Abstract
The gallbladder can be subject to an entire spectrum of noninflammatory tumor-like changes. A typical example is cholesterolosis of the gallbladder, defined by a mucosal lesion characterized by accumulation of cholesteryl esters and triglycerides in foamy macrophages. Multiple mucosal yellowish to white lesions produce a macroscopically striking lesion termed strawberry gallbladder. Large accumulations of foamy cells give rise to cholesterol polyps that may protrude into the gallbladder lumen and mimic a neoplastic polyp. These polyps may undergo ulceration followed by secondary inflammatory changes. The adipose tissue situated around the gallbladder can undergo steatonecrosis followed by formation of numerous lipogranulomas. The gallbladder is rarely involved with malakoplakia, a reactive lesion more commonly occurring in the urogenital tract. Gallbladder malakoplakia presents in the form of yellowish plaques or nodules and histologically consists of large macrophages with distinct calcifications, the Michaelis-Gutmann bodies. The gallbladder is the site of endometriosis, endometrioma, several types of metaplasia, and tissue ectopias, whereby misplaced pancreatic tissue is the most common variant.
Access provided by CONRICYT-eBooks. Download reference work entry PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
Cholesterolosis of the Gallbladder
Introduction
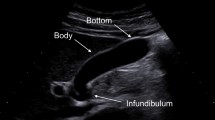
Cholesterolosis (synonyms: cholesterosis, cholesterinosis) is defined by a mucosal lesion of the gallbladder characterized by accumulation of cholesteryl esters and triglycerides in macrophages, which show the morphology of foam cells. Cholesterolosis was first described by Rudolf Virchow in 1857 (Virchow 1857). Typically, cholesterolosis displays a multifocal mucosal distribution, with multiple yellowish and sharply delineated foci distributed on the dark red-green background of the mucosa, a phenotype which somewhat resembles the morphology of the skin of a strawberry (“strawberry gallbladder ”). Cholesterolosis can, however, produce endophytic lesions manifest as polyps, lesions that may radiologically mimic true gallbladder tumors.
Selected References:
MacCarty 1919; Judd and Mentzer 1927a; Mackay 1937; Arnell 1941; Lewis and Peterson 1943; Womack and Haffner 1944; Mitty and Rousselot 1957; Reid 1962; Salmenkivi 1964; Heino and Ritama 1965; Andersson and Bergdahl 1971; Jacyna and Bouchier 1987; Csendes et al. 1998; Izzo et al. 2001; Owen and Bilhartz 2003.
Epidemiology
Cholesterolosis of the gallbladder is a common lesion. In a study of 633 consecutive necropsies, 134 strawberry gallbladders (diffuse cholesterolosis), 61 polypous forms, and 29 combined forms were found. None of the persons was under 15 years, and only three were under 20 years of age (Mentzer 1925). In a study of 1,000 surgical cases of cholesterolosis of the gallbladder, 26 % of the stone-free and 82 % of the gallstone cases occurred in females. The age incidence in the two groups was essentially alike, the greatest number of cases in each group occurring between the ages of 35 and 40 years (Judd and Mentzer 1927a). In 1,323 cholecystectomy preparations, cholesterolosis was detected in 15.6 % of cases (Celoria et al. 1994). In a more recent hospital-based retrospective study, 549 patients underwent cholecystectomy and hepatic resection for hepatocellular carcinoma, the prevalence of cholesterolosis of the gallbladder was 6.6 %, and the prevalence of cholesterol polyp of the gallbladder was 0.9 % (Lai 2011).
Among 549 consecutive patients who had cholecystectomies for various gallbladder disorders, 13.4 % had cholesterolosis. Cholesterolosis with coexistent gallstones was documented in 63.3 %, and 85.1 % of the cases were reported to have abnormally high fasting serum cholesterol levels (Khairy et al. 2004). The prevalence of cholesterolosis is higher in obese patients, being 38 % in obese vs. 6 % in nonobese patients in one study (Dittrick et al. 2005) and 37 % in another (Csendes et al. 2003). In a study of 1,000 cases of gallbladder cholesterolosis, stones were present in half of the specimens, and multiple stones were found in 69 %, 99 % of the stones being cholesterol stones. Cholesterolosis alone, as the typical strawberry gallbladder, was the diagnosis in 53 % of the stone-free group and in 82 % of the stone group, and polypous cholesterolosis was detected in 47 % and 18 %, respectively. Diffuse and local/polypous forms were combined in 34 % of the stone-free and in 10 % of the stone-containing specimens (Judd and Mentzer 1927b).
Clinical and Imaging Features
Isolated cholesterolosis is clinically silent in most cases. In the era preceding CT and MRI studies, imaging for cholesterolosis using X-ray was associated with a high percentage of errors (Judd and Mentzer 1927b; Damore et al. 2001), although other authors consider ultrasonography as an efficient tool (Price et al. 1982; Sandri et al. 2003). In an ultrasonography study of 853 patients who underwent laparoscopic cholecystectomy, 56 had gallbladder polyps, including cholesterolosis polyps, 75 % of them being smaller than 10 mm. Overall US-based diagnosis of gallbladder polyp was inaccurate in 82 % (Akyürek et al. 2005).
Pathology
Macroscopy
Macroscopically, cholesterolosis of the gallbladder shows small yellowish elevations when the mucosa is inspected in the fresh specimen (Fig. 1). These elevations form circumscribed lesions or coalesce to form short bar-like or hook-like structures or an incomplete network reflecting the fine mucosal fold pattern of the gallbladder (Cooke 1931; Lewis and Peterson 1943; Feldman and Feldman 1954). The lesions, which occupy the tips of mucosal ridges, are 1 mm in diameter or less. Seventy-eight percent of cases showed grossly visible fatty changes (cholecystosteatosis) in the wall of the gallbladder (Judd and Mentzer 1927a).
Histopathology
The histology of gallbladder cholesterolosis has been studied in detail (Guerra et al. 1963). The lamina propria of the mucosa and in particular the tips of the delicate mucosal folds are densely infiltrated by large and clear, markedly vacuolated macrophages (foamy cells, Figs. 2 and 3). Cholesterolosis is often associated with papillary epithelial hyperplasia of the gallbladder (Elfving et al. 1968; Celoria et al. 1994) and sometimes with adenomyosis (Helpap and Huegel 1988). The foamy macrophage accumulations can develop regressive changes, associated with release of cholesteryl esters into the extracellular space, an inflammatory response of the foreign-body type, and formation of cholesterol crystals and cholesterol granulomas, with foreign-body giant cells apposed to crystals (Womack and Haffner 1944). In old lesions, collections of cholesterol granulomas, sometimes in band-like formations, may be noted in deep layers of the gallbladder, in part of the cases associated with formation of lymph follicles and germinal centers. This process can induce mucosal ulceration, granulation tissue, and scarring, sometimes followed by inspissated bile depositions and dystrophic calcifications of the gallbladder wall (Womack and Haffner 1944). Exceptionally, osseous metaplasia can develop in such lesions (Ortiz-Hildalgo and Baquera-Heredia 2000). Released cholesterol and its esters may be transported to locoregional lymph nodes, where they can form “metastatic” cholesterol granulomas in the subcapsular sinus (Womack and Haffner 1944). Rarely, cholesterolosis has been found in association with carcinoma in situ (Akiyama et al. 1996).
Electron Microscopic Findings
Cholesteryl ester-laden macrophages in cholesterolosis show numerous protruded processes, which also contain organelles, lipid droplets, and abundant lysosomes. In foam cells, the cytoplasm is filled with large lipid droplets containing cholesteryl esters. Adjacent epithelial cells also show ultrastructural signs of high cholesterol content, with small lipid droplets and a well-developed agranular endoplasmic reticulum (Nevalainen and Laitio 1972; Koga 1985; Satoh and Koga 1997). Epithelial cells of the cystic duct in cholesterolosis of the gallbladder showed mucous secretory granules that appear dilated, and peculiar intracellular cholesterol deposits are detectable in the apical and subapical region of cells and around condensed mitochondria (Gilloteaux et al. 1997), suggesting that the cystic duct mucosa may participate in cholesterolosis of precursor lesions of this condition.
Tumor-Like Cholesterol Polyps of the Gallbladder
In clinico-radiologic work-ups , tumorous lesions of the gallbladder larger than 10 mm have a high incidence of malignancy. In rare instances, cholesterol polyps of the gallbladder can grow to sizes exceeding 1 cm, forming large papillary masses with a diameter of up to 3 cm and thus mimicking gallbladder cancer (Kaido et al. 2004).
Pathogenesis
Cholesterolosis of the gallbladder is considered to be multifactorial, and first ideas about pathogenic pathways date back to more than 50 years (Graham and Elman 1932). As part of investigations documented a correlation between high serum cholesterol levels and the prevalence of cholesterolosis of the gallbladder (Khairy et al. 2004), excess of cholesterol production was regarded as a pathogenic factor. However, in a study on 446 patients with cholesterolosis associated with gallstones and 190 patients without stones, cholesterolosis was not associated with high plasma cholesterol levels (Méndez-Sanchez et al. 1997). In patients with cholesterolosis, a positive correlation was obtained between the cholesterol saturation of bile and the content of esterified cholesterol in the gallbladder mucosa (Sahlin et al. 1995). Free sterols can be transferred from bile to the gallbladder mucosa (Tilvis et al. 1982), where free cholesterol is esterified within cells, mainly mucosal macrophages. Cholesteryl ester accumulation is not caused by reduced efflux of cholesterol due to a defective sterol 27-hyroxylase mechanism (Strömsten et al. 2004). There is evidence that cholesteryl ester synthesis of gallbladder mucosa might play a role in the pathogenesis of cholesterolosis, as the activity of acylCoA-cholesterol ester acyltransferase is increased (Watanabe et al. 1998).
Steatonecrosis, Panniculitis, and Lipogranulomas of the Gallbladder
Severe transmural and specifically fistulating and perforating cholecystitis can induce necrosis and inflammation in the pericholecystic adipose tissue (panniculitis). This process can lead to numerous and in part cystic lipogranulomas, eventually producing a mass effect. Fat necrosis (steatonecrosis, adiponecrosis) of the gallbladder is uncommonly found in patients with acute pancreatitis (Chitkara 1995). It presents as whitish flecks or patches in the subadventitional adipose tissue, identical to the lesions found in peripancreatic adipose tissue. Steatonecrosis of the gallbladder may be marked and associated with direct extension from fat necrosis of the hepatoduodenal ligament, causing an ill-defined mass (Schein et al. 1993). A rare instance of membranous fat necrosis of the gallbladder has been reported (Ohtsuki et al. 2012). Membranous fat necrosis (synonyms: membranocystic change, lipomembranous panniculitis) is usually observed in skin-related diseases but may become a systemic alteration. The tissue contains wavy sudanophilic fluffy membranes that can elicit vigorous foreign-body reactions with giant cells. Gallbladder panniculitis can rarely be a manifestation of the panniculitis disorder, Weber-Christian disease (Ishida et al. 1993).
Cholecystosteatosis (Nonalcoholic Fatty Gallbladder Disease)
Obesity may cause fatty infiltration of multiple internal organs, including liver, heart, kidney, and pancreas, associated with organ and tissue dysfunction. Adipose tissue and tissues having fat overload form a dynamic endocrine organ regulating energy expenditure and adipokine turnover. Obesity is also associated with cholecystosteatosis. Cholecystosteatosis (synonym: nonalcoholic fatty gallbladder disease, NAFGBD) denotes increased fat deposition in the gallbladder wall (review, Pitt 2007). Increased gallbladder tissue lipids comprise free fatty acids, phospholipids, and triglycerides (Goldblatt et al. 2006; Mathur et al. 2008). It has been found that there is a relation between the type of cholecystitis and total gallbladder wall fat. Patients with acalculous and calculous cholecystitis have increased gallbladder fat compared to nondiseased controls, and this increased fat may lead to poor gallbladder emptying and biliary symptoms and signs (Al-Azzawi et al. 2007). Increased lipids enhance inflammatory reactions of the gallbladder (steatocholecystitis) resulting in an abnormal wall structure and decreased contractility (review: Tsai 2009).
