Abstract
Genetic diversity keeps the soil fertile, recycles all nutrients, and cleans the air and water. The richer the genetic baggage, the higher shall be the capacity to fight different fungi, virus, or bacteria. Like other essentials, rubber is an industrial commodity that is indispensable to humans with more than 55,000 vivid products made from it. Hevea, rubber originated in Amazon, where 1652 plants belonging to 107 species in 37 different families are found in about 630 m2. Hevea—rubber originated in such a biologically diversified environment. From the story of the first transfer of rubber seeds from Brazil to Asia, it is difficult to evaluate how narrow the genetic base initially was for what has now become the “Wickham” domesticated population. Much importance was conferred to a small number of 22 seedlings disseminated from Singapore to Malaysia after 1876, and the original Wickham stock was collected in only one Brazilian site, Boïm, on the Western banks of the Tapajoz River. Though generation wise assortative mating as the prime breeding tool was applied to these accessions and Wickham collection, much genetic variation could not be tapped for commercial purpose. The molecular marker systems (all three generations markers) are being applied in Hevea rubber. Of these, SNPs are the new generation markers used for Marker-Assisted Selection (MAS). A saturated linkage map of Hevea brasiliensis has been accomplished and the whole genome size was calculated as 6 × 108 base pairs. Selection was indirectly toward nuclear DNA polymorphism, while evolving modern clones. mtDNA of Wickham clones has lesser variation because their female progenitors are all primary clones (either PB 56 or Tjir 1). Chloroplast genomes are sufficiently large and complex to include structural and point mutations that are useful for evolutionary studies from intraspecific to interspecific levels. Populations were subjected to several rounds of controlled crossing that further narrowed the diversity. But the strategy followed by the breeders to select only the desirable genotypes and to reject the unwanted ones (without assessing the utility other than yield) is the main reason that reduced diversity. Much work at the molecular level had been carried out like for Tapping Panel Dryness, latex production, defense genes and alike. Setting up of a molecular library for Hevea and scientists working worldwide contributing to this library will be a good option to study and document molecular diversity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
6.1 Introduction
Nearly 10,000 different plant species have been used by humans for food and fodder production since the agriculture began 12,000 years ago (Wadley and Martin 1993). Farming first began in the Fertile Crescent, which stretches from Israel north to southeast Turkey and then curves southeast to the Persian Gulf (Zeder 2008). (see http://www.ngdc.noaa.gov/paleo/ctl/10k.html for further details). However, agriculture was invented independently in other parts of the world as well. World population was only five million then, which is seven billion today. But as of now, just 150 crops feed most human beings on the planet, and just 15 crops provide 90 % of food energy—wherein, wheat, rice, maize, and potato alone provide 60 % (Xu 2010; Ji et al. 2013). Most of a crop’s varietal diversity has been lost through genetic erosion due to extensive cultivation of a few so-called high yielding varieties. In these species, a combination of genes defines the characteristics of species morphology and their capacity to interact with the world. The gene content and diversity among plant species are intriguing. The total amount of genes differs as in bacteria with as many as 1600 genes (Cole et al. 2001) and mammals with 30,000 (Waterston et al. 2002). Among flowering plants, rice has 41,000 genes (Sterck et al. 2007), with Paris japonica having the biggest genome (Pellicer et al. 2010). This genetic baggage is passed through each generation and causes a species to evolve ways and means to confront with natural selection.
Genetic diversity plays a crucial role in the stability of our ecological system. Every species fulfills a role in the earth’s biosphere and assures ecological survival. By this, biodiversity keeps the soil fertile, recycles all nutrients, and cleans the air and water. The richer the genetic baggage, the higher shall be the capacity to fight different fungi, virus, or bacteria. It is the diversity of genetic baggage that makes natural extinction so rare. Basically, biodiversity provides everything humans need to survive like food, fresh air, clean water, clothing, medicine, wood, and various raw materials for industrial uses. A rich ecological environment is indeed very complex, and is impossible for humans to recreate. Genetic erosion is wiping out millions of years of evolution and a loss in biodiversity is irreparable. In recent years, there is a fillip which contests that Genetically Modified Organisms (GMOs) can bring in genetic diversity. With the advent of transgenic plants, the problem of gene flow that may turn as a cause for ecological risk stems special significance (Hammer and Teklu 2008). This contention requires ample debate.
Genetic erosion is the loss of genetic diversity—often magnified or accelerated by human activities. In native plant populations, genetic erosion results from habitat loss and fragmentation, but it also can result from a narrow genetic base in the original collections or by practices that reduce genetic diversity. The loss of biological diversity has been measured traditionally by frequency of species extinctions. In general, genetic erosion is loss of genetic diversity within a species. It can represent the loss of entire populations genetically differentiated from others, the loss or change in frequency of specific alleles within populations or over the species as a whole, or the loss of allele combinations. Quite often, cultivation of a limited number of high-yielding genotypes can also lead to genetic erosion.
6.2 Genetic Diversity in Hevea Rubber
Like other essentials, rubber is an industrial commodity that is indispensable to humans with more than 55,000 vivid products made from it. The para rubber tree, the Euphorbiaceous Hevea brasiliensis (Willd. ex A. Juss.) Müll Arg, is the chief contributor to natural rubber production worldwide (Priyadarshan and Clément-Demange 2004). Rubber is synthesized in over 2500 plant species, confined to 300 genera of seven families: Euphorbiaceae, Apocynaceae, Asclepiadaceae, Asteraceae, Moraceae, Papaveraceae, and Sapotaceae (Backhaus 1985; Lewinsohn 1991; Cornish et al. 1993). At least two fungal species (Lactarius deceptiva and Peziza sp.) are also known to make natural rubber (Stewart et al. 1955). The Euphorbiaceae family is extremely diverse and considered to be polyphyletic (Webster 1994). Much of the diversity of the center of origin (Amazon basin, Brazil) is being lost due to extensive deforestation (Fig. 6.1).
Today, the Amazon River is the most voluminous river on Earth, (eleven times the volume of Mississippi) that drains an area equivalent in size of United States. Every day, up to 500 billion cubic feet of water (5,787,037 cu ft/s) flows into the Atlantic. The Amazon River Basin is home to the largest rainforest on Earth. The basin covers some 40 % of the South American continent and includes parts of nine South American countries, viz., Brazil, Bolivia, Peru, Ecuador, Colombia, Venezuela, Guyana, Suriname, and French Guiana. The Amazon basin consists of enormous trees, some exceeding a height of 100 m, with an incredible number of species growing side by side in the greatest profusion arranged in different strata. For example, in Manaus (Brazil), 1652 plants belonging to 107 species in 37 different families were found in about 630 m2. Ducke (1941) estimated 2500 tree species and as many as 100 arboreal species have been counted on a single acre of forest with hardly any one of them occurring more than once. Papers of Seibert (1947) and Schultes (1945) further confirm this enormous diversity. Latest analysis suggests that lowland Amazonia harbors 3.9 × 1011 trees and ~16,000 tree species (Steege et al. 2013). According to the mathematical model used in the study, roughly 6,000 tree species in the Amazon have populations of lesser than 1000 individuals, which automatically qualifies them for inclusion in the International Union for Conservation of Nature (IUCN) Red List of Threatened Species. The problem is that these species are so rare that scientists may never find them. The Amazon forest has a strikingly layered structure. The canopy of sun-loving giants, soar to as much as 40 m above the ground and a few, known as emergents, rise beyond such canopies, frequently attaining heights of 70 m. Their straight, whitish trunks are covered with lichens and fungus. A characteristic of these giant trees is the buttresses, or basal enlargements of their trunks, which presumably help stabilize the top heavy trees during infrequent heavy winds. Further characteristics of the canopy trees are their narrow, downward-pointing “drip-tip” leaves that easily shed water. Flowers are inconspicuous. Among the canopy species, prominent members include the rubber tree (H. brasiliensis), the silk cotton (Ceiba pentandra), the Brazil nut (Bertholletia excelsa), the sapucaia (Lecythis), and the sucupira (Bowdichia). Many creatures, including monkeys and sloths, spend their entire lives in this sunlit canopy.
The Amazon basin covers a surface area of 4,100,000 km2 (1,583,000 square miles), of which around 3.4 million km2 (1.3 million square miles) are presently forested (Schroth et al. 2004). Accounting for parts of the Amazon outside Brazil, the total extent of the Amazon is estimated at 8,235,430 km2 (3,179,715 square miles). By comparison, this is equivalent to the land area of the USA (including Alaska and Hawaii) which is 9,629,091 km2 (3,717,811 square miles). In total, the Amazon River drains about 6,915,000 km2 (2,722,000 square miles), or roughly 40 % of South America (Schroth et al. 2003).
Amazonian evergreen forests account for about 10 % of the world’s terrestrial primary productivity and 10 % of the carbon stores in ecosystems (Melillo et al. 1993)—of the order of 1.1 × 1011 t of carbon (Tian et al. 2000). Amazonian forests are estimated to have accumulated 0.62 ± 0.37 t of carbon ha−1 year−1 between 1975 and 1996 (Tian et al. 2000). Fires related to Amazonian deforestation have made Brazil one of the top greenhouse gas producers. Brazil produces about 300 million tons of CO2 a year; 200 million of these come from logging and burning in the Amazon. Despite this, Brazil is listed as one of the lowest per capita (rank 118) in CO2 emissions according to the US Department of Energy’s Carbon Dioxide Information Analysis Center (CDIAC).
From the story of first transfer of rubber seeds from Brazil to Asia (Dean 1987; Baulkwill 1989), it is difficult to evaluate how narrow the genetic base initially was for what has now become the “Wickham” domesticated population. Much importance was conferred to a small number of 22 seedlings disseminated from Singapore to Malaysia after 1876; but a significant part of the Wickham seedlings which germinated in Kew Gardens was then sent to Ceylon (now Sri Lanka), raised and disseminated to different countries, especially India (Fig. 6.2). However, it must be underlined that the original Wickham stock was collected in only one Brazilian site, Boïm, on the Western banks of the Tapajoz River, not far from Santarem. All these contentions are debatable (Thomas 2001). Directional selection applied to such populations for more than a century, and the limitation of the low fruit-set in Hevea probably further contributed to reduce the genetic base. Genetic diversity can now be compared to that of the available wild Amazonian populations by use of molecular genetic markers (MGMs).
The “Wickham” population developed in Asia and issued from the collection of seeds in Brazil by Wickham in 1876, has been the basis for rubber domestication and was reputed to have a narrow genetic base.
This justified the organization of other collections and transfers of wild germplasm from Amazonia to the main rubber producing countries, mainly for H. brasiliensis but also for allied species. Moreover, Ford and Firestone in Latin America, as well as Brazilian research contributed to create a stock of selected “Amazonian” and “Wickham × Amazonian” germplasm (F, FX, MDF, FDR, IAN, and IAC clones).
Many other introductions from Brazil to Asia and also Africa were carried out between 1876 and 1974, including some species different from H. brasiliensis (Dijkman 1951; Brookson 1956; Baptist 1961; Wycherley 1968; Hallé and Combe 1975; Nicolas 1976; Ong et al. 1983; Tan 1987; Ong and Tan 1987). All collections were quantitatively rather limited, especially for non-brasiliensis species.
