Abstract
Epigenetic modifications, including DNA methylation, covalent histone modifications, and small noncoding RNAs, play a key role in regulating the gene expression. This regulatory mechanism is important in cellular differentiation and development. Recent advances in the field of epigenetics extended the role of epigenetic mechanisms in controlling key biological processes such as genome imprinting and X-chromosome inactivation. Aberrant epigenetic modifications are associated with the development of many diseases. The role of epigenetic modifications in various neurodegenerative disorders including Alzheimer’s disease, Parkinson’s disease, Huntington disease, epilepsy, and multiple sclerosis is rapidly emerging. The use of epigenetic modifying drugs to treat these diseases has been the interest in recent years. A number of natural products having diverse mechanism of action are used for drug discovery. For many years, natural compounds have been used to treat various neurodegenerative diseases, but the use of such compounds as epigenetic modulators to reverse or treat neurological diseases are not well studied. In this chapter, we mainly focus on how various epigenetic modifications play a key role in neurodegenerative diseases, their mechanism of action, and how it acts as a potential therapeutic target for epigenetic drugs to treat these diseases will be discussed.
An erratum to this chapter can be found at http://dx.doi.org/10.1007/978-3-319-28383-8_24
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- DNA methylation
- Histone deacetylases (HDACs)
- Neurological diseases
- Epigenetic modulators
- Dietary products
Introduction
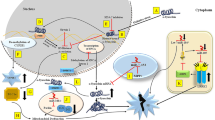
The eukaryotic genome is organized and packed into chromatin, which is a complex structure composed of DNA, histone, and nonhistone proteins. Chromatin remodeling is a dynamic process that modulates gene expression. Chromatin exists either in a condensed, inactive, transcriptionally repressive state called heterochromatin or transcriptionally active state called euchromatin. The term epigenetics is defined as heritable change in gene expression without altering the DNA sequence. Epigenetic modifications regulating gene expression are reversible and have long lasting effects. These epigenetic modifications control the gene expression during cellular development through DNA methylation, histone code modifications, and small noncoding RNAs (Costa 2008; Kouzarides 2007). All the three mechanisms regulate gene expression without altering the DNA sequence. There is a complex interplay between these three processes to regulate the gene expression (Fig. 1). These epigenetic modifications are involved in a number of essential cellular processes such as transcription, cellular differentiation, development, X-chromosome inactivation, gene imprinting, and cellular responses to environmental stimuli (Guibert and Weber 2013; Klose and Bird 2006; Smith and Meissner 2013; Subramaniam et al. 2014). Aberrant epigenetic modifications have been extensively reported in cancer. In recent years, the upcoming interest is developing on studying the role of epigenetic modifications in a number of neuropsychiatric and neurodegenerative diseases including schizophrenia (Grayson et al. 2005; Veldic et al. 2004, 2005) epilepsy, Alzheimer’s disease (AD), Huntington’s, and Parkinson’s diseases (PD). Abnormal epigenetic mechanisms also have been reported in a number of mental disorders such as Rett, ICF, Fragile-X, and ATRX syndrome (Egger et al. 2004).
DNA Methylation
DNA methylation is one of the most classically studied epigenetic modifications and involves covalent modification of cytosine residue in the CpG back ground by the addition of a methyl group at the fifth carbon position on its pyrimidine ring. The CpG dinucleotide rich regions are called CpG islands and are found in the promoter regions of the gene. The Promoter CpG island methylation plays an important role in regulating the gene expression by preventing transcription factors binding on to the promoter and thereby recruiting transcriptional repressors on to the promoter regions (Cedar and Bergman 2012). DNA methylation is also involved in silencing of imprinted genes in which only one allele, either paternal or maternal, is expressed (Reik et al. 2001; Edwards and Ferguson-Smith 2007). It is also involved in X chromosome inactivation in women (Jaenisch and Bird 2003). DNA methylation is also associated with maintenance of chromosomal stability and translocation prevention (Bird 2002). In human genome, most of the methylated CpGs occur in repetitive sequences such as long interspersed transposable element 1 (LINE1) and Alu repeats (Edwards and Myers 2008).
DNA Methyl Transferases
DNA methyl transferases catalyze the addition of a methyl group onto the cytosine nucleotide by utilizing S-adenosyl methionine (SAM) as the methyl donor. There are about five DNA methyl transferases reported that play a crucial role in establishing and maintaining DNA methylation. They are DNMT1, DNMT3A, DNMT3B, DNMT3L, and DNMT2. They share common structural similarities of having an N terminal regulatory and C terminal catalytic domain. Of all the five members of the DNMT family, DNMT1, 3A, and 3B are required to establish and maintain the genome methylation (Fig. 2).
A schematic representation of DNA methyl transferases (DNMTs). The five member family of DNMTs includes DNMT1, DNMT2, DNMT3A, 3B, and 3L. They all share conserved methyl transferase motifs indicated by roman numerals in the catalytic domain at C terminus. The regulatory N terminal domain is represented with NLS (nuclear localization signal), RFT (replication foci targeting domain), BAH (bromo-adjacent homology domain), PWWP (proline–tryptophan–tryptophan–proline) motif, and PHD (plant homeodomain). DNMT3L lacks methyl transferase motifs and is catalytically in active. DNMT2 lacks N terminal regulatory domain
DNMT1 is most abundant in mammalian cells and first murine DNMT cloned (Bestor 1988). DNMT1 plays an important role in methylating newly synthesized DNA during replication. It has a preference toward hemimethylated DNA and is responsible for copying preexisting methylation patterns to the newly synthesized DNA strand. It is also called maintenance methyl transferase. It is a large protein with around 1620 amino acids in length. It has an N terminal regulatory domain and C terminal catalytic domain. DNMT1 plays an essential role in development. DNMT1 knockout mice results in early embryonic lethality (Li et al. 1992).
DNMT 3A and 3B are other groups of DNMTs that effectively methylate unmethylated DNA de novo. They are considered as de novo DNA methyl transferases. They are encoded by two different genes and have structural homology with N terminal regulatory domain and C terminal catalytic domain. They play an important role in germ cell development and embryogenesis. In addition to redundancy in de novo methylation, these enzymes have different functional roles. DNMT3A is ubiquitously expressed and DNMT3B is expressed at low levels except in testis, thyroid, and bone marrow (Xie et al. 1999). DNMT3B expression is increased in tumor cell lines and focused on methylating CpGs in repetitive sequences of pericentric regions of the chromosome (Hansen et al. 1999; Xu et al. 1999).
DNMT3L is another member of DNA methyl transferase that lacks the methyl transferase activity. It may cooperate with other de novo DNMTs and thereby increase the activity of that enzyme. It plays an important role in genomic imprinting since targeted disruption of DNMT3L resulted in biallelic expression of genes imprinted and expressed from one parental origin (Bourc’his et al. 2001).
DNMT2 was cloned based on its sequence homology with other DNMTs. It is the most conserved and its targeted disruption in ES cells did not detect any effect on global methylation suggesting it is not an essential for DNA methylation (Okano et al. 1998).
