Abstract
Isolated limb infusion (ILI) using melphalan and dactinomycin (actinomycin D) was developed as a simplified and minimally invasive alternative to the traditional, more invasive, and elaborative isolated limb perfusion (ILP) to treat unresectable metastatic melanoma confined to the limb. An increasing number of centers around the world have reported their results using the procedure. Reports from different centers have shown that the procedure is safe, with mild-to-moderate regional toxicity, and results in satisfactory response rates. When comparing ILI and ILP, it must be borne in mind that ILI is often performed in significantly older patients and in patients with higher stages of disease, which decreases the likelihood of a favorable response. Even in this era of effective systemic therapies for metastatic melanoma, ILI is still worthwhile and a relatively straightforward, single-treatment option to treat locally recurrent or in-transit metastatic melanoma involving a limb. Due to its minimally invasive nature, ILI is an ideal platform to test new drugs and drug combinations. Potential exists to further improve ILI response rates when combined with novel therapies.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Introduction and Historical Perspective
The delivery of chemotherapy to a limb using hyperthermic isolated limb perfusion (HILP) has been shown to be an effective limb-sparing treatment for patients with in-transit melanoma metastases of an extremity. Centers undertaking HILP have reported complete response rates exceeding 50% in treated limbs, after a simple procedure, with overall response rates of 80–90% (Nieweg and Kroon 2014; Moreno-Ramirez et al. 2010). Although there are now systemic therapies that are effective for metastatic melanoma, the response rates after HILP are still higher that can be achieved with these therapies or with other forms of locoregional therapy (Grünhagen et al. 2015; Raigani et al. 2017). HILP is undoubtedly effective, but it is a complex, costly, and invasive procedure. This means that patient selection criteria for HILP must be rigorous, and those with severe comorbidities are often not considered suitable for the procedure. Another disadvantage is that repeat procedures when melanoma recurrence occurs are difficult to undertake due to scarring around previously cannulated vessels; the overall complication rate increases from 28% for a single procedure to 51% for a repeat procedure (Vrouenraets et al. 1998).
In the past, numerous attempts have been made to find a simpler and less invasive method of achieving the benefits of high-dose regional chemotherapy, as in HILP, without incurring its major disadvantages. These attempts have included direct intra-arterial drug infusion and a technique that was described as “tourniquet infusion,” with partial venous outflow restriction (Karakousis et al. 1979, 1982; Bland et al. 1989). However, these procedures failed to achieve results that were comparable to those achieved by HILP.
In 1994, Thompson et al. reported a simplified form of HILP that had been developed at the Sydney Melanoma Unit (SMU), now Melanoma Institute Australia (MIA), and which appeared to achieve results similar to those achieved by HILP (Thompson et al. 1994a). To differentiate this procedure from HILP, it was termed isolated limb infusion (ILI). Essentially, ILI is a low-flow HILP using percutaneously placed arterial and venous catheters and performed under hypoxic conditions (i.e., without oxygenation of the blood in the isolated limb). Clinical experience with ILI at multiple centers around the world has established that ILI can produce outcomes similar to those that have been reported after HILP (Kroon et al. 2014a, 2016; Beasley et al. 2009).
Patient Selection for Isolated Limb Infusion
The primary indication for ILI is the same as the indication for HILP, namely, unresectable in-transit melanoma metastases of an extremity, i.e., AJCC disease stages IIIB–IIID (Gershenwald et al. 2017). The ILI procedure is well-tolerated, even by medically compromised, frail, and elderly patients, making it feasible to treat many who would be considered unsuitable for treatment by HILP (Kroon et al. 2017). After ILI, elderly patients experience limb toxicity and response rates that are similar to those that occur in younger patients.
As with HILP, good results are achieved following a single ILI procedure. A planned second ILI after 4 weeks after a first ILI increases toxicity without increasing efficacy and is therefore not recommended (Lindner et al. 2004). However, a repeat ILI can be considered for limb recurrences after an initial HILP or ILI with minimally increased toxicity (Noorda et al. 2006; Kroon et al. 2009a). ILI has also been utilized as palliative treatment for patients with systemic metastases (AJCC stage IV) disease who have limb tumors that are troublesome because of pain, ulceration, or bleeding, even if systemic metastases are present (provided life expectancy is expected to be more than a few months) (Kroon et al. 2009b).
ILI with melphalan and dactinomycin (actinomycin D) has also been used in patients with advanced extremity sarcoma (Mullinax et al. 2017), squamous cell carcinoma, Merkel cell carcinoma (Turaga et al. 2011), refractory warts of the hands (Damian et al. 2001), refractory chromomycosis (Damian et al. 2006), and localized refractory cutaneous T-cell lymphoma (Elhassadi et al. 2006).
Technical Details of the Isolated Limb Infusion Procedure
Preoperative Assessment and Management
Routine preoperative evaluation for surgery is undertaken, and antithrombotic prophylaxis is recommended: oral aspirin 300 mg daily and subcutaneous heparin 5,000 IU every 8 h, commencing on the day of surgery. This prophylaxis is continued for the duration of the patient’s hospital stay, and aspirin is continued for 3 months after the ILI.
There are two commonly performed methods of estimating limb volume preoperatively. The first is the water displacement technique, as described by Wieberdink et al. and originally used for HILP (Wieberdink et al. 1982). Marks that indicate tissue volumes are made on the limb at multiple levels preoperatively so that when the tourniquet is applied to perform the ILI, the volume of infused tissue can be estimated by extrapolation from these marks and appropriate drug dose calculations can be made. A second method for calculating limb volume involves taking circumferential measurements at 1.5 cm longitudinal intervals, encompassing the entire region of the extremity that is to be infused. The most proximal measurement is taken where the limb tourniquet is likely to be positioned. These measurements are then entered into a limb volume calculation program using Excel (Microsoft Corp., Redmond, WA) (Beasley et al. 2008).
Insertion and Positioning of Arterial and Venous Catheters
Catheter insertion is undertaken in the radiology department or in a hybrid theater by the radiologist or vascular surgeon on the morning of the ILI procedure. Small-caliber radiologic catheters are percutaneously inserted into the common femoral artery and vein via the contralateral groin (Kroon et al. 2014b). A standard Seldinger technique is used, with advancement of the catheters along guide wires into the axial artery and vein of the affected limb. IV heparin (5,000–10,000 units) is given at the time of catheter placement. The venous catheter normally used is a straight 8F catheter with 10 side holes near the tip (William A. Cook Pty. Ltd., Brisbane, Australia), while some centers use 6F venous catheters. If a venous catheter of smaller caliber is used, satisfactory venous return from the limb may be difficult to achieve. For the artery, a straight 6F catheter with a single end hole is used. The arterial catheter is usually inserted through a standard radiologic sheath. However, when angulation of the arterial catheter occurs as it crosses the aortic bifurcation and kinking at this point is thought likely, an extra-long sheath that itself crosses the aortic bifurcation can be used to increase rigidity and prevent kinking (Cassumbhoy and Pitman 2007). In patients without occlusive vascular disease, the catheter tips for a lower limb ILI are positioned in the popliteal artery and vein at a level just proximal to the knee joint (Fig. 1). If the superficial femoral artery is occluded due to atherosclerosis, however, it is possible to perform an ILI by passing the catheter down the profunda femoral artery and positioning the tip as far distally in the thigh as possible. Similarly, if the superficial femoral vein is occluded because of previous venous thrombosis, the venous catheter can be placed in the profunda femoral vein, sometimes using the ipsilateral common femoral vein. The ability to perform an ILI in the presence of arterial or venous occlusion relies on flow through collateral vessels distal to the tourniquet. An upper limb ILI can be performed by positioning the catheter tips in the brachial artery and basilic vein just above the level of the elbow joint, also via the common femoral artery and vein. Placement of the venous catheter is sometimes difficult for both lower and upper limb ILIs because valves may be encountered near the root of the limb. However, it is usually possible to negotiate these valves by first passing a guide wire through them.
After the catheters have been inserted, low-dose heparin infusions are started through them and continued until the start of the ILI procedure. It is important that the patient is kept as warm as possible during the catheter insertion procedure and during transfer to the operating room or preoperative ward because low body and limb temperatures on arrival in the operating room make it more difficult to achieve adequate heating of the limb during the ILI procedure, limiting the chance of a favorable response.
Procedure in the Operating Room
From the time the patient arrives in the anesthesia room, continuous precautions are taken to ensure that body and limb temperatures are maintained. These include covering the entire body with a hot-air blanket (Bair Hugger; Augustine Medical, Inc., Eden Prairie, Minnesota, USA) and setting the room temperature at 28–30 °C, if possible. If these precautions are not taken, initial subcutaneous temperatures at the start of the procedure can be as low as 34–35 °C, which is unsatisfactory.
General anesthesia is normally used, although it is possible to perform a lower limb ILI using spinal anesthesia, provided that an atraumatic spinal tap is achieved, since full systemic heparinization is required for the ILI. A single dose of a 5-HT3 antagonist such as ondansetron or tropisetron is given intravenously as prophylaxis against postoperative nausea and vomiting.
