Abstract
Induced pluripotent stem cells (iPSCs) are a type of experimentally produced pluripotent stem cell (PSC), which share similar features with embryonic stem cells (ES) isolated directly from early embryos. Shinya Yamanaka’s lab in Kyoto, Japan was the first to develop iPSCs in 2006 by the introduction of four genes that encode transcription factors of PSC into mouse embryonic fibroblasts—a process known as “reprogramming”. Later on, different animal and human fetal or adult somatic cell types have been converted into iPSCs using this technology, demonstrating similarities and slight differences between iPSCs lines, which are known to depend on the origin of the cells used in reprogramming. The present chapter will provide an overview of iPSCs derived from dental stem cells (DSCs), such as stem cells isolated from apical papilla (SCAPs), stem cells from exfoliated deciduous teeth (SHEDS), from pulp of third molars and adult permanent teeth (DPSCs). We will discuss the origin of the cells used for reprogramming, factors which may favor or hinter the reprogramming process, methods and efficiency of cell reprogramming; the differentiation ability of iPSCs derived from DSCs; their safety, tolerance by the host and regenerative potential in preclinical models, as well as the use of these cells in toxicological studies, disease modeling and drug discovery. The possible use of iPSCs obtained from DSCs as a new tool for regenerative therapy will also be shortly discussed.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Reprogramming
- Pluripotency
- Dental pulp
- Apical papilla
- Toxicological studies
- Regenerative medicine
- Cell therapy
7.1 Introduction
Currently, fetal, neonatal, or adult fibroblasts are the main cell types used for reprogramming of human somatic cells into the pluripotent state . These cell sources seem to be highly accessible for generating human iPSCs and already show great promise in future clinical applications. However, dermal fibroblasts, as well as foreskin or hair keratinocytes and, as a consequence, adult stem cells derived from these sources, are strongly exposed to environmental factors, which can compromise their use as genetic models and therapeutic tools [1, 2]. The question then becomes “which source of donor cells is the best for iPSCs generation”. The biological and functional characteristics of donor cells, their ability to produce iPSCs and to differentiate efficiently into cell types of interest in therapeutics, as well as safety and tolerance concerns after transplantation, initially as determined in preclinical models, will help us to answer this question.
Findings demonstrate that successful generation of iPSCs may be easier if these iPSCs originate from actively dividing cells rather than from slow or non-dividing cells [3]. Dental tissues (DTs) contain mesenchymal stem cells (MSCs), which are multipotent cells of neural crest origin similar to dermal fibroblasts, and foreskin or hair keratinocytes [4, 5]. These cells rapidly proliferate in vitro and are able to differentiate into mesenchymal tissues such as bone, cartilage, muscle, ligament, tendon and adipose tissue [6]. Additionally, they are able to produce ectopic dentin and related pulp tissues, and neural cells. A variety of SC sources derived from DTs have been studied. SCs from deciduous teeth are especially useful because they are young and mainly healthy cells, which is good for clinical use, plus they can be used to study pediatric diseases [6–8].
7.2 DTs as a Source of Stem Cells (SCs)
The embryonic counterparts of adult DSCs are cranial neural crest derived multipotent dental MSCs. These cells, after neurulation, migrate away from the neural tube into developing craniofacial tissues . Following the developmental courses of determination and differentiation, they give rise to all structures of the tooth and its supporting tissues, except enamel. They also robustly contribute to central nervous system (CNS) formation. Thus, DTs as well as CNS both derive from the embryonic ectoderm [9].
Primary (baby) teeth start their development during prenatal life, and are formed between the 6–8th week of fetal development. Nonetheless, SCs derived from pulp of deciduous teeth, due to ethical considerations, can only be isolated when baby teeth start to spontaneously fall out, which usually happens when children are between 5–10 years old, within a relatively short time interval in comparison to the human normal life span. Baby teeth are habitually discarded, thus dental pulp derived from these teeth represents a healthy, available source of SCs, which is free from the ethical considerations associated with human embryonic stem (ES) cells isolation [6].
The process known as exfoliation occurs when the last primary tooth falls out and permanent teeth start to form, usually at 11 to 12 years of age. Extraction of permanent teeth is ethically more problematic then that of baby teeth, and can happen only if such procedure is necessary for oral health, as determined by the dentist. The extraction of permanent teeth is uncomfortable for donors and requires medical attention. Generally, dental pulp is extracted from vital teeth of healthy adults; however, the majority of these teeth are extracted due to severe periodontal disease, the need to fabrication complete dentures, etc. All of the above is also true in respect to third molars (also called “wisdom teeth”), which are a type of permanent teeth frequently extracted because of decay, pain or impaction. Impaction occurs when an “impacted” tooth has failed to fully emerge in its expected position and needs to be extracted.
Dental follicles, periodontal ligament tissue, gingiva and apical papilla are additional DT sources that can be used for stem cell isolation. The periodontal ligament (PDL), the supporting tooth structure, is differentiated from the dental follicle (sac containing the developing tooth) and consists of the cementum, periodontal ligaments, gingiva and alveolar bone. PDL stem cells (PDLSCs) can be collected from the root surface after permanent, deciduous or third molar tooth extraction, while SCs from gingival tissues can be obtained from remnant or discarded tissue following routine dental procedures from human donors with relatively healthy periodontium and without previous history of periodontal disease. Finally, SCs can be isolated from the apical papilla (SCAPs), which contributes to tooth formation. This tissue is known as “apical” because as the root continues to develop after the bell stage (fetal stage of tooth development), the dental papilla localization is apical to the pulp tissue (reviewed by [10, 11]).
As mentioned before, SCs are usually extracted from vital DT of healthy adults, nevertheless the majority of the teeth themselves are extracted due to medical indication and severe inflammation occurring in DT, which can impair the quality of isolated SCs, debilitating their differentiation capacity into bone, for example [12]. The age of the donor of DT can also be important for the quality of SCs, as previously reported [13]. Although every type of DSCs can be used for iPSCs generation, to date, the SCs isolated are from apical papilla-SCAPs, from pulp tissue of primary deciduous teeth, such as SHEDs (human exfoliated deciduous teeth) and iDPSCs (immature dental pulp stem cells), as well as pulp tissues from permanent (DPSCs) and from wisdom teeth (TGSCs) were used [14–23].
