Abstract
Isolated perfusion is a treatment option when metastases are located on a limb and they are too numerous or when they recur too frequently for excision, even in the presence of distant metastases. Isolated limb perfusion enables treatment of an entire extremity with drugs without exposing the rest of the body to the medication. In the isolated limb, drug concentrations of up to 20 times the level that would be tolerated in the rest of the body may be reached. Perfusion provides the opportunity to treat not only the lesions that are evident but also occult lesions that could become evident later. Continuous monitoring can detect systemic leakage at an early stage. This is needed in situations when systemic leakage is known to occur and when tumor necrosis factor is administered. The detection of systemic leakage was classically performed by measurement of radiotracer concentrations in blood samples from the perfusate and systemic circulation. Radioguided monitoring of potential systemic leakage has been accomplished by using a precordial scintillation detector, a handheld gamma ray probe, or a mobile small field-of-view gamma camera in the operating room.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
These keywords were added by machine and not by the authors. This process is experimental and the keywords may be updated as the learning algorithm improves.
1 Introduction
The biology of melanoma has particular features that are not found in other types of cancer. Satellite and in-transit dissemination are typical of this disease and occur in a small percentage of the patients. These metastases originate from tumor cells that are caught in lymph vessels in the skin or in the subcutaneous tissues. Such metastases are typically excised, but they often recur in larger numbers (Fig. 23.1). Isolated perfusion is a well-established treatment option when such metastases are located on a limb and they are too numerous or when they recur too frequently for excision, even in the presence of distant metastases. A range of other options is available for inoperable in-transit disease, like intralesional injection with BCG, Rose Bengal, or T-VEC. Options for cutaneous lesions are topical application of dinitrochlorobenzene, diphencyprone, or monobenzone and imiquimod. Other options are diathermy, cryotherapy, carbon dioxide laser ablation, electrochemotherapy, and radiotherapy. New effective systemic drugs may provide another option. With all these alternatives available, amputation is rarely performed these days in patients with locally or regionally advanced melanoma [1, 2].
2 Definitions and Prognosis
The nomenclature used for recurrences in melanoma can be confusing because terms such as locoregional recurrence, satellitosis, and in-transit disease have all been used with varying definitions and intentions. Local recurrence is preferably defined as the regrowth of the primary melanoma in the excision scar or graft [3, 4].
The most recent American Joint Committee on Cancer (AJCC) and Union International Contre le Cancer (UICC) staging system for melanoma define in-transit metastases as any skin or subcutaneous metastases that are more than 2 cm from the primary lesion but are not beyond the regional nodal basin. Satellite metastases are defined as cutaneous or subcutaneous metastases occurring within 2 cm of the primary melanoma. The staging classification does not differentiate between in-transit lesions and satellitosis in the assignment of stage, both being designated as N2 disease, or N3 if regional nodes are also involved [5, 6].
Satellites and in-transit metastases are associated with a 5-year survival rate of 69 % and a 10-year survival rate of 52 %. These numbers are somewhat better than the 59 % 5-year and 43 % 10-year survival for stage IIIB patients overall [7].
3 Isolated Limb Perfusion
Isolated limb perfusion enables treatment of an entire extremity with systemic drugs without exposing the rest of the body to the medication. Isolating the limb from the main circulation and creating a separate blood circuit accomplish this goal. The rationale is that melanoma is sensitive to chemotherapy, but requires a higher dose than is customary in other types of cancer. In the isolated limb, drug concentrations of up to 20 times the level that would be tolerated in the rest of the body may be reached. Perfusion provides the opportunity to treat not only the lesions that are evident but also occult lesions that could become evident later.
An 80 % response rate is accomplished with perfusion with a complete response rate of 54 % [8]. Approximately half of the patients with a complete response recur in the perfused limb after a median interval of 6 months. In 70 % of these patients, such recurrences can be managed by simple local treatment modalities like excision, or laser ablation, or radiotherapy. The 10-year survival rate in patients with a complete response is 49 % [9]. Repeat perfusion can be considered for patients who recur following an initial response and is often effective [10]. Long-term survivors have a better quality of life than comparable control individuals [11, 12].
