Abstract
V-ATPases are highly conserved proton pumps that are found in all eukaryotic cells. They play vital housekeeping roles in cell physiological processes by performing their classical functions in acidifying luminal compartments of a variety of endomembrane organelles. Recently, it has become evident that V-ATPases also have nonclassical roles that require their direct interaction, apart from their proton translocating function. Moreover, V-ATPases can have specialized tissue-specific functions in organisms, where V-ATPase mutations or inappropriate expression can result in pathological conditions. Because of their multi-subunit structure and numerous subunit variants, V-ATPase expression and function may be uniquely fine-tuned for specific, biologically significant roles. From an interventionist point of view, these same traits potentially make V-ATPases uniquely selectively targetable, both within an organism and among different species. Recent examples, that have at least provided proof of principle for this notion, span fields ranging from medicine to agriculture. The study of V-ATPases in the last three decades has produced thousands of publications and many dozens of review articles. The present work seeks to provide a concise overview of the more recent works on structure and function of V-ATPases, their occurrence and importance, how they are regulated, and how they might be targeted. We focus on recent primary literature, but historical papers of interest and important reviews are also cited. In the areas of targeted pharmaceutical and pesticidal intervention we present published strategies for drug discovery and also provide relevant proofs of concept for targeting V-ATPases to the benefit human health and prosperity.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Biological membranes
- Drug discovery
- Drug screening
- pH regulation
- Proton transport
- Regulation of expression
- Small-molecule inhibitors
- Species-targeted pesticides
- Therapeutic targeting
- Vacuolar-type proton pump
1 Introduction
1.1 Classical V-ATPase Function
V-ATPases are highly conserved, multi-subunit molecular motors that hydrolyze ATP to pump protons across biological membranes against a pH gradient [1]. V-ATPases are found in all eukaryotes and manifestations of their activity that involve pH regulation or proton gradient formation are thought of as their “classical” functions as proton pumps. These functions can be subcategorized as “housekeeping” or “specialized.” Without intracellular housekeeping functions of the V-ATPases, eukaryotic cells and organisms cannot survive in a native environment [2, 3]. Housekeeping functions include energizing membrane compartments to drive proton gradient (ΔH+)-coupled transporters, and maintaining the acidic luminal pH required for the functions of the Golgi, lysosomes, and endomembrane organelles involved in vesicular trafficking, endocytosis, and secretion [3–5]. V-ATPases also contribute to intracellular pH homeostasis [6–8].
Specialized functions are not required for cell survival, but are crucial to the development and ongoing health of organisms. Apart from their ubiquitous housekeeping functions, intracellular V-ATPases perform tissue-specific functions, such as driving ΔH+-coupled neurotransmitter loading of synaptic vesicles [9]. When they are localized to the plasma membrane, V-ATPases are involved in numerous tissue-specific functions involving acidification of extracellular compartments. Examples include osteoclast resorption lacunae involved in bone resorption [10, 11], luminal spaces of epididymal tubules involved in sperm maturation [10, 11], kidney tubules, where V-ATPases plays a role in systemic acid-base balance through proton secretion into the urine [12–15], and the coronary arterial endothelium, where V-ATPases maintain an acidic extracellular environment that enables lipid raft formation required for regulatory redox signaling crucial to endothelial function in the coronary circulation [16].
1.2 Nonclassical V-ATPase Function
V-ATPases are now recognized as having “nonclassical” functions that involve more than proton pumping activity. For example, V-ATPases are involved in regulation of vesicular trafficking and membrane fusion, which necessitates generation of vesicular pH gradients, but also requires the direct participation of some V-ATPase subunits [17–22]. V-ATPases have been shown to recruit cytohesin-2 in a luminal pH-sensitive manner, implying that they can act as pH sensors [22, 23]. V-ATPase-bound cytohesin-2 recruits ARF6 to the early endosomes and plasma membrane [24], where the complex may regulate endocytic vesicular trafficking, cytoskeletal organization, and cell adhesion [22]. Endosome recycling is dependent on the latter process, and it is thought that the V-ATPase undergoes conformational changes in response to luminal pH to facilitate cytohesin-2 docking [25, 26]. V-ATPase apparently can also sense the pH of secretory vesicles that it acidifies, allowing discrimination of fully loaded and partially loaded vesicles [27]; however, whether the pH gradient, or direct involvement of V-ATPase components, is the primary factor governing subsequent membrane fusion, remains controversial [17, 28]. In receptor-mediated signaling, V-ATPase is required for acidification of early endosomes for ligand dissociation and receptor recycling, or lysosomal acidification for protein degradation. For Wnt signaling, however, a direct interaction of LRP6 co-receptor with V-ATPase is also required for signal transmission, and for Notch signaling V-ATPase assembly factors play an important role [29, 30].
V-ATPase appears also to be involved in regulation of autophagy and cell growth, by engaging in amino acid- and “ragulator complex”-dependent, recruitment of the mammalian target of rapamycin cytoplasmic 1 complex, mTORC1 [31]. The mTORC1 complex essentially ensures that an adequate supply of resources is available before the cell commits to proliferation. It senses cellular energy and redox status, and amino acid supply in late endosomal/lysosomal compartments, and inhibits growth and promotes autophagy if any one of these prerequisites is inadequate, operating essentially as an anabolic/catabolic switch that is also influenced by insulin and growth hormones [32–35]. Abnormal function has been implicated in pathologies including neurodegenerative diseases, diabetes, and cancer. V-ATPase plays an important role in sensing free amino acid status, and conveys this information by recruiting mTORC1 to the lysosomal membrane through its interaction with the multicomponent ragulator complex [36, 37]. Recent evidence suggests that amino acid sensing likely also requires the involvement of lysosomal amino acid transporters [38, 39]. Interestingly, mTORC1 coordinately regulates the activity of TFEB, a transcription factor that is a master regulator of lysosome biogenesis, thereby regulating expression of a host of mTORC1-responsive genes, including V-ATPase subunit genes [32]. All of this may be independent of the classical function of V-ATPase as a proton pump [36], although this notion remains controversial [40].