Since the introduction of rubber to southeast Asian countries by Wickham and Cross in 1877 through Kew Botanic Gardens, there have been attempts to collect new material and increase the genetic diversity. Between 1945 and 1982, at least 10 collections from Brazil (mostly Rondonia) were undertaken (Gonçalves et al. 1982). During 1951–1952, 1614 seedlings of five Hevea species (H. brasiliensis, H. guianensis, H. benthamiana, H. spruceana, and H. pauciflora) were introduced in Malaysia (Tan 1987). Seeds of different Hevea species were also imported from the Schultes Museum at Belem, Brazil in 1966 to Malaysia. In Sri Lanka, 11 clones of H. brasiliensis and H. benthamiana and 105 hybrid materials were imported during 1957–1959, through collaboration of USDA, IAN (Brazil), and Liberia. Many of these clones were later given to Malaysia which were used for further breeding programs at RRIM (Tan 1987). It is very evident that Hevea rubber was evolved because of evolution spanning over thousands of years. Today, the cultivation of Hevea rubber spans several continents and new environments that are both ideal and with constraints (Priyadarshan 2003) (see Table 6.1).
6.2.1 IRRDB Explorations
With the initiatives taken up by the International Rubber Research and Development Board (IRRDB), 64,734 seeds, 1413 m of budwood from 194 high-yielding trees and 1160 seedlings were collected during 1981 from Acre, Rondonia, and Mato Grosso states of Brazil, from 60 different locations spread to 16 districts (Nicolas 1981; Nouy 1982; Tan 1987; Simmonds 1989). Of this, 37.5 % of the seeds went to Malaysia and 12.5 % to Côte d’Ivoire while half of the collections were retained in Brazil. The clonal selections were brought to Malaysia and Côte d’Ivoire after quarantine measures (of one year in Guadalupe Island) for South American Leaf Blight (SALB—Microcyclus ulei). IRRDB supports germplasm centers based in Malaysia and Côte d’Ivoire to conserve these materials. After the establishment of two IRRDB Germplasm Centres in Malaysia and Côte d’Ivoire, other IRRDB member countries were supplied with budwood from this material according to their request. Malaysia alone established 8900 seedlings and 109 clones from this exploration (Pushparajah 2001). Crosses between Wickham and Amazonian accessions could introduce more variation. Breeding at Institut de Recherches sur le Caoutchouc en Afrique (IRCA), Côte d’Ivoire, under the auspices of CIRAD, involve utilization of Amazonian accessions (Clement-Demange et al. 1998).
The field evaluation of this wild Amazonian germplasm showed that the latex yield was as low as about 10 % of GT1, one of the most cultivated clones. Attempts to improve it through Wickham × Amazonian crosses resulted in recombinants with a still low yield, ranging between 30 and 50 % of the level of GT1, probably due to the important genetic gap lying between the two populations. Conversely, a wide variability was found within these crosses for growth, enabling the selection of very vigorous Wickham × Amazonian clones. A clear difference in branching habit could be observed between accessions from Acre and Rondonia, which more often have tall trunks with poor branching located at high height, and those from Mato Grosso, which display abundant branching at low height. Obviously, this wild Amazonian germplasm is bearing an important genetic burden in terms of unfavorable alleles. From the evaluation of the IRRDB 1981 germplasm in Côte d’Ivoire, a working population of 287 accessions was selected, taking into account genetic diversity but mainly based on yield; the average yield level of this population is estimated at 36 % of the level of GT1 (Nicolas et al. 1988; Clément-Demange et al. 1998). Four genetic groups of this population could be the base of a population pre-breeding work aimed at improving their yield level before testing them by crossing with the Wickham population.
In 1995, an expedition was launched by RRIM to collect rubber seeds from Brazil. From this collection, about 50,231 seedlings were planted in Malaysia, including allied species (RRIM Annual Report 1997; MRB Annual report 1999). In order to enlarge genetic variability of Hevea, some research was carried out on mutation breeding (Ong and Subramaniam 1973; Markose et al. 1977), and on polyploidization of the 2n = 36 H. brasiliensis species’ (Mendes and Mendes 1963; Shepherd 1969; Zheng et al. 1980, 1981). An artificial triploid has been produced by crossing a diploid and a tetraploid (Saraswathyamma et al. 1988). Naturally occurring triploids have been reported (Nazeer and Saraswathyamma 1987). The existence of some putative genetically dwarf or semi-dwarf genotypes was mentioned (Ong et al. 1983); H. camargoana would have a dwarf growth habit (Gonçalves et al. 1982). It was attempted to associate some MGMs with the dwarfing trait (Venkatachalam et al. 2004).
6.3 Hevea as a Species Complex
The genus Hevea is basically comprised of 10 species: H. brasiliensis, H. guianensis, H. benthamiana, H. pauciflora, H. spruceana, H. microphylla, H. rigidifolia, H. nitida, H. camporum, and H. camargoana (Webster and Paardekooper 1989; Wycherley 1992; Schultes 1990). All species originated in Brazil. Seven species are found in the upper Rio Negro region, considered to be the centre of origin of the genus. H. brasiliensis is found in Southern areas outside of this centre, in the upper Rio Madeira, where five other species are represented. It has generally been assumed that the species are freely inter-compatible (Seibert 1947; Baldwin 1947). Pires (1981) observed natural hybrids of H. camargoana × H. brasiliensis, and Gonçalves et al. (1982) analyzed progenies issued from hand pollination from this type of crossing. Consequently, Hevea species might be considered as a species complex, due to the absence of a strict barrier to recombination between species. Many efforts led to the identification of certain types which were formerly presented as other possible species. H. paludosa was identified in Brazil by Ule in 1905 and is often considered as an eleventh species (Gonçalves et al. 1990; Priyadarshan and Gonçalves 2003). An elaborate description of taxonomical and botanical aspects of Hevea has been reviewed by Schultes (1977, 1987, 1990) and Wycherley (1992).
All Hevea species have 2n = 36 chromosomes, with the exception of one triploid clone of H. guianensis (2n = 54) and the existence of one genotype of H. pauciflora with 2n = 18 (Baldwin 1947; Majumder 1964). Although Hevea behaves as a diploid, it is believed to be an amphidiploid (2n = 36; x = 9) that stabilized during the course of evolution. This contention is supported by the observance of tetravalents during meiosis (Raemer 1935; Ong 1975; Wycherley 1976). In situ hybridization studies revealed two distinct 18S-25S rDNA loci and one 5S rDNA locus, suggesting a possible allotetraploid origin with the loss of 5S rDNA during the course of evolution (Leitch et al. 1998). But locus duplications are infrequent in Hevea genome, and they could have occurred due to chromosomal modifications posterior to the polyploidization event (Seguin et al. 2003); consequently, the two unknown ancestral genomes of Hevea would have strongly diverged (Priyadarshan and Clément-Demange 2004; Priyadarshanet al. 2008).
The species are inter-crossable (Clement-Demange et al. 2000). Schultes (1977) and Wycherley (1992) refer the readers to excellent reviews on this subject. The taxonomic considerations from 1874 to 1970 delineated the genus with several species at different occasions. Though even 24 species were considered during 1906, the species concept crystallized with nine species in 1970 (Schultes 1977). A tenth species, H. camargoana was added during 1971 (Schultes 1987). Brazil considers 11 species including H. paludosa (Pires 1973; Goncalves et al. 1990). Three botanists shall be considered principal workers on species delineation—Baldwin, Seibert, and Schultes—who during their classical exploratory studies contributed significantly toward the botany of Hevea. A Harvard University Gazette Archives says “Schultes’ field work, conducted mostly in the Colombian Amazon beginning in 1941, made him a leading voice in the field and one of the first in the 1960s to warn about destruction of the rainforests and disappearance of their native people” (see www.harvard.edu).
A summary of the salient features of different species of Hevea is presented in Table 6.2. All species have 36 chromosomes (2n = 36 x = 9). H. brasiliensis behaves as an amphidiploid (Ong 1979). However, this contention is disputed at the molecular level. In situ hybridization studies revealed two distinct 18S-25S rDNA loci and one 5S rDNA locus (Leitch et al. 1998), suggesting a possible allotetraploid origin with the loss of 5S rDNA during the course of evolution. Hence, as long as a potential ancestor with 2n = 18 is unknown, rubber tree will be considered as an amphidiploid. The genus Hevea could eventually be considered as a species complex.
6.3.1 Distribution of Allied Species
The distribution of allied species of Hevea is wide among the countries of South America (Fig. 6.3a, b). Hevea species are indigenous to Bolivia, Brazil, Colombia, French Guiana, Guayana, Peru, Surinam, and Venezuela. All species occur in Brazil, the center of origin. Four species have been found in Colombia and three occur in Venezuela. Two occur in Bolivia and French and British Guyanas. H. guianensis is the most widely adapted species (Fig. 6.3a) (Priyadarshan and Goncalves 2003). These species of Hevea were evolved in Amazonian forests over 100 thousand years ago (Clement-Demange et al. 2000). It is pertinent that species adaptation to a particular area is as per climatic and edaphic requirements. Species like H. camporum, H. paludosa and H. rigidifolia shows only limited adaptation. The specific adaptation needs to be closely studied, with reference to climatic and edaphic factors, when clones are to be developed for new environments especially for marginal areas. It is worthwhile to note that except H. benthamiana (F 4512, F 4542), none of the other species has been actively utilized for the improvement of rubber tree.
6.4 Molecular Diversity and Genomics
The association between DNA sequence variation and heritable attributes has helped to define variations in plants at the molecular level. However, identification and utilization of recombinants with desirable traits is time consuming and laborious in rubber due to long generation time and larger size of the crop. With the advent of DNA markers, localization of desirable traits has become routine. The molecular marker systems can be broadly classified into three viz., first generation (RFLPs, RAPDs and modifications); second generation (simple sequence repeats—SSRs, Amplified Length Polymorphism—AFLPs) and third generation markers (Expressed Sequence Tags—ESTs, Single Nucleotide Polymorphism—SNPs) (Gupta et al. 2001). Of these, SNPs are the new generation markers used for Marker-Assisted Selection (MAS). All marker systems, except SNPs have been applied in Hevea to facilitate identification and characterization of genes (Saha and Priyadarshan 2012). Recently, a saturated linkage map of H. brasiliensis has been accomplished (Lespinasse et al. 2000a). Efforts were on for breeding Hevea at the molecular level ever since Low and Bonner (1985) characterized nuclear genomes containing 48 % of most slowly annealing DNA (putative single copy) and 32 % middle repetitive sequences with remaining highly repetitive or palindromic ones. Also, the whole genome size was calculated as 6 × 108 base pairs.
Low and Bonner (1985) characterized Hevea nuclear genome as containing 48 % of slowly annealing DNA (putative single copy) and 32 % middle repetitive sequences with remaining highly repetitive or palindromic DNA. The whole nuclear genome size was first estimated as 6 × 108 base pairs. Estimation with flux cytometry demonstrated 1.9 × 109 base pairs for H. brasiliensis, H. benthamiana, H. guianensis, H. pauciflora, and H. spruceana (Seguin et al. 2003). The evolution of cytoplasmic genome was slower, due to the lack of genetic recombination through meiosis. The estimated mean molecular size of chloroplast DNA (ct DNA) is 152 kb (Fong et al. 1994). Differentiation of the genus into species appears to be linked with the evolution of the Amazonian forest over the last one hundred thousand years. Alternations of humid and semi-arid periods responsible for the forest extension or fragmentation resulted in the formation of forest islets. These are assumed to have become zones of protection and differentiation under local selection pressures.
