Abstract
Isolated limb perfusion with melphalan has a well-established role in the treatment of unresectable extremity melanoma manifestations. Tumour necrosis factor α (TNFα) can be added to melphalan in patients with bulky disease. Literature indicates a 54 % average complete response rate after perfusion. Additional excision, CO2 laser ablation or radiotherapy can eradicate tumour remnants in most partially responding patients. Further limb recurrence develops in approximately 44 % of the patients with a complete response, after a relatively short median interval of 5–10 months. Repeat perfusion for recurrent limb melanoma after a first perfusion frequently results in a renewed complete response. Patients with recurrent limb melanoma combined with regional lymph node metastases have an increased risk of dying within 1 year after perfusion. Adjuvant perfusion after excision of melanoma lesions may have a role in repeatedly recurring disease, since it decreases the number of recurrences and lengthens the limb recurrence-free interval significantly. Perfusion is valuable and safe in elderly patients with similar results as in younger people. When risk factors are taken into account and leakage is controlled adequately, regional toxicity and systemic toxicity after perfusion are mild. Nevertheless, considerable long-term treatment-related morbidity can occur. It has been shown that isolated limb infusion, a minimally invasive alternative to isolated limb perfusion, can also effectively and safely be used to treat advanced melanoma confined to a limb.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Complete Response Rate
- Isolate Limb Perfusion
- Extracorporeal Circuit
- Isolate Limb Infusion
- Recurrent Melanoma
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
25.1 Introduction
The technique of isolated limb perfusion, utilising an oxygenated extracorporeal circuit, was pioneered by Creech et al. at Tulane University, New Orleans, in 1957 [1]. A complete response was achieved in the 76-year-old male patient with extensive recurrent melanoma of his leg, and this lasted until he died at the age of 92. The advantage of this intriguing treatment modality is that a high dose of a cytostatic drug can be delivered to a tumour-bearing limb without the risk of systemic side effects. Isolated limb perfusion is a valuable therapeutic option in patients with an unresectable limb tumour and especially in lesions with a high tendency for locoregional recurrence, as is sometimes the case in melanoma [2].
25.2 Perfusion Methodology
25.2.1 Technique
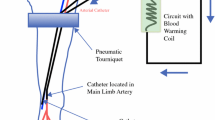
Isolation of the blood circuit of a limb is achieved by open access to the major artery and vein supplying the limb and by ligating the collateral vessels. A tourniquet is applied around the limb proximal to the region to be perfused in order to compress the smaller vessels in the muscles and subcutaneous tissues. After insertion of a catheter in the major artery and vein, the isolated limb is perfused by an extracorporeal circulation, oxygenated and propelled by a heart-lung machine (Fig. 25.1). The cytostatic drug is administered into this circuit. The priming volume of the extracorporeal circuit is about 750 ml and is composed of 300 ml autologous whole blood diluted with physiological electrolyte solution to a haematocrit of about 30 %. The autologous blood is taken from the venous catheter, just before the start of the isolation. The flow-driven extracorporeal circulation aims for the highest flow rate possible without increasing the venous pressure more than 10 cm above the starting point. The venous pressure in the limb can be monitored in a subcutaneous vein at the distal end of the extremity. Physiological blood gas values can be obtained with flow rates of 30–40 ml/min/l tissue [3].
Perfusion may be conducted in the lower limb at the external iliac level, at the femoral or popliteal level and in the upper limb at the axillary or brachial level. For iliac perfusion, the iliac and obturator lymph nodes are removed. Two recent descriptions of the surgical technique have been published [4, 5].
25.2.2 Drugs
Melphalan is the cytostatic drug of choice for melanoma. The dose of melphalan is guided by the limb volume to be perfused, and this volume can be determined by immersion of the limb in a cylinder filled with water [3]. The maximum dose associated with an acceptable risk of toxicity in the normal tissues is approximately 10 mg/l perfused tissue for the lower limb and 13 mg/l for the upper limb [6]. This difference in dosing is due to the change in drug concentration in the different circulating volumes that are used in upper and lower limb perfusions.
Recombinant tumour necrosis factor alpha (TNFα) is sometimes added to the melphalan in the circuit. The main indication for this biological response modifier is the presence of bulky, well-vascularised melanoma lesions, since TNFα has a selective destructive effect on newly formed vessels. This typically leads to thrombosis with acute necrosis of tumour tissue and to a selectively increased melphalan concentration in tumour tissue due to an enhanced permeability of the vessel walls [7–9]. Objective response data using other drugs like actinomycin D, cisplatin, vindesine, DTIC, fotemustine, interleukin-2 and lymphokine-activated killer cells are not encouraging [10–14]. It appears that none of these drugs is as effective as melphalan, neither as single agents nor in combination with other agents.
25.2.3 Monitoring Leakage
The extremely high cytostatic drug dosages in the limb could be fatal when they reach vital organs. The limbs, for example, can endure melphalan concentrations up to 20 times higher than would be tolerated in the rest of the body [6]. The potential leakage into the systemic circulation thus requires careful monitoring. To this end, a small dose of a radiopharmaceutical is administered into the extracorporeal circuit, and a detector placed over the heart registers radioactivity that reaches the main circulation. An experienced team can generally keep the leakage rate below 5 %. In 438 procedures, we measured a mean cumulative systemic leakage of 0.9 % (range 0.0–15.6 %) after 60 min of perfusion [15].
