Abstract
Increasing numbers of therapeutic genes and cellular targets are available for gene therapy. Many clinical trials using virus-derived delivery systems are devoted to combat cancer, to correct single-gene malfunctions or to regenerate tissues. To develop gene delivery vectors with high efficiency through target cell selectivity, in particular under in situ conditions, remains a major challenge. The most widely used vector systems to transduce cells are based on adenoviruses. Recent approaches to develop selective adenoviral vectors (Ad) that exclusively target cells or tissues of interest without interfering with all others have focused on the modification of the broad natural tropism of adenoviruses. A popular way of Ad targeting is attained by directing vector particles towards distinct cellular receptors. Retargeting can be accomplished by linking custom-made peptides with unique specificity and reasonable affinity to cellular surface protein moieties via genetic alteration, chemical coupling or bridging with dual-specific adaptor molecules. Ideally, target-specific vectors are incapable of entering cells via their native receptors. Such altered vectors offer new opportunities to delineate functional genomics in the native environment and should enable efficient systemic therapeutic approaches. This review provides a summary of current state-of-the-art techniques to target specifically adenovirus-derived gene delivery vector systems.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
2.1 Introduction
2.1.1 The Concept of Gene Therapy
Somatic gene therapy is defined as the transfer of nucleic acids into a patient’s cells to achieve a lasting therapeutic effect without influencing the germline. A myriad of conditions has been tackled where a cure is envisaged by intervening at the genetic level rather than via conventional application of drugs. Thereby, influence on or correction of inherited or acquired malfunctioning genes should be lasting. The best known tools to affect a cell’s genetic makeup are virus-based vectors as the vehicles of choice to intervene on the gene expression profile of cells and ideally, to correct a malfunction, to suppress the expression of an undesired cellular gene or to aim at quantitative elimination of a population of cells out of control. Still, therapeutic approaches to alter inherited or acquired genetic disorders as well as those to eliminate neoplastic cells once disseminated in the patient are still far from regular clinical application.
Effective in vivo transfer of a gene using an adenoviral vector was shown in 1991 by Ronald Crystal’s group (Rosenfeld et al. 1991), and the first clinical application of a vector delivering an intact cystic fibrosis transmembrane conductance regulator gene was done in 1993 as reviewed by Crystal (2014). In his review, the author reiterates in retrospect the success and feasible future applications for adenovirus-based vectors in human gene therapy. Due to the strong immune response against vector proteins, Crystal concludes that Ads may serve best in temporary applications to build new structures, to selectively destroy cells or as vehicles to induce immunity against a transgene. The authors of this chapter put the focus on the concepts of targeted adenoviral gene transfer by ablating the native tropism and reroute Ad vectors by altering their physical composititon or by covering the native particle with moieties directed against selected cell plasma surface structures. At the time of assembling this article, cumulatively over 2200 gene therapy trials in different clinical phases were completed or are ongoing with more than half of these clinical trials being dedicated to combat cancer. Amongst those, Ads are the most widely used gene transfer vehicles (Wirth et al. 2013; Das et al. 2015).
2.1.2 The Tools for Gene Therapy
The isolation of adenoviruses dates back to 1953 (Rowe et al. 1953). The family of Adenoviridae consists of four accepted genera (Davison et al. 2003; Cupelli and Stehle 2011) with some species awaiting assignment. Adenoviruses infect numerous vertebrates including man. Nowadays, about 57 human adenoviruses have been identified and were subclassified on the basis of parameters regarding classical standard methods, and more recently, by comparison of the adenoviral genomes (Seto et al. 2011). In the genus Mastadenovirus, the human pathogenic species are further divided into the subgroups A to G (Russel 2000; Berk 2013). The virions are built up of an icosahedral capsid that carries a single molecule of double-stranded DNA as the viral genome. The particles are studded with 12 fibers consisting of trimeric rigid structural elements linked to the icosahedron’s vertices. The capsid components forming the vertices are called penton bases and the fibers are non-covalently linked to these structures. Most virus serotypes bind to their target cells via the interaction of the fiber knob, the most distal part of the fiber with their counterpart, a protein structure on the cell plasma membrane. This moiety, the coxsackie adenovirus receptor (CAR) represents the main high-affinity receptor for many adenoviruses. CAR is a 46-kDa transmembrane protein in the superfamily of immunoglobulins (Bergelson et al. 1997; Roelvink et al. 1998; Bewley et al. 1999). Internalization of the virus depends on the subsequent secondary interaction of an Arginine-Glycine-Aspartate (RGD) protein structure on the penton base with αVβ3 or αVβ5 integrins also present on the cell plasma surface (Wickham et al. 1993). CAR is highly abundant in many tissues and therefore, adenoviruses display a wide tropism and infect a broad spectrum of cells (Wickham 2000; Arnberg 2009; Chailertvanitkul and Pouton 2010).
All of these characteristics drew an early focus on this virus family as masterpieces for gene therapy approaches. Unlike other viral vector systems, Ad virions have a high packaging capacity and easily replicate to high titers. Moreover, Ad-derived vectors maintain high stability in vivo and transduce both, dividing and non-dividing cells (Douglas 2004). Once internalized into the cell, the genome predominantly persists as episomal DNA with an extremely low frequency of integration into the host genome (Rauschhuber et al. 2012), and thereby, insertional mutagenesis does not occur. Considering this safety aspect, adenovirus based vectors are particularly attractive for gene therapy applications, where temporary gene expression is desired or preferred over permanent genetic modifications. Due to their apathogenicity and non-oncogenic properties, the most commonly utilized Ad vectors for gene therapy are derivatives from adenovirus Serotype 5 (Ad5) in the subgroup C of human adenoviruses (Arnberg 2009; Armendariz-Borunda et al. 2011). What ultimately converts the replicating agent into a one way delivery system is the purposeful removal of a part of the viral genome (Haj-Ahmad and Graham 1986).
Tissue tropism of adenoviral vectors is greatly influenced by the viral serotype and receptor presence and density, depending on a cell type’s provenance (Wickham et al. 1993; Hashimoto et al. 1997; Takayama et al. 1998; Havenga et al. 2002; Arnberg 2009). Ad5 are best explored and most widely used in preclinical studies as well as clinical trials. Ad5 particles bind exclusively to CAR. However, in the case of Ads made from Serotype 41, a subgroup F member, only one of its two distinct types of fibers can recognize CAR (Russel 2000; Zhang and Bergelson 2005). Some serotypes enter host cells via other receptors such as CD46, desmoglein 2, CD80 or CD86 or the sialic acid moiety (reviewed by Sharma et al. 2009). Altogether, Ad5 vectors are efficient vehicles for delivering foreign genes into target cells in vitro and in vivo (Haj-Ahmad and Graham 1986; Pützer et al. 1997). Based on the favourable attributes, Ad5 remains the vector of choice in human gene therapy trials due to their proven safety profile (Hedley et al. 2006; Das et al. 2015).
Whenever the utilization of adenoviral vectors as targeted gene delivery systems is desired, the Ad5 vector’s broad tropism for a wide range of cells and tissues is a challenging obstacle (Coughlan et al. 2010). If a gene transfer system is required that exclusively alters a single cellular compartment or a particular kind of tissue that spares all other cells and tissues from transduction, alteration of vector particles becomes essential. Another major obstacle after systemic adenoviral vector administration is that 80 % of circulating particles are sequestered in the liver after an interaction with coagulation factors (Shayakhmetov et al. 2005; Stone et al. 2007), and thereby, most particles may not reach the tissue to be addressed. A number of researchers demonstrated that after the virus is recognized by the coagulation system, the immune system is activated, and in turn, an acute inflammatory response is initiated (Reynolds et al. 2001; Shayakhmetov et al. 2004; Khare et al. 2011; Doronin et al. 2012; Xu et al. 2013). In addition, regarding Ad transport in the bloodstream, Duffy and colleagues (2013) identified a number of small molecules capable of efficiently blocking the intracellular virus transport independently of factor X-associated inactivation. Considering these hurdles, a selective gene transfer by wild-type adenoviral vectors imposes an increased risk of toxicity due to vector dissemination to non-targeted cells, even if the particles are administered close to or directly into the tissue of interest. Other undesired side effects of systemic virus administration are virus-associated immunogenic toxicity, thrombocytopenia, intense periportal polymorphonuclear lymphocyte infiltration and elevated liver enzyme secretion (Morral et al. 2002; Thacker et al. 2009a; Coughlan et al. 2012).
The reverse obstacle is the question of how to reach cell types refractory to adenoviral infection, due to their lack of or insufficient CAR expression. Such cells include, for instance, many cancer cells, as well as hematopoietic and neural stem cells (McConnell and Imperiale 2004; Schmidt et al. 2005). To achieve gene transfer into those cell types and to ensure efficient integrin receptor-mediated virus uptake, extremely high vector doses are required. High vector doses in turn increase inadvertent side effects, like viral sequestering in Kupffer cells in the liver (Haisma et al. 2009), and once vectors surpass the latter’s binding capacity, hepatocytes will absorb the remaining vector particles.
The restrictions outlined above can be overcome by strategies to modify the vector’s cellular tropism , as reviewed by Beatty and Curiel (2012). Redirecting vectors towards cells of interest can also enhance the therapeutic potential with increased safety by reduction of immune responses, since simultaneous re- and de-targeting allows lower vector doses to be administered systemically (Schmidt et al. 2007; Dorer und Nettelbeck 2009; Haisma et al. 2010; Schmidt et al. 2011). In this review, we present and discuss three different methods to alter the natural Ad vector tropism. We describe genetic integration of peptide sequences into the fiber, peptide conjugation via chemical modification using polyethylene glycol (PEG) and bridging the vector and cell of choice with bispecific adaptor molecules. The advantages and benefits, as well as restrictions and limitations of these technological approaches are reported and debated. The initial considerations towards targeting, however, relate to the identification of suitable moieties on the plasma membrane of the cells or the tissue to be addressed that fulfil the following characteristics: singularity, abundance, and affinity.
2.2 Screening Methods for Cell-Specific Ligands
The ultimate gene “taxi” for systemic gene therapy purposes should exclusively recognize the cells to be treated and leave all others unaffected. Directed gene delivery can be achieved by addressing selective moieties on the cells of interest. Specifically selected peptides possess appropriate properties to serve as targeting agents and are valid alternatives to antibody-based targeting approaches, since unique cellular receptors are often unknown.
The simplest way to design a specific binding peptide for a receptor is to analyse structural data and the expression profile of cells to identify candidate peptides. Often however, structural data are not available or incomplete. To solve this, the phage display method is a frequently used technique to determine specific binding peptides. We and others have used the phage display technology to screen for and to identify tissue- or cell-specific ligands in cell culture systems and animal models (Böckmann et al. 2005a, b; Vives et al. 2008, Reetz et al. 2013). Already in 1990, researchers constructed an epitope library that yielded a mixture of filamentous phage clones with each one displaying a single peptide sequence on the virion’s surface (Scott and Smith 1990). After the interaction of the phage with the specific binding partner, the expansion of the phage comprehends several rounds of infection followed by selection. The display of polypeptide repertoires on the surface of phages, together with the efficient enrichment and amplification of the desired binding specificities was shown to be a valuable route towards isolation of unique peptides suitable to serve as vehicles for targeting applications (Arap et al. 1998; Nicklin et al. 2000; Essler and Ruoslahti 2002; Dias-Neto et al. 2009; Chen et al. 2010; Kügler et al. 2013). Taken together, the phage display technique identifies peptides in a range from 7 to 12 amino acids (Vives et al. 2008).