7.3 Expression of Pluripotent Markers in DSCs
Pluripotent transcription factors, such as, KLF4, a member of the Krüppel-like factor (KLF) family, OCT-4 (octamer-binding transcription factor 4) also known as POU5F1 (POU domain, class 5, transcription factor 1) and SOX-2 (SRY (sex determining region Y)-box 2) are highly expressed in ES cells, thus regulating the developmental signaling network necessary for ES cell pluripotency. The overexpression of these factors induces reprogramming of both mouse and human somatic cells into the embryonic stage [24]. The expression of PSC markers such as OCT-4, NANOG, SOX-2 and STAT-3 is observed during different fetal stages of tooth germ development [25, 26]. During adulthood, in situ expression of OCT-4 has been found in the dental pulp of deciduous teeth in the cell-rich zone which contains fibroblasts and undifferentiated mesenchymal cells, and also in the cell-free zone, which is rich in both capillaries and nerve networks [27]. Recently, this finding was confirmed by another group [10]. Interestingly, OCT-4 expression is also maintained in a small amount of iDPSCs (≥10–20 %) after isolation and in vitro cultivation. The OCT-4 transcription factor is critical for pluripotency and multilineage differentiation potential of SCs and its expression, even at low levels, in DSCs may play a critical role in reprogramming [5, 6, 10].
7.4 DSC Reprogramming
Freshly isolated DSCs are plastic-adherent, present common fibroblast-like morphology (Fig. 7.1a), are clonogenic and express a set of markers, which, as the scientific community has determined, is typical of MSCs. Furthermore, these cells are able to differentiate into osteoblasts, adipocytes and chondroblasts in vitro [28]. The majority of SCs isolated from dental pulp seems to be of multipotent MSCs rather than of multipotent neural crest cells. Such multipotent MSCs derived from DTs preserve their main characteristics, such as immunophenotype, proliferation rate and differentiation potentials, unchanged over several passages (up to 25) of in vitro cultivation [5]. However, culture characteristics certainly depend on method of isolation, enzymatic digestion or explant culture, and cultivation-different culture media can be used supplemented, or not, with different growth factors [5].
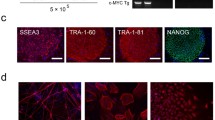
Immature dental pulp derived stem cell (iDPSC) -derived induced pluripotent stem cells (iPSCs). This figure depicts (a) iDPSCs before reprogramming; (b) Morphological changes observed in iDPSCs after reprogramming; (c) A colony of DSC-derived iPSCs; (d) Multiple iDPSC-derived iPSC colonies. (e–g) Expression of transcription factors, such as, OCT-4 (e), NANOG (f) and SOX-2 (g) in the nuclei (green) and cytoplasm (red) SSEA-4 (f), of iDPSC-derived iPSCs. (h) In vitro differentiation, through embryoid body (EB) formation, of iDPSC-derived iPSCs. Differentiated cells can be observed around EB. (i) Haematoxylin- and Eosin-stained teratoma sections obtained 3 months after intramuscular injection of iDPSC-derived iPSCs into nude mice. (a–d, h, i) Light microscopy and Phase contrast (except of i). (e–g) Epifluorescence. Scale bars: (a, b, e, g) = 50 μm; (f) = 10 μm; (c, d, h) = 100 μm; (i) = 200 μm. PI; Propidium iodide (red) and DAPI; 4′,6-diamidino-2-phenylindole (blue), DNA dyes. Methodology described in Beltrao-Braga et al. [14]
The first study on iPSCs generation was focused on SHEDs and SCAPs. For reprogramming, a lentiviral vector carrying C-MYC (Myc proto-oncogene protein), KLF4, OCT-4 and SOX-2 was used. However, this vector failed to reprogram these cells. Next, another lentiviral vector carrying four factors, this time LIN28 (LIN-28 homolog A), NANOG, OCT-4, and SOX-2, was constructed to obtain iPSCs, but the efficiency of colony generation from SHEDs and SCAPs was still very low. To improve reprogramming efficiency, a retroviral vector carrying the same genes initially studied: C-MYC, KLF4, OCT-4, and SOX-2, was employed and, after a second round of transduction, iPSCs colonies were finally produced. Nevertheless, human fibroblasts, used as a control in this study, were not able to undergo reprogramming under such conditions [22].
Other authors reported reprogramming of DSCs from deciduous teeth iDPSCs, which are different from SHEDs due to method of isolation [6], using a retroviral vector expressing four of Yamanaka’s factors (KLF4, OCT-4, C-MYC and SOX-2) [24]. The derived cells so far express a low level of OCT-4 and NANOG [5]. The successful reprogramming of these cells occurs after one round of transduction, with satisfying efficiency: 0.1–1 % or even higher [14] (Fig. 7.1a–i). More recently, an alternative polycistronic lentiviral vector encoding OCT-4, SOX-2, KLF4, and C-MYC [29] with addition of the dTomato reporter gene that allows real-time monitoring of transduction efficiency and silencing of transgenes, was used for successful reprogramming DSCs from deciduous teeth [16].
Reprogramming of DSCs from wisdom teeth, deciduous teeth and human dermal fibroblasts (HDFs) has also been carried out using retroviruses expressing four (OCT-4, SOX-2, KLF4, and C-MYC) or three (without C-MYC) factors [15, 18, 21]. In order to avoid an integration of an RNA virus into the host genome, a vector based on Sendai virus [30] was constructed to generate iPSCs that express the transcription factors encoded by OCT-4, SOX-2, KLF4, and C-MYC from permanent teeth-derived DSCs [19].