Melphalan is the standard drug for isolated limb perfusion. The addition of tumor necrosis factor (TNF)-α to the perfusate results in tumor vasculature destruction, increased cytotoxicity, and modulation of the immune response. TNF-α augments the response rate, but can cause serious morbidity when substantial leakage occurs [13–15].
Systemic toxicity can be avoided by adequate isolation of the limb. For this purpose, a tourniquet around the base of the limb is used and is combined with ligation of collateral vessels. Systemic leakage can be limited by avoiding a high flow rate of perfusion and limiting the venous pressure. Continuous monitoring can detect systemic leakage at an early stage. This is needed in situations when systemic leakage is known to occur and when high dose TNF-α is administered.
The aim of this chapter is to describe the current options for intraoperative radioguided monitoring of systemic leakage during isolated limb perfusion.
4 Description of Perfusion Technique
Isolated limb perfusion requires a multidisciplinary effort by the surgeon, the perfusionist, the nuclear medicine physician, and the operating room team. A detailed description of the technique can be found elsewhere [16]. There is variation in the perfusion technique between institutions. A summary of our protocol at Hospital Clinic of Barcelona is described here.
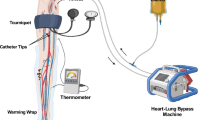
General anesthesia is used. Perfusion can be performed in the lower limb at the level of the external iliac vessels, at the femoral level, or the popliteal level and in the upper limb at the axillary or brachial level. A tourniquet, for subsequent compression, is placed at the base of the limb under sterile conditions. After disinfection of the entire limb, an arterial line is inserted in the dorsal pedal (or radial) artery to assess the mean arterial pressure, reflecting the perfusion pressure. Subsequently, the main artery and vein are dissected at the base of the limb. Collateral vessels are ligated to prevent leakage to and from the systemic circulation. Arteriotomy and venotomy are performed and cannulae are inserted and connected to the perfusion circuit lines (Fig. 23.2). The blood draining from the venous cannula is propelled by a pump through an oxygenator and a heat exchanger and reintroduced via the arterial cannula, with a pressure lower than the mean systemic arterial pressure. Isolation of the limb is finalized by wrapping a rubber bandage or inflatable tourniquet around its root to compress the smaller vessels in the muscles and subcutaneous tissue. Thermal probes are inserted into the subcutaneous tissue and a muscle compartment to monitor the temperature.
For leakage monitoring, a small dose of a radiopharmaceutical like 99mTc‐labeled serum human albumin (i.e., Vasculocis ®) is added to the perfusion circuit. Leakage of this tracer into the systemic circulation is continuously monitored by a gamma ray detector (gamma camera) placed over the heart. After establishing the absence of venous blood leakage to the main circulation, the perfusion of cytostatic drugs (melphalan 1.50 mg/kg – TNF-alpha 3 mg for the arm and 4 mg/kg for the leg) is initiated. At the end of the perfusion, the limb is washed out with 1 l (arm) to 4 l (iliac perfusion) of physiological saline solution, the tourniquet is released, and all the blood from the venous line is drained. Subsequently, all vascular cannulae are removed and the arteriotomy and venotomy are closed with vascular suture material.
5 Systemic Leakage Monitoring
Originally, the detection of systemic leakage was performed by measurement of radiotracer concentrations in blood samples from the perfusate and systemic circulation with intervals of several minutes. The different volumes involved in the procedure of extracorporeal perfusion are total blood volume (Vt) as the sum of systemic blood volume (Vs) and the blood volume of the perfused extremity (Ve).
For extracorporeal circulation (Vc), the overall volume consists of Ve plus the volume of the pump oxygenator with the connecting tubes (Vo).
Usually, the value for Vo is known. Vt can be extracted from medical tables and Ve values are estimated to be 5 % of the Vt for the upper limbs and 10 % for the lower limbs. For a more exact determination of Vt, Ve, or Vc, it is important to do a dilution analysis. Thus, a known quantity of a blood pool tracer in a known volume must be injected into an unknown volume. After a mixing period, a blood sample is drawn from the unknown volume and the concentration is measured.