Phage display was successfully employed to acquire peptides that specifically recognize human embryonic progenitor cells (Bignone et al. 2013) and bind normal or aberrant tissues, like vascular endothelium (Pasqualini and Ruoslahti 1996, 2000; White et al. 2001), lymphatic vessels (Laakkonen et al. 2002), kidney tubules (Odermatt et al. 2001), hepatocytes (Piccolo et al. 2014), and several others (Barry et al. 1996; Ravera et al. 1998; Ivanenkov et al. 1999; Mazzucchelli et al. 1999; Cheung et al. 2013; Sclavons et al. 2013). Furthermore, the lack of gene transfer systems that are potent in selectively targeting tumor tissue prompted the search for cancer-specific peptide molecules for yet unknown tumor-associated receptors (Araki et al. 2010; Deutscher et al. 2010; Ahmadvand et al. 2011; Nishimoto et al. 2012). Many novel peptides homing to angiogenic vessels showed cross-affinity with several tumor types (Liu and Wu 2008). In this regard, we conducted biopanning on human medullary thyroid carcinoma (MTC) cells in vitro and transplanted tumor xenografts in vivo (Böckmann et al. 2005a). MTC , which is caused by dominant activating mutations in the RET proto-oncogene encoding a transmembrane tyrosine kinase receptor, is characterized by aggressive growth and early metastasis and therefore, provides a perfect model for targeting disseminated cancer cells (Drosten and Pützer 2006). The selected phages bound with highest specificity to and were internalized by these tumor cells in culture as well as after systemic injection into nude mice (Böckmann et al. 2005a). The same 7-mer cyclic phage peptide library was injected into the tail vein of RET oncogene transgenic mice carrying bilateral orthotopic tumors in their thyroid glands (Böckmann et al. 2005b). This ligand that also binds efficiently to human MTC cells was covalently linked to adenoviral capsids carrying a RET oncogene inhibitor as therapeutic gene. Systemic delivery of this peptide-tagged adenovirus led to a substantial growth reduction of orthotopic and disseminated xenograft tumors, while the interaction with other organs, such as the liver, was abolished (Schmidt et al. 2011). This precedent opens a promising new road towards using peptide-mediated adenoviral gene transfer to achieve an efficient and selective therapeutic response also against circulating and metastasis initiating tumor cells detected in an advanced stage of metastatic disease that possess features of cancer stem cells. Beyond that, other researchers took the first step in developing a molecular map of the human vasculature by screening a peptide library in patients (Arap et al. 2002; Chang et al. 2009; Seung-Min et al. 2009). Rangel and co-workers developed a novel technique that enables receptor-independent phage particle entry into mammalian cells. Phage particles provide a unique discovery platform for combinatorial intracellular targeting of organelle ligands along with their corresponding receptors and for fingerprinting functional protein domains in living cells (Rangel et al. 2012, 2013).
An alternative approach that aims at detecting molecules with high affinity, adequate specificity and suitable pharmacokinetic properties for in vivo applications is represented by single-stranded nucleic acid ligands, termed aptamers. Aptamers are isolated by the Systematic Evolution of Ligands by Exponential Enrichment (SELEX ) technology . Applying this technology against whole-living cells in culture or in vivo allows direct selection of aptamers even against rare antigens without prior purification of membrane-bound targets, access to membrane proteins in their native conformation and identification of targets related to a specific phenotype. Their thermal stability, low cost, unlimited applications and high binding affinity to disease-associated proteins or non-protein targets (Song et al. 2012; Wang et al. 2014; Mahlknecht et al. 2015) make them attractive, even in clinical trials for the treatment of distinct medical conditions, as reviewed extensively elsewhere (Sundaram et al. 2013; Tan et al. 2013; Zimbres et al. 2013). The potential of aptamers as tools for tumor targeting gene delivery systems with high transduction efficiency was summarized by others (Zhu et al. 2012; Hu and Zhang 2013). In this perspective, an innovative step towards targeted therapies would certainly be a combination of both technologies, cell-specific aptamers and adenoviral vectors.
2.3 Methods to Specifically Target Adenovirus-Derived Vectors
2.3.1 Genetic Fiber Engineering
To increase the selectivity of adenovirus for target tissues, novel approaches in Ad vector design exploit the concept of tissue-specific expression of therapeutic transgenes or virus replication. With the fiber being the major determinant of adenovirus tropism, the development of genetically targeted vectors has, consequently, focused on this distant protruding entity of the particle. The fiber is a rigid homotrimeric protein structure characterized by a domain organization with an N-terminal tail domain anchoring it in the Ad capsid. A C-terminal globular domain, termed the knob, mediates binding to CAR and a central shaft domain extends the knob away from the particle. Any manipulations of the fiber knob per se significantly reduce the transduction efficiency of CAR-positive cells by Ad vectors (Wickham et al. 1997; Krasnykh et al. 1998; Einfeld et al. 2001). Different strategies of adenovirus fiber modification have been employed, like genetic replacement of the fiber or ligand incorporation into the fiber knob. In this regard, the development of a fiber phage display system (Nishimoto et al. 2012) or a fiber-shuttle library for the adenoviral knobs (Wu et al. 2010) provide tools to alter Ad vector binding specificity.
Several studies narrowed down the insertion positions for targeting peptides within the fiber knob to two locations where the vector system as such tolerates the genetic alterations without structural impairment. The sites of choice for targeting ligand incorporation are the fiber knob’s HI loop, which connects the ß-strands H and I, and the C-terminus of the protein (Dmitriev et al. 1998; Krasnykh et al. 1998; Mizuguchi et al. 2001; Koizumi et al. 2003; Glasgow et al. 2004; Nettelbeck et al. 2004; Coughlan 2009; Wang et al. 2011). These findings indicated that ligands whose sizes exceed 25 to 30 amino acid residues cannot be configured into the carboxy-terminus of the fiber, as they destabilize the fiber structure (Wickham et al. 1997) and thus, limit the range of potential ligand candidates to short peptides. The structural properties of the HI loop of the Ad fiber, however, favor the insertion of larger ligands and expand the size of potential targeting moieties. Meanwhile Behr and colleagues (2014) elaborated on insertion of a peptide ligand to target the receptor tyrosine kinase EphA2 by introducing a previously selected peptide into different positions of a chimeric fiber derived from Ad5 and Ad 41 with striking permissiveness concerning the insertion site into several loops of the fibers.
When testing the resilience of fiber modification, Belousova and co-workers (2002) incrementally increased the size of the peptides integrated in the HI loop and generated Ad vectors with fiber inserts ranging from 13 to 83 amino acid residues. The authors concluded that the incorporation of heterologous sequences in the examined size ranges was essentially tolerated without a negative impact or compromising the production yield or transductional properties of the vectors. HI-loop incorporation of rather short 7- and 9-mer peptides was performed to transduce CAR-deficient primary tumor cells, such as ovarian cancer cells, vascular endothelia, vascular smooth muscle cells, and brain microcapillary endothelia in culture (Dmitriev et al. 1998; Nicklin et al. 2000, 2003; Xia et al. 2000; Work et al. 2004). The last step of Ad entry into target cells depends on the interaction between RGD motif at the penton base protein and the host cell integrins (Cao et al. 2012). Ad vectors containing this RGD peptide in the HI loop of the fiber showed higher yields of gene transfer than vectors containing the identical peptide attached at the fiber’s C-terminus, due to the easy access to the receptor (Kurachi et al. 2007; Tanaka et al. 2007; Schmidt et al. 2011). Several researchers efficiently transduced different types of tumor cells by inserting this RGD motif into the HI loop of the Ad fiber in vitro (Terao et al. 2009) and in vivo (Bayo-Puxan et al. 2009; Katayama et al. 2011). Rojas et al. (2012) improved systemic antitumor therapy with oncolytic adenoviruses by replacing the fiber shaft heparin sulfate glycosaminoglycan-binding domain with RGD in order to achieve simultaneously liver de- and tumor re-targeting.
Each fiber knob monomer forms an eight-stranded antiparallel ß-sandwich structure. The ß-strands are connected with turns and loops (Bewley et al. 1999). To further reduce the transduction efficiency of Ad vectors to CAR-positive cells, elaborate mutation studies on the AB, DE or FG loop of the fiber knob have been reported (Roelvink et al. 1999; Jakubczak et al. 2001; Leissner et al. 2001). In order to completely ablating a vector particle’s tropism from CAR binding and eventually entering the cell, genetic fiber modification is not sufficient. Rather, the secondary minor interaction of the RGD motif at the penton base with the αv-integrin receptor should be depleted as well (Mizuguchi et al. 2002), to fully ablate Ad vectors from the native binding moieties. Whereas the dual mutation markedly reduces the retention of the vector in the liver (Einfeld et al. 2001; Koizumi et al. 2003), single mutations in the fiber knob or penton base did not alter the biodistribution of adenoviral vectors injected into mice (Alemany and Curiel 2001; Leissner et al. 2001; Nakamaura 2003; Smith et al. 2003a, b). In order to use Ads in cancer gene therapy, gene transduction to tumor cells is limited by the weak expression of CAR on these cells, as reviewed by (Tanaka et al. 2007). Magnusson and co-workers (2007) efficiently transduced human ovarian and breast cancer cell lines with a vector that carried a tandem repeat of the human epidermal growth factor receptor 2 (HER2/neu) reactive affibody molecule in the HI loop of a CAR ablated fiber knob. Later, the group generated a vector with dual specificity by incorporating the HER2/neu-binding (ZH) and Taq polymerase-binding (ZT) sequences at different positions within the HI-loop. Receptor-binding studies revealed that ZT in the first position and ZH in the second position bound to both receptors, whereas the reverse order of both motifs was devoid of binding to HER2/neu (Myhre et al. 2009). Subsequently, these researchers designed a vector to transduce efficiently HER2/neu-presenting cell lines, by altering the RGD motif to EGD (Glu-Gly-Asp) and substituting the KKTK motif in the third shaft repeat to RKSK (Arg-Lys-Ser-Lys). This new vector at last gained the ability to efficiently infect prostate cancer cells in vitro (Magnusson et al. 2012).
Genetic modification also covers the replacement of the entire fiber or just the knob domain with that derived from other adenovirus serotypes (Wu et al. 2002). Belousova et al. (2003) targeted an Ad vector with bacteriophage T4 fibritin to the CD40 receptor. The tropism was modified by incorporating into the virion capsid a recombinant protein comprising structural domains of the Ad Serotype 5 fiber, phage T4 fibritin, and the human CD40 ligand. The authors achieved specific gene delivery in monocyte-derived dendritic cells in vivo. In a pilot vaccination study, Thacker and colleagues (2009b) successfully targeted these cells in a canine model by integrating the CD40 ligand into the fiber knob. The same group reported later that Ad vectors bound to the CD40 ligand failed to infect integrin-deficient canine lymphoma cells. This study demonstrates that the lack of virus internalization signals can impair targeting approaches (O’Neill et al. 2011).
Yu and co-workers (2011) modified the Ad5 hexon protein by inserting the protein transduction domain from the HIV-1 Tat protein. The resulting viral vector showed significantly higher transduction efficiency on many tumor cells compared to the parental vector. In the next step, this group increased the infection efficiency of human primary cell types even further after swapping wild-type Ad5 fiber against a Serotype 35 fiber specific for the CD46 receptor, a surface marker often upregulated in malignant tumors. This surface modified Ad vector was developed to transduce cells that are otherwise difficult to transduce in basic, pre-clinical, and clinical research (Yu et al. 2013). Another strategy to reroute adenoviral vectors from healthy towards cancer tissue is the utilization of recombinant adenovirus. The Lieber group constructed a capsid-modified adenovirus that expresses the TNF-related apoptosis-inducing ligand (TRAIL) and specifically replicates in tumor cells (Sova et al. 2004). Their Ad capsid contains the Serotype 35-derived short-shafted fibers, which recognize the CD46 receptor. In combination with TRAIL , expression of this oncolytic vector induces apoptosis in tumor cell lines derived from human colorectal, prostate, lung, and liver cancer. Both, the cell culture and xenograft tumor models tested in these experiments showed efficient intratumoral spread of the virus.
