Abstract
In this chapter, performance assessment of a mini class turboprop engine is presented. Exergy analysis is used for this purpose on the basis of applicability on thermal systems. As a result of the component-based exergy analysis, relative irreversibility of the combustion chamber is higher relatively. Exergy destruction rates within the air compressor, combustion chamber and gas turbine components are 24.08 kW, 100.76 kW and 15.80 kW respectively. Additionally, exergy efficiencies of the components are 74.11, 69.68 and 98.99 % in order of air compressor, combustion chamber and gas turbine.
Access provided by CONRICYT-eBooks. Download chapter PDF
Similar content being viewed by others
Keywords
1 Introduction
Performance assessment of thermal systems enables us to see the current situation and improvement aspects of the system. However, many methods are presented in the literature, but thermodynamic-based method is well accepted by many researchers and industries as a result of explanation of their working principle by thermodynamics. On the other hand, lack of energy sources proportional to population growth and energy demand on the earth, thermodynamically performance assessment gains importance. As a fact, most of the commonly used devices, which facilitate our daily life, are invented on the basis of thermodynamics such as refrigerators, air conditioners, engines of automobiles, electricity generating power plants and aircraft engines [1–3].
Evaluating any type of thermal system thermodynamically is required in the present century to utilize energy sources more efficiently as mentioned above. Thermodynamic evaluation bases upon two fundamental laws. First of them discusses energy conversation within the system. According to the first law of thermodynamics, energy changes form but neither comes from nothing nor vanishes from nothing. First law analysis enables us to understand quantity of energy utilization within the system while revealing the form changes of the energy. Second law explains the working principles of heat machines and presents the limitations by Kelvin–Planck and Clausius statements. Kelvin–Planck statement asserts the impossibility of operating a heat generation system which absorbs energy in the form of heat from a single thermal sink and delivers an equivalent amount of work. In other words, any energy system operating with 100 % efficiency is not able to be built. Clausius statement explains the direction of the heat transfer. On the basis of Clausius statement, it is impossible for any system to operate in such a way that the sole result would be an energy transfer by heat from a cooler to a hotter sink [2–5].
Advances in thermal engineering emerged exergy term. Therewith, performance assessment of thermal systems could make sense. Exergy or availability as the first name can help us to compare the current situation of the system with achievable maximum performance under operating conditions. In exergy analysis despite of energy analysis, second law of thermodynamics is used in addition to first law. Thus, energy utilization quality within the examined system is possible. In the end, improvement possibilities, environmental impact and economic aspect of the system can be cleared by exergy analysis [1, 6–8].
In this chapter, a mini class turboprop engine is evaluated by thermodynamic performance assessment method. The examined engine can be used to meet power demand of an unmanned aerial vehicle, micro cogeneration system or a small-scale energy utilization system. It is aimed to present the performance assessment methodology of the engine by thermodynamic laws. The main goal and originality of this study can be written as follows:
-
Evaluating a gas turbine system which can be integrated to a micro cogeneration system,
-
Revealing improvement potential of the engine and pinpointing primary components for improvement,
-
Finding second law efficiencies of the system components.
2 Thermodynamic Fundamentals
In this section of the chapter, basic terms are defined and discussed to understand the exergy analysis of a mini class turboprop engine better. For this purpose, first and second laws of thermodynamics are explicated in detail respectively.
2.1 First Law Approach
Mass and energy balance equations are used to evaluate energy conservation within a system or through a process. For a steady-state, steady-flow system mass balance can be formulated as follows, whereas \( \dot{m} \) denotes mass flow rate of the system inlet and outlet [9, 10]:
Energy balance equation for a steady-state, steady-flow system is [9, 10]:
Also it can be expanded as following [9, 10]:
Here \( h \), \( {\text{ke}} \), \( {\text{pe}} \), \( \dot{Q} \) and \( \dot{W} \) notate enthalpy, kinetic energy, potential energy, heat transfer rate across the boundary layer of the system and work rate gained from the system, respectively. Besides, in many analysis, change in kinetic and potential energies is disregarded in addition to adiabatic system assumption. Thus, total energy rate of the flow is considered to be equivalent of enthalpy of the flow. Enthalpy of an ideal gas can be found by [9, 10]:
In Eq. 4, \( {\text{d}}h \) denotes enthalpy change of the ideal gas while \( {\text{d}}T \) is the temperature change within the gas and \( c_{p} \) is specific heat capacity under constant pressure.