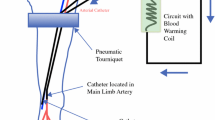
A hot-air or liquid warming blanket is placed under and around the affected limb forming a cocoon, and heat is also applied from above using an overhead radiant heater (PW820 SunTouch Surgical Warmer; Fisher & Paykel Healthcare, Auckland, New Zealand) (Kroon et al. 2014b). Needle probes to monitor subcutaneous and intramuscular limb temperatures are inserted into the calf or forearm, and a pneumatic tourniquet is positioned at the appropriate level around the root of the limb (Fig. 2). Alternatively, an Esmarch tourniquet can be used when disease is present more proximally to include a larger infusion field. After tourniquet inflation, papaverine (30–60 mg) is injected directly into the arterial catheter to promote vasodilation in the skin and thereby improve the prompt flow of the cytotoxic infusate to cutaneous and subcutaneous tumor deposits. If there is no disease in the distal extremity, the hand or foot can be excluded using an additional Esmarch or pneumatic tourniquet placed around the distal portion of the extremity. Then, the patient is fully heparinized to achieve a target activated clotting time (ACT) ≥350 s, and the arterial and venous catheters are connected to the external circuit which passes through a heat exchanger and has three-way stopcocks on the arterial and venous catheters. Circulation is performed manually by repeatedly drawing blood from the venous catheter using a 20cc syringe and reinjecting it into the arterial catheter, via the extracorporeal circuit. Once circulation of blood via the catheters is confirmed to be adequate, the pneumatic or Esmarch tourniquet is inflated or tightened around the proximal portion of the limb. Circulation is continued after tourniquet placement to ensure an adequate limb temperature before drug infusion.
On the basis of the preoperative volume measurements, the volume of limb tissue to be infused is determined, allowing drug dosages to be calculated. The dose of melphalan is 7.5 mg/L for a lower extremity and 10 mg/L for an upper extremity, with a maximum total dose of 100 mg for lower and 50 mg for upper extremity. The dactinomycin dose is 75 mcg/L for a lower extremity and 100 mcg/L for an upper extremity. Some centers correct dosages for ideal body weight (IBW) with the objective of minimizing toxicity, the rationale for which is discussed below in the section dealing with melphalan pharmacokinetics (Beasley et al. 2008; McMahon et al. 2009). The cytotoxic drugs are diluted in 400 mL heparinized normal saline solution, pre-warmed to 40 °C. When the subcutaneous temperature in the extremity is at least 37.0 °C, the chemotherapy is infused as rapidly as possible (in 2–5 min) through the arterial line, using an intravenous fluid pump set, fed from a pressurized infusion bag. Once the infusion is complete, a 30-min circulation of the chemotherapy through the limb via the external circuit commences. With a blood warmer set at 41 °C incorporated into the extracorporeal circuit, the infusate is continuously recirculated using the 20cc syringe, which is attached to a wide-bore, high-flow, three-way tap attached to the venous catheter (Level 1 Technologies, Inc., Rockland, Mass., USA).
Specimens for circuit blood gas analysis are taken from the venous line at the start of the procedure and at 25 and 30 min after the infusion of chemotherapy to analyze the degree of hypoxia and acidosis. During this 30-min period, it is usually possible to increase limb temperatures by 1–2 °C. This means that limb temperatures of at least 38.0–38.5 °C can be achieved by the end of the procedure.
After 30 min of drug exposure, the limb is flushed with Hartmann’s solution or normal saline via the arterial catheter, and as much blood as possible is extracted from the venous catheter. Low pressure suction attached to the venous catheter assists in the efficient extraction of blood. This effluent from the limb is discarded into a cytotoxic waste disposal container. The washout process is terminated when the effluent is clear, normally after infusion of 800–1,000 mL Hartmann’s solution. Protamine is administered intravenously to reverse residual heparin, and the arterial and venous catheters are withdrawn. Bleeding at the arterial catheter exit site is controlled either with direct digital pressure or via use of an inflatable occluder (Femostop II; Radi Medical Systems, Uppsala, Sweden), with pressure maintained until satisfactory hemostasis has been obtained (15–20 min), or by using a Perclose™ or Angioseal™ device to occlude the catheter exit site. The ILI circuit in diagrammatic form is shown in Fig. 3. Figure 4 shows an ILI in progress in the operating room for both an upper limb and a lower limb ILI.
(a) Isolated limb infusion procedure in progress in the operating room. (b) Recirculation of infusate using a 20 mL syringe through a high-flow, three-way tap. (c) Lower limb isolated limb infusion. Note the Esmarch bandage on around the foot to exclude it from the circulation, the overhead heater placed over the limb, and the blood-warming coil incorporated in the extracorporeal circuit (on the right). (d) Upper limb isolated limb infusion
Postoperative Course and Care
Bed rest is maintained for the first 24 h postoperatively for lower limb ILIs, after which most patients are allowed bathroom privileges. Limb toxicity is normally limited to erythema and edema of the skin and subcutaneous tissues; these develop within 24 h and usually subside quickly after reaching a peak at 4–5 days. A patch of inflammation often develops in the skin surrounding or overlying tumor nodules within 48 h. Rarely there is discomfort in the limb, requiring analgesia. The limb is carefully examined at least twice a day, particularly for evidence of a developing compartment syndrome. Peripheral pulses are assessed, and muscular compartments are monitored. Serum creatine kinase (CK) levels are measured daily, and if the serum CK exceeds 1,000 IU/L, systemic corticosteroids are administered: dexamethasone 4 mg intravenously every 6 h, with dose reduction once the serum CK level starts to fall (Kroon et al. 2014b). If there is concern that a compartment syndrome might be developing despite corticosteroid therapy, a subcutaneous fasciotomy is performed. After ILI the need for fasciotomy is rare (Kroon et al. 2014a; Beasley et al. 2009).
Similarities and Differences Between Isolated Limb Infusion and Hyperthermic Isolated Limb Perfusion
Both HILP and ILI involve vascular isolation and perfusion of an extremity with chemotherapy to achieve regional drug concentrations several orders of magnitude higher than can be attained by systemic drug administration. Because of adequate isolation of the limb from the rest of the body while it is exposed to the high-dose chemotherapy, serious systemic side effects are avoided effectively in ILI as they are in HILP (Vrouenraets et al. 1998; Beasley et al. 2009; Kroon et al. 2009c; Katsarelias et al. 2018).
ILI differs from HILP in that it is a minimally invasive procedure with small-caliber catheters whereas HILP is a large invasive surgical procedure with open blood vessel cannulation using large-caliber catheters. Due to the difference in catheter caliber between both procedures, blood circulates in the isolated extremity at a much slower rate than HILP (150–1,000 mL/min in HILP vs. 50–100 mL/min in ILI), and drug exposure time is 30 min compared to 60 min (Koops et al. 2004; Kroon et al. 2008). Theoretically the low-flow system of ILI may lead to lower melphalan uptake by tumor cells although this has not been shown (Roberts et al. 2001a). Another major difference is that during ILI the extremity is not oxygenated, leading to marked hypoxia and acidosis, in contrast to HILP where a pump oxygenator maintains oxygenation and a normal acid/base status of the extremity (Table 1). The hypoxic conditions that develop during ILI may in fact be advantageous by enhancing the cytotoxic effect of melphalan since there is good evidence that alkylating agents such as melphalan are more effective under these conditions (Kroon et al. 2008; Siemann et al. 1991; Skarsgard et al. 1995; Van der Merwe et al. 1993). Whereas blood transfusions are normally required during HILP to prime the extracorporeal circuit, this is not necessary in ILI. Furthermore, if a patient has had previous groin or axillary surgery, for instance, a lymph node dissection, catheter insertion via the contralateral groin for ILI is usually straightforward, whereas both venous and arterial access for HILP can be technically difficult, with both short-term and long-term risks to vessel patency. Similarly, surgical access to the vessels for a repeat HILP procedure is often difficult due to scarring around the previous vascular access sites, but the percutaneous catheter insertion for a subsequent ILI normally does not present problems. These and other differences between ILI and HILP are detailed in Table 2.
Drugs Used in Isolated Limb Infusion
While different drugs have been utilized in regional therapy studies, the alkylating agent L-phenylalanine mustard , also known as melphalan or L-PAM, has been the drug of choice for HILP and ILI for decades (Kroon et al. 2014a; Fletcher et al. 1994; Bonenkamp et al. 2004; Cornett et al. 2006). Its mechanism of action is through alkylation of DNA bases resulting in breaks in DNA molecules, ultimately inducing cellular damage (Hansson et al. 1987). When delivered systemically, melphalan is ineffective due to the lower tolerable concentrations to avoid dose-related myelosuppression (Defty and Marsden 2012). However, when administered regionally, higher doses (10- to 100-fold higher doses than systemic regimens) can be utilized. In ILI, dactinomycin, an inhibitor of DNA transcription, is often administered in addition to melphalan, based on results from MIA utilizing the combination during HILP, where an OR rate of 73% was achieved, without increasing limb toxicity (Lindner et al. 2002).
