Abstract
To determine the difference in the velocity parameters of cerebrospinal fluid flow in patients with varying severity of communicating hydrocephalus compared to a group of healthy volunteers without hydrodynamic disorders. The study involved 35 subjects with communicating hydrocephalus (25 subjects with Evans index of 0.31; 10 subject with Evans index of 0.46) and 62 healthy volunteers. The mean, volume, and peak flow velocities were determined at the different intracranial levels. Also were made an assessment of gender and age differences. Analysis of the differences between the mean values showed the progressive inhibition of cerebrospinal fluid outflow from the cranial cavity [in moderate communicating hydrocephalus—at 1.5 times (p < 0.05), in severe communicating hydrocephalus at 2–2.5 times (p < 0.01)], depending on the severity of enlargement of the ventricular system and, most likely, related to inhibition of its reabsorption. These changes may explain the clinical symptoms of subjects and serve as diagnostic criteria. Also it was revealed a significant influence of the factor of age on speed characteristics of the cerebrospinal fluid flow (F = 5.3303, p = 0.0003, for mean velocity).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Changes in the cerebrospinal fluid (CSF) system accompany many neurological and neurosurgical diseases; therefore, its comprehensive study is of great interest today. Such conditions as hydrocephalus, malformations of the brain and ventricles, Chiari malformation, tumours of the brain and spinal cord, and subarachnoid cysts often lead to a shift in the haemostatic system of cerebrospinal fluid (a discrepancy between its production, flow, and absorption). However, diagnostic assessment of changes that are in the process of manifesting presents certain difficulties, and existing methods either do not allow for a good assessment of the anatomical and morphological features of the liquor-containing structures or have a number of side effects that limit their use [1]. That is why neurosurgeons, neurologists, and neuroradiologists are acutely interested in the improvement of existing, and the introduction of new, non-invasive diagnostic procedures for liquor assessment into everyday clinical practice.
In addition, the large number of existing patients with communicating hydrocephalus (CH) needing bypass surgery as well as the emergence of new surgical treatment methods require more precise diagnostic criteria and clarity in the pathological physiology of the disorders in hydrodynamics that occur [2–4]. To date, MRI is the most informative method of neuroimaging. Application of supplementary techniques making possible a functional evaluation of morphological parameters (phase-contrast MRI) is enabling it to be more widely introduced. However, an assessment of the efficiency of these techniques and their possible use in diagnostic practice is required [5, 6].
At the same time, there is no information about the comprehensive assessment and possibilities for diagnosing changes in liquor dynamics at various intracranial levels as a single system of cerebrospinal fluid spaces in normal condition as well as in the case of communicating hydrocephalus. In addition, there is not enough research available on the brain basal cisterns level as one of the systems implementing the compensatory potential of the brain in homeostasis disorders. Therefore, the main focus of our work was to examine the change in velocity characteristics of CSF flow dynamics in patients with varying degrees of severity of hydrocephalus with using noninvasive advanced MRI applications, and to further define the diagnostic criteria and possible prognostic factors, from which a forecast can be made for the progression of CSF circulation disorders and the development or worsening of clinical symptoms.
The aim of our work was to study the quantitative characteristics of cerebrospinal fluid dynamics in the head and neck in patients with varying degrees of severity of communicating hydrocephalus using phase-contrast magnetic resonance imaging.
Materials and methods
To study the dynamic characteristics of the movement of cerebrospinal fluid as well as to assess the morphometric parameters of liquor-containing spaces in the head and neck, MRI was performed on 89 patients. All patients were divided into two groups: 62 healthy ones (with no history of neurological disorders and no current neurological conditions, with no signs of cerebral haemodynamic or CSF circulation problems detected via MRI, and with no signs of volumetric or focal lesions of brain tissue detected via MRI) and 35 patients with communicating hydrocephalus (CH). All patients underwent tomographic examination in an outpatient setting, so the main criteria for selection were the radiological data. However, before the survey was conducted questioning with the explanation of the collection of complaints and neurological history. The criteria for selection was the presence of internal non-obstructive hydrocephalus at varying degrees of severity. All patient were divided into two groups by clinical signs, MR-signs, the Evans index of anterior horns. The patients with moderate/severe CH had the following clinical manifestations: general cerebral symptoms observed in varying degrees of severity in all patients, headaches of varying severity in 17/8; reduced visual acuity and visual field loss in 2/3, cognitive impairment in 16 elderly patients and in 9 patients under 40; dysuria in 0/6; ambulation disorder in 5/10, and complete Hakim–Adams syndrome in 0/6. According to available data (extract from the neurological department) in five patients with severe clinical symptoms (cognitive disorders, urinary incontinence, gait disturbance) present a positive tap test (short-term improvement in the breeding of about 40 ml of CSF during lumbar puncture). MRI determined expansion of the ventricles of the brain, with no evidence of blockage of cerebrospinal fluid pathways. We excluded patients with atrophic changes in the brain (hydrocephalus ex vacuo). For all patients, the Evans index is calculated as the ratio of the maximum distance between the outer walls of the anterior horns of lateral ventricles to the maximum biparietal diameter of the skull [7, 8]. A value up to 0.29–0.30 is considered normal; in the patient group, the index exceeded 0.31.