1.3 Overview of V-ATPase Structure
The default discussion in this review concerns human V-ATPases or, more generally, mammalian V-ATPases, and often examples will be taken from the field of bone research, especially osteoclasts, as this is the authors’ area of expertise. Much of our understanding of V-ATPases, however, comes from work done in yeast (esp. Saccharomyces cerevisiae), insect (esp. Manduca sexta), and other nonmammalian systems; this will be noted where observations may not be generalized to mammalian V-ATPases.
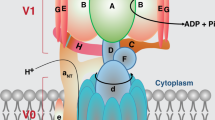
The functional V-ATPase complex, the holoenzyme, comprises two subcomplexes: a peripherally bound cytoplasmic sector, V1 (subunits A–H, empirically organized as (AB)3DF(EG)3CH), and an integral membrane sector, V0 (subunits a, c, c″, d, and e (and accessory proteins Ac45 and M8-9 in vertebrates; yeast have an additional subunit, c′), empirically organized as aed(c 5 c″)(Ac45, M8-9); aed(c 4 c′c″) in yeast) [3, 41, 42]. The mammalian V-ATPase is depicted diagrammatically in Fig. 20.1 with 27 subunits, derived from at least 15 different genes. Many of the mammalian V-ATPase subunits have multiple isoforms encoded by paralogous genes [3, 43], and their posttranscriptional variants [44, 45]. These are listed in Table 20.1. Thus, many different combinations of subunit isoforms can potentially be found within a given holoenzyme, allowing the assembly and expression of many different isoenzymes, or “isocomplexes,” of V-ATPase, which may have subtle influences on cellular organelle-specific, or tissue-specific, function or localization [3, 45–49].
Structure and function of V-ATPase. (a) Diagrammatic representation of the organization of a generic mammalian V-ATPase complex. The cytoplasmic V1 sector consists of a catalytic headpiece (three each of alternating A and B subunit pairs forming a toroidal “barrel”) that hydrolyzes ATP to drive a central rotor shaft (D and F subunits). The headpiece is held immobile against the torque that it generates by a stator complex (three pairs of E and G heterodimers attached to the Fig. 20.1 (continued) catalytic headpiece, supported by a “collar” consisting of the C and H subunits and the N-terminal domain, NTa, of the a subunit) that is anchored to the membrane via continuity with the cytoplasmic domain, CTa, of the a subunit of the V0 sector. The bifurcation of NTa, as shown here, is speculative [189]. The V0 sector is largely inserted into the membrane bilayer, consisting of the rotor (a heterohexameric ring of 5 c subunits and a c″ subunit; one of the c subunits is replaced with a c′ subunit in yeast) and a d subunit, which couples the c-ring rotor to the ATP-driven DF central rotor shaft of the V1 sector. The a subunit provides both a stator function, by interacting with the V1 sector and anchoring it to the membrane, and a proton channel function. There are some additional subunits associated with the a subunit, whose locations and functions are as yet poorly understood, viz. the e subunit, and the accessory proteins Ac45 (AP1) and M8-9 (AP2) that are found in some specialized tissues. (b) One mechanism for control of V-ATPase activity is reversible disassembly, which at the least involves dissociation of the C subunit from the V1V0 complex and possibly also conformational changes in the NTa domain that destabilize the complex (depiction here is speculative) [136]. (c) Many published works have shown that regulatory reversible disassembly results in further dissociation of V1 from V0 [130]; however, this may be an in vitro experimental artifact, though overall conclusions regarding V-ATPase regulation likely remain reliable [136]. (d) The theory of V-ATPase transmembrane proton translocation suggests that protons diffuse into a cytoplasmic hemichannel formed by the CTa domain of the a subunit to protonate a glutamate residue (blue dot with proton, “H”) on a subunit of the c-ring rotor. This is carried by ATP-driven rotation nearly 360° (clockwise as viewed from the cytoplasm, as indicated), until a luminal hemichannel is encountered where the proton can dissociate into the lumen [3]. A charged residue barrier within CTa (green sphere, “+”) is thought to prevent carryover of the proton back into the cytoplasmic hemichannel. This latter event can, however, occur under some circumstances and is referred to a “slip” [172]. The orange subunit represents the single c″ subunit of the mammalian c-ring. Modified from Ref. [189] with permission of ©The American Society for Biochemistry and Molecular Biology
1.4 Health Impact of V-ATPases: Disease-Causing Mutations of Subunits
Why V-ATPases are of interest as therapeutic targets becomes clear when one realizes the astonishing range of important functions that they perform, many of which came to light when disease causing mutations were mapped to V-ATPase subunit genes. The first such report was for B1, by Karet et al. [50]. B1 is highly expressed in the kidney, where it is involved in systemic pH homeostasis, and the inner ear, where it maintains the pH of the fluid environment of sensory hair cells. Thus, the consequences of mutations are typically distal renal tubular acidosis (dRTA) with sensorineural deafness. Recently, loss of B1 function in a mouse model has also been shown to result in impaired olfactory function [51]. The majority of disease-causing mutations of V-ATPases, however, involve the a subunit. Knockout of a1, which plays a role in neural transmission, appears to be embryonic lethal [18], but loss of function of a2 results in cutis laxa, characterized by aberrant Golgi function leading to glycosylation defects and abnormal elastin processing that affects the skin and other organs [52, 53]. Loss of function of a3 in osteoclasts results in autosomal malignant osteopetrosis, characterized by dense, brittle bone due to diminished bone resorption [54]. Loss of a4 function results in dRTA with occasional hearing loss [55, 56]. Loss of d2 function in mice results in ineffective osteoclast precursor fusion, resulting in mild osteopetrosis [57], but no equivalent mutations in human d2 have been characterized. In rare cases of X-linked Parkinsonism with spasticity (XPDS) Korvatska et al. [58] reported the causative role of a variant transcript of the M8-9 accessory protein. It displayed a high incidence of exon 4 skipping, yielding a protein with a 32 amino acid deletion and a consequent reduction in crucial V-ATPase function in autophagy in brain cells. A mutation with similar splicing consequences has also been reported that results in impaired ERK1/2 activation and resultant X-linked mental retardation with epilepsy [59].