25.2.4 Tissue Temperatures
As we aim for physiological conditions, concentrating only on drug effectiveness, tissue temperatures of the limb are kept at normothermic levels between 37 and 38 °C during the whole drug circulation time. This is called “controlled” normothermia because special measures such as warming the perfusate and applying a warm water blanket around the limb must be taken to prevent the limb from cooling down during the preparative surgery [2].The drug is added to the perfusate when a temperature of 37 °C is indicated by all four temperature probes inserted into proximal and distal subcutis and muscle. We question the benefit of the widely used so-called “mild” hyperthermia method (with tissue temperatures between 39 and 40 °C), since the specific cell-killing effect of heat is mainly obtained at temperatures exceeding 41.5 °C [16]. A retrospective analysis showed similar results for perfusions with normothermia and perfusions with mild hyperthermia [17]. “True” hyperthermia means heating the tissues to temperatures between 41.5 and 43 °C. Such high temperatures are no longer applied in perfusion because of unacceptable potentiation of melphalan toxicity by heat, although encouraging antitumour effects have been obtained [18]. The administration of true hyperthermia by a perfusion without cytostatics is, however, an option. Such a perfusion is based only on the cell-killing effect of high-dose hyperthermia [19]. To exploit this option, a double perfusion schedule was tested in our institute. A 2-h true hyperthermic (tissue temperatures between 42 and 43 °C) perfusion without melphalan was followed 1 week later by a 1-h normothermic perfusion with melphalan at a dose of 10 mg/l tissue [20]. The intention of the perfusion with high-dose hyperthermia was to kill cells in the hypoxic parts of the tumour, and the normothermic perfusion with high-dose melphalan was given to attack the residual well-perfused part of the tumour [21]. With this sequential schedule, both treatment modalities, high-dose hyperthermia and high-dose cytostatic, were given at their maximum dosage without an increase in toxicity, which the heat would have caused if used simultaneously with melphalan. Using this schedule, a high complete response rate (63 %) and a low limb recurrence rate (27 %) were seen in 17 patients with extensive, recurrent melanoma [22]. Toxicity was only mild. This regimen could be an alternative to perfusion with the combination of melphalan and TNFα in patients with extensive or bulky lesions.
25.3 Toxicity and Morbidity
25.3.1 Regional
Acute regional toxicity after isolated limb perfusion typically consists of slight oedema, erythema, and pain in a warm limb, commonly developing within 48 h after the procedure and resolving within 14 days (Fig. 25.2). The redness fades to a brown hue that gradually fades over a period of 3–6 months. Usually there is no visible evidence of any change after approximately 6 months. Other local manifestations are sometimes seen (e.g. temporary loss of nails (Fig. 25.3), drying or blistering of skin of the palm of the hand or sole of the foot, inhibition of hair growth on the extremity or transient neuralgia), but all subside over time. More severe toxicity may occur in the form of blistering and muscle damage. Damage to the deep tissues can cause permanent loss of function and may also result in a threatening or manifest compartment syndrome, which necessitates amputation on rare occasions. The grading system of these toxic reactions of the normal tissues after perfusion with melphalan was introduced by Wieberdink et al. in 1982 (Table 25.1) [3]. From published data of large series, one can conclude that moderate to severe acute skin or soft tissue reactions (grades III–V according to Wieberdink) occur in 7–37 % of patients. The degree of acute regional injury has a significant effect on the incidence of long-term morbidity [23]. This long-term morbidity mainly consists of stiffness, muscle atrophy and functional impairment. Restricted range of ankle motion is seen in 25 % of the patients [24–26]. Severe chronic pain as a result of the procedure is reported in 5–8 % of the patients [23, 26]. The occurrence of long-term neuropathy was studied at the authors’ institute and was encountered in 20 % of the patients after perfusion at the axillary level and in 2 % after perfusion at the iliac level [27]. This morbidity is usually the result of the tourniquet being applied too tight during the procedure. The most important risk factors for severe acute regional toxicity are tissue temperatures over 40 °C, a high melphalan peak concentration in the perfusate, female gender and obesity [18, 28]. The reason why obese extremities are prone to develop toxicity is the higher melphalan uptake in muscle compared to fat. Because the melphalan dosage is based on limb volume, muscle tissue in obese extremities is thus exposed to a relatively high drug dose [29]. Some strategies to lower the toxic reactions have been recommended. The melphalan peak concentration can be lowered without decreasing the absolute drug dose by using a larger priming volume and by prolonged or fractionated administration [30, 31]. Obese people often receive a 10 % reduction in melphalan dose [27].
Normal acute regional toxicity reaction after isolated limb perfusion (Grade II) (Reproduced from the Textbook of Melanoma, 2004, with permission of Taylor and Francis [86])
25.3.2 Systemic
Systemic toxicity should be absent or mild, when leakage from the isolated limb is adequately controlled. Careful ligation of collateral vessels, the avoidance of high flow rates and high venous pressures and a thorough wash-out of the isolated circuit at the end of the procedure are particularly important in this respect. With these precautions, systemic toxicity was found to be absent or mild in patients perfused at the authors’ institute, both after perfusions with melphalan alone and after perfusions in which the combination with TNFα was used [32, 33].
In the melphalan-alone group, nausea and vomiting were the most frequently encountered side effects. Fever immediately following the operation was common if TNFα was added. A systemic inflammatory response syndrome with hypotension and respiratory distress, as reported by others [34], was not observed in our TNFα-perfused patients.