In this way, all the subjects were divided according to the severity of hydrocephalus, 25 patient with moderate communicating hydrocephalus; ten patients with severe communicating hydrocephalus.
The age of all subjects ranged from 15 to 63 years (the average age in the control group was 33.31 ± 3.16 years and in the group of patients—37.29 ± 5.02 years). All patient were divided into five age groups (Table 1) and by gender (15 men and 20 women).
The procedure of involving patients in the study was designed strictly in accordance with international standards, which include the awareness of the subject, his or her consent to participate in the study in its entirety, and guarantees of confidentiality. All of the studies conformed to ethical standards developed in accordance with the Helsinki Declaration of the World Medical Association as amended in 2000. In addition, the studies were supervised by the Institutional Review Board.
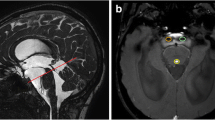
The work was conducted using a 1.5 T MRI Achieva (Philips). The study started with routine testing, which included standard T1, T2-WI, FLAIR, and non-contrast MR myelography and angiography. Next, five sequences of phase-contrast MRI (PC-MRI) for five different levels were conducted. The methodology used the following parameters: slice thickness—4 mm, velocity encoding factor—15 cm/s for the cerebral aqueduct and interpeduncular cistern level, 5 cm/s for the fourth ventricle and prepontine cistern, 10 cm/s for the Magendie foramen and cerebellomedullary cistern as well as the subarachnoid space of the foramen magnum and cervical level (level C2–C3); the scanning time for one level was 2 min 47 s. The values estimated were the average for the cardiac cycle: mean, flux, and peak flow rates. Cross-sections for velocity imaging were positioned as perpendicular as possible to the CSF flow along the chosen structure on the sagittal T1-WI (Fig. 1a).
a Slice orientation on T1 sagittal imaging: 1 Sylvian aqueduct and interpeduncular cistern, 2 fourth ventricle and prepontine cistern, 3 Magendie’s foramen and cerebellomedullaris cistern, 4 anterior and posterior subarachnoid spaces of the foramen magnum, 5 anterior and posterior subarachnoid spaces of cervical level. Also shown is a conventional division into two topographic systems of the CSF spaces: internal (broken line) and external (continuous line); b to trace manually circuits studied structures along the border areas with hypo/hyperintense signal (pulsating CSF) to create irregular geometric shapes (ROI)
The processing of obtained information on the workstation included tracing the cross-sections of the obtained structures along the border areas with hypo/hyperintense signal (pulsating CSF flow) to create irregular geometric shapes (region of interest, ROI), and the subsequent automatic transfer of the geometry to each of the remaining 14 phases of the cardiac cycle (Fig. 1b).
The data were processed, and the average value of the confidence interval for each indicator was calculated. The parameters of antegrade and retrograde flow of cerebrospinal fluid under normal and pathological conditions were compared using Mann–Whitney test (U test). To assess the influence of age and gender factors on flow velocities, non-parametric test Kruskal-N-Wallis was performed for each level.
Results
For a more detailed assessment of the results, the averaged parameters of the cerebrospinal fluid flow were divided into two topographical groups (Fig. 1a):
-
1.
The internal system: cerebral aqueduct, fourth ventricle, Magendie foramen, and the posterior subarachnoid space of foramen magnum and neck region (as an indicator of CSF production in the intraventricular choroid plexuses).
-
2.
The external system: interpeduncular, prepontine, and cerebellomedullary cisterns, the front subarachnoid space of the foramen magnum area, and cervical C2–C3 level (as an indicator of the evacuation of CSF from the brain cavities into the intrathecal space of the spinal cord).
