Abstract
This work covers the use of solution-processed metal oxides as interface layers for organic solar cells. To study the interface properties, intrinsic and Al-doped ZnO x were chosen as reference systems. From the class of n-type metal oxides, ZnO x was chosen because it can be doped when it is solution processed. Furthermore, the influence of thin modification layers applied on top of the metal oxides is investigated.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
- Aluminum-doped zinc oxide
- Barium hydroxide
- Charge transport in nanoparticular films
- Conjugated polyelectrolyte
- Device structure
- Diketopyrrolopyrrole-quinquethiophene
- Doped zinc oxide
- Electronic surface properties
- Energy diagram
- Energy-level diagrams
- EQE spectra
- Inferface layers for organic solar cells
- Interface layers
- Interface modification layers
- Inverted organic solar cells
- J-V characteristics
- Low temperature
- Molybdenum oxide
- Mott–Schottky capacitance
- Oxygen
- P3HT
- PCDTBT
- Phosphonic acid anchored fullerene SAM
- Polyvinylpyrrolidone
- Poole–Frenkel effect
- Reference solar cells
- SAM modification
- Stability of interface materials
- Stabilization of AZO nanoparticles
- Summary
- Thickness dependence
- Trioxadecanoic acid
- Water
- Zinc oxide
1 Introduction
Organic solar cells have been processed in two different geometries: (1) in the inverted geometry, where the ZnO layer is directly processed on the transparent conducting oxide electrode as well as (2) in the normal cell structure, where the ZnO is deposited on top of the semiconductor and beneath the top cathode. This was possible because in addition to the classical sol-gel-processed ZnO x layers we were able to process intrinsic and Al-doped ZnO x layers via a nanoparticle suspension without high-temperature annealing processes that harm the polymeric active layer. The main objectives were to systematically vary the doping level and to build a correlation between the device functionality and the work function of the interface layer: its conductivity and carrier concentration, respectively.
Finally, these investigations provided valuable insight into the origin of the second diode and surface recombination in organic solar cells. Second diodes are internal diodes in solar cells that are connected in series to the photovoltaic junction and cause a kink in the J–V curve in the fourth quadrant and a delayed opening of the diode in the first quadrant [1].
The main aspect of this work was the development, advancement, design, characterization, and understanding of interface layers for a solution-processed polymer–fullerene solar cell. Although the development of the full solar cell (including the semiconductor, top electrode, and packaging) was not the focus of this work, this research delivered further important input to the architecture and design criteria for the rest of the solar cell’s stack.
The first part of this work focused on the development and characterization of solution-processed ZnO at various bulk doping levels. The second part focused on modifying the electronic surface properties of doped and intrinsic ZnO. A description of the work’s progress and the results obtained are summarized in the following sections.
1.1 Reference Solar Cells
Reference solar cells were built in the normal architecture. Figure 1 displays representative J–V curves of solar cells and the standard architecture used with different donor polymers in the active layer.
1.2 Fundamentals and Mechanisms
1.2.1 Charge Transport in Nanoparticle Thin Films of ZiZnO and Aluminum-Doped ZnO
According to the first part of the work package, the synthesis, characterization, and testing of reference materials, namely ZnO, TiOx, and a first aluminum-doped ZnO (AZO) precursor according to known routes taken from the literature [2–4], started. The lattice and crystallite structure of the materials was investigated through X-ray diffraction measurements. We obtained anatase TiOx with a crystallite size of 12.5 nm and wurtzite ZnO and AZO with crystallite sizes of 4.2 and 4.0 nm, respectively. A slight shift in the peak of the 002 plane of the AZO in comparison to the ZnO is a sign for interstitial doping. With optical measurements, the transmission and bandgap of the materials were investigated and found to be in a suitable range for a window layer in solar cell applications. The transmission of all materials is over 90 % in the visible part of the spectrum. This is a result of the wide optical bandgaps: 3.85 eV for TiOx, 3.44 eV for ZnO, and 3.34 eV for AZO. Electrical characterization showed conductivities on the order of 10−4 S/cm for TiOx and 10−6 S/cm for ZnO, whereas AZO had the highest conductivity, on the order of 10−3 S/cm.
