Abstract
Thomas Willis in 1664 suggested that the cerebral ventricles contain fluid produced by the choroid plexus, but this was not established until the 19th century. Dandy and Blackfan produced experimental hydrocephalus and reported that choroid plexus (CP) ablation decreased its severity; this strongly implicated CP in generating CSF and ventricular pressure. Choroid plexuses produce most cerebrospinal fluid (CSF), proteins, and small molecules, regulate the entry of ions and vitamins into the central nervous system, and have immunological and endocrine regulatory functions. CP is the single most important brain-immune interface; the CSF is its channel of communication with the brain. Substantial loss of CP may harm CSF-brain biochemical interactions. Early attempts to control human hydrocephalus by CP ablation produced poor outcomes. Currently, however, combined choroid plexus coagulation (CPC) and endoscopic third ventriculostomy (ETV) are used for resolving infant hydrocephalus and protecting brain against elevated ICP. Still, does addition of CPC to ETV significantly improve long-term outcomes? How does the brain compensate, if it does, for loss of CP homeostatic mechanisms involving endocrine and immunologic phenomena? Clinical studies of CPC should examine possible effects on synaptic plasticity, neurogenesis, and cognition.
Access provided by Autonomous University of Puebla. Download reference work entry PDF
Similar content being viewed by others
Keywords
- Choroid plexus
- Ion transport
- Cerebrospinal fluid secretion
- Blood-CSF
- Barrier
- Hydrocephalus
- Ependyma
- Homeostasis
- Neurogenesis
- Intracranial pulsation
- Brain development
Overview
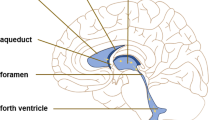
Hydrocephalus has been known since classical times. The presence of fluid within the skull was known, but its location within the ventricular system was not established until the eighteenth century. Thomas Willis suggested in 1664 that the ventricles were filled with CSF that came from the choroid plexuses, but his opinion was slighted (Willis 1664). Links between hydrocephalus and choroid plexus (CP) were established in the early twentieth century by Dandy and Blackfan, who produced experimental hydrocephalus in dogs by blocking the cerebral aqueduct. They also found that CP ablation or coagulation decreased the severity of human or experimental hydrocephalus (Various terms are used for destruction (ablation) or removal of the choroid plexus. Most early removals employed surgical excision, but by the 1930s most involved electrocoagulation of the plexus. We shall therefore use choroid plexus coagulation (CPC) for all forms unless otherwise specified.).
Choroid plexus ablation was used to treat hydrocephalus before effective shunting procedures existed. It was used much less once effective shunts were introduced in the 1960s. Milhorat was an influential voice stressing the ineffectiveness of CPC (Milhorat 1974). However, endoscopic neurosurgery has improved markedly, and there has been a resurgence of CPC in combination with endoscopic third ventriculostomy (ETV), notably in parts of Africa (Warf et al. 2009) where specialized treatment centers are few and the consequences of shunt failure grave. It has also been advocated and used for treatment of several forms of infant hydrocephalus in North America (Zhu and DiRocco 2013; Kulkarni et al. 2010; Kulkarni et al. 2014; Pindrik et al. 2016). Benjamin Warf has been a major advocate for CPC and has presented data relevant to the benefit, cost (including developmental status), and possible explanations for its benefit (Mandell et al. 2015; Warf 2005; Warf et al. 2012). See section “Choroid Plexus and CNS Infection/Immune Functions” for further discussion. Randomized studies are needed to establish the benefit of combined CPC and ETV in the developed world. Such studies should include CSF analysis or freezing for later analysis. Analyses might include vitamins and the regulators that we review, plus exosomes and some microRNAs (miRNAs). Pretreatment CSF analyses might correlate with outcome.
Risk/benefit analysis of CPC requires consideration of possible harm from loss of some of its many functions that go far beyond electrolyte transport and are most important for very young animals. These other functions include CNS nutrition and ciliary “tasting” or chemical monitoring of the CSF (Tomas et al. 2016). The choroid plexus secretes hundreds of proteins and smaller molecules. Embryonic and adult CSF can support the growth of adult neural stem cells as neurospheres (Lehtinen et al. 2011). The choroid plexus probably secretes many responsible factors, although other tissues (e.g., ependyma and arachnoid epithelial cells) undoubtedly also contribute. The choroid plexus is also a major gateway regulating cellular entry into the CNS. Unrestricted entry of cells or molecules into the CNS is harmful (Weber and Tuomanen 2007; Stranahan et al. 2016).
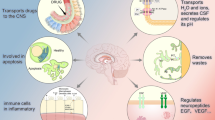
The important CP role in CSF production and simplistic hydraulic analogies have diverted attention from its increasing number of regulatory roles (Johanson et al. 2008). Harm from loss of these special growth and developmental functions is unlikely to be detectable in short-term studies of small infants, who have so few behaviors. CPC has not been proven to offer additional long-term benefit beyond that from ETV alone. Figure 1 illustrates today’s broader concepts of choroid plexus and CSF function.
Introduction
Modern ideas of hydrocephalus began with Dandy and Blackfan at Johns Hopkins University, with a series of papers, most notably their 77-page paper, “Internal Hydrocephalus-An Experimental, Clinical And Pathological Study,” published in the American Journal of Diseases of Children (Dandy and Blackfan 1914). They stated, “It is evident that internal hydrocephalus is due to an abnormality either in the formation or in the absorption of cerebrospinal fluid or possibly in both.” They showed that blocking the aqueduct of Sylvius in dogs produced chronic hydrocephalus with enlarging ventricles and symptoms of increased intracranial pressure (ICP). Removal of the CP from both lateral ventricles at the time of surgery resulted in less severe hydrocephalus. Their work suggested a division of internal hydrocephalus into two forms, depending on the presence or absence of obstruction to flow of CSF through the subarachnoid space. Dandy and Blackfan were greatly influenced by their colleague Harvey Cushing, who moved from Baltimore to Boston in 1912. Cushing described fluid emerging from the CP at operation, which ceased if the feeding vessels were ligated (Cushing 1914). Moreover, Cushing characterized the CSF as the third circulation, parallel to the vascular and lymphatic circulations. He discussed the possible excretion of metabolic products into the CSF and local differences in CSF composition.
The concept of CSF as a third circulation is useful. The blood brings most nutrients derived from the diet to the brain and takes away many waste products. Since immune cells and antibodies travel through vascular channels, the vascular system and the blood-brain (BBB) and blood-cerebrospinal fluid (BCSFB) barriers are important parts of the immune system. The lymphatic system is both an excretory system and an important part of the immune system. The choroid plexus-CSF system is very complex anatomically and physiologically. Although simplistic statements that the brain lacked lymphatics were common in the past, reports of materials injected into the CSF and found in the cervical lymphatics go back to at least to 1927 (Davson 1962; Bradbury and Cole 1980). The CSF has a hydraulic dimension, providing buoyancy and mechanical support for the fragile brain, and is subject to blockage. It delivers vitamins and some hormones and nutrients to brain and is a major source of regulatory molecules, especially in the young. It is also an important vehicle for removing metabolic wastes. The choroid plexus is the most important producer and regulator of the CSF. The choroid plexus and CSF are major parts of the neuroimmune system as well (Kunis et al. 2013). Wilcox found that the choroid plexus was important in the marked difference in susceptibility of neonates and adults to Herpes simplex virus (HSV) encephalitis (Wilcox et al. 2016). Because of the overlap and coordination between the vascular, lymphatic, and CSF-CP systems, it is useful to consider how they work together rather than thinking of them as completely separate entities. We stress this overlap in Fig. 1.
The choroid plexus is often compared to the kidney. Both are now known to have major immune functions (Kurts et al. 2013). If CPC is no longer used to treat hydrocephalus someday, the CP-CSF system will remain important for hydrocephalus, aging, and neurological disorders. The CSF is much more than a balanced salt solution, as shown by Bjorefeldt et al. (2016). It contains many growth factors (the exact number depends on the definition of a growth factor) and many regulators of brain growth, differentiation, and plasticity. It is likely that dysfunctional CSF regulatory signals contribute to some diseases including some of the many forms of hydrocephalus.
History of Understanding the Choroid Plexus
The choroid plexus was described by writers in the Hippocratic era. Leonardo da Vinci produced the first accurate drawings of the brain ventricular system, but its nature was misunderstood. The great Fabricius described the ventricles as air-containing spaces that fill during inspiration and contain the animal spirit. The writings of some anatomists, including Costanzo Varolio, Francis Glisson, and Albrecht Haller, seem to imply the existence of liquid CSF, but the Venetian Niccolo Massa was the first to clearly describe a “mass of fluid” that he encountered within the ventricles while performing an autopsy, in 1536. This did not end the debate, however. In 1764, Domenico Felice Antonio Cotugno was the first to discover and write clearly about CSF. Faivre suggested in 1854 that CSF was formed in the ventricles by the CP. The introduction of lumbar puncture in 1891 made much more information available. Cushing observed weeping of fluid from the surface of the CP during surgery, supporting Faivre’s view. Dandy’s 1918 experimental work showed that the lateral ventricular dilation expected after occlusion of the foramen of Monro did not occur if the CP was excised from that ventricle (Dandy 1918). Putnam subsequently confirmed these findings by decreasing or eliminating infant hydrocephalus by CPC. The Johns Hopkins workers were not the first to treat hydrocephalus by destroying the choroid plexus. Chicago urologist L’ Espinasse used a rigid cystoscope to cauterize the choroid plexus in two hydrocephalic infants in 1910, one of whom died immediately while the other one survived for 5 years. Dandy’s influential studies involved very few animals, and later workers could not reproduce their findings in some cases (Milhorat 1975).
Lina Stern, then working in Geneva, was the first to suggest a nutritional role for the CSF and the first to use the phrase “barriere hemato-encephalique” (Stern 1921). However, Magendie had written about both “liquide cephalo-rachiden” and “liquide cephalo-spinal” in 1842. Magendie’s ideas were disputed and his work was disparaged, but known to Stern. Most early workers ignored Stern’s work. They saw the blood-brain barrier as a purely defensive barrier, functioning to keep materials out of the CNS. Notions that environmental signals could get through the CNS barriers and have positive effects on brain development and function began to appear only after more and more CP-CSF transporters were discovered. CNS malfunction and maldevelopment was identified in germ-free animals, and immune proteins found to be intimately involved in brain plasticity. Miyan and colleagues understood the important role of CSF in brain development in 2003 when they proposed, “the CSF is vital in controlling development of the nervous system along the whole length of the neural tube” (Miyan et al. 2003). Veening in 2010 also spoke of molecules sent to brain regions by CSF to regulate behavior and suggested “the flowing CSF is involved in more than just nutrient and waste control, but is also used as a broadcasting system consisting of coordinated messages to a variety of nearby and distant brain areas.” Those were unconventional ideas in 2010. Today they are supported by much more evidence.
Blood-CSF Barrier Development and Structure
Choroid Plexus Anatomy and Embryology
The choroid plexuses of the third and fourth ventricles differ from those in the lateral ventricles during development, but they become more similar postnatally (Netsky and Shuangshoti 1975). The basic CP structure is conserved in all vertebrates. It consists of a fenestrated vasculature core, an interstitial stromal layer, and a single layer of polarized cuboidal epithelia.
The choroid plexus is rich in mitochondria and highly vascularized with much higher blood flow than adjacent brain (Spector et al. 2015a). Endothelial-derived and epithelial-derived basement membranes flank the stroma providing semipermeable filtration. Except for teleosts, the vertebrate brain contains four choroid plexuses, one in each ventricle. These leaflike structures are much more impressive suspended in the CSF in vivo than after removal from the brain. The choroid plexus has an exceptionally large surface area to volume ratio (Spector et al. 2015a).