Malakoplakia of the Gallbladder
Malakoplakia is a rare and unusual inflammatory process first described in the early 1900s (see the chapter on malakoplakia of the liver). Very few cases of gallbladder malakoplakia have been reported (Hanada et al. 1981; Charpentier et al. 1983; Hide et al. 2001; Agnarsdottir et al. 2004; Di Tommaso et al. 2005; Vaiphei et al. 2012). Gallbladder malakoplakia has been found in association with diabetes mellitus type 2 (Vaiphei et al. 2012). Macroscopically, yellowish plaques or nodules were noted. Histopathologically, accumulation of large macrophages with von Kossa-positive Michaelis-Gutmann bodies, associated with lymphocytic infiltration, is the hallmark. In hematoxylin and eosin-stained sections, Michaelis-Gutmann bodies appear as targetoid cytoplasmic inclusions, and these bodies are PAS positive with and without diastase treatment and are also positive for the colloidal iron stain.
In the presence of typical targetoid, von Kossa-positive Michaelis-Gutmann bodies, the macrophage-rich lesions can hardly be confounded with other granulomatous inflammatory lesions.
Endometriosis and Endometrioma of the Gallbladder
Introduction
Endometriosis is defined as the presence of functioning endometrial tissue outside the uterine cavity. The prevalence of endometriosis has been estimated to be between 8 % and 18 % in young women. Endometriosis outside the pelvic cavity and ovaries mainly involves the abdominal wall, the gastrointestinal tract, and the urinary tract. Rare locations include muscle tissue, inguinal canal, umbilicus, mediastinum, bronchi, pleura, and even nasal region (nasolacrimal endometriosis). Endometriosis, which is well documented for the liver (see the respective paragraph), very rarely occurs in the gallbladder (Saadat-Gilani et al. 2007; Saldaña et al. 2010; Iafrate et al. 2013). Clinically, gallbladder endometriosis was manifest as chronic and vague or colicky abdominal pain, most severe in the right hypochondrium and accentuated during menstruation, and eventually an upper abdominal mass (Iafrate et al. 2013). The cyclic pain is thought to be caused by intrafocal bleeding during menstruation. Gallbladder endometriosis may be an isolated manifestation of this disorder or may be accompanied by endometriotic nodules situated elsewhere, e.g., the abdominal wall (Iafrate et al. 2013).
Pathology
Macroscopically, endometriotic foci may be situated in any part of the gallbladder but predominate in the fundus. The foci may adhere to the gallbladder surface or form internal nodules. The lesions may grow to macroscopic size and mimic cancer (gallbladder endometrioma; Saldaña et al. 2010). Histopathologically, endometriotic foci show preserved or markedly altered endometrial tissue consisting of endometrial glands in various phases of proliferation or secretion and the typical cellular stroma. Secondary changes mainly comprise fresh and old hemorrhage, with accumulation of hemosiderin-containing macrophages and free hemosiderin granules, necrosis, and fibrosis.
As endometriosis is an estrogen-dependent disease, endometriotic foci express estrogen receptors, a phenomenon which may help in the correct classification of stromal foci in the hepatobiliary tract (review: Burns and Korach 2012). Biologically, active estrogens are available to endometriotic tissue via several mechanisms, specifically aromatase activity. The rapid estrogen effects on endometriotic tissue are mediated by both membrane-associated estrogen receptors alpha and G protein-coupled receptor 30/GPER (Plante et al. 2012; Samartzis et al. 2012). Estrogen receptor-beta levels are more than 100 times higher in endometriosis than in normal endometrial tissue (Bulun et al. 2012). Also the estrogen-regulated genes, GREB1, c-MYC, and cyclin D1, are overexpressed in endometriotic foci (Pellegrini et al. 2012).
Differential Diagnosis
Endometriosis occurs in the liver and can be situated close to the gallbladder. Endometriosis sometimes develops on the undersurface of the diaphragm (Triponez et al. 2010).
Osseous Metaplasia of the Gallbladder
Introduction
Osseous metaplasia (heterotopic bone) denotes a tissue alteration characterized by the development of immature and/or mature bone within connective tissue of various organs. Osseous metaplasia of the gallbladder wall is an uncommon finding and has mainly been observed in the setting of chronic fibrosing cholecystitis (Indyk and Shipton 1957; Duchini 1967; Yosepovich et al. 2002; Nelson and Kahn 2009; Rege and Vargas 2011).
Pathology
Histologically, one most often notes a delicate network of osteoid trabecules lined by osteoblasts, embedded in a collagenous matrix with or without associated lymphocytic infiltration (“cholecystitis ossificans”). Mature mineralized bone may also develop in part of the cases. Osseous metaplasia sometimes exclusively involves the gallbladder mucosa (Nelson and Kahn 2009). In one patient with cholecystitis and cholelithiais, osseous metaplasia of the gallbladder wall was associated with a fasciitis-like fibroblastoid proliferation containing osteoclast-like giant cells (Rege and Vargas 2011).
Differential Diagnosis
Ossifications occur in part of gallbladder carcinosarcomas (Nakagawa et al. 1996). Impacted calcified gallstones may mimic circumscribed osseous metaplasia.
Pancreatic Ectopia of the Gallbladder
Introduction
Misplaced pancreatic tissue (ectopic pancreas, heterotopic pancreas) can occur in the wall of the gallbladder. This alteration was first described by Poppi in 1916. Since then, numerous descriptions of this clinicopathologic entity have appeared in the literature.
Selected References:
Mutschmann 1946; Elfving 1959; Monfreda et al. 1967; Dolan et al. 1974; Qizilbash 1976; Ben-Baruch et al. 1986; Lai and Tompkins 1986; Collard et al. 1989; Jarde et al. 1989; Murakami and Tsutsumi 1989; Jeng et al. 1991; Hadzi-Nikolov et al. 1997; Kondi-Paphiti et al. 1997; Bhana and Chetti 1999; Mboti et al. 2003; Meshikhes et al. 2003; Pilloni et al. 2006; Beltran et al. 2007; Elpek et al. 2007; Neupert et al. 2007; Shiwani and Gosling 2008; Bromberg et al. 2009; Al-Shraim et al. 2010; Cerullo et al. 2011; Gucer et al. 2011; Klimis et al. 2011; Sroczynski et al. 2013.
Instead of pancreatic heterotopia or ectopia, the term pancreatic choristoma of the gallbladder is employed to denote this lesion (Beltran et al. 2007). At least half of the cases are located to the gallbladder neck, which embryologically is more close to the pancreas anlage.
Clinical Features
In the majority of cases, heterotopic pancreatic tissue in the gallbladder is an asymptomatic, incidentally found alteration. In part of patients, the lesion can induce, or be associated with, acute or chronic cholecystitis in part of patients (Bhana and Chetty 1999; Mboti et al. 2003; Elpek et al. 2007; Shiwani and Gosling 2008; Bromberg et al. 2009; Al-Shraim et al. 2010; Klimis et al. 2011; Elhence et al. 2012; Sroczynski et al. 2013). Cholecystitis may be related to obstruction, as it was found in pancreatic heterotopia located to the gallbladder neck (Weppner et al. 2009; Limaiem et al. 2012). The lesion rarely presents with symptoms and signs of pancreatitis, as the ectopic pancreatic tissue can undergo acute inflammation, similar to the orthotopic organ (Qizilbash 1976; Pilloni et al. 2006). Sometimes, pancreatic heterotopia gives rise to a suspicious tumefaction (Collard et al. 1989; Foucault et al. 2012), and in one patient, the heterotopia was associated with hypertrophic ectopic pancreatic ducts mimicking an adenomyoma (Pilloni et al. 2006). One case with malignant change of pancreatic heterotopia of the gallbladder was reported (Jeng et al. 1991). The lesion may be associated with high levels of amylasuria (Klimis et al. 2011) or may cause an elevation of pancreatic enzymes in gallbladder bile (Sato et al. 2012).
Pathology
Macroscopically, pancreatic heterotopia is usually manifest as a mere thickening of the gallbladder wall, with circumscribed nodules of pancreatic tissue embedded in fibrous stroma. The heterotopia can, however, also present as gross, tumor-like nodules of 1 cm diameter or even more (Mboti et al. 2003). Histologically, all cellular systems of the normal pancreas can be present, including acinar cells, duct cells, and endocrine islet-type cells or fully developed islets of Langerhans with expression of insulin and somatostatin (Pilloni et al. 2006; Beltram et al. 2007). In some cases, large parts of the gallbladder wall are involved by pancreatic exocrine tissue, the pancreatic ductules and ducts resembling Rokitansky-Aschoff sinuses (Pilloni et al. 2006). Pancreatic heterotopia can be associated with synchronous heterotopic gastric mucosa of the gallbladder (Jaerve and Meurman 1964).
Ectopic Liver of the Gallbladder
Introduction
Ectopic liver tissue (liver choristoma, accessory liver, hepar succenturiatum) is a rare condition that most often involves the pancreas, stomach, gastrohepatic ligament, umbilical ligament, gallbladder, omentum, adrenal glands, esophagus, and thoracic cavity, including mediastinum, lung, and heart. Ectopic liver of the gallbladder is a rare clinical entity that is usually asymptomatic and observed incidentally in the setting of laparoscopy, cholecystectomy for other reasons, or autopsy. Ectopia of liver tissue in the gallbladder wall was first described in 1922 under the term supernumerary liver lobe implanted on the inferior surface of the gallbladder (Corsy 1922).
Epidemiology
Overall, the incidence of ectopic liver in the abdominal and thoracic cavities has been estimated from 0.24 % to 0.47 %. The incidence of ectopic liver of the gallbladder is probably low but seems to be the most common intra-abdominal site of liver ectopia (Griniatsos et al. 2002; Algin et al. 2008; Triantafyllidis et al. 2009). In a study of 5,500 autopsies, only three cases were detected (0.05 %; Eiserth 1940). In a more recent investigation on 1,060 laparoscopies, three cases were identified (0.28 %; Watanabe et al. 1989).
Clinical Features
Most cases of ectopic liver of the gallbladder are incidental findings without symptoms and signs. However, ectopic liver can produce a mass lesion that may be confounded with a gallbladder tumor (Hamdani and Baron 1994). In unusual situations, ectopic liver can undergo secondary changes that are symptomatic, including torsion of pedunculated lesions, acute hemorrhage, compression, obstruction of the gallbladder, or malignant transformation.
Selected References:
Eiserth 1940; Klein 1955; Bassis and Izenstark 1956; Horanyi and Fuesy 1963; Ashby 1969; Costero et al. 1975; Collan et al. 1978; Torchio and Maconi 1978; Natori et al. 1986; Fellbaum et al. 1987; Tejada and Danielson 1989; Watanabe et al. 1989; Castro Viera et al. 1990; Iacconi and Masoni 1990; Svane and Knudtzon 1991; Hamdani and Baron 1994; Kodama and Yokoyama 1996; Sato et al. 1998; Djuricic et al. 1999; Acar et al. 2002; Griniatsos et al. 2002; Sakarya et al. 2002; Lundy et al. 2005; Wang and Liu 2006; Koh and Hunt 2007; Triantafyllidis et al. 2009; Catani et al. 2011; Dettmer et al. 2011; Nagar et al. 2011; Martinez et al. 2013.
Pathology
Macroscopy
Most cases of liver ectopia of the gallbladder showed liver tissue attached to the outer surface of the organ. The lesion may be pedunculated and forming a polypoid structure, with a thick or thin stalk of variable length connecting it with the gallbladder and containing blood vessels (Lundy et al. 2005; Triantafyllidis et al. 2009). Very few reports documented the presence of ectopic liver tissue in inner parts or the gallbladder wall (intramural ectopia) or even the mucosa (Torchio and Maconi 1978; Natori et al. 1986). Ectopic liver tissue of the gallbladder usually manifests as small brownish nodules measuring from a few mm to 1 or 2 cm (Natori et al. 1986), but lesions measuring several cm in diameter have also been observed (Lundy et al. 2005). Due to circulation failure, ectopic liver tissue can undergo necrosis associated with acute hemorrhage, the lesion presenting as a dark red nodule on the gallbladder surface (Nagar et al. 2011).