2.2 Second Law Approach
In many texts, exergy is stated to be composed of kinetic, potential, physical and chemical exergies [11]:
If kinetic and potential exergies are disregarded, thermal exergy is found by summing up the physical and chemical exergies [11]:
Exergy balance equation for a system or process can be written as following [9, 11]:
Also, exergy loss is generally neglected. So that [9, 11]
Here mass flow, heat transfer and work related exergies can be found as follows [9, 11]:
In Eq. 12, \( {\text{ex}} \) is specific flow exergy whereas \( s \) represents entropy [9–11]:
Various formulations for finding exergy efficiency are presented in the literature until today. Exergy efficiency of a thermal system can be calculated by [1, 9]
2.3 Some Useful Exergy Parameters
Some useful parameters are defined in former studies to evaluate performance of a thermal system with exergetic approach [12, 13]:
-
Improvement potential,
-
Relative irreversibility,
-
Fuel depletion rate,
-
Productivity rate.
Maximum improvement within a system or process exergetically can be achieved by minimizing exergy loss or irreversibility. Thus, improvement potential may be expressed as [12]
Relative irreversibility, fuel depletion rate, and productivity rate can be found by following formulations respectively [13]:
3 General Description of the Engine
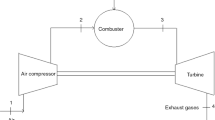
The examined mini class turboprop engine, which is a single shaft turboprop engine, consists of an axial diffuser and single stage centrifugal air compressor, through flow annular combustion chamber, single stage axial high pressure turbine and exhaust nozzle. The engine was designed primarily for technology demonstration purposes; having performed bench tests the project has been converted to aim to be used for unmanned aerial vehicles, micro cogeneration systems or a small scale energy utilization systems. The engine was integrated with closed loop lubrication system one of a kind in its class such that the engine is able to run at maximum power levels for at least couple of hours. The engine generates 177 N thrust with 125 g N−1 h−1 specific fuel consumption at sea level conditions. The engine can be operated by kerosene. Principle scheme of the engine is demonstrated in Fig. 1.
Following assumptions were made to evaluate engine with the aid of exergy:
-
The engine was considered to be operated under steady-state, steady test conditions.
-
The air compressor, the gas turbine and the combustion chamber components were assumed adiabatic.
-
The fuel injected into combustion chamber was kerosene.
-
The changes in the kinetic energy and exergy, the potential energy and exergy for each component and overall engine were disregarded.
-
The temperature and the pressure of the ambient were 303.15 K and 90.64 kPa, respectively, at test cell.
-
The air was assumed to be composed of 75.76 % nitrogen, 20.35 % oxygen, 0.0345 carbon dioxide, 3.03 % water vapour and 0.8255 % other gases [14].
-
The air and combustion gases were assumed ideal gas.
4 Exergy Analysis of the Mini Class Turboprop Engine
Exergy analysis of the mini class turboprop engine is performed as mentioned in Sect. 2 with regard to assumptions given in Sect. 3 previously. Component-based exergy analysis of the each component is explained in detail as following.