The choroidal tissues are shaggy due to many blood vessels and lush epithelial villi on their external surfaces. The ependymal and choroidal epithelial cells are ciliated. Cilia are easily seen in fetal tissue but disappear a few hours after death in adults. Their physical fragility delayed our understanding of their important role in the function of the choroid plexuses and ependymal surface (Liu et al. 2014; Narita and Takeda 2015). Most human cells have cilia (Satir and Christensen 2007). Many diseases or ciliopathies with abnormal ciliary function involving multiple cell types have been discovered in recent years. Most involve the nervous system (Pruski et al. 2016) and often include hydrocephalus (Yamasaki and Kanemura 2015).
The stroma of the choroid plexus lies between the two basement membranes (Johanson et al. 2011) and contains many cell types, including fibroblasts, telocytes, stem cells, dendritic cells, leukocytes, macrophages, and T cells (Lun et al. 2015). The choroid plexus stromal T cell population is variable and generally enriched with effector memory CD4+ T cells specific for CNS antigens in normal rodents (Baruch and Schwartz 2013) and humans. Dendritic cells of bone marrow origin are especially important because they are the most potent antigen-presenting cells and activators of T lymphocytes, critical immune cells. The extracellular (interstitial plus vascular) volume comprises about 20–25% of CP tissue volume. The stroma is thought to be a stem cell niche with the nestin-positive fibroblasts and telocytes supporting myeloid progenitors and neural stem cells, respectively (Kaur et al. 2016; Popescu et al. 2012). Grafts of choroid plexus and stem cells derived from CP have been used in treatment of experimental models of neurological disease.
The choroid plexus endothelial cells are fenestrated and contribute little to molecular BCSFB function (Wolburg and Paulus 2010). The BBB has been more extensively studied and is more restrictive and better understood than is the BCSFB (Tietz and Engelhardt 2015). BBB also has more surface area, but the difference with BCSFB is less than originally thought. Peripheral blood cells have relatively easy access to the perivascular space within the CP (Prodinger et al. 2011) but then face an inner epithelial barrier limiting access to CSF and the brain. The choroid plexus epithelial cells are joined by tight junctions that are less restrictive than the tight junctions of the BBB. The epithelium and membrane junctions between cells impede or regulate the movement of substances across the barrier.
The epithelial cell layer makes up 70–80% of the CP tissue volume. The choroid plexus epithelium is a major CNS barrier system (Spector et al. 2015a; Wolburg and Paulus 2010; Kaur et al. 2016), and the blood-cerebrospinal fluid barrier (BCSFB) is generally considered as an occluding interface between the blood and the ventricular CSF (Abbott et al. 2010). The tight junctions and basolateral membrane are both impermeant to diffusion. However, many solutes and drugs may cross the BCSFB by transport processes or pharmacological mechanisms. The tight junctions of the BBB and the BCSFB (the CP endothelial cells have some tight junctions that are not limited to the apical portions of cells and are intermixed with adherens junctions) are not static, even in the normal state. For example, sheep and many photoperiodic animals (photoperiodism is evident to a varying extent in most plants and animals and regulates many processes such as reproductive cycles on the basis of length of day) show altered choroid plexus permeability to some hormones as the length of day changes. These changes are explained by physical changes in the tight junctions, as shown in Fig. 2, showing that the levels of occludin, zonula occludens (ZO) proteins ZO-1 and ZO-2, afadin, and cadherin were significantly higher during short days. Other proteins remained unchanged during long and short days. Later studies of the CSF proteome or protein constituents showed that 41 of 103 identified proteins had different concentrations depending upon the photoperiod.
Stable and modifiable tight junction proteins (From Lagaraine et al. 2011 reproduced with permission)
There is no equivalent barrier between the CSF and the brain parenchyma in adult mammals, although a transient barrier in the developing brain of sheep and mice is formed by so-called “strap” junctions connecting the neuroependymal cells lining the ventricular wall (Wolburg and Paulus 2010; Liddelow 2015; Whish et al. 2015). Similar barriers may exist in prenatal humans. The brain interstitial fluid (ISF) and the CSF are similar but not identical, in composition, even though they communicate freely at several locations (Abbott 2004; Abbott et al. 2010). The ependymal cell layer and the pia mater and glia limitans, the latter two separating the brain parenchyma from the CSF in the subarachnoid space, may be considered leaky “cellular barriers” compared to the TJ-based “molecular barrier” located at the choroid plexus epithelial cells. After ventricular surgery or infection, the ependymal barrier generally remains incomplete.
Fundamental differences exist between the BBB for ions and molecules and that for cells. Reviews of brain and CSF cellular content identify a two-step process controlling entry into the CNS (Owens et al. 2008; Prodinger et al. 2011), whereas molecules face a single barrier made up of tight junctions. Leukocytes must slow down by binding to surface adhesion molecules on veins before leaving the circulation. Then they begin rolling, searching for points of exit that are usually between cells. This first stage is similar in all tissues, and the second (that controls exit from the perivascular space) is specific to the BBB (Prodinger et al. 2011; Bentivoglio and Kristensson 2014). Leukocytes leave the vascular system only from veins, while most molecular influx involves capillary walls. The surface area of the capillary-CNS interface is much larger than the corresponding venous interface. A healthy CNS must control both molecular and cellular entry; the CP is critical for both.
The choroid plexuses form early in embryonic life, but the neural tube contains fluid before they appear. This is initially amniotic fluid but within 2 days, with choroid plexus still absent, the fluid within the neural tube changes considerably (Chau et al. 2015). This embryonic spinal fluid is rich in proteins and other morphogens (Bueno and Garcia-Fernandez 2016). The subcommissural organ (SCO) is a specialized neuroepithelial structure found in the dorsal midline beneath the posterior commissure and above the aqueduct of chordates, including man. The SCO appears before the choroid plexuses do and secretes complex proteins into the CSF, such as SCO-spondin (a member of the thrombospondin superfamily) that is found in complexes of different molecular weights. SCO-spondin promotes neurogenesis in the very early brain (Guerra et al. 2015). Insoluble glycoprotein complexes including SCO-spondin form Reissner’s fiber, a structure in the third ventricle extending down into the central canal of the spinal cord. First described in 1860, Reissner’s fiber binds monoamines, but its functional significance is still debated. The SCO and SCO-spondin illustrate the concept that CSF growth factors and other signaling molecules may precede the appearance of the CP. Abnormalities of the SCO and Reissner’s fiber are found in some forms of murine hydrocephalus due to mutations. However, species differences between mouse and human SCO are marked; no definite role can be currently ascribed to the SCO in human hydrocephalus (Huh et al. 2009). We focus on the choroid plexus, but we take note of other sources for CSF regulatory signals and the complex relationship among CP, circumventricular organs, and CSF. The circumventricular organs are part of the BCSFB but are not involved in the BBB. Figure 3, taken from Dempsey’s 1968 paper, shows the close proximity of the third ventricular CP to ependyma and the subfornical organ, another midline circumventricular organ then called the intercolumnar tubercle (Dempsey 1968). Dempsey stated that ependymal cells, in contrast to the CP epithelium, lack cilia. We now know that all ependymal cells have multiple cilia that beat in a coordinated manner (Kishimoto and Sawamoto 2012).
Detailed studies of choroid plexus development are available. Kappers (1958) and Netsky and Shuangshoti (1975) focused on humans, while Dziegielewska defined four stages in the development of the choroid plexuses in both humans and sheep (Dziegielewska et al. 2001). The fourth ventricular plexus forms first in all species studied. In some species, such as marsupials, most choroid plexus development occurs postnatally, but its ultimate structure is similar to that of humans. The human fourth ventricle choroid plexus begins to appear in the 6th or 7th week of embryonic life. The plexuses develop from ependyma in the roof plate of the neural tube. It’s interesting to note that intercellular tight junctions are prominent in even very early choroid plexuses (Tennyson and Pappas 1968), while the characteristic cuboidal epithelium appears only in the 17th or 18th week (De Spiegelaere et al. 2008).
Choroid plexus epithelium is derived from neuroepithelial cells, the multipotent stem cells of the nervous system, whereas the stromal component is thought to originate from head mesenchymal cells. Specification of CP epithelium from neuroepithelial cells seems to require the repression of neural cell fate. The number of stages is somewhat arbitrary. The rapidly increasing ratio of CP to lateral ventricle volume in the first trimester and the rapidly increasing CP secretory function in early gestation are more important than the somewhat arbitrary stages reported by embryological studies. Tu and colleagues (1990) found that sheep CP produced high levels of mRNA for transthyretin, the proteinase inhibitor cystatin C, and beta A4 amyloid precursor protein early in gestation and continued to do so throughout gestation.
Choroid Plexus Transport Functions and Physiology
The choroid plexus, like the kidney, contains many transport proteins, with a unique transport profile that differs from the BBB endothelium. We will see in the next section that multiple transport functions in both CP and kidney are regulated by the klotho protein (Sopjani and Dermaku-Sopjani 2016). Ion transport is essential to secretion of CSF, but the CP transport capabilities include additional categories of nonionic transport (e.g., hormones, growth factors, neurotrophins) (Johanson et al. 2008; Spector et al. 2015b). Basolateral (plasma-facing) Na+ and Cl− enter the CP epithelium by membrane-bound exchangers (Spector et al. 2015a). Apical Na+/K+ ATPase uses energy released by ATP hydrolysis to drive CSF fluid formation and maintain a choroidal transcellular Na+ gradient. Cotransport mechanisms carry Cl− into the CSF, while HCO3− is carried by electrogenic NaHCO3 cotransport. To complete the CSF secretory process, ion channels allow conductance of Na+, Cl−, and HCO3− across the apical membrane, down specific electrochemical gradients (Millar et al. 2007). Water then follows, primarily through aquaporin 1 channels (Papadoupos and Verkman 2013).
Many choroid plexus transporters are now known. We refer the reader to past reviews of CP ion transport (Spector 2015a, b) to discuss metal and antibiotic transport before moving on to nutritive and regulatory CP transport functions (Johanson and Johanson 2016). Cerebrospinal fluid calcium and magnesium concentrations are regulated; these ions have great influence on neuronal excitability (Fig. 4).
Age and CSF constituents (From Saunders et al. 2016, reproduced under creative commons article. It stresses the age dependency of ionic differences between plasma and CSF)
We briefly discuss zinc and iron transport as examples of important divalent ions. Iron and zinc are the two most abundant trace metals in living organisms. They are concentrated in gray matter, while calcium and magnesium are most abundant in white matter. Table 1 from Saunders show an impressive number of metal transporter transcripts, grouped by embryo or adult preponderance (Saunders et al. 2015). The same paper shows large numbers of other transporter transcripts, displayed by age, including ions, sugars, and amino acids.
Zinc fingers are important in biology today. A zinc finger is a DNA binding protein or transcription factor; humans have hundreds of zinc finger proteins that regulate DNA transcription. Zinc is especially important for nervous system function. A subset of CNS glutamatergic synapses contains zinc sequestered into synaptic vesicles, where it colocalizes with transmitter glutamate. Zinc has an important although only partly understood roles in synaptic function, neural development, and plasticity. However, zinc in high concentration kills neurons. Unlike iron and copper, zinc is redox neutral, meaning that it has only one oxidation state. Zinc is an essential cofactor for six different classes of enzymes. Zinc concentration in CSF (0.15–0.2 μM) is about 1% of that in plasma. Zinc concentrations in gray matter are much higher and similar to those of copper, iron, and magnesium, about 0.4–0.55 mM (Lovell et al. 1998). Zinc is not a trace metal in the CNS. Radioactive zinc is highly concentrated in the choroid plexus 1 h after intravenous injection. Zinc transporters ZnT1, ZnT3, ZnT4, and ZnT6 are highly expressed in CP epithelium, and Zn concentration in the CSF is tightly regulated (Wang et al. 2004). Stressors such as lead poisoning are known to alter levels of these transporters.