Histopathology
Histologically, the ectopic liver tissue usually shows a normal architecture, although the lobules may be deformed and/or undersized (Griniatsos et al. 2002). In at least part of the cases, ectopic liver contained portal tracts with small bile ducts, arteries, and venous branches. The ectopic liver tissue is sometimes cholestatic, with accumulation of bile in canaliculi (Svane and Knudtzon 1991). Interestingly, these livers or liverlets do however not always show cholestasis, although a connection to the gallbladder lumen or the cystic duct cannot, or not easily, be identified. Hepatocytes located in ectopic liver may undergo changes similar to that of orthotopic hepatocytes, apart from cholestasis, such as fatty change (Eiserth 1940), hemosiderosis (Tejada and Danielson 1989), and cirrhosis (Watanabe et al. 1989). In one case of ectopic liver localized to the gallbladder fundus, retention of alpha-1-antitrypsin was detected in the ectopic hepatocytes (Dettmer et al. 2011).
Ectopic Liver and Malignancy
Ectopic liver tissue has a propensity to develop hepatocellular carcinoma/HCC, especially in oriental patients (see the respective chapter; Arakawa et al. 1999; Caygill and Gatenby 2004; Leone et al. 2004). In the study of Arakawa and coworkers (1999), which focused at ectopias other than those of the gallbladder, 22 out of 48 cases developed HCC. HCC can also develop in ectopic liver of the gallbladder (Tamura et al. 1985; Arakawa et al. 1999). Interestingly, the incidence of HCC is much lower in ectopic liver tissue of the gallbladder: only one of 33 cases developed cancer (Arakawa et al. 1999). The reason for this striking difference is unknown. Ectopic liver of the gallbladder may have less time to undergo carcinogenesis, because the involved gallbladders may be removed early. It has also been suggested that the difference might be related to the finding that ectopic liver attached to the gallbladder is an anomaly occurring later in ontogenesis and is thus well differentiated and composed of more stable tissue (Griniatsos et al. 2002).
Pathogenic Pathways
It is assumed that ectopic liver of the gallbladder arises from residual liver primordial cells located in the caudal part of the liver primordium.
Thyroid Ectopia of the Gallbladder
In the course of thyroid anlage descent, groups or clusters of thyrocyte precursors can lodge at various non-eutopic sites and thus give rise to ectopic tissue. The most common sites of ectopic thyroid tissue are lingual, sublingual, thyroglossal, laryngotracheal, and lateral cervical sites. Thyroid ectopia in the gallbladder wall has been reported few times (Harach 1998; Ihtiyar et al. 2003; Venditti et al. 2007; Cassol et al. 2010; Liang et al. 2010; review, Klubo-Gwiezdzinska et al. 2011).
Macroscopically, thyroid ectopia can produce a gallbladder mass (Liang et al. 2010), but this is a highly unusual event as ectopic thyroid tissue is usually detected histologically as an incidental finding. Histology is characterized by normal-looking thyroid tissue, with or without a lobular texture, follicles sometimes being embedded in a collagenous matrix. A potential differential diagnosis of thyroid ectopia is gallbladder metastasis of well-differentiated follicular thyroid carcinoma.
Pathogenic Pathways
Similar to thyroid ectopia in the liver (see the respective paragraph), ectopic thyroid tissue in the gallbladder wall is thought to arise via aberrant migration of thyroidocyte progenitors in the course of the thyroid anlage descent from the foramen cecum to the mediastinum. The descending thyroid anlage is, in a certain phase of embryogenesis, in close contact with the mesenchyme of the future septum transversum, and cell exchange may occur during this developmental phase.
Adrenocortical Ectopia
Very rarely, ectopic adrenal cortex was found in the form of small nodular structures in the subserosal space of the gallbladder (Busuttil 1974).
Gastric Mucosal Heterotopia in the Gallbladder
Introduction
Gastric mucosal heterotopia (GMH) of the gallbladder is a congenital abnormality characterized by the presence of usually circumscribed areas of gastric mucosa replacing the original gallbladder mucosa. Gallbladder GMH was first described in 1934, based on a polypoid lesion (Egyedi 1934), and relatively few observations have been documented since.
Selected References:
Williams and Humm 1953; Curtis and Sheahan 1969; Summers et al. 1970; Bentivegna and Hirschl 1972; Keramidas et al. 1977; Mooney et al. 1979; Adam et al. 1989; Pradines et al. 1989; Boyle et al. 1992; Vallera et al. 1992; Schimpl et al. 1994; Uchiyama et al. 1995; Hamazaki and Fujiwara 2000; Inoue et al. 2000; Xeropotamos et al. 2001; Isik et al. 2002; Lombay et al. 2003; Madrid et al. 2003; Sciumè et al. 2005; Cöl et al. 2007; Triki et al. 2008; Hayama et al. 2010; Bulus et al. 2012; Liang et al. 2013.
GMH of the gallbladder is an uncommon condition, while pseudopyloric or pyloric gland metaplastic epithelium in the gallbladder is a common finding, detectable in 66–84 % of cholecystectomy specimens, whereas intestinal metaplasia is present in 12–52 % of gallbladders and is often associated with pyloric metaplasia (review, Xeropotamos et al. 2001). Gallbladder GMH is almost equally distributed among sexes, with an age range at diagnosis of 6–77 years, a considerable proportion of cases being reported for the pediatric age group. In almost a third of cases, gallstones were present. GMH can lead to mucosal defects, including peptic ulcerations (Kehrer and De Minjer 1951; Larsen et al. 1985), sometimes causing massive hemobilia (Adam et al. 1989; Yoon et al. 2005), and hematemesis and melena (Larsen et al. 1985). GMH may occur in gallbladders with preexisting anatomical abnormalities, including duplicate gallbladder (Bailie et al. 2003), or anomalous union of the pancreatobiliary duct (Wakiyama et al. 1998). Apart from the gallbladder, GMH can develop in the cystic duct (Orizio et al. 2011). GMH and intestinal metaplasia of the gallbladder are considered to be precancerous (Yamagiwa and Tomiyama 1986). Etiology and pathogenesis of gastric mucosal heterotopias are not known.
Pathology
Macroscopy
Macroscopically, gallbladder GMH can present as a flat or plaque-like lesion, but GMH growing as pedunculated or sessile polypous masses is also well known (Yamamoto et al. 1988, 1989; Schimpl et al. 1994; Uchiyama et al. 1995; Leyman et al. 1996; Sciumè et al. 2005; Hayama et al. 2010). In case of polypoid lesions, gallbladder carcinoma may suspected based on imaging results (Hayama et al. 2010), also because polypous GMH may grow to a size exceeding 2 cm. Gallbladder GMH can also present as a firm nodular mass or as a multiloculated lesion (Xeropotamos et al. 2001). Large GMH masses can cause symptom-producing tumors (Bentivegna and Hirschl 1972). On CT images, GMH appears as a slightly high density area which is intermediately enhanced early after bolus injection of contrast medium (Inoue et al. 2000). The lesions are often located in the gallbladder fundus, but GMH also occurs in the gallbladder neck (Sciumè et al. 2005).
Histopathology
Histologically, GMH reveals a gastric-type superficial epithelium and associated gastric glands, including pyloric glands, corpus glands, and fundic glands, chief cells and parietal/oxyntic cells being present (Runge et al. 1978). The epithelium overlying the glandular structures may show hyperplastic changes. Neuroendocrine/APUD cells have been found in part of the cases (Vallera et al. 1992). GMH can be associated with extensive adjacent pyloric and/or pseudopyloric metaplasia, staining red with the Alcian blue-PAS stain (Xeropotamos et al. 2001), or intestinal gallbladder metaplasia (Tavli et al. 2005). It can undergo secondary changes, such as cystic change (Popkharitov et al. 2008) or squamous metaplasia (Daud et al. 2007). Gallbladder GMH can be associated with other types of heterotopia/ectopia, such as pancreas and thyroid tissue (Murakami and Tsutsumi 1999), or with adenoma of the gallbladder (Summers et al. 1970). On gastric-type epithelium of the gallbladder, no Helicobacter pylori was detected (Arnaout et al. 1990).
Ectopic Gallbladder
Ectopic gallbladder, albeit a rare condition, has clinical significance because it can lead to misdiagnosis and misinterpretation as a tumorous lesion. Several types of gallbladder ectopia are known (Table 1).
A retroposed gallbladder (retrohepatic gallbladder) may suggest the presence of a cystic tumor on the underside of the liver (Feldman and Venta 1988; Chowbey et al. 2004). A retrohepatic gallbladder can be contained in the coronary ligament (Principe et al. 1979). Ectopic gallbladder may be situated within the liver substance, with or without stones (Glasionov 1961; Schulz et al. 1975; Velchik and Noel 1987; Lobo et al. 2007), and can mimic an intrahepatic cystic tumor (Schneider et al. 1979). If it is situated away from the peritoneum, signs of acute cholecystitis may be absent. In some cases, ectopic gallbladder has an own mesenterium (mesovesicula) containing feeding vessels (Popli et al. 2010), the ectopic gallbladder then being a hanging lesion that can undergo torsion and gangrenous infarction (the “floating gallbladder”; Havrilla et al. 1978). A floating gallbladder on a long “mesovesicula” can also herniate through the foramen of Winslow into the lesser sac, with signs of strangulation (Blanton et al. 1974). In rare instances, the gallbladder is situated in a suprahepatic position (Faintuch et al. 1979; Youngwirth et al. 1983; Sheu et al. 1995), sometimes associated with malformations of the right liver (Hsu et al. 1994) or inverted liver (Hibbs and Ahmad 2010). It can also occur intrathoracically (Labitzke 1991), and in few instances, the gallbladder was transposed to the left underside of the liver (gallbladder transposition; Duimstra and Greenfield 1977; Keller et al. 1982; Wong et al. 2001; Dhulkotia et al. 2002) or was malpositioned in the region of extrahepatic bile ducts (gallbladder interposition; Walia et al. 1986). The gallbladder can be situated on the left side of the common bile duct and the cystic duct, arising from the right hepatic duct (Chung et al. 1997).
Reactive Vascular Mass Lesions of the Gallbladder
Introduction
Similar to extrahepatic and intrahepatic bile ducts, the gallbladder can be involved in a variety of reactive vascular alterations that may produce mass effects or pseudotumors mimicking neoplastic disease. The most important changes include gallbladder varices and pseudoaneurysms of the cystic artery.
Gallbladder Varices
In part of patients with portal hypertension, ectopic varices (varicose veins) as dilated venous collaterals can develop in the wall of the gallbladder.
Selected References:
Malusev 1951; Salam et al. 1979; Lebrec and Benhamou 1985; West et al. 1991; Chawla et al. 1994, 1995; Safadi et al. 1996; Gabata et al. 1997; Palazzo et al. 2000; Chu et al. 2002; Radhi 2003; Ito et al. 2009; de Alcantara et al. 2013.
It is estimated that the incidence of gallbladder varices in cirrhotic and non-cirrhotic portal hypertension amounts to up to 30 % (West et al. 1991; Chawla et al. 1994; Helbich et al. 1994; Rathi et al. 1996). Gallbladder varices have also been described in the pediatric age group (Helbich et al. 1994; Rathi et al. 1996). The varices may or may not be associated with extrahepatic portal vein occlusion (West et al. 1991) but are sometimes associated with portal vein cavernoma (Lebrec and Benhamou 1985). Varices cause fixed filling defects (Rosen and Wilson 1980) and thickening of the gallbladder wall suspicious of malignancy (Saigh et al. 1985). Rupture of varices leads to variceal bleeding, sometimes followed by life-threatening or fatal abdominal hemorrhage (Holmlund and Lundström 1977; Chu et al. 2002; Kevans et al. 2009; Vilallonga et al. 2012). The dilated gallbladder veins can be visualized by means of color Doppler sonography (Kainberger et al. 1990; Helbich et al. 1994; Safadi et al. 1996; Mishin 2005). A direct communication of the varices to intrahepatic portal vein branches can be demonstrated by Doppler sonography and CT (Gabata et al. 1997).
Aneurysmatic Changes and Related Vascular Disorders
The most common alteration in this group is cystic artery pseudoaneurysm (CAP). Pseudoaneurysms (synonyms: false aneurysm, aneurysma spurium, aneurysma falsum) are characterized by a periarterial hematoma following a tear in the arterial wall involving intima and media but leaving the adventitia intact in classical cases. However, in some of these aneurysms, the hematoma will break through the adventitia with time. CAP is most often observed in the setting of acute cholecystitis (Machida et al. 2008; Hague et al. 2010; Dewachter et al. 2012; Fung et al. 2013) and can develop as a complication of xanthogranulomatous cholecystitis (Ahmed et al. 2010). CAP can protrude into the gallbladder lumen, producing masses of up to 2 cm in diameter (Ahmed et al. 2010). This vascular lesion can cause acute internal hemorrhage (Fung et al. 2013) and hemoperitoneum (Ghoz et al. 2007), sometimes with fatal outcome (Olbrycht 1965). Pseudoaneurysms can also develop in the right hepatic artery and rupture into the gallbladder (Schubert et al. 1980; Lin et al. 2010). Percutaneous liver biopsy can be complicated by arterial-portal fistula causing a gallbladder polyp as a manifestation of hemorrhage (Lin et al. 2005).