DNA Demethylation
DNA methylation has long thought to be a permanent epigenetic mark and is irreversible. However, it is a dynamic process during early mammalian development and alteration of methylation is also important for normal development (Shemer and Razin 1996). There is considerable evidence supporting genome-wide active demethylation found in zygotes (Mayer et al. 2000; Oswald et al. 2000), primordial germ cells (Morgan et al. 2005; Hajkova et al. 2002), and locus-specific active demethylation observed in somatic cells such as neurons (Ma et al. 2009) and T lymphocytes (Bruniquel and Schwartz 2003). However, the mechanism of active demethylation is not clearly understood. Recent studies showed 5-hydroxymethylcytosine (5hmC) is likely to have an important implication in mammalian genome for active demethylation. The substantial amount of 5hmC has been detected in mouse purkinje neurons (Kriaucionis and Heintz 2009) and in ES cells (Tahiliani et al. 2009) In humans, TET family of proteins, TET1, TET2, and TET3, have been identified to catalyze the conversion of 5mC to 5hmC (Tahiliani et al. 2009).
Histone Code Modifications
In the eukaryotic cell nucleus, DNA is packed with histone octomer composed of two copies each of H2A, H2B, H3, and H4. Chromatin remodeling in the brain is characterized by posttranslational modification of histones. The specific amino acid residues such as lysine, arginine, serine, and threonine at the N terminal tail of histones subjected to potential modifications such as acetylation, methylation, phosphorylation, ubiquitination, and sumoylation. These modifications are associated with transcriptional activation or repression depending on the site of residue and the type of modification thereby forms the histone code. Posttranslational modification of histones is a dynamic and reversible process mediated by two different sets of enzyme complexes that add or remove a particular chemical group in a site-specific manner.
Histone Acetylation and Deacetylation
Histone acetylation is associated with positive transcription. Histone acetylation of lysine residue is one of the well-studied histone modifications. Histone H3 and H4 acetylation increases gene expression by promoting open configuration of chromatin. It is mainly catalyzed by histone acetyl transferases (HATs). They catalyze the transfer of acetyl group from acetyl co-A to lysine residues of histones. These acetyl groups neutralize positive histone charge, thereby opening up the chromatin for transcriptional activation. Some of the HATs include GCN5-related N acetyl transferases, MYST HATS, p300/CBP HATS, TATA binding protein-associated factor II (TAF II), RE1 silencing transcription factor (REST), nuclear factor kappa B (NFkB), etc. Acetylation is a transient mark and is vital for precise temporal transcription control. There are a number of acetylation sites on histone residues dynamically regulated by HATs and Histone deacetylases (HDACs). The most important acetylation sites of histone H3 are H3K9, K14, K18, and K56. The histone H4 acetylation sites are H4K5, K8, K12, and K16. Another histone H2B lysine residues are also acetylated at K7, K16, and K17. All these histone code modifications play a role in transcriptional activation (Strahl and Allis 2000). Histone deacetylation involves removal of acetyl groups of lysine residues in the conserved tails of core histone proteins, thereby altering the negative to the positive charge. This results in the tight interaction of histones with negatively charged DNA, thereby facilitating the closed chromatin structure. It is associated with transcriptional repression catalyzed by HDACs. There are two major family proteins with HDAC activity. Sir2 (silent information regulator-2) or sirtuin (sir2-like protein) family of NAD-dependent HDACs (Class III HDACs) and classical HDAC family protein (De Ruijter et al. 2003; Yang and Seto 2008). The classical HDAC family proteins comprise three different classes such as class I, II, and IV. The class I HDACs includes HDAC1, 2, 3, and 8 which are smaller proteins. The class II HDACs includes HDAC 4, 5, 6, 7, 9, and 10 which are larger proteins (Bjerling et al. 2002; Fischle et al. 2002). The class IV HDAC member includes HDAC11 which has sequence similarity to class I and II HDACs (Gregoretti et al. 2004).
Histone Methylation and Demethylation
Histone methylation is associated with both transcriptional activation and repression depending on the modified amino acid residues. It occurs mainly on lysine and arginine residues either as mono-, di- and, trimethylation. Methylation of H3K4 and H3K36 are associated with transcriptional activation. H3K9, K27, and H4K20 methylations are associated with transcriptional repression (Barski et al. 2007). Histone methyl transferases (HMTs) which catalyze H3K9, K27, and H4K20 include G9a, GLP (Tachibana et al. 2002, 2005), SUV39H1, EZH2 (Cao et al. 2002), and PR-SET7 (Nishioka et al. 2002). However, SET7/9 mediates H3K4-specific methylation (Wang et al. 2003). These enzymes catalyze the transfer of the methyl group from S-adenosyl-l-methionine to the lysine residues of histones. Like other histone modifications, histone demethylation also plays an important role in the regulation of gene expression. Lysine-specific demethylase1 (LSD1) is the first reported histone demethylase which act on mono- and dimethylations (Shi et al. 2004). Jumanji domain containing protein is another histone demethylase that acts on trimethylated as well as mono- and dimethylated lysine’s (Tsukada et al. 2006; Klose et al. 2006).
The overall epigenetic modification machinery including DNA methylation, demethylation, and various histone code modifications regulate gene expression either positively or negatively constitute the whole epigenome as it is represented in Fig. 3.
Euchromatin- and heterochromatin-associated epigenetic modifications. Transcriptionally active euchromatin is represented in blue at top. DNA wrapped around histones. Unmethyl cytosine residues are represented in open circles on DNA. The hydroxyl methyl cytosine was represented as pentagon structure on the DNA. The N terminal tail of histone3 (H3) is represented with methyl group (filled blue inverted triangle) at K4 and acetyl group (filled blue circles) at K9 and K27. Transcriptionally inactive heterochromatin is represented in red at bottom. DNA wrapped around histones. Methyl cytosine residues are represented in closed circles (filled black circles). The N terminal tail of H3 is represented with methyl group (filled red inverted triangle) at K9 and K27
Epigenetic Dysregulation in Neurological Diseases
DNA methylation has been implicated in regulation of gene activity in the adult brain. It is linked to activation or repression of genes by synaptic activity. Such mechanisms regulate the expression of specific sets of neuronal genes that are important for neural activity, survival, and morphology of neurons. DNA methylation patterns were altered in schizophrenia, Alzheimer’s, Parkinson’s, and other related psychiatric diseases. There is growing evidence that DNA methylation is involved in the pathophysiological mechanism of depression and addiction (Table 1).
Schizophrenia
Schizophrenia is a psychiatric disorder with the positive symptoms such as delusions, hallucinations and disorganized thoughts, social withdrawal, and apathy. There is an evidence that epigenetic mechanisms are involved in pathogenesis of schizophrenia disease. One of the global methylome study identified numerous DNA methylation changes at differentially methylated regions in schizophrenia and bipolar disorder (Xiao et al. 2014). GADD67 and Reelin genes were extensively studied in psychiatric disorders. These genes are downregulated in GABA neurons of the prefrontal cortex of schizophrenia and bipolar disorder patients (Impagnatiello et al. 1998; Guidotti et al. 2000; Fatemi et al. 2000). The downregulation of these gene expressions leads to a decrease in GABAergic transmission, which is an important pathological mechanism that underlies the clinical manifestation of schizophrenia and bipolar disorders (Akbarian et al. 1995; Guidotti et al. 2005; Eastwood and Harrison 2006). Hypermethylation of promoters of GADD67 and Reelin are associated with reduced expression of these genes in GABAergic neurons. There is a characteristic overexpression of DNMT1 in GABAergic neurons responsible for downregulation of GADD67 and Reelin in schizophrenia and bipolar disorder patients (Veldic et al. 2004, 2005; Grayson et al. 2005; Ruzicka et al. 2007). DNMTs utilizes the SAM as a substrate to transfer the methyl group to cysteine there by converting SAM to S-adenosyl l-homocysteine (SAH) which is subsequently hydrolyzed to form homocysteine. The plasma homocysteine levels were also reported to be increased in schizophrenia patients (Applebaum et al. 2004; Levine et al. 2002; Adler Nevo et al. 2006). The accumulation of homocysteine has been shown to cause neural damage and cognitive dysfunctions (Krebs et al. 2009).