2 Broader Disease-Related Implications
V-ATPases are even more generally implicated as potential targets in a wide variety of disease processes. Targeting the ruffled border V-ATPase in osteoclasts, for example, has been investigated as a means of controlling bone loss diseases, like osteoporosis [60–62]. Furthermore, the potential for intervention in rare cases of osteopetrosis, by targeting a protein-folding mutant a3 subunit, has also been identified [63]. Gharanei et al. [64] have shown that the Wolfram syndrome 1 protein (WFS1 ), characteristic of that neurodegenerative disorder, binds the A subunit and destabilizes it, with consequences for granular acidification. This likely is a contributing factor for Wolfram syndrome, but whether targeting this association might alleviate symptoms is presently unknown. Inappropriate expression of plasma membrane V-ATPases containing a3 or a4 subunits in tumor cells may lead to tumor progression, metastasis and chemotherapy resistance [15, 34, 65–69], but their cell surface expression makes them potential therapeutic targets, as is discussed elsewhere within this volume.
There are also numerous correlations that require further investigation: V-ATPases containing a3 isoform subunits are present in insulin-secretory granules of pancreatic β cells and appear to play a regulatory role in insulin secretion [21]. It has also been found that downregulation of H subunit expression correlates strongly with type 2 diabetes, though in what capacity remains to be determined [70, 71]. Loss of V-ATPase function in the autophagy-lysosome pathway has been implicated in aberrant metabolism of proteins that accumulate in neurodegenerative diseases , like Alzheimer’s dementia and Parkinson’s disease [72]. Loss of function of the VMA21 chaperone protein that is required for V-ATPase assembly, though it is not a part of the mature complex, also disrupts lysosomal acidification, leading to X-linked myopathy with excessive autophagy (XMEA) [73]. V-ATPase may also play a role in cardiovascular disease , possibly as an indirect consequence of excessive V-ATPase activity in osteoclasts, leading to calcification of arteries [74]. It is thought also that impaired endothelial cell plasma membrane V-ATPase function in diabetes may play a role in defective angiogenesis [75, 76].
V-ATPases of pathogenic organisms can also be of clinical importance. Parasitic nematodes , for example, place a significant burden on both human health and agriculture. It has been argued that V-ATPase, which performs many crucial functions within the Caenorhabditis elegans model parasitic organism, might serve as a useful target for controlling them [77]. Similarly, the fungal V-ATPase appears to be essential for virulence and it has been suggested that it may be an appropriate target for controlling Candida albicans and other fungal pathogens [78]. Dengue fever is transmitted by mosquitoes and 100 million people are infected annually, with half the world population at risk. A mosquito V-ATPase has been identified as a required host factor in Aedes aegypti ; its targeted inhibition could effectively control dengue virus transmission [79].
V-ATPases also play a role in viral infection . The H subunit binds the adaptor-related protein complex 2 (AP-2) μ2 chain (AP2M2) of coated endocytic vesicles and also the HIV Nef protein. Thus, the H subunit acts as a connector between HIV and the trafficking mechanism that carries endosomes to lysosomes, thereby contributing to HIV infectivity [80]. More generally, because of its involvement in viral processing, it has been suggested that targeting V-ATPase might provide an alternative means of preventing the spread of pandemic avian influenza, and a treatment modality that avoids selection for resistant strains [81].
2.1 Other Impacts
Agriculture has always been plagued by insect pests [82] and we consider in a following section ways that insect V-ATPases might be targeted to provide novel insecticides with high specificity for target species and the potential to significantly improve global agricultural yields. It is worth noting also that, globally, a considerable fraction of potentially arable land is inaccessible to high-yield agriculture due to excessive soil salt concentrations [83], and plant salt tolerance depends in part on V-ATPase expression [84]. Further understanding of this process, and engineering ways of exploiting the V-ATPase-dependent ion and osmotic stress response could improve agricultural yields, a growing concern as human population continues to expand.
3 Factors Affecting V-ATPase Activity
Expression is normally thought of as being under the control of promoters and transcription factors, which account for differential tissue distribution and assembly of V-ATPase isocomplexes with “customized” subunit isoform composition. However, differential sorting and trafficking , which determine subcellular localization, are also crucial to expression, as the V-ATPases have a uniquely diverse range of functions in various organelles. Additionally, modulation of function is an important acute form of regulation of in situ V-ATPase activity, in immediate response to various stimuli. Understanding V-ATPase regulation is a prerequisite for finding therapeutic solutions to diseases that involve a particular V-ATPase isocomplex , which will invariably be found in a background of vital housekeeping isocomplexes. The following is a brief survey of V-ATPase regulation, and there are many recent reviews of broader scope [3, 26, 45, 46, 85, 86].