25.4 Indications and Results
25.4.1 Locally Advanced Melanoma
Isolated perfusion with melphalan is the treatment of choice for patients with locally unresectable melanoma of a limb (Figs. 25.4, 25.5 and 25.6). In the past, these patients frequently had to undergo amputation, a mutilation that is now seldom necessary [35]. When the lesions are bulky and well vascularised, high response rates have been reported when melphalan is combined with TNFα [36, 37]. In a recent preclinical study in mice, selective targeting of VE-cadherin was found to be one of the mechanisms in which TNFα destroys the integrity of the tumour vasculature [38]. In our hands, a complete response rate of 59 % could be obtained with the combination of melphalan and TNFα in a group of 130 patients with truly unresectable lesions (i.e. more than ten nodules or nodules with a size larger than 3 cm) [39]. With melphalan alone, the complete response rate was 45 %. Approximately half of the patients with a complete response recurred in the perfused region after a median interval of 6 months after treatment. The recurrences could be managed by simple local treatment modalities, such as excision, laser ablation or radiotherapy in 70 % of the patients. Approximately 50 % of the patients without a complete response could also be managed by simple local limb-sparing treatment. The limb-saving rate in the study population was 97 %, a satisfying percentage considering the fact that the disease had been truly unresectable in all cases. Literature shows the complete response rate after melphalan-alone perfusions for advanced limb melanoma to be on average 54 %, which is higher than the 45 % at our institution [40, 41]. This difference can be explained by a difference in tumour load. It has been shown that the number of lesions and the total tumour surface area are important prognostic factors for response after isolated limb perfusion [42, 43]. The published, complete response rates after isolated limb perfusion with the drug combination vary from 26 % to 90 %, with the higher percentages probably being attributable to a generally lower tumour load [37, 44–49]. In patients with a complete response, other investigators found subsequent limb recurrence rates comparable to ours: 44 % of the patients recur after a median interval of 5–10 months [40].
(a) Multiple in-transit metastases of the left thigh. (b) Ongoing remission, 6 weeks after perfusion with melphalan. Complete remission was achieved some weeks later (Reproduced from the Textbook of Melanoma, 2004, with permission of Taylor and Francis [86])
(a) Neglected primary melanoma with bone involvement. (b) Complete remission 6 months after perfusion (Reproduced from the Textbook of Melanoma, 2004, with permission of Taylor and Francis [86])
Perfusion may also be applied in the presence of distant melanoma metastases. Unresectable symptomatic locoregional limb melanoma is rare in these patients, but if present, perfusion can achieve satisfactory palliation [49]. The procedure has to be considered, especially in patients with distant skin metastases, distant subcutaneous metastases or distant nodal metastases, since these patients tend to survive longer than a year.
A potential drawback of perfusion is the fact that it cannot be repeated frequently due to technical reasons. Still, a double perfusion schedule with intervals of 3–4 weeks between the treatments is feasible and has been investigated at the authors’ institute [50]. Although 77 % of these patients obtained a complete response using such a schedule, half of them recurred in the perfused area after a median period of 5 months. It is noteworthy that in this study patients with a complete response had a significantly shorter time interval between their perfusions than patients without a complete response.
25.4.2 Isolated Limb Perfusion as an Adjunct in Primary and Recurrent Melanoma
Isolated limb perfusion as an adjunct after excision of high-risk primary melanoma (Breslow thickness ≥1.5 mm) was studied in a large multicentre randomised clinical trial, comprising 852 patients. The patients underwent either wide local excision or wide local excision and perfusion [51]. Locoregional recurrences occurred less frequently in the perfusion group (3.3 % versus 6.6 %, P = 0.05). The disease-free survival was better in the perfusion group during the first 2–3 years after treatment (P = 0.02), but no long-term effect was observed. There was no beneficial effect of perfusion on overall survival.
There is only one randomised prospective isolated limb perfusion study in patients with resectable recurrent melanoma [52]. In this rather small study, 69 patients with local recurrences, satellites and/or in-transit metastases were randomised to excision only or excision combined with perfusion. In the perfusion group, a lower locoregional recurrence rate of 45 % was seen versus 67 % in the excision-only group (P = 0.13). The median disease-free interval was prolonged to 17 months after perfusion compared with 10 months after excision only (P = 0.04). No significant difference in overall survival was observed, with 5-year survival rates of 44 % and 39 %, respectively (P = 0.28).
The investigators of both studies concluded that isolated limb perfusion with all its costs and associated morbidity cannot be recommended as an adjunct treatment option, because no overall survival benefit was observed., However, it is interesting that perfusion reduced the number of recurrences and increased disease-free survival in both studies, suggesting a destructive effect on micrometastastic disease. Adjuvant perfusion may thus provide valuable locoregional disease control in patients with frequently recurring and multiple resectable lesions. This hypothesis was tested at the authors’ institute in 43 patients, who had their first perfusion for a third or subsequent limb recurrence [53]. The median limb recurrence-free interval in these patients had decreased significantly over time from the primary excision to the third or fourth limb recurrence, for which perfusion was performed (P < 0.001). After perfusion, the median limb recurrence-free interval was 4.7 times longer compared with the last interval before perfusion (P < 0.001). The mean number of lesions was 2.6-fold lower compared with patients before perfusion (P < 0.001). Perfusion in this study thus lengthened the limb recurrence-free interval and decreased the number of recurrences significantly. We concluded that perfusion is a valuable adjunct to excisional surgery of repeated locoregional melanoma recurrences in patients whose limb recurrence-free intervals tend to shorten over time [53].
25.5 Repeat Isolated Limb Perfusion
After successful treatment of locoregionally recurrent limb melanoma with isolated perfusion, further recurrences develop in 46–54 % of patients [54]. Treatment options in these situations vary depending on the extent of the disease and consist among others of further excision, CO2 laser ablation, [55] intra-lesional chemo- or immunoablation, [56] and radiotherapy with or without local hyperthermia [57]. If lesions are too large or too numerous, repeat perfusion seems the only alternative to amputation. In 1996 the authors published their results of repeat isolated limb perfusion with melphalan, using various schedules, both single and multiple, normothermic and hyperthermic (tissue temperatures over 41.5 °C) [58]. A high complete response rate was obtained (74 %) with a limb recurrence-free interval of 9 months. The associated regional toxicity was substantial and was explained by the use of intensive schedules. Data on repeat perfusion using the combination of melphalan and TNFα were published in 2006 [59]. The complete response rate was 62 %, and the median limb recurrence-free survival was 13 months. Regional toxicity was mild and comparable with the first perfusion, a finding that was also reported by others [60]. Repeat isolated limb perfusion with melphalan and TNFα seems feasible with a relatively high complete response rate, a considerable limb recurrence-free survival and mild regional toxicity.