Human serum albumin (HSA) tagged with a radioactive tracer is one of the radiopharmaceuticals that can be administered to measure this potential leakage. HSA labeled with 125I or 131I (half-life 60 and 8 days, respectively) was used for this purpose in the past. Thyroid uptake of the radioiodine released after HSA catabolism should be prevented with potassium iodide or sodium perchlorate given 1 or 2 days before the procedure, and these should be continued for 1 or 2 weeks. HSA is also available for labeling with 99mTc-pertechnetate and this isotope is currently used. The labeling efficiency should be greater than 90 %.
When radioactive blood pool indicators are used, their presence is expressed in counts per minute (cpm). The count rate is proportional to the concentration of the indicator. Thus, systemic leakage is calculated as the quotient of the difference between tracer concentration in the systemic circulation at the beginning and at the end of one perfusion time frame and the total amount of tracer in the perfusion circulation at the beginning of that interval.
6 Radioguided Monitoring
Intraoperative radioguided monitoring of systemic leakage during isolated limb perfusion surgery is well described in the literature [17–28], and can be performed using a precordial scintillation detector, a handheld gamma ray probe, or a mobile small field-of-view gamma camera in the operating room. During perfusion, this device is positioned in the precordial region to acquire the background count rate from the blood pool within the heart. A small amount of radiopharmaceutical is injected into systemic circulation to calibrate the system before monitoring. Subsequently, a larger dose (usually ten times greater) of the same tracer is added to the perfusion circuit. The background count rate over the heart is nearly proportional to the activity in the systemic circulation, and the latter can thus be monitored continuously. A rapid calculation of leakage is possible at any time.
The basic principles of this radioguided technique were described by Stehlin et al. using a single, large, overhead-mounted scintillation detector that displayed continuous tracing results in cpm, on a rectilinear recorder for detecting 131I-HSA [17]. This same principle using a more modern handheld gamma detection probe system was later described by Sardi et al. [18]. The system described by Sardi et al. [18] consisted of two handheld gamma ray detectors, one positioned over the precordial area and one positioned over the distal aspect of the thigh. Each patient received nearly 30 MBq of 99mTc-pentetate through the volume of perfusion pump. The percentage of leakage was calculated by a simultaneous reading of the two gamma ray detection probes at 1-min intervals. Identical percentages of leakage were detected when this approach was compared to a method of intermittent simultaneous blood sampling from the perfusate and systemic circulations at intervals of several minutes. In contrast to the intermittent (i.e., every 15 min) blood sampling from the perfusate and systemic circulations, the minute-by-minute monitoring of the two handheld gamma probe system allowed for a real-time indication of any fluctuations in the percentage of leakage.
Since then, variations of the radioguided systemic leakage monitoring technique have been described [19–28]. For example, Manner et al. described the use of a two-probe system (precordial region and thigh) with 18.5 MBq of 111In-labeled red blood cells added to the perfusate [19]. Sprenger et al. used a three-probe system (precordial, thigh, and perfusate circuit) with low dose (0.15 MBq) of 111In-labeled red blood cells injected into the systemic circulation to establish a minimum baseline reference activity within the systemic circulation. A subsequent high dose (12 MBq) of 111In-labeled red blood cells was injected into the perfusate [20]. Barker et al. used a one-probe precordial system with a low dose (0.74 MBq) of 131I-labeled human serum albumin injected into the systemic circulation and a subsequent tenfold higher dose (7.4 MBq) of 131I-HSA injected into the perfusate circulation [21]. Van Ginkel et al. described the use of a precordial one-probe system and a combination of two radionuclides. They administered a low dose (0.5 MBq) of 131I-HSA and 10 MBq of 99mTc-HSA into the systemic circulation and a subsequent tenfold higher dose (5 MBq) of 131I-HSA injected into the perfusion circuit [22].