The air compressor work rate can be found with regard to first law of thermodynamics [9, 15]:
During the exergy analysis of the air compressor (AC), specific flow exergy of the air may be expressed as [16]
whereas \( i \), \( 0 \) and \( R \) represents station conditions, ambient conditions and gas constant of the air respectively. However, heat capacity of the air under constant pressure can be found as following [16]:
As mentioned before, the engine is fed with kerosene fuel. The chemical formula, lower heating value (\( {\text{LHV}} \)) of the fuel are considered to be C11H21 and 43370.596 kJ/kg respectively. In this framework, chemical exergy of the fuel may be formulated as following [1, 10, 16]:
Besides, following equations are used to calculate exergy amount of the combustion gaseous while \( N \), \( M \), \( j \) and \( \bar{R} \) notate mole number, molar weight, jth constituent of the combustion gaseous and universal gas constant respectively [16, 17]:
Equations (24)–(26) are used to find out exergy measure of the combustion gaseous at inlet and outlet sections of the gas turbine (GT). In addition to that, generated work rate by gas turbine (GT) can be calculated as following [9, 15]:
Exergy measures of the mini class turboprop engine found as a result of the exergy analysis are summarized in Table 1. Exergy destruction rates within the air compressor, combustion chamber and gas turbine components are 24.08 kW, 100.76 kW and 15.80 kW respectively. It can be stated that, the highest exergy destruction rate within the combustion chamber is indicative of irreversibilities and inefficiencies over the course of combustion process. The lowest exergy efficiency of the combustion chamber is 69.68 % properly. Also minimum exergy destruction occurs within the gas turbine component in consequence of 98.99 % exergy efficiency of the component. Distribution of the exergy destruction within the overall engine is illustrated in Fig. 2 depending on the engine components.
On the other hand, highest improvement potential is appertained to the combustion chamber as a result of the maximum exergy destruction and minimum exergy efficiency. In Fig. 3, that case situation is clearly indicated. Additionally, 98.99 % exergy efficiency value of the gas turbine component causes minimum improvement potential value of 0.16 kW for the gas turbine component.
According to Table 1, fuel depletion rate of air compressor, combustion chamber and turbine are 9.14 %, 38.24 % and 6.00 % respectively. Depending on exergy destructions in the engine, the highest fuel depletion occurs within the combustion chamber properly with exergy destruction rate. Also, productivity rates of air compressor, combustion chamber and turbine are in order of 34.93, 43.49 and 11.66 %. As same as fuel depletion rate, the highest productivity rate is appertained to the combustion chamber as a result of the maximum exergy destruction within the component.
5 Conclusion
In this chapter of the book, performance of a mini class turboprop engine is evaluated with the aid of exergy. The results of the analysis presents that, the combustion chamber among all components of the mini class turboprop engine has the maximum exergy consumption within the engine, due to the irreversibilities and inefficiencies associated with the combustion process. The main conclusions drawn from the results of the present study are as follows:
-
Exergy analysis notates that, the most irreversible component of the mini class turboprop engine is combustion chamber. Especially irreversibilities of the chemical process causes that result.
-
Most efficient component of the mini class turboprop engine is found to be gas turbine in accordance of minimum exergy destruction rate.
-
To improve system thermodynamically, it is essential to focus on combustion chamber component.
Abbreviations
- \( \dot{E} \) :
-
Energy rate (kW)
- \( {\dot{\text{E}}\text{x}} \) :
-
Exergy rate (kW)
- \( {\dot{\text{I}}\text{P}} \) :
-
Improvement potential (kW)
- \( \dot{Q} \) :
-
Heat rate (kW)
- \( \dot{W} \) :
-
Work rate (kW)
- \( \dot{m} \) :
-
Mass flow rate (kg s−1)