Arteriovenous and Other Vascular Malformations of the Gallbladder
Arteriovenous malformations of the gallbladder are very unusual alterations characterized with the presence of serpentine around and within the gallbladder wall. Angiographically, dilated and tortuous cystic artery branches, a racemose vascular network, and early filling branches of the cystic vein have been noted (Tajima et al. 1997; Osada et al. 2007). The gallbladder is rarely involved in the setting of Osler-Weber-Rendu disease, with multiple telangiectasias in the gallbladder wall (Baba et al. 1995).
Gallbladder Hemorrhage and Hematoma
Introduction
Gallbladder hematomas can occur under various conditions, including trauma, inflammation, coagulation disorders, vascular accidents, and malignant neoplasms. More commonly, blood accumulates within the gallbladder lumen and produces a hemocholecyst, but various conditions also cause intramural gallbladder bleeding which results in wall thickening, mass effect, and sometimes extensive wall dissection. Gallbladder hemorrhages and hematomas can be divided into several anatomical categories (Table 2).
Hemorrhagic Cholecystitis
Gallbladder hemorrhage is a relatively rare complication of hemorrhagic cholecystitis (Parekh and Corvera 2010) and is sometimes associated with pathological coagulopathy or the administration of anticoagulative therapies (Morris et al. 2008; Chen et al. 2010), uremia (Lai and Tarng 2009), or cytostatic therapy. Hemorrhagic cholecystitis can result in hemobilia (Bazzoni et al. 1993) or in gallbladder rupture with massive intraperitoneal bleeding (Tavernaraki et al. 2011). Hemorrhagic cholecystitis, with its alteration in wall structure and contractility, may simulate gallbladder carcinoma (Gremmels et al. 2004).
Intraluminal Hematoma (Hemocholecyst)
Acute or continuous bleeding into the gallbladder lumen causes the formation of a blood clot that may completely fill the lumen (hemocholecyst , gallbladder hematocele, Scharling and Geisinger 1993) and which reveals characteristic sonographic and CT features (Grant and Smirniotopoulos 1983; Kauzlaric and Barmeir 1985). Intraluminal blood masses may be mixed with bile, gallstones, mucus, exudate, and tissue debris. Intraluminal blood escapes through the cystic duct and hence causes hemobilia in more distal parts of the biliary tract. In severe and rapidly progressing hemorrhage, gallbladder rupture may result. Important causes of hemocholecyst mainly comprise blunt trauma with gallbladder contusion (Sandblom 1948; Saad et al. 1979; Fröschle et al. 1990; McNabney et al. 1990; Erb et al. 1994), gallbladder malignancy (Faure et al. 1969; Uchiyama et al. 1998; John et al. 1999; Heise et al. 2000; Kubota et al. 2000), coagulation disorders (e.g., hemophilia; Shimura et al. 2000), complications of anticoagulant therapy (Brawner et al. 1966; Mikou et al. 2004; Zangrandi et al. 2009), chemotherapy, radiofrequency ablation therapy of malignant liver tumors (Yamamoto et al. 2003; Shin et al. 2011), benign tumors and polyps (Cappell et al. 1993), hemorrhagic cholecystitis (Ku et al. 2004), ruptured artery and venous aneurysms and pseudoaneurysms (Barzilai and Kleckner 1956; Hakami et al. 1976; Miura et al. 1998), and venous hemorrhage in portal hypertension (Krustev et al. 2002). In the setting of portal hypertension, gallbladder varices may develop, followed by variceal hemorrhage (Chu et al. 2002; Kevans et al. 2009). Intraluminal gallbladder hematoma was also induced by percutaneous liver biopsy (Kwon et al. 2002). Hemocholecyst can present as a tumorous mass and may mimic a gallbladder neoplasm (Jung et al. 2011).
Intramural Hematoma
Intramural hematoma of the gallbladder is less common than hemocholecyst (Tesler and Cantor 1957; Pilling 1979). Hematoma confined to the wall results in a mass that exerts pressure. The hematoma may remain confined to the wall or may rupture into the gallbladder lumen or through the serosal covering into the peritoneal cavity. Hematoma of the gallbladder wall may be associated with infiltrating hematoma of the hepatic pedicle (Dao et al. 1989). Intramural hematoma as a “pushing” or infiltrating mass can mimic a gallbladder neoplasm on sonographic, CT, or MR images (Tan et al. 2005; Jung et al. 2011).
Hemorrhagic Tumors
Malignancies of the gallbladder can give rise to hemorrhage, both hemocholecyst and mural hematomas at the site of the tumor, whereby the tumors themselves may be hemorrhagic (Petrin 1966; Faure et al. 1969; Piotrowski et al. 1975; Calmat et al. 1979; Fourdan et al. 1994; Osawa et al. 1996; Jones et al. 1997). Metastases to the gallbladder also cause hemorrhage and hemobilia, e.g., metastases of renal cell carcinoma (Fullarton and Burgoyne 1991) or of hepatocellular carcinoma (Chang et al. 1998). In addition to malignant tumors, also benign gallbladder neoplasms can give rise to tumor hemorrhage (Cho et al. 2001).
Pathology
Macroscopy
In acute hemorrhagic cholecystitis, the gallbladder wall is thickened, with edema and bleeding in the extramuscular tissue. The mucosa has lost its fine texture and appears as a dark red to blackish surface with overlying blood coagula and exudate on ulcerated parts. On cut sections, the wall has lost its layers and is visualized as a dark red and often friable tissue. Hemocholecyst is macroscopically characterized by liquid or coagulated blood filling the gallbladder lumen. In recently developing cases, the blood is easily removable from the mucosa, while with time the coagulated blood may adhere to the mucosal surface. In formalin-fixed specimen, the blood forms a dark and hard mass that usually falls off when cutting through the organ. Intramural hematomas, which are either an isolated phenomenon or are combined with hemocholecyst, appear as blood masses of variable size that dissect the gallbladder wall and may bulge into the lumen, mimicking a hemorrhagic tumor.
References
Acar T, Taçyildiz R, Karakayali S (2002) Ectopic liver tissue attached to the gallbladder. Acta Chir Belg 102:210–211
Adam R, Fabiani B, Bismuth H (1989) Hematobilia resulting from heterotopic stomach in the gallbladder neck. Surgery 105:564–569
Agnarsdottir M, Willén R, El Hag IA (2004) Three cases of malakoplakia of the gallbladder. Ups J Med Sci 109:255–259
Ahmed I, Tanveer UH, Sajjad Z, Munazza B, Azeem UD, Basit S (2010) Cystic artery pseudo-aneurysm: a complication of xanthogranulomatous cholecystitis. Br J Radiol 83:e165–e167
Akiyama T, Sahara H, Seto K, Saitou H, Kiriyama M, Tomita F, Kosaka T, Kita I et al (1996) Gallbladder cancer associated with cholesterosis. J Gastroenterol 31:470–474
Akyürek N, Salman B, Irkörücü O, Sare M, Tatliciolglu E (2005) Ultrasonography in the diagnosis of true gallbladder polyps: the contradiction in the literature. HPB (Oxford) 7:155–158
Al-Azzawi HH, Nakeeb A, Saxena R, Maluccio MA, Pitt HA (2007) Cholecystosteatosis: an explanation for increased cholecystectomy rates. J Gastrointest Surg 11:835–842
Algin et al. (2008) http://www.ncbi.nlm.nih.gov/pubmed/21490853
Al-Shraim M, Rabie ME, Elhakeem H, Kandeel A, Shah MT, Jamil S (2010) Pancreatic heterotopia in the gallbladder associated with chronic cholecystitis. A rare combination. JOP 11:464–466
Andersson A, Bergdahl L (1971) Acalculous cholesterosis of the gallbladder. Arch Surg 103:342–344
Arakawa M, Kimura Y, Sakata K, Kubo Y, Fukushima T, Okuda K (1999) Propensity of ectopic liver to hepatocarcinogenesis: case reports and a review of the literature. Hepatology 29:57–61
Arnaout AH, Abbas SH, Shousha S (1990) Helicobacter pylori is not identified in areas of gastric metaplasia of gall bladder. J Pathol 160:333–334
Arnell O (1941) Cholesterolosis vesicae felleae. Acta Chir Scand 85:511–524
Ashby EC (1969) Accessory liver lobe attached to the gall-bladder. Br J Surg 56:311–312
Baba R, Hashimoto E, Yashiro K, Nagasako K, Hayashi N, Nishikawa T, Ludwig J (1995) Multiple abdominal telangiectases and lymphangiectases. A limited form of Osler-Weber-Rendu disease? J Clin Gastroenterol 21:154–157
Bailie AG, Wyatt JI, Sheridan MB, Stringer MD (2003) Heterotopic gastric mucosa in a duplicate gallbladder. J Pediatr Surg 38:1401–1403
Barzilai R, Kleckner MS (1956) Hemocholecyst following ruptured aneurysm of portal vein: report of a case. AMA Arch Surg 72:725–727
Bassis ML, Izenstark JL (1956) Ectopic liver: its occurrence in the gall bladder. AMA Arch Surg 73:204–206
Bazzoni C, Serini M, Ongari M, Squazzini C, Alleva M, Lombardi C (1993) Massive hemobilia caused by necrotic hemorrhagic cholecystitis. Report of a case (in Italian). Minerva Chir 48:857–860
Beltran et al. (2007) http://www.ncbi.nlm.nih.gov/pubmed/18180840
Ben-Baruch D, Sandbank Y, Wollock Y (1986) Heterotopic pancreatic tissue in the gallbladder. Acta Chir Scand 152:557–558
Bentivegna S, Hirschl S (1972) Heterotopic gastric mucosa in the gallbladder presenting as a symptom-producing tumor. A case report. Am J Gastroenterol 57:423–428
Bhana BD, Chetty R (1999) Heterotopic pancreas – an unusual cause of cholecystitis. S Afr J Surg 37:105–107
Blanton DE, Bream CA, Mandel SR (1974) Gall bladder ectopia: a review of anomalies of position. AJR Am J Roentgenol 121:396
Boyle L, Gallivan MV, Chun B, Lack EE (1992) Heterotopia of gastric mucosa and liver involving the gallbladder. Report of two cases with literature review. Arch Pathol Lab Med 116:138–142
Brawner J, Trivedi H, Sataline LR (1966) Hemocholecyst. Report of a case associated with anticoagulation therapy. Ohio State Med J 62:1028–1030
Bromberg SH, Franco MI, Franca LCM, Neto CC (2009) Pancreatic heterotopia in the gallbladder: a case report and literature review. Einstein J Biol Med 7:215–218
Bulun SE, Monsavais D, Pavone ME, Dyson M, Xue Q, Attar E, Tokunaga H et al (2012) Role of estrogen receptor-b in endometriosis. Semin Reprod Med 30:39–45
Bulus H, Yildiz M, Alimogullari M, Simsek G, Köklü S, Koçak E (2012) Gastric heterotopia within the gallbladder wall in a patient with chronic cholecystitis. Am Surg 78:E51–E52
Burns KA, Korach KS (2012) Estrogen receptors and human disease: an update. Arch Toxicol 86:1491–1504
Busuttil A (1974) Ectopic adrenal within the gall-bladder wall. J Pathol 113:231–233