All these classic real-time leakage determination methods displayed continuous tracing results on a rectilinear recorder. The percentage of leakage was manually calculated every minute, making this procedure tedious and increasing the possibility of mistakes. Also, the intraoperative equipment (detector + recorder) used to perform this procedure was bulky (Fig. 23.3). In order to overcome some drawbacks associated with the properties of 131I, a procedure based on HSA labeled with 99mTc in combination with a handheld gamma ray detection probe was developed. Sandrock et al. used a portable gamma probe with digital display and investigated the physical properties in a phantom study simulating blood pool activity at different angles of the probe to the surface and at different distances. In twenty patients, the limb circulation was surgically separated from the systemic blood circulation, and the limb was then selectively perfused for 1 h. Initially, 15 MBq 99mTc-labeled autologous red blood cells were injected into the limb circulation, and an equal amount was kept as a standard. Every 10 min, blood samples were drawn from the body circulation and count rates were simultaneously measured using the probe system in the precordial area. All blood samples were counted for calculation of leakage in terms of percent of the injected dose, and the results were compared with the intraoperative count rates of the probe system. They found a high correlation between the two techniques (r = 0.92) [23].
Lately, Casara et al. advocated a precordial one-probe system following well-defined steps. Firstly, 48–72 h before perfusion, a 99mTc-HSA dose corresponding to 10 % of the dose calculated for perfusion (i.e., 0.05 MBq/kg body weight) was administered to the patient. The maximum count-rate zone detected on the precordial area was marked on patient’s skin. During the perfusion procedure, a 99mTc-HSA dose of 0.5 MBq/kg body weight was injected into the perfusion circuit before TNF-α administration. A handheld gamma probe, usually employed in sentinel lymph node procedure, was placed over the precordial area in the zone premarked during the simulation test. A 60-min time-activity curve corresponding to the circulating 99mTc-HSA radioactivity effective decay was calculated to compensate for the leakage systemic counting observed during perfusion.
A good correlation was found (R 2 = 0.965, P < 0.01) when the results of handheld gamma probe monitoring were compared with the results of patient blood and perfusion circuit samples taken simultaneously every 5 min. So, this approach appeared to be technically simple and accurate enough for the real-time monitoring of perfusion leakage [24, 25].
Orero et al. developed a method using a portable gamma camera (Sentinella S102 (ONCOVISION, Valencia, Spain)), specially designed for intraoperative use [26]. This device is a compact scintillation camera with a CsI (Na) crystal optically coupled to a flat panel-type position-sensitive photomultiplier tube [27]. A USB port connects the camera to a computer. The gamma camera is mounted on a lightweight support to facilitate transport to the operating theater (Fig. 23.4). A parallel multi-hole collimator was developed because monitoring of tracer activity requires a higher sensitivity than can be provided by a pinhole collimator (Fig. 23.5). Software to acquire the data needed to detect leakage from the perfusion limb to the systemic circulation was designed. This program measures activity data in the precordial region at 30-s intervals throughout the procedure. The basal activity in this region is measured after the radiotracer measurements in both circuits have reached a steady state and before the drug is administered. Knowing the injected activity in the body A b, in the isolated extremity A e, and the measured basal counts B, a detection increase Δ corresponds to a certain leakage L. An approximate value for the leakage, L a , can be obtained by using the formula:
Orero et al. [27] concluded from their pilot work that the monitoring system could give reliable values for the leakage. Using this approach, the percentage of blood leakage to the systemic vascular territory is calculated and displayed on the computer screen for the duration of the procedure (Figs. 23.6, 23.7, and 23.8). The initial experience was obtained in sixteen melanoma patients in whom the percentage of leakage ranged from 0.5 to 8 % (mean = 3.1 %).
Example of software developed for monitoring systemic leakage. The upper row shows tracer activity (in counts). The lower row depicts the percentage of systemic activity variation from the baseline readings as histograms. This example is of a patient with a stable count rate during radiotracer injection (TNF-alpha administration and melphalan perfusion)
Same patient as in Fig. 23.6 with a more prolonged monitoring (after 30 min of melphalan perfusion). Systemic leakage was maintained below 5 % with minimal peaks of systemic leakage higher than this value
Our group has performed 45 perfusions with this radioguided system. The median leakage percentage demonstrated was 3.5 % (range 0–10 %). During the immediate postoperative period (1–7 days after the operation), there were two vascular complications. Fourteen patients presented bone marrow toxicity (eight leukopenia and six thrombocytopenia) that was successfully managed using colony-stimulating factor. Complete regional response was achieved in 50 % of cases, with a nearly 30 % of the remaining patients showing partial response (unpublished data, Ramon Rull, 2014).