7.5 Factors Relevant in Reprogramming
In general, reprogramming requires the use of mouse embryonic fibroblasts (MEF) as a feeder layer for iPSC growth. MEF have been employed in all studies with DSCs reprogramming except one, which succeeded in establishing and propagating iPSCs under feeder-free conditions on matrigel-coated dishes (Fig. 7.1b–d) [14], thus avoiding the contamination of human cells with zoonoses derived from mice cells. This is an essential step in iPSC technology development, especially when iPSCs are obtained for their potential use in cell therapy.
Fetal bovine serum (FBS) is also considered an undesirable component of culture media to obtain SCs for therapeutic use. Although currently FBS can be purchased from companies whose product is originated from FDA-approved regions, where production is followed by extensive inspection and rigorous quality control (www.pan-biotech.com), chemically-defined media protocols that avoid the use of FBS, have already been developed: first, for the isolation and growth of DSCs used for iPSC generation and, second, for generation, growth and differentiation of iPSCs [19]. From a practical point of view, the great disadvantage of this protocol is that, even before iPSC generation, DSCs cultured under chemically-defined conditions show delay in growth dynamics and generate significantly lower number of primary colonies than those obtained in the presence of FBS. Most importantly, under FBS-free conditions, growth of these cells is strongly donor-dependent, and the use of DNA array analyses demonstrates that gene expression patterns are robustly altered in DSCs grown under chemically-defined conditions in comparison with the cells grown in FBS. Another disadvantage of the protocol used is that despite all the care that was taken to avoid FBS use, the iPSCs obtained in this study, before they were transferred onto matrigel, were grown on SNL Feeder Cells, which are clonally derived from a mouse fibroblast STO cell line. Therefore, potential contamination with mouse-derived zoonoses can still occur by using this protocol [19].
Next, as hypoxia enhances the reprogramming efficiency of HDFs into iPSCs [31], DSCs were also submitted to early and transient hypoxia (3 % O2) during reprogramming, and under such conditions, the transition of SCs to iPSCs was 3.3- to 5.1-fold higher as compared to that of cells cultured in normoxia (21 % O2). Interestingly, in contrast to what is observed during HDF reprogramming to iPSCs, when DSC-derived iPSCs are treated with 3 % O2 during the later stage of reprogramming (from day 6 to day 21), the generation of iPSCs under such conditions is strongly inhibited. There is still no rational explanation for such phenomenon and the authors speculate that metabolic changes may be involved [17].
The process of iPSC isolation is still very costly, largely because of low reprogramming efficiency. It seems that less differentiated somatic cells can be reprogrammed more efficiently than terminally differentiated cells, and even require fewer viruses than fibroblasts for efficient reprogramming [32–34]. Additional factors that may influence reprogramming efficiency are the age of cell donor, cell type and number of transcription factors used [34, 35]. Thus, the efficiency of reprogramming of young DSCs is higher than those obtained from their adult counterparts as well as than that of HDFs and human primary gingival fibroblasts. Additionally, a comparative study between immature and mature teeth derived DSCs converted into pluripotent states has been carried out in order to understand the low reprogramming efficiency of mature human iPSC. This study has shown that only two factors, OCT-4 and SOX-2, are needed for immature teeth SC reprogramming and these factors are not sufficient to convert mature teeth DSCs to iPSCs [14, 16, 18, 21, 22]. The comparison of gene expression profiles between these two DSC groups (immature and mature) unveiled a new transcript factor, distal-less homeobox 4 (DLX4), which is highly expressed in immature teeth DSCs in comparison to mature ones. The suppression of this gene by transforming growth factor beta (TGF-β) impairs iPSC generation. This gene may be the first candidate that can substitute already known transcription factors (e.g., C-MYC oncogene) and make this process safer due to lower cancer risk [20].
Another issue that must be considered when deriving iPSC from DSCs is the system used for gene delivery. The use of lentivirus and retrovirus is efficient; but this technique is not safe since these viruses can integrate into the host DNA, potentially altering gene expression and leading to cancer. Adenoviral delivery of these genes is safer because adenoviral DNA does not integrate into the genome [21]. Experimental approaches, however, have demonstrated that this is not a rule and that retroviral vectors can be transcriptionally silent in iPSCs [18] and that transgene expression from retroviral vectors can even be lost for some reason during reprogramming [14].
Overall, iPSCs derived from DSCs show stable karyotype after reprogramming, although the methylation status of cytosine guanine dinucleotides (CpG) in these cells remains to be clarified due to controversies found in the literature and to the few data available for analysis [14, 21–23]. Another important marker of undifferentiated PSCs is telomerase activity, which is found to be restored in iPSCs in comparison with the original cells used for iPSC generation [18, 22].
7.6 Transcription Factor Expression in DSC-Derived iPSCs
A very important issue for the potential clinical applications of iPSCs, which has been less studied, is that of transcription factor expression in iPSCs after reprogramming in comparison with naturally developed human ES cells derived from human embryos. Two recent studies reported that transcription factor expression in iPSCs is similar to that of human ES cells [17, 20]. Nevertheless, we have previously demonstrated that although immunofluorescence analyses reveal expression of OCT-4, NANOG and SOX-2 in iPSCs derived from iDPSCs of deciduous teeth (Fig. 7.1e–g), molecular analysis shows a low level of expression of all factors (OCT-4, NANOG and SOX-2), and, especially, of NANOG (Fig. 7.1f) in iPSCs [14]. This low level of expression of PSC factors apparently does not affect their ability to form embryoid bodies (EBs , spherical structures with cystic cavities resembling early embryos, albeit, chaotically organized) and teratomas upon in vivo implantation-a “gold standard” to test pluripotency of ES cells. In spite of the lower expression of transcription factors, as compared with previous studies [17, 20], these iPSCs demonstrate very robust differentiation within teratomas and strong neuronal commitment in vitro and in vivo [14].
7.7 Differentiation Potential
The differentiation potential of iPSCs derived from DSCs has been studied using conventional models: in vitro formation and differentiation of EB (Fig. 7.1h); and in vivo teratoma generation (Fig. 7.1i). All authors have reported the capacity of these cells to produce the wide spectrum of cells derived of the three germ layers (mesoderm, endoderm and ectoderm) (Fig. 7.1i); moreover, neuronal differentiation is a “trademark” of DSCs derived iPSCs [14, 15].