Calmat A, Riquet M, Clot JP, Kariotakis E, Mercadier M (1979) Acute haemocholecystitis due to carcinoma of the gallbladder (in French). Ann Chir 33:371–373
Cappell MS, Marks M, Kirschenbaum H (1993) Massive hemobilia and acalculous cholecystitis due to benign gallbladder polyp. Dig Dis Sci 38:1156–1161
Cassol CA, Noria D, Asa SL (2010) Ectopic thyroid tissue within the gall bladder: case report and brief review of the literature. Endocr Pathol 21:263–265
Castro Viera GA, Sanuy ME, Camara HA, Cordoba OC (1990) Accessory liver in gallbladder. Apropos of a case report (in Spanish). Rev Esp Enferm Dig 77:295–296
Catani M, De Milito R, Romagnoli F, Mingazzini P, Silvestri V, Usai V, Romeo V, Modini C (2011) Ectopic liver nodules: a rare finding during cholecystectomy. G Chir 32:255–258
Caygill CP, Gatenby PA (2004) Ectopic liver and hepatocarcinogenesis. Eur J Gastroenterol Hepatol 16:727–729
Celoria GC, Rodriguez Otero JC, Proske SA, Vallilengua C (1994) Papillary hyperplasia and cholesterolosis of the gallbladder. Medicina (B Aires) 54:31–34
Cerullo G, Marrelli D, Di Mare G, Onorati M, Tripodi S, Neri A, Roviello F (2011) Heterotopic pancreatic tissue in the gallbladder. Two case reports and brief review of the literature. G Chir 32:259–262
Chang KM, Jeng KS, Yang FS, Chen BF (1998) Metastatic hepatocellular carcinoma presenting as hemocholecyst with perforation: a case report. Zhonghua Yi Xue Za Zhi (Taipei) 61:613–618
Charpentier P, Prade M, Bognel C, Gadenne C, Duvillard P (1983) Malakoplakia of the gallbladder. Hum Pathol 14:827–828
Chawla Y, Dilawari JB, Kataiya S (1994) Gallbladder varices in portal vein thrombosis. AJR Am J Roentgenol 162:643–645
Chawla A, Dewan R, Sarin SK (1995) The frequency and influence of gallbladder varices on gallbladder functions in patients with portal hypertension. Am J Gastroenterol 90:2010–2014
Chen YY, Yi CH, Chen CL, Huang SC, Hsu YH (2010) Hemorrhagic cholecystitis after anticoagulation therapy. Am J Med Sci 340:338–339
Chitkara YK (1995) Pathology of the gallbladder in gallstone pancreatitis. Arch Pathol Lab Med 119:355–359
Cho YU, Kim JY, Choi SK, Hur YS, Lee KY, Kim SJ, Ahn SI, Hong SC, Woo ZH et al (2001) A case of hemorrhagic gallbladder paraganglioma causing acute cholecystitis. Yonsei Med J 42:352–356
Chowbey PK, Wadhwa A, Sharma A, Khullar R, Soni V, Baijal M (2004) Ectopic gallbladder: laparoscopic cholecystectomy. Surg Laparosc Endosc Percutan Tech 14:26–28
Chu EC, Chick W, Hillebrand DJ, Hu KQ (2002) Fatal spontaneous gallbladder variceal bleeding in a patient with alcoholic cirrhosis. Dig Dis Sci 47:2682–2685
Chung CC, Leung KL, Lau WY, Li AK (1997) Ectopic gallbladder revisited, laparoscopically: a case report. Can J Surg 40:464–466
Cöl C, Boran C, Turkeli V, Dinler K, Kordon O, Erkol H, Sengul N (2007) Heterotopic gastric mucosa in gallbladder associated with kidney agenesis and congenital hip dysplasia. Acta Clin Belg 62:120–122
Collan Y, Hakkileuoto A, Hastgacka J (1978) Ectopic liver. Ann Chir Gynaecol 67:27–29
Collard P, Mazy V, Jardon-Jeghres C, Focan C (1989) Local tumefaction of the gallbladder wall due to pancreatic heterotopia. J Belge Radiol 72:471–473
Cooke HH (1931) A pathological study of the “strawberry” gallbladder. Thesis, University of Minnesota
Corsy F (1922) Lobe supérnumeraire du foie implanté sur la face inférieure de la vésicule biliaire. C R Seances Soc Biol 86:695–697
Costero C, Quilantan R, Meléndez J (1975) Ectopic liver located in the gallbladder (in Spanish). Rev Invest Clin 27:55–58
Csendes A, Smok G, Burdiles P, Diaz JC, Maluenda F, Korn O (1998) Histological findings of gallbladder mucosa in 95 control subjects and 80 patients with asymptomatic gallstones. Dig Dis Sci 43:931–934
Csendes A, Burdiles P, Smok G, Csendes P, Burgos A, Recio M (2003) Histologic findings of gallbladder mucosa in 87 patients with morbid obesity without gallstones compared to 87 control subjects. J Gastrointest Surg 7:547–551
Curtis LE, Sheahan DG (1969) Heterotopic tissues in the gallbladder. Arch Pathol 88:677–683
Damore LJ, Cook CH, Fernandez KL, Cunningham J, Ellison EC, Melvin WS (2001) Ultrasonography incorrectly diagnoses gallbladder polyps. Surg Laparosc Endosc Percutan Tech 11:88–91
Dao TH, Rotman N, Mathieu D, Vasile N (1989) Infiltrating hematoma of the hepatic pedicle and gallbladder wall. Ultrasonographic and x-ray computed tomographic aspects (in French). J Radiol 70:431–433
Daud MS, Salomao FC, Salomao EC, Salomao BC (2007) Gastric heterotopia together with squamous metaplasia in the gallbladder. Acta Gastroenterol Latinoam 37:164–167
De Alcantara RV, Yamada RM, Cardoso SR, de Fatima M, Servidoni CP, Hessel G (2013) Ultrasonography predictors of esophageal varices. J Pediatr Gastroenterol Nutr 57:700–703
Dettmer M, Cathomas G, Willi N (2011) Alpha-1-antitrypsin retention in an ectopic liver. Diagn Pathol 6:16
Dewachter L, Dewaele T, Rosseel F, Crevits I, Aerts P, De Man R (2012) Acute cholecystitis with pseudoaneurysm of the cystic artery. JBR-BTR 95:136–137
Dhulkotia A, Kumar S, Kabra V, Shukla HS (2002) Aberrant gallbladder situated beneath the left lobe of liver. HPB (Oxford) 4:39–42
Di Tommaso L, Arizzi C, Roncalli M (2005) Malacoplakia of the gallbladder. Histopathology 46:474–475
Dittrick GW, Thompson JS, Campos D, Bremers D, Sudan D (2005) Gallbladder pathology in morbid obesity. Obes Surg 15:238–242
Djuricic S, Zlatkovic M, Stankonic I, Plamenac P (1999) Heterotopic liver tissue in the fundus of the gallbladder. Srp Arh Celok Lek 127:412–415
Dolan RV, Remine WH, Dockery MB (1974) The fate of heterotopic pancreatic tissue. Arch Surg 109:762–765
Duchini L (1967) Apropos of a case of ossification of the gallbladder. Contribution to the study of heterotopic ossifications (in Italian). Arch de Vecchi Anat Patol 50:537–552
Duimstra F, Greenfield RE (1977) Left liver lobe gall bladder. S D J Med 30:7–9
Egyedi L (1934) Case of polypus of gallbladder containing gastric mucosa membrane tissue. Gyogyaszat 74:596–599
Eiserth P (1940) Beiträge zur Kenntnis der Nebenlebern. Virchows Arch A Pathol Anat Histopathol 307:307–313
Elfving G (1959) Heterotopic pancreatic tissue in the gall bladder wall: report of a case. Acta Chir Scand 118:32–36
Elfving G, Palmu A, Teir H (1968) Cholesterolosis and mucosal hyperplasia of gallbladder. Ann Chir Gynaecol Fenn 57:28–30
Elhence P, Bansal R, Agrawal N (2012) Heterotopic pancreas in gall bladder associated with chronic cholecystolithiasis. Int J Appl Basic Med Res 2:142–143
Elpek GO, Bozova S, Küpesiz GY, Ogüs M (2007) An unusual cause of cholecystitis: heterotopic pancreatic tissue in the gallbladder. World J Gastroenterol 13:313–315
Erb RE, Mirvis SE, Shanmuganathan K (1994) Gallbladder injury secondary to blunt trauma: CT findings. J Comput Assist Tomogr 18:778–784
Faintuch J, Machado MC, de Mendonça LK, das Neves MM, Raia AA (1979) Suprahepatic gallbladder – a rare ectopia of the gallbladder (in Portuguese). Rev Hosp Clin Fac Med Sao Paulo 34:260–264
Faure GJ, Maupin JM, Barbier H (1969) Acute hemobilia and hemoperitoneum caused by cancer of the gallbladder (in French). J Chir (Paris) 98:529–532
Feldman M, Feldman M (1954) Cholesterosis of the gallbladder; an autopsy study of 165 cases. Gastroenterology 27:641–648
Feldman L, Venta L (1988) Percutaneous cholecystostomy of an ectopic gallbladder. Gastrointest Radiol 13:256–258
Fellbaum C, Beham A, Schmid C (1987) Isolated accessory liver (hepar succenturiatum) at the neck of the gallbladder. Case report with review of the literature (in German). Wien Klin Wochenschr 99:825–827
Foucault A, Veilleux H, Martel G, Lapointe R, Vandenbroucke-Menu F (2012) Heterotopic pancreas presenting as suspicious mass in the gallbladder. JOP 13:700–701
Fourdan O, Prat F, Fritsch J, Brocheriou I, Choury D, Etienne JP (1994) Hemobilia revealing calculosis and cancer of the gallbladder. Value of retrograde choledochoscopy (in French). Gastroenterol Clin Biol 18:901–902
Fröschle G, Brümmer P, Gebhardt J (1990) Isolated gallbladder hematoma and covered perforation following blunt abdominal trauma (in German). Zentralbl Chir 115:365–368
Fullarton GM, Burgoyne M (1991) Gallbladder and pancreatic metastases from bilateral renal carcinoma presenting with hematobilia and anemia. Urology 38:184–186
Fung AK, Vosough A, Olson S, Aly EH, Binnie NR (2013) An unusual cause of acute internal haemorrhage: cystic artery pseudoaneurysm secondary to acute cholecystitis. Scott Med J 58:e23–e26
Gabata T, Matsui O, Kadoya M, Yoshikawa J, Ueda K, Nobata K, Kawamori Y, Takashima T (1997) Gallbladder varices: demonstration of direct communication to intrahepatic portal veins by color Doppler sonography and CT during arterial portography. Abdom Imaging 22:82–84
Ghoz A, Kheir E, Kotru A, Halazun K, Kessel D, Patel JJ, Lodge JP (2007) Hemoperitoneum secondary to rupture of cystic artery pseudoaneurysm. Hepatobiliary Pancreat Dis Int 6:321–323
Gilloteaux J, Hawkins WS, Gilloteaux LC, Jasso J, Kelly TR (1997) Ultrastructural aspects of human cystic duct epithelium as a result of cholelithiasis and cholesterolosis. Microsc Res Tech 39:22–38
Glasionov IM (1961) On intrahepatic localization of the gall-bladder (in Russian). Khirurgiia (Mosk) 37:116–117
Goldblatt MI, Swartz-Basile DA, Al-Azzawi HH, Trans KQ, Nakeeb A, Pitt HA (2006) Nonalcoholic fatty gallbladder disease: the influence of diet in lean and obese mice. J Gastrointest Surg 10:193–201
Graham EA, Elman R (1932) The pathogenesis of the ‘strawberry’ gallbladder. Arch Surg 24:14–22
Grant EG, Smirniotopoulos JG (1983) Intraluminal gallbladder hematoma: sonographic evidence of hemobilia. J Clin Ultrasound 11:507–509
Gremmels JM, Kruskal JB, Parangi S, Kane RA (2004) Hemorrhagic cholecystitis simulating gallbladder carcinoma. J Ultrasound Med 23:993–995
Griniatsos J, Riaz AA, Isla AM (2002) Two cases of ectopic liver attached to the gallbladder wall. HPB (Oxford) 4:191–194
Gucer H, Bagci P, Coskunoglu EZ, Karadag C (2011) Heterotopic pancreatic tissue located in the gallbladder wall. A case report. JOP 12:152–154
Guerra L, Perna AM, Lotti G, Siccardi A (1963) Cholesterolosis of the gallbladder. Anatomopathological study (in Italian). Pathologica 55:471–484