Yet others designed Ad vectors presenting the short fibers of Ad41 as a ligand insertion tool, achieving higher infection efficiency when compared to viruses presenting the same ligand cloned into another part of the fiber (Hesse et al. 2007). Even an enhanced transduction efficiency of recombinant adenovirus Serotype 5 vectors with Serotype 35 fibers was observed by Matsui and co-workers (2011). Using a feasible in vitro ligation, the group incorporated two copies of the RGD peptide in two different loops of the fiber 35 knob and observed high infection efficiencies in CD46-positive cells.
Overall and despite these positive results, genetic modifications on the native Ad5 fiber knob remain a laborious technical challenge and its benefit is hard to calculate. The repertoire of incorporable ligands to yield functional retargeted vectors for gene therapy is restricted to a small number of peptides that do not impair correct folding and assembly of the fiber trimer (Magnusson et al. 2002). A general limitation of this approach is the necessity to tediously re-engineer a given Ad vector for every further target cell. On the other hand, if common motifs were found for instance on the surface of cancer cells, corresponding metastases, and/or circulating cancer stem cells, the workload and more fine-tuning on fiber manipulations may pay off. With oncolytic viruses, the agents engineered from wild-type replicating pathogens and designed to specifically deplete neoplastic cells, both, in solid tumors and straying through the body, chimeric fibers gain further attention. Oncolytic viruses are by definition restricted to replicate only in neoplastic cells and supposed to deliver a payload that attracts the immune system or restores apoptotic pathways (Dias et al. 2012; Schipper et al. 2014) and many ongoing clinical trials rely on the power of this concept (Pol et al. 2014; Jiang et al. 2015). To target pancreatic cancer, Nishimoto and co-workers (2009) screened a peptide-display adenovirus library. They came up with a selected peptide incorporated in the fiber of a virus ablated for CAR interaction. The technique based on a previous paper of the same group showing the validity of incorporating random sections of genomic DNA and screening of the resulting viruses for specifity for a desired cell type (Miura et al. 2007). The virus yielded potent cancer cell killing than the parental virus only deficient for CAR binding.
More recent setups towards fiber modification came for instance from the Curiel group. In a think out of the box approach, the researchers utilized antibodies from a member or the camelid family, the alpaca. Immunoglobulin of these animals consists of heavy chains only. The group immunized the animals with the human carcinoembryonic antigen and, in brief, replaced the adenovirus knob and part of the shaft with protein fragments directed against the antigen which transduced target cells that carried the original epitope (Kaliberov et al. 2014b). Jose and colleagues (2014) made a tumor selective adenovirus altering the fiber in a way that it gets activated by cancer-specific proteases. This approach can be fruitful for both, vector design and the development of virotherapeutic agents.
2.3.2 Alteration of Vector Tropism by PEGylation
Targeting an adenoviral vector can be achieved without altering the fiber by genetic modification. One option to ablate and redirect an adenoviral vector’s tropism is to coat the otherwise native particle via covalently attached small molecules. This can be achieved by means of bispecific non-toxic spacer molecules. Polyethylene glycol (PEG) is a linear hydrophilic polymer and often used in pharmaceutic formulations involving proteins and peptides to prolong their blood persistence (Mizuguchi and Hayakawa 2004). In addition, PEG is, therefore, considered as a bridging entity between the capsid and peptide of choice for a given cell plasma membrane target. Optimized transduction by targeted Ad vectors can be accomplished by linking cell-specific peptides, antibodies or antigens to the particle’s surface by a chemical process called PEGylation (Kreppel and Kochanek 2008). PEG forms a covalent bridge between the proteins of the virion’s surface and the targeting molecule of choice, resulting in a vector coated all over with the desired ligand. This approach of redirecting viral vectors does not require genetic modification, including the efforts to ablate the native tropism. Additional benefits of PEGylated vectors are reduced immunogenicity (Elkon et al. 1997; Zaiss et al. 2002; Croyle et al. 2004; Mok et al. 2005), fewer hepatotoxic side effects (Gao et al. 2007), less cytokine secretion and prolongation of the vector plasma half-life (Alemany et al. 2000).
Such PEG-driven Ad vector modifications have been adapted in a number of targeting approaches (O’Riordan et al. 1999; Alemany et al. 2000; Croyle et al. 2002; Lee et al. 2005; Hofherr et al. 2008; Eto et al. 2010; Wonagan and Croyle 2010). The success of such approaches might depend on the ligand size. Romanczuk and co-workers (1999) were the first to link biologically selected peptides to Ads surface via PEGylation. For instance, coupling of a short RGD motif to the tip of PEG has shown both, high in vitro transduction efficiency (Lanciotti et al. 2003; Eto et al. 2004, 2005¸ Ogawara et al. 2004) and overall improvement of systemic gene delivery (Xiong et al. 2006; Gao et al. 2007). To target ovary cancer cells, the full-length fibroblast growth factor 2 (FGF2) was linked to an Ad vector by PEGylation. This vector mediated increased transgene expression in tumor tissue and reduced localization of adenovirus to non-target cells when compared to the native vector (Lanciotti et al. 2003). To silence the proinflammatory activation status of endothelial cells, Kuldo and colleagues (2013) demonstrated the potential of an E-selectin targeted adenoviral vector to deliver a therapeutic transgene into microvascular endothelial cells in inflammation and downregulate the endothelial adhesion molecule. Besides ablating the native tropism and to redirect a vector to another cellular receptor, the PEGylation treatment prolongs the circulation time and decreases the formation of neutralizing antibodies as shown by Kim and colleagues (2011). A PEGylated Ad that recognizes Her2/neu receptor-positive cancer cells showed longer circulation times than the unmodified control and decreased the level of neutralizing antibodies. These observations raise positive expectations for future therapeutic applications of PEGylated vectors against late-stage cancer diseases. Exploring the suitability of PEGylated Ad vectors to address metastatic tumors, a dual cancer-specific strategy was described using this technology for transductional targeting with transgene expression under control of the telomere reverse transcriptase promoter for transcriptional targeting (Yao et al. 2009).
With regard to the conclusion that the molecular weight of PEG and the PEG modification ratio significantly affects the characteristics of conjugates (Kaneda et al. 2004), Eto and colleagues (2010) optimized adenovirus PEGylation in a way that after systemic administration of PEGylated adenoviral vector expressing tumor necrosis factor-alpha, an antibody reduction against Ad and an increased therapeutic response was observed against metastatic cancer. In a rather elegant experimental setup, Yao and co-workers demonstrated that the CGKRK (Cys-Gly-Lys-Arg-Lys) peptide conjugated to Ad with PEG was highly selective and yielded good gene expression in tumors and tumor vasculatures after systemic administration (Yao et al. 2011, 2012). Their results indicate an important aspect to consider if working with Ads coated with PEG. The appropriate ratio between PEG and targeting ligand concentration is crucial to achieve specific tissue transduction. As described above, the latest success in the treatment of disseminated tumors in mice was made by injecting a low dose with 108 plaque forming units per animal of Ad vector encoding RET oncogene inhibitor coated with MTC-specific 7-mer peptide via PEG into the tail vein, which led to the regression of multiple orthotopic and xenograft tumors in mouse models (Schmidt et al. 2011). The same Ad-PEGylation approach using a short artificial peptide selected by phage display, which in this case, specifically binds neural stem cells isolated from the hippocampus of adult mice, was highly effective after injecting the vector into the brain (Schmidt et al. 2007). Such tools could eventually serve to exclusively manipulate neural stem cells either by direct injection in the brain or systemic vector application with the potential as a delivery system for therapeutic genes to treat various disorders of the central nervous system.
Another chemical Ad modification using diblock copolypeptides as an alternative for PEG was first described by Jiang and co-workers (2013). Copolypeptides are well-defined polypeptide sequences (Deming 1997) which provide non-covalent Ad vector modification resulting in efficiently altering Ad tropism with an obvious potential to target cancer metastases. PEGylation has also been used to link cell-penetrating peptides to adenoviral particles to overcome obstacles with the CAR ablation (Nigatu et al. 2015). Whether this treatment will have advantages when targeting the particles to specific cells remains to be seen.
2.3.3 Bifunctional Non-Covalently Linked Adaptor Molecules
Another way for re-directing and widening Ad vector tropism is the application of bifunctional adaptor molecules or other bispecific protein fragments often derived from antibodies . Such adaptors recognize with one binding site the vector and the other the desired structure on the cell membrane. Bispecific antibodies are usually composed of an anti-fiber binding portion and a component specific for a cell-specific receptor or secondary antibody conjugated with a peptide moiety against a selected cell surface antigen. Since CAR does not play any role in virus internalization, the Ad fiber knob’s CAR binding domain accessibility is dispensable and therefore, the candidate of choice to link heterologous binding sites, for instance a bispecific adaptor molecule. A fully studded Ad vector particle with a bridging molecule prevents any interaction with CAR and thus, ablates Ad’s native tropism (Curiel 1999; Everts and Curiel 2004; Glasgow et al. 2006).
In an initial demonstration of CAR-independent targeting, a conjugate consisting of folate and a fragment derived from an antibody directed against the fiber was used as a recombinant protein to bind the Ad fiber as well as the target, the folate receptor, which is widely expressed on the surface of numerous malignant cells (Douglas et al. 1999). In a similar strategy, a conjugated FGF was used to target ovarian carcinoma cells (Rancourt et al. 1998). The approach reached a clinical trial, where FGF2-conjugated Ad vector expressing human herpes simplex virus thymidine kinase was applied in patients (Bauerschmitz et al. 2002). Reynolds and colleagues (2000) succeeded in targeting pulmonary endothelial cells in vivo by i.v. injection of Ad vectors bearing fibers studded with a bispecific antibody directed against the Ad fiber knob and the angiotensin-converting enzyme.
In light of the development of new therapeutic strategies for diseases in which angiogenesis plays an important role and considering that physiological barriers for high molecular weight components prevent the transduction of the majority of tumor cells, vascular targeting became a worthwhile approach in cancer gene therapy (Griffioen and Molema 2000). Targeting of adenovirus to endothelial cells by a bispecific fusion protein directed against the human endoglin CD105 receptor for antivascular cancer gene therapy was published by Nettelbeck and coworkers (2001). In 2004, the same group designed a single-chain adaptor molecule that binds the fiber protein and the high molecular weight melanoma-associated antigen. This antigen is widely and specifically expressed on the surface of melanoma cells and its expression is associated with tumor development and progression (Nettelbeck et al. 2004). Other bispecific constructs directing Ad fibers to cells were developed for endothelial receptors (Haisma et al. 2010), the epidermal growth factor receptor (Haisma et al. 2000; van Beusechem et al. 2000), and the lymphocyte antigen 6 complex (van Zeeburg et al. 2010). An elegant approach uses a truncated soluble form of CAR as the virus attachment site fused to human epidermal growth factor (EGF) to direct a vector against cancer cells that express the EGF receptor (Dmitiriev et al. 2000; Kashentseva et al. 2002; Hemminki et al. 2002). In addition, a number of authors described the adaptor-based strategy to target CAR-deficient dendritic cells as a therapeutic vaccination against cancer or infectious diseases (Tillmann et al. 2000; Pereboev et al. 2004; Kim et al. 2010; Echeverria et al. 2011; Hangalapura et al. 2012; Williams et al. 2012). Figure 2.1 provides a schematic representation of all three strategies used to alter virus tropism described above.