The HOX proteins participate in many common developmental processes during normal embryogenesis and the vertebrate nervous system is a major site of HOX gene expression and function. Furthermore, they play a key role in extending our understanding of the CNS development [36]. Additional evidence that DSC-derived iPSCs have predominantly a neuronal fate was recently provided. Expression profiling of HOX genes by neurons differentiated from DSC-derived iPSCs and HDF-iPSCs was compared and showed a high degree of correlation for the two sources of neurons; nevertheless, they differ in the expression of some important genes, especially in several members of the HOX gene families. Lower levels of expression for genes involved in hindbrain development are observed in the neurons differentiated from DSC-derived iPSCs as compared with HDF-iPSCs. In contrast, several transcription factors involved in the forebrain development are considerably increased, such as FOXP2 (Forkhead box 2 encoded by the FOXP2 gene, also known as CAGH44, SPCH1 or TNRC10, and required for proper development of speech and language), OTX1 (orthodenticlehomeobox 1, which encodes a member of the bicoid sub-family of homeodomain-containing transcription factors and may play a role in brain and sensory organ development), and LHX2 (LIM/homeoboxprotein that specifies cortical identity and suppresses hippocampal organization fate). These transcription factors are involved in the development of communicative and linguistic neural networks [37–41]. Such difference might influence the decision to use DSC-or HDF-derived iPSCs in neuropsychiatric disorder studies and treatments, such as in schizophrenia and autism spectrum disorders [42].
Interestingly, spontaneous (without the use of specific differentiation inducing agents in culture medium) robust neuronal and endothelial differentiations of DSC-derived iPSCs have been demonstrated [16]. Furthermore, when a chemically-defined protocol to isolate DSC-iPSCs focusing on their safe establishment was used, significantly less primary colony formation was observed with respect to the protocols using FBS. Although the DNA array analyses indicate that the culture conditions robustly alter DSCs gene expression patterns, DSC-derived iPSCs grown under defined conditions show a donor-dependent growth capacity but the differentiation capacity of these cells is not changed in comparison to that of DSC-derived iPSCs grown with FBS [19, 43].
Regarding cell differentiation to improve vascularization, two factors, OCT-4 and SOX-2, have been used to produce iPSCs (2 factors (2F)–iPSCs). After the addition of basic fibroblast growth factor (bFGF) and vascular endothelial growth factor A (VEGF A) to the culture medium, the effective in vitro differentiation of DSC-derived iPSCs into functional endothelial progenitor cells (EPCs) and smooth muscle cells has been shown. The global transcriptomic analysis of iPSC-derived EPCs and endothelial cells (ECs) demonstrates limited variations in gene expression similar to those of EPCs and ECs derived from human ES cells. However, evaluation of the expression of CD31 in iPSCs-derived EPCs and ECs suggests that they are highly heterogeneous in respect to the presence of the cell populations : arterial, venous, and lymphatic cell subtypes. Such heterogeneity is genetically controlled by the multistep regulatory system associated with key signaling pathways and transcription factors before circulation begins [43]. Therefore, to validate this method, it should be determined whether iPSC-derived EPCs cultured in vitro are associated with the same signaling pathways that control cells during early embryonic development in vivo. On the other hand, in order to understand heterogeneity, we should determine functional benefits of each subtype of arterial, venous, and lymphatic endothelial cells to establish the protocol for optimal differentiation of iPSC-derived EPCs. The heterogeneity aforementioned also strongly indicates the immature state of iPSC-derived EPCs, which increases the possibility of teratoma formation or even tumor development. Regarding endothelial cell differentiation, it is also worth mentioning the matrigel plug angiogenesis assay, which is a simple in vivo technique to detect newly formed blood vessels in transplanted gel plugs in nude mice. This assay was used and confirmed the angiogenic and neovasculogenic capacities of 2F-hEPCs [23].
7.8 DSC-Derived iPSCs and Disease Modeling
Neurodegenerative diseases combine a wide range of pathogeneses which affect neurons in the human brain and spinal cord . When neurons become damaged or die, they cannot be replaced rapidly by natural sources of SCs in the human body. Such diseases result in progressive degeneration, which causes problems with movement (ataxias), or mental functioning (dementias). The list of such diseases includes Parkinson’s, Alzheimer’s, and Huntington’s diseases. They are incurable and the lack of effective treatments for various neurodegenerative disorders has placed an enormous burden on society. iPSC technology has emerged as a powerful tool for in vitro modeling of neurodegenerative diseases, the study of their cellular and molecular mechanisms, and drug screening and cell therapy for their treatment (Fig. 7.2). Before iPSC technology became a reality, researchers used post-mortem tissues, which often are not available and frequently obtained at the last stage of disease, or, alternatively, from transgenic animals. Both approaches are not able to fully reproduce the course of human disease or development of cells with neural phenotypes [44, 45]. The establishment of iPSC lines from patients has been an essential and novel step in medicine and biotechnology (Fig. 7.2). Thus, in 2008, the first iPSCs derived from patients with genetic diseases, including Parkinson’s and Huntington’s diseases, were obtained. The majority of iPSC lines were able to maintain the patient genotype and phenotype in vitro, while, for other diseases, further phenotypic confirmation is needed [37, 46–48]. Moreover, because of the non-invasive method of DT isolation, DSCs are important source of iPSC for modeling and investigating pediatric diseases [49].