Iron homeostasis in the brain, CP, and CSF is complex and interesting. Iron in the blood is bound to transferrin. Cerebrospinal fluid iron concentrations are normally much lower than in plasma (0.25–1 μM vs. ~20 μM, Codazzi et al. 2015), while CSF ascorbate concentrations are very much higher than plasma. CSF transferrin is completely saturated with iron. This causes a reducing atmosphere and is only one of several benefits from very high CSF ascorbate levels (Spector and Johanson 2014). Ascorbate perfusion of the subarachnoid space to prevent oxidative injury and late complications of subarachnoid hemorrhage has been advocated but never validated in humans. Iron released from erythrocytes stimulates free radical production and oxidative stress as discussed below. Choroid plexus both transports and synthesizes transferrin, and transferrin receptors are found on cerebral capillaries, CP, and neurons. Most iron enters the brain from the capillaries by transferrin receptor-mediated endocytosis. Iron entry into the CNS by the CP is quantitatively less important than that for zinc entry, but the CP has high concentrations of iron regulatory molecules (Rouault et al. 2009). Divalent metal transporter 1 (DMT1) is essential for iron entry into most cell types, but is not found in astrocytes. It participates in iron efflux although ferroportin is the primary iron efflux transporter. DMT1 is abundant in brain capillary endothelial cells and CP endothelium. Iron deficiency or excess is dangerous to the nervous system. Iron deficiency impairs development of the nervous system and leads to upregulation of CP and BBB iron transport. Iron excess leads to formation of damaging free radicals and oxidative stress. Late complications of subarachnoid hemorrhage include vasospasm, meningeal fibrosis, and hydrocephalus. Hemoglobin and methemoglobin released from cells appear to be major causes of these complications. Various preventive treatments have been advocated including perfusion of the subarachnoid spaces with ascorbate, iron chelators, and free radical scavengers. Excessive brain iron content is reported in several diseases, including restless legs syndrome, where CP tissue was reported to contain reduced iron content with increased content of DMT, transferrin receptor, and ferroportin (Connor et al. 2011). Lactoferrin is an iron protein structurally similar to transferrin. Lactoferrin receptors found in the choroid plexus are involved in lactoferrin transport into the CSF. To add to the complexity, at least two zinc transporters can also transport iron.
Most workers agree that the choroid plexuses produce most of the cerebrospinal fluid, although some fluid is produced at other sites (Damkier et al. 2013; Spector et al. 2015b). We will not therefore discuss the work of Oreskovic, Klarica, and colleagues, whose work denying significant CP-CSF production has been reviewed and rejected by many authorities (see also Brinker et al. 2014; Hladky and Barrand 2014).
Many nutritive CP functions have been demonstrated in recent years, some of which are shared with other cell types. For example, glucose transport is a property of many cell types, including the BBB endothelium. Loss of CP may not be significant for glucose entry into brain tissues. Insulin-like growth factors I and II are very important growth regulators in the brain and elsewhere. Both factors are taken up from the blood by CP and locally produced in CP as well. They both stimulate adult neurogenesis. This illustrates the great capacity and complexity of transport in the CP-CSF system and the CP role in regulating brain growth and metabolism.
Complex Regulatory Signals Sent from Choroid Plexus to Brain
The brain is the master organ for adaptation to environmental change. The complex regulation of multiple CNS processes involves learning, plasticity, neurogenesis, and related matters, which can be discussed only briefly. The discovery that complement and other immune proteins have a major role in regulating brain development and plasticity was unexpected (Stephan et al. 2012).
Neurogenesis is related to learning and plasticity. We’ve seen that SCO-spondin originates from the subcommissural organ, which is a less important brain-immune interface than is the choroid plexus. We briefly review three other complex regulatory signals produced by the CP and sent via the CSF to the brain to illustrate the complex CP role in brain development (Zappaterra and Lehtinen 2013): folate transport proteins, the klotho protein, the oct2 protein, and brain-derived neurotrophic factor (BDNF). The choroid plexus contributes to the regulation of neurogenesis and subsequent differentiation in young animals, in terms of neuroblast production, survival, and alignment of new neurons. Cell migration is a prenatal phenomenon, human neurogenesis decreases substantially after birth, while neuronal differentiation, synaptogenesis, and shaping of synapses (and more complex networks involving glial cells) are more active postnatally (Benoit et al. 2015).
Folate Transport and Folate Receptors
Folates are water-soluble vitamins found in several isoforms within the body and essential to normal CNS function. Their membrane transport is complex, and their CSF concentration closely regulated. Active transport at the CP-CSF interface was demonstrated in the 1970s (Spector and Lorenzo 1975). Choroid plexus contains two different folate carriers, the reduced folate carrier (RFC) and the proton-coupled folate transporter (PCFT) (Zhao and Goldman 2013). The folate receptor provides a third pathway. It binds plasma folates that then cross the CP epithelium by endocytosis. Folate receptor (FRα) is found in the choroid plexus, while FRβ is the folate receptor found in some peripheral tissues. Vectorial folate transport from blood to CSF requires both PCFT and FR; deletion of either one markedly reduces CSF folate. Choroid plexus contains much higher concentrations of FRα than other parts of the CNS; FRα is a high-affinity folate binder (Kb 1–10 nM) matching the 5-methyltetrahydrofolate concentration found in human plasma (Grapp et al. 2013). A very rare FRα mutation causes low CSF folate and a progressive neurodegenerative disorder (Steinfeld et al. 2009). A much more common folate deficiency disorder results from autoantibodies against FRα and is treated with high doses of reduced folates to take advantage of the RFC.
Folate within the CP is released into the CSF where it is found in a bound fraction, in exosomes, that are small vesicles. These exosomes are then transported to the brain and within a few hours are found inside cerebral astrocytes and neurons. Exosomes negative for FRα penetrate less well into the brain, most remain close to the ependyma, and some are found inside microglia. Grapp and colleagues found that 36% of all CSF exosomes contained FRα. Figure 5, from Grapp’s interesting paper (it is Fig. 7), portrays the complex mechanisms for delivering folate to brain.
The hydrocephalic Texas rat (H-Tx) demonstrates the CSF influence on cortical growth and neurogenesis and is also a folate-responsive form of hydrocephalus. The H-Tx rat arose in a normal rat colony as a spontaneous mutation and has been subsequently maintained by brother-sister mating. Its genetic basis is unresolved. About 40% of newborn pups have hydrocephalus and develop increased intracranial pressure. Obstruction and blockage of the aqueduct develops at embryonic day 18. After aqueductal obstruction develops, neurogenesis in the cortical mantle becomes abnormal with decreased cell proliferation and failure to retain proliferating cells in the germinal layer. The cortex becomes thin and malformed. Tissue from the cortical mantle grows normally in culture, whereas CSF from the obstructed third ventricle is toxic and kills neural progenitors from normal rats maintained in culture (Mashayekki et al. 2002). Subsequent work showed that affected H-Tx rats also had low CSF levels of the folate enzyme, 10-formyltetrahydrofolate dehydrogenase. Supplementation of the pregnant dams with a combination of tetrahydrofolic and 5-formyl-tetrahydrofolic acid reduced the incidence of hydrocephalus and improved brain development. By contrast, folic acid supplementation increased the incidence of congenital hydrocephalus in this model (Cains et al. 2009). This model illustrates congenital hydrocephalus with complex etiology and also shows that not all forms of folic acid are brain equivalent.
Klotho Protein
Klotho is a calcium homeostasis regulating protein that is highly expressed in the choroid plexus and kidney (Watanabe et al. 2004; Abraham et al. 2016). In the choroidal epithelium, klotho is localized to the perinuclear membrane as well as the apical (CSF-facing) membrane (Semba et al. 2014). The extracellular domain (fragment) of this transmembrane protein is cleaved and shed into the CSF (Akimoto et al. 2012) to act as a hormone with endocrine targets in the brain. Klotho (associated with Na+-K+-ATPase) has several modulating functions, including regulation of ion channels and transporters (Na+ and Ca++). It also promotes growth hormone release. Klotho is essential to brain well-being; the reduced expression of klotho leads to Ca++-related proteolysis (Semba et al. 2014), cell death, premature aging, and cognitive loss. CSF concentration of klotho declines in aging and Alzheimer’s disease (Huang 2010). Conversely, enhanced expression and secretion of klotho increases longevity and slows brain aging as well as cognitive diminution. Small organic molecules to enhance klotho expression may eventually find application in neural diseases associated with defects in the klotho gene.
Orthodenticle Homeobox 2 (otx2) Protein
The otx2 homeobox gene is a major gene shaping vertebrate forebrain induction and head formation (Beby and Lamonerie 2013). Homeobox genes are a large gene family that directs the formation of many body structures during early embryonic development. Over 200 human homeobox genes are known; Hox genes are a subgroup of homeobox genes. Otx2 has multiple roles in retinal development, from the embryo to the adult, where it has been extensively studied. Homeoproteins are a class of transcription factors containing a conserved DNA sequence. Transcription factors are proteins that regulate the expression of other genes (Philippidou and Dasen 2013; Prochiantz and Di Nardo 2015).
Unbalanced early visual experience, for example, by temporarily closing one eye changes neuronal responses in the visual cortex in many mammals from mice to humans. This includes pruning of closed eye-associated axons and dendritic spines and strengthening of open eye-associated input. The unbalanced experience reduces visual acuity of the closed eye (amblyopia), a state that is difficult to remedy in adulthood after the critical period (Morishita and Hensch 2008). The primary visual cortex is the best-known model of developmental plasticity since the initial Nobel Prize-winning work of Hubel and Wiesel (Wiesel and Hubel 1963). The transfer of homeoprotein otx2 into parvalbumin-expressing inhibitory neurons (PV-cells) in the visual cortex regulates cortical plasticity (Bernard et al. 2016). Other genes and factors also contribute. No otx2 is made in visual cortex. Where does it come from?
This has been studied in detail. Not only is otx2 produced by the choroid plexus and released into the CSF, but also immune reduction of otx2 protein has demonstrable effects of adult visual cortical function in mice. We have no direct evidence about otx2 and human amblyopia yet, but otx2 mutations produce a similar kind of retinal dystrophy in mice and men (Vincent et al. 2014).
Brain-Derived Neurotrophic Factor
Brain-derived neurotrophic factor (BDNF) , a well-known regulator of neuronal growth and function (Benarroch 2015), is secreted into the CSF by CP. It is also made by glial and other cells and is highly expressed in the kidney. There are no studies of CPC in experimental animals that might elucidate the importance of decreased levels of these factors for subsequent brain development. Cerebrospinal fluid levels of klotho, FRα, otx2, and BDNF all decline with age, as do many cerebral functions. Are the neurochemical and behavioral changes causally related? Loss of regulation by these CSF factors might follow surgical procedures such as CPC or spontaneous disease. Such losses might be demonstrable with specific in vivo biomarkers of neurogenesis, synaptogenesis, and network architecture. A “neurogenesis-specific” peak at 1.28 ppm in the adult human hippocampus was reported.