Recently, there has been interest in using temozolomide, an imidazotetrazine derivative of dacarbazine, during ILI (Ueno et al. 2004). Like melphalan, the mechanism of action of temozolomide involves disruption of DNA replication through alkylation. A multicenter phase I dose-escalation trial of ILI using temozolomide demonstrated a favorable safety profile. OR rates were low (15.8%), but the study population was small and not designed to evaluate drug efficacy (Beasley et al. 2015). Therefore, further studies involving large sample sizes are required to assess the efficacy of temozolomide during ILI.
Pharmacokinetics of Melphalan During Isolated Limb Infusion
The plasma concentration of melphalan in the limb during ILI falls in a monoexponential fashion, suggesting rapid uptake by the tissues (Roberts et al. 2001b). This is in keeping with in vitro studies, demonstrating uptake of melphalan into melanoma cells as a rapid, active, temperature-dependent process that achieves saturation after 10 min (Parsons et al. 1981). The mean residence time and elimination half-life of melphalan during ILI were 21–35 min and 15–25 min, respectively (Roberts et al. 2001b).
In vitro studies have shown that the hypoxic conditions during ILI enhance the cytotoxic effect of melphalan by a factor of approximately 1.5 and the combination of hypoxia and acidosis can increase the effect by a factor of 3 (Siemann et al. 1991; Skarsgard et al. 1995). HILP studies have also shown that by administering glucose to the isolated circuit, the intracellular pH in the tumor can be decreased, with a concomitant increase in the response rate (Van der Merwe et al. 1993). However, conflicting data from a HILP in vitro model, examining the effect of a variety of factors on the sensitivity of melanoma cells to melphalan, showed that a pH as low as 6.0 had no significant impact on cell survival (Clark et al. 1994). On the basis of these reports, it appears that the significance of hypoxia and acidosis during ILI remains to be fully elucidated.
Studies of the pharmacokinetics of HILP and ILI have demonstrated similar wide variations of plasma melphalan concentrations. Cheng et al. examined pharmacokinetics by obtaining plasma melphalan drug levels during HILP (Cheng et al. 2003). Five of 14 patients suffered Wieberdink limb toxicity grade III/IV (Wieberdink et al. 1982), and marked differences in melphalan plasma concentrations were observed despite using similar dosing guidelines. The strongest predictor of toxicity was the ratio of estimated limb volume (Vesti) to steady-state (Vss) limb drug volume of distribution, whereas the area under the curve and peak plasma concentration failed to correlate with toxicity. All toxicity was seen in patients whose Vesti/Vss ratio was over 4: five of seven patients with a ratio greater than 4 had grade III/IV limb toxicity. In an initial experience with drug pharmacokinetics at Duke University Medical Center (Duke UMC), similar variability was found. Toxicity was also related to overestimation of limb volume compared to steady-state limb drug volume of distribution (Beasley et al. 2008). An analysis of 185 ILIs from MIA found that patients with a body mass index (BMI) of >25 kg/m2 experienced greater limb toxicity (grade III/IV) (Kroon et al. 2009c). This finding may also indicate an overestimation of the volume of distribution. Since melphalan uptake is higher in muscle as opposed to fat, the skin and subcutaneous tissues are exposed to a relatively higher dose of melphalan when concentrations are based on limb volumes only because overweight patients have a lower muscle-to-fat ratio (Kroon et al. 2008, 2009c; Klaase et al. 1994; Scott et al. 1990).
Melphalan Dosage and Ideal Body Weight
The observations discussed above suggest that the therapeutic index of melphalan could be optimized through a better understanding of its pharmacokinetics in individual patients, with patients who fit the profile for a high Vesti/Vss given a lower melphalan dose. In view of this, some centers have modified melphalan dosage according to IBW (Beasley et al. 2008). This calculation is performed by multiplying the melphalan dose (7.5 mg/L for a lower limb; 10 mg/L for an upper limb) by the ratio of IBW to actual body weight. Patients at Duke UMC who had their melphalan dose corrected for IBW experienced less variability in melphalan plasma concentrations, and a significant decrease in toxicity was observed (p = 0.024) when melphalan dose was corrected for IBW without adversely affecting tumor response (McMahon et al. 2009).
Use of Microdialysis During Isolated Limb Infusion
Microdialysis is a technique that enables drug concentrations to be monitored in various tissues to investigate the relationships between melphalan concentrations in plasma, the interstitium, and tumor tissue (Wu et al. 2000). In patients undergoing ILI at MIA, microdialysis catheters (CMA60/CMA70; CMA, Solna, Sweden) were inserted subcutaneously into normal and tumor tissues before the start of ILI (Thompson et al. 2001). A microdialysis pump (CMA 106; CMA) maintained a constant infusion of fluids while melphalan concentrations in the samples were measured using high-performance liquid chromatography (Wu et al. 1995). The study showed that the peak melphalan concentrations in plasma were higher than in subcutaneous tissues and tumor tissues. This technique enables melphalan concentrations to be monitored in subcutaneous tissues and tumor deposits and therefore may help to optimize ILI conditions and improve tumor response; however, further studies involving larger sample sizes are required.
Toxicity and Side Effects Following Isolated Limb Infusion
Locoregional Side Effects of Isolated Limb Infusion
In general, ILI is a well-tolerated procedure. As with HILP, superficial desquamation of the skin often occurs after 2–3 weeks, and residual pigmentation of the limb may persist for months. If the foot or hand has not been excluded by an Esmarch bandage or distal pneumatic tourniquet, as is often possible, loss of the epidermis of the sole of the foot or palm of the hand may occur, leaving a delicate and sensitive new skin surface exposed for weeks until the area is again covered by normal plantar or palmar skin. Furthermore, loss of toe or fingernails of the treated limb may occur 3–4 months after treatment, as well as the loss of hair in the limb (Thompson et al. 1994b).
Limb Toxicity Following Isolated Limb Infusion
The Wieberdink toxicity scale, historically used for HILP, is also applicable after ILI (Table 3) (Wieberdink et al. 1982). At MIA, ILI usually results in mild-to-moderate limb toxicity ; 56% and 39% of patients experienced Wieberdink grade II and grade III limb toxicity, respectively, while only 3% experienced grade IV toxicity (Kroon et al. 2009c). Although this incidence of limb toxicity is at the higher end of the spectrum of that reported following HILP, long-term morbidity is less frequently observed and less severe after ILI compared to HILP. Fasciotomies due to threatened or actual severe limb toxicity, for instance, are rarely necessary after ILI, and from all reported series, only one patient has required an amputation due to toxicity following ILI (grade V limb toxicity) (Kroon et al. 2009c, 2014a; 2016; Beasley et al. 2008, 2012; Brady et al. 2009; Dossett et al. 2016; Santillan et al. 2009; O’Donoghue et al. 2017). Large, contemporary series have reported grade III limb toxicity or higher in less than 30% (Kroon et al. 2016; Beasley et al. 2012; O’Donoghue et al. 2017). An Australian multicenter study evaluating 316 ILI procedures reported grade III limb toxicity in 27% and grade IV toxicity in 3% of the patients, with no amputations due to toxicity, and a recent single-center study from Moffitt Cancer Center (MCC) reported grade III or higher in 12% of the patients.
At Duke UMC, toxicity has been assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 3 (CTCAEv3; Table 4) (Beasley et al. 2008; Common Terminology Criteria for Adverse Events 2006). Using the CTCAEv3, the severity of limb toxicity was similar to that reported by other series using the Wieberdink toxicity scale (Table 5). At Duke UMC, ILI was associated with significantly less limb toxicity compared to HILP, after which more patients experienced grade III limb toxicity or higher (44% after HILP vs. 18% after ILI; p = 0.009) including nine compartment syndromes and two amputations. They reported that limb toxicity was further reduced by melphalan dose correction for IBW (Beasley et al. 2008).
Following ILI, no relationship has been found between more severe limb toxicity and complete response (CR), duration of response, or overall survival (OS), but a relationship was observed between Wieberdink grade III/IV limb toxicity and overall response (OR) at MIA (Beasley et al. 2008; Kroon et al. 2008, 2009c). It is interesting to note that the partial response (PR) rate at Duke UMC (14%), which largely accounted for their lower OR rate (44%), was much lower than the PR rate at MIA (46%; OR 84%). One hypothesis that would explain this lower PR rate is that, while the major determinant of a CR is tumor chemosensitivity, a PR may be related to maximal chemotherapy, a delivery which is associated with more limb toxicity. Since at Duke UMC the majority of patients who had chemotherapy dose correction for IBW experienced less toxicity, it is possible that they may have had a favorable response with a higher melphalan dose.