Next, these materials were incorporated in inverted polymer solar cells with a usual thickness of a few tens of nanometers (20–40 nm). It was found that all three materials worked comparably and as well as electron extraction layers (EELs) in polymer solar cells (Figs. 2 and 3, right). Interestingly, doped ZnO turned out to be superior to intrinsic ZnO in the thick-film limit, that is, for extraction layers with a thickness of 100 nm and above (Fig. 3, right). The solar cells with thick AZO EELs maintained their performance compared with the thin EELs, but the efficiency of the devices with thick intrinsic ZnO EELs deteriorated (Table 1). Thick and robust films are a prerequisite to reliable large-scale EEL printing.
In subsequent work, the thickness of the interface layer was increased to even 600 nm without limiting the device performance.
As one of our goals was to build more environmentally stable devices, it was necessary to replace the commonly used acidic and hygroscopic PEDOT:PSS as the p-type interlayer, which is known to decrease device lifetimes. Therefore, we developed a solution process to deposit the more stable MoO3 as the anode interlayer, which yields solar cells with a performance comparable to that of PEDOT:PSS.
1.2.2 Charge Transport in Nanoparticle Thin Films of ZnO and Aluminum-Doped ZnO
To improve our understanding of the above results, we carried out a charge-transport study on intrinsic and doped zinc oxide with commercially available ZnO/AZO dispersions from Nanograde Ltd. While the J–V characteristics at low voltage obey Ohm’s law, transport in the high-voltage data regime is controlled by the Poole–Frenkel effect. The occurrence of the Poole–Frenkel effect is related to coulombically bound electrons, which have to overcome a field-dependent barrier to the next nanoparticle. Also, the conduction mechanism was demonstrated to be equivalent for the higher conductive Al-doped ZnO particles, where Al acts as a shallow donor. To the best of our knowledge, this is the first time that the Poole–Frenkel effect was unambiguously demonstrated as the dominant transport mechanism in a nanoparticle thin film (Fig. 4).
1.3 Processing of Interface Materials
Following our work program, the formulation design of doped metal oxide inks was started. As the primary prototype, the metal oxide ZnO was chosen since it has been shown that ZnO [4–8]
-
can be processed from solution
-
can be doped (e.g., with In, Ag, Ga, Mg, but especially Al)
-
has been demonstrated as a functional layer in organic photovoltaic (OPV) cells when it was processed as an intrinsic semiconductor from solution
-
has been demonstrated as a functional electrode for organic light-emitting diodes when it was processed from vacuum (atomic layer deposition, sputtering, etc.)
A variety of coating procedures have been well established for many different kinds of applications. In this work, we used sol-gel processes [9] because of their various advantages. Sol-gel precursors are easy to handle in atmospheric conditions, a defined band-doping of the processed materials is possible [10], they are cost-efficient, and they can be deposited by various techniques, including dip coating, spin coating, or several printing and coating processes [11]. There are two different sol-gel processes: the so-called high-temperature and low-temperature paths [12].
In the high-temperature method, the ZnO x sol is prepared using zinc-acetate-2-hydrate, diethanolamine, and isopropyl alcohol (ISP). Aluminum-nitrate-9-hydrate is added as dopant [13]. The functional layer is formed by annealing the thin-film layer at temperatures over 250 °C [14]. This classic sol-gel process resulted in AZO functional layers with a charge carrier density of 4.2 × 1019 cm−3, a specific resistivity of 3.1 × 10−3 Ω/cm, and a transmittance of up to 0.88 at a layer thickness of 1250 nm.