25.6 Isolated Limb Perfusion in Elderly Patients
The mean life expectancy at age 75 is 8.5 years for males and 11 years for females [61]. Maintaining and improving the quality of life in this age group is desirable. Therefore, effective perfusion with low morbidity is important also in this phase of life. A study of 202 patients was performed combining data from the authors’ institute and from Erasmus Medical Center–Daniel den Hoed Cancer Center, Rotterdam, the Netherlands. Toxicity, complications and long-term morbidity were similar in patients younger than 75 years of age and patients over 75 years of age [62]. Responses were also similar with 56 % complete responses in the older patients and 58 % complete responses in the younger patients. Hospital stay was somewhat longer in the older patients. Approximately half of the patients with a complete response achieved long-term locoregional disease control in either age category. It was concluded that older patients can undergo perfusion safely and derive the same benefit as younger people. Age is not a contraindication for isolated limb perfusion.
25.7 Prognostic Factors for Poor Survival After Perfusion
Patients with recurrent melanoma have 5-year survival rates varying between 27 and 56 %. Some of these patients do not survive longer than 1 year after treatment. Considering the usual 3–6 months duration of locoregional toxicity after perfusion and the maximum response of the lesions at a median of 4 months after the procedure, identification of a tool to select patients who will live long enough to experience the benefits of perfusion would be desirable.
In an attempt to identify prognostic factors for short survival, a study was performed in 439 patients with recurrent melanoma at our institute [63]. Sixty-nine of them (16 %) died within 1 year, 64 of metastatic melanoma. Patients with regional lymph node metastases or with regional lymph node metastases combined with satellites and/or in-transit metastases had a relative risk of 4.6 (95 % CI 2.0–6.6) and 3.6 (95 % CI 2.1–10) of dying within 1 year from perfusion, respectively (p < 0.001). In patients with distant disease, the relative risk was 22 (95 % CI 3.8–127, p = 0.001). Therefore, patients with limb melanoma have an increased risk of dying within 1 year after perfusion when regional lymph node or distant metastases are present. In these patients, the indication for perfusion should be carefully considered.
25.8 Quality of Life in Long-Term Survivors After Perfusion
Isolated limb perfusion can result in long-term morbidity in up to 40 % of the patients. We examined the impact of long-term morbidity on the quality of life. Fifty-one long-term disease-free survivors, perfused for recurrent melanoma (mean age 71 (38–90), median time after perfusion 14 (3–25) years), were selected from our database [64]. Quality of life assessment in this group was compared with an age- and gender-matched group of the Dutch population. It was demonstrated that the study group scored better on all domains of quality of life. The difference was statistically significant regarding bodily pain, general health perception, and overall mental and physical health scores. Nevertheless, the perfused patients reported considerable disease- and treatment-related morbidity, fear of recurrent disease and cosmetic problems. This counterintuitive outcome can be due to the composition of the study group, a favourable selection of long-term survivors, and “response shift”, meaning that patients have changed their perspectives over time and accommodated these to their complaints.
25.9 Isolated Limb Infusion as an Alternative to Isolated Limb Perfusion
Isolated limb infusion was designed as a simplified and minimally invasive alternative to isolated limb perfusion [65]. Similar to isolated limb perfusion, it involves a method of vascular isolation to be able to distribute high concentrations of cytotoxic drugs to the extremity (Fig. 25.7).
Schematic drawing of the isolated infusion circuit (Reproduced from Cancer Management and Research, 2013, with permission of Dove Press [87])
During isolated limb infusion, arterial and venous access are obtained percutaneously using a standard Seldinger technique, obviating the need for an open surgical procedure [66]. Via the contralateral femoral artery and vein, the tips of the catheters are placed in the disease-bearing limb. In the operating room, the catheters are connected to an extracorporeal circuit primed with saline solution, incorporating a heat exchanger but no mechanical pump or oxygenator. In order to achieve reliable isolation, a pneumatic tourniquet is inflated proximally around the affected limb. A low blood flow can be achieved in this isolated circuit by repeated aspiration from the venous catheter and reinjection into the arterial catheter, using a syringe. This results in a lower blood flow compared to isolated limb perfusion (150–1,000 ml/min for isolated limb perfusion vs. 50–100 ml/min for isolated limb infusion). The lack of oxygenation during isolated limb infusion results in a hypoxic and acidotic environment, rendering the melphalan more potent [67]. Great care is given to heating the limb with the goal to raise the temperature to mild hyperthermia, which is achieved by a heat exchanger in the external circuit, a warm air blanket placed around the limb and a radiant heater placed above it. The cytotoxic drug combination of choice for isolated limb infusion is melphalan and actinomycin D [68]. A relatively short circulation time of 30 min is sufficient given the 15–20 min half-life of melphalan and the quick tissue absorption of both melphalan and actinomycin D [68–70]. Real-time leakage control of the cytotoxic drugs to the systemic circulation is not required because the flow and the pressure in the isolated limb circuit are low and the pneumatic tourniquet secures effective isolation. After 30 min, the limb vasculature is flushed, and the normal circulation to the limb is restored by removing the tourniquet and the catheters.
