Abstract
This study deals with exergetic and environmental analyses of turbojet engine used on the military training aircrafts. In the analysis, the engine data measured in the Engine Test Cell at First Air Maintenance and Factory Directorate of Turkish Air Forces in Eskisehir, Turkey are utilized. The exergy balance equations are derived for each component of the engine along with the overall the engine. Several thermodynamic parameters (the fuel exergy depletion ratio, the productivity lack ratio, the relative exergy consumption ratio, exergetic improvement potential, exergetic improvement potential ratio, relative exergetic improvement potential, exergetic fuel-product ratio, and sustainability index) are used to evaluate the performance of the engine and its main components (the air compressor, the combustion chamber, the gas turbine, the exhaust forward duct, the aft exhaust duct, and the mechanical shaft). Exergy losses and destructions are investigated to determine thermodynamic inefficiencies. The exergetic efficiency of the engine is determined to be 18.77%. The highest exergy destruction rate of 2921.01 kW in the engine occurs within the combustion chamber. The mechanical shaft of the engine has the maximum sustainability index of 100.65. An environmental analysis of the engine is also performed.
Access provided by Autonomous University of Puebla. Download conference paper PDF
Similar content being viewed by others
Keywords
1 Introduction
Using the energy of fuel to produce flights is the job of both the military and civil aviation propulsion system. The military aircraft is used to maximize aerodynamic performance, in which case it complies with some operational constraints and uses fuel less efficiently than an efficient aircraft. High-performance start-up, maximum performance climbing and retrofitting are significantly less fuel-efficient than driving performance [1]. For cost-effective and environmental-friendly aviation, system efficiency should be kept maximum, while minimizing cost and environmental impacts of aircraft engines. In order to achieve these objectives, the engine must be operated in optimum operating mode, the best quality fuel should be selected, the fuel consumption rate and the exergetic consumption (losses and destruction) rate should be reduced and the cost of capital should be diminished. In this context, thermodynamic exergy, exergoeconomic, sustainability, and environmental (exergoeconomic, environmental damage cost) analysis methods are used to evaluate the performance of aircraft engines [2]. The main objective of this study can be summarized as follows:
-
Exergetic analyses of J69 turbojet engine used on the military training aircrafts.
-
Environmental analyses of J69 turbojet engine used on the military training aircrafts.
The exergy balance equations derived for each component of the engine along with the overall the engine and exergoenvironomic balance equations are given below section.
2 Methodology
2.1 General Description of TJE with Afterburner
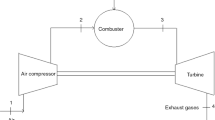
A schematic of the investigated TJE is given in (Fig. 1). This system consists of an air compressor (AC), a combustion chamber (CC), a gas turbine (GT), an exhaust forward duct (EFD), an exhaust aft duct (EAD), and a gas turbine mechanical shaft (GTMS).
2.2 Assumptions
In this study, the assumptions made are listed below:
-
The TJE operates in a steady-state.
-
The ideal gas principles are applied to air and combustion gas.
-
The combustion reaction is complete.
-
The changes in the kinetic exergy and potential exergy are assumed negligible.
-
The temperature and pressure of dead state are 288.15 K and 99.85 kPa, respectively.
-
The exergetic analyses are made on the lower heating value (LHV) basis of liquid JP-8 fuel.
-
The chemical formula of jet fuel is assumed as C12H23.
-
While the engine operates in military operation, air/fuel mass ratio is equal to 65.
2.3 The Exergy Equilibrium Equations
General exergy equilibrium equation is defined in Eq. (1) as under mentioned:
where \(\dot{Q}_{k}\) represents the heat transfer rate through the boundary at temperature \(T_{k}\) at the location k, \(\dot{W}\) is the work rate, \({\dot{\text{E}}}{\text{x}}\) is the exergy rate of stream, and \({\dot{\text{E}}}{\text{x}}_{\text{D}}\) is the exergy destruction rate.
The total exergy for a system can be given in Eq. (2) as under mentioned:
where the terms \({\dot{\text{E}}}{\text{x}}_{kn}\), \({\dot{\text{E}}}{\text{x}}_{\text{pt}}\), \({\dot{\text{E}}}{\text{x}}_{\text{ph}}\), and \({\dot{\text{E}}}{\text{x}}_{\text{ch}}\) denote the kinetic exergy, potential exergy, physical exergy, and chemical exergy, respectively. In the present study, the changes in the kinetic exergy and potential exergy within the system are assumed negligible.