The low-temperature path is based on nanoparticle suspensions [15]. These suspensions consist of crystalline ZnO or AZO nanoparticles with a primary particle size between 15–40 nm. The nanoparticles are redispersed in solvents such as ethanol, diethylene glycol, or ISP and stabilized with surface-modifying agents [e.g., carbon acids such as pentanoic or trioxadecanoic acid (TODS)] [14]. The surface-modifying agents are added to the suspension to prevent aggregation and agglomeration of the nanoparticles. Additionally, an ultrasonic dissolver and stirrer are used for deagglomeration. In addition to the surface-modifying agents, a binder phase is necessary to provide a sufficient mechanical stability of the coating by interconnecting the particles on the one hand and to adhere the coating to the substrate on the other hand [16].The binder phase may also include functional groups that enable a curing of the coating at temperatures below 130 °C by using, for instance, ultraviolet radiation and photostarter. The state-of-the-art are polymeric binder phases based on glycidoxypropyltrimethoxysilane or methacryloxypropyl-trimethoxysilane [17].
The nanoparticles themselves can, for example, be prepared by a surfactant-assisted sol-gel method using zinc nitrate and citric acid as starting materials [18]. A polyesterification reaction due to the Pechini process can also be used for the preparation of ZnO and AZO powders. This synthesis process is based on the chelation of metal ions by an α-hydroxyl acid such as citric acid in order to form stable homogeneous solutions. The powder can be obtained via calcination [19]. The various processes described were completed by a high-temperature calcination (300 °C) and a subsequent particle surface modification to result in stable suspensions with a particle size below 100 nm. These systems can be processed at low temperatures and are compatible with temperature-sensitive substrates such as polymers.
To date there has been only one paper describing the stabilization of AZO nanoparticles in ethylene glycol [20]. As ethylene glycol affects the performance of the solar cells negatively, it could not be used in our work. We have managed to stabilize AZO nanoparticles with at least four different surface-modifying agents in at least three solvents, respectively, solvent mixtures. Therefore, a series of ZnO solutions has been prepared and was successfully integrated in polymer solar cells.
A solution containing AZO particles, TODS surfactant, and ISP was identified as one candidate for yielding suitable films for solar cell integration. Only 140 °C is necessary to anneal these films, so they can be incorporated onto the temperature-sensitive organic active layer. The electrical functionality of the obtained AZO film was confirmed in standard solar cells with the layer stack displayed in Fig. 5 (left). The J–V characteristics in Fig. 5 (right) show that the AZO forms the electron-selective contact with the proper work function to achieve the expected high open-circuit voltage, V OC. The high fill factor (FF) shows the good quality of the AZO EEL.
Further investigations have been performed on redispersed AZO nanoparticle solutions with different stabilizers, and we researched mixtures of stabilizers to find a solution with long-term stability and improved properties within organic solar cells.
Stable suspensions in IPS could be prepared with the surfactants TODS and polyvinylpyrrolidone (PVP) as well as with a mixture of TODS and PVP and with acetylacetone (AcAc), 1,4-butanediol, hexamethylenetetramine, and ε-caprolactone. The smallest particle sizes could be reached by using the surfactants TODS, TODS + PVP, and AcAc, which are shown in Table 2. Usually, the hydrodynamic particle sizes are higher than the primary particle sizes due to agglomeration and aggregation of the primary particles. The particle sizes are similar, which confirms the long-term stability of the prepared AZO nanoparticle solutions.
The surface roughness of the thin films prepared at low temperatures reached values down to 20 nm with an electrical conductivity of up to 10−2 S/cm for layers prepared directly after dispersing the AZO nanoparticles as well as for layers prepared with a three-month-old AZO nanoparticle suspension. Finally, inverted polymer–fullerene solar cells have been built with an AZO interface layer using these AZO nanoparticle solutions (Fig. 6, left). The performance of the devices built was found to depend on the surfactant used, with the best obtained with TODS and PVP (Fig. 6, right). The effect can be attributed to a smaller particle size in the dispersion and better layer morphology.
Finally, redispersed AZO nanoparticle solutions with long-term stability have been developed and can successfully be applied to organic solar cells to investigate their properties and improve the performance.
1.4 Interface Modification Layers
One of the weak points of metal oxide–based EELs frequently discussed is their reliability and reproducibility when processed at low temperature (i.e., at 140 °C). Low processing temperatures may yield incomplete ligand removal and thus result in bad control of the electronic surface properties. For our investigated EEL materials, the performance gap between standard and inverted architecture is small. However, for active layers containing other high-performance donor polymers (e.g., poly[2,6-(4,4-bis-(2-ethylhexyl)dithieno[3,2-b:2′,3′-d]silole)-alt-4,7-(2,1,3-benzothiadiazole)] (Si-PCPDTBT); Fig. 7) or small molecules, the gap can be large.