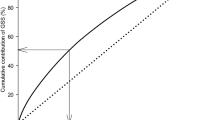
Seguin et al. (2003) proposed a general organization of H. brasiliensis germplasm with 6 genetic groups: group 1 made up with the two districts AC/T (Tarauaca) and AC/F (Feijo) in the western part of Acre, and with the Calima component of the Schultes collection; group 2 made up with the three districts AC/B (Brasileia), AC/S (Sena Madureira), and AC/X (Xapuri) in the eastern part of Acre; group 3 made up with the six following districts of Rondonia: RO/A (Ariquemenes), RO/C (Calama), RO/CM (Costa Marques), RO/J (Jaru), RO/JP (Jiparana), RO/OP (Ouro Preto), the district MT/VB (Vila Bella) of Mato Grosso, and accessions Madre de Dios Firestone (MDF) from the Firestone collection in Peru; group 4 made up with three districts MT/A (Aracatuba), MT/C (Juruena), and MT/IT (Itauba) of Mato Grosso, and the district RO/PB (Pimenta Bueno) of Rondonia; group 5 made up with the Palmira component of the Schultes collection; and group 6 made up with the domesticated Wickham population (Fig. 6.4). Even if no prediction can be made about the progenies of crosses between these groups, they can be used as a base for managing the genetic variability in the long term and organizing the recombination process. Methodological researches have been carried out in order to select the genotypes for making up a collection of reduced size of the Amazonian germplasm, representative of the predominant part of the total variability of this germplasm, according to the concept of “core collection” (Hamon et al. 1998). The germplasm characterization and diversity analysis studies coordinated by CIRAD were funded by the European Union from 1985 to 1997. On the contrary, Lekawipat et al. (2003) used 12 microsatellite markers to detect DNA polymorphism among 108 accessions of H. brasiliensis including 40 Wickham clones and 68 wild accessions (1981 Amazonian accessions). Genetic similarity values between genotypes calculated from all the microsatellite markers were used to produce a dendrogram of the relationship among accessions, using the unweighted pair-groups method with arithmetic average. A total of 170 alleles were detected. The number of alleles ranged from 5 to 21, with an average of 14 alleles per marker. The results clearly demonstrated that wild accessions are more polymorphic than cultivated Wickham clones and could be divided into three clusters, depending on the geographical origin of collection areas such as Acre, Rondonia, and Mato Grosso state. Despite the narrow genetic basis of Wickham clones, their high level of polymorphism could be detected.
Molecular genetic grouping of Amazonian accessions (after Besse et al. 1994)
6.4.1 Gene Flow and Paternity Identification
Pre-breeding of the Amazonian genetic groups was considered based on recombination through seed gardens. For methodological purposes, one seed garden made up with 50 Amazonian genotypes and GT1 clone, planted at CNRA (Côte d’Ivoire) was subjected to the analysis of gene flux and paternity identification with isozymes and microsatellites (Blanc et al. 2001; Lidah 2005). Paternity identification with microsatellites was carried out with the Cervus software (Marshall et al. 1998). A high level of confidence was found for paternity identification carried out with eight microsatellite markers. The distribution of the contribution of the different genotypes to pollination was found highly unequal, with four genotypes accounting for 40 %, 14 genotypes accounting for 80 %, and 25 genotypes accounting for 95 % of the total fertilization of the seed garden. The variation of selfing rate was assessed among the genotypes with an average of 5 %, and no selfing was found on GT1 as expected for a male-sterile clone. The isolation of the seed garden was confirmed since no allele other than those belonging to the parental population was found. The efficiency in paternity identification which is made possible by microsatellites suggests the new possibility to exercise selection on seedlings raised from natural pollination and to identify paternity a posteriori only on the best trees. Here, male-sterile clones GT 1 and BPM 24 can be used to ensure cross-pollination.
6.4.2 Breeding Without Breeding (BwB)
The classical breeding methods used by tree breeders rely on pre-determined mating designs. El-Kassaby et al. (2006) has introduced a scheme of Breeding without Breeding (BwB) that allows the assemblage of full-sib (FS) and half-sib (HS) families from seed orchards’ naturally pollinated offspring without conducting any crosses. This scheme circumvents artificial mating, focusing instead, on a subset of randomly sampled, maternally known but paternally unknown offspring to delineate their paternal parentage. This method calls for highly informative molecular markers (e.g., SSRs), for pedigree reconstruction (El-Kassaby and Lstibůrek 2009). SSRs are now in a development stage in Hevea rubber (Garcia et al. 2011; Li et al. 2012). But this situation shall improve with time. In Hevea, well organized breeding orchards that permit pollen from only hetero-neighbors can be subjected for raising such FS and HS families. A three-dimensional hetero-neighbors’ layout, as proposed by Simmonds (1986), can be well suitable for such an exercise (Fig. 6.5). This can be used for both breeding FS offspring and for collection of polyclonal seeds. For this, large polyclonal orchards are necessary that can produce thousands of seeds every year. Alternately, a clone evaluation garden laid under completely randomized design (CRD) can also be used for collecting HS seeds. Such HS families shall be raised in closer spacing (2–3 m) that can be subjected for yield screening upon attainment of 50 cm girth. A mistake usually being committed by the breeders is to select the early yielding genotypes and reject the ones that are yet to be tapped. This exercise has indirectly culminated in the selection of clones with faster girth increment that reduces gestation period. However, a point to be remembered here is that the left over set may contain high-yielding recombinants, which may attain maturity a little late. While exercising clone selection, both early yielding and late yielding clones are a necessity, to present before the planters an array of clones with vivid attributes to choose from. If SSRs that are linked to QTLs for high yield can be used, then, the exercise can minimize screening process to a great extent. In this way, this method allows the capture of 75–85 % of the genetic response to selection attained through conventional programs without the need to do any controlled pollination or possibly no experimental field testing: both considered to be the most resource-demanding activities in breeding programs. The selections borne out of these HS evaluations can be further confirmed through clonal nursery trials following line RBD with a reference clone. Simultaneously, these selections can be propagated and given for block trials in government owned areas with a reference clone. In this way, a quick derivation of clone can be achieved. For all this, DNA profiles of all available clones is a prerequisite to ascertain the parentage.
A three dimensional hetero-neighbours’ layout for breeding orchard (after Simmonds 1986)
6.4.3 Genetic Mapping
The availability of numerous MGMs (Molecular Genetic Markers) led to the development of genetic linkage mapping based on the analysis of the percentage of crossing-over between the loci of two markers during meiosis (a genetic and not a physical distance), and the ranking of the different loci on the different chromosomes of one species. Due to the heterozygotic nature of rubber clones, the construction of genetic linkage maps in Hevea requires specific methodology. Unlike annual crops, a cross between two heterozygous parents in Hevea can yield information up to four alleles, which are segregated further. The first comprehensive genetic linkage map of H. brasiliensis has been built recently, mainly by use of RFLP markers but also AFLPs, microsatellites and isozymes (Lespinasse et al. 2000a). This was accomplished through a double pseudo-test cross as per the methodology of Grattapaglia and Sederoff (1994) and a map was constituted separately for each parent. Further, homologous markers segregating in both parents were ascertained and consensus map prepared. The parents used were PB260 (PB5/51 PB49) and RO38 (F4542 AVROS363). F4542 is a clone of Hevea benthamiana species. The F1 synthetic map of 717 markers was distributed in 18 linkage groups (LG) corresponding to the 18 chromosomes. This comprised of 301 RFLP, 388 AFLP, 18 microsatellite and 10 isozyme markers. The genetic length of the 18 chromosomes was fairly homogeneous, with an average map length per chromosome of 120 cm. Many AFLP markers were seen in clusters, which were attributed as reduced recombination frequency regions. Though the RFLP markers were well distributed all over the 18 LG, these were insufficient to saturate the map. AFLPs and a few microsatellites together contributed to saturating the map. A partially nonrandom arrangement of duplicate loci observed in RFLP profiles indicate that they have homology descending from a common ancestor (Lespinasse et al. 2000a). The origin of such duplications is still unknown and H. brasiliensis continues to behave as a diploid.
In yet another study, Souza et al. (2011) found 603 microsatellite markers, with 309 of them (51 %) showing polymorphism. Chi-square test was carried out on the genotyping polymorphic loci showed that 110 loci followed a segregation ratio of 1:1, 28 followed a ratio of 1:2:1 and 87 (38.7 %) followed a ratio of 1:1:1:1. The map consists of 225 markers, distributed in 23 LG and 2471.2 cm in length with an average genetic distance of 11 cm between adjacent markers. The largest group has 215.9 cm (18 markers) and the smallest has 2.71 cm (2 markers). This reflects a real polymorphism in a FS cross.
Genetic linkage maps associated to phenotyping studies (field evaluation of the genotypes) can generate phenotypic comparisons between a huge number of classes of alleles and lead to the identification of QTLs. The research developed on the cross PB260 × RO38 was targeted to understanding the genetic determinism of the resistance of this cross to SALB, first with manual infection at the laboratory level (Lespinasse et al. 2000b). Eight QTLs, with one predominant on linkage group g13, were identified for resistance in RO38 map through Kruskel-Wallis marker-by-marker test and interval mapping method (Lander and Botstein 1989; Oojen van et al. 1992). The F1 consensus map confirmed results obtained in parental maps. It was further rationalized that the resistance alleles of RO 38 have inherited from its wild grandparent (H. benthamiana) and no favorable allele came from AVROS 363, the Wickham parent. Eight different QTLs for five strains of fungus were found available in RO38, with specificity of resistance to different strains. Field evaluation against the pool of Microcyclus strains available in French Guyana was carried out under the real infestation conditions, and it confirmed the presence of the predominant QTL in g13 previously found under controlled infestation (Le Guen et al. 2003). Then it was shown that this major QTL was no more efficient against two widely virulent and highly aggressive strains; for one of them, another QTL located on the linkage group g12 was able to reduce the aggressiveness. This genetic mapping and QTL approach is currently being continued with other crosses for analyzing the genetic determinism to different sources of South American Leaf Blight (SALB) resistance. Research for identifying and cloning the real genes responsible for this QTL in linkage group g13 is undertaken at CIRAD in the framework of the building of a bacterial artificial chromosome (BAC) bank and of a physical map of the rubber tree genome based on the clone RO38 that inherited the resistance trait from F4542. Among other applications, this will make possible the search for the DNA fragments bearing the QTL g13 and the development of the “chromosome walking” technique towards genes associated with QTL g13 on these fragments. This physical map with a high density of MGMs (fine mapping) will also allow one to assess the stability of linkage between the neighboring genetic markers.
Mantello et al. (2012) studied new genomic microsatellite markers developed and characterized in H. brasiliensis and evaluated their transferability to other Hevea species. They constructed di- and trinucleotide-enriched libraries. From these two libraries, 153 primer pairs were designed and initially evaluated using nine genotypes of H. brasiliensis. A total of 119 primer pairs had a good amplification product, 90 of which were polymorphic. A total of 46 polymorphic markers were characterized in 36 genotypes of H. brasiliensis. The expected and observed heterozygosities ranged from 0.1387 to 0.8629 and 0.0909 to 0.9167, respectively. The polymorphism information content (PIC) values ranged from 0.097 to 0.8339, and the mean number of alleles was 6.4 (2–17). The microsatellites were also tested in six other Hevea species. The percentage of transferability ranged from 82 to 87 %. Locus duplication was found in H. brasiliensis and also in five of other species in which transferability was tested. Six other species from the genus Hevea (H. guianensis, H. rigidifolia, H. nitida, H. pauciflora, H. benthamiana and H. camargoana) being two different genotypes of H. pauciflora, were used to evaluate the transferability of the markers. All loci were tested under the same PCR conditions used for H. brasiliensis. Of the 46 loci tested, 40 (87 %) were amplified for H. guianensis and H. pauciflora—(112CNSG), 39 (85 %) were amplified for H. camargoana, H. nitida and H. pauciflora—(116CNSG), and 38 (82 %) were amplified for H. benthamiana. This high percentage of transferability may be useful in the evaluations of genetic variability and to monitor introgression of genetic variability from different Hevea species into breeding programs.