However, further work shows that in vivo imaging of human hippocampal neurogenesis has not been reliably attained (Ho et al. 2013). There are no current in vivo neurogenesis biomarkers, but magnetic resonance and electrophysiological probes for synaptogenesis and network development appear to be close to realization. Human adult neurogenesis differs from that in most other mammals. Olfactory bulb neurogenesis probably does not occur, but hippocampal and striatal neurogeneses have been reproducibly demonstrated. Both of these regions are often damaged in hydrocephalic syndromes. The effects of CP ablation on CSF levels of BDNF and other complex trophic/modulatory molecules await study, particularly in the context of neurogenesis in periventricular regions. Remember that progenitor cells in CNS neurogenic niches are intimately exposed to both blood and CSF .
Innervation of the Choroid Plexus
The choroid plexuses are richly innervated by fibers of the autonomic nervous system. Some fibers envelop choroidal blood vessels, others penetrate spaces surrounding the epithelial cells, and others contact cells with neuronal properties (Prasongchean et al. 2015). Aminergic and other nerves innervate mammalian choroid plexus.
Stimulation of the superior cervical ganglion and administration of aminergic drugs such as norepinephrine decrease CSF production (Lindvall and Owman 1981). The effect is blocked by propranolol and considered to be mediated by CP β adrenoceptors. Carbachol and other cholinergic agents are also known to reduce CSF secretion (Ellis et al. 2000). Nerves containing neuropeptide Y (NPY) and other peptides are found in the choroid plexus, with some species variation. There are indications that this innervation or the receptors for its messengers differ between various parts of the same choroid plexus. Choroid plexus neurotransmitters have many roles affecting CP blood flow, epithelial enzyme and transporter activity, and CSF formation rate. If or when increased ICP damages the CP, this may increase CNS deficits.
Choroid Plexus Pathology and Tumors
Choroid plexus pathology is a relatively obscure subject except for tumors, which are rare but of considerable interest. Early pathological studies reported that various abnormalities were more frequent in mentally ill adults, but provided no numbers or real evidence (Findlay 1899). Interest remains in the CP-CSF brain interface in mental illness, but proof of a primary role in the etiology of mental illness remains elusive (Demeestere et al. 2015). Findlay did not mention hydrocephalus. Little has been published about CP pathology in human hydrocephalus and how this affects outcomes. The choroid plexuses have important roles in CNS infectious and inflammatory disorders and are major sites for leukocyte entry into the CNS in both health and disease.
Choroid plexus tumors have a peak incidence in the first 2 years of life. They are most often found in the lateral ventricle of children and the fourth ventricle of adults. The most common clinical manifestation is with signs and symptoms of intracranial hypertension. They are histologically classified as plexus papilloma, atypical plexus papilloma, and plexus carcinoma. A few CP papillomas have been found in fetal life.
Plexus papillomas may be associated with hydrocephalus produced by obstruction or by excessive CSF production, which is mostly limited to pediatric cases. Reports of possible CSF hypersecretion appeared even before World War II. The suggestive evidence of CSF hypersecretion included prompt and persistent elimination of hydrocephalus after surgical resection (progression of the hydrocephalus in spite of working shunts if the tumor was not removed) and drainage of very large CSF volumes. These neurosurgeons could only suspect that excessive CSF production was present. Proof came in the 1970s when two groups measured CSF production by ventriculocisternal perfusion (Eisenberg et al. 1974; Milhorat et al. 1976). Milhorat and colleagues repeated the perfusion study 8 weeks after operation and showed that CSF production had declined to 19% of the abnormally high preoperative value. They also found evidence of obstructed CSF absorption pathways, i.e., there was more than one cause of hydrocephalus.
Xanthogranulomas or xanthomas of the choroid plexus are often found at autopsy and only occasionally symptomatic. These are not true neoplasms, but are lipid-containing granulomatous tissue (Netsky and Shuangshoti 1975).
Choroid plexus hyperplasia is a very rare cause of hydrocephalus (Welch et al. 1983; Fujimoto et al. 2004; Warren et al. 2009). Villous hyperplasia is usually considered to be an increased number of normal-sized cells; the pathological distinction between hyperplasia and papilloma may be difficult. The existence of hyperplastic and neoplastic CP with CSF hyperproduction is analogous to the hypersecretion seen with endocrine tumors and hyperplastic syndromes (e.g., Horvath et al. 1999 in the case of the pituitary).
Choroid plexus cysts are often discovered as incidental findings by fetal ultrasound imaging. Most fetal cysts regress or disappear after birth (Fong et al. 2011; Maher and Platt 2015). Small CP cysts are often found at autopsy (Shuangshoti and Netsky 1966). The importance of fetal CP cysts is that they may be associated with trisomies, large chromosomal deletions, and other malformation syndromes or may be a residual of congenital virus infection. However, in most cases they do not imply future problems. Cysts attached to the choroid plexus are considered to arise from the plexus. Choroid plexus cysts occasionally obstruct the ventricular system and cause hydrocephalus in fetal or postnatal life (Eboli and Danielpour 2011). Cysts near the foramen of Monro may cause monoventricular hydrocephalus.
Choroid plexus hemorrhage is moderately common in neonates and may be the source of intraventricular hemorrhage (Reeder et al. 1982). Nodular choroid plexus pathology is reported in Hurler’s syndrome and other mucopolysaccharidoses (Lach and Haust 2011). Hydrocephalus may occur in Hurler’s syndrome, but does not appear to be related to these CP abnormalities. Several different types of abnormalities are reported in patients with Leigh syndrome and other mitochondrial disorders, including increased numbers of mitochondria. Cystinosis is often associated with the presence of crystals in the CP. These patients may develop hydrocephalus, but there is no evidence that the CP crystals are responsible. Choroid plexus calcification is seen in apparently normal children during the first decade of life, including some children only a few months old (Whitehead et al. 2015).
Choroid plexus enlargement associated with complex regional pain syndrome has been reported by a Finnish group (Zhou et al. 2015). This would be interesting, if confirmed, because of the implication of an expanded role for the choroid plexus. Klosovski (1963) reported that removal of all choroid plexus tissue in young dogs was often fatal, but some surgical brain injury was likely involved. This surgery did not utilize endoscopic techniques. Experimental immune destruction of the CP is possible without surgery but has not been reported in humans. In vitro destruction of CP tissue with ricin-conjugated antibodies is also reported (Del Bigio and Di Curzio 2016). Moreover, the elimination of certain CP transport functions, by gene knockout in animals or by mutation in humans, is associated with major pathology or death (Spector and Johanson 2014). Severe injury after mutations, gene knockouts, or surgery further emphasizes vital CP functions beyond CSF formation.
Subarachnoid hemorrhage is a major problem in clinical medicine. It is complicated by acute hydrocephalus in a minority of patients. These patients have a worse prognosis than others without it; many papers consider the cause and treatment of this complication. Late hydrocephalus probably has a somewhat different pathogenesis, related in part of obliteration of the subarachnoid space as already mentioned. Most work uses the model produced by blood injected into the cisterna magna; several injections are often given. Some have measured CSF production by ventriculocisternal perfusion, often 10–15 days after the first injection, and found it normal or nearly so. Kanat and colleagues gave a single injection of blood into the fourth ventricle and cisterna magna of rabbits, killing some 2 days after injection and others at 14 days for histological study of the brain and CP. They found large differences between the two experimental groups. The 2-day specimens showed a large increase in the number of water-filled vesicles within the CP epithelium, while the 14-day group showed many fewer vesicles and atrophic CP epithelial cells. They did not measure CSF production but assumed that the increased number of vesicles meant increased CSF production. They recommended treatment of early hydrocephalus with acetazolamide and furosemide plus lumbar CSF drainage (Kanat et al. 2013). Others rejected their assumption that these vesicles meant increased CSF production. There was agreement that the CP is damaged in the late stages of experimental hydrocephalus.
The hydrocephalic process produces CP injury and pathology even if the plexuses are not involved in the genesis of hydrocephalus. This is easier to demonstrate in animal models than in human material. Tirapelli and colleagues report ultrastructural changes in rat CP after kaolin-induced hydrocephalus (Tirapelli et al. 2007). Netsky concluded that CP changes in kaolin-induced hydrocephalus were primarily due to pressure and developed slowly. Different kinds of changes (increased numbers of lysosomes and abnormal Golgi apparatus) were found in hydrocephalus induced by vitamin A deficiency (Netsky and Shuangshoti 1975). Both hypo- and hypervitaminosis A produce hydrocephalus and choroid plexus pathology (Becker and Sutton 1963). The common models of experimental hydrocephalus may be analogous to only some forms of human hydrocephalus.
Many papers report human CP changes in patients with hydrocephalus. They suggest that CSF production may decrease in chronic hydrocephalus. Some patients probably show this change, either from feedback mechanisms to control CSF production or because of damaged CP epithelium (Silverberg et al. 2002). Silverberg reported 30 adult patients, 6 with acute hydrocephalus and 10 with chronic hydrocephalus. The chronic cases had somewhat lower rates of CSF production, measured by the Masserman technique of removing CSF and measuring the time needed for CSF pressure to return to its original value; evidently, no patients in this series underwent sequential analyses. Rather than performing additional sequential CSF production studies before and after treatment, we stress the limitations of the Masserman technique, including its need for sedation and risk to patients. More refined techniques (especially noninvasive) are needed to quantify CSF formation rate in humans.
Magnetic resonance techniques are noninvasive and easier to repeat. Silverberg noted that MR techniques generally produce higher CSF production rates than the older techniques (Silverberg et al. 2001). There are many different techniques that are not strictly comparable (Bogomyakova et al. 2016); some studies are simulations with phantoms or extrapolations from a single adult volunteer. None involve small infants, although useful dynamic MR imaging studies in infants are reported (Capel et al. 2014). We emphasize the still incompletely understood differences between infant and adult CSF physiology. The fontanel alters CSF dynamics; infants cannot hold their breath to demonstrate respiratory effects on CSF flow, etc.
The effects of various kinds of CP pathology on CSF content of neurochemical regulators in infants deserve more attention. Choroid plexus tumors, cysts, and the acute phase changes discussed below likely disturb the metabolism and secretion of the CP epithelium. This in turn may alter CSF composition and brain development as well as function.
Choroid Plexus and CNS Infection/Immune Functions
Infections of the CNS are often accompanied by or followed by hydrocephalus.
The choroid plexus plays an important role in CNS infection (Schwerk et al. 2015) and is a proven site of entry for many bacteria and viruses (Levine 1987). Repeated peripheral inflammatory stimuli (such as endotoxin or lipopolysaccharide) alter CP gene expression (Marques et al. 2009; Marques and Sousa 2015). Genes promoting cell adhesion and chemoattraction are upregulated at the BCSFB, the products of which may be harmful to the host. The acute phase reaction is a classical medical entity in which liver changes in protein synthesis cause changes in glucose tolerance, leukocytosis, ceruloplasmin, fibrinogen, etc. and fever that is usually cerebral in origin. These are nonspecific responses to inflammation, tissue injury, or infection and are often measured by the C-reactive protein test. Marques et al. urge us to think of dozens of proteins synthesized by the choroid plexus as “choroid plexus acute phase reactants,” similar nonspecific reactions of the innate immune system. Acute phase reactions have both beneficial and harmful consequences, depending on the details of the patient and the problem.
Virtually all cases of hydrocephalus associated with infection are due to obstruction (or obliteration) of access to sites of reabsorption, because CSF hypersecretion is rare, as noted. Hyperthermia may increase CSF production, and rare cases of infection-associated hydrocephalus may include elements of temperature-related hypersecretion. In general, infection-associated hydrocephalus responds well to shunting procedures if the shunt continues to function. The choroid plexuses are sites of pathogen entry into the CSF-CNS and also sites for lymphocyte entry in normal and diseased brain.