- \( h \) :
-
Specific enthalpy (kJ kg−1)
- \( M \) :
-
Molar weight (kg kmol−1)
- \( N \) :
-
Mole number (kmol)
- \( P \) :
-
Pressure (kPa)
- \( R \) :
-
Gas constant (kJ kmol−1 K−1)
- \( \bar{R} \) :
-
Universal gas constant (kJ kmol−1 K−1)
- \( T \) :
-
Temperature (K)
- \( c \) :
-
Specific heat capacity (kJ kg−1 K−1)
- \( {\text{d}} \) :
-
Differential
- \( {\text{ex}} \) :
-
Specific exergy (MJ kg−1)
- \( {\text{ke}} \) :
-
Specific kinetic energy (kJ kg−1)
- \( {\text{pe}} \) :
-
Specific potential energy (kJ kg−1)
- \( s \) :
-
Specific entropy (kJ kg−1 K−1)
- \( \delta \) :
-
Fuel depletion rate
- \( \varepsilon \) :
-
Exergy efficiency
- \( \xi \) :
-
Productivity rate
- \( \chi \) :
-
Relative irreversibility
- \( 0 \) :
-
Ambient conditions
- \( {\text{heat}} \) :
-
Heat transfer related
- \( {\text{air}} \) :
-
Air
- \( {\text{dest}} \) :
-
Destruction
- \( {\text{fuel}} \) :
-
Fuel
- \( {\text{gas}} \) :
-
Combustion gaseous
- \( {\text{in}} \) :
-
Inlet
- \( j \) :
-
jth constituent of the combustion gas
- \( {\text{loss}} \) :
-
Loss
- \( {\text{mass}} \) :
-
Mass transfer related
- \( {\text{out}} \) :
-
Outlet
- \( p \) :
-
Constant pressure
- \( {\text{work}} \) :
-
Work related
- \( {\text{CH}} \) :
-
Chemical
- \( {\text{KE}} \) :
-
Kinetic
- \( {\text{PE}} \) :
-
Potential
- \( {\text{PH}} \) :
-
Physical
- \( {\text{TH}} \) :
-
Thermal
- AC:
-
Air compressor
- CC:
-
Combustion chamber
- GT:
-
Gas turbine
References
Dincer I, Rosen MA (2007) Exergy: energy, environment, and sustainable development. Elsevier, Oxford
Dincer I, Cengel YA (2001) Energy, entropy and exergy concepts and their roles in thermal engineering. Entropy 3:116–149
Rosen MA, Dincer I (1999) Thermal storage and exergy analysis: the impact of stratification. Trans Can Soc Mech Eng 23:173–186
Šılhavý M (1982) On the second law of thermodynamics II. Inequalities for cyclic processes. Czech J Phys B 32:1073–1099
Dreyer W, Müller WH, Weiss W (2000) Tales of thermodynamics and obscure applications of the second law. Continuum Mech Thermodyn 12:151–184
Cengel YA, Wood B, Dincer I (2002) Is bigger thermodynamically better. Exergy Int J 2:62–68
Tsatsaronis G (1993) Thermoeconomic analysis and optimization of energy systems. Prog Energy Combust Sci 19:227–257
Bejan A (1982) Second-law analysis in heat transfer and thermal design. Adv Heat Transf. 15:1–58
Cengel YA, Boles MA (2011) Thermodynamics: an engineering approach. McGraw-Hill, New York
Bejan A, Tsatsaronis G, Moran MJ (1996) Thermal design and optimization. Wiley
Romero JC, Linares P (2014) Exergy as a global energy sustainability indicator a review of the state of the art. Renew Sustain Energy Rev 33:427–442
Van Gool W (1992) Exergy analysis of industrial processes. Energy 17:791–803
Xiang JY, Cali M, Santarelli M (2004) Calculation for physical and chemical exergy of flows in systems elaborating mixed-phase flows and a case study in an IRSOFC plant. Int J Energy Res 28:101–115
Moran MJ, Shapiro HN, Boettner DD, Bailey MB (2010) Fundamentals of engineering thermodynamics. Wiley, New York
Aydin H, Turan O, Midilli A, Karakoc TH (2013) Energetic and exergetic performance assessment of a turboprop engine at various loads. Int J Exergy 13:543–564
Kotas TJ (1985) The exergy method of thermal plant analysis. Anchor Brendon Ltd.
Heywood JB (1988) Internal combustion engine fundamentals. McGraw-Hill, New York
Acknowledgments
The authors gladly thank the Faculty of Aeronautics and Astronautics, Anadolu University for supporting the study.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2017 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Coban, K., Şöhret, Y., Sogut, M.Z., Turan, O., Karakoc, T.H. (2017). Exergy Approach to Evaluate Performance of a Mini Class Turboprop Engine. In: Zhang, X., Dincer, I. (eds) Energy Solutions to Combat Global Warming. Lecture Notes in Energy, vol 33. Springer, Cham. https://doi.org/10.1007/978-3-319-26950-4_23
Download citation
DOI: https://doi.org/10.1007/978-3-319-26950-4_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-26948-1
Online ISBN: 978-3-319-26950-4
eBook Packages: EnergyEnergy (R0)