6.4.4 Expressed Genes in Hevea
Lekawipat (2004) performed a genetic diversity analysis of H. brasiliensis germplasm over 66 Amazonian and 40 Wickham accessions, by use of non-expressed MGMs (12 microsatellites) and also 17 markers of expressed genes (Single Strand Confirmation Polymorphism—SSCP, based on PCR and the secondary conformation structure of single strand DNA on non-denaturing acrylamide gel, aimed at mutation detection in expressed genes). It was found that microsatellites could detect higher polymorphism than gene specific primers of SSCP in rubber accessions. SSCP markers could not differentiate the Wickham and the Mato Grosso accessions.
In reproductive biology, rubber flower and inflorescence development have been characterized; one important gene regulating flower induction and development (leafy/floricaula) was cloned and its expression was analyzed and localized by in situ hybridization (Dornelas and Rodriguez 2005). In post-germination changes in rubber seeds, proteomics (2D-Page and mass spectrometry methods) were implemented for examining the changes in protein expression from the mature seed to the germinated seed (Wong and Abubakar 2005). NMR spectroscopy was used for characterizing cassiicolin, the toxin of Corynespora (Barthe et al. 2007). Suppression Substractive Hybridization (SSH) technique is currently widely implemented between different couples of mRNA samples for the production of molecular resources by Real-Time-Polymerase Chain Reactions (RT-PCR) in the form of substracted cDNA libraries. SSH is enormously useful in addressing the issues related to Tapping Panel Dryness (TPD). Although a great deal of effort has been made to study TPD in rubber tree, the molecular mechanisms underlying TPD remain poorly understood. Identification and systematic analyses of the genes associated with TPD are the prerequisites for elucidating the molecular mechanisms involved in TPD. Li et al. (2010) made an attempt to decipher the intricacies of TPD with the help of SSH. To identify the genes related to TPD in rubber tree, forward and reverse cDNA libraries from the latex of healthy and TPD trees were constructed using SSH method. Among the 1106 clones obtained from the two cDNA libraries, 822 clones showed differential expression in two libraries by reverse Northern blot analyses. Sequence analyses indicated that the 822 clones represented 237 unique genes; and most of them have not been reported to be associated with TPD in rubber tree. The expression patterns of 20 differentially expressed genes were further investigated to validate the SSH data by reverse transcription PCR (RT-PCR) and real-time PCR analysis. According to the Gene Ontology (GO) convention, 237 unique genes were classified into 10 functional groups, such as stress/defense response, protein metabolism, transcription and post-transcription, rubber biosynthesis, etc. Among the genes with known function, the genes preferentially expressed were associated with stress/defense response in the reverse library, whereas metabolism and energy in the forward one. Systematic analyses of the genes related to TPD suggest that the production and scavenging of reactive oxygen species (ROS), ubiquitin proteasome pathway, programmed cell death and rubber biosynthesis might play important roles in TPD.
MicroRNAs (miRNAs) are set of RNAs that are induced by abiotic stress and regulate gene expression by targeting the cleavage or translational inhibition of target messenger RNAs. Gébelin et al. (2013) studied sequences of miRNAs expressed in latex cells to identify TPD-related putative targets. Deep sequencing of small RNAs was carried out on latex from trees affected by TPD using Solexa technology. The most abundant small RNA class size was 21 nucleotides for TPD trees compared with 24 nucleotides in healthy trees. By combining the LeARN pipeline, data from the Plant MicroRNA database and Hevea EST sequences, 19 additional conserved and four putative species-specific miRNA families were identified that were not found in previous studies. The relative transcript abundance of the Hbpre-MIR159b gene increased with TPD. This study revealed a small RNA-specific signature of TPD-affected trees. Both RNA degradation and a shift in miRNA biogenesis are suggested to explain the general decline in small RNAs and, particularly, in miRNAs.
Collection of Expressed Sequence Tags (ESTs, or small and partial 5’-end-sequences of expressed genes) that are related with varied metabolic aspects are developed to study genes expressed in latex cells (Garcia et al. 2011; Triwitayakorn et al. 2011; Li et al. 2012; Salgado et al. 2014; Cubry et al. 2014). Entries of these banks are compared with public databases of already known genes for identifying the putative functions of the corresponding genes. These EST banks will also create the way for macro- or microarray-based studies of Hevea gene expression. The ‘Latex Lambda Triplex’ EST-cDNA library (Ko et al. 2003) published in the EMBL/GenBank databases (858 entries) showed that about 16 % of the database matched ESTs encoding rubber biosynthesis-related proteins. Rubber biosynthesis-related genes appeared to be expressed at the maximum, followed by defense-related genes and other protein-related genes (Han et al. 2000). Another EST bank was developed from a latex cDNA library in Malaysia (Chow et al. 2001), with a current number of more than 10,000 entries. Published DNA sequences of the latex allergens were matched against these ESTs, thereby indirectly providing a ranking of the allergens depending on their concentration in the latex. More than 1000 ESTs matched with the sequences of rubber elongation factor (REF, or Hev.b.1) and small rubber particle protein (SRPP, or Hev.b.3).
Genes responsible for the synthesis of rubber transferase, the key enzyme for polymerisation of polyisoprene (natural rubber), appears to be among the most abundantly expressed genes in the latex (Cornish and Xie 2012). By using sequence information from the conserved regions of cis-prenyl chain elongating enzymes that were cloned earlier, Asawatreratanakul et al. (2003) isolated and characterized cDNAs from H. brasiliensis for a functional factor participating in natural rubber biosynthesis. Sequence analysis revealed that all of the five highly conserved regions among cis-prenyl chain elongating enzymes were found in the protein sequences of the Hevea cis-prenyltransferase. Northern blot analysis indicated that the transcript(s) of the Hevea cis-prenyltransferase were expressed predominantly in the latex as compared with other Hevea tissues. Hevein, a chitin-binding protein, one of the defense proteins that play a crucial role in the protection of wound sites from fungal attack, is also involved in the coagulation process; it belongs to a multigene family, and the specificity of its expression in the latex is under investigation (Broekaert et al. 1990; Pujade-Renaud et al. 2005). Nearly 12.6 % of the proteins available in the latex are defense related (Han et al. 2000). Among 200 distinct polypeptides (Posch et al. 1997), mainly three rubber synthesis-related genes are expressed in the latex: REF (Dennis and Light 1989; Goyvaerts et al. 1991), hydroxy-3-methylglutaryl coenzyme A reductase (HMGR) (Chye et al. 1992), and SRPP (Oh et al. 1999). The most abundantly expressed gene is REF (6.1 %) and then SRPP (3.7 %) (Han et al. 2000). References and partial or full-length sequences of these cloned genes can be found in the EMBL/GenBank databases.
Unlike photosynthetic genes, transcripts involved in rubber biosynthesis are 20–100 times greater in laticifers than in leaves (Kush et al. 1990). On the other hand, transcripts for chloroplastic and cytoplasmic forms of glutamine synthetase are restricted to leaves and laticifers, respectively (Kush et al. 1990), indicating thereby that the cytoplasmic form of G. synthetase plays a decisive role in amino acid metabolism of laticifers. The transcript levels of hydrolytic enzymes viz., polygalacturonase and cellulase, might be taken as indicators for a better development of the laticifers. Genes expressed in the latex of Hevea can be divided into three groups based on the proteins they encode: (1) defense-related proteins such as hevein, chitinase, β-1,3-glucanase, and HEVER; (2) rubber biosynthesis-related proteins such as REF, HMGR (hydroxymethylglutaryl-coA reductase) and hydroxymethylglutaryl-coA synthase (HMGS), cis-prenyltransferase (CIS), geranylgeranyl diphosphate (GGPP) synthase, small rubber particle protein (SRPP), isopentenyl diphosphate (IPP) isomerase; and (3) latex allergen proteins such as Hev.b.3, Hev.b.4, Hev.b.5, Hev.b.7. Biological functions of the allergenic proteins are largely unknown (Oh et al. 1999).
Mantello et al. (2014) performed RNA sequencing (RNA-seq) of H. brasiliensis bark on the Illumina GAIIx platform, which generated 179,326,804 raw reads on the Illumina GAIIx platform. A total of 50,384 contigs that were over 400 bp in size were obtained and subjected to further analyses. A similarity search against the non-redundant (nr) protein database returned 32,018 (63 %) positive BLASTx hits. The transcriptome analysis was annotated using the clusters of orthologous groups (COG), GO, Kyoto Encyclopedia of Genes and Genomes (KEGG), and Pfam databases. A search for putative molecular marker was performed to identify SSRs and SNPs. In total, 17,927 SSRs and 404,114 SNPs were detected. Finally, we selected sequences that were identified as belonging to the mevalonate (MVA) and 2-C-methyl-D-erythritol 4-phosphate (MEP) pathways, which are involved in rubber biosynthesis, to validate the SNP markers. A total of 78 SNPs were validated in 36 genotypes of H. brasiliensis. This new dataset represents a powerful information source for rubber tree bark genes and will be an important tool for the development of microsatellites and SNP markers for use in future genetic analyses such as genetic linkage mapping, quantitative trait loci identification, investigations of linkage disequilibrium and marker-assisted selection.
6.5 Nuclear versus Cytoplasmic Genetic Diversity
Besse et al. (1994), using 92 clones of Amazonian origin and 73 Wickham clones did an assessment of RFLP profiles. Interestingly, accessions of Brazil Amazonia could be categorized into genetic groups according to their geographic origin (Acre, Rondonia, Mato Grosso). On the other hand, cultivated clones conserved relatively high level of polymorphism, despite narrow genetic base and continuous assortative mating and selection. As expected, polymorphism is very prudent among allied species of Hevea. A comparison of isozyme analysis (Lebrun and Chevallier 1990) with DNA markers showed much similarity (Besse et al. 1994). Identification of all Wickham clones could be done with 13 probes associated with restriction enzyme Eco RI (Besse et al. 1993). The cultivated clones are genetically close to the Mato Grosso genotypes. Rondonia and Mato Grosso clones are more polymorphic as per RFLP data (Besse et al. 1994). A Rondonia clone (RO/C/8/9) showed eight specific restriction fragments and a unique malate dehydrogenase (MDH) allele, indicating this clone is of interspecific origin. Such molecular markers are useful in rubber tree breeding since no distinct morphological traits exist. Mitochondrial DNA (mtDNA) polymorphism was analyzed in 345 Amazonian accessions, 50 Wickham clones and two allied species (H. benthamiana, H. pauciflora) (Luo et al. 1995). While the variation in wild accessions was considerable, the cultivated clones formed only two clusters.