Near and Not-So-Near Future of dental stem cell (DSC)-Derived induced pluripotent stem cells (iPSCs). Near: This figure depicts that DSC-derived iPSCs can originate from healthy or diseased donors. Both these cell types can be used in toxicological studies, disease modeling and drug discovery . The great advantage of iPSCs is that they can be used undifferentiated and as precursors of cell lineages of many types upon differentiation. In toxicological studies, multiple endpoints can be evaluated in two dimension culture systems (organotoxicity) or 3D systems (embryotoxicity, embryoid body models), other cell culture models and even germ cells, which can be produced in vitro; iPSCs derived from donors with diseases provide unique tools to study molecular and cellular mechanisms of diseases of interest, which help to understand the etiology of this disease and forms of treatment. To study neurodegenerative diseases , DSCs-derived iPSCs are particularly important tools, since the original cells tend to be committed to neural differentiation. DSC-derived iPSCs are potentially important in drug discovery and can be used for cytotoxic endpoint assays, as well as to test a variety of drugs in both undifferentiated and differentiated cells, and especially to evaluate the drug effect on differentiation pathways throughout the daisy chain of intermediates. Not-So-Near Future: DSCs-derived iPSCs without disease are of great interest in regenerative medicine. However, many questions still need to be answered. Patient-specific iPSCs can be used in autologous treatments, however when genetic disease issues need to be addressed, the use of autologous cells is not welcome and therefore, allogenic iPSCs without disease are recommended. In addition, patients with a family history of ischemic vascular disease should likewise avoid the use of autologous iPSCs. In both cases, need an additional in vitro iPSCs manipulation in order to produce more mature and safe precursors. Finally, we would like to emphasize once again that DSCs-derived iPSCs use is highly recommended to treat neurological diseases
DSCs present clear advantages over commonly used skin fibroblasts and other somatic cell types because of the easy access to DT with minimum discomfort for the patient, their rapid cell proliferating, young donor age, and lower exposure to environmental factors such as ultraviolet irradiation [6]. Clonal variation among pluripotent SCs is also a very important factor, which seems to be less problematic in iPSCs from DSCs [23]. Furthermore, normal ES cells derived from human blastocysts and iPSCs derived from fibroblasts of the same donor show the variable neuronal differentiation potentials [50, 51]. In vitro differentiation of iPSCs derived from DSCs occurs practically spontaneously [14, 15]. Despite high neuronal commitment and great potential in research in neurological conditions and disorders, only one study used DSCs to generate patient-specific iPSCs for modeling of non-syndromic autism and to investigate the impact of TRPC6 (transient receptor potential cation channel, subfamily C, member 6) disruption in human neurons [52]. This group identified the disruption of the TRPC6 gene by a balanced de novo translocation in a non-syndromic autism spectrum disorder (ASD) individual. This gene is involved in the regulation of axonal guidance, dendritic spine growth and excitatory synapse formation [53, 54]. Generation of DSC-derived iPSCs from the ASD individual allowed this group to explore the functional consequences of TRPC6 disruption in human neuronal cells . Overall, they demonstrated that patient-specific iPSC-derived neurons can be used to associate novel variants to ASD patients to study the etiology of these disorders [52]. Finally, the importance of DSC-derived iPSCs for neuropsychiatric disorders , such as schizophrenia and ASD, has also been suggested [42].
7.9 Drug Discovery and Cytotoxicity Studies
Drug discovery today has been very unsuccessful considering time and capital investment, with several drugs failing in the clinical trials phases due to lack of efficiency or safety. This should not be happening, considering the major progress associated with chemical synthesis technologies, the large amount of data derived from “omics” initiatives (genomics, epigenomics, transcriptomics, proteomics, metabolomics, among others), the advances in analytical sciences and the possibility of high-throughput screening. However, the absence of adequate models of human disease for drug screening, which include cell lines, is probably one of the reasons for the high failure rate in this field.
Traditional drug discovery uses animal and human cell lines established long ago that poorly reflect the in vivo biology. Drug screening and toxicological studies often require cells from highly differentiated tissues or from tissues where there is no cell proliferation, such as neurons, cardiomyocytes and some gland cells. Toxicological studies, which are carried out in parallel with drug discovery to weed out highly toxic drug candidates, require, for instance, hepatocytes and cardiomyocytes. Liver cells are important since they receive high quantities of drugs in the first pass stage of distribution, and also because they carry out drug metabolism, which may lead to the generation of highly toxic compounds. Hepatocytes are known to be highly capable of proliferation. However, hepatocyte-derived cell lines rarely maintain all metabolic routes necessary for drug metabolism and safety screening. As for cardiomyocytes, these cells typically do not proliferate in vivo after development, which is why it has been hard to establish cardiomyocyte models. However, they are of major importance in drug discovery and toxicity studies, given the many forms of heart disease on one hand, and the fact that heart damage by drugs being a reason for abandonment of drug development projects. Likewise, drug screening and toxicity studies lack good models of neuronal cells, which are also hard to establish in spite of their need in the discovery of drugs that are used to treat central nervous system disorders and in toxicological protocols to prevent neurotoxicity of new drugs.
Most of the above considerations can be addressed using patient-specific iPSCs. iPSCs with genetic disorders are important models which allow, upon differentiation, studying the effect of genetic mutations on cell function and drug discovery screening on these diseased models (Fig. 7.2). In particular, drug discovery can profit from iPSCs and differentiated cells derived from diseased individuals, whereby the total diversity of genetic background of the individual can be considered (Fig. 7.2). This is particularly true nowadays, when whole genomic sequencing, or, at least, polymorphism screening, are straightforward. Most common diseases are associated with combinations of genetic polymorphisms [55], and the development of cell lines obtained from many patients can be useful in drug discovery and toxicity by addressing this complex genetic background in vitro (Fig. 7.2). It is likely that such cell lines can improve drug screening success by indicating which subpopulations will respond to drugs and which will be unresponsive or even not tolerate the medication due to toxic side effects. These studies are still in their “infancy” and need to be further improved. Thus, Muotri’s group provides insights supporting the testing of novel drugs in patient-specific ASD DSC-derived iPSCs such as hyperforin, a drug that specifically activates TRPC6, or insulin growth factor-1 (IGF-1), which is expected to increase not only TRPC6 pro tein levels but also other synaptic components [52].
