
Demircan PÇ, Sarıboyacı AE, Ünal ZS, Gacar G, Subaşı C, Karaoz E (2011) Immunoregulatory effects of human dental pulp-derived stem cells on T cells: comparison of transwell co-culture and mixed lymphocyte reaction. Cytotherapy 13(10):1205–2005
Kerkis I, Kerkis A, Dozortsev D et al (2006) Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs 184:105–116
Karaoz E, Doğan BN, Aksoy A, Gacar G, Akyüz S, Ayhan S, Genç ZS, Yürüker S, Duruksu G, Demircan PC, Sarıboyacı AE (2010) Isolation and in vitro characterisation of dental pulp stem cells from natal teeth. Histochem Cell Biol 133(1):95–112
Akpinar G, Kasap M, Aksoy A, Duruksu G, Gacar G, Karaoz E (2014) Phenotypic and proteomic characteristics of human dental pulp derived mesenchymal stem cells from a natal, an exfoliated deciduous, and an impacted third molar tooth. Stem Cells Int. doi:10.1155/2014/457059
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676
Takahashi K, Tanabe K, Ohnuki M et al (2007) Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872
Yamanaka S (2012) Induced pluripotent stem cells: past, present, and future. Cell Stem Cell 10(6):678–684
Wan M, Du W, Zhou X, Xu X, Zheng L (2015) Stem cell-based tooth engineering and their potential in dental medicine. Curr Stem Cell Res Ther 10(5):443–449
Cai J, Cho SW, Kim JY, Lee MJ, Cha YG, Jung HS (2007) Patterning the size and number of tooth and its cusps. Dev Biol 304(2):499–507
Cai J, Zhang Y, Liu P, Chen S, Wu X, Sun Y, Li A, Huang K, Luo R, Wang L, Liu Y, Zhou T, Wei S, Pan G, Pei D (2013) Generation of tooth-like structures from integration-free human urine induced pluripotent stem cells. Cell Regen 2(1):6
Menendez L, Kulik MJ, Page AT et al (2013) Directed differentiation of human pluripotent cells to neural crest stem cells. Nature Protocols 8(1):203–212
Liu P, Zhang Y, Chen S, Cai J, Pei D (2014) Application of iPS cells in dental bioengineering and beyond. Stem Cell Rev 10(5):663–670
Hynes K, Menicanin D, Mrozik K, Gronthos S, Bartold PM (2014) Generation of functional mesenchymal stem cells from different induced pluripotent stem cell lines. Stem Cells Dev 23(10):1084–1096
Hynes K, Menicanin D, Gronthos S, Bartold MP (2014) Differentiation of iPSC to mesenchymal stem-like cells and their characterization. Methods Mol Biol. doi:10.1007/7651_2014_142
Hynes K, Menicanin D, Han J, Marino V, Mrozik K, Gronthos S, Bartold PM (2013) Mesenchymal stem cells from iPS cells facilitate periodontal regeneration. J Dent Res 92(9):833–839
Yang H, Aprecio RM, Zhou X, Wang Q, Zhang W, Ding Y, Li Y (2014) Therapeutic effect of TSG-6 engineered iPSC-derived MSCs on experimental periodontitis in rats: a pilot study. PLoS One 9(6):1–7
Hynes K, Menichanin D, Bright R, Ivanovski S, D.W. Hutmacher DW, Gronthos S, Bartold PM (2015) Induced pluripotent stem cells: a new frontier for stem cells in dentistry. J Dent Res. doi:10.1177/0022034515599769
Ozeki N, Mogi M, Kawai R, Yamaguchi H, Hiyama T, Nakata K, Nakamura H (2013) Mouse-induced pluripotent stem cells differentiate into odontoblastlike cells with induction of altered adhesive and migratory phenotype of integrin. PLoS One 8(11):1–13
Beikler T, Flemmig TF (2015) EAO consensus conference: economic evaluation of implant-supported prostheses. Clin Oral Implants Res. doi:10.1111/clr.12630
Gjengedal H, Dahl L, Lavik A, Trovik TA, Berg E, Boe OE, Malde MK (2012) Randomized clinical trial comparing dietary intake in patients with implant-retained overdentures and conventionally relined denture. Int J Prosthodont 25(4):340–347