Histone code modifications are another epigenetic regulators which plays a role in schizophrenia disease. Increased levels of GAD1 promoter H3K9 and H3R17 di- and trimethylation are associated with its reduced expression in cortical neurons and adjacent nonneuronal cells of post-mortem tissue of schizophrenia patients and are typically associated with neuronal metabolism (Akbarian 2010). GAD67 a GABA synthesis enzyme expression is downregulated in cerebral and cerebellar cortex of schizophrenia, depression, or autism patients and may be contributing to desynchronization of cortical networks and cognitive dysfunction due to defective GABAergic inhibition. The promoter that regulates GAD67 expression is associated with altered histone code modifications, including loss of H3K4 methylation marks and excess of repressive marks such as H3K27 methylation (Huang et al. 2007). The three HMTs G9a, GLP, and SETDB1 that mediate H3K9 di- and trimethylations are increased across the genome in lymphocytes from schizophrenia patients (Wang et al. 2003; Zee et al. 2010). H3K4 methylation levels are reduced at nearly 600 loci, including near multiple NMDA receptor subunits and genes involved in neurodevelopment. HDAC1 expression is increased in the prefrontal cortex of schizophrenia patients (Sharma et al. 2008).
Alzheimer’s Disease
Alzheimer’s disease (AD) is the age-related most common type of dementia with characteristic features of loss of memory, language, ability to focus, reasoning skills, and visual perception (Blennow et al. 2006). The amyloid precursor protein (APP) which is a membrane protein that is expressed throughout the brain and particularly concentrated in neuronal synapses cleaved to produce β-amyloid plaques is a hallmark of AD. The hyperphosphorylated microtubule-associated protein tau that is expressed in neurons is capable of forming neurofibrillary tangles is another hallmark of AD (Voss and Gamblin 2009). There is a growing evidence suggesting epigenetic mechanisms mediate the risk for AD. Genome-wide hypomethylation has been reported in AD patients (Mastroeni et al. 2011). Global DNA hypomethylation was reported in the entorhinal cortex of AD patients compared to controls (Mastroeni et al. 2010). Studies also reported that folate and SAM levels were significantly reduced in AD (Bottiglieri et al. 1990; Morrison et al. 1996). CpG islands become more methylated with aging, while loci not in CpG islands were hypomethylated (Christensen et al. 2009). Repetitive Alu elements were also hypomethylated with aging, but not the repetitive LINE-1 elements (Bollati et al. 2009). APP and tau genes involved in pathophysiology of AD are affected by epigenetic regulation. In addition to DNA methylation, histone code modification plays an important role in AD. The cleavage of APP generates APP C-terminal peptide (AICD) in addition to Aβ peptide. AICD translocates to the nucleus and acts on specific genes and modify their expression. Over expression of AICD in rat primary cortical neurons associated with increased acetylation of histones H3K14 and H4K5. Fe65 is a binding partner of APP and its interaction with AICD recruits Tip60 to DNA strand breaks. Tip60 an HAT acetylates H4 which is necessary for the correct repair of DNA and this process could be important in AD (Stante et al. 2009). In AD, an accumulation of phospho-H2AX, an indicator of DNA strand breaks, has been described (Myung et al. 2008). HDAC2 deficiency results in increased synapse number and memory facilitation supporting the role of histone acetylation and deacetylation in human diseases associated with memory impairment such as AD (Guan et al. 2009).
Parkinson’s Disease
Parkinson’s disease (PD) is another common neurodegenerative disease characterized by progressive loss of substantia nigra dopamine neurons and striatal projections. The typical symptoms include muscle rigidity, tremor, bradykinesia, and postural instability. Genome-wide DNA methylation studies in the brain and blood samples of PD patients were reported to have differential methylation pattern of several genes associated with PD pathology supporting the role of epigenetic dysregulation in PD (Masliah et al. 2013). The presence of Lewy bodies (structures containing aggregates of α-synuclein encoded by gene SNCA) which accumulate at sites where neuronal loss is found is a hallmark of PD. The epigenetic regulation of SNCA gene plays an important role in the pathogenesis of PD. The increased α-synuclein production is associated with PD may result from increased expression of the SNCA gene as a consequence of hypomethylation of this gene (Ammal Kaidery et al. 2013). It has also been reported that α-synuclein sequesters DNMT1 in cytoplasm, leading to global DNA hypomethylation in PD and dementia with characteristic accumulation of Lewy bodies found in post-mortem brains and in transgenic mouse models (Desplats et al. 2011). Another study reported hypomethylation of TNFα promoter and its overexpression induces dopaminergic neuronal cell death in substantia nigra in PD (Pieper et al. 2008).
α-Synuclein can associate with histones and inhibit their acetylation. It is largely associated with Sirt2, a type of NAD-dependent class III HDAC. The inhibition of Sirt2 using siRNA rescued α-synuclein toxicity (Outeiro et al. 2007). Another epigenetic hallmark associated with PD is dopamine depletion observed in this disease is associated with reduction in H3K4me3. Over all epigenetic regulation might have an important role in the pathogenesis of this disease.
Epilepsy
Epilepsy is another common brain disorder affecting millions of people worldwide. In epilepsy, certain brain regions such as the hippocampus is susceptible to electrical discharge that promote some morphological changes such as cell death in the CA1 and mossy fiber sprouting and dispersion of granule cell layer that are thought to be involved in recurrent excitatory circuits that contribute to seizure susceptibility (Heck et al. 2004). DNA methylation is one of the epigenetic modifications involved in epilepsy (Kobow et al. 2009; Miller-Delaney et al. 2012; Zhu et al. 2012). Kobow et al. 2013 reported global DNA methylation pattern in chronic epileptic rats using methyl seq and showed global hypermethylation of the DNA. They also confirmed hypermethylation of calcium calmodulin-dependent protein kinase with its reduced expression involved in calcium signaling in pilocarpine induced epileptic rat model.
Histone modifications have also been altered in epilepsy induced animal models. In kainic acid induced animal model of epilepsy, transient phosphorylation of histone H3 and sustained acetylation of histone H4 were observed in hippocampal neurons (Sng et al. 2006). Other promoter-specific histone code modifications in epilepsy include hyperacetylation of histone H4 on BDNF promoter which correlate with its increased expression. Histone H3 and H4 are rapidly deacetylated at the promoter of glutamate receptor subtype GluR2 correlated with its reduced expression after seizure development (Huang et al. 2002).
The importance of DNA methylation in association with other neurological disorders such as Rett syndrome and the ICF syndrome has been reported. Rett syndrome is one of the most common mental retardation diseases in females. Mutations of methyl CpG binding protein2 (MeCP2) have been found in 80 % of Rett syndrome patients (Amir et al. 1999). Mutations of DNMT3b have been reported in about 60 % of ICF syndrome patients (Hansen et al. 1999). ICF syndrome patients are characterized with immune defects, chromosomal instability, and neurological defects including mental retardation.