Viruses and bacteria must cross CNS barrier systems to enter the CSF-brain and produce infection. The access of many pathogens to the brain via the BBB and BCSFB is well described (Kim 2008; Schwerk et al. 2015). Although there is good evidence for the CP as a primary entry site for numerous pathogens into the CSF-CNS, the site (s) of CNS entry is not known for most viruses.
Zika virus infection is currently very topical. Zika virus is a flavivirus, only recently discovered to have neurotropic properties. The most common cause of viral encephalitis in the United States has been West Nile virus, another flavivirus transmitted by mosquitos. We have many reports in 2016 of microcephaly associated with Zika virus infection, something that was not previously reported. Driggers et al. recently reported Zika virus infection of the prenatal CNS, including choroid plexus pathology (Driggers et al. 2016)
Pathogens can either utilize transcellular penetration through the cell, or they may open tight junctions for paracellular entry. They can also hijack infected phagocytic host cells and penetrate into the CNS via a “Trojan horse” strategy (Kim 2008). After crossing these barriers, pathogens elicit brain inflammation characteristic of meningitis and meningoencephalitis. Many different microorganisms use the BCSFB to reach the CNS, including viruses, bacteria, fungi, and parasites. The brain entry of African trypanosomes, that cause sleeping sickness, has been extensively studied. Wolburg and Paulus found ultrastructural evidence that they enter the brain via CP. Mogk and coworkers showed that the trypanosomes are attracted to tight junctions in the choroid plexus epithelium through which they pass by still unknown mechanisms (Mogk et al. 2014). Trypanosomes do not remain long in the CSF, because they pass into the adjacent pia mater.
Leukocyte trafficking across the BCSFB is important in health and disease. The effect of hydrocephalus on such migration is unknown. The choroid plexus contains resident macrophages and dendritic cells. Special immune Kolmer epiplexus cells in the ventricle, at the epithelial apical lining, attach themselves to CP for immunosurveilance (antigen-presenting roles) of the CSF. Bacterial meningitis represents a group of very destructive infections, often causing disability or death. It has become clear that the host immune reaction to the infection is a major factor in the poor outcomes that often result (Weber and Tuomanen 2007). Reducing the number of leukocytes that enter the CNS in experimental infection leads to better outcomes. This has led to suggestions that patients with meningitis should be treated with corticosteroids and other agents such as anti-selectins, anti-CD 18 integrin, and anti-interleukin 1-β receptor treatments to reduce leukocyte penetration into the CNS. Many studies of steroid-treated human meningitis have been done; their results are variable. A recent Cochrane review (Brouwer et al. 2015) concluded that studies in the developed countries showed reductions in hearing loss and neurological complications, without improvements in mortality, a modest benefit from adding steroid treatment of bacterial meningitis. No consistent benefit was found in studies done in underdeveloped countries. Attempts to reduce damaging leukocyte entry without impairing infection control have had very limited success thus far.
It is increasingly clear that the choroid plexus has major immunological roles. This information has not been integrated with older information concerning causes and treatment of hydrocephalus. During development, microglia from bone marrow enter the brain through the CP and remain closely linked to the latter. Both the microglia and CP cells contribute to regulation of periventricular neuronal stem cell growth and neuroplasticity as well as network pruning and synaptic functions. Circulating lymphocytes, and particularly CD4+ T cells specific for brain self-antigens, were shown to have a major role in supporting brain plasticity, both in health and in response to CNS trauma (Hendrix and Nitsch 2007; Wolf et al. 2009). The regulation of lymphocyte entry across the BCSFB is especially important in management and pathogenesis of autoimmune disease. Interferon production by choroid plexus, and its secretion into CSF, probably alters brain immune functions in aging and neural disease (Deczkowska et al. 2016). We need data relevant to the control of the traffic of leukocytes and chemical mediators in early development. This is especially important in regard to infant hydrocephalus.
CSF Cellular Content
The normal CSF contains cells. Just as with the ionic content of CSF and blood, differences in the cellular profile between blood and CSF are instructive. Normal CSF contains mostly lymphocytes, which are largely CD4+ memory T lymphocytes (De Graaf et al. 2011). The cellular composition and lymphocyte subtypes of normal CSF differ markedly from peripheral blood. B lymphocytes and natural killer cells are rare in normal CSF. Small numbers of dendritic cells can be found.
Resident microglia provide the primary surveillance and control of intruders in brain and spinal cord parenchyma, while peripheral immune cells patrol specialized CNS compartments located outside the parenchyma such as the ventricular and subarachnoid CSF (Hickey et al. 1991; Ousman and Kubes 2012). How do peripheral immune cells gain access to the normal CNS? Most normal CSF lymphocytes probably enter through the choroid plexus and meninges (Kivisakk et al. 2003; Muller 2009). Surveying macrophages and dendritic cells present antigens to and activate roaming T cells. Approximately 80% of CSF immune cells of healthy individuals are T cells that have probably penetrated the choroid plexus and meninges. We therefore may expect loss or dysregulation of T cell penetration into the CSF after CPC.
Therapeutic Drugs and Choroid Plexus-CSF Functions
Pharmacologic interest in the choroid plexus has centered around two main topics: the role of CP transporters and barrier properties in determining drug levels in the CSF and, secondly, the impact of diuretic-type agents to reduce CSF formation rate and pressure. First, the BCSFB greatly restricts plasma water-soluble agents from entering the CSF; moreover, some drugs that penetrate the CSF, albeit slowly, are then actively reabsorbed back across the epithelium into the choroidal circulation. In this regard, problems in maintaining therapeutic concentrations of certain drugs (e.g., antibiotics) in CSF have been mostly overcome by specific pharmacologic strategies (Spector 2010). A few agents such as transferrin and insulin enter the CSF via receptor-mediated endocytosis. It is possible to inhibit certain efflux pumps (for extrusion from CSF) with drugs such as probenecid and verapamil, which inhibit OAT-mediated antibiotic efflux and the p-glycoprotein (P-gp) efflux mechanism found in many cells and often called multidrug resistance or MDR1, respectively. However, the efflux inhibitors may cause additional problems. Glycosylation is a relatively simple chemical manipulation. Glycosylated opioid peptides have been shown to enter human CSF readily (Mabrouk et al. 2012).
Problems of cancer chemotherapy have stimulated many studies of the CNS P-gp efflux system. Substrates such as verapamil, and even more so cyclosporin and trifluoperazine, may increase net CNS entry, but often cause problems due to their own toxicity. Newer approaches have used other mechanisms to bypass the P-gp or MDR1 efflux system with some success. We’ll mention three of the many new developments on the horizon. Nanocarriers are colloidal systems including polymeric micelles, nanoparticles, lipid nanocapsules, and liposomes. Many such systems have been developed to enhance net brain uptake of drugs and bioactive substances by modulating P-gp transporter activity. Transferrin-conjugated nanoparticles are effective for delivery of chemotherapeutic drugs like taxanes across the BBB (Gan and Feng 2010). Dendrimers are synthetic macromolecules with a specific configuration designed to inhibit P-gp mechanisms by endocytosis and passage into the cell interior. Dendrimers introduce drugs and nucleic acid into the brain (Li et al. 2016) but some are toxic. Exosomes can be isolated from the patient’s own cells in culture and loaded with drugs, RNA, or other compounds (Alvarez-Erviti et al. 2011).
Diuretics and other drugs have been used to inhibit various enzymes or transporters in choroid plexus that normally mediate CSF formation. Diuretics have been widely used to modify CSF dynamics in pediatric and adult patients (Johanson et al. 2008). The carbonic anhydrase inhibitor acetazolamide (Diamox) was shown to produce reliable decreases in CSF production in the 1950s, especially for acute hypertensive episodes in congenital hydrocephalus. Diamox has been extensively used for over 60 years to acutely lower ICP, but systemic side effects (e.g., acidosis) have discouraged chronic usage. Drugs that inhibit ion transporters or Na+/K ATPase (e.g., digoxin) also lower CSF production. Other choroid plexus-inhibiting agents have been used to control CSF fluid balance; among them are furosemide, amiloride, and topiramate. Topiramate is as effective as acetazolamide for lowering ICP in intracranial hypertension (pseudotumor cerebri) but is not proven to reduce CSF formation and may introduce additional complications (Table 2).
Attempts have been made to control hydrocephalus with drug treatment, but with limited success (Del Bigio and Di Curzio 2016). Hydrocephalus with substantially elevated ICP damages the CP, both its structural elements (Weaver et al. 2004) and transporter capacity, e.g., less chloride extrusion transport into CSF (Knuckey et al. 1993). Newer approaches, replacing the moderately effective diuretic drugs with natural peptidergic agents such as atrial natriuretic peptide (ANP), have been proposed. ANP is elevated in the CSF of pediatric hydrocephalus patients, putatively as a homeostatic response to raised ICP; moreover in animals, exogenously administered ANP reduces ICP. Supplemental ANP to raise the CSF hormone level has been considered as a novel approach to control ICP with an endogenous peptide (Johanson et al. 2006).
Abnormal production and secretion of cytokines and other regulators, such as klotho (see above) by the choroid plexus, secondary to hydrocephalus, trauma, and other insults, may progressively damage the CNS. Although the choroid plexus mouse transcriptome is similar to the human, differences in expression (especially in neurodevelopmental control) warrant caution when comparing gene functions in hydrocephalus across species (Janssen et al. 2013). Systematic analysis of gene expression in CP and the associated proteins in CSF, at various stages of hydrocephalus, has not been done. We now know that there are also substantial interindividual differences in the CSF proteome, which adds another layer of complexity. Begcevic and colleagues used a sensitive analytical approach including mass spectrometry to identify all CSF proteins in the CSF of six apparently healthy adults. They identified 2615 different proteins and an even greater number of peptides. Figure 6 from their paper shows the large interindividual variation in proteins that they found (Begcevic et al. 2016). Only 21% of the 2615 proteins were detected in all six samples. Such differences complicate the interpretation of data from individual patients.
Fluid Formation, Intracranial Pressure, and Hydrocephalus
Choroid Plexus Involvement in Hydrocephalus
Edgar Bering suggested in 1955 that arterial pulsations of the choroid plexus contribute to ventricular enlargement in pathological states (Bering 1955). This concept has been accepted as valid by many authors including Warf (2005), Kahle et al. (2016), and others (Egnor et al. 2002). However, the issue is complex. There is evidence against the pulsation assumption (Milhorat 1975; Matsumae et al. 2014). Evidently no relevant studies have included infants. More work is needed to elucidate the intracranial pressure pulse factors that trigger or exacerbate hydrocephalus disorders. Phase-contrast magnetic resonance imaging techniques have shown that the respiratory cycle has a profound effect on CSF flow in the living brain (Dreha-Kulaczewski et al. 2015), much greater than that of the cardiac cycle in normal adults. Newer imaging techniques, including MR time-SLIP technology, have provided much more information about CSF flow and pulsation and have included some patients with hydrocephalus (Kelly and Yamada 2016; Yamada et al. 2013). The Nedergaard group who developed the glymphatic theory has also studied CSF pulsations by a somewhat different method. They studied healthy adult volunteers and showed that respiratory pulsations were substantial and opposite in direction from arterial pulsations (Kiviniemi et al. 2016). They did not refer to any of Yamada’s papers. Neither group considered postural effects on CSF pulsation and flow that are not easily studied in magnetic resonance scanners. Since CSF hydrodynamics differs greatly between adults and infants (who, e.g., lack arachnoid granulations), we must not presume that these findings apply to infants with hydrocephalus. We expect more information about different kinds of CSF pulsations, but only data from infants will resolve the significance of the Bering hypothesis (Bering 1962) for infancy; moreover, findings in normal infants will not necessarily apply to congenital hydrocephalus.