Methods to alter adenovirus tropism. Wild-type adenovirus enters target cells after binding the coxsackie-adenovirus receptor (CAR), an entity present on a wide number of cell types (a). Ablation of CAR binding and re-directing adenovirus-derived vectors towards the cells of choice by means of specific peptides can be achieved by genetically integrating the peptide into the fiber knob (b), chemically coating the vector particle with bi-specific polyethylene glycol (c) or through bridging by means of a bifunctional adapter molecule (d)
Watkins and colleagues (1997) utilized a construct that encodes a fusion protein derived from a single-chain neutralizing anti-adenovirus fiber antibody, designated S11, fused to a specific peptide ligand directed against cellular receptors, termed the bispecific adaptor molecule. Coating virus with this adaptor molecule ablates CAR binding and directs the viral particle to the desired cellular receptor. S11 can be produced in eukaryotic, as well as prokaryotic cells . By means of its 6-His-tag, purification and concentration of the fusion protein can be easily performed by nickel-affinity chromatography. This procedure ensures the high yield of pure protein without the loss of activity.
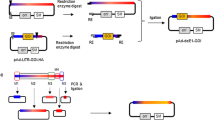
Based on the S11 strategy , we intended to specifically transduce in vitro and in vivo activated hepatic stellate cells (HSCs), whose number is increased in fibrotic livers (Reetz et al. 2013). Therefore, we picked a peptide derived from nerve growth factor (NGFp) with specific affinity for the p75 neurotrophin receptor (p75NTR) present on activated HSCs. Coating the GFP-expressing Ad vector with NGFp was done either via chemical conjugation using bifunctional PEG or, alternatively, by molecular bridging with an S11-based fusion protein specific for viral fiber knob and p75NTR (S11-NGFp; Fig. 2.2). After systemic administration of the targeted viral particles, we observed that Ad.GFP-S11-NGFp transduced activated HSCs better than Ad.GFP-PEG-NGFp. The latter’s low transduction potential could be explained either by an improper ratio between PEG and targeting ligand concentration that prevented successful and specific tissue transduction or due to the ablation of the viral internalization signals such as the RGD motif by the chemical procedure. These experiments contributed to the development of a targeted gene transfer system to specifically deliver antifibrotic compounds into activated HSCs by systemically applied adenoviral vectors modified by the NGFp ligand. In our study, we demonstrate that adenoviral-mediated targeting of HSCs via p75NTR, concurrently avoiding its binding to hepatocytes, provides a potentially feasible and effective strategy for therapeutic gene delivery to activated HSCs in the liver in vivo and the technique may be useful to support approaches to regenerate liver tissue (Best et al. 2015; Salazar-Montez et al. 2015). Beyond, Haisma and co-workers (2010) observed a selective targeting of Ad5 to the endothelial receptors in vitro and obtained viral transgene expression only in tumors infected with adenobody retargeted adenovirus from mice bearing subcutaneously colon carcinoma cell derived tumors.
Specific targeting of hepatic stellate cells (HSC). Shown are two different HSC-specific retargeting strategies for adenoviral vectors by linking NGFp. This peptide has specificity for p75NTR, an entity expressed by HSC but not hepatocytes. NGFp was attached to the vector’s surface via chemical coupling by PEGylation or as part of the bispecific single-chain immunoglobulin-derived adapter molecule S11. Top: Wild-type vectors enters target cells after binding the coxsackie-adenovirus receptor (CAR), the receptor present on a wide range of cell types including hepatocytes and HSC. Center, left: the edging formed by PEGylation shields the entire vector surface including RGD-binding motifs and exposes NGFp. Center, right: the S11 adapter molecule is stoichiometrically attached to the fiber knobs. Bottom, left: p75NTR-binding via PEG-NGFp. Bottom, center: S11-NGFp-mediated binding of the HSC-specific moiety. Bottom, right: both coupling methods ablate the interaction of the vector particle with CAR (Reetz et al. 2013)
Most recently, we utilized the S11-based Ad targeting method to transduce exclusively neural stem cells in the subventricular zone of adult mice (Reetz et al. 2015). Like in our previous in vivo study on HSC transduction, a relevant peptide to be fused to S11 was beforehand detected with the phage-display and biopanning technique (Schmidt et al. 2007). For easy production of the fusion protein, we established by means of lentiviral transduction, a eukaryotic cell line that permanently secretes the bivalent adaptor. Thinking further, bivalent vector targeting approaches may also allow the creation of so-called mosaic modified vectors for instance for patients suffering from metastatic cancer in analogy to work by Pereboeva and colleagues (2004, 2007). The researchers targeted EGFR with a metabolically biotinylated fiber-mosaic adenovirus and demonstrated enhanced binding of vectors with heterologous fiber trimming. Utilizing heterologous fibers, a recent pancreatic cancer therapy approach improved the effect of conditionally replicating adenovirus-based oncolytic virus (Kaliberov et al. 2014a). This approach opens applications adenoviral vectors to target cells where single targeting molecules are sparce. Different bivalent adaptors on the same particle may enhance transduction of such cells.
A potential alternative to targeting approaches by single-chain antibodies are Ad vectors coated with adaptor molecules based on designed ankyrin repeat proteins (DARPins) . DARPins differ from antibodies in size, structure, binding pattern, and stability. These properties paired with high-yield, easy production in E. coli makes them promising candidates for targeting purposes. Dreier and colleagues (2011, 2013) designed an adaptor molecule consisting of two DARPin modules fused to each other. One binding site anchors the molecule to the Ad fiber knob and the other enables the particle to attach to tumor cell markers, like the human epidermal growth factor receptor or the epithelial cell adhesion molecule. In their work, the authors convincingly demonstrate that DARPins are high-affinity adaptor molecules that allow efficient gene transfer and are a promising tool to rapidly target Ad vectors to any desired receptor.
To alter the tropism of adenoviral vectors, the recombinant fusion protein technology offers a number of technical advantages compared to the methods of chemical conjugation. The conveniences of rerouting adenoviral tropism using recombinant proteins include simplified production in prokaryotic or, preferably, in eukaryotic expression systems, as well as vector purification. In addition, this approach may allow the application of different fusion proteins suitable for retargeting Ad to other receptors, simply by the substitution of the peptide ligand. This procedure offers, according to our experience, the method of choice to retarget Ad vectors.
2.4 Conclusions
In conclusion, adenoviral vectors have been proven to serve as efficient tools for gene delivery when temporary gene expression is beneficial. The major challenge towards applying the technology remains the development of a target system for specific gene delivery that reaches a high level of efficiency. Genetic approaches to modify the fiber require tediously re-engineering of a given Ad vector, and PEGylation causes decreased transduction efficiencies due to improper PEG to ligand ratios as well as RGD ablation, bifunctional adaptor molecules seem to be the most favorable targeting approach. An expeditious and simple production followed by a broad portfolio of different fusion proteins suitable to retarget Ad by substitution peptide ligands offers a standardized method to retarget vectors for both in vitro and/or in vivo applications. Moreover, engineering of bifunctional adaptors may be customized more easily than fiber modifications and chemical treatment of vector preparations. Increased knowledge of adenovirus biology and powerful techniques such as the phage display method to identify new cell or tissue specific targets as well as unique receptors on neoplastic cells provide the opportunity to develop innovative strategies for gene therapy. Custom-made gene shuttles that exclusively deliver into cells to be addressed a therapeutic nucleic acid or a potent inhibitor of pathogenic genes may, in future, allow success in the treatment of patients with systemic disease first and foremost with metastasized cancer.
References
Ahmadvand D, Rahbarizadeh F, Moghimi SM (2011) Biological targeting and innovative therapeutic interventions with phage-displayed peptides and structured nucleic acids (aptamers). Curr Opin Biotechnol 22:832–838
Alemany R, Curiel DT (2001) CAR-binding ablation does not change biodistribution and toxicity of adenoviral vectors. Gene Ther 8:1347–1353
Alemany R, Suzuki K, Curiel DT (2000) Blood clearance rates of adenovirus type 5 in mice. J Gen Virol 81:2605–2609
Araki K, Yamashita T, Reddy N, Wang H, Abuzeid WM, Khan K, O’Malley BW Jr, Li D (2010) Molecular disruption of NBS1 with targeted gene delivery enhances chemosensitisation in head and neck cancer. Br J Cancer 103:1822–1830
Arap W, Pasqualini R, Ruoslahti E (1998) Cancer treatment by targeted drug delivery to tumor vasculature in a mouse model. Science 279:377–380
Arap W, Kolonin MG, Trepel M, Lahdenranta J, Cardo-Vila M, Giordano RJ, Mintz PJ, Ardelt PU, Yao VJ, Vidal CI, Chen L, Flamm A, Valtanen H, Weavind LM, Hicks ME, Pollock RE, Botz GH, Bucana CD, Koivunen E, Cahill D, Troncoso P, Baggerly KA, Pentz RD, Do KA, Logothetis CJ, Pasqualini R (2002) Steps toward mapping the human vasculature by phage display. Nat Med 8:121–127
Armendariz-Borunda J, Bastidas-Ramirez BE, Sandoval-Rodriguez A, Gonzalez-Cuevas J, Gomez-Meda B, Garcia-Banuelos J (2011) Production of first generation adenoviral vectors for preclinical protocols: amplification, purification and functional titration. J Biosci Bioeng 112:415–421
Arnberg N (2009) Adenovirus receptors: implications for tropism, treatment and targeting. Rev Med Virol 19:165–178