6.5.1 Potentiality of mtDNA
The aforesaid observations amply indicate that the selection was indirectly towards nuclear DNA polymorphism, while evolving modern clones. Luo et al. (1995) argue that the geographic specificity towards nuclear and mtDNA polymorphisms are due to great level of genetic structuring among natural populations in the Amazon forests in relation to hydrographic network. In wild accessions, seed dispersal and selection are as per the environmental conditions. If this is true, we observe that much of the variations produced in the natural habitat are being lost due to selection pressure of environmental factors. This is a matter of concern since the wild accessions have not rendered much contribution in evolving high yielding clones so far, after introduction to other parts of the globe. On the other hand, Wickham clones exhibited high nuclear DNA polymorphism, perhaps due to breeding under different climates. It is presumable that the nuclear genome has been forced to enhance variation to suit the diverse hydrothermal situations of newly introduced areas, resulting in selection of rightly adapted clones under a given environment. mtDNA of Wickham clones has lesser variation because their female progenitors are all primary clones, naturally bred under the similar environmental conditions of Malaysia and Indonesia. These clones were introduced later into India and Sri Lanka for further breeding programmes. Moreover, cytoplasmic donors for most of the improved clones are either PB 56 or Tjir 1 (Fig. 6.6). While the cytoplasm of PB 56 is transferred through PB 5/51, the cytoplasm of Tjir 1 was through RRII 105, RRIM 600 and RRIM 605. In conventional breeding systems followed in rubber, the best parents of one generation are used as parents for the next cycle of breeding (Simmonds 1989). Obviously, this is the reason for the mtDNA profile showing only two clusters. A possible explanation for greater polymorphism in mtDNA in wild accessions is that they must have been evolved through interspecific hybridization. mtDNA polymorphism in wild accessions needs to be exploited fully. A molecular survey of available Amazon accessions and isolation of competent molecular variants in their progeny are the possible exercises that would give meaningful results.
Plant mitochondrial genomes encode tRNAs, rRNAs, proteins and ribosomal proteins and range in size from 200 Kb in Brassica hirta (Palmer and Herbon 1987) to 2.74 Mb in Cucumis melo (Rodríguez-Moreno et al. 2011). Mitochondrial genome expansion in land plants is primarily due to large intergenic regions, repeated segments, intron expansion and incorporation of foreign DNA such as plastid and nuclear DNA (Turmel et al. 2003; Bullerwell and Gray 2004). Accumulation of repetitive sequences in plant mitochondrial genomes cause frequent recombination events and dynamic genome rearrangements within a species (Chang et al. 2011; Allen et al. 2007). Several mutations by gene rearrangement of the mitochondrial genes were found associated with cytoplasmic male sterility (CMS) such as the T-urf13 gene in maize (Dewey et al. 1981), pcf gene (a fusion of atp9 and cox2 portions) in petunia Young and Hanson 1987), cox1 in rice (Wang et al. 2006) and mutations in ATPase subunits in sunflower (Laver et al. 1991) and Brassica (Landgren et al. 1996). RNA processing also plays an important role in controlling CMS as evidenced in orf355/orf77 (atp9) and T-urf13 in maize (Gallagher et al. 2002; Dill et al. 1997). With the development of next generation sequencing (NGS) technologies, new strategies have been used to obtain plant mitochondrial genomes. A combination approach of shotgun and paired-end NGS sequencing from non-enriched whole genome DNA libraries have been successfully used to obtain the mitochondrial genomes.
Clone BPM 24 exhibits CMS, inherited from the variety GT 1. Shearman et al. (2014) constructed the rubber tree mitochondrial genome of a cytoplasmic male sterile variety, BPM 24, using 454 sequencing, including 8 kb paired-end libraries, plus Illumina paired-end sequencing. They further annotated this mitochondrial genome with the aid of Illumina RNA-seq data and performed comparative analysis. Shearman et al. (2014) then compared the sequence of BPM 24 to the contigs of the published rubber tree, variety RRIM 600, and identified a rearrangement that is unique to BPM 24 resulting in a novel transcript containing a portion of atp9 (Fig. 6.7). The novel transcript is consistent with changes that cause CMS through a slight reduction to ATP production efficiency. The exhaustive nature of the search rules out alternative causes and supports previous findings of novel transcripts causing CMS.
Annotated representation of the rubber tree mitochondrial genome (after Shearman et al. 2014)
6.5.2 Potentiality of cpDNA
Chloroplast genomes are sufficiently large and complex to include structural and point mutations that are useful for evolutionary studies from intraspecific to interspecific levels (Neale et al. 1988; McCauley 1992; Graham and Olmstead 2000; Provan et al. 2001). Since the first complete chloroplast (cp) genome sequence of liverwort (Marchantia polymorpha) was reported in 1986 (Ohyama et al. 1986), more than 150 chloroplast genomes have been sequenced and characterized thus disclosing an enormous amount of evolutionary and functional information of chloroplasts. In chloroplasts, transcripts undergo a series of RNA processing steps such as intron splicing, polycistronic cleavage, and RNA editing. RNA editing is a mechanism to change genetic information at the transcript level by nucleotide insertion, deletion or conversion (Bock 2000; Knoop 2010). The chemical composition of natural rubber is cis-polyisoprene, a high-molecular weight polymer formed from sequential condensation of isopentenyl diphosphate (IDP) units catalyzed by the action of rubber transferase (Cornish 2001). IDP is also an important intermediate for biosynthesis of essential oils, abscisic acid, cytokinin, phytoalexin, sterols, chlorophyll, carotenoids and gibberellins (Chappell 1995a; McGarvey and Croteau 1995; Lichtenthaler et al. 1997; Cornish 2001). There are two IDP biosynthesis pathways: the MVA pathway which occurs in cytosol (Chappell 1995b); and the 1-deoxy-D-xylulose 5-phosphate/2-C-methyl-Derythritol 4-phosphate (MEP) pathway which occurs in plastids (Lichtenthaler 1999; Ko et al. 2003). One approach to improving rubber production in H. brasiliensis would be to engineer chloroplasts and modify metabolic flux to produce more biosynthetic intermediates. The availability of the complete chloroplast genome sequence should also facilitate the chloroplast transformation technique. The improved transformation efficiency and foreign gene expression can be achieved through utilization of endogenous flanking sequences and regulatory elements (Birch-Machin et al. 2004; Maliga 2004; Tangphatsornruang et al. 2010). Transformation of chloroplast genome offers a number of advantages over nuclear transformation including a high level of transgene expression, polycistronic transcription, lack of gene silencing or positional effect and transgene containment (Daniell et al. 2002; Maliga 2002, 2004; Bock 2007). Tangphatsornruang et al. (2011) reported the complete chloroplast genome sequence of rubber tree as being 161,191 bp in length including a pair of inverted repeats of 26,810 bp separated by a small single copy region of 18,362 bp and a large single copy region of 89,209 bp. The chloroplast genome contains 112 unique genes, 16 of which are duplicated in the inverted repeat. Of the 112 unique genes, 78 are predicted protein-coding genes, four are ribosomal RNA genes and 30 are tRNA genes. Relative to other plant chloroplast genomes, Tangphatsornruang et al. (2011) observed a unique rearrangement in the rubber tree chloroplast genome: a 30-kb inversion between the trnE(UUC)-trnS(GCU) and the trnT(GGU)-trnR(UCU). A comparison between the rubber tree chloroplast genes and cDNA sequences revealed 51 RNA editing sites in which most (48 sites) were located in 26 protein-coding genes and the other 3 sites were in introns. Phylogenetic analysis based on chloroplast genes demonstrated a close relationship between Hevea and Manihot in Euphorbiaceae.
Shotgun genome sequencing of H. brasiliensis using pyrosequencing technology revealed the complete chloroplast genome sequence (Tangphatsornruang et al. 2011). Gene content and structural organization of the rubber tree chloroplast genome is similar to that of M. esculenta, with an exception of the 30-kb fragment rearrangement. By comparing the rubber tree chloroplast genes and the cDNA sequences, the distribution and the location of RNA editing sites in the chloroplast genome could be determined (Tangphatsornruang et al. 2011). The phylogenetic relationships among angiosperms, based on ct DNA sequences including those of the rubber tree ct DNA provided a strong support for a monophyletic group of the eurosid I and demonstrated a close relationship between Hevea, Manihot, Jatropha and Populus in Malpighiales.
As a synthesis of these diversity studies, good relationships were found between the results issued from the different genetic markers. Even if the contribution of isozymes is important by itself, molecular markers provided important clarifications for the distinction of different groups. There would be no barrier to migration of Hevea genes within the Amazonian basin. However, the wideness of the area and the limited dispersion of Hevea seeds allowed the preservation of the current structure, which is assumed to have initially resulted from the fragmentation of the Amazonian forest during the pleistocene period, according to the refuge theory presented by Haffer (1982). Moreover, the Hevea germplasm genetic structure clearly appears as geographically structured in relationship with the hydrographic network of the Amazonian forest, which confirms the role of rivers and inundated zones in the transport of seeds and dissemination of the species (Besse et al. 1993; Luo et al. 1995; Seguin et al. 1996). The mtDNA of Wickham population has lesser variation since their female progenitors are restricted to a very small set of primary clones. Cytoplasm donors for most of the improved clones are either PB56 or Tjir1. Obviously, this is the reason for the mtDNA profile showing only two clusters (Priyadarshan and Gonçalves 2003). Possible explanation for greater polymorphism in mtDNA of wild accessions is that many might have been evolved through interspecific hybridization.
6.6 Conclusions and Prospects
As said earlier, genetic erosion can result from a narrow genetic base in the original collections or by practices that reduce genetic diversity. That the original 22 seedlings of Wickham collection, as it is believed till date, is the base population from which the day-to-day Hevea clones were evolved had been genetically narrow to enrich the Hevea gene pool. In addition, these populations were subjected to several rounds of controlled crossing that further narrowed the diversity. Moreover, the strategy followed by the breeders to select only the desirable genotypes and to reject the unwanted ones (without assessing the utility other than yield) is the main reason that reduces diversity. Concerted efforts to infuse the Amazonian germplasm through controlled crossings never met with enriching the diversity as desired as expected. This is because selection was, and is always been in favor of higher yield only. Preserving other genotypes/entries can not be accomplished due to space constraints unlike annual species. This drawback needs to be addressed resolutely if the diversity of Hevea rubber is to be increased. Genetic diversity not produced or preserved is equivalent to genetic diversity lost. The total number of clones is not more than hundred that are being cultivated world wide for natural rubber production.
Molecular characterization of Hevea has not been done systematically. Only molecular diversity of Amazonian accessions and a few clones had been studied to an extent. A very systematic study of all Hevea clones at molecular level is appreciable, since the wisdom of understanding differences in morphological and molecular diversity has accumulated of late. QTL mapping is yet another area that needs to be undertaken with international coordination. As mentioned in this article, much work at the molecular level had been carried out like for TPD, latex production, defense genes and alike. Only growth related traits have been attempted for QTL mapping (Souza et al. 2013). But a sincere and systematic effort to tie up other variations with QTLs for yield had not been done so far. This exercise is difficult but not impossible. This systematic exercise can only elucidate the intricacies underlying diversity of Hevea rubber. Such an exercise can lead to setting up of a molecular library for Hevea and scientists working worldwide can contribute to this molecular library. The deposition of microsatellites, SSRs and ESTs is not enough, but a library that includes genes for QTLs is most warranted. The contribution of Rahman et al. (2013) on gene sequencing of Hevea is a sincere and systematic step towards this. The attempts of Saldago et al. (2014) did transcriptome analysis in Hevea. Such investigations with modern methodologies are encouraging, since utilization of such technologies almost rarely happens in tree research. This tempo needs to be accelerated further, should there be a comprehensive gene library for Hevea rubber.
One of the early contributors to the science of plant genetic resources, Harlan (1970) remarked: ‘The varietal wealth of the plants that feed and clothe the world is slipping away before our eyes, and the human race simply cannot afford to loose it’, and he also predicted a ‘genetic wipe out of centers of diversity’ (Harlan 1975). Genetic wipe out has not really happened but the modern varieties have replaced traditional varieties or land races. One of the primary duties of a Plant Breeder is to evolve, document and manage genetic diversity. As such, there are no land races in Hevea rubber, but only modern clones. In this context, how much genetic diversity is getting conserved, cataloged and utilized and how much genetic erosion happens are the options left to one’s own wisdom.