Various pharmacokinetic variables have been shown to predict limb toxicity after ILI. An analysis at MIA revealed that a high peak melphalan level, a high final melphalan level, and a larger melphalan concentration area under the curve in the isolated limb were significantly associated with more severe limb toxicity (Kroon et al. 2009c). Also, a smaller increase of the CO2 level in the isolated circuit during ILI was found to be significantly associated with increased limb toxicity. This finding was surprising given the fact that a bigger rise in CO2 had earlier shown to improve response rates (Kroon et al. 2008). These results, demonstrating the abovementioned effect of hypoxia and acidosis both on toxicity and response following melphalan ILI, clearly require further investigation.
Postoperatively a high peak serum creatine kinase (CK) level has been shown to be a strong predictor of limb toxicity. In an Australian multicenter study, patients who experienced grade III/IV limb toxicity had a significantly higher peak CK compared to patients with grade I/II limb toxicity (median 2,553 IU/L vs. 217 IU/L, respectively; p < 0.001), and in the US multicenter study, a median peak CK level of >563 U/L was a significant predictor for developing severe acute regional toxicity (p < 0.01) (Kroon et al. 2016; Santillan et al. 2009). For this reason the ILI protocol recommends the administration of high-dose steroids to patients with CK levels of >1,000 U/L in an attempt to avoid severe limb toxicity (Kroon et al. 2014b).
Systemic Toxicity and Complications of Isolated Limb Infusion
Serious systemic side effects are rare after ILI, with no occurrence of bone marrow depression and only occasional mild postoperative nausea and vomiting, which resolves quickly with conservative management. The reasons for the small number of patients suffering from systemic toxicity after ILI are mainly attributed to the low influx of chemotherapy from the isolated limb to the systemic circulation. Influx of chemotherapy to the systemic circulation is prevented by the reliability of limb isolation with the pneumatic tourniquet, the thorough flushing of the limb after completion of the ILI, and the low pressure in the isolated circuit, which is much lower than the systemic blood pressure. At MIA, systemic melphalan was detected at a very low rate in a minority of the patients: <1% of the infused melphalan dose was detected systemically in 14 patients (8%), and 1 patient had a systemic leakage of 6%. None of these patients experienced systemic side effects that were of any concern (Kroon et al. 2009c).
Clinical Results of Isolated Limb Infusion
Response Rates Following Isolated Limb Infusion
In the majority of patients, cutaneous tumor deposits begin to show signs of involution within 7–14 days following ILI. Sometimes, however, it can take several weeks before tumor deposits decrease appreciably in size. Interval photographs of a patient who had a CR to ILI are shown in Fig. 5.
The main goal of ILI is to achieve a CR, as this improves the quality of life markedly, but achieving a PR also substantially improves the patient’s quality of life (Jiang et al. 2015). Early studies of ILI using melphalan +/− dactinomycin for melanoma reported OR rates of 44–100% (Thompson et al. 1994a, b; Beasley et al. 2008; Kroon et al. 2008; Brady et al. 2009; Mian et al. 2001). While the majority of the early series were limited by small patient numbers, there was still considerable variability in efficacy among subsequent larger studies. The largest single institution involved 185 patients and reported a CR rate of 38% and OR rate of 84% (Kroon et al. 2008). These results were not reproduced by the first three single-center experiences in the USA, all of which had fewer patients (Beasley et al. 2008; Brady et al. 2009; Wong et al. 2013). Recently, there have been multiple single and multicenter studies and a systematic review that have further expanded the ILI experience (Table 6) (Kroon et al. 2008, 2014a, 2016; Beasley et al. 2008, 2009; Defty and Marsden 2012; O’Donoghue et al. 2017; Mian et al. 2001; Wong et al. 2013; Coventry et al. 2014; Muilenburg et al. 2015; Brady et al. 2006). An updated single-center study of 148 patients at MCC reported an OR of 59% (CR 25.7%, PR 33.3%) (O’Donoghue et al. 2017). Three multicenter studies, one from Australia and two from the USA, reported similar results. In the Australian multicenter study, five institutions reported their combined ILI experience in 316 patients with an OR rate of 75% (CR 33%, PR 42%) (Kroon et al. 2016). In the US multicenter studies, while response rates were not as high as in the Australian study, OR rates remained promising and ranged from 57% to 64% (Beasley et al. 2009; Muilenburg et al. 2015). Lastly, a systematic review consisting of 576 patients showed an OR rate of 73% (CR 33%, PR 40%) (Kroon et al. 2014a).
Differences in treatment efficacy between these newer studies must be interpreted with great caution as they may be due to differences in patient populations, experience and modifications to the ILI protocol, as well as lack of consistent criteria by which responses are assessed. A greater percentage of patients at Duke UMC and MCC, for instance, suffered from higher disease stages than at MIA. All patients in these studies had AJCC stage IIIB or higher, whereas in the MIA study, 11% of all patients suffered from lower disease stages (Beasley et al. 2008; Kroon et al. 2008; O’Donoghue et al. 2017). Since stage of disease is a recognized predictor of response to ILI, the lower response rates (CR 30%, PR 14%) seen at Duke UMC compared to MIA may therefore be partially attributable to the inclusion of patients with higher stages of disease.
Furthermore, a study performed at MIA showed that increased experience in performing ILI had a positive effect on outcome in patients treated in the more recent years (Huismans et al. 2011). Despite a significantly greater tumor load (a known prognostic factor for a worse response) in the more recently treated patients, response rates and OS were similar compared to the earlier treated patients.
Variations in the ILI protocol also existed among the studies. The Memorial Sloan Kettering Cancer Center (MSKCC) used a 20-min drug exposure time with a median tourniquet time of 32 min (Brady et al. 2006). This is significantly shorter than the drug exposure and tourniquet times used in other centers and may have had an effect on response to ILI. MIA used a drug circulation time of 20–30 min and initially reported that a tourniquet time longer than 40 min was associated with increased OS, but not with response or duration of response (Lindner et al. 2002). However, subsequent analysis including larger patient numbers could not confirm that outcome was improved after longer tourniquet times (Kroon et al. 2008). Duke UMC and MCC both used 30-min drug exposure times, and the Duke UMC study found no association between tourniquet time and response rates (p = 0.491) (Beasley et al. 2008; O’Donoghue et al. 2017). Furthermore, as the MSKCC study has shown, shorter drug exposure and tourniquet times also lead to lower limb temperatures during ILI, which may negatively affect response to ILI as well (Kroon et al. 2008; Chang et al. 1978; Kroon 1988). An additional protocol variable is the use of papaverine after tourniquet insufflation to promote vasodilatation in the skin of the isolated limb to improve the prompt flow of the cytotoxic infusate to cutaneous and subcutaneous tumor deposits. Papaverine was used at MIA, MSKCC, and MCC, but not routinely at Duke UMC. Finally, the venous catheters used at Duke UMC and MCC are 6F, while the venous catheters used at MIA are 8F. The size of the catheter may be important for optimal circulation and drug exposure.
Finally, no uniform objective response criteria have been used in the various studies. In the US series, the response to ILI has been assessed at 3 months using the “Response Evaluation Criteria In Solid Tumors” (RECIST) criteria version 1.1, with the highest OR rate of 72% reported by MCC (CR 32%, PR 40%) (O’Donoghue et al. 2017; Eisenhauer et al. 2009). At MIA it was found that there was a wide range of the time between ILI and best response ranging from 1 to 84 weeks (median 6 weeks) (Kroon et al. 2008). Therefore, in the Australian centers, responses have been assessed according to the standard World Health Organization criteria (World Health Organization 1979). These define a CR as the disappearance of all measurable disease, determined by two observations more than 4 weeks apart, and a PR as a ≥50% decrease in total tumor size determined by two observations more than 4 weeks apart and no appearance of new lesions or progression of any lesion. Assessing response can also be difficult when the treated limb bears a large number of tumor nodules and when pigment remains after treatment. Additionally, response assessment can be complicated by the appearance of systemic disease, in which case patients often receive other forms of treatment that may have an effect on the magnitude or durability of response in the extremity. Overall, standardization of objective response criteria will aid future studies and will be necessary for valid comparisons between different studies.
Limb Recurrence-Free Interval and Overall Survival Following Isolated Limb Infusion
In terms of durability of response and OS, initial response to treatment portends improved limb recurrence-free interval (LRFI) and OS (Kroon et al. 2016; Beasley et al. 2008; O’Donoghue et al. 2017; Brady et al. 2006). The reported median duration of response following ILI is around 12–13 months. At MIA, median LRFI following a CR was 22 months. Of all 114 patients in whom relapses in the treated limb were documented, 107 (94%) occurred within 24 months. The median OS was 38 months, whereas patients achieving a CR experienced a median OS of 53 months, which was significantly longer than in the group of patients in whom a PR or no response occurred. In the MCC series, patients who had either a CR or PR following ILI had a significantly increased LRFI (14.1 vs. 3.2 months, p < 0.0001), distant metastatic free survival (not reached vs. 25.8 months, p = 0.006), and OS (56 vs. 26.7 months, p = 0.0004) compared to non-responders (O’Donoghue et al. 2017). Similarly, in the large Australian multicenter study, a CR resulted in a median OS of 80 months (Fig. 6) (Kroon et al. 2016).