Barry MA, Dower WJ, Johnston SA (1996) Toward cell-targeting gene therapy vectors: selection of cell-binding peptides from random peptide-presenting phage libraries. Nat Med 2:299–305
Bauerschmitz GJ, Barker SD, Hemminki A (2002) Adenoviral gene therapy for cancer: from vectors to targeted and replication competent agents (review). Int J Oncol 21:1161–1174
Bayo-Puxan N, Gimenez-Alejandre M, Lavilla-Alonso S, Gros A, Cascallo M, Hemminki A, Alemany R (2009) Replacement of adenovirus type 5 fiber shaft heparan sulfate proteoglycan-binding domain with RGD for improved tumor infectivity and targeting. Hum Gene Ther 20:1214–1221
Beatty MS, Curiel DT (2012) Adenovirus strategies for tissue-specific targeting. Adv Cancer Res 115:39–67
Behr M, Kaufmann JK, Ketzer P, Engelhardt S, Mück-Häusl M, Okun PM, Petersen G, Neipel F, Hassel JC, Ehrhardt A, Enk AH, Nettelbeck DM (2014) Adenoviruses using the cancer marker EphA2 as a receptor in vitro and in vivo by genetic ligand insertion into different capsid scaffolds. PLoS One 9:e95723
Belousova N, Krendelchtchikova V, Curiel DT, Krasnykh V (2002) Modulation of adenovirus vector tropism via incorporation of polypeptide ligands into the fiber protein. J Virol 76:8621–8631
Belousova N, Korokhov N, Krendelshchikova V, Simonenko V, Mikheeva G, Triozzi PL, Aldrich WA, Banerjee PT, Gillies SD, Curiel DT, Krasnykh V (2003) Genetically targeted adenovirus vector directed to CD40-expressing cells. J Virol 77:11367–11377
Bergelson JM, Modlin JF, Wieland-Alter W, Cunningham JA, Crowell RL, Finberg RW (1997) Clinical coxsackievirus B isolates differ from laboratory strains in their interaction with two cell surface receptors. J Infect Dis 175:697–700
Berk AJ (2013) Adenoviridae. In: Knipe DM, Howley PM (eds) Field’s virology, 6th edn. Lippincott Williams & Wilkins, Philadelphia, pp 1704–1731
Best J, Manka P, Syn WK, Dolle L, van Grunsven LA, Canbay A (2015) Role of liver progenitors in liver regeneration. Hepatobiliary Surg Nutr 4:48–58
Bewley MC, Springer K, Zhang YB, Freimuth P, Flanagan JM (1999) Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science 286:1579–1583
Bignone PA, Krupa RA, Sternberg H, Funk WD, Snyder EY, West MD, Larocca D (2013) Identification of human embryonic progenitor cell targeting peptides using phage display. PLoS One 8:e58200
Böckmann M, Drosten M, Pützer BM (2005a) Discovery of targeting peptides for selective therapy of medullary thyroid carcinoma. J Gene Med 7:179–188
Böckmann M, Hilken G, Schmidt A, Cranston AN, Tannapfel A, Drosten M, Frilling A, Ponder BA, Pützer BM (2005b) Novel SRESPHP peptide mediates specific binding to primary medullary thyroid carcinoma after systemic injection. Hum Gene Ther 16:1267–1275
Cao C, Dong X, Wu X, Wen B, Ji G, Cheng L, Liu H (2012) Conserved fiber-penton base interaction revealed by nearly atomic resolution cryo-electron microscopy of the structure of adenovirus provides insight into receptor interaction. J Virol 86:12322–12329
Chailertvanitkul VA, Pouton CW (2010) Adenovirus: a blueprint for non-viral gene delivery. Curr Opin Biotechnol 21:627–632
Chang DK, Chiu CY, Kuo SY, Lin WC, Lo A, Wang YP, Li PC, Wu HC (2009) Antiangiogenic targeting liposomes increase therapeutic efficacy for solid tumors. J Biol Chem 284:12905–12916
Chen CY, May SM, Barry MA (2010) Targeting adenoviruses with factor x-single-chain antibody fusion proteins. Hum Gene Ther 21:739–749
Cheung CS, Lui JC, Baron J (2013) Identification of chondrocyte-binding peptides by phage display. J Orthop Res 31:1053–1058
Coughlan L, Vallath S, Saha A, Flak M, McNeish IA, Vassaux G, Marshall JF, Hart IR, Thomas GJ (2009) In vivo retargeting of adenovirus type 5 to αvβ6 integrin results in reduced hepatotoxicity and improved tumor uptake following systemic delivery. J Virol 83:6416–6428
Coughlan L, Alba R, Parker AL, Bradshaw AC, McNeish IA, Nicklin SA, Baker AH (2010) Tropism-modification strategies for targeted gene delivery using adenoviral vectors. Viruses 2:2290–2355
Coughlan L, Vallath S, Gros A, Gimenez-Alejandre M, van Rooijen N, Thomas GJ, Baker AH, Cascallo M, Alemany R, Hart IR (2012) Combined fiber modifications both to target αvβ6 and detarget the coxsackievirus-adenovirus receptor improve virus toxicity profiles in vivo but fail to improve antitumoral efficacy relative to adenovirus serotype 5. Hum Gene Ther 23:960–979
Croyle MA, Chirmule N, Zhang Y, Wilson JM (2002) PEGylation of E1-deleted adenovirus vectors allows significant gene expression on readministration to liver. Hum Gene Ther 13:1887–1900
Croyle MA, Callahan SM, Auricchio A, Schumer G, Linse KD, Wilson JM, Brunner LJ, Kobinger GP (2004) PEGylation of a vesicular stomatitis virus G pseudotyped lentivirus vector prevents inactivation in serum. J Virol 78:912–921
Crystal RG (2014) Adenovirus: the first effective in vivo gene delivery vector. Hum Gene Ther 25:3–11
Cupelli K, Stehle T (2011) Viral attachment strategies: the many faces of adenoviruses. Curr Opin Virol 1:84–91
Curiel DT (1999) Strategies to adapt adenoviral vectors for targeted delivery. Ann N Y Acad Sci 886:158–171
Das SK, Menezes ME, Bhatia S, Wang X-Y, Emdad L, Sarkar D, Fisher PB (2015) Gene therapies for cancer: strategies, challenges and successes. J Cell Physiol 230:259–271
Davison AJ, Benko M, Harrach B (2003) Genetic content and evolution of adenoviruses. J Gen Virol 84:2895–2908
Deming TJ (1997) Facile synthesis of block copolypeptides of defined architecture. Nature 390:386–389
Deutscher SL (2010) Phage display in molecular imaging and diagnosis of cancer. Chem Rev 110:3196–3211
Dias JD, Hemminki O, Diaconu I, Hirvinen M, Bonetti A, Guse K, Escutenaire S, Kanerva A, Pesonen S, Löskog A, Cerullo V, Hemminki A (2012) Targeted cancer immunotherapy with oncolytic adenovirus coding for a fully human monoclonal antibody specific for CTLA-4. Gene Ther 19:988–998
Dias-Neto E, Nunes DN, Giordano RJ, Sun J, Botz GH, Yang K, Setubal JC, Pasqualini R, Arap W (2009) Next-generation phage display: integrating and comparing available molecular tools to enable cost-effective high-throughput analysis. PLoS One 4:e8338
Dmitriev I, Krasnykh V, Miller CR, Wang M, Kashentseva E, Mikheeva G, Belousova N, Curiel DT (1998) An adenovirus vector with genetically modified fibers demonstrates expanded tropism via utilization of a coxsackievirus and adenovirus receptor-independent cell entry mechanism. J Virol 72:9706–9713
Dmitriev I, Kashentseva E, Rogers BE, Krasnykh V, Curiel DT (2000) Ectodomain of coxsackievirus and adenovirus receptor genetically fused to epidermal growth factor mediates adenovirus targeting to epidermal growth factor receptor-positive cells. J Virol 74:6875–6884
Dorer DE, Nettelbeck DM (2009) Targeting cancer by transcriptional control in cancer gene therapy and viral oncolysis. Adv Drug Deliv Rev 61:554–571
Doronin K, Flatt JW, di Paolo NC, Khare R, Kalyuzhniy O, Acchione M, Sumida JP, Ohto U, Shimizu T, Akashi-Takamura S, Miyake K, MacDonald JW, Bammler TK, Beyer RP, Farin FM, Stewart PL, Shayakhmetov DM (2012) Coagulation factor X activates innate immunity to human species C adenovirus. Science 338:795–798
Douglas JT (2004) Adenovirus-mediated gene delivery to skeletal muscle. Methods Mol Biol 246:29–35
Douglas JT, Miller CR, Kim M, Dmitriev I, Mikheeva G, Krasnykh V, Curiel DT (1999) A system for the propagation of adenoviral vectors with genetically modified receptor specificities. Nat Biotechnol 17:470–475
Dreier B, Mikheeva G, Belousova N, Parizek P, Boczek E, Jelesarov I, Forrer P, Plückthun A, Krasnykh V (2011) Her2-specific multivalent adapters confer designed tropism to adenovirus for gene targeting. J Mol Biol 405:410–426
Dreier B, Honegger A, Hess C, Nagy-Davidescu G, Mittl PR, Grütter MG, Belousova N, Mikheeva G, Krasnykh V, Plückthun A (2013) Development of a generic adenovirus delivery system based on structure-guided design of bispecific trimeric DARPin adapters. Proc Natl Acad Sci U S A 110:E869–E877
Drosten M, Pützer BM (2006) Mechanisms of disease: cancer targeting and the impact of oncogenic RET for medullary thyroid carcinoma therapy. Nat Clin Pract Oncol 3:564–574
Duffy MR, Parker AL, Kalkman ER, White K, Kovalskyy D, Kelly SM, Baker AH (2013) Identification of novel small molecule inhibitors of adenovirus gene transfer using a high throughput screening approach. J Control Release 170:132–140
Echeverria I, Pereboev A, Silva L, Zabaleta A, Riezu-Boj JI, Bes M, Cubero M, Borras-Cuesta F, Lasarte JJ, Esteban JI, Prieto J, Sarobe P (2011) Enhanced T cell responses against hepatitis C virus by ex vivo targeting of adenoviral particles to dendritic cells. Hepatology 54:28–37
Einfeld DA, Schroeder R, Roelvink PW, Lizonova A, King CR, Kovesdi I, Wickham TJ (2001) Reducing the native tropism of adenovirus vectors requires removal of both CAR and integrin interactions. J Virol 75:11284–11291
Elkon KB, Liu CC, Gall JG, Trevejo J, Marino MW, Abrahamsen KA, Song X, Zhou JL, Old LJ, Crystal RG, Falck-Pedersen E (1997) Tumor necrosis factor α plays a central role in immune-mediated clearance of adenoviral vectors. Proc Natl Acad Sci U S A 94:9814–9819
Essler M, Ruoslahti E (2002) Molecular specialization of breast vasculature: a breast-homing phage-displayed peptide binds to aminopeptidase P in breast vasculature. Proc Natl Acad Sci U S A 99:2252–2257
Eto Y, Gao JQ, Sekiguchi F, Kurachi S, Katayama K, Mizuguchi H, Hayakawa T, Tsutsumi Y, Mayumi T, Nakagawa S (2004) Neutralizing antibody evasion ability of adenovirus vector induced by the bioconjugation of methoxypolyethylene glycol succinimidyl propionate (MPEG-SPA). Biol Pharm Bull 27:936–938
Eto Y, Gao JQ, Sekiguchi F, Kurachi S, Katayama K, Maeda M, Kawasaki K, Mizuguchi H, Hayakawa T, Tsutsumi Y, Mayumi T, Nakagawa S (2005) PEGylated adenovirus vectors containing RGD peptides on the tip of PEG show high transduction efficiency and antibody evasion ability. J Gene Med 7:604–612
Eto Y, Yoshioka Y, Ishida T, Yao X, Morishige T, Narimatsu S, Mizuguchi H, Mukai Y, Okada N, Kiwada H, Nakagawa S (2010) Optimized PEGylated adenovirus vector reduces the anti-vector humoral immune response against adenovirus and induces a therapeutic effect against metastatic lung cancer. Biol Pharm Bull 33:1540–1544