7.10 Regenerative Medicine and Cell Therapy
The main goal of regenerative medicine is to obtain unlimited numbers of a specific cell type, which can be achieved by reprogramming of DSCs into pluripotent state , and establishment of differentiation protocols, which allow direct differentiation of DSC-derived iPSCs into “pure” populations of precursors, which, when introduced back into the organism, are able to produce therapeutically significant numbers of mature differentiated cells with functional capacities that allow total restoration of lost cells, tissues and organs function. However, it seems that, currently, researchers are mainly focused on the methods of iPSCs derivation and cultivation, and less on differentiation potential of these cells, which needs to be more extensively studied from the perspective of iPSC future applications in regenerative medicine.
The neural precursor is a cell type consistently obtained from iPSCs and great progress has been made in the area of neuronal lineage specification, which is highly dependent on imitating in vitro the early patterning signals that convey axial coordinates during neural development. However, in vivo replacement of nerve cells in traumatic or degenerative disorders of the central nervous system (CNS) is still at early stages of development. Over the years, embryoid body formation, co-culture on neural inducing feeders and direct neural induction have been used in the field of directed neural differentiation, which are still complex, long lasting and time consuming [56]. Therefore, the finding that DSC-derived iPSCs are able to undergo spontaneous differentiation into neurons and endothelial cells is of great importance. From a practical point of view, developing protocols for purification of neural and endothelial precursors obtained as a result of spontaneous differentiation of DSC-derived iPSCs may be much more interesting than in vitro induced differentiation. However, this would be nice, if not for a small detail, that we don’t know how immature or mature these precursors are. The study, which demonstrates the derivation of EPCs from DSC-derived iPSCs, is an alert for the scientists that we still need to study a lot about iPSC differentiation. These cells have a very strong differentiation potential which is abundant in different cell types, when compared with DSC-derived MSCs from post-natal tissues. Therapeutic use of iPSCs expects that their differentiation occurs not in a “dish”, but in particular tissue and organ, and under the complete control of an organism. Hence, teratogenicity, as well as, short- and long-term tumorigenicity of iPSC-derived precursors must be thoroughly evaluated before any clinical application (Fig. 7.2).
Another popular field of regenerative medicine regards treatment of peripheral arterial disease (PAD) . All over the world, PAD affects many people; only in the United States, there are about 10 million individuals who suffer from PAD. The murine hind-limb ischemia preparation is a model of PAD, and is useful for testing new therapies. The advantages of this model are the ease of access to the femoral artery and the low mortality rates. It has been shown that 2F hEPC-iPSCs have a strong ability to produce angiogenic and vasculogenic EPCs. The therapeutic effects of 2F hEPC-iPSC transplantation were verified in mouse models of hind-limb ischemia and myocardial infarction. The 2F-EPCs efficiently incorporated into newly formed vascular structures and enhanced neovascularization in both experimental models. This study recommends a follow up investigation of the use of EPC derived from iPSCs in patient-specific therapies, especially in ischemic vascular diseases [23] (Fig. 7.2).
The origin of cell types is an important factor, which can influence the molecular and functional properties of SCs. Thus, upon reprogramming, iPSCs generally gain new characteristics, but they habitually hold a ‘footprint’ of the tissue of origin [57–59]. The use of SCs in regenerative medicine and cell therapies, therefore, may dependent on SC origin, which could have significant effects on the outcome, for example, in efficient differentiation and functional properties of cells. DSCs can differentiate into odontoblasts, osteoblasts, endotheliocytes, smooth muscle cells, adipocytes and chondrocytes [5], however, due to their neural crest origin, they may also be a very important source of SCs to be used to repair spinal cord injuries and to prevent or even treat patients with neurological disorders [42] (Fig. 7.2). The dental tissue origin also suggests that DSC-derived iPSCs can potentially have a major impact on oral health [60, 61]. Regeneration of the tooth structure may avoid or delay the loss of the whole tooth. A preclinical study has already focused on tissue engineering of tooth-like structures, although it was developed using non-odontogenic SCs as a cell source [62]. On the other hand, this study is interesting and important, and will allow the comparison of the capacity of non-odontogenic SCs and odontogenic SCs to regenerate tooth structures (Fig. 7.2).
7.11 Near and Not-So-Near Future
The advantage of iPSCs as compared to ES cell lines includes ethical issues-the first are harvested from discarded teeth, without harm to the donors, whereas the later come from non-implanted embryos, which sometimes can be associated with social, ethical and legal complications. The risk aspect is the teratogenic potential of both these cell types as well as the efficiency of inducing differentiation into definite cell types, which can be isolated as pure progenitor populations that will not produce cells with negative features, such as hyperproliferation, tumorigenicity, and ectopic tissue formation. All SCs which are potentially tumorigenic should be eliminated in order to prevent recurrence of malignancies. The safety of iPSCs and iPSC-derived progenitors can be, for example, evaluated by genome and epigenome analyses , and this may minimize the risk to a level acceptable for clinical trials, but nobody can confirm how the cells will respond until clinical trials are completed.
The iPSCs can be relatively rapidly obtained from DSCs, and this process seems to have good reproducibility, which means a high variety of cell lines will soon be available representing human genetic diversity. Since the human donor is known and alive, the cell lines can be associated with many phenotypic traits that can be useful additional data. Whereas human ES cells can also represent genetic diversity, one cannot have further information on the “donor”, since it is destroyed in the process of cell line establishment. Furthermore, iPSCs derived from the DSCs of patients, or close relatives, can differentiate to tissues associated with the disorder (i.e. lung tissue, Langerhans cells, cardiomyocytes, etc.) and can be used for therapeutic purposes as well as to screen for effective drugs for that given patient. Currently, such approach is too complex and expensive for the general public. Nevertheless, automation and protocol standardization will probably soon follow, rendering this form of drug “pre-screening” more viable in order to find effective and low-toxicity treatments, especially for very fragile patients.