(a) Overall survival (months) following isolated limb infusion in 316 Australian patients (Kroon et al. 2016). (b) Survival (months) of patients after a complete response (CR; solid line) compared with a partial response (PR; dotted line) after isolated limb infusion (p = 0.014; HR 2.42; 95% CI 1.67–3.09) (Kroon et al. 2016)
Prognostic Factors for Outcome Following Isolated Limb Infusion
Multiple patient-related factors have been reported as predictors of response and OS. In addition to stage of disease being a predictor of response and OS, patients treated at MIA who had tumors extending below the level of the deep fascia had a shorter OS compared to those who had cutaneous or subcutaneous lesions only (p = 0.029), but no difference in response rates was observed. Breslow thickness of the primary melanoma and a BMI ≥26 were also found to be predictors of OS and again not of response (Kroon et al. 2008). In several studies, burden of disease (BOD) and the number of tumors have also been reported to predict response and OS. At MIA, patients with only one lesion compared to patients with 2–5 lesions or >5 lesions had improved OS rates (p = 0.010) (Kroon et al. 2008). In a US multicenter study, BOD was defined as being low if patients had less than ten tumors with none greater than 2 cm, and patients were classified as having a high BOD if they had more than ten lesions or any single lesion larger than 2 cm (Muilenburg et al. 2015). On multivariate analysis, patients with a low BOD were 3.5 times more likely than high BOD patients to have a favorable response to ILI (odds ratio 3.5, p < 0.001). Moreover, patients with a low BOD experienced a significantly increased median LRFI of 6.9 months compared to 3.8 months for high BOD patients (p = 0.047), although this finding did not translate into improved OS. The findings that several of the patient factors were independent predictors of LRFI and OS, including depth of tumor infiltration, number of lesions/BOD, and Breslow thickness of the primary melanoma, can possibly be explained by the fact that they are derivatives of stage of disease, which has been shown to be a prognostic factor for duration of response and OS (Kroon et al. 2008, 2016). The longer OS of patients who achieved a CR following ILI could be explained by the fact that they tended to have a lower stage of disease and suggests that the underlying tumor biology may be associated with greater chemosensitivity (Kroon et al. 2008; Aloia et al. 2005; Sanki et al. 2007). At Duke UMC it was shown that patients who responded to ILI had significantly smaller limb volumes (6.4 ± 2.2 L) than those who did not respond (8.1 ± 3.4 L) (p = 0.043), and at MCC an increased response was seen after upper limb ILI compared to lower limb ILI (Beasley et al. 2008; O’Donoghue et al. 2017). These observations again suggest an important role of drug distribution, as seen in the microdialysis studies that demonstrated that drug distribution was better achieved in smaller limbs than larger limbs, leading to a better drug delivery to the skin and subcutaneous tissues in patients with smaller limbs (Kroon et al. 2009c; Klaase et al. 1994; Thompson et al. 2001). Ultimately, the identification of both favorable tumor and patient-related factors that influence outcomes will help to stratify patients into groups likely to derive the greatest benefit from ILI.
In the MIA series, several intraoperative factors with a predictive value for response were identified. A higher melphalan concentration at the conclusion of the procedure was associated with significantly improved CR (p = 0.013) and OR (p = 0.022) (Kroon et al. 2008). A greater difference in pCO2 in the isolated circuit between the commencement and the conclusion of the procedure was also associated with an improved CR rate (p = 0.017), and a tourniquet time >40 min was a prognostic factor for OS and showed a trend toward an increased OR rate (p = 0.074) (Lindner et al. 2002; Kroon et al. 2008). As previously mentioned, these findings could possibly be related to the synergism of hypoxia and acidosis with melphalan.
There was a trend toward a higher OR in patients who had a larger increase from the initial to the final subcutaneous temperatures in the limb during ILI (p = 0.062). Hyperthermia and its synergistic cytotoxic effect with melphalan were first described over 40 years ago (Cavaliere et al. 1967; Stehlin 1969). During ILI, however, it is usually not possible to achieve true hyperthermia (i.e., tissue temperatures exceeding 41.0 °C) in the treated limb because of the low-flow rate due to the high resistance of the small-caliber catheters in the isolated circuit. However, previous HILP studies have suggested that it is mainly the maintenance of normothermia and the avoidance of hypothermia that are most important, rather than the attainment of high limb temperatures. In view of this, the mildly hyperthermic limb temperatures of 38–39 °C achieved during ILI may actually be beneficial, as hyperthermic limb temperatures have the disadvantage of causing more rapid melphalan degradation and greater limb toxicity (Chang et al. 1978; Kroon 1988).
Postoperatively, a high CK level showed a significant association with OR rate (p = 0.029), and limb toxicity grade predicted the OR rate (p = 0.002) but not the CR rate, LRFI, or OS. In contrast, the Duke UMC series reported that neither limb temperatures, tourniquet time, nor postoperative CK level predicted outcomes (Beasley et al. 2008). Larger studies will be needed to further investigate these relationships.
Special Isolated Limb Infusion Regimens and Indications
Special ILI regimens include a planned double ILI, a repeat ILI procedure for disease recurrence, ILI for palliation in patients with AJCC stage IV disease, and ILI as induction therapy.
One study examined a planned double ILI at a median of 2–8 weeks apart (Lindner et al. 2004). This protocol achieved a CR of 41% and a PR of 47%, with a median duration of response of 18 months in 47 patients. Because a planned double ILI increased limb toxicity without a significant increasing efficacy, it was concluded that performance of a single ILI remained the preferred treatment option for melanoma confined to a limb (Lindner et al. 2004).
A repeat procedure after an initial ILI in patients with disease recurrence or progression may be beneficial, especially if there had been a favorable response to the initial ILI. For patients who did respond to an initial ILI, a repeat procedure is unlikely to provide much benefit. In those cases, systemic therapies will need to be considered. As mentioned before, the minimally invasive character of ILI allows a repeat procedure to be performed with relative ease, in contrast to HILP (Kroon et al. 2009a; Chai et al. 2012; Raymond et al. 2011). A multi-institutional series by Chai et al. evaluated the use of repeat HILP versus repeat ILI after disease progression: 3 patients (7%) had a repeat HILP, 10 (23%) had a HILP following an initial ILI, and 12 (27%) had an ILI following an initial HILP (Chai et al. 2012). Most patients tolerated repeat regional chemotherapy well without increased toxicity or LOS, and no statistical difference in response rates or OS was noted when comparing repeat ILI or HILP procedures. Another series showed that patients who experienced regional recurrences after an initial regional treatment were more likely to achieve a CR after repeat HILP (50%, n = 10) compared with repeat ILI (28%, n = 18) (Raymond et al. 2011). However, the likelihood of grade IV limb toxicity was greater after HILP (2 of 62) than after ILI (0 of 122). Given that response rates and duration of response following ILI and HILP are generally similar and ILI results in fewer complications due to its minimally invasive nature and causes less limb toxicity grades, many centers use ILI and consider repeat ILI for patients who had a good response to the initial ILI (Dossett et al. 2016).
Because of its minimally invasive nature, low limb toxicity, and low complication rates, ILI can also be considered as a palliative procedure to avoid limb amputation in patients with both symptomatic limb disease and distant melanoma metastases in order to achieve limb salvage and increase quality of life. In an ILI MIA study in patients with AJCC stage IV disease, limb preservation was achieved in 86% of the patients (n = 37) (Kroon et al. 2009b). In this time of effective systemic therapy for melanoma metastases, combination treatment of locoregional therapy through ILI and systemic therapy will very likely be considered in the near future in this subset of patients for increased efficacy both on systemic and limb disease. In the two paragraphs below, ILI in combination with systemic therapies is discussed.
Another mechanism by which ILI can help achieve limb preservation is by using it as induction therapy. This can convert unresectable disease into resectable disease, and simple local treatment of the remaining lesions, such as excision, laser ablation, electrodessication, and injection with Rose Bengal (PV-10), can then achieve effective disease control (Thompson et al. 2015; Huismans et al. 2016). After a PR, for instance, resection of residual limb disease achieved LRFI and OS rates similar to those observed following a CR after ILI alone (Wong et al. 2014).
Finally, ILI can safely and effectively be used in the elderly and in patients with upper extremity melanoma. Particularly in elderly patients, ILI appears to be an attractive and safe procedure compared to HILP, since older patients experienced less limb toxicity compared with younger patients (Wieberdink grade III/IV toxicity 36% vs. 51%; p = 0.009), but efficacy, systemic toxicity, complications, and long-term morbidity were similar in a recent multicenter Australian study (Kroon et al. 2017). ILI for upper extremity melanoma is associated with similar CR rates but lower toxicity than lower limb ILI despite comparable methods, suggesting a particularly important role for ILI in the management of upper extremity disease (Beasley et al. 2012; O’Donoghue et al. 2017).