References
Allen JO, Fauron CM, Minx P, Roark L, Oddiraju S, Lin GN, Meyer L, Sun H, Kyung Kim K, Wang C, Du F, Xu D, Gibson M, Cifrese J, Clifton SW, Newton KJ et al (2007) Comparisons among two fertile and three male-sterile mitochondrial genomes of maize. Genetics 177:1173–1192
Asawatreratanakul K, Asawatreratanakul K, Zhang Y, Wititsuwannakul D, Wititsuwannakul R, Takahashi S, Rattanapittayaporn A, Koyama T (2003) Molecular cloning, expression and characterization of cDNA encoding cis-prenyltransferases from Hevea brasiliensis: a key factor participating in natural rubber biosynthesis. Eur J Biochem 270:4671–4680
Backhaus RA (1985) Rubber formation in plants—mini review. Isr J Bot 34:283–293
Baldwin JJT (1947) Hevea: a first interpretation. A cytogenetic survey of a controversial genus, with a discussion of its implications to taxonomy and to rubber production. J Hered 38:54–64
Baptist EDC (1961) Breeding for high yield and disease resistance in Hevea. In: Proceedings of the natural rubber conference. Kuala Lumpur,1960, pp 430–445
Barthe P, Pujade-Renaud V, Breton F, Gargani D, Thai R, Roumestand C, de Lamotte F (2007) Structural analysis of cassiicolin, a host-selective protein toxin from Corynespora cassiicola. J Mol Biol 367:89–101
Baulkwill WJ (1989) The history of natural rubber production. In: Webster CC, Baulkwill WJ (eds) Rubber. Longman Scientific and Technical, Essex, pp 1–56
Besse P, Seguin M, Lebrun P, Lanaud C (1993) Ribosomal DNA variations in wild and cultivated rubber tree (Hevea brasiliensis). Genome 36:1049–1057
Besse P, Seguin M, Lebrun P, Chevallier MH, Nicolas D, Lanaud C (1994) Genetic diversity among wild and cultivated populations of Hevea brasiliensis assessed by nuclear RFLP analysis. Theor Appl Genet 88:199–207
Birch-Machin I, Newell CA, Hibberd JM, Gray JC (2004) Accumulation of rotavirus VP6 protein in chloroplasts of transplastomic tobacco is limited by protein stability. Plant Biotechnol J 2:261–270
Blanc G, Rodier-Goud M, Lidah YJ, Clément-Demange A, Seguin M (2001) Study of open pollination in Hevea using microsatellites. Plantations, recherche, développement 68–71 (ISSN 1254-7670)
Bock R (2000) Sense from nonsense: how the genetic information of chloroplasts is altered by RNA editing. Biochimie 82:549–557
Bock R (2007) Plastid biotechnology: prospects for herbicide and insect resistance, metabolic engineering and molecular farming. Curr Opin Biotechnol 18:100–106
Brazil (1971) Ministério da Indústria e Comércio. Superintendência da Borracha. O gênero Hevea, descrição das espécies e distribuição geográfica. Rio de Janeiro, Sudhevea, 1971 (Plano Nacional da Borracha, anexo 7)
Broekaert N, Lee H, Kush A, Chua NH, Raikhel N (1990) Wound induced accumulation of mRNA containing a hevein sequence in laticifer of rubber tree (Hevea brasiliensis). Proc Natl Acad Sci USA 87:7633–7637
Brookson EV (1956) Importation and development of new strains of Hevea brasiliensis by the Rubber Research Institute of Malaya. J Rubb Res Inst Malaya 14:423–448
Bullerwell CE, Gray MW (2004) Evolution of the mitochondrial genome: protist connections to animals, fungi and plants. Curr Opin Microbiol 7:528–534
Chang S, Yang T, Du T, Huang Y, Chen J, Yan J, He J, Guan R (2011) Mitochondrial genome sequencing helps show the evolutionary mechanism of mitochondrial genome formation in Brassica. BMC Genom 12:497
Chappell J (1995a) The biochemistry and molecular biology of isoprenoid metabolism. Plant Physiol 107:1–6
Chappell J (1995b) Biochemistry and molecular biology of the isoprenoid biosynthetic pathway in plants. Annu Rev Plant Physiol Plant Mol Biol 46:521–547
Chow KS, Sunderasan E, Tan SH, Harikrishna K, Yeang HY (2001) Analysis of latex expressed sequence tags (ESTs) in Hevea brasiliensis. In: Sainte-Beuve J (ed) Annual IRRDB meeting. CIRAD, Montpellier
Chye ML, Tan CT, Chua NH (1992) Three genes encode 3-hydroxy-3-methyl glutaryl-coenzyme A reductase in Hevea brasiliensis. hmg1 and hmg3 are differentially expressed. Plant Mol Biol 19:473–484
Clément-Demange A, Legnaté H, Chapuset T, Pinard F, Seguin M (1998) Characterization and use of the IRRDB germplasm in Ivory Coast and French Guyana: status in 1997. p. 71–88. In: Cronin ME (ed) Proceedings of the IRRDB symposium natural rubber in Vietnam, 13–15 Oct 1997, vol 1. International Rubber Research and Development Board (IRRDB), Hertford
Clément-Demange A, Legnate H, Seguin M, Carron MP, Le Guen V, Chapuset T, Nicolas D (2000) Rubber tree. In: Charrier A, Jacquot M, Hamon S, Nicolas D (eds) Tropical plant breeding. Collection Reperes. CIRAD-ORSTOM, Montpellier, pp 455–480
Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, Wheeler PR, Honoré N, Garnier T, Churcher C, Harris D, Mungall K, Basham D, Brown D, Chillingworth T, Connor R, Davies RM, Devlin K, Duthoy S, Feltwell T, Fraser A, Hamlin N, Holroyd S, Hornsby T, Jagels K, Lacroix C, Maclean J, Moule S, Murphy L, Oliver K, Quail MA, Rajandream MA, Rutherford KM, Rutter S, Seeger K, Simon S, Simmonds S, Skelton J, Squares R, Squares S, Stevens K, Taylor K, Whitehead S, Woodward JR, Barrell BG (2001) Massive gene decay in the leprosy bacillus. Nature 409:1007–1011
Cornish K (2001) Similarities and differences in rubber biochemistry among plant species. Phytochemistry 57:1123–1134
Cornish K, Xie W (2012) Natural rubber biosynthesis in plants: rubber transferase. Methods Enzymol 515:63–82
Cornish K, Siler D, Grosjen O, Goodman N (1993) Fundamental similarities in rubber particle architecture and function in three evolutionarily divergent plant species. J Nat Rubb Res 8:275–285
Cubry P, Pujade-Renaud V, Garcia D, Espeout S, Leguen V, Granet F, Seguin M (2014) Development and characterization of a new set of 164 polymorphic EST-SSR markers for diversity and breeding studies in rubber tree (Hevea brasiliensis Mull Arg). Plant Breeding. 1–8
Daniell H, Khan MS, Allison L (2002) Milestones in chloroplast genetic engineering: an environmentally friendly era in biotechnology. Trends Plant Sci 7:84–91
de Gonçalves PS, Fernando DM, Rossetti AG (1982) Interspecific crosses in the genus Hevea. A preliminary progeny test of SALB resistant dwarf hybrids. Pesq Agropec Brasileira 17:775–781
de Gonçalves PS, Cardoso M, Ortolani AA (1990) Origin, variability and domestication of Hevea—a review. Pesq Agropec Brasileira 25(2):135–156
Dean W (1987) Brazil and the struggle for rubber. Cambridge University Press, Cambridge
Dennis MS, Light DR (1989) Rubber elongation factor from Hevea brasiliensis Identification, characterization and role in rubber biosynthesis. J Biol Chem 264:18608–18617
Dewey RE, Levings CS III, Timothy DH (1981) Novel recombinations in the maize mitochondrial genome produce a unique transcriptional unit in the Texas male-sterile cytoplasm. Cell 44:439–449
Dijkman MJ (1951) Hevea: thirty years of research in the Far East. University Miami Press, Coral Gables
Dill CL, Wise RP, Schnable PS (1997) Rf8 and Rf* mediate unique T-urf13-transcript accumulation, revealing a conserved motif associated with RNA processing and restoration of pollen fertility in T-cytoplasm maize. Genetics 147:1367–1379
Dornelas MC, Rodriguez APM (2005) The rubber tree (Hevea brasiliensis Muell Arg) homologue of the LEAFY/FLORICAULA gene is preferentially expressed in both male and female floral meristems. J Expt Bot 56:1965–1974
Ducke A (1941) Revisão de gênero Hevea, principalmente das espécies brasileiras. Departamento de Publicações do Estado do Amazonas, Manaus 42p
El-Kassaby YA, Lstibůrek M (2009) Breeding without breeding. Genet Res 91:111–120
El-Kassaby YA, Lstibůrek M, Liewlaksaneeyanawin C, Slavov GT, Howe GT (2006) Breeding without breeding: approach, example, and proof of concept. In: Proceedings of the IUFRO, low input breeding and genetic conservation of forest tree species, Antalya, pp 43–54
Fong CK, Lek KC, Ping CN (1994) Isolation and restriction analysis of chloroplast DNA from Hevea. J Nat Rubb Res 9:278–288
Gallagher LJ, Betz SK, Chase CD (2002) Mitochondrial RNA editing truncates a chimeric open reading frame associated with S male-sterility in maize. Curr Genet 42:179–184
Garcia D, Carels N, Koop DM, de Sousa LA, de Andrade SJ Jr, Pujade-Renaud V, Mattos CRR, Cascardo JCM (2011) EST profiling of resistant and susceptible Hevea infected by Microcyclus ulei. Physiol Mol Plant Pathol 76:126–137. doi:10.1016/j.pmpp.2011.07.006
Gébelin V, Leclercq J, Argout X, Chaidamsari T, Hu S, Tang C, Sarah G, Yang M, Montoro P (2013) The small RNA profile in latex from Hevea brasiliensis trees is affected by tapping panel dryness. Tree Physiol 33:1084–1098. doi:10.1093/treephys/tpt076
Goyvaerts E, Dennis M, Light D, Chua NH (1991) Cloning and sequencing of the cDNA encoding the Rubber Elongation Factor of Hevea brasiliensis. Plant Physiol 97:317–321
Graham SW, Olmstead RG (2000) Utility of 17 chloroplast genes for inferring the phylogeny of the basal angiosperms. Am J Bot 87:1712–1730
Grattapaglia D, Sederoff R (1994) Genetic linkage maps of Eucalyptus grandis and Eucalyptus urophylla using a pseudo-test cross mapping strategy and RAPD markers. Genetics 137:1121–1137
Gupta PK, Roy JK, Prasad M (2001) Single nucleotide polymorphism for molecular marker technology and DNA polymorphism detection with emphasis on their use in plants. Curr Sci 80:524–535
Haffer J (1982) General aspects of the refuge theory. In: Prance GT (ed) Biological diversification in the tropics. Columbia University Press, New York, pp 6–26
Hallé F, Combe CC (1975) Mission en Amazonie brésilienne pour la récolte de matériel génétique nouveau destiné à l’amélioration de l’Hevea. 17 Sept 11, Nov 1974. Rapport Interne IRCA
Hammer K, Teklu Y (2008) Plant genetic resources: selected issues from genetic erosion to genetic engineering. J Agri Rural Develop Tropics Subtropics 109:15–50