Everts M, Curiel DT (2004) Transductional targeting of adenoviral cancer gene therapy. Curr Gene Ther 4:337–346
Gao JQ, Eto Y, Yoshioka Y, Sekiguchi F, Kurachi S, Morishige T, Yao X, Watanabe H, Asavatanabodee R, Sakurai F, Mizuguchi H, Okada Y, Mukai Y, Tsutsumi Y, Mayumi T, Okada N, Nakagawa S (2007) Effective tumor targeted gene transfer using PEGylated adenovirus vector via systemic administration. J Control Release 122:102–110
Glasgow JN, Kremer EJ, Hemminki A, Siegal GP, Douglas JT, Curiel DT (2004) An adenovirus vector with a chimeric fiber derived from canine adenovirus type 2 displays novel tropism. Virology 324:103–116
Glasgow JN, Everts M, Curiel DT (2006) Transductional targeting of adenovirus vectors for gene therapy. Cancer Gene Ther 13:830–844
Griffioen AW, Molema G (2000) Angiogenesis: potentials for pharmacologic intervention in the treatment of cancer, cardiovascular diseases, and chronic inflammation. Pharmacol Rev 52:237–268
Haisma HJ, Grill J, Curiel DT, Hoogeland S, van Beusechem VW, Pinedo HM, Gerritsen WR (2000) Targeting of adenoviral vectors through a bispecific single-chain antibody. Cancer Gene Ther 7:901–904
Haisma HJ, Boesjes M, Beerens AM, van der Strate BW, Curiel DT, Plüddemann A, Gordon S, Bellu AR (2009) Scavenger receptor A: a new route for adenovirus 5. Mol Pharm 6:366–374
Haisma HJ, Kamps GK, Bouma A, Geel TM, Rots MG, Kariath A, Bellu AR (2010) Selective targeting of adenovirus to αvβ3 integrins, VEGFR2 and Tie2 endothelial receptors by angio-adenobodies. Int J Pharm 391:155–161
Haj-Ahmad Y, Graham FL (1986) Development of a helper-independent human adenovirus vector and its use in the transfer of the Herpes simplex thymidine kinase gene. J Virol 57:267–274
Hangalapura BN, Timares L, Oosterhoff D, Scheper RJ, Curiel DT, de Gruijl TD (2012) CD40-targeted adenoviral cancer vaccines: the long and winding road to the clinic. J Gene Med 14:416–427
Hashimoto Y, Kohri K, Akita H, Mitani K, Ikeda K, Nakanishi M (1997) Efficient transfer of genes into senescent cells by adenovirus vectors via highly expressed αvβ5 integrin. Biochem Biophys Res Commun 240:88–92
Havenga MJ, Lemckert AA, Ophorst OJ, van Meijer M, Germeraad WT, Grimbergen J, van den Doel MA, Vogels R, van Deutekom J, Janson AA, de Bruijn JD, Uytdehaag F, Quax PH, Logtenberg T, Mehtali M, Bout A (2002) Exploiting the natural diversity in adenovirus tropism for therapy and prevention of disease. J Virol 76:4612–4620
Hedley SJ, Auf der Maur A, Hohn S, Escher D, Barberis A, Glasgow JN, Douglas JT, Korokhov N, Curiel DT (2006) An adenovirus vector with a chimeric fiber incorporating stabilized single chain antibody achieves targeted gene delivery. Gene Ther 13:88–94
Hemminki A, Zinn KR, Liu B, Chaudhuri TR, Desmond RA, Rogers BE, Barnes MN, Alvarez RD, Curiel DT (2002) In vivo molecular chemotherapy and noninvasive imaging with an infectivity-enhanced adenovirus. J Natl Cancer Inst 94:741–749
Hesse A, Kosmides D, Kontermann RE, Nettelbeck DM (2007) Tropism modification of adenovirus vectors by peptide ligand insertion into various positions of the adenovirus serotype 41 short-fiber knob domain. J Virol 81:2688–2699
Hofherr SE, Shashkova EV, Weaver EA, Khare R, Barry MA (2008) Modification of adenoviral vectors with polyethylene glycol modulates in vivo tissue tropism and gene expression. Mol Ther 16:1276–1282
Hu M, Zhang K (2013) The application of aptamers in cancer research: an up-to-date review. Future Oncol 9:369–376
Ivanenkov VV, Felici F, Menon AG (1999) Targeted delivery of multivalent phage display vectors into mammalian cells. Biochim Biophys Acta 1448:463–472
Jakubczak JL, Rollence ML, Stewart DA, Jafari JD, Von Seggern DJ, Nemerow GR, Stevenson SC, Hallenbeck PL (2001) Adenovirus type 5 viral particles pseudotyped with mutagenized fiber proteins show diminished infectivity of coxsackie B-adenovirus receptor-bearing cells. J Virol 75:2972–2981
Jiang ZK, Koh SB, Sato M, Atanasov IC, Johnson M, Zhou ZH, Deming TJ, Wu L (2013) Engineering polypeptide coatings to augment gene transduction and in vivo stability of adenoviruses. J Control Release 166:75–85
Jiang H, Gomez-Manzano C, Rivera-Molina Y, Lang FF, Conrad CA, Fueyo J (2015) Oncolytic adenovirus research evolution: from cell-cycle checkpoints to immune checkpoints. Curr Opin Virol 13:33–39
Jose A, Rovira-Rigau M, Luna J, Gimenez-Alejandre M, Vaquero E, Garcia de la Torre B, Andreu D, Alemany R, Fillat C (2014) A genetic fiber modification to achieve matrix-metalloprotease-activated infectivity of oncolytic adenovirus. J Control Release 192:148–156
Kaliberov SA, Kaliberova LN, Buchsbaum DJ, Curiel DT (2014a) Experimental virotherapy of chemoresistant pancreatic carcinoma using infectivity-enhanced fiber-mosaic oncolytic adenovirus. Cancer Gene Ther 21:264–274
Kaliberov SA, Kaliberova LN, Buggio M, Tremblay JM, Shoemaker CB, Curiel DT (2014b) Adenoviral targeting using genetically incorporated camelid single variable domains. Lab Invest 94:893–905
Kaneda Y, Tsutsumi Y, Yoshioka Y, Kamada H, Yamamoto Y, Kodaira H, Tsunoda S, Okamoto T, Mukai Y, Shibata H, Nakagawa S, Mayumi T (2004) The use of PVP as a polymeric carrier to improve the plasma half-life of drugs. Biomaterials 25:3259–3266
Kashentseva EA, Seki T, Curiel DT, Dmitriev IP (2002) Adenovirus targeting to c-erbB-2 oncoprotein by single-chain antibody fused to trimeric form of adenovirus receptor ectodomain. Cancer Res 62:609–616
Katayama K, Furuki R, Yokoyama H, Kaneko M, Tachibana M, Yoshida I, Nagase H, Tanaka K, Sakurai F, Mizuguchi H, Nakagawa S, Nakanishi T (2011) Enhanced in vivo gene transfer into the placenta using RGD fiber-mutant adenovirus vector. Biomaterials 32:4185–4193
Khare R, Chen CY, Weaver EA, Barry MA (2011) Advances and future challenges in adenoviral vector pharmacology and targeting. Curr Gene Ther 11:241–258
Kim YS, Kim YJ, Lee JM, Han SH, Ko HJ, Park HJ, Pereboev A, Nguyen HH, Kang CY (2010) CD40-targeted recombinant adenovirus significantly enhances the efficacy of antitumor vaccines based on dendritic cells and B cells. Hum Gene Ther 21:1697–1706
Kim PH, Sohn JH, Choi JW, Jung Y, Kim SW, Haam S, Yun CO (2011) Active targeting and safety profile of PEG-modified adenovirus conjugated with herceptin. Biomaterials 32:2314–2326
Koizumi N, Mizuguchi H, Sakurai F, Yamaguchi T, Watanabe Y, Hayakawa T (2003) Reduction of natural adenovirus tropism to mouse liver by fiber-shaft exchange in combination with both CAR- and αv integrin-binding ablation. J Virol 77:13062–13072
Krasnykh V, Dmitriev I, Mikheeva G, Miller CR, Belousova N, Curiel DT (1998) Characterization of an adenovirus vector containing a heterologous peptide epitope in the HI loop of the fiber knob. J Virol 72:1844–1852
Kreppel F, Kochanek S (2008) Modification of adenovirus gene transfer vectors with synthetic polymers: a scientific review and technical guide. Mol Ther 16:16–29
Kügler J, Zantow J, Meyer T, Hust M (2013) Oligopeptide m13 phage display in pathogen research. Viruses 5:2531–2545
Kuldo JM, Asgeirsdottir SA, Zwiers PJ, Bellu AR, Rots MG, Schalk JA, Ogawara KI, Trautwein C, Banas B, Haisma HJ, Molema G, Kamps JA (2013) Targeted adenovirus mediated inhibition of NF-κB-dependent inflammatory gene expression in endothelial cells in vitro and in vivo. J Control Release 166:57–65
Kurachi S, Koizumi N, Sakurai F, Kawabata K, Sakurai H, Nakagawa S, Hayakawa T, Mizuguchi H (2007) Characterization of capsid-modified adenovirus vectors containing heterologous peptides in the fiber knob, protein IX, or hexon. Gene Ther 14:266–274
Laakkonen P, Porkka K, Hoffman JA, Ruoslahti E (2002) A tumor-homing peptide with a targeting specificity related to lymphatic vessels. Nat Med 8:751–755
Lanciotti J, Song A, Doukas J, Sosnowski B, Pierce G, Gregory R, Wadsworth S, O’Riordan C (2003) Targeting adenoviral vectors using heterofunctional polyethylene glycol FGF2 conjugates. Mol Ther 8:99–107
Lee GK, Maheshri N, Kaspar B, Schaffer DV (2005) PEG conjugation moderately protects adeno-associated viral vectors against antibody neutralization. Biotechnol Bioeng 92:24–34
Leissner P, Legrand V, Schlesinger Y, Hadj DA, van Raaij M, Cusack S, Pavirani A, Mehtali M (2001) Influence of adenoviral fiber mutations on viral encapsidation, infectivity and in vivo tropism. Gene Ther 8:49–57
Liu Z, Wu K (2008) Peptides homing to tumor vasculature: imaging and therapeutics for cancer. Recent Pat Anticancer Drug Discov 3:202–208
Magnusson MK, Hong SS, Henning P, Boulanger P, Lindholm L (2002) Genetic retargeting of adenovirus vectors: functionality of targeting ligands and their influence on virus viability. J Gene Med 4:356–370
Magnusson MK, Henning P, Myhre S, Wikman M, Uil TG, Friedman M, Andersson KME, Hong SS, Hoeben RC, Habib NA, Stahl S, Boulanger P, Lindholm L (2007) Adenovirus 5 vector genetically re-targeted by an Affibody molecule with specificity for tumor antigen HER2/neu. Cancer Gene Ther 14:468–479
Magnusson MK, Kraaij R, Leadley RM, de Ridder CM, van Weerden WM, van Schie KA, van der Kroeg M, Hoeben RC, Maitland NJ, Lindholm L (2012) A transductionally retargeted adenoviral vector for virotherapy of Her2/neu-expressing prostate cancer. Hum Gene Ther 23:70–82
Mahlknecht G, Sela M, Yarden Y (2015) Aptamer targeting the ERBB2 receptor tyrosine kinase for applications in tumor therapy. Methods Mol Biol 1317:3–15
Matsui H, Sakurai F, Katayama K, Kurachi S, Tashiro K, Sugio K, Kawabata K, Mizuguchi H (2011) Enhanced transduction efficiency of fiber-substituted adenovirus vectors by the incorporation of RGD peptides in two distinct regions of the adenovirus serotype 35 fiber knob. Virus Res 155:48–54
Mazzucchelli L, Burritt JB, Jesaitis AJ, Nusrat A, Liang TW, Gewirtz AT, Schnell FJ, Parkos CA (1999) Cell-specific peptide binding by human neutrophils. Blood 93:1738–1748
McConnell MJ, Imperiale MJ (2004) Biology of adenovirus and its use as a vector for gene therapy. Hum Gene Ther 15:1022–1033
Miura Y, Yoshida K, Nishimoto T, Hatanaka K, Ohnami S, Asaka M, Douglas JT, Curiel DT, Yoshida T, Aoki K (2007) Direct selection of targeted adenovirus vectors by random peptide display on the fiber knob. Gene Ther 14:1448–1460
Mizuguchi H, Hayakawa T (2004) Targeted adenovirus vectors. Hum Gene Ther 15:1034–1044