Hadzi-Nikolov D, Resl M, Herzig B, Svetlik M (1997) Heterotopic pancreatic tissue in the wall of the gallbladder (in Czech). Cesk Patol 33:146–148
Hague J, Brennand D, Raja J, Amin Z (2010) Cystic artery pseudoaneurysms in hemorrhagic acute cholecystitis. Cardiovasc Intervent Radiol 33:1287–1290
Hakami M, Beheshti G, Amirkhan A (1976) Hemobilia caused by rupture of cystic artery aneurysm. Am J Proctol 27:56–57
Hamazaki K, Fujiwara T (2000) Heterotopic gastric mucosa in the gallbladder. J Gastroenterol 35:376–381
Hamdani SD, Baron RL (1994) Ectopic liver simulating a mass in the gallbladder wall: imaging findings. AJR Am J Roentgenol 162:647–648, 16, 294–297
Hanada M, Tujimura T, Kimura M (1981) Cholecystic granulomas in gallstone disease. A clinicopathologic study of 17 cases. Acta Pathol Jpn 31:221–231
Harach HR (1998) Ectopic thyroid tissue adjacent to the gallbladder. Histopathology 32:90–91
Havrilla TR, Reich NE, Haaga JR, Seidelmann FE, Cooperman AM, Alfidi RJ (1978) Computed tomography of the gall bladder. AJR Am J Roentgenol 130:1059
Hayama S, Suzuki Y, Takahashi M, Hazama K, Fujita M, Kondo S, Katoh H (2010) Heterotopic gastric mucosa in the gallbladder: report of two cases. Surg Today 40:783–787
Heino AE, Ritama V (1965) Observations on cholesterosis of the gall-bladder. An autopsy study. Ann Chir Gynaecol Fenn 54:387–391
Heise CP, Giswold M, Eckhoff D, Reichelderfer M (2000) Cholecystitis caused by hemocholecyst from underlying malignancy. Am J Gastroenterol 95:805–808
Helbich T, Breitenseher M, Heinz-Peer G, Vergesslich K, Granditsch G, Kainberger F (1994) Color Doppler ultrasound of gallbladder varicose veins in children. A rare sign of portal hypertension. Ultraschall Med 15:126–130
Helpap B, Huegel A (1988) Cholesterosis and adenomyomatosis of the gallbladder (cholecystosis) (in German). Pathologe 9:70–77
Hibbs H, Ahmad U (2010) Inverted liver with suprahepatic, anteriorly displaced gallbladder. J La State Med Soc 162:150–152
Hide G, Desai S, Bloxham CA (2001) Malakoplakia of the gall-bladder: imaging and histological features. Clin Radiol 56:326–328
Holmlund D, Lundström B (1977) Extrahepatic obstruction of the portal vein with bleeding from the gallbladder. Report of a case. Acta Radiol Diagn (Stockh) 18:680–684
Horanyi J, Fuesy F (1963) Accessory liver in the wall of the gallbladder (in Hungarian). Magy Seb 16:294–297
Hsu KL, Chou FF, Shern JY, Yang AD (1994) Suprahepatic gallbladder with agenesis of the right lobe of the liver: report of a case. J Formos Med Assoc 93:320–323
Iacconi P, Masoni T (1990) Accessory liver. Report of 2 cases. Acta Chir Belg 90:228–230
Iafrate F, Ciolina M, Iannitti M, Baldassari P, Pichi A, Rengo M, De Cecco CN, Laghi A (2013) Gallbladder and muscular endometriosis: a case report. Abdom Imaging 38:120–124
Ihtiyar E, Isikoy S, Algin C, Sahin A, Erkasap S, Yasar B (2003) Ectopic thyroid in the gallbladder: report of a case. Surg Today 33:777–780
Indyk JS, Shipton EA (1957) Heterotopic bone formation in the gall-bladder. Med J Aust 44:9–11
Inoue Y, Shibata T, Niinobu T, Ishida T, Sato T, Hanada M (2000) Heterotopic gastric mucosa in the gallbladder: sonographic and CT findings. Abdom Imaging 25:198–200
Ishida T, Nakamura H, Hori S, Marukawa T, Mitani T, Murakami T, Nishikawa M, Nakanishi K et al (1993) Acalculous cholecystitis (panniculitis) associated with Weber-Christian disease. Clin Imaging 17:56–58
Isik I, Sezer C, Dursun A (2002) Gastric heterotopia in the gallbladder: a case report. Turk J Gastroenterol 13:172–174
Ito K, Fujita T, Shimizu A, Sasaki K, Tanabe M, Matsunaga N (2009) Imaging findings of unusual intra- and extrahepatic portosystemic collaterals. Clin Radiol 64:200–207
Izzo L, Boschetto A, Brachini G, Binda B, Lamazza A, Caramanico L, Corigliano N et al (2001) “Strawberry” gallbladder: review of the literature and our experience (in Italian). G Chir 22:33–36
Jacyna MR, Bouchier IAD (1987) Cholesterolosis: a physical cause of “functional” disorder. Br Med J 295:619–620
Jaerve and Meurman (1964) http://www.ncbi.nlm.nih.gov/pubmed/14188064
Jarde O, Barrat JP, Degardin P, Maingueux P, Tardif B (1989) Aberrant pancreas in the gallbladder. J Chir 126:476–477
Jeng KS, Yang KC, Kuo SH (1991) Malignant degeneration of heterotopic pancreas. Gastrointest Endosc 37:196–198
John A, Ramachandran TM, Ashraf S, Nair MS, Devi RS (1999) Carcinoma of gallbladder presenting as hemobilia. Indian J Gastroenterol 18:88–89
Jones RL, Mackie G, Taylor SA (1997) Case report: gallbladder carcinoma – an unusual cause of haemobilia. Clin Radiol 52:962–963
Judd ES, Mentzer SH (1927a) Cholesterosis of the gall bladder. I. A clinical study. Calif West Med 27:337–339
Judd ES, Mentzer SH (1927b) Cholesterosis of the gall bladder. II. The surgical aspects. Cal West Med 27:487–489
Jung YM, Son BK, Ahn SB, Kim DH, Kim EK (2011) Intramural gallbladder hematoma mimicking gallbladder neoplasm in a 55-year-old male patient. J Korean Surg Soc 81:216–220
Kaido T, Kano M, Suzaki S, Yanagibashi K, Shiota M (2004) Large cholesterol polyp of the gallbladder mimicking gallbladder carcinoma. Abdom Imaging 29:100–101
Kainberger FM, Vergesslich KA, Eilenberger M, Poeltner S, Ponhold W (1990) Color-coded Doppler evaluation of cholecystic varices in portal hypertension. Pediatr Radiol 21:71–72
Kauzlaric D, Barmeir E (1985) Sonography of intraluminal gallbladder hematoma. J Clin Ultrasound 13:291–294
Kehrer JK, De Minjer A (1951) Peptic ulcer of the gall-bladder. Arch Chir Neerl 3:151–156
Keller D, Gayral F, Larrieu H (1982) Malposition of the gallbladder under the left lobe of the liver. A case report (in French). Ann Chir 36:243–245
Keramidas DC, Skondras C, Anagnostou D, Doulas N (1977) Gastric heterotopia in the gallbladder. J Pediatr Surg 12:759–762
Kevans D, MacNicholas R, Norris S (2009) Gallbladder wall variceal haemorrhage with associated rupture: a rare cause of mortality in the cirrhotic patient. Eur J Gastroenterol Hepatol 21:955–957
Khairy GA, Guraya SY, Murshid KR (2004) Cholesterolosis. Incidence, correlation with serum cholesterol level and the role of laparoscopic cholecystectomy. Saudi Med J 25:1226–1228
Klein F (1955) Accessory liver of microscopic size in the gallbladder wall (in German). Zentralbl Allg Pathol 93:466–469
Klimis T, Roukounakis N, Kafetzis I, Mouziouras V, Karantonis I, Andromanakos N (2011) Heterotopic pancreas of the gallbladder associated with chronic cholecystitis and high levels of amylasuria. JOP 12:458–460
Klubo-Gwiezdzinska J, Manes RP, Chia SH, Burman KD, Stathatos NA, Deeb ZE et al (2011) Clinical review: ectopic cervical thyroid carcinoma – review of the literature with illustrative case series. J Clin Endocrinol Metab 96:2684–2691
Kodama T, Yokoyama T (1996) Heterotopic tissues in the gallbladder (heterotopic gastric mucosa, heterotopic intestinal epithelium, heterotopic pancreatic tissue and ectopic liver in the gallbladder (in Japanese). Ryoikibetsu Shokogun Shirizu 9:360–362
Koga A (1985) Fine structure of the human gallbladder with cholesterosis with special reference to the mechanism of lipid accumulation. Br J Exp Pathol 66:605–611
Koh CE, Hunt R (2007) Ectopic liver encountered during laparoscopic cholecystectomy. Asian J Surg 30:227–229
Kondi-Paphiti A, Antoniou AG, Kotsis T, Polimeneas G (1997) Aberrant pancreas in the gallbladder wall. Eur Radiol 7:1064–1066
Krustev N, Mendizova A, Velev M (2002) Hemobilia caused by gallbladders’s polyps in liver cirrhosis (in Bulgarian). Khirurgiia (Sofia) 58:42–44
Ku J, DeLaRosa J, Kang J, Hoyt D, Coimbra R (2004) Acute cholecystitis with a hemocholecyst as an unusual presentation of gallbladder cancer: report of a case. Surg Today 34:973–976
Kubota H, Kageoka M, Iwasaki H, Sugimoto K, Higuchi R, Honda S, Watanabe F et al (2000) A patient with undifferentiated carcinoma of gallbladder presenting with hemobilia. J Gastroenterol 35:63–68
Kwon TK, Jeon SH, Park HW, Jung WJ, Hwang JY, Park KS, Cho KB, Hwang JS et al (2002) A case of intraluminal gallbladder hematoma after percutaneous liver biopsy (in Korean). Taehan Kan Hakhoe Chi 8:486–489
Labitzke R (1991) Congenital intrathoracic ectopic gallbladder – a currently unreported developmental disorder. Attempt at a classification of abnormal gallbladder sites (in German). Chirurg 62:755–758
Lai SW (2011) Pathologic evaluation of gallbladder in patients who underwent cholecystectomy and hepatic resection for hepatocellular carcinoma. Am J Med Sci 341:305–307
Lai YC, Tarng DC (2009) Hemorrhagic acalculous cholecystitis: an unusual location of uremic bleeding. J Chin Med Assoc 72:484–487
Lai EC, Tompkins RK (1986) Heterotopic pancreas. Review of a 26 year experience. Am J Surg 151:697–700
Larsen EH, Diederich PJ, Sorensen FB (1985) Peptic ulcer in the gallbladder. A case report. Acta Chir Scand 151:575–576
Lebrec D, Benhamou JP (1985) Ectopic varices in portal hypertension. Clin Gastroenterol 14:105–121
Leone N, De Paolis P, Carrera M, Carucci P, Musso A, David E, Brunello F, Fronda GR et al (2004) Ectopic liver and hepatocarcinogenesis: report of three cases with four years’ follow-up. Eur J Gastroenterol Hepatol 16:731–735
Lewis KM, Peterson CW (1943) Cholesterosis of the gallbladder: observations on twenty-five cases without stones. Ann Surg 117:450–455
Leyman P, Saint-Marc O, Hannoun L, Parc R (1996) Heterotopic gastric mucosa presenting as gallbladder polyps. Acta Chir Belg 96:128–129
Liang K, Liu JF, Wang YH, Tang GC, Teng LH, Li F (2010) Ectopic thyroid presenting as a gallbladder mass. Ann R Coll Surg Engl 92:W4–W6
Liang YL, Liang X, Wang YF, Cai XJ (2013) Heterotopic gastric mucosa with mild dysplasia in the gallbladder. Chin Med J (Engl) 126:978–979
Limaiem F, Jedidi S, Hassan F, Korbi S, Aloui S, Lahmar A, Bouraoui S, Mzabi S (2012) Pancreatic heterotopia in the gallbladder neck associated with chronic cholecystitis. Pathologica 104:446–448
Lin CL, Chang JJ, Lee TS, Lui KW, Yen CL (2005) Gallbladder polyp as a manifestation of hemobilia caused by arterial-portal fistula after percutaneous liver biopsy: a case report. World J Gastroenterol 11:305–307