Local toxicity following isolated limb infusion is also scored according to the Wieberdink classification [3]. On average, toxicity following infusion is limited and manageable [71]. A systematic review revealed that 2 % of the patients suffered from a threatening or manifest compartment syndrome after infusion [72]. Isolated limb infusion can also safely be performed in elderly patients, and repeat procedures are possible without increased toxicity [73, 74].
Isolated infusion limb was pioneered at the Melanoma Institute Australia, and their response percentages appear somewhat less than those achieved by isolated limb perfusion, but this may be due to patient selection. Following isolated limb infusion, a complete response rate of 38 % and a partial response rate of 46 % are seen with a median duration of response of 22 and 13 months, respectively [75]. Recent reports from other centres show a somewhat lower fraction of responders [76, 77]. A multicenter US study achieved a 31 % complete response rate and a 33 % partial response rate [78]. Another multicenter study reported the responses in Australian centres, excluding the Melanoma Institute Australia. The complete response rate was 27 % and the partial response 36 % [79]. A recent systematic review including all original isolated limb infusion papers showed a complete response in 33 % of the patients and a partial response in 40 % [72]. Patient selection, slight technical differences and difficulties of standardising a response reporting system for in-transit metastases may play a role in the differences between the various reports [72, 80, 81]. Isolated limb infusion, for instance, is frequently performed in older and medically more compromised patients, both of which are statistically independent prognostic factors for an inferior response [75]. Similarly to isolated limb perfusion, isolated limb infusion does not alter survival as this is mostly dictated by tumour biology. Patients with a complete response to isolated limb infusion have a median survival of 53 months. Median survival times are significantly shorter in those with a partial response or stable disease [75]. More additional mature data from centres other than the Melanoma Institute Australia are needed to define the exact place of isolated limb infusion as a minimally invasive alternative procedure compared to isolated limb perfusion.
25.10 Future
Isolated limb perfusion has a significant effect on micrometastatic disease. Its application in resectable primary and recurrent melanoma lesions may therefore be useful. Tools to identify patients with a high risk of limb recurrence and a limited risk of distant metastases whose disease is susceptible to perfusion are desirable. Perhaps, genetic testing can select such patients. To lengthen the limb recurrence-free interval after perfusion, promising preliminary results of consolidation systemic biotherapy have been published. This combined regional and systemic approach deserves further study [82, 83].
Since 2010, a number of targeted systemic therapies and immunotherapies, such as B-Raf, MEK and KIT inhibitors, anti-CTLA4 antibodies and PD-1 pathway inhibitors, have become available and result in considerable response rates and improved survival in patients with inoperable stage III and IV melanoma [84]. Currently, however, the high 54 % complete response rate of perfusion and its modest morbidity compare favourably to the limited complete response rates and the substantial morbidity of these new drugs. We feel that perfusion (and infusion) is still the first choice of treatment for patients with extensive disease confined to a limb [85]. For patients who also have distant metastases, systemic therapy with the new drugs may be a more attractive option. Combining systemic targeted therapy with high drug concentrations administered by isolated limb perfusion in these patients is another interesting option to be explored.
References
Creech OJ, Krementz ET, Ryan RF, Winbald JN. Chemotherapy of cancer: regional perfusion utilizing an extracorporeal circuit. Ann Surg. 1958;148:616–32.
Kroon BBR. Regional isolation perfusion in melanoma of the limbs; accomplishments, unsolved problems, future. Eur J Surg Oncol. 1988;14:101–10.
Wieberdink J, Benckhuijsen C, Braat RP, van Slooten EA, Olthuis GAA. Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol. 1982;18:905–10.
Nieweg OE, Imhof O, Kroon BBR. Isolated limb perfusion. In: Mulholland MW, Hawn MT, Hughes SJ, Albo D, Sabel MS, Dalman RL, editors. Operative techniques in surgery. Riverwoods: Wolters Kluwer; 2014. p. 1647–55.
Boesch CE, Meyer T, Waschke L, et al. Long-term outcome of hyperthermic isolated limb perfusion (HILP) in the treatment of locoregionally metastasized malignant melanoma of the extremities. Int J Hyperthermia. 2010;26:16–20.
Benckhuijsen C, Kroon BBR, van Geel AN, Wieberdink J. Regional perfusion treatment with melphalan for melanoma in a limb: an evaluation of drug kinetics. Eur J Surg Oncol. 1988;14:157–63.
Lejeune FJ. High dose recombinant tumour necrosis factor (rTNF alpha) administered by isolation perfusion for advanced tumours of the limbs: a model for biochemotherapy of cancer. Eur J Cancer. 1995;31A:1009–16.
De Wilt JH, ten Hagen TL, de Boeck G, et al. Tumour necrosis factor alpha increases melphalan concentration in tumour tissue after isolated limb perfusion. Br J Cancer. 2000;82:1000–3.
Van Etten B, de Vries MR, van IJken MG, et al. Degree of tumour vascularity correlates with drug accumulation and tumour response upon TNF-alpha-based isolated hepatic perfusion. Br J Cancer. 2003;88:314–9.
Aigner K, Hild P, Henneking K, Paul E, Hundeiker M. Regional perfusion with cis-platinum and dacarbazine. Recent Results Cancer Res. 1983;86:239–45.
Vaglini M, Belli F, Marolda R, Prada A, Santinami M, Cascinelli N. Hyperthermic antiblastic perfusion with DTIC in stage IIIA–IIIAB melanoma of the extremities. Eur J Surg Oncol. 1987;13:127–9.
Vaglini M, Belli F, Santinami M, et al. Isolation perfusion in extracorporeal circulation with interleukin-2 and lymphokine-activated killer cells in the treatment of in-transit metastases from limb cutaneous melanoma. Ann Surg Oncol. 1995;2:61–70.