Removal/Coagulation of Choroid Plexus
Choroid plexus ablation reduces CSF secretion, may lower “water hammer pulsations,” and eliminates beneficial gene regulatory mechanisms such as the klotho cascade (see above). CPC may attenuate nutritional support for the brain and possibly impairs subsequent development. This is difficult to study because of the great heterogeneity among hydrocephalic patients with variable causes and intervention at various points in their history. Warf and colleagues have provided some outcome studies, comparing patients with and without choroid plexus coagulation (Mandell et al. 2015). There are many confounding variables, and simple comparison of postoperative CSF volumes is inadequate for evaluation of outcomes and child development.
Since the choroid plexuses are major sites of the CNS barrier systems, their injury or removal may leave physical gaps in the BCSFB and therefore dysregulate lymphocyte entry into the CNS. We are unaware of any special propensity to CNS infection or central autoimmunity after choroid plexus ablation.
Choroid plexus coagulation has also been used in treatment of hydranencephaly (Sandberg et al. 2012). Hydranencephaly represents a heterogeneous group of problems related to hydrocephalus where control of head size is desired and functional improvements are not expected.
Summary
The choroid plexus plays vital roles in the development, maintenance, and aging of the brain. We can now go beyond simple enumeration of choroid plexus transport systems, secreted proteome, etc. to identify how its molecules regulate function and gene expression in CNS regions some distance away. Neuroendocrine and immunologic functions of the CP-CSF are integrated with those of the hypothalamus and circumventricular organs and are important for CNS transport modeling in health and disease. New pharmacologic agents may help control CSF formation and pressure; and smarter shunt systems may better balance the altered brain fluids and distorted metabolism in hydrocephalus. While CSF pharmacology and shunting are being improved, there may be a place for alternative ways to lower elevated ICP, e.g., CPC has been used for almost a century. However, the remarkably complex range of choroid plexus functions was unknown until recently, and few of those papers are known by clinicians.
Remaining choroid plexus third and fourth ventricular tissue after CPC may be sufficient to maintain most nutritive and regulatory functions of the BCSFB after partial removal. Even if this is true, differences between the brain tissue adjacent to lateral ventricles compared to that adjacent to the third and fourth ventricles might be demonstrable by sophisticated imaging and spectroscopic techniques. The overall risk/benefit ratio from CPC + ETV, as compared to ETV alone or shunting, is not clear at this time. We agree with Warf that the situation and risk analysis in sub-Saharan Africa is unique with so few neurosurgical facilities and high risk of catastrophic outcomes upon shunt failure. Recent data suggest that the success of combined ETV and CPC may be predictable if the involved choroid plexuses are shown to disappear on postoperative imaging studies (Pindrik et al. 2016). However, current MR imaging approaches have limited ability to demonstrate all choroid plexus tissue. Standardization of MR techniques for estimating CSF production and CP volumes is needed.
Studies of CPC in experimental animals can help establish the effects of reducing the volume of CP tissue. Analyses of CSF levels of nutrients such as vitamin C and folic acid and regulators like FRα, klotho, otx2, and BDNF protein following CPC would be valuable. Long-term cognitive development after CPC is a serious concern. Even if CPC were to be abandoned at some future time, studies of the CP role in hydrocephalus and other diseases would remain important. Human hydrocephalus has many causes, only some of which are known. The possibility that the CP-CSF system produces “toxic CSF” that accelerates neuronal death, as in the Texas (H-Tx) rat, in certain forms of human hydrocephalus, requires further study. It is likely that “toxic CSF” is a factor in some other neurological diseases, but this awaits further elucidation. Many have wondered about a role for the CP in Alzheimer’s disease and other late life dementias.
Choroid plexus histology is abnormal in old age; most or possibly all of the molecules that stimulate neurogenesis and network maintenance decrease markedly in the CSF of the elderly. However we lack evidence that CP dysfunction is primary in any late life dementias. We also note that Bjorefeldt and colleagues studied human CSF from both older adults and normal controls and found that both had similar complex effects on hippocampal neuronal firing and membrane properties. We may speculate that non-choroid plexus-CSF components become more important with age as the structure-building CP functions decline. Bjorefeldt speculates about wake-up functions of the CSF based on molecules like β-endorphin (coming from naked CSF endings of the arcuate nucleus) and orexins (coming from the infundibulum); see Bjorefeldt et al. 2016). If confirmatory data were available, drugs to enhance production of the most important regulators (as discussed in the klotho section) or enhance its CNS penetration might be beneficial. We lack knowledge on the most important CSF regulators. They are surely different for various stages of life, and their production in CP versus other sites may change in aging.
The CP transmits information to the brain, via CSF and altered barrier function, about peripheral inflammation and gut microbiota. We don’t know the consequences of CPC on these functions. Finally, reduced CP-CSF “homeostatic reserve,” following CPC, may compromise the ability of the brain to recover from trauma and diseases in later life.
References
Abbott NJ (2004) Evidence for bulk flow of brain interstitial fluid: significance for physiology and pathology. Neurochem Int 45:545–552
Abbott NJ, Patabendige AA, Dolman DE et al (2010) Structure and function of the blood-brain barrier. Neurobiol Dis 37:13–25
Abraham CR, Mullen PC, Tucker-Zhou T et al (2016) Klotho is a neuroprotective and cognition-enhancing protein. Vitam Horm 101:215–238
Akimoto T, Shiizaki K, Sugase T et al (2012) The relationship between the soluble Klotho protein and the residual renal function among peritoneal dialysis patients. Clin Exp Nephrol 16:442–447
Alvarez-Erviti L, Seow Y, Fang HY et al (2011) Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol 29:341–347
Ariens Kappers J (1958) Structural and functional changes in the telencephalic choroid plexus during human ontogenesis. In: Wolstenholme GEW, O’Connor CM (eds) Ciba foundation symposium – the cerebrospinal fluid: production, circulation and absorption. Little Brown, Boston, pp 3–31
Baruch K, Schwartz M (2013) CNS-specific T cells shape brain function via the choroid plexus. Brain Behav Immun 34:11. pii: S0889-1591 (13) 00142-6
Beby F, Lamonerie T (2013) The homeobox gene Otx2 in development and disease. Exp Eye Res 111:9–16
Becker NH, Sutton CH (1963) Pathologic features of the choroid plexus. 1. Cytochemical effects of hypervitaminosis A. Am J Pathol 43:1017–1030
Begcevic I, Brinc D, Drabovich AP et al (2016) Identification of brain-enriched proteins in the cerebrospinal fluid proteome by LC-MS/MS profiling and mining of the human protein atlas. Clin Proteomics 13:11
Benarroch EE (2015) Brain-derived neurotrophic factor: regulation, effects, and potential clinical relevance. Neurology 84:1693–1704
Benoit J, Ayoubb AE, Rakic P (2015) Transcriptomics of critical period of visual cortical plasticity in mice. Proc Natl Acad Sci U S A 112:8094–8099
Bentivoglio M, Kristensson K (2014) Tryps and trips: cell trafficking across the 100-year-old blood-brain barrier. Trends Neurosci 37:325–333
Bering EA Jr (1955) Choroid plexus and arterial pulsation of cerebrospinal fluid; demonstration of the choroid plexuses as a cerebrospinal fluid pump. AMA Arch Neurol Psychiatry 73:165–172
Bering EA Jr (1962) Circulation of the cerebrospinal fluid. Demonstration of the choroid plexuses as the generator of the force for flow of fluid and ventricular enlargement. J Neurosurg 19:405–413
Bernard C, Vincent C, Testa D et al (2016) A mouse model for conditional secretion of specific single-chain antibodies provides genetic evidence for regulation of cortical plasticity by a non-cell autonomous homeoprotein transcription factor. PLoS Genet 12:e1006035. https://doi.org/10.1371/journal.pgen.1006035
Bjorefeldt A, Wasling P, Zetterberg H et al (2016) Neuromodulation of fast-spiking and non-fast-spiking hippocampal CA1 interneurons by human cerebrospinal fluid. J Physiol 594:937–952. https://doi.org/10.1113/JP271553
Bogomyakova O, Stankevich Y, Mesropyan N et al (2016) Evaluation of the flow of cerebrospinal fluid as well as gender and age characteristics in patients with communicating hydrocephalus, using phase-contrast magnetic resonance imaging. Acta Neurol Belg 116(4):495–501
Bradbury MW, Cole DF (1980) The role of the lymphatic system in drainage of cerebrospinal fluid and aqueous humour. J Physiol 299:353–365
Brinker T, Stopa E, Morrison J et al (2014) A new look at cerebrospinal fluid circulation. Fluids Barriers CNS 11:10. https://doi.org/10.1186/2045-8118-11-10
Brouwer MC, McIntyre P, Prasad K et al (2015) Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev 12:CD004405
Bueno D, Garcia-Fernàndez J (2016) Evolutionary development of embryonic cerebrospinal fluid composition and regulation: an open research field with implications for brain development and function. Fluids Barriers CNS 13:5. https://doi.org/10.1186/s12987-016-0029-y
Cains S, Shepherd A, Nabiuni M et al (2009) Addressing a folate imbalance in fetal cerebrospinal fluid can decrease the incidence of congenital hydrocephalus. J Neuropathol Exp Neurol 68:404–416
Capel C, Makki M, Gondry-Jouet C et al (2014) Insights into cerebrospinal fluid and cerebral blood flows in infants and young children. J Child Neurol 29:1608–1615
Chau KF, Springel MW, Broadbelt KG et al (2015) Progressive differentiation and instructive capacities of amniotic fluid and cerebrospinal fluid proteomes following neural tube closure. Dev Cell 35:789–802
Codazzi F, Pelizzoni I, Zacchetti D et al (2015) Iron entry in neurons and astrocytes: a link with synaptic activity. Front Mol Neurosci 8:18. https://doi.org/10.3389/fnmol.2015.00018
Connor JR, Ponnuru P, Wang XS et al (2011) Profile of altered brain iron acquisition in restless legs syndrome. Brain 134(Pt 4):959–968
Cushing H (1914) Studies on the cerebro-spinal fluid. J Med Res 31:1–19
Damkier HH, Brown PD, Praetorius J (2013) Cerebrospinal fluid secretion by the choroid plexus. Physiol Rev 93:1847–1892