Novel Isolated Limb Infusion Regimens
Given its minimally invasive character and the easy visual assessment of tumor response and access for biopsies of in-transit metastases, ILI is an ideal model to explore novel therapeutic agents and therapy approaches (Lidsky et al. 2014). Using temozolomide as an alternative to melphalan was explored in a phase I study in patients who had previously failed ILI with melphalan. Patients treated with the maximum tolerated temozolomide dose experienced low regional toxicity, but without an increase in OR (Beasley et al. 2015).
Another interesting strategy is the use of systemic modulators to augment the cytotoxic effects of regional chemotherapy administered by ILI. In a prospective multicenter phase II trial, 45 patients received 2 doses of systemic ADH-1 (N-cadherin antagonist) in combination with a standard melphalan ILI. CR was seen in 38% of the patients and an OR in 60% without increased toxicity, compared with an OR of 40% with melphalan only previously achieved at the same institution (Beasley et al. 2011a). Following promising results of systemic use of the multi-tyrosine kinase inhibitor sorafenib in combination with dacarbazine, another study used systemic sorafenib in combination with ILI. Results, however, were disappointing: in 20 patients the addition of sorafenib did not augment the response to ILI, and an increase in limb toxicity was observed (McDermott et al. 2008; Beasley et al. 2011b).
Over the last few years, immune checkpoint inhibitors have improved the prognosis for patients with advanced melanoma, making ILI plus checkpoint inhibition a novel strategy for patients with limb melanoma (Callahan et al. 2018; Howie et al. 2015). A phase II trial by Ariyan et al. explored the use of ILI followed by the CTLA-4 blocking antibody, ipilimumab (Ariyan et al. 2018). The concept is that ILI can generate immune cell infiltration and increase the efficacy of CTLA-4 blockade. In 26 patients a CR was seen in 62% and a PR in 23% with a 58% progression-free survival at 1 year. Although these results are promising, 38% of the patients experienced significant ipilimumab systemic side effects, similar to the 45% reported in large trials (Weber et al. 2017).
Future of Isolated Limb Infusion
The approval of multiple new, effective systemic therapies for melanoma has dramatically changed treatment strategies for patients with metastatic melanoma. To understand the role of ILI in this new era, a brief review of these new therapies is necessary.
Because of the side effects of CTLA-4 inhibitors as mentioned in the previous paragraph, potentially safer strategies may involve ILI in combination with PD-1 blockade or with more specific tyrosine kinase inhibition, such as BRAF+MEK inhibitors that have been proven effective clinically (McArthur et al. 2014; Chapman et al. 2011; Hauschild et al. 2012). Examples include mutation-based targeted therapies such as vemurafenib, a BRAF inhibitor, in combination with trametinib, a MEK inhibitor (Long et al. 2017). For the 50% of melanoma patients with a BRAF mutation, impressive initial responses occur in the majority, but resistance usually develops within 6–9 months (Grob et al. 2015). Immune checkpoint inhibitors represent another category of recently developed systemic therapies that appear to have greater long-term efficacy, as they do not appear to be as susceptible to the development of resistance as mutation-based therapy. Also, they are not restricted to patients harboring specific mutations. Therefore, PD-1 based therapy has now become the cornerstone of systemic therapy for patients with advanced melanoma, yet the development of both primary and late resistance remains a problem (Franklin et al. 2017). Dual checkpoint inhibitor therapy can result in higher response rates, but toxicity can be considerable and is a concern (Callahan et al. 2018).
Another strategy particularly for cutaneous melanoma recurrences are injectable therapies including oncolytic immunotherapy. Talimogene laherparepvec (T-VEC) was found to be effective in a phase III randomized trial showing durable response rates of 16.3% in the T-VEC arm compared to 2.1% in the GM-CSF arm (Andtbacka et al. 2015). Also, intralesional injection of cutaneous and subcutaneous melanoma deposits with PV-10 resulted in a CR of 26% and an OR of 51% (Thompson et al. 2015). Additionally, many other novel injectable agents including other oncolytic viruses and immunocytokines are currently being developed (Brown et al. 2017).
Despite these important advances in the treatment of metastatic melanoma, the durable response rates achieved are suboptimal, and novel combination therapies with higher response rates may come with unacceptable toxicity. To date, ILI for metastatic limb melanoma is still an effective option, with only low regional toxicity (Grünhagen et al. 2015). Series from Australian and US centers in nearly 800 patients in total report consistent CR rates following ILI of 35%, with a median durability of 12 months (Kroon et al. 2014a, 2016; Beasley et al. 2009; O’Donoghue et al. 2017). Thus, while much in terms of durability remains unknown for novel therapies, ILI remains an important option for patients with unresectable melanoma confined to the limb. However, if in the future systemic therapies do achieve more durable results with less toxicity, ILI may become important as second-line therapy after failure of systemic therapy, with the advantages of a single, minimally invasive treatment associated with low toxicity and minimal systemic side effects. Additionally, as discussed above, combination strategies of ILI in addition to systemic therapies may increase the efficacy of both procedures when administered individually. Finally, for patients with major comorbidities or elderly patients who are thought to be unfit for systemic treatment or HILP, ILI will remain an effective treatment option.
Conclusions
Therapeutic options for patients with melanoma metastases confined to a limb continue to evolve, in parallel with systemic therapies, now being used also as adjuvant and neoadjuvant treatments. Since its first introduction by Thompson et al. over 25 years ago as a minimally invasive alternative to HILP, ILI has been widely applied in patients with in-transit melanoma confined to a limb. In this chapter, we have discussed indications, patient selection, technique, toxicity, and results following ILI. The technique has been used to study the role of hypoxia and hyperthermia in cancer therapeutics and as a model to explore novel combination strategies. In recent years, many effective therapies for patients with metastatic melanoma have been developed and have dramatically increased treatment options. While ILI remains an important and effective option for patients with unresectable limb melanoma, selection is more than ever of utmost importance to select those patients who will benefit most from the procedure, either as an initial therapeutic option, as a second-line treatment, or in combination with systemic therapies.
Cross-References
-
Axillary and Epitrochlear Lymph Node Dissection for Melanoma
-
Evolving Role of Chemotherapy-Based Treatment of Metastatic Melanoma
-
Inguinofemoral, Iliac/Obturator, and Popliteal Lymphadenectomy for Melanoma
-
Neoadjuvant Systemic Therapy for High-Risk Melanoma Patients
-
Novel Immunotherapies and Novel Combinations of Immunotherapy for Metastatic Melanoma
-
Sequencing and Combinations of Molecularly Targeted and Immunotherapy for BRAF-Mutant Melanoma
References
Aloia TA, Grubbs E, Onaitis M et al (2005) Predictors of outcome after hyperthermic isolated limb perfusion: role of tumor response. Arch Surg 140:1115–1120
Andtbacka RH, Kaufman HL, Collichio F et al (2015) Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol 33:2780–2788
Ariyan CE, Brady MS, Siegelbaum RH et al (2018) Robust antitumor responses result from local chemotherapy and CTLA-4 blockade. Cancer Immunol Res 6: 189–200
Beasley GM, Petersen RP, Yoo J et al (2008) Isolated limb infusion for in-transit malignant melanoma of the extremity: a well-tolerated but less effective alternative to hyperthermic isolated limb perfusion. Ann Surg Oncol 15:2195–2205
Beasley GM, Caudle A, Petersen RP et al (2009) A multi-institutional experience of isolated limb infusion: defining response and toxicity in the US. J Am Coll Surg 208:706–715
Beasley GM, Riboh JC, Augustine CK et al (2011a) Prospective multicenter phase II trial of systemic ADH-1 in combination with melphalan via isolated limb infusion in patients with advanced extremity melanoma. J Clin Oncol 29:1210–1215