The physical exergy for air and combustion gaseous with constant specific heat is obtained in Eq. (3) as under mentioned [3]:
The chemical exergy of liquid fuels as CaHb on a unit mass basis can be determined in Eq. (4) as under mentioned:
where \(\gamma_{f}\) denotes the liquid fuel exergy grade function. The chemical formula of jet fuel is assumed as C12H23. \(\gamma_{f}\) is calculated as 1.0596 for this fuel. The sum of fuel chemical exergy and the fuel physical exergy gives fuel energy in Eq. (5) as follows:
2.4 The Exergy Efficiency and Thermodynamic Performance Parameters
The exergy efficiency of the system or subsystems can be defined as the ratio of the exergy in outputs products to the exergy in inputs. The exergy efficiency of air compressor is obtained as under mentioned:
The exergy efficiencies of the n’th component of a system are calculated in Eq. (7) as under mentioned:
The exergy efficiency of whole system is obtained as under mentioned:
The thermodynamic parameters such as the fuel depletion rate, relative irreversibility, and productivity lack, are used in evaluating the exergetic performance of the system [4]. These are given in Eqs. (9)–(16) as follows:
The fuel exergy depletion ratio is written as the ratio of the exergy consumption of n’th component to the fuel exergy rate input the TJE such as:
The productivity lack ratio is written as the ratio of the exergy consumption of n’th component to the exergy of products as:
The relative exergy consumption ratio is defined as the ratio of the exergy consumption of n’th component to the exergy consumption of the TJE system as:
Van Gool also stated that maximum improvement in the exergy efficiency for a process or system could be achieved when the exergy consumption is minimized. Consequently, he suggested that it is useful to employ the concept of an exergetic improvement potential when analyzing different processes, as applied by some investigators [5]. The exergetic improvement potential can be written as follows [2]:
The exergetic improvement potential ratio:
The relative exergetic improvement potential:
Exergetic fuel-product ratio:
Sustainability index:
2.5 The Specific Heat Capacity of Air and Combustion Gases
Combustion equilibrium equation for the engine is given as under mentioned:
The specific heat capacity of the combustion gases:
The specific heat capacity of air is a function of temperature [6]:
2.6 The Exergy Equilibrium Equations of the TJE and Its Components
The exergy equilibrium equations for the TJE and its primary segments are shown in Eqs. (20)–(29):
For air compressor:
For combustion chamber:
For gas turbine:
For exhaust forward duct:
For aft exhaust duct:
For mechanical shaft:
Work rate distribution:
For the whole engine:
2.7 Exergoeconomic Analysis
The economic analysis, conducted as part of the exergoeconomic analysis, provides the appropriate monetary values associated with the investment, operation, maintenance, and fuel costs of the system being analyzed [7, 8]. These values are used in the cost balances [9].
2.8 Exergoenvironomic Analysis
To minimize the environmental impacts, a primary target is to increase the efficiency of energy conversion processes and, thus, decreases the amount of fuel and the related overall environmental impacts, especially the release of carbon dioxide, which is one of the main components of greenhouse gas [10]. In this study, three steps were applied to carry out the exergoenvironomic analysis of gas turbine system. The first step is the determination of pollutant emission (CO and NOx) in grams per kilogram of fuel, the estimation of the total cost rate of product and environmental impact and CO2 emission calculation.
2.9 Determination of Pollutant Emission
In order to determine the pollutant emission in grams per kilogram of the fuel, the adiabatic flame temperature in the combustion chamber has to be computed first. The adiabatic flame temperature in the primary zone;
where π is a dimensionless pressure P2/Pref (P2 being the combustion pressure and Pref = 101,300 Pa); θ is a dimensionless temperature T2/Tref (T2 being the inlet temperature and Tref = 298.15 K); ψ is the H/C atomic ratio (ψ = 4); σ = ∅ for ϕ ≤ 1 (ϕ is the fuel to air equivalent ratio), and σ = ϕ − 0.7 for ϕ ≥ 1. Moreover, x, y, and z are quadratic functions of σ based on the following equations [11]:
where parameters A, α, β, λ, ai, bi, and ci are constant parameters. These parameters are given in Table 1 regarding Eqs. (31)–(33) [12]. The calculated exergy rate and other thermodynamic parameters of the components of the TJE are given in Table 2.
The amount of CO and NOx produced in the combustion chamber and combustion reaction depends on the adiabatic flame temperature [11]. Accordingly, to determine the pollutant emission in grams per kilogram of the fuel were used in this study.
where τ is the residence time in the combustion zone (τ is assumed constant and is equal to 0.002 s); Tpz is the primary zone combustion temperature; P2 is the combustor inlet pressure; ∆P2/P2 is the non-dimensional pressure drop in the combustion chamber.