Inverted solar cell with an aluminum-doped ZnO electron extraction layer and an Si-PCPDTBT:PC70BM active layer (structure similar to Fig. 4)
1.4.1 Self-assembled Monolayer Modification
Decorating metal oxides with self-assembled monolayers (SAMs) is one way to achieve predefined surface properties. The experiment depicted in Fig. 8 with the key parameters listed in Table 3 demonstrates how varying the interface properties influences the device performance of inverted solar cells based on poly(3-hexylthiophene-2,5-diyl) (P3HT). The phosphonic acid-anchored aliphatic SAM (SAM-C6) forms a very unfortunate surface on the AZO, which decreases the device performance significantly and inflicts a second diode behavior in the J–V curve. In contrast, the fullerene-functionalized SAMs [SAM(C6-C60) and SAM(C18-C60)] as termination groups for the surface of the doped ZnO films improve the device efficiency [21].
Left: layer stack of the investigated solar cells including the chemical structure of the self-assembled monolayers (SAMs) and the results of the contact angle measurements to confirm the proper SAM deposition on aluminum-doped ZnO (AZO) and reference AlOx layers. Right: J–V characteristics of solar cells with SAM-decorated AZO electron extraction layers
1.4.2 Barium Hydroxide Modification
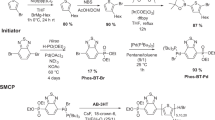
Inverted organic solar cells with air-stable interface materials and top electrodes and an efficiency of 6.01 % are achieved by inserting a barium hydroxide [Ba(OH)2] layer between the AZO EEL and the active layer. A low-bandgap diketopyrrolopyrrole-quinquethiophene alternating copolymer (pDPP5T-2) and phenyl-C61-butyric acid methyl ester (PC60BM) were chosen as the active layer compounds (Fig. 9). Compared to the control device without Ba(OH)2, inserting a 10-nm-thick Ba(OH)2 layer results in an enhanced V OC of 10 %, a short-circuit current density, J SC, of 28 %, an FF of 28 %, and a power conversion efficiency (PCE) of 80 % (Fig. 10 and Table 4). Modifying AZO with a solution-processed low-cost Ba(OH)2 layer increased the efficiency of the inverted device by dominantly reducing the energy barrier for electron extraction from PCBM (see schematic in Fig. 11); consequently, a higher built-in voltage and increased surface charge density are observed (see Fig. 12). The effects of a Ba(OH)2 layer on the improvement in the device performance stem from the potential surface doping by diffusion of Ba2+ ions through the surface of the active layer, the reduced charge recombination, the decreased energy barrier for electron extraction and transport via inserting an interface dipole layer, and the increased built-in potential from a corresponding increase in the surface charge density [22].
1.4.3 Conjugated Polyelectrolyte Modification
The device structure, the molecular structure of poly[3-(6-trimethylammoniumhexyl)thiophene] (P3TMAHT), and the energy levels of the components used in the devices are shown in Fig. 13. Detailed methods for the syntheses of N(Ph-2T-DCN-Et)3 and P3TMAHT are described in the literature [23, 24]. Through application of the P3TMAHT layer on top of the AZO, the work function of AZO is reduced to from 4.2 to 3.8 eV. Indeed, the increased polarity due to the accumulation of ions and the formation of a dipole layer at the interface of AZO is observed as described in the references [25, 26].