3.1 Regulation of Transcription
Differential expression of V-ATPase isocomplexes is driven by the regulation of transcription of subunit isoform genes. Some V-ATPase isoforms, however, are ubiquitously expressed. For example, though the B2 subunit is one of the subunit isoforms characteristic of the osteoclast ruffled-border V-ATPase, it is widely expressed and its promoter contains a TATA-less, GC-rich regulatory region containing “CpG islands,” with multiple Sp1 and AP-2-like binding sites [87, 88]. Similar promoter regions are seen for the C1, c″, and c subunits, where also Oct1 motifs are present [89, 90]. CpG islands are common in promoter regions of ubiquitously expressed genes, and genes expressed in early embryogenesis, though otherwise they are infrequent [91–93]. Thus, expression of ubiquitous V-ATPase subunits is regulated largely through such promoter regions [45, 94], and cytosine methylation in CpG islands may allow further epigenetic fine-tuning of expression [95].
There are examples also of regulation of tissue-restricted, specialized V-ATPase expression. In renal intercalated cells, the plasma membrane V-ATPase requires the B1 subunit isoform, whereas in osteoclasts it is the B2 isoform of the V1 B subunit; these are paired with a4, or a3, respectively, of the V0 a subunit. B1 expression is largely restricted to a family of forkhead-related epithelial (FORE) cells that occur in the kidney, epididymis and inner ear, where not only B1, but also E2 and a4 subunits are under control of the forkhead box (FOX) transcription activator FOXI1, which acts as a master regulator of specialized plasma membrane V-ATPase expression in FORE cells [95–97]. It has been noted that some existing drugs may modulate expression of other FOX proteins [98]; whether FORE cell V-ATPase expression might be amenable to similar therapeutic manipulation is as yet unclear.
In osteoclasts, a3 expression is under control of an NF-κB-induced transcription factor complex containing NFATC1, the master regulator of osteoclast differentiation [99, 100]. The a3 subunit gene bears a RANKL-responsive NFATC1 promoter 1.6 kb upstream of the start codon. Basal transcription is downregulated by poly(ADP-ribose) polymerase-1 (PARP-1) binding to the promoter. RANKL stimulation results in PARP-1 degradation, causing upregulation of a3 transcription [101]. A second PARP-1 site is a few hundred bases downstream, adjacent to an AP-1 site [102].
The d2 isoform, which is part of the osteoclast ruffled border V-ATPase is also upregulated in osteoclasts, through the NFATC1 promoter [103] and co-activation by myocyte enhancer factor 2 (MEF2) and microphthalmia-associated transcription factor (MITF) [104]. RANKL-induced osteoclast differentiation, through NFATC1, turns on not just specific V-ATPase subunit isoform genes, but also a host of ancillary genes required for bone resorption, to express proteins like CLC7, the chloride counterion shunt without which V-ATPase could not effectively pump protons, and proteases like MMP9 and cathepsin K, required for degradation of the organic component of bone [100].
3.2 Messenger RNA Stability
Lee et al. [87] observed that in human macrophage/monocyte differentiation, where V-ATPase expression is upregulated, only B2 transcription is elevated. It was suggested that other subunits must be upregulated by post-transcriptional mechanisms. Furthermore, in kidney, transcript ratios do not equal their corresponding protein ratios for V-ATPase subunits [105], yet in osteoclasts the ratios are equal [106], suggesting that regulation in kidney must also involve mRNA stability, translation rates, or protein turnover rates. In a similar vein, Wang et al. [89] showed that promoter activity was similar for c subunit mRNA transcription in murine macrophage and fibroblast cell lines, despite a six- to eightfold difference in expression. In the high-expression macrophages, stability of mRNA was shown to be higher for B2, E1, F, a1, and c transcripts. Jeyaraj et al. [107] later showed that stability was determined by an AU-rich element (ARE), a common regulator of mammalian mRNA stability, near the 3′-UTR polyadenylation site [108]. AREs tend to be destabilizing, by involvement of microRNA binding; in contrast, HuR binding promotes transcript stability and translation efficiency [109, 110]. HuR has been shown, along with a second regulatory protein, hnRNP, to bind E1, G1, c, and c″ mRNA [107, 111, 112]. Though regulation of mRNA stability may play an important role in V-ATPase isocomplex expression and subunit selection, a more complete understanding will require further investigation.
3.3 MicroRNA Regulation
MicroRNAs are conserved, short-hairpin RNAs that can bind mRNA targets and repress their expression, either by directly causing their cleavage, destabilizing them by shortening their polyA tail, or interfering with translation [113, 114]. It has been shown by Stark et al. [115] that the muscle microRNA, miR-1, binds human A, B2, C1, a1, and c subunit transcripts, and also D. melanogaster and C. elegans homologues of E, G, and d, possibly regulating the coordinated expression of ubiquitous subunits. In a specific example of relevance to human health, O’Connor et al. [116] showed that catestatin processing from the prohormone chromogranin A was variable, depending on a sequence polymorphism, T+3246C, residing in the 3′-UTR of the a1 transcript. This C variant resulted in lowered plasma catestatin levels, leading to lower blood pressure and a reduced risk of hypertensive disease. This was later found to be due to regulation by the miR-637 microRNA, which preferentially binds the C variant a1 mRNA, inhibiting its translation [117]. This causes a reduction in vacuolar V-ATPase activity, an increase in luminal pH, and a consequent decrease in chromogranin A processing to catestatin [116]. Although there is a great deal more to be learned about V-ATPase regulation by microRNAs, it seems evident that engineered microRNAs may have a future in therapeutic targeting of specific V-ATPase isocomplexes.