Patients from the CH group manifested an expanded ventricular system and an increase in the anterior–posterior size of the cerebral aqueduct and Magendie foramen, prepontine, and cerebellomedullary cisterns compared to the control group (p < 0.05). Evans index in patients with moderate communicating hydrocephalus was 0.31 ± 0.01, in patients with severe communicating hydrocephalus an index of anterior horns was 0.46 ± 0.05. The patients had the following clinical manifestations: general cerebral symptoms observed in varying degrees of severity in all patients, headaches of varying severity in 72 %; reduced visual acuity and visual field loss in 14 %, cognitive impairment in 95 % of elderly patients and in 25 % of patients under 40; dysuria in 18 %; ambulation disorder in 42 %, and complete Hakim–Adams syndrome in 16 %.
PC-MRI showed the flow of cerebrospinal fluid in the studied levels as a hyper- or hypointensive pulsating signal in the selected ROI. The antegrade (caudal flow) flow of CSF was defined as negative values and the retrograde flow (cranial flow) as positive values.
For the internal and external systems of cerebrospinal fluid spaces, the results are presented as complex graphic charts showing dynamic changes in the CSF dynamics parameters for the studied levels under normal conditions and moderate and severe CH, indicating the significance of differences between the average values of velocity parameters in the control group and the patient group (Figs. 2, 3).
Character of changes of the velocity characteristics of CSF flow in the internal system (Sylvian aqueduct—fourth ventricle—Magendie’s foramen—posterior subarachnoid space of the foramen magnum—posterior subarachnoid space of cervical level) in control subjects and patients with moderate and severe communicating hydrocephalus: a mean velocity, b flux velocity, c peak velocity, d cross-sectional area
Character of changes of the velocity characteristics of CSF flow in the external system (interpeduncular cistern—prepontine cistern—cerebellomedullaris cistern—anterior subarachnoid space of the foramen magnum—anterior subarachnoid space of cervical level) in control subjects and patients with moderate and severe communicating hydrocephalus: a mean velocity, b flux velocity, c peak velocity, d cross-sectional area
The most significant differences in liquor dynamics parameters were identified for the external system of cerebrospinal fluid spaces.
In addition, the impact of gender and age factors on the velocity characteristics of the cerebrospinal fluid flow was assessed. In assessing the effect of age on the mean, flux, and peak velocities, it was noted that in patients with moderate CH the smallest velocities are observed in the first age group which then increase, with the third age group (31–40 years) and with a subsequent decrease in the fourth and fifth groups (F = 7.0120, p = 0.0001; F = 9.6583, p = 0.0000; F = 12.4620, p = 0.0000, respectively). In the group of patients with severe CH, the highest values of velocity parameters are observed in the first age group (15–20 years old) with a subsequent gradual reduction of parameters until the fourth group, followed by a slight increase in the fifth group (F = 7.5982, p = 0.0001; F = 3.1339, p = 0.0223; F = 6.1912, p = 0.0004, respectively), Fig. 4a, b.
The following patterns were identified in the external system of cerebrospinal fluid spaces: patients of the first, second, and third age groups manifested higher mean, flux, and peak flow rates in both the moderate and the severe CH groups. However, in the older age groups the phase synchronicity of the graphs discontinues: in patients with moderate CH, the velocity characteristics continue to decline gradually in the fourth and fifth age groups with the difference in values between the third and fourth groups being small. In patients with severe CH, the fourth group manifests a sharp fall in the velocity parameters, and the fifth group—a small increase. These changes can be explained by the sufficiently stable state of the patients with moderate CH. The dramatic decrease of values in the 41–50 age group in patients with severe CH can be linked to a change in the rheological properties of blood and the reduction of compensatory capacity. And, since the formation and absorption of cerebrospinal fluid is inextricably linked with the state of the circulatory system, the changes that take place can be attributed to the restructuring of the vascular bed, more severe in this age group, Fig. 5a, b.
The Kruskal–Wallis test did not show a significant effect of the gender factor in the analysed symptoms (p > 0.05).
Discussion
In assessing the significance of differences in speed parameters in the internal system, significant differences were found only on some levels. Thus, in patients with moderate CH, moderate acceleration of the flux flow rate at the level of the Magendie foramen and the cerebral aqueduct was identified (p < 0.05). For patients with severe CH, the 2–2.5 times acceleration was manifested in the flux and mean velocities at the level of the cerebral aqueduct, fourth ventricle, and Magendie foramen (p < 0.05). Such data can be explained by the fact that this system is sufficiently monomorphic, consists of tubular structures, has relatively stable anatomical and topographic parameters in the population, and is therefore the least susceptible to any changes in the case of pathology [9, 10].