Hamon S, Dussert S, Deub M, Hamon P, Seguin JC, Glaszmann L, Grivet J, Chantereau MH, Chevallier A, Flori P, Lashermes H, Legnate Noirot M (1998) Effects of quantitative and qualitative principal component score strategies on the structure of coffee, rubber tree, rice and sorghum core collections. Gen Select Evol 30:237–258
Han KH, Shin DH, Yang J, Kim IJ, Oh SK, Chow KS (2000) Gene expresion in latex of Hevea bralisiensis. Tree Physiol 20:503–510
Harlan JR (1970) Evolution of cultivated plants. In: Frankel OH, Bennett E (eds) Genetic resources in plants—IBP handbook no 11. International Biological Programme, London, pp 19–32
Harlan JR (1975) Our vanishing genetic resources. Science 188:618–621
Ji Q, Xu X, Wang K (2013) Genetic transformation of major cereal crops. Int J Dev Biol 57:495–508
Knoop V (2010) When you can’t trust the DNA: RNA editing changes transcript sequences. Cell Mol Life Sci 68:567–586
Ko JH, Chow K, Han K (2003) Transcriptome analysis reveals novel features of the molecular events occurring in the laticifers of Hevea brasiliensis (para rubber tree). Plant Mol Biol 53:479–492
Kush AE, Goyvaerts E, Chye ML, Chua NH (1990) Laticifer specific gene expression in Hevea brasiliensis (rubber tree). Proc Natl Acad Sci USA 87:1787–1790
Lander ES, Botstein D (1989) Mapping mendelian factors underlying quantitative traits using RFLP linkage maps. Genetics 121:185–199
Landgren M, Zetterstrand M, Sundberg E, Glimelius K (1996) Alloplasmic male-sterile Brassica lines containing B. tournefortii mitochondria express an ORF 3’ of the atp6 gene and a 32 kDa protein. Plant Mol Biol 32:879–890
Laver HK, Reynolds SJ, Moneger F, Leaver CJ (1991) Mitochondrial genome organization and expression associated with cytoplasmic male sterility in sunflower (Helianthus annuus). Plant J 1:185–193
Lebrun P, Chevallier MH (1990) Starch and Polyacrylamide Gel Electrophoresis of Hevea brasiliensis: a Laboratory Manual. IRCA/CIRAD, Montpellier, France
Le Guen V, Lespinasse D, Lover G, Rodier-Goud M, Pinard F, Seguin M (2003) Molecular mapping of genes conferring field resistance to South American Leaf Blight (Microcyclus ulei) in trubber tree. Theor Appl Genet 108:160–167
Leitch AR, Lim KY, Leitch IJ, O’Neill M, Chye M, Low F (1998) Molecular cytogenetic studies in rubber Hevea brasiliensis Muell Arg (Euphorbiaceae). Genome 41:464–467
Lekawipat N (2004) Comparison of gene and non-gene specific molecular markers for evaluating genetic diversity in rubber (Hevea brasiliensis Muell. Arg). Diss., Doctor of Philosophy (Tropical Agriculture), Graduate School, Kasetsart University, Thailand
Lekawipat N, Teerawatannasuk K, Rodier-Goud M, Seguin M, Vanavichit A, Toojinda T, Tragoonrung S (2003) Genetic diversity analysis of wild germplasm and cultivated clones of Hevea brasiliensis Muell Arg by using microsatellite markers. J Rubb Res 6:36–47
Lespinasse D, Rodier-Goud M, Grivet L, Leconte A, Legnaté H, Seguin M (2000a) A saturated genetic linkage map of rubber tree (Hevea spp.) based on RFLP, AFLP, microsatellite and isozyme markers. Theor Appl Genet 100:127–138
Lespinasse D, Grivet L, Troispoux V, Rodier-Goud M, Pinard F, Seguin M (2000b) Identification of QTLs involved in the resistance to South American Leaf Blight (Microcyclus ulei) in the rubber tree. Theor Appl Genet 100:975–984
Lewinsohn TM (1991) The geographical distribution of plant latex. Chemoecology 2:64–68
Li D, Deng Z, Qin B, Liu X, Men Z (2012) De novo assembly and characterization of bark transcriptome using Illumina sequencing and development of EST-SSR markers in rubber tree (Hevea brasiliensis Muell Arg). BMC Genom 13:192. doi:10.1186/1471-2164-13-192
Lichtenthaler H (1999) The 1-deoxy-D-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 50:47–65
Lichtenthaler HK, Schwender J, Disch A, Rohmer M (1997) Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Lett 400:271–274
Lidah YJ (2005) Contribution à l’amélioration génétique de l’hévéa (Hevea brasiliensis Muell Arg) par l’étude du mode de reproduction de populations sauvages en vergers à graines. Thèse de doctorat soumise à l’Université de Cocody, Abidjan, Côte d’Ivoire
Low FC, Bonner J (1985) Characterization of the nuclear genome of Hevea brasiliensis. In: Proceedings of the international rubber conference. Kuala Lumpur, p 1–9
Luo H, Van Coppenolle B, Seguin M, Boutry M (1995) Mitochondrial DNA polymorphism and phylogenetic relationships in Hevea brasiliensis. Mol Breed 1:51–63
Majumder SK (1964) Chromosome studies of some species of Hevea. J Rubb Res Inst Malay 18:269–273
Maliga P (2002) Engineering the plastid genome of higher plants. Curr Opin Plant Biol 5:164–172
Maliga P (2004) Plastid transformation in higher plants. Annu Rev Plant Biol 55:289–313
Mantello CC, Suzuki FI, Souza LM, Gonçalves PS, Souza AP (2012) Microsatellite marker development for the rubber tree (Hevea brasiliensis): characterization and cross-amplification in wild Hevea species. BMC Res Notes 5:329. doi:10.1186/1756-0500-5-329
Mantello CC, Cardoso-Silva CB, da Silva CC, de Souza LM, Scaloppi Junior EJ, Gonçalves PS, Vicentini R, de Souza AP (2014) De Novo assembly and transcriptome analysis of the rubber tree (Hevea brasiliensis) and SNP markers development for rubber biosynthesis pathways. PLoS ONE 9(7):e102665. doi:10.1371/journal.pone.0102665
Markose VC, Panikkar AON, Annamma Y, Nair VKB (1977) Effect of gamma rays on rubber seeds, germination, seedling growth and morphology. J Rubb Res Inst Sri Lanka 54:50–64
Marshall TC, Slate J, Kruuk LEB, Pemberton JM (1998) Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol 7:639–655
McCauley DE (1992) The use of chloroplast DNA polymorphism in studies of gene flow in plants. Trends Ecol Evol 10:198–202
McGarvey DJ, Croteau R (1995) Terpenoid metabolism. Plant Cell 7:1015–1026
Melillo JM, McGuire AD, Kicklighter DW, Moore B III, Vorosmarty CJ, Schloss AL (1993) Global climate change and terrestrial net primary production. Nature 363:234–240
Mendes LOT, Mendes AJ (1963) Poliploidia artificial em seringueria (Hevea brasiliensis Muell Arg). Bragantia 22:383–392
MRB (1999) Annual Report 1999. Malaysian Rubber Board, p 27
Nazeer MA, Saraswatyamma CK (1987) Spontaneous triploidy in Hevea brasiliensis (Willd. ex. A. de. Juss) Muell Arg J Plant. Crops 15:69–71
Neale DB, Saghai-Maroof MA, Allard RW, Zhang Q, Jorgensen RA (1988) Chloroplast DNA diversity in populations of wild and cultivated barley. Genetics 120:1105–1110
Nicolas D (1976) Mission en amazonie, transfert du matériel génétique nouveau du Brésil aux relais phytosanitaires, 16 Jan 12 Fév Rapport IRCA
Nicolas D (1981) Prospection et récolte de matériel végétal Hevea dans la forêt amazonienne. Fév-Mars 1981. Rapport IRCA
Nicolas D, Chevallier M-H, Clément-Demange A (1988) Contribution to the study and evaluation of new germplasm for use in Hevea genetic improvement. In: Compte-rendu du Colloque Exploitation-Physiologie et Amélioration de l’Hevea. Colloque Hevea 88 IRRDB. Irca-Cirad, Paris, pp 335–352
Nouy B (1982) Status report on new Hevea germplasm collected from Brasil. IRRDB African Germplasm Centre. Status: Sept 1982
Oh SK, Kang HS, Shin DS, Yang J, Chow KS, Yeang HY, Wagner B, Breiteneder H, Han KH (1999) Isolation, characterization and functional analysis of a novel cDNA clone encoding a small rubber particle protein (SRPP) from Hevea brasiliensis. J Biol Chem 274:17132–17138
Ohyama K, Fukuzawa H, Kohchi T, Shirai H, Sano T, Sano S, Umesono K, Shiki Y, Takeuchi M, Chang Z, Aota S, Inokuchi S, Ozeki H (1986) Chloroplast gene organization deduced from complete sequence of liverwort Marchantia polymorpha chloroplast DNA. Nature 322:572–574
Ong SN (1975) Chromosome morphology at pachytene stage in Hevea brasiliensis: a preliminary report. In: Sripathi B (ed) Proceedings of the international rubber conference. Rubber Research Institute of Malaysia, Kuala Lumpur, pp 3–12
Ong SH (1979) Cytotaxonomic investigation of the genus Hevea. PhD thesis, University of California
Ong SH, Subramaniam S (1973) Mutation breeding in Hevea brasiliensis Muell Arg. Induced mutations in vegetatively propagated plants. IAEA, Vienna
Ong SH, Tan H (1987) Utilization of Hevea genetic resources in the RRIM. Malays Appl Biol 16(1):145–155
Ong SH, Ghani MNA, Tan AM, Tan H (1983) New Hevea germplasm: its introduction and potential. In: Proceedings of the rubber research institute of malaysia rubber planters’ conference, Kuala Lumpur. pp 1–14
Palmer JD, Herbon LA (1987) Unicircular structure of the Brassica hirta mitochondrial genome. Curr Genet 11:565–570
Pellicer J, Fa MF, Leitch IJ (2010) The largest eukaryotic genome of them all? Bot J Linn Soc 164:1–10
Pires JM (1973) Revisão do gênero Hevea: descrição da espécies e distribuição geográfica. Relatório Anual, 1972. Belém, Instituto de Pesquisa Agropecuária do Norte, 1973, pp 6–66 (Projeto de Botânica—Subprojeto revisão do gênero Hevea. Sudhevea/Dnpea (Ipean)
Pires JM (1981) Euphorbiaceae: Hevea camargoana sp. Notas de herbario I. Museu Emilio Goeldi, Belem, pp 4–8
Posch A, Chen Z, Wheeler C, Dunn MJ, Raulf-Heinsoth K, Baur X (1997) Characterization and identification of latex allergens by two-dimensional electrophoresis and protein micro-sequencing. J Allerg Chem 99:385–395
Priyadarshan PM (2003) Breeding Hevea brasiliensis for environmental constraints. Adv Agron 79:351–400 (Review Article—Academic Press)
Priyadarshan PM, Clément-Demange A (2004) Breeding Hevea rubber: formal and molecular genetics. Adv Genet 52:51–115
Priyadarshan PM, de Goncalves PS (2003) Hevea gene pool for breeding. Genet Resour Crop Evol 50:101–114
Priyadarshan PM, de Goncalves PS, Omokhafe K (2008) Rubber. In: Kole CK (ed) Genome mapping and molecular breeding in plants, vol 6. Springer, Berlin, pp 143–174
Provan J, Powell W, Hollingsworth PM (2001) Chloroplast microsatellites: new tools for studies in plant ecology and evolution. Trends Ecol Evol 16:142–147