References
Boehnke K, Falkowska-Hansen B, Stark HJ, Boukamp P (2012) Stem cells of the human epidermis and their niche: composition and function in epidermal regeneration and carcinogenesis. Carcinogenesis 33(7):1247–1258
Mouret S, Forestier A, Douki T (2012) The specificity of UVA-induced DNA damage in human melanocytes. Photochem Photobiol Sci 11(1):155–162
Haase A, Olmer R, Schwanke K et al (2009) Generation of induced pluripotent stem cells from human cord blood. Cell Stem Cell 5(4):434–441
Achilleos A, Trainor PA (2012) Neural crest stem cells: discovery, properties and potential for therapy. Cell Res 22(2):288–304
Kerkis I, Kerkis A, Dozortsev D et al (2006) Isolation and characterization of a population of immature dental pulp stem cells expressing OCT-4 and other embryonic stem cell markers. Cells Tissues Organs 184(3–4):105–116
Kerkis I, Caplan AI (2012) Stem cells in dental pulp of deciduous teeth. Tissue Eng Part B Rev 18(2):129–138
Kerkis I, Kerkis A, Lizier NF, Wenceslau CV (2015) Dental stem cells: risk and responsibilities. In: Bhattacharya N, Stubblefield FG (eds) Regenerative medicine using non-fetal sources of stem cells. Springer, London, pp 171–175
Lizier NF, Kerkis I, Wenceslau CV (2013) Generation of induced pluripotent stem cells from dental pulp somatic cells, pluripotent stem cells. In: Bhartiya D (ed) Pluripotent stem cells. inTech, Rijeka. doi:10.5772/55856
Shakhova O, Sommer L (2008) Neural crest-derived stem cells. In: Gage F, Watt F (eds) StemBook. The Stem Cell Research Community, Boston, http://www.ncbi.nlm.nih.gov/books/NBK44752/
Liu J, Yu F, Sun Y et al (2015) Concise reviews: characteristics and potential applications of human dental tissue-derived mesenchymal stem cells. Stem Cells 33(3):627–638
Ponnaiyan D (2014) Do dental stem cells depict distinct characteristics? - Establishing their “phenotypic fingerprint”. Dent Res J (Isfahan) 11(2):163–172
Park JC, Kim JM, Jung IH et al (2011) Isolation and characterization of human periodontal ligament (PDL) stem cells (PDLSCs) from the inflamed PDL tissue: in vitro and in vivo evaluations. J Clin Periodontol 38(8):721–731
Zhang J, An Y, Gao LN, Zhang YJ, Jin Y, Chen FM (2012) The effect of aging on the pluripotential capacity and regenerative potential of human periodontal ligament stem cells. Biomaterials 33(29):6974–6986
Beltrao-Braga PC, Pignatari GC, Maiorka PC et al (2011) Feeder-free derivation of induced pluripotent stem cells from human immature dental pulp stem cells. Cell Transplant 20(11–12):1707–1719
Chang YC, Li WC, Twu NF et al (2014) Induction of dental pulp-derived induced pluripotent stem cells in the absence of c-Myc for differentiation into neuron-like cells. J Chin Med Assoc 77(12):618–625
Dambrot C, van de Pas S, van Zijl L et al (2013) Polycistronic lentivirus induced pluripotent stem cells from skin biopsies after long term storage, blood outgrowth endothelial cells and cells from milk teeth. Differentiation 85(3):101–109
Iida K, Takeda-Kawaguchi T, Hada M et al (2013) Hypoxia-enhanced derivation of iPSCs from human dental pulp cells. J Dent Res 92(10):905–910
Oda Y, Yoshimura Y, Ohnishi H et al (2010) Induction of pluripotent stem cells from human third molar mesenchymal stromal cells. J Biol Chem 285(38):29270–29278
Takeda-Kawaguchi T, Sugiyama K, Chikusa S et al (2014) Derivation of iPSCs after culture of human dental pulp cells under defined conditions. PLoS One 9(12):e115392. doi:10.1371/journal.pone.0115392
Tamaoki N, Takahashi K, Aoki H et al (2014) The homeobox gene DLX4 promotes generation of human induced pluripotent stem cells. Sci Rep 4:7283. doi:10.1038/srep07283
Tamaoki N, Takahashi K, Tanaka T et al (2010) Dental pulp cells for induced pluripotent stem cell banking. J Dent Res 89(8):773–778
Yan X, Qin H, Qu C, Tuan RS, Shi S, Huang GT (2010) iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cells Dev 19(4):469–480
Yoo CH, Na HJ, Lee DS et al (2013) Endothelial progenitor cells from human dental pulp-derived iPS cells as a therapeutic target for ischemic vascular diseases. Biomaterials 34(33):8149–8160
Takahashi K, Yamanaka S (2006) Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126(4):663–676
da Cunha JM, da Costa-Neves A, Kerkis I, da Silva MC (2013) Pluripotent stem cell transcription factors during human odontogenesis. Cell Tissue Res 353(3):435–441
Tompkins K (2006) Molecular mechanisms of cytodifferentiation in mammalian tooth development. Connective tissue research 47(3):111–118
Lizier NF, Kerkis A, Gomes CM et al (2012) Scaling-up of dental pulp stem cells isolated from multiple niches. Plos One 7(6):e39885. doi:10.1371/journal.pone.0039885
Dominici M, Le Blanc K, Mueller I et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8(4):315–317
Warlich E, Kuehle J, Cantz T et al (2011) Lentiviral vector design and imaging approaches to visualize the early stages of cellular reprogramming. Mol Ther 19(4):782–789
Fusaki N, Ban H, Nishiyama A, Saeki K, Hasegawa M (2009) Efficient induction of transgene-free human pluripotent stem cells using a vector based on Sendai virus, an RNA virus that does not integrate into the host genome. Proc Jpn Acad Ser B Phys Biol Sci 85(8):348–362
Yoshida Y, Takahashi K, Okita K, Ichisaka T, Yamanaka S (2009) Hypoxia enhances the generation of induced pluripotent stem cells. Cell Stem Cell 5(3):237–241