Epigenetic Modulatory Drugs to Treat Neurological Diseases
The most widely studied epigenetic modulatory drugs include DNA methylation inhibitors and HDAC inhibitors. 5-AZA deoxycytidine, zebularine are DNA methylation inhibitors more widely used to treat cancer. These drugs incorporate in to target DNA and bind to DNMT1 during DNA replication to inhibit its activity and therefore require DNA replication to be active (Wu and Santi 1985). However, the use of these drugs for treating brain disorders has limitation since most of the neurons are postmitotic. The mechanism of action of demethylation in postmitotic neurons is not clearly understood. However, the recent study used RG108, a small molecule DNA methylation inhibitor in epilepsy therapeutics suggesting a novel epigenetic modulatory drug to treat neurological diseases (Machnes et al. 2013).
HDAC inhibitors show a promise for cognitive improvement and are being considered for drug development in neurological diseases. Hence, HDAC inhibitors could be used as promising therapeutic agents for diseases associated with dementia and cognitive impairments. The class I HDAC inhibitors such as sodium valproate and sodium butyrate improve memory in AD mouse model (Kilgore et al. 2010). Valproic acid is also used as an anticonvulsant in epileptic patients and as a mood stabilizer in bipolar disorder patients (Phiel et al. 2001). Sodium butyrate and Sirtuin HDAC siRNA inhibitors are effectively used in a Drosophila model of PD (St Laurent et al. 2013; Outeiro et al. 2007). The use of Valproic acid in the treatment of schizophrenia and bipolar disorders has also been reported (Weaver et al. 2006).
Natural Products as Epigenetic Modulators in Neurological Diseases
Most of the human diseases are significantly influenced by diet in a varying degree. Except the inherited one, some are almost purely influenced by dietary components like the vitamin and mineral deficiency. In the last decade, a new wealth of information has emerged to explain how nutrition and diet affect different diseases to varying degrees. Nutrition and diet have little impact on some diseases, but strongly affects others. Considering the role of epigenetics in human diseases; here, we discuss how the diet affects long-term health by altering the epigenome and how one can prevent degenerative conditions by consuming of diets rich in antioxidants and anti-inflammatory components. This part of the chapter mainly focuses to discuss the promising epigenetic effects of dietary factors (phytochemicals) and their effects in neurodegeneration and neuroprotection.
Phytochemicals
Phytochemicals are considered as nonessential complex chemicals found in plants, particularly in fruits and vegetables. Even though, phytochemicals do not fall under the category of essential nutrients for humans, they are contributing greatly to health and well-being. So far, in nature more than 3000 phytochemicals have been discovered and here we have provided a noncomprehensive list of phytochemicals and examples of how they are broadly subclassified (Liu 2004).
-
1.
Terpenoids (Isoprenoids)
-
(a)
Carotenoid terpenoids (lycopene, beta-carotene, alpha-carotene, lutein, zeaxanthin, and astaxanthin)
-
(b)
Noncarotenoid terpenoids (perillyl alcohol, saponins, terpeneol, and terpene limonoids)
-
(a)
-
2.
Polyphenolics
-
(a)
Flavonoid polyphenolics (anthocyanins, catechins, isoflavones and hesperetin, naringin, rutin, quercetin, silymarin, tangeretin, and tannins)
-
(b)
Phenolic acids (ellagic acid, chlorogenic acid, P-coumaric acid (para-coumaric acid), phytic acid, ferulic acid, vanillin, cinnamic acid, and hydroxycinnamic acids)
-
(c)
Nonflavonoid polyphenolics (curcumin, resveratrol, pterostilbene, lignans, and coumestans)
-
(a)
-
3.
Glucosinolates
-
(a)
Isothiocyanates (phenethyl isothiocyanate and sulforaphane)
-
(b)
Indoles [Indole-3-Carbinol (I3C)]
-
(a)
-
4.
Thiosulfonates
-
5.
Phytosterols (beta-sitosterol)
-
6.
Anthraquinones
-
(a)
Senna
-
(b)
Barbaloin
-
(c)
Hypericin
-
(a)
-
7.
Capsaicin
-
8.
Piperine
-
9.
Chlorophyll (chlorophyllin)
-
10.
Betaine
-
11.
Pectin
-
12.
Oxalic acid
In recent years, the role of epigenetic modifications in neurodiseases and degeneration has been well established through systematic studies. Many studies have shown that essential nutritional compounds like vitamin B12 or folic acid play key role in the modulation of epigenetic changes. Increasing scientific evidence suggests that oxidative stress (cellular and metabolic) has crucial implications for the pathogenesis of many neurodegenerative diseases, including PD, AD, and many others via epigenetic changes (Andersen 2004). Therefore, nonnutritional compounds such as polyphenols have attracted the scientific world as epigenetic modulators mainly through its antioxidant properties. In addition to plant based natural products (compounds), certain secondary metabolites derived from marine and terrestrial micro- and macro-organisms are discovered as drugs that have epigenetic targets such as HDACs. Although, many natural compounds have been shown to possess potential epigenetic modulatory effects in diseases like cancer and atherosclerosis, only fewer natural products inhibitors have been documented in modulating epigenetic pathways in brain-related diseases.
Dietary Factors as Modulators of HDAC Activity
Diet derived bioactive components are able to modulate epigenetic events and their epigenetic targets. For example, genistein, diallyl sulfide, vitamin D3, or all-trans retinoic acids have been shown to impact DNA methylation by altering histones and chromatin structure (Bassett and Barnett 2014). Here, we provided a brief introduction of some important bioactive natural compounds and how they target the epigenetic events in disease conditions. Table 2 gives a partial list of dietary compounds that are known to modulate HDAC activity.
Genistein
Genistein is mainly a soy-derived compound classified under the category of isoflavones (Fig. 4). Many anticancer studies have shown that genistein affects tumorigenesis/carcinogenesis through epigenetic regulations (Zhang and Chen 2011). Reports suggest that genistein may be involved in inhibiting the DNMTs and can regulate gene expression by erasing DNA methylation at promoter levels. Genistein has been proved to prevent breast cancer risk and promotes DNA demethylation of SF1 promoter in endometrial stromal cells (Khan et al. 2012), a type of cells that is present in the endometrium (the innermost lining) of the uterus. Recently, it has been shown that genistein, inhibits neuroblastoma (NB) growth and tumor microvessel formation in vivo by decreasing hypermethylation levels of tumor suppressor genes (TSGs) such as CHD5 and enhances the expression of CHD5 as well as p53 (Li et al. 2012). In addition, genistein acts as an inhibitor to significantly decrease the expression of DNMT3b in NB model and thereby suggest that genistein could be used as an adjuvant therapeutic agent for NB treatment.
Also, genistein induces chromatin remodeling and DNA methylation, which leads to the activation of TSGs and thereby suppression of the cancer cell survival. Genistein has also been shown to inhibit the DNMT activity, which causes inhibition of DNA methylation and thus may be acting as an anticancer agent (Li and Tollefsbol 2010). Genistein has been observed to enhance the acetylation of histones H3 and H4 in the transcription sites of p21 and p16, thereby, it upregulates the TSGs in prostate cancer cells (Zhang and Chen 2011). Prenatal exposure to genistein possesses estrogenic activity and affects the erythropoiesis in the fetus and alters the gene expression and DNA methylation in hematopoietic cells (Vanhees et al. 2011). Also, genistein causes modulation of the HAT activity and extents the acetylation of histone (Piaz et al. 2011). In breast and prostate cancer cell lines, genistein has been shown to inhibit the proliferation (Moyad 1999) and to compete with estrogen, it prevents the estrogen receptor mediated cell growth (Wang et al. 1996). Another study shows that genistein is positively associated with modulation of DNA methylation at CpG islands of certain genes in prostate of a mouse. Genistein seems to reduce the hypermethylation status of RARβ, p16, and MGMT genes in vitro and inhibit the activity of DNA methyltransferases dose dependently, showing that genistein can reactivate methylation-silenced genes, via inhibiting DNA methyltransferase (Fang et al. 2005). Supplementation of genistein during the gestation period modulated the site-specific DNA methylation of offspring and changed coat color of heterozygous yellow agouti (Avy/a) pups to black pseudoagouti by reducing the DNA methylation status of the agouti locus (Dolinoy et al. 2006).