Mizuguchi H, Koizumi N, Hosono T, Utoguchi N, Watanabe Y, Kay MA, Hayakawa T (2001) A simplified system for constructing recombinant adenoviral vectors containing heterologous peptides in the HI loop of their fiber knob. Gene Ther 8:730–735
Mizuguchi H, Koizumi N, Hosono T, Ishii-Watabe A, Uchida E, Utoguchi N, Watanabe Y, Hayakawa T (2002) CAR- or αv integrin-binding ablated adenovirus vectors, but not fiber-modified vectors containing RGD peptide, do not change the systemic gene transfer properties in mice. Gene Ther 9:769–776
Mok H, Palmer DJ, Ng P, Barry MA (2005) Evaluation of polyethylene glycol modification of first-generation and helper-dependent adenoviral vectors to reduce innate immune responses. Mol Ther 11:66–79
Morral N, O’Neal WK, Rice K, Leland MM, Piedra PA, Aguilar-Cordova E, Carey KD, Beaudet al, Langston C (2002) Lethal toxicity, severe endothelial injury, and a threshold effect with high doses of an adenoviral vector in baboons. Hum Gene Ther 13:143–154
Myhre S, Henning P, Friedman M, Stahl S, Lindholm L, Magnusson MK (2009) Re-targeted adenovirus vectors with dual specificity; binding specificities conferred by two different Affibody molecules in the fiber. Gene Ther 16:252–261
Nakamura T, Sato K, Hamada H (2003) Reduction of natural adenovirus tropism to the liver by both ablation of fiber-coxsackievirus and adenovirus receptor interaction and use of replaceable short fiber. J Virol 77:2512–2521
Nettelbeck DM, Miller DW, Jerome V, Zuzarte M, Watkins SJ, Hawkins RE, Müller R, Kontermann RE (2001) Targeting of adenovirus to endothelial cells by a bispecific single-chain diabody directed against the adenovirus fiber knob domain and human endoglin (CD105). Mol Ther 3:882–891
Nettelbeck DM, Rivera AA, Kupsch J, Dieckmann D, Douglas JT, Kontermann RE, Alemany R, Curiel DT (2004) Retargeting of adenoviral infection to melanoma: combining genetic ablation of native tropism with a recombinant bispecific single-chain diabody (scDb) adapter that binds to fiber knob and HMWMAA. Int J Cancer 108:136–145
Nicklin SA, White SJ, Watkins SJ, Hawkins RE, Baker AH (2000) Selective targeting of gene transfer to vascular endothelial cells by use of peptides isolated by phage display. Circulation 102:231–237
Nicklin SA, Dishart KL, Buening H, Reynolds PN, Hallek M, Nemerow GR, Von Seggern DJ, Baker AH (2003) Transductional and transcriptional targeting of cancer cells using genetically engineered viral vectors. Cancer Lett 201:165–173
Nigatu AS, Vupputuri S, Flynn N, Ramsey JD (2015) Effects of cell-penetrating peptides on transduction efficiency of PEGylated adenovirus. Biomed Pharmacother 71:153–160
Nishimoto T, Yoshida K, Miura Y, Kobayashi A, Hara H, Ohnami S, Kurisu K, Yoshida T, Aoki K (2009) Oncolytic virus therapy for pancreatic cancer using the adenovirus library displaying random peptides on the fiber knob. Gene Ther 16:669–680
Nishimoto T, Yamamoto Y, Yoshida K, Goto N, Ohnami S, Aoki K (2012) Development of peritoneal tumor-targeting vector by in vivo screening with a random peptide-displaying adenovirus library. PLoS One 7:e45550
O’Neill AM, Smith AN, Spangler EA, Whitley EM, Schleis SE, Bird RC, Curiel DT, Thacker EE, Smith BF (2011) Resistance of canine lymphoma cells to adenoviral infection due to reduced cell surface RGD binding integrins. Cancer Biol Ther 11:651–658
O’Riordan CR, Lachapelle A, Delgado C, Parkes V, Wadsworth SC, Smith AE, Francis GE (1999) PEGylation of adenovirus with retention of infectivity and protection from neutralizing antibody in vitro and in vivo. Hum Gene Ther 10:1349–1358
Odermatt A, Audige A, Frick C, Vogt B, Frey BM, Frey FJ, Mazzucchelli L (2001) Identification of receptor ligands by screening phage-display peptide libraries ex vivo on microdissected kidney tubules. J Am Soc Nephrol 12:308–316
Ogawara K, Rots MG, Kok RJ, Moorlag HE, van Loenen AM, Meijer DK, Haisma HJ, Molema G (2004) A novel strategy to modify adenovirus tropism and enhance transgene delivery to activated vascular endothelial cells in vitro and in vivo. Hum Gene Ther 15:433–443
Pasqualini R, Ruoslahti E (1996) Organ targeting in vivo using phage display peptide libraries. Nature 380:364–366
Pasqualini R, Koivunen E, Kain R, Lahdenranta J, Sakamoto M, Stryhn A, Ashmun RA, Shapiro LH, Arap W, Ruoslahti E (2000) Aminopeptidase N is a receptor for tumor-homing peptides and a target for inhibiting angiogenesis. Cancer Res 60:722–727
Pereboev AV, Nagle JM, Shakhmatov MA, Triozzi PL, Matthews QL, Kawakami Y, Curiel DT, Blackwell JL (2004) Enhanced gene transfer to mouse dendritic cells using adenoviral vectors coated with a novel adapter molecule. Mol Ther 9:712–720
Pereboeva L, Komarova S, Mahasreshti P, Curiel DT (2004) Fiber-mosaic adenovirus as a novel approach to design genetically modified adenoviral vectors. Virus Res 105:35–46
Pereboeva L, Komarova S, Roth J, Ponnazhagan S, Curiel DT (2007) Targeting EGFR with metabolically biotinylated fiber-mosaic adenovirus. Gene Ther 14:627–637
Piccolo P, Annunziata P, Mithbaokar P, Brunetti-Pierri N (2014) SR-A and SREC-I binding peptides increase HDAd-mediated liver transduction. Gene Ther 21:950–957
Pol J, Bloy N, Obrist F, Eggermont A, Galon J, Cremer I, Erbs P, Limacher JM, Preville X, Zitvogel L, Kroemer G, Galluzzi L (2014) Trial watch: oncolytic viruses for cancer therapy. Oncoimmunology 3:e28694
Pützer BM, Hitt M, Muller WJ, Emtage P, Gauldie J, Graham FL (1997) Interleukin 12 and B7-1 costimulatory molecule expressed by an adenovirus vector act synergistically to facilitate tumor regression. Proc Natl Acad Sci U S A 94:10889–10894
Rancourt C, Rogers BE, Sosnowski BA, Wang M, Piche A, Pierce GF, Alvarez RD, Siegal GP, Douglas JT, Curiel DT (1998) Basic fibroblast growth factor enhancement of adenovirus-mediated delivery of the herpes simplex virus thymidine kinase gene results in augmented therapeutic benefit in a murine model of ovarian cancer. Clin Cancer Res 4:2455–2461
Rangel R, Guzman-Rojas L, le Roux LG, Staquicini FI, Hosoya H, Barbu EM, Ozawa MG, Nie J, Dunner K Jr, Langley RR, Sage EH, Koivunen E, Gelovani JG, Lobb RR, Sidman RL, Pasqualini R, Arap W (2012) Combinatorial targeting and discovery of ligand-receptors in organelles of mammalian cells. Nat Commun 3:788
Rangel R, Dobroff AS, Guzman-Rojas L, Salmeron CC, Gelovani JG, Sidman RL, Pasqualini R, Arap W (2013) Targeting mammalian organelles with internalizing phage (iPhage) libraries. Nat Protoc 8:1916–1939
Rauschhuber C, Noske N, Ehrhardt A (2012) New insights into stability of recombinant adenovirus vector genomes in mammalian cells. Eur J Cell Biol 91:2–9
Ravera MW, Carcamo J, Brissette R, Alam-Moghe A, Dedova O, Cheng W, Hsiao KC, Klebanov D, Shen H, Tang P, Blume A, Mandecki W (1998) Identification of an allosteric binding site on the transcription factor p53 using a phage-displayed peptide library. Oncogene 16:1993–1999
Reetz J, Genz B, Meier C, Kowtharapu BS, Timm F, Vollmar B, Herchenröder O, Abshagen K, Pützer BM (2013) Development of adenoviral delivery systems to target hepatic stellate cells in vivo. PLoS One 8:e67091
Reetz J, Hildebrandt S, Schmidt A, Meier C, Herchenröder O, Gläser A, Witt M, Pützer BM, Wree A (2015) Novel subventricular zone early progenitor cell-specific adenovirus for in vivo therapy of central nervous system disorders reinforces brain stem cell heterogeneity. Brain Struct Funct. doi:10.1007/s00429-015-1025-8
Reynolds PN, Zinn KR, Gavrilyuk VD, Balyasnikova IV, Rogers BE, Buchsbaum DJ, Wang MH, Miletich DJ, Grizzle WE, Douglas JT, Danilov SM, Curiel DT (2000) A targetable, injectable adenoviral vector for selective gene delivery to pulmonary endothelium in vivo. Mol Ther 2:562–578
Reynolds PN, Nicklin SA, Kaliberova L, Boatman BG, Grizzle WE, Balyasnikova IV, Baker AH, Danilov SM, Curiel DT (2001) Combined transductional and transcriptional targeting improves the specificity of transgene expression in vivo. Nat Biotechnol 19:838–842
Roelvink PW, Lizonova A, Lee JG, Li Y, Bergelson JM, Finberg RW, Brough DE, Kovesdi I, Wickham TJ (1998) The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J Virol 72:7909–7915
Roelvink PW, Mi Lee G, Einfeld DA, Kovesdi I, Wickham TJ (1999) Identification of a conserved receptor-binding site on the fiber proteins of CAR-recognizing adenoviridae. Science 286:1568–1571
Rojas JJ, Gimenez-Alejandre M, Gil-Hoyos R, Cascallo M, Alemany R (2012) Improved systemic antitumor therapy with oncolytic adenoviruses by replacing the fiber shaft HSG-binding domain with RGD. Gene Ther 19:453–457
Romanczuk H, Galer CE, Zabner J, Barsomian G, Wadsworth SC, O’Riordan CR (1999) Modification of an adenoviral vector with biologically selected peptides: a novel strategy for gene delivery to cells of choice. Hum Gene Ther 10:2615–2626
Rosenfeld MA, Siegfried W, Yoshimura K, Yoneyama K, Fukayama M, Stier LE, Pääkkö PK, Gilardi P, Stratford-Perricaudet LD, Perricaudet M, Jallat S, Pavirani A, Lecocq JP, Crystal RG (1991) Adenovirus-mediated transfer of a recombinant α1-antitrypsin gene to the lung epithelium in vivo. Science 252:431–434
Rowe WP, Huebner RJ, Gilmore LK, Parrott RH, Ward TG (1953) Isolation of a cytopathogenic agent from human adenoids undergoing spontaneous degeneration in tissue culture. Proc Soc Exp Biol Med 84:570–573
Russell WC (2000) Update on adenovirus and its vectors. J Gen Virol 81:2573–2604
Salazar-Montes AM, Hernandez-Ortega LD, Lucano-Landeros MS, Armendariz-Borunda J (2015) New gene therapy strategies for hepatic fibrosis. World J Gastroenterol 21:3813–3825
Schipper H, Alla V, Meier C, Nettelbeck DM, Herchenröder O, Pützer BM (2014) Eradication of metastatic melanoma through cooperative expression of RNA-based HDAC1 inhibitor and p73 by oncolytic adenovirus. Oncotarget 15:5893–5907
Schmidt A, Böckmann M, Stoll A, Racek T, Pützer BM (2005) Analysis of adenovirus gene transfer into adult neural stem cells. Virus Res 114:45–53
Schmidt A, Haas SJ, Hildebrandt S, Scheibe J, Eckhoff B, Racek T, Kempermann G, Wree A, Pützer BM (2007) Selective targeting of adenoviral vectors to neural precursor cells in the hippocampus of adult mice: new prospects for in situ gene therapy. Stem Cells 25:2910–2918
Schmidt A, Eipel C, Fürst K, Sommer N, Pahnke J, Pützer BM (2011) Evaluation of systemic targeting of RET oncogene-based MTC with tumor-selective peptide-tagged Ad vectors in clinical mouse models. Gene Ther 18:418–423