3.4 Splice Variants
The majority of V-ATPase subunit splice variant transcripts inferred in Table 20.1 remain uncharacterized, but their potential importance is highlighted by some examples: Poëa-Guyon et al. [118] characterized rat brain expression of subunit a2 and four splice variants of subunit a1, a1-I (C variant), a1-II (N variant), a1-III (canonical), and a1-IV (N+C variant). The mRNA splice variants result in a seven amino acid insertion, peptide N, between the translated exons 4 and 5 (in the cytoplasmic NTa domain shown in Fig. 20.1a) and/or a six amino acid insertion, peptide C, between exons 17 and 18 (in the cytoplasmic loop between transmembrane helices 6 and 7 in the integral membrane domain, CTa, shown in Fig. 20.1a). Subunits a2, a1-I and a1-II were found to be endogenously co-expressed in rat hippocampal neurons. The peptide C-containing a1 variants appeared to be specific to neuronal expression and were upregulated during neuronal synaptogenic differentiation. The three neuronal a subunit variants, epitope tagged and recombinantly expressed in cultured neuronal cells, were found to sort to different subcellular compartments; the ubiquitous a2 to the soma, likely the Golgi, as is typical for a2, the a1-I variant to nerve terminals, and the a1-IV variant to dendritic processes. It appears that the peptide C insert determines sorting specific to neurotransmitter storage, and this may be modified by the addition of peptide N to target the plasma membrane. Peptide C also introduces a PEST motif that likely reduces the biological half-life of the a1-I and a1-IV subunit variants, but the significance of this remains unclear.
It has been shown that mouse and human a4 have alternate first exons [119, 120]. In mouse, this results in differential embryonic and adult expression, though this has not been shown for human a4. The C2 subunit has an alternate exon [121, 122] resulting in lack of a 46 amino acid insert; C2+ (C2a) is expressed in lung, whereas C2− (C2b) is expressed in kidney. Additionally, there is an example of alternate start codon usage that results in non-V-ATPase expression of the a3 subunit. The TIRC7 membrane protein involved in T cell activation is derived from an a3 transcript utilizing a start codon within exon 5 of ATP6V0A3 (historically, TCIRG1, ATP6I) [123]. TIRC7 is expressed on the surface of lymphocytes, whereas a3 is highly expressed on the ruffled border of osteoclasts; alternate promoter usage must account for this differential expression. Thus, splice variants may account for differential tissue expression, sorting to various subcellular compartments, and proteins of alternate function.
3.5 Assembly and Reversible Disassembly
In yeast, the V1 sector of V-ATPase appears to self-assemble in the cytoplasm [124]. For V0 there are at least three ER chaperones, Vma12p, Vma21p, and Vma22p that are essential for assembly [124, 125]; there is also an ER-resident accessory chaperone, Pkr1p, that enhances V0 assembly efficiency [126]. Vma21p additionally escorts the V0 sector to the Golgi, but is then recycled to the ER; none of the aforementioned proteins are retained as part of the functional V-ATPase complexes at their final destinations. In higher plants, the ER quality control chaperones, calnexin and BiP, have been coimmunoprecipitated with the full V-ATPase holoenzyme, suggesting that they too are involved as chaperones and in quality control of V-ATPase assembly and, moreover, that the entire V1V0 holoenzyme is assembled at the ER [127]. In humans the process of V0 assembly appears to be conserved, but the chaperones have diverged considerably, although a putative ortholog of Vma21p has been identified [73].
Considerably more needs to be understood about assembly of mammalian V-ATPases before therapeutic intervention can be considered; however, their regulated disassembly may be more tractable. It has long been proposed that under certain types of stress, particularly cellular glucose deprivation, the V1 sector of V-ATPase dissociates from the V0 sector (Fig. 20.1c), resulting in V-ATPase inactivation (reviewed in Refs. [4, 128–130]). This process also results in the reversible loss of the C subunit from the V1 sector (Fig. 20.1b, c). Regulation by reversible disassembly has been described most thoroughly in the yeast (S. cerevisiae) and in the insect (M. sexta) systems [85, 130–132]. Its evolutionary rationale may be to spare ATP for more immediately essential cellular processes, under starvation conditions. V-ATPase is known to associate with aldolase, by direct interaction with subunits B, E and a, which contributes to V-ATPase stability [23, 133–135] and is part of a glycolytic metabolon that dissociates on glucose starvation [136]. As part of this metabolon, V-ATPase also interacts with phosphofructokinase-1, which may also stabilize the complex [137]. This glycolytic metabolon senses and responds to metabolic status, making ATP and protons from glycolysis proximally available to the proton pump, but it also shuts down V-ATPase activity to regulate intracellular pH, or to respond to restricted cellular energy status.
V-ATPase disassembly in this process requires the involvement of microtubules, and it has been suggested that ATP/ADP binding and phosphorylation of the C subunit may destabilize V-ATPase structure in a regulatory manner by altering its affinity for actin, or components of the V-ATPase stator [26, 138, 139]. The NTa domain of the a subunit recruits cytohesin-2 in a pH-dependent manner, which in turn recruits ARF6. It has been speculated that the activity of this cytohesin guanine nucleotide exchange factor (GEF) signaling complex, which may be further modulated by aldolase binding, affects the interaction of NTa with stator EG heterodimers, resulting in instability leading to the regulatory disassembly of the C subunit and of V1–V0 [26].