Pujade-Renaud V, Sanier C, Cambillau L, Arokiaraj P, Jones H, Ruengsri N, Tharreau D, Chrestin H, Montoro P, Narangajavana J (2005) Molecular characterization of new members of the Hevea brasiliensis hevein multigene family and analysis of their promoter region in rice. Biochem Biophys Acta 1727:151–161
Pushparajah E (2001) Natural rubber. In: Last FT (ed) Tree crop ecosystems (Ecosytems of the world series), vol 19. Elsevier Science, Amsterdam, pp 379–407
Raemer H (1935) Cytology of Hevea. Genetics 17:193
Rahman AYA et al (2013) Draft genome sequence of the rubber tree Hevea brasiliensis. BMC Genom 14:75. http://www.biomedcentral.com/1471-2164/14/75
Rodríguez-Moreno L, González VM, Benjak A, Marti MC, Puigdomènech P, Aranda MA, Garcia-Mas J (2011) Determination of the melon chloroplast and mitochondrial genome sequences reveals that the largest reported mitochondrial genome in plants contains a significant amount of DNA having a nuclear origin. BMC Genom 12:424
RRIM (1997) Rubber Research Institute of Malaysia Annual report 1997, p 13
Saha T, Priyadarshan PM (2012) Genomics of Hevea rubber. In: Schnell RJ, Priyadarshan PM (eds) Genomics of tree crops. Springer, Berlin, pp 261–298
Salgado LR, Koop DM, Pinheiro DG, Rivallan R, Le Guen V, Nicolás MF, de Almeida LGP, Rocha VR, Magalhães M, Gerber AL, Figueira A, de Mattos Cascardo JC, Tereza A, de Vasconcelos R, Silva WA, Coutinho LL, Garcia D (2014) De novo transcriptome analysis of Hevea brasiliensis tissues by RNA-seq and screening for molecular markers. BMC Genom 15:236. doi:10.1186/1471-2164-15-236
Saraswathyamma CK, Nazeer MA, Premakumari D, Licy J, Panikkar AON (1988) Comparative cytomorphological studies on a diploid, a triploid and a tetraploid clone of Hevea brasiliensis (Willd. ex. Adr. De. Juss) Müll Arg. Ind J Nat Rubb Res 1:1–7
Schroth G, Coutinho P, Moraes VHF, Albernaz AL (2003) Rubber agroforests at the Tapajós River, Brazilian Amazonia: environmentally benign land use systems in an old forest frontier region. Agric Ecosyst Environ 97:151–165
Schroth G, Fonseca GAB, Harvey CA, Gascon C, Lasconcelos HL, Izac AN (2004) Agroforestry and biodiversity conservation in tropical landscapes. Island Press, Washington DC 575 p
Schultes RE (1945) Estudo preliminary del genero Hevea em Colombia. Revista de la Academia Colombiana de Ciências Exatas Fricas y Naturales. Bogotá 61:331–338
Schultes RE (1977) Wild Hevea: an untapped source of germpalsm. J Rubb Res Inst Sri Lanka 54:227–257
Schultes RE (1987) Studies in the genus Hevea VIII. Notes on intrageneric variants of Hevea brasiliensis (Euphorbiaceae). Econ Bot 41:125–147
Schultes RE (1990) Taxonomic nomenclature and ethnobotanic notes on Elaeis. Elaeis 2:172–187
Seguin M, Besse P, Lespinasse D, Lebrun P, Rodier-Goud M, Nicolas D (1996) Hevea molecular genetics. Plantations, Recherche, Développment 3:77–88
Seguin M, Flori A, Legnaté H, Clément-Demange A (2003) Rubber tree. In: Hamon P, Seguin M, Perrier X, Glaszmann JC (eds) Genetic diversity of cultivated tropical plants. Cirad, Ird, Collection “Repères”, pp 277–306. ISBN 2-87614-541-3
Seibert RJ (1947) A study of Hevea in republic of Peru. Ann Mo Bot Gard 34:261–352 (Saint Lows)
Shearman J, Sangsrakru D, Ruang-areerate P, Sonthirod C, Uthaipaisanwong P, Yoocha T, Poopear S, Theerawattanasuk K, Tragoonrung S, Tangphatsornruang S (2014) Assembly and analysis of a male sterile rubber tree mitochondrial genome reveals DNA rearrangement events and a novel transcript. BMC Plant Biol 14:45. doi:10.1186/1471-2229-14-45
Shepherd H (1969) Aspects of Hevea breeding and selection. Investigations undertaken on Prang Besar Estate. RRIM Planters’ Bulletin No: 104, pp 206–216
Simmonds NW (1986) Theoretical aspects of synthetic/polycross populations of rubber seedlings. J Nat Rubb Res 1:1–15
Simmonds NW (1989) Rubber breeding. In: Webster CC, Baulkwill WJ (eds) Rubber. Longman Scientific and Technical, Essex, pp 85–124
Souza LM, Mantello CC, Suzuki F, Gazaffi R, Garcia D, Le Guen V, Garcia AAF, Souza AP (2011) Development of a genetic linkage map of rubber tree (Hevea braziliensis) based on microsatellite markers. BMC Proceedings 2011, 5 (Suppl 7): P39, http://www.biomedcentral.com/1753-6561/5/S7/P39
Souza LM, Gazaffi R, Mantello CC, Silva CC, Garcia D, Le Guen V, Cardoso SEA, Garcia AAF, Souza AP (2013) QTL mapping of growth-related traits in a full-sib family of rubber tree (Hevea brasiliensis) evaluated in a sub-tropical climate. PLoS ONE 8(4):e61238. doi:10.1371/journal.pone.0061238
Steege H et al (2013) Hyperdominance in the Amazonian tree flora. Science 342:1243092. doi:10.1126/science.1243092
Sterck L, Rombauts S, Vandepoele K, Rouzé P, Van de Peer Y (2007) How many genes are there in plants (… and why are they there)? Curr Opin Plant Biol 10:199–203
Stewert WD, Watchel WL, Shipman JJ, Hanks JA (1955) Synthesis of rubber by fungi. Science 122:1271
Tan H (1987) Strategies in rubber tree breeding. In: Abbott AJ, Atkin RK (eds) Improving vegetatively propagated crops. Academic Press, London, pp 28–54
Tangphatsornruang S, Birch-Machin I, Newell CA, Gray JC (2010) The effect of different 3’ untranslated regions on the accumulation and stability of transcripts of a gfp transgene in chloroplasts of transplastomic tobacco. Plant Mol Biol 76(3–5):385–396. doi:10.1007/s11103-010-9689-1 (Epub)
Tangphatsornruang S, Sangsrakru D, Chanprasert J, Uthaipaisanwong P, Yoocha T, Jomchai N, Tragoonrung S (2011) Characterization of the complete chloroplast genome sequence of Hevea brasiliensis reveals genome rearrangement, RNA editing sites and phylogenetic relationships among angiosperms. Gene 475:104–112
Thomas KK (2001) Role of Clement Robert Markham in the introduction of Hevea rubber into the British India. The Planter 77:287–292
Tian H, Melillo JM, Kicklighter DW, Mcguire AD, Helfrich J III, Moore B III, Vörösmarty CJ (2000) Climatic and biotic controls on annual carbon storage in Amazonian ecosystems. Glob Ecol Biogeogr 9:315–335
Triwitayakorn K, Chatkulkawin P, Kanjanawattanawong S, Sraphet S, Yoocha T, Sangsrakru D, Chanprasert J, Ngamphiw C, Jomchai N, Therawattanasuk K, Tangphatsornruang S (2011) Transcriptome sequencing of Hevea brasiliensis for development of microsatellite markers and construction of a genetic linkage map. DNA Res 18:471–482
Turmel M, Otis C, Lemieux C (2003) The mitochondrial genome of Chara vulgaris: insights into the mitochondrial DNA architecture of the last common ancestor of green algae and land plants. Plant Cell 15:1888–1903
van Oojen JW, Sandbrink H, Purimahua C, Vrielink R, Verkerk R, Zabel P, Lindhout D (1992) Mapping quantitative genes involved in a trait assessed on an ordinal scale: a case study with bacterial canker in Lycopersicon peruvianum. In: Yoder JI (ed) Molecular biology of tomato. Technomic, Lancaster, pp 59–74
Venkatachalam P, Priya P, Saraswathyamma CK, Thulaseedharan A (2004) Identification, cloning and sequence analysis of a dwarf genome-specific RAPD marker in rubber tree Hevea brasiliensis Muell Arg. Plant Cell Rep 23:327–332
Wadley G, Martin A (1993) The origins of agriculture: a biological perspective and a new hypothesis. Aust Biologist 6:96–105
Wang Z, Zou Y, Li X, Zhang Q, Chen L, Wu H, Su D, Chen Y, Guo J, Luo D, Long Y, Zhong Y, Liu Y (2006) Cytoplasmic male sterility of rice with boro II cytoplasm is caused by a cytotoxic peptide and is restored by Two related PPR motif genes via distinct modes of mRNA silencing. Plant Cell Online 18:676–687
Waterston RH, Lindblad-Toh K, Birney E, Rogers J, Brent MR, Collins FS, Guigó R, Hardison RC, Haussler D, Jaffe DB, Kent WJ, Miller W, Ponting CP, Smit A, Zody MC, Lander ES (2002) Initial sequencing and comparative analysis of the mouse genome. Nature 420:520–562
Webster CL (1994) Classification of the Euphorbiaceae. Ann Missouri Bot Gard 81:3–32
Webster CC, Paardekooper EC (1989) Botany of the rubber tree. In: Webster CC, Baulkwill WJ (eds) Rubber. Longman Scientific and Technical, Essex, pp 57–84
Wong PF, Abubakar S (2005) Post-germination changes in Hevea brasiliensis seeds proteome. Plant Sci 169:303–311
Wycherley PR (1968) Introduction of Hevea to the orient. The Planter 4:1–11
Wycherley PR (1976) Rubber. In: Simmonds NW (ed) Evolution of crop plants. Longman, London, pp 77–80
Wycherley PR (1992) The genus Hevea—botanical aspects. In: Sethuraj MR, Mathew NM (eds) Natrual rubber: biology, cultivation and technology. Elsevier, Amsterdam, pp 50–66
Li D, Deng Z, Chen C, Xia, Z, Wu M, He P, Chen S (2010) Identification and characterization of genes associated with tapping panel dryness from Hevea brasiliensis latex using suppression subtractive hybridization. BMC Plant Biol 10: 140 http://www.biomedcentral.com/1471-2229/10/140
Xu Y (2010) Molecular plant breeding CABI. UK. 734 pages
Young EG, Hanson MR (1987) A fused mitochondrial gene associated with cytoplasmic male sterility is developmentally regulated. Cell 50:41–49
Zeder MA (2008) Domestication and early agriculture in the Mediterranean Basin: origins, diffusion, and impact. Proc Natl Acad Sci USA 105:11597–11604
Zheng X, Zeng X, Chen X, Yang G (1980) A further report on induction and cytological studies on polyploid mutants of Hevea. (I). Chin J Trop Crops 1:27–31
Zheng X, Zeng X, Chen X, Yang G (1981) A further report on induction and cytological studies on polyploid mutants of Hevea (II). Chin J Trop Crops 2:1–9
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Priyadarshan, P.M. (2016). Genetic Diversity and Erosion in Hevea Rubber. In: Ahuja, M., Jain, S. (eds) Genetic Diversity and Erosion in Plants. Sustainable Development and Biodiversity, vol 8. Springer, Cham. https://doi.org/10.1007/978-3-319-25954-3_6
Download citation
DOI: https://doi.org/10.1007/978-3-319-25954-3_6
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-25953-6
Online ISBN: 978-3-319-25954-3
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)