Dandy WE (1918) Extirpation of the choroid plexus of the lateral ventricles in communicating hydrocephalus. Ann Surg 68:569–579
Dandy WE, Blackfan KD (1914) Internal hydrocephalus an experimental, clinical and pathological study. Am J Dis Child 8:406–482
Davson H (1962) The blood-cerebrospinal fluid and blood-brain barriers. Ergeb Physiol 52:20–73, 1963
de Graaf MT, Smitt PA, Luitwieler RL et al (2011) Central memory CD4+ T cells dominate the normal cerebrospinal fluid. Cytometry B Clin Cytom 80(1):43–50
De Spiegelaere W, Casteleyn C, Van den Broeck W et al (2008) Electron microscopic study of the porcine choroid plexus epithelium. Anat Histol Embryol 37:458–463
Deczkowska A, Baruch K, Schwartz M (2016) Type I/II interferon balance in the regulation of brain physiology and pathology. Trends Immunol 37:181–192
Del Bigio MR, Di Curzio D (2016) Nonsurgical therapy for hydrocephalus: a comprehensive and critical review. Fluids Barriers CNS 13:3. https://doi.org/10.1186/s12987-016-0025-2
Demeestere D, Libert C, Roosmarijn E et al (2015) Therapeutic implications of the choroid plexus–cerebrospinal fluid interface in neuropsychiatric disorders. Brain Behav Immun 50:1–13. https://doi.org/10.1016/j.bbi.2015.06.010
Dempsey EW (1968) Fine structure of the rat’s intercolumnar tubercle and its adjacent ependyma and choroid plexus, with especial reference to the appearance of its sinusoidal vessels in experimental argyria. Exp Neurol 22:568–589
Dreha-Kulaczewski S, Joseph AA, Merboldt KD et al (2015) Inspiration is the major regulator of human CSF flow. J Neurosci 35:2485–2491
Driggers RW, Ho CY, Korhonen EM et al (2016) Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med 374:2142–2151
Dziegielewska KM, Ek J, Habgood MD et al (2001) Development of the choroid plexus. Microsc Res Tech 52:5–20
Eboli P, Danielpour M (2011) Acute obstructive hydrocephalus due to a large posterior third ventricle choroid plexus cyst. Pediatr Neurosurg 47:292–294
Egnor M, Zheng L, Rosiello A et al (2002) A model of pulsations in communicating hydrocephalus. Pediatr Neurosurg 36:281–303
Eisenberg HM, McComb JG, Lorenzo AV (1974) Cerebrospinal fluid overproduction and hydrocephalus associated with choroid plexus papilloma. J Neurosurg 40:381–385
Ellis DZ, Nathanson JA, Sweadner KJ (2000) Carbachol inhibits Na(+)-K(+)-ATPase activity in choroid plexus via stimulation of the NO/cGMP pathway. Am J Physiol Cell Physiol 279:C1685–C1693
Engelhardt B, Sorokin L (2009) The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol 31:497–511
Faraci FM, Mayhan WG, Heistad DD (1990) Effect of vasopressin on production of cerebrospinal fluid: possible role of vasopressin (V1)-receptors. Am J Phys 258:R94–R98
Findlay JW (1899) The choroid plexuses of the lateral ventricles of the brain, their histology, normal and pathological, (in relation specially to insanity). Brain 22:11–25
Fong K, Chong K, Toi A et al (2011) Fetal ventriculomegaly secondary to isolated large choroid plexus cysts: prenatal findings and postnatal outcome. Prenat Diagn 31:395–400
Fujimoto Y, Matsushita H, Plese JP et al (2004) Hydrocephalus due to diffuse villous hyperplasia of the choroid plexus. Case report and review of the literature. Pediatr Neurosurg 40:32–36
Gan CW, Feng SS (2010) Transferrin-conjugated nanoparticles of poly(lactide)-D-alpha-tocopheryl polyethylene glycol succinate diblock copolymer for targeted drug delivery across the blood-brain barrier. Biomaterials 31:7748–7757
Grapp M, Wrede A, Schweizer M et al (2013) Choroid plexus transcytosis and exosome shuttling deliver folate into brain parenchyma. Nat Commun 4:2123. https://doi.org/10.1038/ncomms3123
Guerra MM, Gonzalez C, Caprile T et al. (2015) Understanding how the subcommissural organ and other periventricular secretoary structures contribute via the cerebrospinal fluid to neurogenesis. Front Cell Neurosci 9:480. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4689152/
Hendrix S, Nitsch R (2007) The role of T helper cells in neuroprotection and regeneration. J Neuroimmunol 184:100–112
Hickey WF, Hsu BL, Kimura H (1991) T-lymphocyte entry into the central nervous system. J Neurosci Res 28:254–260
Hladky SB, Barrand MA (2014) Mechanisms of fluid movement into, through and out of the brain: evaluation of the evidence. Fluids Barriers CNS 11:26. https://doi.org/10.1186/2045-8118-11-26:26
Ho NF, Hooker JM, Sahay A et al (2013) In vivo imaging of adult human hippocampal neurogenesis: progress, pitfalls and promise. Mol Psychiatry 18:404–416
Horvath E, Kovacs K, Scheithauer BW (1999) Pituitary hyperplasia. Pituitary 1:169–179
Huang CL (2010) Regulation of ion channels by secreted Klotho: mechanisms and implications. Kidney Int 77:855–860
Huh MS, Todd MAM, Picketts DJ (2009) SCO-ping out the mechanisms underlying the etiology of hydrocephalus. Physiology 24:117–126
Janssen SF, van der Spek SJ, Ten Brink JB et al (2013) Gene expression and functional annotation of the human and mouse choroid plexus epithelium. PLoS One 8:e83345
Johanson CE, Johanson NLW (2016) Merging transport data for choroid plexus with blood-brain barrier to model CNS homeostasis and disease more effectively. CNS Neurol Dis- Drug Targets 15:1151–1180
Johanson CE, Donahue JE, Spangenberger A et al (2006) Atrial natriuretic peptide: its putative role in modulating the choroid plexus-CSF system for intracranial pressure regulation. Acta Neurochir Suppl 96:451–456
Johanson CE, Duncan JA 3rd, Klinge PM et al (2008) Multiplicity of cerebrospinal fluid functions: new challenges in health and disease. Cerebrospinal Fluid Res 5:10
Johanson CE, Stopa EG, McMillan PN (2011) The blood-cerebrospinal fluid barrier: structure and functional significance. Methods Mol Biol 686:101–131
Kahle KT, Kulkarni AV, Limbrick DD Jr et al (2016) Hydrocephalus in children. Lancet 387:788–799
Kanat A, Turkmenoglu O, Aydin MD et al (2013) Toward changing of the pathophysiologic basis of acute hydrocephalus after subarachnoid hemorrhage: a preliminary experimental study. World Neurosurg 80:390–395
Kaur C, Rathnasamy G, Ling EA (2016) The choroid plexus in healthy and diseased brain. J Neuropathol Exp Neurol 75:198. pii: nlv030. [Epub ahead of print]
Kelly EJ, Yamada S (2016) Cerebrospinal fluid flow studies and recent advancements. Semin Ultrasound CT MR 37:92–99
Kim KS (2008) Mechanisms of microbial traversal of the blood-brain barrier. Nat Rev Microbiol 6:625–634
Kishimoto N, Sawatomo K (2012) Planar polarity of ependymal cilia. Differentiation 83:S86–S90
Kiviniemi V, Wang X, Korhonen V et al (2016) Ultra-fast magnetic resonance encephalography of physiological brain activity – Glymphatic pulsation mechanisms? J Cereb Blood Flow Metab 36:1033–1045
Kivisakk P, Mahad DJ, Callahan MK et al (2003) Human cerebrospinal fluid central memory CD4+ T cells: evidence for trafficking through choroid plexus and meninges via P-selectin. Proc Natl Acad Sci U S A 100:8389–8394
Klosovski BN (1963) The development of the brain and its disturbance by harmful factors. Macmillan, New York
Knuckey NW, Preston J, Palm D et al (1993) Hydrocephalus decreases chloride efflux from the choroid plexus epithelium. Brain Res 618:313–317
Kulkarni AV, Shams I, Cochrane DD et al (2010) Quality of life after endoscopic third ventriculostomy and cerebrospinal fluid shunting: an adjusted multivariable analysis in a large cohort. J Neurosurg Pediatr 6:11–16
Kulkarni AV, Riva-Cambrin J, Browd SR et al (2014) Endoscopic third ventriculostomy and choroid plexus cauterization in infants with hydrocephalus: a retrospective hydrocephalus clinical research network study. J Neurosurg Pediatr 14:224–229
Kunis G, Baruch K, Rosenzweig N et al (2013) IFN-γ-dependent activation of the brain’s choroid plexus for CNS immune surveillance and repair. Brain 136:3427–3440
Kurts C, Panzer U, Anders H-J et al (2013) The immune system and kidney disease: basic concepts and clinical implications. Nat Rev Immunol 13:738–753
Lach B, Haust MD (2011) Nodular lesions of choroid plexus in Hurler disease. Fetal Pediatr Pathol 33:189–198
Lagaraine C, Skipor J, Szczepkowska A et al (2011) Tight junction proteins vary in the choroid plexus of ewes according to photoperiod. Brain Res 1393:44–51
Lehtinen MK, Zappaterra MW, Chen X et al (2011) The cerebrospinal fluid provides a proliferative niche for neural progenitor cells. Neuron 69:893–905
Levine S (1987) Choroid plexus: target for systemic disease and pathway to the brain. Lab Investig 56:231–233
Li X, Tsibouklis J, Weng T et al (2016) Nano carriers for drug transport across the blood–brain barrier. J Drug Target. https://doi.org/10.1080/1061186X.2016.1184272
Liddelow SA (2015) Development of the choroid plexus and blood-CSF barrier. Front Neurosci 9:32
Lindvall M, Owman C (1981) Autonomic nerves in the mammalian choroid plexus and their influence on the formation of cerebrospinal fluid. J Cereb Blood Flow Metab 1:245–266
Liu T, Jin X, Prasad RM (2014) Three types of ependymal cells with intracellular calcium oscillation are characterized by distinct cilia beating properties. J Neurosci Res 92:1199–1204
Lovell MA, Robertson JD, Teesdale WJ et al (1998) Copper, iron and zinc in Alzheimer’s disease senile plaques. J Neurol Sci 158:47–52
Lun MP, Monuki ES, Lehtinen MK (2015) Development and functions of the choroid plexus-cerebrospinal fluid system. Nat Rev Neurosci 16:445–457
Mabrouk OS, Falk T, Sherman SJ et al (2012) CNS penetration of the opioid glycopeptide MMP-2200: a microdialysis study. Neurosci Lett 531:99–103
Maher CO, Platt JH Jr (2015) Incidental findings on brain and spine imaging in children. Pediatrics 135:e1084–e1096. https://doi.org/10.1542/peds.2015-0071
Mandell JG, Kulkarni AV, Warf BC (2015) Volumetric brain analysis in neurosurgery: part 2. Brain and CSF volumes discriminate neurocognitive outcomes in hydrocephalus. J Neurosurg Pediatr 15:125–132
Marques F, Sousa JC (2015) The choroid plexus is modulated by various peripheral stimuli: implications to diseases of the central nervous system. Front Cell Neurosci 9:136
Marques F, Sousa JC, Coppola G et al (2009) The choroid plexus response to a repeated peripheral inflammatory stimulus. BMC Neurosci 10:135
Mashayekhi F, Draper CE, Bannister CM (2002) Deficient cortical development in the hydrocephalic Texas (H-Tx) rat: a role for CSF. Brain 125(Pt 8):1859–1874
Matsumae M, Hirayama A, Atsumi H et al (2014) Velocity and pressure gradients of cerebrospinal fluid assessed with magnetic resonance imaging. J Neurosurg 120:218–227
Milhorat TH (1974) Failure of choroid plexectomy as treatment for hydrocephalus. Surg Gynecol Obstet 139:505–508
Milhorat TH (1975) The third circulation revisited. J Neurosurg 42:628–645
Milhorat TH, Hammock MK, Chien T et al (1976) Normal rate of cerebrospinal fluid formation five years after bilateral choroid plexus extirpation. Case report. J Neurosurg 44:735–739