For V-ATPase reassembly, Chan and Parra [140] have shown in S. cerevisiae that reassociation of the C subunit is dependent on the Pfk2p subunit of the glycolytic enzyme, phosphofructokinase-1. V1V0 reassembly is modulated by glucose-sensitive association of V-ATPase with aldolase [134, 141], and with the yeast “regulator of H+-ATPase of vacuolar and endosomal membranes” (RAVE; or equivalent rabconnectins in mammalian cells), a complex that interacts with EG heterodimers and the C subunit. RAVE-dependent assembly in yeast may be specific to the a subunit isoform, required for V-ATPases containing Vph1p and not for those containing Stv1p [142]. Similarly, Tuttle et al. [143] have suggested that different rabconnectins may pair with specific a subunit isoforms to influence cell type-specific trafficking and signal processing in vertebrates. Signaling pathways dependent on cytosolic pH, as determined by glycolytic activity [144], and involving phosphatidylinositol 3-kinase (PKI3) [145] and protein kinase A (PKA) [146], have been shown to be involved in recruitment and assembly of V-ATPases. Reassembly or stability also seem to be promoted by high extracellular pH [147]. Both disassembly and reassembly are rapidly reversible, and the catalytic ATPase and proton translocation activities of the isolated V1 and V0 sectors, respectively, are inhibited [132, 148, 149]. The H subunit inhibits the ATPase activity of the V1 sector, likely by interaction with the F subunit of the DF “crankshaft,” which protrudes from the catalytic headpiece of dissociated V1 [148, 150, 151]. Interestingly, in S. cerevisiae it has been shown that the V0 a subunit lysosomal isoform, Vph1p, is more responsive for reversible disassembly than the Golgi-localized Stv1p, with both V-ATPase subunit composition and local membrane environment contributing to the difference [152, 153].
The first mammalian report of V1V0 disassembly was for dendritic cells responding to maturation signals [154]. In immature dendritic cells, lysosomal acidification is depressed by V-ATPase dissociation to preserve antigen integrity. On maturation, V-ATPase reassembles, the lysosome is acidified, and antigen is processed. Recent evidence suggests that this reassembly is controlled by the PI3K/mTOR signaling pathway [155]. Type II alveolar cells stimulated with surfactant secretagogues also disassemble a1/B2-containing lamellar body V-ATPase [156]. These examples are not the result of glucose deprivation, and their mechanisms are poorly understood. The first evidence of mammalian glucose-dependent reassembly was found in cultured kidney cells [145, 157]. Glucose treatment promotes both reassembly of V1V0 after starvation and translocation of V-ATPase to the apical plasma membrane from a cytoplasmic vesicle pool, and both of these processes are dependent on PI3K activity. Reversible disassembly also appears to be regulated by extracellular pH [144, 147], by salt stress in plants [158] and, in insect cells, transient phosphorylation of the C subunit might mediate reassociation of the C subunit with V1 and consequent reassembly of V1V0 [159].
Tabke et al. [136] have suggested recently that in vivo disassembly of V1V0 into independent sectors does not occur, but rather only the C subunit dissociates from the complex, rendering it reversibly inactive. Their in vivo FRET analyses, using fluorescent protein-tagged V-ATPase subunit expression in S. cerevisiae show that yeast V1 and V0 sectors remain in close proximity to the vacuolar membrane upon cellular glucose starvation. Instead of V1 dissociation, dissociation of the C subunit alone is observed, and this appears to depend on direct interaction of microtubules with the C subunit, though reassembly is microtubule-independent. Tabke et al. [136] further argue that when the C subunit dissociates, the V1V0 holoenzyme is destabilized to the extent that further in vitro histo/cytochemical or biochemical manipulation results in artifactual V1V0 dissociation, in proportion to the prior loss of C subunit. These FRET analyses also reveal a change in distance between the B and a subunits during glucose starvation, in a manner that suggests conformational changes in the V-ATPase that could plausibly account for its apparent concurrent instability upon C subunit loss. One might perhaps think of in vitro V1–V0 dissociation as an assay for in vivo C subunit dissociation, with few conclusions in the published literature being substantially affected by the distinction. Regardless of the precise in vivo mechanism of “reversible disassembly,” it results in regulatory inactivation/reactivation of V-ATPase as an ATP hydrolytic enzyme and as a proton pump. Tapping into this regulatory mechanism by way of therapeutic intervention will, however, require a more detailed understanding relevant to human tissues.
3.6 Regulation by Localization : Recruitment and Redistribution
V-ATPases depend on vesicular trafficking to arrive at the various destination membrane compartments where they perform their functions. Failure to target correctly, whether in a regulatory manner, as a result of pathology, or due to therapeutic intervention, negates the intended V-ATPase function. How normal targeting is regulated remains obscure, but in at least some cases it is likely that the V-ATPase subunit composition encodes signals that predetermine its localization. In yeast, for example, the two a subunit isoforms Vph1p and Stv1p are localized to the vacuole and the Golgi apparatus, respectively, by virtue of polypeptide targeting signals within their NTa domains [47–49, 160].