2.10 Cost of Environmental Impact
The cost of environmental impact expresses the environmental impact as the total pollution damage ($/h) due to CO and NOx emission by multiplying their respective flow rates by their corresponding unit damage cost (CCO, and \(C_{{{\text{NO}}_{x} }}\) are equal to 0.02086 $/kgCO and 6.853 $/kgNOx) [8]. In the present work, the cost of pollution damage is considered to be added directly to the expenditures that must be paid. Where, Zk, Cf, CD, and Cenv are the purchase cost of each component, fuel cost, cost of exergy destruction, and cost of environmental impact, respectively.
2.11 CO2 Emissions Calculation
Using the combustion equations, the normalized CO2 emission is expressed as below [13]:
The effect of CO2 emissions is of considerable significance, such that reduction of its harmful release is twofold. The first is obviously related to communal and environmental health. The second, as suggested in many references, is improvement in reduction of harmful emissions in the combustion chamber can lead to improvements of gas turbine cycle efficiency. Reduction of the harmful emissions in the combustion chamber to the environment has proven its benefits in increasing system efficiency, which in turn increases sustainability by lengthening the lives of the fuel resources. A depletion number Dp could characterize the efficient fuel consumption.
The relationship between the depletion number and the exergy efficiency and SI are described by:
3 Results and Discussion
The exergoeconomic parameters considered in this study include average costs per unit of fuel exergy CF and product exergy CP, rate of exergy destruction ĖD, cost rate of exergy destruction ĊD, investment and O&M costs rate Ż, and exergoeconomic factor f. In analytical terms, the components with the highest value of Żk + ĊDk are considered the most significant components in terms of an exergoeconomic perspective. This provides a means of determining the level of priority a component should be given with respect to the improving of the system. For all the engines considered, the combustion chamber and air compressor have the highest value of the sum Żk + ĊDk. Therefore, they are the most important components from the exergoeconomic viewpoint. The low value of exergoeconomic factor, f, associated with the combustion chamber suggests that the cost rate of exergy destruction is the dominate factor influencing the component. Hence, it is implied that the component efficiency is improved by increasing the capital investment. This can be achieved by increasing gas turbine inlet temperature (GTIT). Table 3 shows the results of exergoenvironmental analysis of this work. The computed exergoenvironmental parameters are CO2 emission, depletion number, sustainability index, cost flow rate of environmental impacts (Ċenv) in $/h, and total cost rates of products (ĊTot) in $/h. The study shows that increasing exergetic efficiency results in CO2 emission reduction. The increase of exergetic efficiency is related to reduction of ambient inlet air temperature into the compressor. The efficiency of the system is directly linked to the entire system. However, it is apparent that the overall exergy destruction of the cycle decreases, while the sustainability index increases with decreasing compressor inlet temperature.
4 Conclusion
Exergy analysis provides useful information about the performance of the turbojet engine.
-
The exergetic efficiency of the engine is accounted for 18.77% with 1191.72 kW as exhaust gases product for thrust.
-
The highest exergy destruction between the components of the engine occurs within the combustion chamber with 2921.01 kW, as expected; hence, the combustion reaction is an irreversible process.
-
The constructional and thermodynamic improvements on the engine can be made to decrease the exergy destruction and losses rate. After this improvements, the exergetic efficiency increases from 18.77 to 46.02%.
-
The results from the exergoeconomic analysis, in common with those from the exergy analysis, show that the combustion chamber has the greatest cost of exergy destruction compared to other components. In addition, the results show that by increasing the turbine inlet temperature (TIT) the gas turbine cost of exergy destruction can be decreased.
-
The finding solidifies the concept that the exergy loss in the combustion chamber is associated with the large temperature difference between the flame and the working fluid. Reducing this temperature difference reduces the exergy loss. Furthermore, cooling compressor inlet air allows the compression of more air per cycle, effectively increasing the gas turbine capacity.
-
The cost rate of environmental impact is 796.54 $/h.
-
The study further shows that increasing exergetic efficiency of gas turbine engine results in CO2 emissions reduction. The increase of exergetic efficiency is related to reduction of ambient inlet air temperature into the compressor. This implies that improvement of a system’s efficiency is twofold. By improving the most inefficient components of the system and utilizing the minimum adequate fuel flow rate ensuring maximum burn. The reduction in wasted unburned fuel and the reduction in overall system inefficiencies results in net CO2 emissions reduction.