Top: J–V characteristics of the conventional and inverted devices based on various interface layers under the illumination of AM 1.5G, 100 mW/cm2. Middle: J–V characteristics of the devices in dark. Bottom: External quantum efficiency spectra of the conventional device and the inverted devices based on various interface layers, and absorption spectra of these blend films of PEDOT/active layer, AZO/active layer, and AZO/P3TMAHT/active layer on the ITO substrates
The current density vs. voltage (J–V) characteristics of N(Ph-2T-DCN-Et)3:PC70BM–based conventional [ITO/PEDOT:PSS/N(Ph-2T-DCN-Et)3:PC70BM/Ca/Ag] and inverted [ITO/AZO/(P3TMAHT)/N(Ph-2T-DCN-Et)3:PC70BM/MoO3/Ag] devices under simulated AM 1.5 and in the dark are shown in Fig. 14 (top and middle), respectively. Device parameters are deduced from the J–V characteristics in the light and dark (summarized in Table 5). The reference conventional cells based on N(Ph-2T-DCN-Et)3:PC[70]BM as active layer have a V OC of 0.939 V, a J SC of 7.79 mA/cm2, an FF of 45.9 %, and a PCE of 3.36 %, while the series resistance (R S) is 1.29 Ω/cm2 and the parallel resistance (R Sh) is 1.84 kΩ/cm2. In contrast, the inverted cells based on AZO as the electron-transporting layer show a PCE of 3.17 % with a V OC of 0.840 V, a J SC of 8.47 mA/cm2, and an FF of 44.5 %. The surface recombination, work function mismatch, or interface barrier might result in a reduced V OC from 0.939 to 0.840 V, thus resulting in a slightly lower PCE of 3.17 %. Consequently, we introduced P3TMAHT as the interface layer on the AZO layer, which facilitates electron injection and transport by reducing the energy barrier between the transparent cathode and active layer. This directly resulted in an increased V OC (from 0.840 to 0.898 V). In addition, compared with the AZO-based inverted devices, the combination of AZO and the P3TMAHT layer slightly enhanced the J SC (from 8.47 to 8.85 mA/cm2) and FF (from 44.5 to 49.2 %) and thus resulted in a higher PCE of 3.91 %.
Considering the improvement in the J SC, Heeger et al. [27] speculated that the increased built-in potential and internal electric field might help the charge carriers to escape shallow traps and reduce trap-assisted recombination. In addition, Kim et al. [28] thought that the smoother and more hydrophobic surface of the buffer layer (AZO/P3TMAHT layer, as shown in Fig. 14, bottom) improved the compatibility between the organic active layer and the AZO layer and that the better contact induced the enhanced J sc. The external quantum efficiency (EQE) spectra of various devices are shown in Fig. 14 (bottom). The EQE results agree with the J SC of various devices mentioned above.
The improved PCE of the device with the AZO/P3TMAHT layer are also consistent with the higher EQE value (>55 %) exhibited in the PC70BM absorption region between 350–500 nm. Although the absorption spectra of blends on the AZO and AZO/P3TMAHT layer are similar, as shown in Fig. 14 (bottom), the higher EQE values of the AZO/P3TMAHT layer-based device in the PC70BM absorption region prove that AZO/P3TMAHT is more efficiently extracting electrons from PC70BM and thus successfully reduces the recombination.
1.5 Stability of Interface Materials
We synthesized and prepared two different types of Al3+-doped ZnO thin transparent films by using a low-cost sol-gel doctor-blade technique. The effect of temperature, adsorption, and diffusion of moisture or moisture vapor on the electrical and optical properties of the films was investigated and correlated to device degradation. The films were placed in a climatic chamber (type C-40/200) for different deposition times. The storage of the samples took place under the following conditions: 40 °C, 90 % relative humidity, 0–140 h. Our results show that the optical properties such as transmittance and bandgap are not influenced by damp-heat testing. Scanning electron microscopy and X-ray diffraction measurements clearly revealed the more amorphous structure of low-temperature AZO thin films with a nanoporous surface morphology. The analyzed Fourier transform infrared spectra confirmed the impact of both adsorbed oxygen and diffused water on AZO layers, promoting the degradation process (see Fig. 15). The electrical properties of both high- and low-temperature AZO films, that is, work function and conductivity, showed evident degradation upon damp-heat exposure, indicating the strong interaction between the metal surface and the ambient conditions that leads to the formation of a depletion layer. Accelerated device degradation under damp-heat testing underlines the formation of a barrier or depletion layer at the interface, reducing V OC, J SC, and FF. The intrinsic stability of metal oxide–based interlayers as well as that of their nanostructure is an essential criterion for the stability of organic electronic devices. Investigations of different metal oxide surfaces are required to better understand how to further improve the electrical integrity of the surface under damp-heat conditions [30].