7 Concluding Remarks
Despite a plethora of innovative therapeutic options, isolated limb perfusion remains a reasonable option for patients with extensive or bulky disease in a limb. The complete response rate is 54 % and the response is durable in half of them. Given the nature and dose of the drugs that are used in the perfusion circuit, monitoring of systemic leakage is of utmost importance. Throughout the years, different approaches have been used for this purpose. The current standard technique involves radiotracers and continuous monitoring. The new detection devices used in other fields of radioguided surgery (e.g., sentinel lymph node localization) have replaced traditional approaches based on blood samples or bulky detectors. Within this framework, several technical variations all appear to have good results.
References
Kapma MR, Vrouenraets BC, Nieweg OE, van Geel AN, Noorda EM, Eggermont AMM, et al. Major amputation for intractable extremity melanoma after failure of isolated limb perfusion. Eur J Surg Oncol. 2005;31:95–9.
Read RL, Haydu L, Saw RPM, Quinn MJ, Shannon K, Spillane AJ, et al. In-transit melanoma metastases: incidence, prognosis, and the role of lymphadenectomy. Ann Surg Oncol. 2015;22:475–81.
Pawlik TM, Ross MI, Johnson MM, Schacherer CW, McClain DM, Mansfield PF, et al. Predictors and natural history of in-transit melanoma after sentinel lymphadenectomy. Ann Surg Oncol. 2005;12:587–96.
Meier F, Will S, Ellwanger U, Schlagenhauff B, Schittek B, Rassner G, et al. Metastatic pathways and time courses in the orderly progression of cutaneous melanoma. Br J Dermatol. 2002;147:62–70.
Balch CM. Melanoma of the skin. In: Edge, S. B., Byrd, D. R., Compton, C. C., Fritz, A. G., Greene, F. L., Trotti, A. (Eds). American Joint Committee on cancer staging manual. 7th ed. New York: Springer; 2010. p. 325.
Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Atkins MB, Byrd DR, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–206.
Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4.
Nieweg OE, Kroon BBR. Isolated limb perfusion with melphalan for melanoma. J Surg Oncol. 2014;109:132–7.
Sanki A, Kam PC, Thompson JF. Long-term results of hyperthermic, isolated limb perfusion for melanoma: a reflection of tumor biology. Ann Surg. 2007;245:591–6.
Klop WM, Vrouenraets BC, van Geel BN, Eggermont AMM, Klaase JM, Nieweg OE, et al. Repeat isolated limb perfusion with melphalan for recurrent melanoma of the limbs. J Am Coll Surg. 1996;182:467–72.
Noorda EM, van Kreij RH, Vrouenraets BC, Nieweg OE, Muller M, Kroon BBR, et al. The health-related quality of life of long-term survivors of melanoma treated with isolated limb perfusion. Eur J Surg Oncol. 2007;33:776–82.
Raymond AK, Beasley GM, Broadwater G, Augustine CK, Padussis JC, Turley R, et al. Current trends in regional therapy for melanoma: lessons learned from 225 regional chemotherapy treatments between 1995 and 2010 at a single institution. J Am Coll Surg. 2011;213:306–18.
Lienard D, Ewalenko P, Delmotte JJ, Renard N, Lejeune FJ. High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol. 1992;10:52–60.
Fraker DL, Alexander HR, Andrich M, Rosenberg SA. Treatment of patients with melanoma of the extremity using hyperthermic isolated limb perfusion with melphalan, tumor necrosis factor, and interferon gamma: results of a tumor necrosis factor dose-escalation study. J Clin Oncol. 1996;14:479–89.