Lin YH, Lee RC, Hsia CY, Chen HC, Chiang JH, Tseng HS, Chang CY (2010) Right hepatic artery pseudoaneurysm ruptured into the gallbladder demonstrated by magnetic resonance angiography. J Chin Med Assoc 73:331–333
Lobo SW, Menezes RG, Mamata S, Baral P, Knachan T, Hunnargi SA, Bodhe AV, Bhat NB (2007) Ectopic partial intrahepatic gall bladder with cholelithiasis – a rare anomaly. Nepal Med Coll J 9:286–288
Lombay B, Kiss A, Szabo L (2003) Heterotopic gastric mucosa in the gallbladder. Pediatr Radiol 33:587
Lundy J, Johnson E, Edwards K, Rivera D (2005) Laparoscopic management of gallbladder-associated ectopic liver. JSLS 9:485–487
MacCarty WC (1919) The frequency of strawberry gallbladder. Ann Surg 69:131–139
Machida H, Ueno E, Shiozawa S, Fujimura M, Tsuchiya A, Kim DH, Ogawa K, Aiba M (2008) Unruptured pseudoaneurysm of the cystic artery with acute calculous cholecystitis incidentally detected by computed tomography. Radiat Med 26:384–387
Mackay WA (1937) Cholesterosis of the gallbladder: a review, supplemented by personal observations on 87 cases. Br J Surg 24:570–594
Madrid C, Berrocal T, Gorospe L, Prieto C, Gamez M (2003) Heterotopic gastric mucosa involving the gallbladder and biliary tree. Pediatr Radiol 33:129–132
Malusev D (1951) Varices of the gallbladder (in Serbian). Srp Arh Celok Lek 49:775–779
Martinez CA, de Resende HC, Rodrigues MR, Sato DT, Brunialti CV, Palma RT (2013) Gallbladder-associated ectopic liver: a rare finding during a laparoscopic cholecystectomy. Int J Surg Case Rep 4:312–315
Mathur A, Al-Azzawi HH, Lu D, Yancey KW, Swartz-Basile DA, Nakeeb A, Pitt HA (2008) Steatocholecystitis: the influence of obesity and dietary carbohydrates. J Surg Res 147:290–297
Mboti F, Maassarani F, de Keuleneer R (2003) Cholecystitis associated with heterotopic pancreas. Acta Chir Belg 103:110–112
McNabney WK, Rudek R, Pemberton LB (1990) The significance of gallbladder trauma. J Emerg Med 8:277–280
Méndez-Sanchez N, Tanimoto MA, Cobos E, Roldan-valadez E, Uribe M (1997) Cholesterolosis is not associated with high cholesterol levels in patients with and without gallstone disease. J Clin Gastroenterol 25:518–521
Mentzer SH (1925) Cholesterosis of the gall bladder. Am J Pathol 1:383–388
Meshikhes AW, Al-Jaroof AH, Atassi R (2003) Heterotopic pancreas in the gall bladder. Saudi Med J 24:907–908
Mikou MM, Mouaffak Y, Benyacob A, Modaddek A, Faroudy M, Ababou A, Lazreq C et al (2004) Haemocholecyst: a rare complication of anticoagulant treatment (in French). Ann Fr Anesth Reanim 23:733–736
Mishin I (2005) Gallbladder varices. Rom J Gastroenterol 14:165–168
Mitty WF, Rousselot LM (1957) Cholesterosis of the gall bladder. Gastroenterology 32:910–916
Miura K, Hoshino T, Komatsu M, Ono T, Sato T, Tanaka J, Masamune O (1998) A case of hemorrhage into the gallbladder probably due to rupture of pseudoaneurysm formed by cystic artery (in Japanese). Nihon Shokakibyo Gakkai Zasshi 95:450–454
Monfreda G, Draghi L, Peloso R (1967) Heterotopic pancreatic tissue. Radiological aspects (in Italian). Quad Radiol 32:449–471
Mooney B, O’Malley E, Dempsey J (1979) Gastric heterotopia in a gall bladder. Ir J Med Sci 148:50–53
Morris DS, Porterfield JR, Sawyer MD (2008) Hemorrhagic cholecystitis in an elderly patient taking aspirin and cilostazol. Case Rep Gastroenterol 2:203–207
Murakami M, Tsutsumi Y (1989) Aberrant pancreatic tissue accompanied by heterotopic gastric mucosa in the gall-bladder. Pathol Int 49:580–582
Murakami M, Tsutsumi Y (1999) Aberrant pancreatic tissue accompanied by heterotopic gastric mucosa in the gall-bladder. Pathol Int 49:580–582
Mutschmann PN (1946) Aberrant pancreatic tissue in the gallbladder wall. Am J Surg 72:282–283
Nagar S, Koffron A, Raofi V (2011) A case of hemorrhagic necrosis of ectopic liver tissue within the gallbladder wall. HPB Surg 2011:389381
Nakagawa T, Yamakado K, Takeda K, Nakagawa T (1996) An ossifying carcinosarcoma of the gallbladder: radiologic findings. AJR Am J Roentgenol 166:1233–1234
Natori T, Hawkin S, Aizawa M, Asai T, Kameda Y, Ikuyohashi K (1986) Intra-cholecystic ectopic liver. Acta Pathol Jpn 36:1213–1216
Nelson JJ, Kahn AG (2009) A case of bone metaplasia of the gallbladder epithelium. South Med J 102:322–324
Neupert G, Appel P, Braun S, Tonus C (2007) Heterotopic pancreas in the gallbladder. Diagnosis, therapy, and course of a rare developmental anomaly of the pancreas (in German). Chirurg 78:261–264
Nevalainen T, Laitio M (1972) Ultrastructure of gallbladder with cholesterolosis. Virchows Arch B Cell Pathol 10:237–242
Ohtsuki Y, Hatano H, Okada Y, Lee GH, Furihata M (2012) Case report: membranous fat necrosis of the gallbladder as the cause of peculiar foreign body reaction in a patients with severe chronic cholecystitis. Biomed Res India 23:457–460
Olbrycht J (1965) Sudden death due to internal hemorrhage into the peritoneal cavity from a ruptured periarteritic aneurysma of the cystic artery (in German). Beitr Gerichtl Med 23:160–170
Orizio P, Villanacci V, Bassotti G, Falchetti D, Torri F, Ekema G (2011) Heterotopic gastric mucosa in the cystic duct. Int J Surg Pathol 19:364–365
Ortiz-Hidalgo C, Baquera-Heredia J (2000) Osseous metaplasia in polypoid cholesterosis. Am J Surg Pathol 24:895
Osada H, Honda N, Takahashi T, Oku S, Watanabe W, Okada T, Ohno H, Hondo M, Nishimura K (2007) Arteriovenous malformation of the gallbladder: CT and angiographic findings. Radiat Med 25:73–75
Osawa H, Mori Y, Inoue F (1996) Case report: malignant haemobilia detected in the gallbladder – retrograde cholangiographic findings. Br J Radiol 69:79–81
Owen CC, Bilhartz LE (2003) Gallbladder polyps, cholesterolosis, adenomyomatosis, and acute acalculous cholecystitis. Semin Gastrointest Dis 14:178–188
Palazzo L, Hochain P, Helmer C, Cuillerier E, Landi B, Roseau G, Cugnenc PH, Barbier JP et al (2000) Biliary varices on endoscopic ultrasonography: clinical presentation and outcome. Endoscopy 32:520–524
Parekh J, Corvera CU (2010) Hemorrhagic cholecystitis. Arch Surg 145:202–204
Pellegrini C, Gori I, Achtari C, Hornung D, Chardonnens E, Wunder D, Fiche M, Canny GO (2012) The expression of estrogen receptors as well as GREB1, c-MYC, and cyclin D1, estrogen-regulated genes implicated in proliferation, is increased in peritoneal endometriosis. Fertil Steril 98:1200–1208
Petrin C (1966) Massive hemobilia in the course of carcinoma and lithiasis of the gallbladder (in Italian). Chir Ital 18:770–782
Pilling DW (1979) Haematoma of the wall of the gall-bladder. Br J Radiol 52:840–841
Pilloni L, Cois A, Uccheddu A, Ambu R, Coni P, Faa G (2006) Complete pancreatic heterotopia of gallbladder with hypertrophic duct simulating an adenomyoma. World J Gastroenterol 12:1786–1787
Piotrowski M, Kostecka W, Dyk T (1975) Hemocholecyst – hemorrhage into the gallbladder in the course of gallbladder carcinoma (in Polish). Wiad Lek 28:1069–1072
Pitt HA (2007) Hepato-pancreatic-biliary fat: the good, the bad and the ugly. HPB (Oxford) 9:92–97
Plante BJ, Lessey BA, Taylor RN, Wang W, Bagchi MK, Yuan L, Scotchie J, Fritz MA et al (2012) G protein-coupled estrogen receptor (GPER) expression in normal and abnormal endometrium. Reprod Sci 19:684–693
Popkharitov AI, Gulubova MV, Dandov AD, Sivrev DP (2008) Heterotopic gastrointestinal cyst mimicking chronic cholecystitis: a case report. J Med Case Rep 2:173
Popli MB, Popli V, Solanki Y (2010) Ectopic gall bladder: a rare case. Saudi J Gastroenterol 16:50
Pradines P, Brauner M, Legrand I, Sibony M, Garin B (1989) Heterotopic gastric mucosa in the gallbladder. AJR Am J Roentgenol 152:432
Price RJ, Stewart ET, Foley WD, Dodds WJ (1982) Sonography of polypoid cholesterolosis. AJR Am J Roentgenol 139:1197–1198
Principe A, Spangaro M, Lapilli A, Vecchi R, Volta L, De Lorenzi P (1979) Congenital anomalies of the gallbladder: a case of retrohepatic gallbladder contained in the coronary ligament (in Italian). Minerva Chir 34:879–884
Qizilbash AH (1976) Acute pancreatitis occurring in heterotopic pancreatic tissue in the gallbladder. Can J Surg 19:413–414
Radhi JM (2003) Gallbladder varices. Pathology 35:358–359
Rathi PM, Soni A, Nanivadekar SA, Sawant P, Bhatnagar MS, Upadhyay AP (1996) Gallbladder varices: diagnosis in children with portal hypertension on duplex sonography. J Clin Gastroenterol 23:228–231
Rege TA, Vargas SO (2011) Cholecystitis and cholelithiasis associated with an intramural fasciitis-like proliferation and osseous metaplasia. Pediatr Dev Pathol 14:80–83
Reid JD (1962) Cholesterolosis of the gallbladder: its nature and pathogenesis. N Z Med J 61:384–391
Rosen IE, Wilson SR (1980) Varices of the gallbladder. J Can Assoc Radiol 31:73–74
Runge et al. (1978) http://www.ncbi.nlm.nih.gov/pubmed/580715
Saad SA, Rush BF, Devanesan JD, Lazaro EJ (1979) Traumatic hematocele of the gallbladder with hemobilia. J Trauma 19:67–69
Saadat-Gilani K, Bechmann L, Frilling A, Gerken G, Canbay A (2007) Gallbladder endometriosis as a cause of occult bleeding. World J Gastroenterol 13:4517–4519
Safadi R, Sviri S, Eid A, Levensart P (1996) Gallbladder varices: a case report and review of the literature. Eur J Med Res 1:506–508
Sahlin S, Stahlberg D, Einarsson K (1995) Cholesterol metabolism in liver and gallbladder mucosa of patients with cholesterolosis. Hepatology 21:1269–1275
Saigh J, Williams S, Cawley K, Anderson JC (1985) Varices: a cause of focal gallbladder wall thickening. J Ultrasound Med 4:371–373
Sakarya A, Erhan Y, Aydede H, Kara E, Ilkgül O, Ciftdogan C (2002) Ectopic liver (choristoma) associated with the gallbladder encountered during laparoscopic cholecystectomy: a case report. Surg Endosc 16:1106
Salam AA, Goldman M, Smith D, Hill HL (1979) Gastric, intestinal, and gallbladder varices: hemodynamic and therapeutic considerations. South Med J 72:402–408
Saldaña DG, de Acosta DA, Aleman HP, Gebrehiwot D, Torres E (2010) Gallbladder endometrioma associated with obstructive jaundice and a serous ovarian cystic adenoma. South Med J 103:1250–1252
Salmenkivi K (1964) Cholesterosis of the gall-bladder. A clinical study based on 269 cholecystectomies. Acta Chir Scand 105(Suppl 324):1–193
Samartzis N, Samartzis EP, Noske A, Fedier A, Dedes KJ, Caduff R, Fink D, Imesch P (2012) Expression of the G protein-coupled estrogen receptor (GPER) in endometriosis: a tissue microarray study. Reprod Biol Endocrinol 10:30