Hoekstra HJ, Schraffordt Koops H, de Vries LG, van Weerden TW, Oldhoff J. Toxicity of hyperthermic isolated limb perfusion with cisplatin for recurrent melanoma of the lower extremity after previous perfusion treatment. Cancer. 1993;72:1224–9.
Bonenkamp JJ, Thompson JF, de Wilt JH, Doubrovsky A, de Faria Lima R, Kam PC. Isolated limb infusion with fotemustine after dacarbazine chemosensitisation for inoperable loco-regional melanoma recurrence. Eur J Surg Oncol. 2004;30:1107–12.
Klaase JM, Kroon BBR, van Geel AN, Eggermont AMM, Franklin HR. Low frequency of isotopically measured systemic leakage in a flow and venous pressure controlled isolated perfusion methodology of the limbs. Br J Surg. 1993;80:1124–6.
Hahn GM, editor. Hyperthermia and cancer. New York: Plenum Press; 1982.
Klaase JM, Kroon BBR, Eggermont AMM, et al. A retrospective comparative study evaluating the results of mild hyperthermic versus controlled normothermic perfusion for recurrent melanoma of the extremities. Eur J Cancer. 1995;31A:58–63.
Vrouenraets BC, Klaase JM, Nieweg OE, et al. Toxicity and morbidity of isolated limb perfusion: a review. Semin Surg Oncol. 1998;14:224–31.
Van der Zee J, Kroon BBR, Nieweg OE, van de Merwe SA, Kampinga HH. Rationale for different approaches to combined melphalan and hyperthermia in regional isolated perfusion. Eur J Cancer. 1997;33:1546–50.
Kroon BBR, Klaase JM, van Geel AN, Eggermont AMM. Application of hyperthermia in regional isolated perfusion for melanoma of the limbs. Reg Cancer Treat. 1992;4:223–6.
der Zee V, Kroon BBR. Isolated limb perfusion for malignant melanoma; possibly better results with high dose hyperthermia. Int J Hyperthermia. 2008;24:602–3.
Noorda EM, Vrouenraets BC, Nieweg OE, Klaase JM, van der Zee J, Kroon BBR. Long-term results of a double perfusion schedule using high-dose hyperthermia and melphalan sequentially in extensive melanoma of the lower limb. Melanoma Res. 2003;13:395–9.
Vrouenraets BC, Klaase JM, Kroon BBR, van Geel AN, Eggermont AMM, Franklin HR. Long-term morbidity after regional isolated perfusion with melphalan for melanoma of the limbs. The influence of acute regional toxic reactions. Arch Surg. 1995;130:43–7.
Van Geel AN, van Wijk J, Wieberdink J. Functional morbidity after regional isolated perfusion of the limb for melanoma. Cancer. 1989;63:1092–6.
Olieman AF, Schraffordt Koops H, Geertzen JH, Kingma H, Hoekstra JH, Oldhoff J. Functional morbidity of hyperthermic isolated regional perfusion of the extremities. Ann Surg Oncol. 1994;15:382–8.
Knorr C, Melling N, Goehl J, Drachsler T, Hohenberger W, Meyer T. Long-term functional outcome after hyperthermic isolated limb perfusion (HILP). Int J Hyperthermia. 2008;24:409–14.
Vrouenraets BC, Eggermont AMM, Klaase JM, van Geel AN, van Dongen JA, Kroon BBR. Long-term neuropathy after regional isolated perfusion with melphalan for melanoma of the limbs. Eur J Surg Oncol. 1994;20:681–5.
Vrouenraets BC, Kroon BBR, Klaase JM, et al. Severe acute regional toxicity after normothermic or ‘mild’ hyperthermic isolated limb perfusion with melphalan for melanoma. Melanoma Res. 1995;5:425–31.
Klaase JM, Kroon BBR, Beijnen JH, et al. Melphalan tissue concentrations in patients treated with regional isolated perfusion for melanoma of the lower limb. Br J Cancer. 1994;70:151–3.
Klaase JM, Kroon BBR, van Slooten GW, et al. Relation between calculated melphalan peak concentrations and toxicity in regional isolated perfusion for melanoma. Reg Cancer Treat. 1992;4:309–12.
Scott RN, Blackie R, Kerr DJ, et al. Melphalan in isolated limb perfusion for malignant melanoma, bolus or divided dose, tissue levels, the pH effect. In: Jakesz R, Rainer H, editors. Progress in regional cancer therapy. Berlin: Springer; 1990. p. 195–20017.
Sonneveld EJA, Vrouenraets BC, van Geel BN, et al. Systemic toxicity after isolated limb perfusion with melphalan for melanoma. Eur J Surg Oncol. 1996;22:521–7.
Vrouenraets BC, Kroon BBR, Ogilvie AC, et al. Absence of severe systemic toxicity after leakage-controlled isolated limb perfusion with high-dose TNFα + melphalan. Ann Surg Oncol. 1999;6:405–12.
Liénard D, Lejeune F, Ewalenko P. In transit metastases of malignant melanoma treated by high dose rTNF in combination with interferon-γ and melphalan in isolation perfusion. World J Surg. 1992;16:234–40.
Kapma MR, Vrouenraets BC, Nieweg OE, et al. Major amputation for intractable extremity melanoma after failure of isolated limb perfusion. Eur J Surg Oncol. 2005;31:95–9.
Fraker DL, Alexander HR, Andrich M, Rosenberg SA. Palliation of regional symptoms of advanced extremity melanoma by isolated limb perfusion with melphalan and high-dose tumour necrosis factor. Cancer J Sci Am. 1995;1:122–30.