Epigallocatechin-3-gallate (EGCG)
Epigallocatechin gallate (EGCG) is a type of catechin (Fig. 5) found highly in green tea and exists in nature as the ester form of epigallocatechin and gallic acid. Trace amounts of (EGCG) are found in plums, onions, hazelnuts, and apple skin. Numerous studies have shown that EGCG has beneficial effects in a broad range of disorders including cancer. Preliminary research shows that EGCG is an inhibitor of various enzymes in epigenetic pathways, such as histone acetyltransferase (Choi et al. 2009) DNA methyltransferase (Choi et al. 2009) or tyrosinase (No et al. 1999).
Decaffeinated green tea and black tea extracts are rich in EGCG and has been shown to inhibit 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone (NNK)-induced DNA methylation in lung cancer model in A/J mice (Shi et al. 1994). In cancer cells, EGCG reactivated methylation-silenced genes by inhibiting DNMTs and suggest the potential use during carcinogenesis (Fang et al. 2003). In addition, a study demonstrated that EGCG and other tea polyphenols (e.g., catechin and epicatechin) and bioflavonoids (quercetin, fisetin and myricetin) can inhibit Dnmt 1-mediated DNA methylation in a concentration-dependent manner (Lee et al. 2005).
Lycopene
Lycopene is a tetraterpene (carotenoid), possess potent antioxidant property (Fig. 6). Lycopene is mainly present in fruits and vegetables, including tomatoes, watermelon, pink grapefruit, pink guava, and papaya. As an antioxidant, lycopene knows to modulate the expression of many genes that are associated with cell cycle, DNA repair, and apoptosis. Also, it has been shown to alter DNA methylation and upregulate the GSTP1 gene in the breast cancer cell line (King-Batoon et al. 2008). It seems that lycopene affects gene expression by modifying gene-specific methylation. It has been reported to demethylate the promoter of RARβ2 and HIN-1 genes (King-Batoon et al. 2008).
Resveratrol
Resveratrol is more famous by the name “French Paradox” by preventing many diseases, including neurological disorders such as AD, PD, and stroke. Studies have shown that the potential beneficial effects of resveratrol (Fig. 7) are not only because of its antioxidant and anti-inflammatory action but also due to activation of sirtuin 1 (SIRT1). It is well known that at least in animal models, caloric restriction has been shown to prevent the development of various cancers through sirtuins as a target. Sirtuins are nicotinamide adenine dinucleotide (NAD(+))-dependent HDACs which are involved in aging and reverted significantly in transformed cells. Resveratrol is known to activate the sirtuin 1 (SIRT1), a class III HDAC (Baur 2010) and thereby preventing aging and cancer cell proliferation.
In cell lines, resveratrol acts as a weak inhibitor of DNMT activity (Paluszczak et al. 2010) and acts synergistically with adenosine analogues to inhibit methylation of retinoic acid receptor beta 2 gene and thereby increases its expression (Stefanska et al. 2010).
Curcumin
Curcumin is the principal active compound of turmeric and known to have an antidisease effect in various animal models and in humans (Fig. 8). Effects of curcumin to induce apoptosis in cancer cell lines are well characterized and recently it has been shown to inhibit certain epigenetic enzymes (such as HATs, HDAC1, HDAC3, and HDAC8) in vitro (Reuter et al. 2011; Vahid et al. 2015). The mode of induction of apoptosis by curcumin may vary from cell to cell. For example, in cervical cancer, curcumin inhibits the acetylation of histone and p53 through specific inhibition of p300/CBP (Balasubramanyam et al. 2004b).
In addition, it also induces histone hypoacetylation, activation of poly (ADP) Ribose polymerase- and caspase-3-mediated apoptosis in brain glioma cells (Kang et al. 2006) were observed. In addition, curcumin decreased histone H3 and H4 acetylation and thereby controls the fate of neural stem cells (Kang et al. 2006).
Anacardic Acid
Anacardic acid falls under the category of phenolic lipids and are highly present in the shell of the cashew nuts (Fig. 9). Anacardic acids seem to arrest the growth of cancer cells by inhibiting acetylation, nuclear translocation of p65 and through modulation of NF-kappaB signaling pathway. (Sung et al. 2008). Anacardic acid can inhibit HATs such as p300, PCAF, and Tip60. Also, anacardic acid has been shown to specifically inhibit HAT (Balasubramanyam et al. 2003; Sun et al. 2006).
Garcinol
Garcinol is a highly cytotoxic polyprenylated benzophenone from the fruit Garcinia indica (Fig. 10). Garcinol is also a potent inhibitor of different HATs, such as p300 and PCAF (Mai et al. 2006; Chandregowda et al. 2009; Balasubramanyam et al. 2004a). Many derivatives of garcinol have been synthesized (1) iso-garcinol (IG), (2) 14-isopropoxy IG (LTK-13), (3) 14-Methoxy IG (LTK-14), and (4) disulfoxy IG (LTK-19). LTK-13, LTK-14, and LTK-19 selectively inhibit p300 and LTK-14 act as a noncompetitive inhibitor of acetyl-coA and histones (Mantelingu et al. 2007).
Plumbagin
Plumbagin is another compound, derived from a root extract of the plant Plumbago rosea (Fig. 11), which has been found to potently inhibit HAT activity (Ravindra et al. 2009). Plumbagin and its derivatives possess HAT inhibitory activity and serves as a noncompetitive inhibitor for p300. The single hydroxyl group seems to be crucial for the HAT inhibitory activity.
Conclusion
A growing body of research suggests that epigenetic defects (epimutations) play a considerable role in human conditions that are strongly influenced by changes in the lifestyle, environment, diet, and pharmacological intervention. Therefore, it is possible that the discovery of novel synthetic and natural dietary compounds or testing the molecules that are already known, may be an effective strategy to treat the epigenetic changes or correct epimutations of various disease states. Although, a variety of compounds have been discovered as epigenetic modulators on various human diseases, such as cancer, obesity, and insulin resistance, only a few compounds (natural or synthetic) are known to target various epigenetic factors in brain disorders which are associated with epigenetic changes. So there is an urgent need in the investigation of phytochemicals as epigenetic modulators in the treatment of neurological diseases.
References
Adler Nevo G, Meged S, Sela BA, et al. Homocysteine levels in adolescent schizophrenia patients. Eur Neuropsychopharmacol. 2006;16:588–91.
Akbarian S. The molecular pathology of schizophrenia—focus on histone and DNA modifications. Brain Res Bull. 2010;83:103–7.
Akbarian S, Kim JJ, Potkin SG, et al. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–66.
Amir RE, Van den Veyver IB, Wan M, et al. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet. 1999;23:185–8.
Ammal Kaidery N, Tarannum S, Thomas B. Epigenetic landscape of Parkinson’s disease: emerging role in disease mechanisms and therapeutic modalities. Neurotherapeutics. 2013;10:698–708.
Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat Med. 2004;10(Suppl):S18–25.
Applebaum J, Shimon H, Sela BA, et al. Homocysteine levels in newly admitted schizophrenic patients. J Psychiatr Res. 2004;38:413–6.
Balasubramanyam K, Altaf M, Varier RA, et al. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J Biol Chem. 2004a;279:33716–26.
Balasubramanyam K, Swaminathan V, Ranganathan A, et al. Small molecule modulators of histone acetyltransferase p300. J Biol Chem. 2003;278:19134–40.
Balasubramanyam K, Varier RA, Altaf M, et al. Curcumin, a novel p300/CREB-binding protein-specific inhibitor of acetyltransferase, represses the acetylation of histone/nonhistone proteins and histone acetyltransferase-dependent chromatin transcription. J Biol Chem. 2004b;279:51163–71.
Barski A, Cuddapah S, Cui K, et al. High-resolution profiling of histone methylations in the human genome. Cell. 2007;129:823–37.
Bassett SA, Barnett MP. The role of dietary histone deacetylases (HDACs) inhibitors in health and disease. Nutrients. 2014;6:4273–301.
Baur JA. Resveratrol, sirtuins, and the promise of a DR mimetic. Mech Ageing Dev. 2010;131:261–9.
Bestor TH. Cloning of a mammalian DNA methyltransferase. Gene. 1988;74:9–12.
Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21.
Bjerling P, Silverstein RA, Thon G, et al. Functional divergence between histone deacetylases in fission yeast by distinct cellular localization and in vivo specificity. Mol Cell Biol. 2002;22:2170–81.
Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368:387–403.
Bollati V, Schwartz J, Wright R, et al. Decline in genomic DNA methylation through aging in a cohort of elderly subjects. Mech Ageing Dev. 2009;130:234–9.
Bottiglieri T, Godfrey P, Flynn T, et al. Cerebrospinal fluid S-adenosylmethionine in depression and dementia: effects of treatment with parenteral and oral S-adenosylmethionine. J Neurol Neurosurg Psychiatry. 1990;53:1096–8.
Bourc’his D, Xu GL, Lin CS, et al. Dnmt3L and the establishment of maternal genomic imprints. Science. 2001;294:2536–9.
Bruniquel D, Schwartz RH. Selective, stable demethylation of the interleukin-2 gene enhances transcription by an active process. Nat Immunol. 2003;4:235–40.
Cao R, Wang L, Wang H, et al. Role of histone H3 lysine 27 methylation in Polycomb-group silencing. Science. 2002;298:1039–43.
Cedar H, Bergman Y. Programming of DNA methylation patterns. Annu Rev Biochem. 2012;81:97–117.
Chandregowda V, Kush A, Reddy GC. Synthesis of benzamide derivatives of anacardic acid and their cytotoxic activity. Eur J Med Chem. 2009;44:2711–9.
Choi KC, Jung MG, Lee YH, et al. Epigallocatechin-3-gallate, a histone acetyltransferase inhibitor, inhibits EBV-induced B lymphocyte transformation via suppression of RelA acetylation. Cancer Res. 2009;69:583–92.
Christensen BC, Houseman EA, Marsit CJ, et al. Aging and environmental exposures alter tissue-specific DNA methylation dependent upon CpG island context. PLoS Genet. 2009;5:e1000602.
Costa FF. Non-coding RNAs, epigenetics and complexity. Gene. 2008;410:9–17.
De Ruijter AJ, van Gennip AH, Caron HN, et al. Histone deacetylases (HDACs): characterization of the classical HDAC family. Biochem J. 2003;370:737–49.
Desplats P, Spencer B, Coffee E, et al. Alpha-synuclein sequesters Dnmt1 from the nucleus: a novel mechanism for epigenetic alterations in Lewy body diseases. J Biol Chem. 2011;286:9031–7.
Dolinoy DC, Weidman JR, Waterland RA, et al. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–72.
Eastwood SL, Harrison PJ. Cellular basis of reduced cortical reelin expression in schizophrenia. Am J Psychiatry. 2006;163:540–2.
Edwards CA, Ferguson-Smith AC. Mechanisms regulating imprinted genes in clusters. Curr Opin Cell Biol. 2007;19:281–9.
Edwards TM, Myers JP. Environmental exposures and gene regulation in disease etiology. Cien Saude Colet. 2008;13:269–81.
Egger G, Liang G, Aparicio A, et al. Epigenetics in human disease and prospects for epigenetic therapy. Nature. 2004;429:457–63.
Fang MZ, Chen D, Sun Y, et al. Reversal of hypermethylation and reactivation of p16INK4a, RARbeta, and MGMT genes by genistein and other isoflavones from soy. Clin Cancer Res. 2005;11:7033–41.
Fang MZ, Wang Y, Ai N, et al. Tea polyphenol (−)-epigallocatechin-3-gallate inhibits DNA methyltransferase and reactivates methylation-silenced genes in cancer cell lines. Cancer Res. 2003;63:7563–70.
Fatemi SH, Earle JA, McMenomy T. Reduction in Reelin immunoreactivity in hippocampus of subjects with schizophrenia, bipolar disorder and major depression. Mol Psychiatry. 2000;5(654–663):571.
Fischle W, Dequiedt F, Hendzel MJ, et al. Enzymatic activity associated with class II HDACs is dependent on a multiprotein complex containing HDAC3 and SMRT/N-CoR. Mol Cell. 2002;9:45–57.
Grayson DR, Jia X, Chen Y, et al. Reelin promoter hypermethylation in schizophrenia. Proc Natl Acad Sci U S A. 2005;102:9341–6.
Gregoretti IV, Lee YM, Goodson HV. Molecular evolution of the histone deacetylase family: functional implications of phylogenetic analysis. J Mol Biol. 2004;338:17–31.
Guan JS, Haggarty SJ, Giacometti E, et al. HDAC2 negatively regulates memory formation and synaptic plasticity. Nature. 2009;459:55–60.
Guibert S, Weber M. Functions of DNA methylation and hydroxymethylation in mammalian development. Curr Top Dev Biol. 2013;104:47–83.
Guidotti A, Auta J, Davis JM, et al. Decrease in reelin and glutamic acid decarboxylase67 (GAD67) expression in schizophrenia and bipolar disorder: a postmortem brain study. Arch Gen Psychiatry. 2000;57:1061–9.
Guidotti A, Auta J, Davis JM, et al. GABAergic dysfunction in schizophrenia: new treatment strategies on the horizon. Psychopharmacology (Berl). 2005;180:191–205.
Hajkova P, Erhardt S, Lane N, et al. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev. 2002;117:15–23.
Hansen RS, Wijmenga C, Luo P, et al. The DNMT3B DNA methyltransferase gene is mutated in the ICF immunodeficiency syndrome. Proc Natl Acad Sci U S A. 1999;96:14412–7.
Heck N, Garwood J, Loeffler JP, et al. Differential upregulation of extracellular matrix molecules associated with the appearance of granule cell dispersion and mossy fiber sprouting during epileptogenesis in a murine model of temporal lobe epilepsy. Neuroscience. 2004;129:309–24.
Huang HS, Matevossian A, Whittle C, et al. Prefrontal dysfunction in schizophrenia involves mixed-lineage leukemia 1-regulated histone methylation at GABAergic gene promoters. J Neurosci. 2007;27:11254–62.