For peak flow velocity in the inner and outer systems, virtually no significant differences were found.
When analyzing the obtained data for the external system, it may be noted that the mean and flux flow rate gradually increase symmetrically towards the exit from the cranial cavity, with the highest values at the level of the cerebellomedullary cistern and the maximum on the neck level (at the same time, there are no significant patterns or any relationship to the difference in lumen area). The peak velocity also increases mostly from the basal cisterns level to the neck level. The cross-sectional area of the prepontine cistern has a higher value in comparison with the control group.
In assessing the significance of differences between groups of patients and control groups, it was found that the most significant differences are manifested in the outer system of CSF spaces (Fig. 5). In patients with moderate CH, there was a decrease of mean and flux flow rate of CSF mostly at the prepontine and cerebellomedullary cisterns level (p < 0.05). In patients with severe CH, there was a decrease of velocity parameters (mean and flux flow rate) at the prepontine and cerebellomedullary cisterns in the front subarachnoid space of the foramen magnum (p < 0.01) as well as the flux velocity at the interpeduncular cistern (p < 0.05). At the same time, a separate comparison of velocity parameters between subgroups of patients with moderate and severe CH was performed. Significant differences were identified for mean and flux flow rates at the prepontine and cerebellomedullary cisterns level (p < 0.05). Thus, a progressive decline of the linear velocity and the flow volume depending on the severity of hydrocephalus can be noted.
The principal known mechanisms leading to the development of hydrocephalus are believed to be: overproduction of cerebrospinal fluid, which is observed in secondary hydrocephalus cases in patients with choroid plexus papilloma’s;
-
decreased reabsorption of cerebrospinal fluid as a result of the blockade arachnoid villi or lymph channels of the cranial and spinal nerves, adventitia of cerebral blood vessels;
-
blockage of cerebrospinal fluid pathways (leading to the development of obstructive hydrocephalus) [1, 3, 11–13]. Also, in the literature there are alternatives that define a mechanism for chronic hydrocephalus because of the increased pulse pressure in the brain capillaries. Chronic hydrocephalus is due to decreased intracranial compliance, causing restricted arterial pulsations and increased capillary pulsations [2].
Considering the criteria for patient selection, the study findings may be related to a progressive decrease in the reabsorption of cerebrospinal fluid into the venous system through pacchionian bodies and capillaries of subarachnoid spaces with simultaneous mostly minor expansion of the basal cisterns. This may indicate increased resistance of the vascular wall. It is also a marked reduction of high-speed characteristics in the level of basal cisterns may be due to a large outflow of cerebrospinal fluid in the subarachnoid space of the spinal cord (as a compensatory mechanism with increased intracranial CSF volume). Gradually decreasing reabsorption results in slower evacuation of fluid from the cranial cavity and, accordingly, its accumulation in the ventricular system. The enlarged ventricles compress the surrounding brain tissue, which leads to worsening of clinical symptoms and a progressive decrease in the ability to work. The findings may help clinicians in assessing the compensatory ability of brain tissue and in forecasting the further development of pathological changes. With the development of CSF circulation disorders, the ventricular system responds later (the expansion of ventricles takes time), but the velocity characteristics of the cerebrospinal fluid flow change sooner. Therefore, the level of compensatory possibilities may be determined on the basis of the data obtained. If the patient (especially a paediatric one) has a moderate expansion of the ventricular system, but there is a significant reduction in mean and flux flow rate in the basal cisterns of the brain we can assume sub- or decompensation of the reabsorption process. This group of patients requires additional attention due to the high probability of increasing severity of changes. Accordingly, such patients require more serious therapeutic treatment and are potential candidates for bypass surgery.
In addition, in patients with severe HC, a decrease of speed parameters at mostly basal cisterns of the brain was manifested. At the same time, there were no significant differences in younger as compared to older patients (i.e. presumably with congenital as opposed to developed damage), which indicates the common pathophysiological mechanism of the reabsorption function disorder.