Abbreviations
- \(c_{\text{P}}\) :
-
Specific heat capacity (kJ kg−1 K−1)
- \({\dot{\text{E}}}{\text{x}}\) :
-
Exergy rate (kW)
- \({\dot{\text{E}}}{\text{xIP}}\) :
-
Exergetic improvement potential (kW)
- \({\dot{\text{E}}}{\text{xIPR}}\) :
-
Exergetic improvement potential rate ratio (%)
- \({\text{LHV}}\) :
-
Lower heating value of fuel (kJ kg−1)
- \(\dot{m}\) :
-
Mass flow rate (kg s−1)
- p :
-
Pressure (kPa)
- \({\text{R}}{\dot{\text{E}}}{\text{xIP}}\) :
-
Relative improvement potential rate (%)
- SI:
-
Sustainability index (–)
- T :
-
Temperature (K)
- \(\dot{W}\) :
-
Work rate or power (kW)
- \(\alpha\) :
-
Fuel exergy depletion ratio (%)
- \(\beta\) :
-
Productivity lack ratio in exergetic term (%)
- \(\chi\) :
-
Relative exergy consumption ratio (%)
- \(\gamma\) :
-
Fuel exergy grade function
- \(\psi\) :
-
Exergy (second law) efficiency (%)
- a:
-
Air
- AC:
-
Air compressor
- C:
-
Consumption
- CC:
-
Combustion chamber
- cg:
-
Combustion gases
- D:
-
Destroyed, destruction
- EAD:
-
Exhaust aft duct
- EFD:
-
Exhaust forward duct
- GT:
-
Gas turbine
- GTMS:
-
Gas turbine mechanical shaft
- in:
-
Input
- out:
-
Output
- P:
-
Pressure
- Pr:
-
Product
- ref:
-
Reference
- T:
-
Temperature
- TJE:
-
Turbojet engine
- AC:
-
Air compressor
- EAD:
-
Exhaust aft duct
- EFD:
-
Exhaust forward duct
- CC:
-
Combustion chamber
- GT:
-
Gas turbine
- GTMS:
-
Gas turbine mechanical shaft
- TJE:
-
Aircraft jet engine
References
Lucia DJ (2011) Cruising in afterburner: air force fuel use and emerging energy policy. Energy Policy 39:5356–5365
Balli O, Hepbasli A (2014) Exergoeconomic, sustainability and environmental damage cost analyses of T56 turboprop engine. Energy 64:582–600
Kotas TJ (1995) The exergy method of thermal plant analyses, Reprint edn. Kieger, Malagar
Xiang JY et al (2004) Calculation for physical and chemical exergy of flows in system elaborating mixed-phase flows and a case study in an IRSOFC plant. Int J Energy Res 28:101–115
Van Gool W (1997) Energy policy: fairly tales and factualities. In: Innovation and technology-strategies and policies, pp 93–105. https://doi.org/10.1007/978-0-585-29606-7_6
Moran MJ, Shapiro HN (1995) Fundamentals of engineering thermodynamics. Wiley, New York
Siahaya Y (2009) Thermoeconomic analysis and optimization of gas turbine power plant. In: Proceedings of the international conference on fluid and thermal energy conversion
Ahmadi P, Dincer I (2011) Thermodynamic and exergoenvironmental analyses, and multi-objective optimization of a gas turbine power engine. Appl Therm Eng 31:14–15
Bejan A, Tsatsaronis G et al (1996) Thermal design and optimization. Wiley, New York
Ahmadi P, Rosen MA et al (2011) Greenhouse gas emission and exergo-environmental analyses of a trigeneration energy system. Int J Greenh Gas Control 5:1540–1549
Ahmadi P, Dincer I (2010) Exergo-environmental analysis and optimization of a cogeneration engine system using multimodal genetic algorithm (MGA). Energy 35:5161–5172
Gulder O (1986) Flame temperature estimation of conventional and future jet fuels. J Eng Gas Turbine Power 108:376–380
Altayib K (2011) Energy, exergy and exergoeconomic analyses of gas turbine based systems. M.Sc. thesis, University of Ontario Institute of Technology
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2020 Springer Nature Switzerland AG
About this paper
Cite this paper
Yuksel, B., Balli, O., Gunerhan, H., Hepbasli, A., Atalay, H. (2020). Exergetic and Environmental Analyses of Turbojet Engine. In: Dincer, I., Colpan, C., Ezan, M. (eds) Environmentally-Benign Energy Solutions. Green Energy and Technology. Springer, Cham. https://doi.org/10.1007/978-3-030-20637-6_21
Download citation
DOI: https://doi.org/10.1007/978-3-030-20637-6_21
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-030-20636-9
Online ISBN: 978-3-030-20637-6
eBook Packages: EnergyEnergy (R0)