A tissue-specific example of targeting is seen in the mammalian intestine, where luminal Cl-/HCO3- equilibrium requires proton secretion mediated by V-ATPase. Here it is the cystic fibrosis transmembrane-conductance regulator, CFTR, that regulates V-ATPase activity by providing a variable chloride counterion shunt. CFTR itself is regulated by the cAMP-dependent protein kinase, PKA, not just in its chloride channel activity, but also in its recruitment to the enterocyte brush border membrane. This recruitment appears to apply to V-ATPase as well, by direct interaction with CFTR, resulting in translocation of the former from the basolateral membrane to the apical brush border [161]. In other examples, it has been shown that cAMP/PKA and Rab11b regulate trafficking of V-ATPase into apical membranes of epithelial cells in the kidney, salivary glands and epididymis by recruitment from subapical endosomal compartments [146, 162, 163]. In mouse cortical collecting duct, angiotensin II initiates a similar redistribution of V-ATPase to apical plasma membranes from subapical vesicles [164]. The A subunit appears to play a direct role in regulation of V-ATPase redistribution, as it has been noted that it can be transiently phosphorylated by PKA at serine 175, resulting in upregulated activity in kidney and epididymal epithelia [165–168]. The A subunit can also be phosphorylated by the 5′-AMP-activated protein kinase, AMPK, at serine 384 [166, 168], although this appears to be inhibitory for plasma membrane V-ATPase activity in kidney. In osteoclasts, V-ATPase is translocated to the ruffled border upon osteoclast activation, from a pool of intracellular lysosomal vesicles. This requires interaction with actin microfilaments that bind the N-terminal domain of V-ATPase B subunits [169, 170]. Therapeutically targeting the interaction of F-actin and V-ATPase B subunits and other specific protein-protein interactions within the osteoclast V-ATPase, to inhibit osteoclastic bone resorption, is discussed further, below.
3.7 Coupling Efficiency
Kawasaki-Nishi et al. [153] showed that yeast V-ATPases with the a subunits Stv1p or Vph1p have similar enzyme kinetic properties, yet Stv1p realizes a four- to fivefold lower coupling efficiency of protons transported per ATP molecule hydrolyzed than Vph1p. It has been suggested that this difference in coupling efficiency accounts for the higher pH observed in the Golgi, compared to the vacuole, and that differences in subunit isoform composition of mammalian V-ATPase isocomplexes may regulate differences in steady state pH within the organelles to which they are targeted. Electrophysiological studies of active transporters have revealed a common “lack of precision” in predicted solute output per molecule of ATP hydrolyzed [171]. For V-ATPases, one possibility for this lack of precision is referred to as “slip,” which may be due to the inability of protons to dissociate into the lumen against a large ∆H+, resulting in carryover, past what is usually an effective charge barrier between the luminal and cytoplasmic proton hemichannels (Fig. 20.1d), and release back into the cytoplasm instead [172]. Slippage, or more generally, proton “shunting,” depends on the current–voltage properties of the proton pump, the transmembrane electrical charge and pH gradient. Shunting alone may account for all of the variable coupling efficiencies that have been observed for V-ATPases and may represent transient thermodynamic behavior of the V-ATPase as an open proton channel [173]. Whether slippage, or shunting, is completely intrinsic to V-ATPase structure, or is influenced by other regulatory elements, or can be pharmaceutically manipulated, is presently not known.
3.8 Lipid Microenvironment
It has recently been shown that the signaling lipid, phosphatidylinositol 3,5-bisphosphate, PI(3,5)P2, directly interacts with the V-ATPase V0 sector and promotes assembly and stability of V-ATPase, possibly by altering the conformation of the N-terminal domain of the a subunit, Vph1p, in yeast [174]. C26 acyl sphingolipids also affect yeast V-ATPase activity, but surprisingly it is the cytosolic V1 sector that is inactive without them. It has been suggested that these lipids support V1 activity indirectly, by affecting the RAVE complex that is required for V1 assembly [175]. In mammalian cells the simple sphingolipid, glucosylceramide, appears to be required to support high levels of V-ATPase activity in melanocyte endomembranes, a necessity for protein sorting and melanosome biogenesis [176]. In plants, tonoplast V-ATPase activity is enhanced in vitro by tonoplast phospholipids, but depressed by tonoplast glycolipids [177]. Yoshida et al. [178] have shown further that in Arabidopsis thaliana vacuoles the organellar membrane consists of microdomains that are characterized by either detergent sensitivity or detergent resistance. V-ATPase was found to associate with detergent resistant microdomains, which have elevated proportions of saturated fatty acids in their phosphatidyl choline and phosphatidyl ethanolamine phospholipids, compared with total vacuolar phospholipids. The authors speculate that since plasma membrane microdomains play a role in signal transduction, vacuolar microdomains may be involved in regulation of membrane transport and signal processing at the vacuolar level; however, direct evidence of this remains lacking.
Although the concept has been largely unexplored in mammalian systems, there is some indication that manipulation of the membrane lipid microenvironment in which V-ATPases find themselves might have therapeutic value. In yeast, membrane ergosterol (which is not found in animal cells) is required for V-ATPase function. V-ATPase is crucial to virulence of pathogenic fungi, and the azole class of antifungal drugs exploits this by inhibiting ergosterol biosynthesis [179].