Sandblom P (1948) Hemorrhage into the biliary tract following trauma; traumatic hemobilia. Surgery 24:571–586
Sandri L, Colecchia A, Larocca A, Vestito A, Capodicasa S, Azzaroli F, Mwangemi C et al (2003) Gallbladder cholesterol polyps and cholesterolosis. Minerva Gastroenterol Dietol 49:217–224
Sato S, Watanabe M, Nagasawa S, Niigaki M, Sakai S, Akagi S (1998) Laparoscopic observations of congenital anomalies of the liver. Gastrointest Endosc 47:136–140
Sato A, Hashimoto M, Sasaki K, Matsuda M, Watanabe G (2012) Elevation of pancreatic enzymes in gallbladder bile associated with heterotopic pancreas. A case report and review of the literature. JOP 13:235–238
Satoh H, Koga A (1997) Fine structure of cholesterolosis in the human gallbladder and the mechanism of lipid accumulation. Microsc Res Tech 39:14–21
Scharling ES, Geisinger KR (1993) Case of the day. Hemobilia: intraluminal gallbladder hematoma. J Ultrasound Med 12:244–245
Schein M, Assalia A, Schmulevski P, Meislin V, Hashmonai M (1993) Infected peri-pancreatic necrosis causing gallbladder necrosis by direct extension. HPB Surg 7:77–79
Schimpl G, Schaffler G, Sorantin E, Ratschek M, Klimpfinger M (1994) Polypoid gastric heterotopia in the gallbladder: clinicopathological findings and review of the literature. J Pediatr Gastroenterol Nutr 19:129–131
Schneider EA, Eisner M, Fridrich R (1979) Scintigraphic demonstration of an intrahepatic gall-bladder presenting as a focal liver lesion. Br J Radiol 52:754–755
Schubert GE, Klasmeier H, Lius W, Roth A (1980) Spurious aneurysm of the hepatic artery in the lumen of the gallbladder with hemobilia. Sonographic, angiographic, and pathological-anatomical findings (in German). Rofo 132:88–90
Schulz RC, Shields JB, Fletcher JW, Donati RM (1975) Liver scanning and the intrahepatic gallbladder: case report. J Nucl Med 16:1029–1030
Sciumè C, Geraci G, Pisello E, Li Volsi E, Facella T, Modoca G (2005) Heterotopic gastric mucosa in the gallbladder: case report and literature review. Ann Ital Chir 76:93–97
Sheu BS, Lin XZ, Chen CY, Chow NH, Lin PW, Tsai HM (1995) Suprahepatic gallbladder and right lobe anomaly of the liver in patients with biliary cancers. Dig Dis Sci 40:2411–2416
Shimura T, Kojima T, Tsutsumi S, Yoshida T, Uchiumi H, Kuwano H (2000) Gallbladder hematoma in a patient with hemophilia B, report of a case. Hepatogastroenterology 47:939–941
Shin KY, Heo J, Kim JY, Lee SJ, Jang SY, Park SY, Jung MK, Cho CM, Tak WY et al (2011) A case of hemocholecyst associated with hemobilia following radiofrequency ablation therapy for hepatocellular carcinoma. Korean J Hepatol 17:148–151
Shiwani MH, Gosling J (2008) Heterotopic pancreas of the gallbladder associated with chronic cholecystitis. JOP 9:30–32
Sroczynski M, Sebastian M, Halon A, Rudnicki J, Sebastian A, Agrawal AK, Piekarz P (2013) Pancreatic heterotopia in the gallbladder: an incidental finding after cholecystectomy. Folia Histochem Cytobiol 51:174–177
Strömsten A, von Bahr S, Bringman S, Saeki M, Sahlin S, Björkhem I, Einarsson C (2004) Studies on the mechanism of accumulation of cholesterol in the gallbladder mucosa. Evidence that sterol 27-hydroxylase is not a pathogenetic factor. J Hepatol 40:8–13
Summers FH, Zinberg SS, Kotkin M, Toch H, Sarkaria DS (1970) Heterotopic gastric mucosa with adenoma of the gall bladder. JAMA 214:597
Svane S, Knudtzon J (1991) Ectopic liver in the gallbladder and cholestasis. Tidsskr Nor Laegeforen 111:2643–2644
Tajima H, Hosaka J, Tajima N, Kumazaki T (1997) Arteriovenous malformation of the gallbladder. Eur Radiol 7:333–334
Tamura S, Yanaginuma N, Fujiwara K (1985) A case of ectopic liver cancer – hepatocellular carcinoma that developed in the gallbladder. Jpn J Gastroenterol 82:2448
Tan SW, Lai SK, Ng KW, Chen P, Chen KH, Jiang CF (2005) Intramural gallbladder hematoma mimicking gallbladder neoplasm in a 33-year-old male. J Chin Med Assoc 68:146–149
Tavernaraki K, Sykara A, Tavernaraki E, Chondros D, Lolis ED (2011) Massive intraperitoneal bleeding due to hemorrhagic cholecystitis and gallbladder rupture: CT findings. Abdom Imaging 36:565–568
Tavli L, Belviranli M, Erikoglu M, Esen H, Toy H (2005) Gastric heterotopia together with intestinal metaplasia in the gallbladder: case report and review of literature. Turk J Gastroenterol 16:160–162
Tejada E, Danielson C (1989) Ectopic or heterotopic liver (choristoma) associated with the gallbladder. Arch Pathol Lab Med 113:950–952
Tesler J, Cantor PJ (1957) Hematoma of the gall bladder. Gastroenterology 33:308–312
Tilvis RS, Aro J, Strandberg TE, Lempinen M, Miettinen TA (1982) Lipid composition of bile and gallbladder mucosa in patients with acalculous cholesterolosis. Gastroenterology 82:607–615
Torchio B, Maconi G (1978) Nodule of ectopic hepatic parenchyma in the wall of the gallbladder. Minerva Chir 33:39–44
Triantafyllidis I, Papapavlou L, Nikoloudis N, Economou A, Andreadis E, Chrissidou M et al (2009) Ectopic liver tissue attached to the gallbladder wall: a case report. Cases J 2:6786
Triki E, Brigand C, Meyer C (2008) Heterotopic gastric mucosa in the gallbladder (in French). J Chir (Paris) 145:376–377
Triponez F, Alifano M, Bobbio A, Regnard JF (2010) Endometriosis-related spontaneous diaphragmatic rupture. Interact Cardiovasc Thorac Surg 11:485–487
Tsai CJ (2009) Steatocholecystitis and fatty gallbladder disease. Dig Dis Sci 54:1857–1863
Uchiyama S, Imai S, Suzuki T, Arita A, Takeda K, Sujino H, Kameda H (1995) Heterotopic gastric mucosa of the gallbladder. J Gastroenterol 30:543–546
Uchiyama K, Aida N, Shibuya T, Tanaka S (1998) Early carcinoma of the gallbladder accompanied by hemobilia: report of a case. Surg Today 28:763–767
Vaiphei K, Singh P, Verma GR (2012) Gallbladder malakoplakia in type 2 diabetes mellitus: a rare entity. BMJ Case Rep 2012. pii: bcr201206601
Vallera DU, Dawson PJ, Path FR (1992) Gastric heterotopia in the gallbladder. Case report and review of the literature. Pathol Res Pract 188:49–52
Velchik MG, Noel AW (1987) False-positive liver scan due to an intrahepatic gallbladder detected by cholescintigraphy. Clin Nucl Med 12:50–52
Venditti M, Hay RW, Kulaga A, Demetrick DJ (2007) Diagnosis of ectopic tissue versus contamination by genetic fingerprinting in a routine surgical pathology specimen. Hum Pathol 38:378–382
Vilallonga R, Gonzalez O, Bergamini S, Fort JM, Armengol M (2012) Gallbladder variceal bleeding in a patient with alcoholic cirrhosis: a rare entity. Rev Esp Enferm Dig 104:153–154
Virchow R (1857) Über das Epithel der Gallenblase und über einen intermediären Stoffwechsel des Fettes. Virchows Arch Pathol Anat Histol 11:574–598
Wakiyama S, Yoshimura K, Shimada M, Kajiyama K, Sugimachi K (1998) Heterotopic gastric mucosa in a gallbladder with an anomalous union of the pancreatobiliary duct: a case report. Hepatogastroenterology 45:1488–1491
Walia HS, Abraham TK, Baraka A (1986) Gall-bladder interposition: a rare anomaly of the extrahepatic ducts. Int Surg 71:117–121
Wang Y, Liu FJ (2006) Ectopic liver tissue in the gallbladder serosa: a case report (in Chinese). Zhonhua Gan Zang Bing Za Zhi 14:369
Watanabe M, Matsura T, Takatori Y, Ueki K, Kobatake T, Hidaka M, Hirakawa H, Fukumoto S et al (1989) Five cases of ectopic liver and a case of accessory lobe of the liver. Endoscopy 21:39–42
Watanabe F, Hanai H, Kaneko E (1998) Increased acylCoA-cholesterol ester acyltransferase activity in gallbladder mucosa in patients with gallbladder cholesterolosis. Am J Gastroenterol 93:1518–1523
Weppner JL, Wilson MR, Ricca R, Lucha PA (2009) Heterotopic pancreatic tissue obstructing the gallbladder neck: a case report. JOP 10:532–534
West MS, Garra BS, Horii SC, Hayes WS, Cooper C, Silverman PM, Zeman RK (1991) Gallbladder varices: imaging findings in patients with portal hypertension. Radiology 179:179–182
Williams MJ, Humm JJ (1953) Heterotopia composed of gastric epithelium and smooth muscle in the wall of the gall bladder; a case report. Surgery 34:133–139
Womack NA, Haffner H (1944) Cholesterolosis: its significance in the badly damaged gallbladder. Ann Surg 119:391–401
Wong LS, Rusby J, Ismail T (2001) Left-sided gall bladder: a diagnostic and surgical challenge. ANZ J Surg 71:557–558
Xeropotamos N, Skopelitou AS, Batsis C, Kappas AM (2001) Heterotopic gastric mucosa together with intestinal metaplasia and moderate dysplasia in the gall bladder: report of two clinically unusual cases with literature review. Gut 48:719–723
Yamagiwa H, Tomiyama H (1986) Intestinal metaplasia-dysplasia-carcinoma sequence of the gallbladder. Acta Pathol Jpn 36:989–997
Yamamoto M, Nakajo S, Tahara E (1988) Histological classification of epithelial polypoid lesions of the gallbladder. Acta Pathol Jpn 38:181–192
Yamamoto M, Murakami H, Ito M, Nakajo S, Tahara E (1989) Ectopic gastric mucosa of the gallbladder: comparison with metaplastic polyp of the gallbladder. Am J Gastroenterol 84:1423–1426
Yamamoto T, Kubo S, Hirohashi K, Tanaka S, Uenishi T, Ogawa M, Sakabe K, Hai S et al (2003) Secondary hemocholecyst after radiofrequency ablation therapy for hepatocellular carcinoma. J Gastroenterol 38:399–403
Yoon AJ, Cowles RA, Stylianos S, O-Toole KM (2005) Heterotopic gastric mucosa in the gallbladder: a rare cause of massive hemobilia. J Pediatr Gastroenterol Nutr 40:606–608
Yosepovich A, Nass D, Zagatsky M, Kopolovic J (2002) Chronic cholecystitis with bone metaplasia. A case report. Pathol Res Pract 198:765–766
Youngwirth LD, Peters JC, Perry MC (1983) The suprahepatic gallbladder. An unusual anatomical variant. Radiology 149:57–58
Zangrandi F, Piotto A, Tregnaghi A, Pelizzo MR (2009) Hemocholecyst associated with antithrombotic therapy. Can J Surg 52:E297–E298
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this entry
Cite this entry
Zimmermann, A. (2017). Noninflammatory Tumor-Like Changes of the Gallbladder. In: Tumors and Tumor-Like Lesions of the Hepatobiliary Tract. Springer, Cham. https://doi.org/10.1007/978-3-319-26956-6_159
Download citation
DOI: https://doi.org/10.1007/978-3-319-26956-6_159
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-26954-2
Online ISBN: 978-3-319-26956-6
eBook Packages: MedicineReference Module Medicine