Huang Y, Doherty JJ, Dingledine R. Altered histone acetylation at glutamate receptor 2 and brain-derived neurotrophic factor genes is an early event triggered by status epilepticus. J Neurosci. 2002;22:8422–8.
Impagnatiello F, Guidotti AR, Pesold C, et al. A decrease of reelin expression as a putative vulnerability factor in schizophrenia. Proc Natl Acad Sci U S A. 1998;95:15718–23.
Jaenisch R, Bird A. Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet. 2003;33(Suppl):245–54.
Kang SK, Cha SH, Jeon HG. Curcumin-induced histone hypoacetylation enhances caspase-3-dependent glioma cell death and neurogenesis of neural progenitor cells. Stem Cells Dev. 2006;15:165–74.
Khan SI, Aumsuwan P, Khan IA, et al. Epigenetic events associated with breast cancer and their prevention by dietary components targeting the epigenome. Chem Res Toxicol. 2012;25:61–73.
Kilgore M, Miller CA, Fass DM, et al. Inhibitors of class 1 histone deacetylases reverse contextual memory deficits in a mouse model of Alzheimer’s disease. Neuropsychopharmacology. 2010;35:870–80.
King-Batoon A, Leszczynska JM, Klein CB. Modulation of gene methylation by genistein or lycopene in breast cancer cells. Environ Mol Mutagen. 2008;49:36–45.
Klose RJ, Bird AP. Genomic DNA methylation: the mark and its mediators. Trends Biochem Sci. 2006;31:89–97.
Klose RJ, Kallin EM, Zhang Y. JmjC-domain-containing proteins and histone demethylation. Nat Rev Genet. 2006;7:715–27.
Kobow K, El-Osta A, Blumcke I. The methylation hypothesis of pharmacoresistance in epilepsy. Epilepsia. 2013;54 Suppl 2:41–7.
Kobow K, Jeske I, Hildebrandt M, et al. Increased reelin promoter methylation is associated with granule cell dispersion in human temporal lobe epilepsy. J Neuropathol Exp Neurol. 2009;68:356–64.
Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705.
Krebs MO, Bellon A, Mainguy G, et al. One-carbon metabolism and schizophrenia: current challenges and future directions. Trends Mol Med. 2009;15:562–70.
Kriaucionis S, Heintz N. The nuclear DNA base 5-hydroxymethylcytosine is present in Purkinje neurons and the brain. Science. 2009;324:929–30.
Lee WJ, Shim JY, Zhu BT. Mechanisms for the inhibition of DNA methyltransferases by tea catechins and bioflavonoids. Mol Pharmacol. 2005;68:1018–30.
Levine J, Stahl Z, Sela BA, et al. Elevated homocysteine levels in young male patients with schizophrenia. Am J Psychiatry. 2002;159:1790–2.
Li E, Bestor TH, Jaenisch R. Targeted mutation of the DNA methyltransferase gene results in embryonic lethality. Cell. 1992;69:915–26.
Li H, Xu W, Huang Y, et al. Genistein demethylates the promoter of CHD5 and inhibits neuroblastoma growth in vivo. Int J Mol Med. 2012;30:1081–6.
Li Y, Tollefsbol TO. Impact on DNA methylation in cancer prevention and therapy by bioactive dietary components. Curr Med Chem. 2010;17:2141–51.
Liu RH. Potential synergy of phytochemicals in cancer prevention: mechanism of action. J Nutr. 2004;134:3479–85s.
Ma DK, Jang MH, Guo JU, et al. Neuronal activity-induced Gadd45b promotes epigenetic DNA demethylation and adult neurogenesis. Science. 2009;323:1074–7.
Machnes ZM, Huang TC, Chang PK, et al. DNA methylation mediates persistent epileptiform activity in vitro and in vivo. PLoS One. 2013;8:e76299.
Mai A, Rotili D, Tarantino D, et al. Small-molecule inhibitors of histone acetyltransferase activity: identification and biological properties. J Med Chem. 2006;49:6897–907.
Mantelingu K, Reddy BA, Swaminathan V, et al. Specific inhibition of p300-HAT alters global gene expression and represses HIV replication. Chem Biol. 2007;14:645–57.
Masliah E, Dumaop W, Galasko D, et al. Distinctive patterns of DNA methylation associated with Parkinson disease: identification of concordant epigenetic changes in brain and peripheral blood leukocytes. Epigenetics. 2013;8:1030–8.
Mastroeni D, Grover A, Delvaux E, et al. Epigenetic changes in Alzheimer’s disease: decrements in DNA methylation. Neurobiol Aging. 2010;31:2025–37.
Mastroeni D, Grover A, Delvaux E, et al. Epigenetic mechanisms in Alzheimer’s disease. Neurobiol Aging. 2011;32:1161–80.
Mayer W, Niveleau A, Walter J, et al. Demethylation of the zygotic paternal genome. Nature. 2000;403:501–2.
Miller-Delaney SF, Das S, Sano T, et al. Differential DNA methylation patterns define status epilepticus and epileptic tolerance. J Neurosci. 2012;32:1577–88.
Morgan HD, Santos F, Green K, et al. Epigenetic reprogramming in mammals. Hum Mol Genet. 2005;14(Spec No 1):R47–58.
Morrison LD, Smith DD, Kish SJ. Brain S-adenosylmethionine levels are severely decreased in Alzheimer’s disease. J Neurochem. 1996;67:1328–31.
Moyad MA. Soy, disease prevention, and prostate cancer. Semin Urol Oncol. 1999;17:97–102.
Myung NH, Zhu X, Kruman II, et al. Evidence of DNA damage in Alzheimer disease: phosphorylation of histone H2AX in astrocytes. Age (Dordr). 2008;30:209–15.
Nishioka K, Rice JC, Sarma K, et al. PR-Set7 is a nucleosome-specific methyltransferase that modifies lysine 20 of histone H4 and is associated with silent chromatin. Mol Cell. 2002;9:1201–13.
No JK, Soung DY, Kim YJ, et al. Inhibition of tyrosinase by green tea components. Life Sci. 1999;65:l241–6.
Okano M, Xie S, Li E. Dnmt2 is not required for de novo and maintenance methylation of viral DNA in embryonic stem cells. Nucleic Acids Res. 1998;26:2536–40.
Oswald J, Engemann S, Lane N, et al. Active demethylation of the paternal genome in the mouse zygote. Curr Biol. 2000;10:475–8.
Outeiro TF, Kontopoulos E, Altmann SM, et al. Sirtuin 2 inhibitors rescue alpha-synuclein-mediated toxicity in models of Parkinson’s disease. Science. 2007;317:516–9.
Paluszczak J, Krajka-Kuzniak V, Baer-Dubowska W. The effect of dietary polyphenols on the epigenetic regulation of gene expression in MCF7 breast cancer cells. Toxicol Lett. 2010;192:119–25.
Phiel CJ, Zhang F, Huang EY, et al. Histone deacetylase is a direct target of valproic acid, a potent anticonvulsant, mood stabilizer, and teratogen. J Biol Chem. 2001;276:36734–41.
Piaz FD, Vassallo A, Rubio OC, et al. Chemical biology of histone acetyltransferase natural compounds modulators. Mol Divers. 2011;15:401–16.
Pieper HC, Evert BO, Kaut O, et al. Different methylation of the TNF-alpha promoter in cortex and substantia nigra: implications for selective neuronal vulnerability. Neurobiol Dis. 2008;32:521–7.