Rossi CR, Foletto M, Mocelli S, Pilati P, Lise M. Hyperthermic isolated limb perfusion with low-dose tumor necrosis factor-α and melphalan for bulky in-transit melanoma metastases. Ann Surg Oncol. 2004;11:173–7.
Menon C, Ghartey A, Canter R, Feldman M, Fraker DL. Tumor necrosis factor-alpha damages tumor blood vessel integrity by targeting VE-cadherin. Ann Surg. 2006;244:781–91.
Noorda EM, Vrouenraets BC, Nieuweg OE, et al. Isolated limb perfusion for unresectable melanoma of the extremities. Arch Surg. 2004;139:1237–42.
Vrouenraets BC, Nieweg OE, Kroon BBR. 35 years of isolated limb perfusion for melanoma: indications and results. Br J Surg. 1996;83:1319–28.
Sanki A, Kam CA, Thompson JF. Long-term results of hyperthermic isolated perfusion for melanoma. A reflection of tumor biology. Ann Surg. 2007;245:591–6.
Di Filippo F, Calabro, Giannarelli D, et al. Prognostic variables in recurrent limb melanoma treated with hyperthermic antiblastic perfusion. Cancer. 1989;63:2551–61.
Klaase JM, Kroon BBR, van Geel AN, et al. Prognostic factors for tumor response and limb recurrence-free interval in patients with advanced melanoma of the limbs treated with regional isolated perfusion with melphalan. Surgery. 1994;115:39–45.
Cornett WR, McCall LM, Petersen RP, et al. Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor: American College of Surgeons Oncology Group Trial Z0020. J Clin Oncol. 2006;25:4196–201.
Grünhagen DJ, Brunstein F, Graveland WJ, van Geel AN, de Wilt JH, Eggermont AM. One hundred consecutive isolated limb perfusions with TNF-alpha and melphalan in melanoma patients with multiple in-transit metastases. Ann Surg. 2004;240:939–47.
Fraker D, Alexander H, Ross M, et al. A phase III trial of isolated limb perfusion for extremity melanoma comparing melphalan alone versus melphalan plus tumor necrosis factor (TNF) plus interferon gamma. Ann Surg Oncol. 2002;9:S8.
Alexander HR, Fraker DL, Bartlett DL, et al. Analysis of factors influencing outcome in patients with in-transit malignant melanoma undergoing isolated limb perfusion using modern treatment parameters. J Clin Oncol. 2010;28:114–8.
Deroose JP, Grünhagen DJ, van Geel AN, de Wilt JH, Eggermont AM, Verhoef C. Long-term outcome of isolated limb perfusion with tumour necrosis factor-α for patients with melanoma in-transit metastases. Br J Surg. 2011;98:1573–80.
Takkenberg RB, Vrouenraets BC, van Geel AN, et al. Palliative isolated limb perfusion for advanced limb disease in stage IV melanoma patients. J Surg Oncol. 2005;91:107–11.
Kroon BBR, Klaase JM, van Geel AN, Eggermont AMM, Franklin HR, van Dongen JA. Results of a double perfusion schedule with melphalan in patients with melanoma of the lower limb. Eur J Cancer. 1993;29A:325–8.
Schraffordt Koops H, Vaglini M, Suciu S, et al. Prophylactic isolated limb perfusion for localized, high-risk limb melanoma: results of a multicenter randomized phase III trial. J Clin Oncol. 1998;16:2906–12.
Hafström L, Rudenstam CM, Blomquist E, et al. Regional hyperthermic perfusion with melphalan after surgery for recurrent malignant melanoma of the extremities. J Clin Oncol. 1991;9:2091–4.
Noorda EM, Takkenberg B, Vrouenraets BC, et al. Isolated limb perfusion prolongs the limb recurrence-free interval after several episodes of excisional surgery for locoregional recurrent melanoma. Ann Surg Oncol. 2004;11:491–9.
Thompson JF, Hunt JA, Shannon KF, Kam PC. Frequency and duration of remission after isolated limb perfusion for melanoma. Arch Surg. 1997;132:903–7.
Strobbe LJA, Nieweg OE, Kroon BBR. Carbon dioxide laser for cutaneous melanoma metastases: indications and limitations. Eur J Surg Oncol. 1997;23:2435–8.
Thompson JF, Hersey P, Wachter E. Chemoablation of metastatic melanoma using intralesional Rose Bengal. Melanoma Res. 2007;18:405–11.
Overgaard J, Gonzalez Gonzalez D, Hulshoff MC, et al. Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastastic malignant melanoma. Society for Hyperthermic Oncology. Lancet. 1995;345:540–3.
Klop WM, Vrouenraets BC, van Geel AN, et al. Repeat isolated limb perfusion with melphalan for recurrent melanoma of the limbs. J Am Coll Surg. 1996;182:467–72.
Noorda EM, Vrouenraets BC, Nieweg OE, et al. Repeat isolated limb perfusion with TNF-alpha and melphalan for recurrent limb melanoma after failure of previous perfusion. Eur J Surg Oncol. 2006;32:318–24.
Grünhagen DJ, van Etten B, Brunstein F, et al. Efficacy of repeat isolated limb perfusions with tumor necrosis factor alpha and melphalan for multiple in-transit metastases in patients with prior isolated limb perfusion failure. Ann Surg Oncol. 2005;12:609–15.
Zenilman ME. Surgery in the elderly. Curr Probl Surg. 1998;35:99–179.
Noorda EM, Vrouenraets BC, Nieweg OE, van Geel AN, Eggermont AMM, Kroon BBR. Safety and efficacy of isolated limb perfusion in elderly melanoma patients. Ann Surg Oncol. 2002;9:968–74.