Beasley GM, Coleman AP, Raymond A et al (2011b) A phase I multi-institutional study of systemic sorafenib in conjunction with regional melphalan for in-transit melanoma of the extremity. Ann Surg Oncol 19: 3896–3905
Beasley GM, Sharma K, Wong J et al (2012) A multi-institution experience comparing the clinical and physiologic differences between upper extremity and lower extremity melphalan-based isolated limb infusion. Cancer 118:6136–6143
Beasley GM, Speicher P, Augustine CK et al (2015) A multicenter phase I dose escalation trial to evaluate safety and tolerability of intra-arterial temozolomide for patients with advanced extremity melanoma using normothermic isolated limb infusion. Ann Surg Oncol 22:287–294
Bland KI, Kimura AK, Brenner DE et al (1989) A phase II study of the efficacy of diamminedichloroplatinum (cisplatin) for the control of locally recurrent and in-transit malignant melanoma of the extremities using tourniquet outflow-occlusion techniques. Ann Surg 209:73–80
Bonenkamp JJ, Thompson JF, de Wilt JH et al (2004) Isolated limb infusion with fotemustine after dacarbazine chemosensitisation for inoperable loco-regional melanoma recurrence. Eur J Surg Oncol 30:1107–1112
Brady MS, Brown K, Patel A et al (2006) A phase II trial of isolated limb infusion with melphalan and dactinomycin for regional melanoma and soft tissue sarcoma of the extremity. Ann Surg Oncol 13:1123–1129
Brady MS, Brown K, Patel A et al (2009) Isolated limb infusion with melphalan and dactinomycin for regional melanoma and soft-tissue sarcoma of the extremity: final report of a phase II clinical trial. Melanoma Res 19:106–111
Brown MC, Holl EK, Boczkowski D et al (2017) Cancer immunotherapy with recombinant poliovirus induces IFN-dominant activation of dendritic cells and tumor antigen-specific CTLs. Sci Transl Med 9(408). http://stm.sciencemag.org/content/9/408/eaan4220.short
Callahan MK, Kluger H, Postow MA et al (2018) Nivolumab plus ipilimumab in patients with advanced melanoma: updated survival, response, and safety data in a phase I dose-escalation study. J Clin Oncol 36:391–398
Cassumbhoy R, Pitman AG (2007) Isolated limb infusion for local control of lower limb melanoma: radiologic aspects. Australas Radiol 51:543–549
Cavaliere R, Ciocatto EC, Giovanella BC et al (1967) Selective heat sensitivity of cancer cells. Biochemical and clinical studies. Cancer 20:1351–1381
Chai CY, Deneve JL, Beasley GM et al (2012) A multi-institutional experience of repeat regional chemotherapy for recurrent melanoma of extremities. Ann Surg Oncol 19:1637–1643
Chang SY, Alberts DS, Farquhar D et al (1978) Hydrolysis and protein binding of melphalan. J Pharm Sci 67: 682–684
Chapman PB, Hauschild A, Robert C et al (2011) Improved survival with vemurafenib in melanoma with BRAF V600E mutation. N Engl J Med 364: 2507–2516
Cheng TY, Grubbs E, Abdul-Wahab O et al (2003) Marked variability of melphalan plasma drug levels during regional hyperthermic isolated limb perfusion. Am J Surg 186:460–467
Clark J, Grabs AJ, Parsons PG et al (1994) Melphalan uptake, hyperthermic synergism and drug resistance in a human cell culture model for the isolated limb perfusion of melanoma. Melanoma Res 4:365–370
Common Terminology Criteria for Adverse Events v3.0 (CTCAE) (2006) https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf. Accessed 22 Aug 2018
Cornett WR, McCall LM, Petersen RP et al (2006) Randomized multicenter trial of hyperthermic isolated limb perfusion with melphalan alone compared with melphalan plus tumor necrosis factor: American College of Surgeons Oncology Group Trial Z0020. J Clin Oncol 24:4196–4201
Coventry BJ, Kroon HM, Giles MH et al (2014) Australian multi-center experience outside of the Sydney Melanoma Unit of isolated limb infusion chemotherapy for melanoma. J Surg Oncol 109:780–785
Damian DL, Barnetson RS, Rose BR et al (2001) Treatment of refractory hand warts by isolated limb infusion with melphalan and actinomycin D. Australas J Dermatol 42:106–109
Damian DL, Barnetson RS, Thompson JF (2006) Treatment of refractory chromomycosis by isolated limb infusion with melphalan and actinomycin D. J Cutan Med Surg 10:48–51
Defty CL, Marsden JR (2012) Melphalan in regional chemotherapy for locally recurrent metastatic melanoma. Curr Top Med Chem 12:53–60
Dossett LA, Ben-Shabat I, Olofsson Bagge R et al (2016) Clinical response and regional toxicity following isolated limb infusion compared with isolated limb perfusion for in-transit melanoma. Ann Surg Oncol 23:2330–2335
Duprat Neto JP, Mauro AC, Molina AS et al (2014) Isolated limb infusion with hyperthermia and chemotherapy for advanced limb malignancy: factors influencing toxicity. ANZ J Surg 84:677–682
Eisenhauer E, Therasse P, Bogaerts J et al (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45: 228–247
Elhassadi E, Egan E, O’Sullivan G et al (2006) Isolated limb infusion with cytotoxic agent for treatment of localized refractory cutaneous T-cell lymphoma. Clin Lab Haematol 28:279–281
Fletcher WS, Pommier R, Small K (1994) Results of cisplatin hyperthermic isolation perfusion for stage IIIA and IIIAB extremity melanoma. Melanoma Res 4(Suppl 1):17–19
Franklin C, Livingstone E, Roesch A et al (2017) Immunotherapy in melanoma: recent advances and future directions. Eur J Surg Oncol 43:604–661
Gershenwald JE, Scolyer RA, Hess KR et al (2017) Melanoma staging: evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin 67:472–492
Grob JJ, Amonkar MM, Karaszewska B et al (2015) Comparison of dabrafenib and trametinib combination therapy with vemurafenib monotherapy on health-related quality of life in patients with unresectable or metastatic cutaneous BRAF Val600-mutation-positive melanoma (COMBI-v): results of a phase 3, open-label, randomised trial. Lancet Oncol 16:1389–1398
Grünhagen DJ, Kroon HM, Verhoef C (2015) Perfusion and infusion for melanoma in-transit metastases in the era of effective systemic therapy. Am Soc Clin Oncol Educ Book 35:e528–e534
Hansson J, Lewensohn R, Ringborg U et al (1987) Formation and removal of DNA cross-links induced by melphalan and nitrogen mustard in relation to drug-induced cytotoxicity in human melanoma cells. Cancer Res 47:2631–2637
Hauschild A, Grob JJ, Demidov LV et al (2012) Dabrafenib in BRAF-mutated metastatic melanoma: a multicentre, open-label, phase 3 randomised controlled trial. Lancet 380:358–365
Howie LJ, Tyler DS, Salama AK (2015) Neoadjuvant use of ipilimumab in locally advanced melanoma. J Surg Oncol 112:841–843
Huismans AM, Kroon HM, Kam PC et al (2011) Does increased experience with isolated limb infusion for advanced limb melanoma influence outcome? A comparison of two treatment periods at a single institution. Ann Surg Oncol 18:1877–1883
Huismans AM, Kroon HM, Kam PC et al (2016) Isolated limb infusion. In: Aigner KR, Stephens FO (eds) Regional therapy of malignant tumors, 2nd edn. Springer, Heidelberg/Berlin, pp 375–390
Jiang BS, Speicher PJ, Thomas S et al (2015) Quality of life after isolated limb infusion for in-transit melanoma of the extremity. Ann Surg Oncol 22:1694–1700
Karakousis CP, Kanter PM, Lopez R et al (1979) Modes of regional chemotherapy. J Surg Res 26:134–141
Karakousis CP, Kanter DM, Park PC et al (1982) Tourniquet infusion versus hyperthermic perfusion. Cancer 49:850–858
Katsarelias D, Rådbo E, Ben-Shabat I et al (2018) The effect of temperature and perfusion time on response, toxicity, and survival in patients with in-transit melanoma metastases treated with isolated limb perfusion. Ann Surg Oncol. https://doi.org/10.1245/s1043401864599
Klaase JM, Kroon BBR, Beijnen JH et al (1994) Melphalan tissue concentrations in patients treated with regional isolated perfusion for melanoma of the lower limb. Br J Cancer 70:151–153
Koops HS, Lejeune KJ, Kroon BBR et al (2004) Isolated limb perfusion for melanoma: technical aspects. In: Thompson JF, Morton DL, Kroon BBR (eds) Textbook of melanoma, 1st edn. Martin Dunitz, London, pp 404–409
Kroon BB (1988) Regional isolation perfusion in melanoma of the limbs; accomplishments, unsolved problems, future. Eur J Surg Oncol 14:101–110
Kroon HM, Moncrieff M, Kam PC et al (2008) Outcomes following isolated limb infusion for melanoma. A 14-year experience. Ann Surg Oncol 15:3003–3013
Kroon HM, Lin DY, Kam PC et al (2009a) Efficacy of repeat isolated limb infusion with melphalan and actinomycin D for recurrent melanoma. Cancer 115: 1932–1940
Kroon HM, Lin DY, Kam PC et al (2009b) Isolated limb infusion as palliative treatment for advanced limb disease in patients with AJCC stage IV melanoma. Ann Surg Oncol 16:1193–1201