Schematic view of induced adsorption of oxygen, and diffusion of water or vapor from the ambient atmosphere in the climatic chamber. (Image modified and adapted from [29])
1.6 Summary
-
Representative solar cells with P3HT, Si-PCPDTBT, and poly[2,6-(4,4-bis(2-ethylhexyl)-4H-cyclopenta[2,1-b;3,4-b′]dithiophene)-alt-4,7(2,1,3-benzothiadi-azole)] with power efficiencies between 3–5 % were processed.
-
The synthesis, characterization, and benchmarking of the reference materials, namely TiOx, ZnO, and AZO for EELs in polymer solar cells, were completed. The relationship between material properties and solar cell performance could be established. Applied in thin films, all three materials performed comparably in the solar cells. AZO performed superior to ZnO in the thick film limit. It is now possible to compare the materials developed in this work to known interface layers.
-
A series of AZO formulations with different compositions was developed and the prepared thin films were characterized. A formulation that yields films with suitable properties for the solar cell application could be identified, and the first solar cells that contain these films were built, with promising results.
-
Furthermore, the experiments focused on the influence of modification layers on device performance. Three different approaches for surface modifications were applied and investigated:
-
SAMs: PA-anchored aliphatic and fullerene-functionalized SAMs were used as termination groups for doped metal oxide films. The surface properties can be modified over a large interval with a huge impact on device performance.
-
BaOH2: A thin modification layer of BaOH2 was applied to AZO. This led to …., which improved the device performance of the respective devices by a large margin.
-
CPE: The conductive polyelectrolyte P3MAHT was deposited on top of AZO to improve the performance of inverted small-molecule–based organic solar cells. The P3MAHT reduced the work function, thus enhancing the contact formation to PCBM.
-
-
Additionally, stable redispersed AZO nanoparticle suspensions for a low-temperature coating process that provided long-term stability together with customized electrical properties (such as the electrical conductivity), a small surface roughness, and a high transmittance were developed.
-
Conclusions
-
Doping of ZnO allowed us to increase the layer thickness of these interface layers to more than 500 nm without deteriorating device performance.
-
Surface modification has a huge impact on device performance. Many different active layer systems can yield improved performance with surface modifications; however, there is no universal solution. A toolbox based on a suitable doped metal oxide with a variety of modifications proved to be the best approach to adjust the interface properties to the active layers’ needs.
-
References
Wagenpfahl A, Rauh D, Binder M, Deibel C (2010) Phys Rev B 82:115306
Wang J, Polleux J, Lim J, Dunn B (2007) J Phys Chem C 111:14925
Alam MJ, Cameron DC (2001) J Vac Sci Technol A 19:1642
Park Y-S, Park HK, Cho SW, Jeong JA, Choi KH, Kim HK, Lee JY, Cho WJ (2008) Electrochem Solid-State Lett 11:J85
Huang H-H, Chu SY, Kao PC, Chen YC (2008) Thin Solid Films 516(16):5664
Yoshida Y, Tanaka S, Hiromttsu I, Fujita Y, Yoshino K (2008) Jpn J Appl Phys 47:867