Sclavons C, Burtea C, Boutry S, Laurent S, Vander Elst L, Muller RN (2013) Phage display screening for tumor necrosis factor-α-binding peptides: detection of inflammation in a mouse model of hepatitis. Int J Pept 2013:348409
Scott JK, Smith GP (1990) Searching for peptide ligands with an epitope library. Science 249:386–390
Seto D, Chodosh J, Brister JR, Jones MS (2011) Members of the adenovirus research community. Using the whole-genome sequence to characterize and name human adenoviruses. J Virol 85:5701–5702
Seung-Min L, Gil-Suk Y, Eun-Sang Y, Tae-Gyun K, In-San K, Byung-Heon L (2009) Application of phage display to discovery of tumor-specific homing peptides: Developing strategies for therapy and molecular imaging of cancer. Methods Mol Biol 512:355–363
Sharma A, Li X, Bangari DS, Mittal SK (2009) Adenovirus receptors and their implications in gene delivery. Virus Res 143:184–194
Shayakhmetov DM, Li Z-Y, Ni S, Lieber A (2004) Analysis of adenovirus sequestration in the liver, transduction of hepatic cells, and innate toxicity after injection of fiber-modified vectors. J Virol 78:5368–5381
Shayakhmetov DM, Gaggar A, Ni S, Li Z-Y, Lieber A (2005) Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J Virol 79:7478–7491
Smith TA, Idamakanti N, Marshall-Neff J, Rollence ML, Wright P, Kaloss M, King L, Mech C, Dinges L, Iverson WO, Sherer AD, Markovits JE, Lyons RM, Kaleko M, Stevenson SC (2003a) Receptor interactions involved in adenoviral-mediated gene delivery after systemic administration in non-human primates. Hum Gene Ther 14:1595–1604
Smith TA, Idamakanti N, Rollence ML, Marshall-Neff J, Kim J, Mulgrew K, Nemerow GR, Kaleko M, Stevenson SC (2003b) Adenovirus serotype 5 fiber shaft influences in vivo gene transfer in mice. Hum Gene Ther 14:777–787
Song KM, Lee S, Ban C (2012) Aptamers and their biological applications. Sensors (Basel) 12:612–631
Sova P, Ren XW, Ni S, Bernt KM, Mi J, Kiviat N, Lieber A (2004) A tumor-targeted and conditionally replicating oncolytic adenovirus vector expressing TRAIL for treatment of liver metastases. Mol Ther 9:496–509
Stone D, Liu Y, Shayakhmetov D, Li Z-Y, Ni S, Lieber A (2007) Adenovirus-platelet interaction in blood causes virus sequestration to the reticuloendothelial system of the liver. J Virol 81:8466–8471
Sundaram P, Kurniawan H, Byrne ME, Wower J (2013) Therapeutic RNA aptamers in clinical trials. Eur J Pharm Sci 48:259–271
Takayama K, Ueno H, Pei XH, Nakanishi Y, Yatsunami J, Hara N (1998) The levels of integrin αvβ5 may predict the susceptibility to adenovirus-mediated gene transfer in human lung cancer cells. Gene Ther 5:361–368
Tan W, Donovan MJ, Jiang J (2013) Aptamers from cell-based selection for bioanalytical applications. Chem Rev 113:2842–2862
Tanaka T, Kuroki M, Hamada H, Kato K, Kinugasa T, Shibaguchi H, Zhao J (2007) Cancer-targeting gene therapy using tropism-modified adenovirus. Anticancer Res 27:3679–3684
Terao S, Acharya B, Suzuki T, Aoi T, Naoe M, Hamada K, Mizuguchi H, Gotoh A (2009) Improved gene transfer into renal carcinoma cells using adenovirus vector containing RGD motif. Anticancer Res 29:2997–3001
Thacker EE, Timares L, Matthews QL (2009a) Strategies to overcome host immunity to adenovirus vectors in vaccine development. Expert Rev Vaccines 8:761–777
Thacker EE, Nakayama M, Smith BF, Bird RC, Muminova Z, Strong TV, Timares L, Korokhov N, O’Neill AM, de Gruijl TD, Glasgow JN, Tani K, Curiel DT (2009b) A genetically engineered adenovirus vector targeted to CD40 mediates transduction of canine dendritic cells and promotes antigen-specific immune responses in vivo. Vaccine 27:7116–7124
Tillman BW, Hayes TL, DeGruijl TD, Douglas JT, Curiel DT (2000) Adenoviral vectors targeted to CD40 enhance the efficacy of dendritic cell-based vaccination against human papillomavirus 16-induced tumor cells in a murine model. Cancer Res 60:5456–5463
van Beusechem VW, van Rijswijk AL, van Es HH, Haisma HJ, Pinedo HM, Gerritsen WR (2000) Recombinant adenovirus vectors with knobless fibers for targeted gene transfer. Gene Ther 7:1940–1946
van Zeeburg HJ, van Beusechem VW, Huizenga A, Haisma HJ, Korokhov N, Gibbs S, Leemans CR, Brakenhoff RH (2010) Adenovirus retargeting to surface expressed antigens on oral mucosa. J Gene Med 12:365–376
Vives E, Schmidt J, Pelegrin A (2008) Cell-penetrating and cell-targeting peptides in drug delivery. Biochim Biophys Acta 1786:126–138
Wang D, Liu S, Mao Q, Zhao J, Xia H (2011) A novel vector for a rapid generation of fiber-mutant adenovirus based on one step ligation and quick screening of positive clones. J Biotechnol 152:72–76
Wang DL, Song YL, Zhu Z, Li XL, Zou Y, Yang HT, Wang JJ, Yao PS, Pan RJ, Yang CJ, Kang DZ (2014) Selection of DNA aptamers against epidermal growth factor receptor with high affinity and specificity. Biochem Biophys Res Commun 453:681–685
Watkins SJ, Mesyanzhinov VV, Kurochkina LP, Hawkins RE (1997) The ‘adenobody’ approach to viral targeting: specific and enhanced adenoviral gene delivery. Gene Ther 4:1004–1012
White SJ, Nicklin SA, Sawamura T, Baker AH (2001) Identification of peptides that target the endothelial cell-specific LOX-1 receptor. Hypertension 37:449–455
Wickham TJ (2000) Targeting adenovirus. Gene Ther 7:110–114
Wickham TJ, Mathias P, Cheresh DA, Nemerow GR (1993) Integrins αvβ3 and αvβ5 promote adenovirus internalization but not virus attachment. Cell 73:309–319
Wickham TJ, Tzeng E, Shears LL 2nd, Roelvink PW, Li Y, Lee GM, Brough DE, Lizonova A, Kovesdi I (1997) Increased in vitro and in vivo gene transfer by adenovirus vectors containing chimeric fiber proteins. J Virol 71:8221–8229
Williams BJ, Bhatia S, Adams LK, Boling S, Carroll JL, Li XL, Rogers DL, Korokhov N, Kovesdi I, Pereboev AV, Curiel DT, Mathis JM (2012) Dendritic cell based PSMA immunotherapy for prostate cancer using a CD40-targeted adenovirus vector. PLoS One 7:e46981
Wirth T, Parker N, Ylä-Herttuala S (2013) History of gene therapy. Gene 525:162–169
Wonganan P, Croyle MA (2010) PEGylated adenoviruses: from mice to monkeys. Viruses 2:468–502
Work LM, Nicklin SA, Brain NJ, Dishart KL, Von Seggern DJ, Hallek M, Büning H, Baker AH (2004) Development of efficient viral vectors selective for vascular smooth muscle cells. Mol Ther 9:198–208
Wu H, Seki T, Dmitriev I, Uil T, Kashentseva E, Han T, Curiel DT (2002) Double modification of adenovirus fiber with RGD and polylysine motifs improves coxsackievirus-adenovirus receptor-independent gene transfer efficiency. Hum Gene Ther 13:1647–1653
Wu P, Kudrolli TA, Chowdhury WH, Liu MM, Rodriguez R, Lupold SE (2010) Adenovirus targeting to prostate-specific membrane antigen through virus-displayed, semirandom peptide library screening. Cancer Res 70:9549–9553
Xia H, Anderson B, Mao Q, Davidson BL (2000) Recombinant human adenovirus: targeting to the human transferrin receptor improves gene transfer to brain microcapillary endothelium. J Virol 74:11359–11366
Xiong Z, Cheng Z, Zhang X, Patel M, Wu JC, Gambhir SS, Chen X (2006) Imaging chemically modified adenovirus for targeting tumors expressing integrin αvβ3 in living mice with mutant herpes simplex virus type 1 thymidine kinase PET reporter gene. J Nucl Med 47:130–139
Xu Z, Qiu Q, Tian J, Smith JS, Conenello GM, Morita T, Byrnes AP (2013) Coagulation factor X shields adenovirus type 5 from attack by natural antibodies and complement. Nat Med 19:452–457
Yao X, Yoshioka Y, Morishige T, Eto Y, Watanabe H, Okada Y, Mizuguchi H, Mukai Y, Okada N, Nakagawa S (2009) Systemic administration of a PEGylated adenovirus vector with a cancer-specific promoter is effective in a mouse model of metastasis. Gene Ther 16:1395–1404
Yao X, Yoshioka Y, Morishige T, Eto Y, Narimatsu S, Kawai Y, Mizuguchi H, Gao JQ, Mukai Y, Okada N, Nakagawa S (2011) Tumor vascular targeted delivery of polymer-conjugated adenovirus vector for cancer gene therapy. Mol Ther 19:1619–1625
Yao XL, Yoshioka Y, Ruan GX, Chen YZ, Mizuguchi H, Mukai Y, Okada N, Gao JQ, Nakagawa S (2012) Optimization and internalization mechanisms of PEGylated adenovirus vector with targeting peptide for cancer gene therapy. Biomacromolecules 13:2402–2409
Yu D, Jin C, Leja J, Majdalani N, Nilsson B, Eriksson F, Essand M (2011) Adenovirus with hexon Tat-protein transduction domain modification exhibits increased therapeutic effect in experimental neuroblastoma and neuroendocrine tumors. J Virol 85:13114–13123
Yu D, Jin C, Ramachandran M, Xu J, Nilsson B, Korsgren O, le Blanc K, Uhrbom L, Forsberg-Nilsson K, Westermark B, Adamson R, Maitland N, Fan X, Essand M (2013) Adenovirus serotype 5 vectors with Tat-PTD modified hexon and serotype 35 fiber show greatly enhanced transduction capacity of primary cell cultures. PLoS One 8:e54952
Zaiss AK, Liu Q, Bowen GP, Wong NC, Bartlett JS, Muruve DA (2002) Differential activation of innate immune responses by adenovirus and adeno-associated virus vectors. J Virol 76:4580–4590
Zhang Y, Bergelson JM (2005) Adenovirus receptors. J Virol 79:12125–12131
Zhu G, Ye M, Donovan MJ, Song E, Zhao Z, Tan W (2012) Nucleic acid aptamers: an emerging frontier in cancer therapy. Chem Commun (Camb) 48:10472–10480
Zimbres FM, Tarnok A, Ulrich H, Wrenger C (2013) Aptamers: novel molecules as diagnostic markers in bacterial and viral infections? Biomed Res Int 2013:731516
Acknowledgments
We apologize to all those colleagues whose important work is not cited because of space constraints. The results of this review article were in part supported by grants from the Deutsche Forschungsgemeinschaft (DFG), the Bundesministerium für Bildung und Forschung (BMBF), the Exzellenzförderprogramm (EFP) Mecklenburg-Vorpommern, and the FORUN (Forschungsförderung der Medizinischen Fakultät der Rostocker Universität) grant program.
Conflicts of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Herchenröder, O., Reetz, J., Pützer, B.M. (2016). Recent Progress in Strategies for Adenovirus Mediated Therapeutic Cell Targeting. In: Steinhoff, G. (eds) Regenerative Medicine - from Protocol to Patient. Springer, Cham. https://doi.org/10.1007/978-3-319-28274-9_2
Download citation
DOI: https://doi.org/10.1007/978-3-319-28274-9_2
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-28272-5
Online ISBN: 978-3-319-28274-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)