Noorda EM, Vrouenraets BC, Nieweg OE, Van Geel AN, Eggermont AMM, Kroon BBR. Prognostic factors for poor survival after isolated limb perfusion for malignant melanoma. Eur J Surg Oncol. 2003;29:916–21.
Noorda EM, van Kreij RH, Vrouenraets BC, et al. The health-related quality of life of long- term survivors of melanoma treated with isolated limb perfusion. Eur J Surg Oncol. 2007;33:776–8.
Thompson JF, Kam PC, Waugh RC, et al. Isolated limb infusion with cytotoxic agents: a simple alternative to isolated limb perfusion. Semin Surg Oncol. 1998;14:238–47.
Kroon HM, Huismans A, Waugh RC, et al. Isolated limb infusion: technical aspects. J Surg Oncol. 2014;109:352–6.
Siemann DW, Chapman M, Beikirch A. Effects of oxygenation and pH on tumor cell response to alkylating chemotherapy. Int J Radiat Oncol Biol Phys. 1991;20:287–9.
Kroon HM, Thompson JF. Isolated limb infusion: a review. J Surg Oncol. 2009;100:169–77.
Wu ZY, Smithers BM, Parsons PG, et al. The effects of perfusion conditions on melphalan distribution in the isolated perfused rat hindlimb bearing a human melanoma xenograft. Br J Cancer. 1997;75:1160–6.
Wu ZY, Smithers BM, Roberts MS. Tissue and perfusate pharmacokinetics of melphalan in isolated perfused rat hindlimb. J Pharmacol Exp Ther. 1997;282:1131–8.
Kroon HM, Moncrieff M, Kam PCA, Thompson JF. Factors predictive of acute regional toxicity after isolated limb infusion with melphalan and actinomycin D in melanoma patients. Ann Surg Oncol. 2009;16:1184–92.
Kroon HM, Huismans AM, Kam PC, et al. Isolated limb infusion with melphalan and actinomycin D for melanoma: a systematic review. J Surg Oncol. 2014;109:348–51.
Kroon HM, Lin DY, Kam PC, Thompson JF. Efficacy of repeat isolated limb infusion with melphalan and actinomycin D for recurrent melanoma. Cancer. 2009;115:1932–40.
Kroon HM, Lin DY, Kam PC, Thompson JF. Safety and efficacy of isolated limb infusion with cytotoxic drugs in elderly patients with advanced locoregional melanoma. Ann Surg. 2009;246:1008–13.
Kroon HM, Moncrieff M, Kam PCA, Thompson JF. Outcomes following isolated limb infusion for melanoma. A 14-year experience. Ann Surg Oncol. 2008;15:3003–13.
Barbour AP, Thomas J, Suffolk J, et al. Isolated limb infusion for malignant melanoma. Predictors of response and outcome. Ann Surg Oncol. 2009;16:3463–72.
Beasley GM, Petersen RP, Yoo J, et al. Isolated limb infusion for in-transit malignant melanoma of the extremity: a well tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol. 2008;15:2195–205.
Beasley GM, Caudle A, Petersen RP, et al. A multi-institutional experience of isolated limb infusion: defining response and toxicity in the United States. J Am Coll Surg. 2009;208:706–15.
Coventry BJ, Kroon HM, Giles MH, et al. Australian multi-center experience outside of the Sydney Melanoma Unit of isolated limb infusion chemotherapy for melanoma. J Surg Oncol. 2014;109:780–5.
Tyler D. Regional therapeutic strategies in melanoma: not just local disease control, but an opportunity to develop novel therapeutic strategies with potential implications for systemic therapy. Ann Surg Oncol. 2008;15:2987–90.
Huismans AM, Kroon HM, Kam PC, Thompson JF. Does increased experience with isolated limb infusion for advanced limb melanoma influence outcome? A comparison of two treatment periods at a single institution. Ann Surg Oncol. 2011;18:1877–83.
Rossi CR, Russano F, Mocellin S, et al. TNF-based isolated limb perfusion followed by consolidation biotherapy with systemic low-dose interferon alpha 2b in patients with in-transit melanoma metastases: a pilot trial. Ann Surg Oncol. 2008;15:1218–23.
Beasley GM, Riboh JC, Augustine CK, et al. Prospective multicenter phase II trial of systemic ADH-1 in combination with melphalan via isolated limb infusion in patients with advanced extremity melanoma. J Clin Oncol. 2011;29:1210–5.
Menzies AM, Long GV. Systemic treatment for BRAF-mutant melanoma: where do we go next? Lancet Oncol. 2014;15:e371–81.
Nieweg OE, Kroon BBR. Isolated limb perfusion with melphalan for melanoma. J Surg Oncol. 2014;109:132–7.
Kroon BBR, Fraker DL, Vrouenraets BC, Thompson JF. Isolated limb perfusion: results and complications. In: Thompson JF, Morton DL, Kroon BBR, editors. Textbook of melanoma. London: Taylor & Francis; 2004. p. 410–28.
Giles MH, Coventry BJ. Isolated limb infusion chemotherapy for melanoma: an overview of early experience at the Adelaide Melanoma Unit. Cancer Manag Res. 2013;5:243–9.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer-Verlag Berlin Heidelberg
About this chapter
Cite this chapter
Kroon, B.B.R. et al. (2016). Isolated Limb Perfusion for Melanoma. In: Aigner, K., Stephens, F. (eds) Induction Chemotherapy. Springer, Cham. https://doi.org/10.1007/978-3-319-28773-7_25
Download citation
DOI: https://doi.org/10.1007/978-3-319-28773-7_25
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28771-3
Online ISBN: 978-3-319-28773-7
eBook Packages: MedicineMedicine (R0)