Zwaveling JH, Maring JK, Clarke FL, van Ginkel RJ, Limburg PC, Hoekstra HJ, et al. High plasma tumor necrosis factor (TNF)-alpha concentrations and a sepsis-like syndrome in patients undergoing hyperthermic isolated limb perfusion with recombinant TNF-alpha, interferon-gamma, and melphalan. Crit Care Med. 1996;24:765–70.
Nieweg OE, Imhof O, Kroon BBR. Isolated limb perfusion. In: Mulholland MW, Hawn MT, Hughes SJ, Albo D, Sabel MS, Dalman RL, editors. Operative techniques in surgery. Riverwoods: Wolters Kluwer; 2014. p. 1647–55.
Stehlin Jr JS, Clark Jr RL, Dewey WC. Continuous monitoring of leakage during regional perfusion. Arch Surg. 1961;83:943–9.
Sardi A, Minton JP, Mojzisik C, Nieroda CA, Ferrara PJ, Hinkle GH, et al. The use of a hand-held gamma detector improves the safety of isolated limb perfusion. J Surg Oncol. 1989;41:172–6.
Manner M, Sinn H, Bubeck H, Kettelhack C, Schlag P. Improved intraoperative leak control in cytostatic drug isolation perfusion of tumors of the extremities. Langenbecks Arch Chir. 1990;375:208–13.
Sprenger HJ, Markwardt J, Schlag PM. Quantitative radionuclide leakage control during isolated limb perfusion. Nuklearmedizin. 1994;33:248–53.
Barker WC, Andrich MP, Alexander HR, Fraker DL. Continuous intraoperative external monitoring of perfusate leak using iodine-131 human serum albumin during isolated perfusion of the liver and limbs. Eur J Nucl Med. 1995;22:1242–8.
van Ginkel RJ, Limburg PC, Piers DA, Koops HS, Hoekstra HJ. Value of continuous leakage monitoring with radioactive iodine-131-labeled human serum albumin during hyperthermic isolated limb perfusion with tumor necrosis factor-alpha and melphalan. Ann Surg Oncol. 2002;9:355–63.
Sandrock D, Horst F, Gatzemeier W, Ghorbani M, Rauschecker H, Munz DL, et al. Leakage measurement during selective limb perfusion using a gamma probe. Eur J Nucl Med. 1996;23:534–8.
Casara D, Rubello D, Pilati PL, Scalerta R, Foletto M, Rossi CR. A simplified procedure for continuous intraoperative external monitoring of systemic leakage during isolated limb perfusion. Tumori. 2002;88:S61–3.
Casara D, Rubello D, Pilati P, Scalerta R, Foletto M, Rossi CR. Optimized procedure of real-time systemic leakage monitoring during isolated limb perfusion using a hand held gamma probe and 99mTc-HSA. Nucl Med Commun. 2004;25:61–6.
Orero A, Vidal-Sicart S, Roé N, Muxí A, Rubí S, Duch J, et al. Monitoring system for isolated limb perfusion based on a portable gamma camera. Nuklearmedizin. 2009;48:166–72.
Sánchez F, Fernández MM, Giménez M, Benlloch JM, Rodríguez-Alvarez MJ, García de Quirós F, et al. Performance tests of two portable mini gamma cameras for medical applications. Med Phys. 2006;33:4210–20.
Povoski SP, Neff RL, Mojzisik CM, O’Malley DM, Hinkle GH, Hall NC, Murrey Jr DA, Knopp MV, Martin Jr EW. A comprehensive overview of radioguided surgery using gamma detection probe technology. World J Surg Oncol. 2009;7:11.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Vidal-Sicart, S., Rull, R., Barriuso, C., Nieweg, O.E. (2016). Radioguided Monitoring of Systemic Leakage During Isolated Limb Perfusion for Melanoma. In: Herrmann, K., Nieweg, O., Povoski, S. (eds) Radioguided Surgery. Springer, Cham. https://doi.org/10.1007/978-3-319-26051-8_23
Download citation
DOI: https://doi.org/10.1007/978-3-319-26051-8_23
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-26049-5
Online ISBN: 978-3-319-26051-8
eBook Packages: MedicineMedicine (R0)