Schimmel M, Katsoulis J, Genton L, Müller F (2015) Masticatory function and nutrition in old age. Swiss Dent J 125(4):449–454
Branemark PI (1983) Osseointegration and its experimental background. J Prosthet Dent 50(3):399–410
Hakki SS, Bozkurt SB, Hakki EE, Korkusuz P, Purali N, Koç N, Timucin M, Ozturk A, Korkusuz F (2012) Osteogenic differentiation of MC3T3-E1 cells on different titanium surfaces. Biomed Mater. doi:10.1088/1748-6041/7/4/045006
Hakki SS, Korkusuz P, Purali N, Korkusuz F, Bozkurt BS, Hakki EE, Onder ME, Gorur I, Nohutcu RM, Timucin M, Ozturk A (2013) Periodontal ligament cell behavior on different titanium surfaces. Acta Odontol Scand 71(3-4):906–916
Zembic A, Kim S, Zwahlen M, Kelly JR (2014) Systematic review of the survival rate and incidence of biologic, technical, and esthetic complications of single implant abutments supporting fixed prostheses. Int J Oral Maxillofac Implants 29:99–116
Sanz AR, Carrión FS, Chaparro AP (2015) Mesenchymal stem cells from the oral cavity and their potential value in tissue engineering. Periodontol 67(1):251–267
Ohazama A, Modino SA, Miletich I, Sharpe PT (2004) Stem-cell-based tissue engineering of murine teeth. J Dent Res 83:518–522
Angelova Volponi A, Kawasaki M, Sharpe PT (2013) Adult human gingival epithelial cells as a source for whole-tooth bioengineering. J Dent Res 92(4):329–334
Oshima M, Inoue K, Nakajima K, Tachikawa T, Yamazaki H, Isobe T, Sugawara A, Ogawa M, Tanaka C, Saito M, Kasugai S, Takano-Yamamoto T, Inoue T, Tezuka K, Kuboki T, Yamaguchi A, Tsuji T (2014) Functional tooth restoration by next-generation bio-hybrid implant as a bio-hybrid artificial organ replacement therapy. Sci Rep 13(4):6044. doi:10.1038/srep06044
Zheng L, Yang F, Shen H, Hu X, Mochizuki C, Sato M, Wang S, Zhang Y (2011) The effect of composition of calcium phosphate composite scaffolds on the formation of tooth tissue from human dental pulp stem cells. Biomaterials 32(29):7053–7059
Gault P, Black A, Romette JL, Fuente F, Schroeder K, Thillou F, Brune T, Berdal A, Wurtz T (2010) Tissue-engineered ligament: implant constructs for tooth replacement. J Clin Periodontol 37(8):750–758
Giannobile WV (2010) Getting to the root of dental implant tissue engineering. J Clin Periodontol 37(8):747–749
Zhao H, Chai Y (2015) Stem cells in teeth and craniofacial bones. J Dent Res. doi:10.1177/0022034515603972
Acknowledgements
This work was supported in part with funding from The Scientific and Technological Research Council (Ankara, Turkey) (TUBITAK/SBAG-110S415, TUBITAK/SBAG-112S587) (SSH) and Selcuk University Research Coordination Office, Konya, Turkey (BAP-10401104) (SSH), TUBITAK/SBAG-108S291 (EK). Authors would like to thank to Prof Ozgur Inan, Selcuk University, Faculty of Dentistry, Department of Prosthodontics, Konya, Turkey, for clinical photographs and Eren Sahin, Ayberk Akat for helping in editing. The authors declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Hakki, S.S., Karaoz, E. (2016). Dental Stem Cells: Possibility for Generation of a Bio-tooth. In: Şahin, F., Doğan, A., Demirci, S. (eds) Dental Stem Cells. Stem Cell Biology and Regenerative Medicine. Springer, Cham. https://doi.org/10.1007/978-3-319-28947-2_9
Download citation
DOI: https://doi.org/10.1007/978-3-319-28947-2_9
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28945-8
Online ISBN: 978-3-319-28947-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)