Ravindra KC, Selvi BR, Arif M, et al. Inhibition of lysine acetyltransferase KAT3B/p300 activity by a naturally occurring hydroxynaphthoquinone, plumbagin. J Biol Chem. 2009;284:24453–64.
Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–93.
Reuter S, Gupta SC, Park B, et al. Epigenetic changes induced by curcumin and other natural compounds. Genes Nutr. 2011;6:93–108.
Ruzicka WB, Zhubi A, Veldic M, et al. Selective epigenetic alteration of layer I GABAergic neurons isolated from prefrontal cortex of schizophrenia patients using laser-assisted microdissection. Mol Psychiatry. 2007;12:385–97.
Sharma RP, Grayson DR, Gavin DP. Histone deactylase 1 expression is increased in the prefrontal cortex of schizophrenia subjects: analysis of the National Brain Databank microarray collection. Schizophr Res. 2008;98:111–7.
Shemer R, Razin A. Epigenetics. In: Russo VEA, Martienssen RA, Riggs AD, editors. Plainview. New York: Cold Spring Harbor Lab. Press; 1996. p. 215–30.
Shi ST, Wang ZY, Smith TJ, et al. Effects of green tea and black tea on 4-(methylnitrosamino)-1-(3-pyridyl)-1-butanone bioactivation, DNA methylation, and lung tumorigenesis in A/J mice. Cancer Res. 1994;54:4641–7.
Shi Y, Lan F, Matson C, et al. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–53.
Smith ZD, Meissner A. DNA methylation: roles in mammalian development. Nat Rev Genet. 2013;14:204–20.
Sng JC, Taniura H, Yoneda Y. Histone modifications in kainate-induced status epilepticus. Eur J Neurosci. 2006;23:1269–82.
St Laurent R, O'Brien LM, Ahmad ST. Sodium butyrate improves locomotor impairment and early mortality in a rotenone-induced Drosophila model of Parkinson’s disease. Neuroscience. 2013;246:382–90.
Stante M, Minopoli G, Passaro F, et al. Fe65 is required for Tip60-directed histone H4 acetylation at DNA strand breaks. Proc Natl Acad Sci U S A. 2009;106:5093–8.
Stefanska B, Rudnicka K, Bednarek A, et al. Hypomethylation and induction of retinoic acid receptor beta 2 by concurrent action of adenosine analogues and natural compounds in breast cancer cells. Eur J Pharmacol. 2010;638:47–53.
Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–5.
Subramaniam D, Thombre R, Dhar A, et al. DNA methyltransferases: a novel target for prevention and therapy. Front Oncol. 2014;4:80.
Sun Y, Jiang X, Chen S, et al. Inhibition of histone acetyltransferase activity by anacardic acid sensitizes tumor cells to ionizing radiation. FEBS Lett. 2006;580:4353–6.
Sung B, Pandey MK, Ahn KS, et al. Anacardic acid (6-nonadecyl salicylic acid), an inhibitor of histone acetyltransferase, suppresses expression of nuclear factor-kappaB-regulated gene products involved in cell survival, proliferation, invasion, and inflammation through inhibition of the inhibitory subunit of nuclear factor-kappaBalpha kinase, leading to potentiation of apoptosis. Blood. 2008;111:4880–91.
Tachibana M, Sugimoto K, Nozaki M, et al. G9a histone methyltransferase plays a dominant role in euchromatic histone H3 lysine 9 methylation and is essential for early embryogenesis. Genes Dev. 2002;16:1779–91.
Tachibana M, Ueda J, Fukuda M, et al. Histone methyltransferases G9a and GLP form heteromeric complexes and are both crucial for methylation of euchromatin at H3-K9. Genes Dev. 2005;19:815–26.
Tahiliani M, Koh KP, Shen Y, et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science. 2009;324:930–5.
Tsukada Y, Fang J, Erdjument-Bromage H, et al. Histone demethylation by a family of JmjC domain-containing proteins. Nature. 2006;439:811–6.
Vahid F, Zand H, Nosrat-Mirshekarlou E, et al. The role dietary of bioactive compounds on the regulation of histone acetylases and deacetylases: a review. Gene. 2015;562:8–15.
Vanhees K, Coort S, Ruijters EJ, et al. Epigenetics: prenatal exposure to genistein leaves a permanent signature on the hematopoietic lineage. FASEB J. 2011;25:797–807.
Veldic M, Caruncho HJ, Liu WS, et al. DNA-methyltransferase 1 mRNA is selectively overexpressed in telencephalic GABAergic interneurons of schizophrenia brains. Proc Natl Acad Sci U S A. 2004;101:348–53.
Veldic M, Guidotti A, Maloku E, et al. In psychosis, cortical interneurons overexpress DNA-methyltransferase 1. Proc Natl Acad Sci U S A. 2005;102:2152–7.
Voss K, Gamblin TC. GSK-3beta phosphorylation of functionally distinct tau isoforms has differential, but mild effects. Mol Neurodegener. 2009;4:18.
Wang H, An W, Cao R, et al. mAM facilitates conversion by ESET of dimethyl to trimethyl lysine 9 of histone H3 to cause transcriptional repression. Mol Cell. 2003;12:475–87.
Wang TT, Sathyamoorthy N, Phang JM. Molecular effects of genistein on estrogen receptor mediated pathways. Carcinogenesis. 1996;17:271–5.
Weaver IC, Meaney MJ, Szyf M. Maternal care effects on the hippocampal transcriptome and anxiety-mediated behaviors in the offspring that are reversible in adulthood. Proc Natl Acad Sci U S A. 2006;103:3480–5.
Wu JC, Santi DV. On the mechanism and inhibition of DNA cytosine methyltransferases. Prog Clin Biol Res. 1985;198:119–29.
Xiao Y, Camarillo C, Ping Y, et al. The DNA methylome and transcriptome of different brain regions in schizophrenia and bipolar disorder. PLoS One. 2014;9:95875.
Xie S, Wang Z, Okano M, et al. Cloning, expression and chromosome locations of the human DNMT3 gene family. Gene. 1999;236:87–95.
Xu GL, Bestor TH, Bourc'his D, et al. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature. 1999;402:187–91.
Yang XJ, Seto E. The Rpd3/Hda1 family of lysine deacetylases: from bacteria and yeast to mice and men. Nat Rev Mol Cell Biol. 2008;9:206–18.
Zee BM, Levin RS, Xu B, et al. In vivo residue-specific histone methylation dynamics. J Biol Chem. 2010;285:3341–50.
Zhang Y, Chen H. Genistein, an epigenome modifier during cancer prevention. Epigenetics. 2011;6:888–91.
Zhu Q, Wang L, Zhang Y, et al. Increased expression of DNA methyltransferase 1 and 3a in human temporal lobe epilepsy. J Mol Neurosci. 2012;46:420–6.
Acknowledgment
We apologize to colleagues whose relevant works were not cited due to space constrains.
Compliance with Ethics Requirements The authors declare that they have no conflicts of interest.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Gangisetty, O., Murugan, S. (2016). Epigenetic Modifications in Neurological Diseases: Natural Products as Epigenetic Modulators a Treatment Strategy. In: Essa, M., Akbar, M., Guillemin, G. (eds) The Benefits of Natural Products for Neurodegenerative Diseases. Advances in Neurobiology, vol 12. Springer, Cham. https://doi.org/10.1007/978-3-319-28383-8_1
Download citation
DOI: https://doi.org/10.1007/978-3-319-28383-8_1
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28381-4
Online ISBN: 978-3-319-28383-8
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)