Millar ID, Bruce JI, Brown PD (2007) Ion channel diversity, channel expression and function in the choroid plexuses. Cerebrospinal Fluid Res 4:8
Miyan JA, Nabiyouni M, Zendah M (2003) Development of the brain: a vital role for cerebrospinal fluid. Can J Physiol Pharmacol 81:317–328
Mogk S, Meiwes A, Boßelmann CM et al (2014) The lane to the brain: how African trypanosomes invade the CNS. Trends Parasitol 30:470–477
Morishita H, Hensch TK (2008) Critical period revisited: impact on vision. Curr Opin Neurobiol 18:101–107
Muller WA (2009) Mechanisms of transendothelial migration of leukocytes. Circ Res 105:223–230
Narita K, Takeda S (2015) Cilia in the choroid plexus: their roles in hydrocephalus and beyond. Front Cell Neurosci 9:39
Netsky MG, Shuangshoti S (eds) (1975) The choroid plexus in health and disease. University Press of Virginia, Charlottesville
Ousman SS, Kubes P (2012) Immune surveillance in the central nervous system. Nat Neurosci 15:1096–1101
Owens T, Bechmann I, Engelhardt B (2008) Perivascular spaces and the two steps to neuroinflammation. J Neuropathol Exp Neurol 67:1113–1121
Papadopoulos MC, Verkman AS (2013) Aquaporin water channels in the nervous system. Nat Rev Neurosci 14:265–277
Philippidou P, Dasen JS (2013) Hox genes: choreographers in neural development, architects of circuit organization. Neuron 80:12–34
Pindrik J, Rocque BG, Arynchyna AA et al (2016) Radiographic markers of clinical outcomes after endoscopic third ventriculostomy with choroid plexus cauterization: cerebrospinal fluid turbulence and choroid plexus visualization. J Neurosurg Pediatr 18:287–295. [Epub ahead of print]
Popescu BO, Gherghiceanu M, Kostin S et al (2012) Telocytes in meninges and choroid plexus. Neurosci Lett 516:265–269
Prasongchean W, Vernay B, Asgarian Z et al (2015) The neural milieu of the developing choroid plexus: neural stem cells, neurons and innervation. Front Neurosci 9:103. https://doi.org/10.3389/fnins.2015.00103
Prochiantz A, Di Nardo AA (2015) Homeoprotein signaling in the developing and adult nervous system. Neuron 85:911–925
Prodinger C, Bunse J, Kruger M et al (2011) CD11c-expressing cells reside in the juxtavascular parenchyma and extend processes into the glia limitans of the mouse nervous system. Acta Neuropathol 121:445–458
Pruski M, Rajnicek A, Yang Z et al (2016) The ciliary GTPase Arl13b regulates cell migration and cell cycle progression. Cell Adhes Migr 10:1–13
Reeder JD, Kaude JV, Setzer ES (1982) Choroid plexus hemorrhage in premature neonates: recognition by sonography. AJNR Am J Neuroradiol 3:619–622
Rouault TM, Zhang D-l, Jeong SY (2009) Brain iron homeostasis, the choroid plexus, and localization of iron transport proteins. Metab Brain Dis 24:673–684
Sandberg DI, Chamiraju P, Zoeller G et al (2012) Endoscopic choroid plexus coagulation in infants with hydranencephaly or hydrocephalus with a minimal cortical mantle. Pediatr Neurosurg 48:6–12
Satir P, Christensen ST (2007) Overview of structure and function of mammalian cilia. Annu Rev Physiol 69:377–400
Saunders NR, Dziegielewska KM, Møllgård K et al (2015) Influx mechanisms in the embryonic and adult rat choroid plexus: a transcriptome study. Front Neurosci 9:123. https://doi.org/10.3389/fnins.2015.00123
Saunders NR, Habgood MD, Møllgård K et al (2016) The biological significance of brain barrier mechanisms: help or hindrance in drug delivery to the central nervous system. F1000Res 5. https://doi.org/10.12688/f1000research.7378. pii: F1000 Faculty Rev-313
Schwerk C, Tenenbaum T, Kim KS et al (2015) The choroid plexus- a multi-role player during infectious diseases of the CNS. Front Cell Neurosci 9:80. https://doi.org/10.3389/fncel.2015.00080
Semba RD, Moghekar AR, Hu J et al (2014) Klotho in the cerebrospinal fluid of adults with and without Alzheimer’s disease. Neurosci Lett 558:37–40
Shuangshoti S, Netsky MG (1966) Neuroepithelial (colloid) cysts of the nervous system: further observations on pathogenesis, location, incidence, and histochemistry. Neurology 16:887–903
Silverberg GD, Heit G, Huhn S et al (2001) The cerebrospinal fluid production rate is reduced in dementia of the Alzheimer’s type. Neurology 57:1763–1766
Silverberg GD, Huhn S, Jaffe RA et al (2002) Downregulation of cerebrospinal fluid production in patients with chronic hydrocephalus. J Neurosurg 97:1271–1275
Sopjani M, Dërmaku-Sopjani M (2016) Klotho-dependent cellular transport regulation. Vitam Horm 101:59–84
Spector R (2010) Nature and consequences of mammalian brain and CSF efflux transporters: four decades of progress. J Neurochem 112:13–23
Spector R, Johanson CE (2014) The nexus of vitamin homeostasis and DNA synthesis and modification in mammalian brain. Mol Brain 7:3. https://doi.org/10.1186/1756-6606-7-3
Spector R, Lorenzo AV (1975) Folate transport in the central nervous system. Am J Phys 229:777–782
Spector R, Keep RF, Snodgrass SR et al (2015a) A balanced view of choroid plexus structure and function: focus on adult humans. Exp Neurol 267:78–86
Spector R, Snodgrass SR, Johanson CE (2015b) A balanced view of the cerebrospinal fluid composition and functions: focus on adult humans. Exp Neurol 273:57–68
Steinfeld R, Grapp M, Kraetzner R (2009) Folate receptor alpha defect causes cerebral folate transport deficiency: a treatable neurodegenerative disorder associated with disturbed myelin metabolism. Am J Hum Genet 85:354–363
Stephan AH, Barres BA, Stevens B (2012) The complement system: an unexpected role in synaptic pruning during development and disease. Annu Rev Neurosci 35:369–389
Stern L (1921) Le liquide céfalo-rachidien au point de vue de ses rapports avec la circulation sanguine et avec les éléments nerveux de l’axe cérébrospinal. Schweiz Arch Neurol Psychiatr 8:215–232
Stranahan AM, Hao S, Dey A et al (2016) Blood-brain barrier breakdown promotes macrophage infiltration and cognitive impairment in leptin receptor-deficient mice. J Cereb Blood Flow Metab 36:2108–2121
Tennyson VM, Pappas GD (1968) The fine structure of the choroid plexus adult and developmental stages. Prog Brain Res 29:63–85
Tietz S, Engelhardt B (2015) Brain barriers: crosstalk between complex tight junctions and adherens junctions. J Cell Biol 209:493–506
Tirapelli DP, Lopes Lda S, Lachat JJ et al (2007) Ultrastructural study of the lateral ventricle choroid plexus in experimental hydrocephalus in Wistar rats. Arq Neuropsiquiatr 65:974–977
Tomás J, Santos CR, Quintela T, Gonçalves I (2016) “Tasting” the cerebrospinal fluid: another function of the choroid plexus? Neuroscience 320:160–171
Tu GF, Cole T, Southwell BR et al (1990) Expression of the genes for transthyretin, cystatin C and beta A4 amyloid precursor protein in sheep choroid plexus during development. Brain Res Dev Brain Res 55:203–208
Vincent A, Forster N, Maynes JT et al (2014) OTX2 mutations cause autosomal dominant pattern dystrophy of the retinal pigment epithelium. J Med Genet 51:797–805
Wang ZY, Stoltenberg M, Jo SM et al (2004) Dynamic zinc pools in mouse choroid plexus. NeuroReport 15:1801–1804
Warf BC (2005) Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. J Neurosurg 103(Suppl 6):475–481
Warf BC, Ondoma S, Kulkarni A et al (2009) Neurocognitive outcome and ventricular volume in children with myelomeningocele treated for hydrocephalus in Uganda. J Neurosurg Pediatr 4:564–570
Warf BC, Tracy S, Mugamba J (2012) Long-term outcome for endoscopic third ventriculostomy alone or in combination with choroid plexus cauterization for congenital aqueductal stenosis in African infants. J Neurosurg Pediatr 10:108–111
Warren DT, Hendson G, Cochran DD (2009) Bilateral choroid plexus hyperplasia: a case report and management strategies. Childs Nerv Syst 25:1617–1622
Watanabe M, Yamada H et al (2004) Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct 29:91–99
Weaver CE, McMillan PN, Duncan JA et al (2004) Hydrocephalus disorders: their biophysical and neuroendocrine impact on the choroid plexus epithelium. In: Hertz L (ed) Non-neuronal cells of the nervous system: function and dysfunction. Advances in molecular and cell biology, vol 31. Elsevier Press, Amsterdam, pp 269–293
Weber JR, Tuomanen EI (2007) Cellular damage in bacterial meningitis: an interplay of bacterial and host driven toxicity. J Neuroimmunol 184:45–52
Welch K, Strand R, Bresnan M et al (1983) Congenital hydrocephalus due to villous hypertrophy of the telencephalic choroid plexuses. Case report. J Neurosurg 59:172–175
Whish S, Dziegielewska KM, Møllgård K et al (2015) The inner CSF-brain barrier: developmentally controlled access to the brain via intercellular junctions. Front Neurosci 9:16
Whitehead MT, Oh C, Raju A, Choudhri AF (2015) Physiologic pineal region, choroid plexus, and dural calcifications in the first decade of life. Am J Neuroradiol 36:575–80
Wiesel TN, Hubel DH (1963) Single-cell responses in striate cortex of kittens deprived of vision in one eye. J Neurophysiol 26:1003–1017
Wilcox DR, Folmsbee SS, Muller WJ, Longnecker R (2016) The type I interferon response determines differences in choroid plexus susceptibility between newborns and adults in herpes simplex virus encephalitis. MBio 7(2):e00437–e00416
Willis T (1664) Cerebri anatome, 2nd edn. Flesher J, London
Wolburg H, Paulus W (2010) Choroid plexus: biology and pathology. Acta Neuropathol 119:75–88
Wolf SA, Steinr B, Akpinarli A et al (2009) CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J Immunol 182:3979–3984
Yamada S, Miyazaki M, Yamashita Y et al (2013) Influence of respiration on cerebrospinal fluid movement using magnetic resonance spin labeling. Fluids Barriers CNS 10:36
Yamasaki M, Kanemura Y (2015) Molecular biology of pediatric hydrocephalus and hydrocephalus-related diseases. Neurol Med Chir (Tokyo) 55:640–646
Zappatera MV, Lehtinen MK (2013) The cerebrospinal fluid: regulator of neurogenesis, behavior, and beyond. Cell Mol Life Sci 69:2863–2878
Zhao R, Goldman ID (2013) The proton-coupled folate transporter: physiological and pharmacological roles. Curr Opin Pharmacol 13:875–880
Zhou G, Hotta J, Lehtinen MK et al (2015) Enlargement of choroid plexus in complex regional pain syndrome. Sci Rep 5:14329. https://doi.org/10.1038/srep14329
Zhu X, Di Rocco C (2013) Choroid plexus coagulation for hydrocephalus. Childs Nerv Syst 29:35–42
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Section Editor information
Rights and permissions
Copyright information
© 2019 Springer Nature Switzerland AG
About this entry
Cite this entry
Snodgrass, R., Johanson, C.E. (2019). Choroid Plexus: Source of Cerebrospinal Fluid and Regulator of Brain Development and Function. In: Cinalli, G., Özek, M., Sainte-Rose, C. (eds) Pediatric Hydrocephalus. Springer, Cham. https://doi.org/10.1007/978-3-319-27250-4_38
Download citation
DOI: https://doi.org/10.1007/978-3-319-27250-4_38
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-27248-1
Online ISBN: 978-3-319-27250-4
eBook Packages: MedicineReference Module Medicine