Park YR, Nam E, Kim YS (2008) Jpn J Appl Phys 47:468
Tsai C-L, Lin YJ, Wu PH, Chen SY, Liu DS, Hong HJH, Liu CJ, Chang HC (2007) J Appl Phys 101(11):113713
Al-Dahoudi N, Aegerter MA (2006) Thin Solid Films 502:193–197
Dislich H (1986) J Non-Cryst Solids 80:115–121
Rydzek M, Reidinger M, Arduini-Schuster M, Manara J (2011) Prog Org Coat 70:369–375
Reidinger M, Rydzek M, Scherdel C, Arduini-Schuster M, Manara J (2009) Thin Solid Films 517:3096–3099
Rydzek M, Reidinger M, Arduini-Schuster M, Manara J (2012) Thin Solid Films 520:4114–4118
Rydzek M, Reidinger M, Scherdel C, Arduini-Schuster M, Manara J (2009) High Temp–High Pressures 38:277–293
Puetz J, Al-Dahoudi N, Aegerter MA (2004) Adv Eng Mater 6:733–737
Al-Dahoudi N (2003) Dissertation, Universität des Saarlandes
Al-Dahoudi N, Aegerter MA (2006) Thin Solid Films 520:193
Zhang YL, Yang Y, Zhao JH, Tan RQ, Cui P, Song WJ (2009) J Sol-Gel Sci Technol 51:198
Serier H, Gaudon M, Ménétrier M (2009) Solid State Sci 11:1192
Zhao J, Tan R, Zhang Y, Yang Y, Guo Y, Zhang X, Wang W, Song W (2011) J Am Ceram Soc 94:725–728
Stubhan T, Salinas M, Ebel A, Krebs FC, Hirsch A, Halik M, Brabec CJ (2012) Adv Energy Mater 2:532
Zhang H, Stubhan T, Li N, Turbiez M, Matt GJ, Ameri T, Brabec CJ (2014) J Mater Chem A 2:18917
Min J, Luponosov YN, Ameri T, Elschner A, Peregudova SM, Baran D, Heumüller T, Li N, Machui F, Ponomarenko S, Brabec CJ (2013) Org Electron 14:219–229
Scherf U, Gutacker A, Koenen N (2008) Acc Chem Res 41:1086–1097
Seo JH, Gutacker A, Sun Y, Wu H, Huang F, Cao Y, Scherf U, Heeger AJ, Bazan GC (2011) J Am Chem Soc 133:8416
He ZC, Zhong CM, Su SJ, Xu M, Wu HB, Cao Y (2012) Nat Photonics 6:591–595
Kyaw AKK, Wang DH, Gupta V, Zhang J, Chand S, Bazan GC, Heeger AJ (2013) Adv Mater 25:2397–2402
Choi H, Park JS, Jeong E, Kim G-H, Lee BR, Kim SO, Song MH, Woo HY, Kim JY (2011) Adv Mater 23:2759–2763
Jeong JK, Won Yang H, Jeong JH, Mo Y-G, Kim HD (2008) Appl Phys Lett 93:123508
Litzov I, Azimi H, Matt G, Kubis P, Stubhan T, Popov G, Brabec CJ Org Electron 15(2):569–576
Oh H, Krantz J, Stubhan T, Litzov I, Brabec CJ (2011) Sol Energy Mater Sol Cells 95:2194
Stubhan T, Oh H, Pinna L, Krantz J, Litzov I, Brabec CJ (2011) Org Electron 12:1539
Stubhan T, Ameri T, Salinas M, Krantz J, Machui F, Halik M, Brabec CJ (2011) Appl Phys Lett 98:253308
Wolf N, Rydzek M, Gerstenlauer D, Arduini-Schuster M, Manara J (2013) Thin Solid Films 532:60–65
Wolf N, Stubhan T, Manara J, Dyakonov V, Brabec CJ (2014) Thin Solid Films 564:213
Sista S, Park M-H, Hong Z, Wu Y, Hou J, Kwan WL, Li G, Yang Y (2010) Adv Mater 22:380
Acknowledgment
This work is supported by the German Science Foundation (DFG), grant numbers BR 4031/1-1, DY 18/7-1, BR 4031/1-2, and DY 18/7-2.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Stubhan, T., Wolf, N., Manara, J., Dyakonov, V., Brabec, C.J. (2017). Controlling the Electronic Interface Properties in Polymer–Fullerene Bulk Heterojunction Solar Cells. In: Leo, K. (eds) Elementary Processes in Organic Photovoltaics. Advances in Polymer Science, vol 272. Springer, Cham. https://doi.org/10.1007/978-3-319-28338-8_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-28338-8_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28336-4
Online ISBN: 978-3-319-28338-8
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)