Muchkaeva IA, Dashinimaev EB, Terskikh VV, Sukhanov YV, Vasiliev AV (2012) Molecular mechanisms of induced pluripotency. Acta Naturae 4(1):12–22
Patel M, Yang S (2010) Advances in reprogramming somatic cells to induced pluripotent stem cells. Stem Cell Rev 6(3):367–680
Zouboulis CC, Adjaye J, Akamatsu H, Moe-Behrens G, Niemann C (2008) Human skin stem cells and the ageing process. Exp Gerontol 43(11):986–997
Banito A, Rashid ST, Acosta JC et al (2009) Senescence impairs successful reprogramming to pluripotent stem cells. Genes Dev 23(18):2134–2139
Nolte C, Krumlauf R (2000) Expression of Hox genes in the nervous system of vertebrates. Madame Curie Bioscience Database [Internet]. Landes Bioscience, Austin (TX)
Larsen KB, Lutterodt MC, Mollgard K, Moller M (2010) Expression of the homeobox genes OTX2 and OTX1 in the early developing human brain. J Histochem Cytochem 58(7):669–678
Salvatierra J, Lee DA, Zibetti C et al (2014) The LIM homeodomain factor Lhx2 is required for hypothalamic tanycyte specification and differentiation. J Neurosci 34(50):16809–16820
Vargha-Khadem F, Gadian DG, Copp A, Mishkin M (2005) FOXP2 and the neuroanatomy of speech and language. Nat Rev Neurosci 6(2):131–138
Zembrzycki A, Perez-Garcia CG, Wang CF, Chou SJ, O’Leary DD (2015) Postmitotic regulation of sensory area patterning in the mammalian neocortex by Lhx2. Proc Natl Acad Sci U S A 112(21):6736–6741
Zhang YF, Liu LX, Cao HT et al (2015) Otx1 promotes basal dendritic growth and regulates intrinsic electrophysiological and synaptic properties of layer V pyramidal neurons in mouse motor cortex. Neuroscience 285:139–154
Chen J, Lin M, Foxe JJ et al (2013) Transcriptome comparison of human neurons generated using induced pluripotent stem cells derived from dental pulp and skin fibroblasts. PLoS One 8(10):e75682. doi:10.1371/journal.pone.0075682
Kume T (2010) Specification of arterial, venous, and lymphatic endothelial cells during embryonic development. Histol Histopathol 25(5):637–646
Phillips W, Michell A, Pruess H, Barker RA (2009) Animal models of neurodegenerative diseases. Methods Mol Biol 549:137–155
Young AB (2009) Four decades of neurodegenerative disease research: how far we have come! J Neurosci 29(41):12722–12728
Park IH, Arora N, Huo H et al (2008) Disease-specific induced pluripotent stem cells. Cell 134(5):877–886
Soldner F, Hockemeyer D, Beard C et al (2009) Parkinson’s disease patient-derived induced pluripotent stem cells free of viral reprogramming factors. Cell 136(5):964–977
Yagi T, Ito D, Okada Y et al (2011) Modeling familial Alzheimer’s disease with induced pluripotent stem cells. Hum Mol Genet 20(23):4530–4539
Zheng GP, Ge MH, Shu Q, Rojas M, Xu J (2013) Mesenchymal stem cells in the treatment of pediatric diseases. World J Pediatr 9(3):197–211
Hu BY, Weick JP, Yu J et al (2010) Neural differentiation of human induced pluripotent stem cells follows developmental principles but with variable potency. Proc Natl Acad Sci U S A 107(9):4335–4340
Osafune K, Caron L, Borowiak M et al (2008) Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol 26(3):313–315
Griesi-Oliveira K, Acab A, Gupta AR et al (2014) Modeling non-syndromic autism and the impact of TRPC6 disruption in human neurons. Mol Psychiatry. doi:10.1038/mp.2014.141
Li Y, Jia YC, Cui K et al (2005) Essential role of TRPC channels in the guidance of nerve growth cones by brain-derived neurotrophic factor. Nature 434(7035):894–898
Zhou J, Du W, Zhou K et al (2008) Critical role of TRPC6 channels in the formation of excitatory synapses. Nat Neurosci 11(7):741–743
Altshuler D, Daly MJ, Lander ES (2008) Genetic mapping in human disease. Science 322(5903):881–888
Tabar V, Studer L (2014) Pluripotent stem cells in regenerative medicine: challenges and recent progress. Nat Rev Genet 15(2):82–92
Kim K, Doi A, Wen B, Ng K, Zhao R, Cahan P et al (2010) Epigenetic memory in induced pluripotent stem cells. Nature 467(7313):285–290
Polo JM, Liu S, Figueroa ME, Kulalert W, Eminli S, Tan KY et al (2010) Cell type of origin influences the molecular and functional properties of mouse induced pluripotent stem cells. Nat Biotechnol 28(8):848–855
Kuijk EW, Chuva de Sousa Lopes SM, Geijsen N, Macklon N, Roelen BA (2011) The different shades of mammalian pluripotent stem cells. Hum Reprod Update 17(2):254–271
Estrela C, Alencar AH, Kitten GT, Vencio EF, Gava E (2011) Mesenchymal stem cells in the dental tissues: perspectives for tissue regeneration. Braz Dent J 22(2):91–98
Machado E, Fernandes MH, Gomes Pde S (2012) Dental stem cells for craniofacial tissue engineering. Oral Surg Oral Med Oral Pathol Oral Radiol 113(6):728–733
Wen Y, Wang F, Zhang W et al (2012) Application of induced pluripotent stem cells in generation of a tissue-engineered tooth-like structure. Tissue Eng Part A 18(15–16):1677–1685
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kerkis, I., Wenceslau, C.V., Pompeia, C. (2016). Induced Pluripotent Stem Cells Derived from Dental Stem Cells: A New Tool for Cellular Therapy. In: Şahin, F., Doğan, A., Demirci, S. (eds) Dental Stem Cells. Stem Cell Biology and Regenerative Medicine. Springer, Cham. https://doi.org/10.1007/978-3-319-28947-2_7
Download citation
DOI: https://doi.org/10.1007/978-3-319-28947-2_7
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28945-8
Online ISBN: 978-3-319-28947-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)