Kroon HM, Moncrieff M, Kam PC et al (2009c) Factors predictive of acute regional toxicity after isolated limb infusion with melphalan and actinomycin D in melanoma patients. Ann Surg Oncol 16:1184–1192
Kroon HM, Huismans AM, Kam PC et al (2014a) Isolated limb infusion with melphalan and actinomycin D for melanoma: a systematic review. J Surg Oncol 109:348–351
Kroon HM, Huismans A, Waugh RC et al (2014b) Isolated limb infusion: technical aspects. J Surg Oncol 109:352–356
Kroon HM, Coventry BJ, Giles MH et al (2016) Australian multicentre study of isolated limb infusion for melanoma. Ann Surg Oncol 23:1096–1103
Kroon HM, Coventry BJ, Giles MH et al (2017) Safety and efficacy of isolated limb infusion chemotherapy for advanced locoregional melanoma in elderly patients: an Australian multicentre study. Ann Surg Oncol 24:3245–3251
Lidsky ME, Speicher PJ, Jiang B et al (2014) Isolated limb infusion as a model to test new agents to treat metastatic melanoma. J Surg Oncol 109:357–365
Lindner P, Doubrovsky A, Kam PC et al (2002) Prognostic factors after isolated limb infusion with cytotoxic agents for melanoma. Ann Surg Oncol 9:127–136
Lindner P, Thompson JF, De Wilt JH et al (2004) Double isolated limb infusion with cytotoxic agents for recurrent and metastatic limb melanoma. Eur J Surg Oncol 30:433–439
Long GV, Hauschild A, Santinami M et al (2017) Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated melanoma. N Engl J Med 377:1813–1823
Marsden J, Samarasinghe V, Duddy M et al (2008) Regional chemotherapy for inoperable limb cancer using isolated limb infusion. Br J Dermatol 159:10
McArthur GA, Chapman PB, Robert C et al (2014) Safety and efficacy of vemurafenib in BRAF(V600E) and BRAF(V600K) mutation-positive melanoma (BRIM-3): extended follow-up of a phase 3, randomised, open-label study. Lancet Oncol 15:323–332
McDermott DF, Sosman JA, Gonzalez R et al (2008) Double-blind randomized phase II study of the combination of sorafenib and dacarbazine in patients with advanced melanoma: a report from the 11715 Study Group. J Clin Oncol 26:2178–2185
McMahon N, Cheng TY, Beasley GM et al (2009) Optimizing melphalan pharmacokinetics in regional melanoma therapy: does correcting for ideal body weight alter regional response or toxicity? Ann Surg Oncol 16:953–961
Mian R, Henderson MA, Speakman D et al (2001) Isolated limb infusion for melanoma: a simple alternative to isolated limb perfusion. Can J Surg 44:189–192
Moreno-Ramirez D, de la Cruz-Merino L, Ferrandiz L et al (2010) Isolated limb perfusion for malignant melanoma: systematic review on effectiveness and safety. Oncologist 15:416–427
Muilenburg DJ, Beasley GM, Thompson ZJ et al (2015) Burden of disease predicts response to isolated limb infusion with melphalan and actinomycin D in melanoma. Ann Surg Oncol 22:482–488
Mullinax JE, Kroon HM, Thompson JF et al (2017) Isolated limb infusion as a limb salvage strategy for locally advanced extremity sarcoma. J Am Coll Surg 224:635–642
Nieweg OE, Kroon BB (2014) Isolated limb perfusion with melphalan for melanoma. J Surg Oncol 109:332–337
Noorda EM, Vrouenraets BC, Nieweg OE et al (2006) Repeat isolated limb perfusion with TNFalpha and melphalan for recurrent limb melanoma after failure of previous perfusion. Eur J Surg Oncol 32:318–324
O’Donoghue C, Perez MC, Mullinax JE et al (2017) Isolated limb infusion: a single-center experience with over 200 infusions. Ann Surg Oncol 24:3842–3849
Parsons PG, Carter FB, Morrison L et al (1981) Mechanism of melphalan resistance developed in vitro in human melanoma cells. Cancer Res 41:1525–1534
Raigani S, Cohen S, Boland GM (2017) The role of surgery for melanoma in an era of effective systemic therapy. Curr Oncol Rep 19:17
Raymond AK, Beasley GM, Broadwater G et al (2011) Current trends in regional therapy for melanoma: lessons learned from 225 regional chemotherapy treatments between 1995 and 2010 at a single institution. J Am Coll Surg 213:306–316
Roberts MS, Wu ZY, Siebert GA et al (2001a) Saturable dose-response relationships for melphalan in melanoma treatment by isolated limb infusion in the nude rat. Melanoma Res 11:611–618
Roberts MS, Wu ZY, Siebert GA et al (2001b) Pharmacokinetics and pharmacodynamics of melphalan in isolated limb infusion for recurrent localized limb malignancy. Melanoma Res 11:423–431
Sanki A, Kam PC, Thompson JF (2007) Long-term results of hyperthermic, isolated limb perfusion for melanoma: a reflection of tumor biology. Ann Surg 245:591–596
Santillan AA, Delman KA, Beasley GM et al (2009) Predictive factors of regional toxicity and serum creatine phosphokinase levels after isolated limb infusion for melanoma: a multi-institutional analysis. Ann Surg Oncol 16:2570–2578
Scott RN, Blackie R, Kerr DJ et al (1990) Melphalan in isolated limb perfusion for malignant melanoma, bolus or divided dose, tissue levels, the pH effect. In: Jakesz R, Rainer H (eds) Progress in regional cancer therapy. Springer, Berlin/Heidelberg, pp 195–200
Siemann DW, Chapman M, Beikirch A (1991) Effects of oxygenation and pH on tumor cell response to alkylating chemotherapy. Int J Radiat Oncol Biol Phys 20:287–289
Skarsgard LD, Skwarchuk MW, Vinczan A et al (1995) The cytotoxicity of melphalan and its relationship to pH, hypoxia and drug uptake. Anticancer Res 15: 219–223
Stehlin JS Jr (1969) Hyperthermic perfusion with chemotherapy for cancers of the extremities. Surg Gynecol Obstet 129:305–308
Thompson JF, Waugh RC, Saw RPM et al (1994a) Isolated limb infusion with melphalan for recurrent limb melanoma: a simple alternative to isolated limb perfusion. Reg Cancer Treat 7:188–192
Thompson JF, Lai DT, Ingvar C et al (1994b) Maximizing efficacy and minimizing toxicity in isolated limb perfusion for melanoma. Melanoma Res 4(Suppl 1):45–50
Thompson JF, Siebert GA, Anissimov YG et al (2001) Microdialysis and response during regional chemotherapy by isolated limb infusion of melphalan for limb malignancies. Br J Cancer 85:157–165
Thompson JF, Agarwala SS, Smithers BM et al (2015) Phase 2 study of intralesional PV-10 in refractory metastatic melanoma. Ann Surg Oncol 22:2135–2142
Turaga KK, Beasley GM, Kane JM 3rd et al (2011) Limb preservation with isolated limb infusion for locally advanced nonmelanoma cutaneous and soft-tissue malignant neoplasms. Arch Surg 146:870–875
Ueno T, Ko SH, Grubbs E et al (2004) Temozolomide is a novel regional infusion agent for the treatment of advanced extremity melanoma. Am J Surg 188: 532–537
Van der Merwe SA, Van den Berg AP, Kroon BB et al (1993) Modification of human tumour and normal tissue pH during hyperthermic and normothermic antiblastic regional isolation perfusion for malignant melanoma: a pilot study. Int J Hyperthermia 9:205–217
Vrouenraets BC, Klaase JM, Nieweg OE et al (1998) Toxicity and morbidity of isolated limb perfusion. Semin Surg Oncol 14:224–231
Weber J, Mandala M, Del Vecchio M et al (2017) Adjuvant nivolumab versus ipilimumab in resected stage III or IV melanoma. N Engl J Med 377:1824–1835
Wieberdink J, Benckhuysen C, Braat RP et al (1982) Dosimetry in isolation perfusion of the limbs by assessment of perfused tissue volume and grading of toxic tissue reactions. Eur J Cancer Clin Oncol 18:905–910
Wong J, Chen YA, Fisher KJ et al (2013) Isolated limb infusion in a series of over 100 infusions: a single-center experience. Ann Surg Oncol 20:1121–1127
Wong J, Chen YA, Fisher KJ et al (2014) Resection of residual disease after isolated limb infusion (ILI) is equivalent to a complete response after ILI-alone in advanced extremity melanoma. Ann Surg Oncol 21:650–655
World Health Organization (1979) WHO handbook for reporting results of cancer treatments. WHO offset publication no. 48. World Health Organization, Geneva
Wu ZY, Thompson MJ, Roberts MS et al (1995) High-performance liquid chromatographic assay for the measurement of melphalan and its hydrolysis products in perfusate and plasma and melphalan in tissues from human and rat isolated limb perfusions. J Chromatogr B Biomed Appl 673:267–279
Wu ZY, Smithers BM, Anderson C et al (2000) Can tissue drug concentrations be monitored by microdialysis during or after isolated limb perfusion for melanoma treatment? Melanoma Res 10:47–54
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this entry
Cite this entry
Beasley, G.M., Miura, J.T., Zager, J.S., Tyler, D.S., Thompson, J.F., Kroon, H.M. (2020). Isolated Limb Infusion for Melanoma. In: Balch, C., et al. Cutaneous Melanoma. Springer, Cham. https://doi.org/10.1007/978-3-030-05070-2_27
Download citation
DOI: https://doi.org/10.1007/978-3-030-05070-2_27
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-05068-9
Online ISBN: 978-3-030-05070-2
eBook Packages: MedicineReference Module Medicine