A limitation of this study was its holding in the outpatient radiology department. Accordingly, the patients who were included in the study, come to us exclusively MRI study with no history of the disease. Before the phase-contrast and MRI study, we collected medical history and clinical application. However, the main emphasis was placed on the radiological evaluation. Actually, the main purpose of the study was to evaluate the change in the CSF parameters in radiological data hydrocephalus. Now being a set of patients in conjunction with the Neurosurgery center, where carried out bypass surgery and performed invasive physiological monitoring. Accordingly, it is planned to conduct a comprehensive analysis. At the time of this study, the main goal was radiological evaluation.
Conclusion
This study illustrates the progressive dysfunctional changes of the outflow of cerebrospinal fluid from the cranial cavity in patients with communicating hydrocephalus, depending on the degree of expansion of the ventricular system and associated with the problems of the CSF reabsorption (not only in the venous system, but in the capillaries and the lymphatic system). These changes may explain the clinical symptoms manifested in patients as well as serve as diagnostic criteria and prognostic factors for neurologists and neurosurgeons in planning therapeutic and surgical treatment (to establish the degree of compensation or decompensation).
References
Teo C, Johnston I (2000) Disorders of CSF hydrodynamics. Child’s Nerv Syst 16:776–799. doi:10.1007/s003810000383
Greitz D (2004) Radiological assessment of hydrocephalus: new theories and implications for therapy. Neurosurg Rev 27(3):145–165. doi:10.1007/s10143-004-0326-9
Johnston I, Howman-Giles R, Whittle I (1984) The arrest of treated hydrocephalus in children. J Neurosurg 61:752–756
Larsson A, Jensen C, Bilting M, Ekholm S, Stephensen H, Wikkelso C (1992) Does the shunt opening pressure influence the effect of shunt surgery in normal pressure hydrocephalus? Acta Neurochir 117:15–22. doi:10.1007/BF01400629
Bhadelia RA, Bogdan AR, Wolpert SM (1995) Analysis of cerebrospinal fluid flow waveforms with gated phase-contrast MR velocity measurements Am. J Neuroradiol 16:389–400
Baledent O, Henry-Feugeas MC, Idy-Peretti I (2001) Cerebrospinal fluid dynamics and relation with blood flow: a magnetic resonance study with semiautomated cerebrospinal fluid segmentation. Invest Radiol 36(7):368–377. doi:10.1097/00004424-200107000-00003
Matsumae M, Kikinis R, Morocz I, Lorenzo A, Albert M, Black P, Lolesz F (1996) Intracranial compartment volumes in patients with enlarged ventricles assessed by magnetic resonance-based image processing. J Neurosurg 84(6):972–981
Toma AK, Holl E, Kitchen ND, Watkins LD (2011) Evans’ index revisited: the need for an alternative in normal pressure hydrocephalus. Neurosurgery 68(4):939–944. doi:10.1227/NEU.0b013e318208f5e0
Huang TY, Chung HW, Chen MY, Giiang LH, Chin SC, Lee CS, Chen CY, Liu YJ (2004) Supratentorial cerebrospinal fluid production rate in healthy adults: quantification with two-dimensional cine phase-contrast MR Imaging with high temporal and spatial resolution. Radiology 233:603–608. doi:10.1148/radiol.2332030884
Nitz WR, Bradley WJ, Watanabe AS, Lee RR, Burgoyne B, O’Sullivan RM, Herbst MD (1992) Flow dynamics of cerebrospinal fluid: assessment with phase-contrast velocity MR imaging performed with retrospective cardiac gating. Radiology 183:395–405
Levine DN (2008) Intracranial pressure and ventricular expansion in hydrocephalus: have we been asking the wrong question? J Neurol Sci 269:1–11. doi:10.1016/j.jns.2007.12.022
Weller RO, Kida S, Harding BN (1993) Aetiology and pathology of hydrocephalus. In: Schurr PH, Polkey CE (eds) hydrocephalus. Oxford University Press, New York, pp 48–91
Williams B (1973) Is aqueduct stenosis a result of hydrocephalus? Brain 96:399–412
Acknowledgments
This work was supported by the Russian Science Foundation (Project N 14-35-00020).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical standard
Institutional review board approval and informed consent were obtained for this study.
Informed consent
For our study informed consent were obtained for all participants.
Rights and permissions
About this article
Cite this article
Bogomyakova, O., Stankevich, Y., Mesropyan, N. et al. Evaluation of the flow of cerebrospinal fluid as well as gender and age characteristics in patients with communicating hydrocephalus, using phase-contrast magnetic resonance imaging. Acta Neurol Belg 116, 495–501 (2016). https://doi.org/10.1007/s13760-016-0608-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13760-016-0608-3