3.9 Ancillary Enzymes
V-ATPases cannot function without a number of supporting enzymes and transport proteins, often also having tissue-specific expression. Intracellular carbonic anhydrase II (CA II) is expressed to rapidly equilibrate the reaction of CO2 and water to produce protons and bicarbonate ions. Though widely distributed, CA II is especially important in highly active acid-secreting cells, like osteoclasts and specialized kidney ductal epithelial cells, to provide a readily accessible pool of protons to the V-ATPase. It also provides bicarbonate ions to power cellular chloride uptake via Cl-/HCO3 - exchange. In osteoclasts, the latter role is performed specifically by the anion exchange protein 2 (AE2) with which CA II directly interacts [180]. Electrogenic proton pumping by V-ATPase would come to a quick halt if the charge gradient were not neutralized by a counterion shunt [181–183]; in the osteoclast this is provided by the chloride channel 7 (CLC7), an electrogenic H+/2Cl- exchanger, which utilizes the chloride ions provided by AE2. Depending on the tissue and subcellular compartment, other isozymes and H+/ion exchangers can take on the roles of those specifically highlighted here.
Mutations in the above ancillary enzymes illustrate their importance to V-ATPase activity. Mutations in CA2, the gene coding for CA II, which is highly expressed in both osteoclasts and kidney, cause combined osteopetrosis and dRTA, similar to what is seen separately for mutations in the V-ATPase subunits a3 and a4, which are also highly expressed in the respective tissues [56, 184]. Mutations in SLC4A2, which codes for AE2 have not been described in humans, but a deletion mutation in bovine SLC4A2 results in osteopetrosis in cattle [185]. Interestingly, mutations in the anion exchange protein 1 (AE1), which is a kidney-specific form, cause a dominant variation of dRTA that is otherwise similar to what is seen for mutations in the kidney-specific V-ATPase B1 and a4 subunit isoforms. Mutations in CLCN7, which codes for the chloride channel CLC7, also cause osteopetrosis, much like mutations in the V-ATPase a3 subunit [186], and a specific inhibitor of CLC7 has been shown to prevent bone loss in ovariectomized rats [187]. Apart from mutations, there is also the observation, as was noted above, that V-ATPase proton extrusion into the gut lumen depends on a specialized, tissue-specific association with CFTR [161]. These examples illustrate the potential for indirect manipulation of V-ATPases by targeting the ancillary proteins upon which their activity depends.
4 Targeting Inhibition of V-ATPase
It is tempting to think that manipulating specific endogenous regulatory mechanisms for V-ATPase expression and function, including those described above, might achieve precise therapeutic targeting. While this may 1 day be the case in some applications, a great deal more needs to be understood regarding these regulatory mechanisms before such an approach can be realized. Furthermore, many of the regulatory molecules described are widely distributed throughout the organism, making a “magic bullet” solution unlikely. Historically, numerous small molecule inhibitors, mostly natural products, have been used to experimentally inhibit V-ATPases, the plecomacrolides bafilomycin A1 and concanamycin A being the most commonly used [188]. These generally are not selective among V-ATPase isocomplexes. Some inhibitors have been described that may be selective for osteoclast V-ATPases, but clinical utility has proved elusive. We briefly review here illustrative examples of strategies used towards trying to achieve specific V-ATPase targeting, with applications ranging from pharmaceutical to agrochemical.
4.1 Novel V-ATPase Inhibitors: Discovery Strategies and Applications
The ultimate goal in targeting V-ATPases clearly is to be able to manage a “surgical strike” against a specific isocomplex without affecting V-ATPases of alternative subunit isoform composition. Recently, strategies have been designed to discover inhibitors of protein interactions that are required for the functions of specific V-ATPase isocomplexes, and RNAi methods to knock down expression of specific subunit isoforms.
4.1.1 Small Molecule Inhibitors of V-ATPase Quaternary Subunit Interaction
Kartner et al. [189] characterized interactions between mouse V-ATPase a and B subunits, using yeast two hybrid screening and pulldown assays of recombinant fusion proteins. They further characterized the interactions between all of the mouse a and B subunit isoform pairs using an ELISA system to generate relative binding curves. This assay lent itself to modification to achieve high-throughput screening for small molecule inhibitors of the a3–B2 interaction, an interaction that has some specificity for osteoclast ruffled-border V-ATPase [190, 191]. Screening of small synthetic compound and natural product libraries led to the discovery of a small synthetic molecule, 3,4-dihydroxy-N′-(2-hydroxybenzylidene)benzohydrazide, that was able to inhibit in vitro osteoclastic bone resorption with an IC50 of 1.2 μM [189].
Crasto et al. [192] used a similar strategy to discover the natural product small molecule inhibitor, luteolin, a plant flavonoid that inhibits the a3–d2 interaction that also occurs in osteoclast ruffled-border V-ATPases. Luteolin inhibited in vitro osteoclast bone resorption with an IC50 of 2.5 μM. Shin et al. [193] have independently shown in vivo that luteolin inhibits prosthetic wear particle osteolysis in mice.
For either of the above compounds, the precise mechanisms of action remain speculative, and metabolic lability and intellectual property issues have impacted on their further development [61, 62]. Nevertheless, these proof-of-concept examples suggest that targeting sites of subunit interaction within the V-ATPase complex may be a viable means of obtaining inhibitors of specific isocomplexes, in these cases those of the osteoclast ruffled border for the purpose of limiting bone-loss diseases like osteoporosis. They also highlight a novel and simple ELISA-based approach to high-throughput random drug screening that can be generalized to potentially identify highly targeted inhibitors of important tertiary, quaternary, or quinary protein structure interactions that can be modeled in vitro. Furthermore, so long as the interacting pair of proteins or appropriate polypeptide segments can be produced or isolated in sufficient quantity, such a screening method can be performed even in the absence of structural information or a precise knowledge of the protein interaction sites.
4.1.2 Small Molecule Inhibitors of V-ATPase Quinary Protein Interaction
In a different screening strategy, Toro et al. [194] exploited the knowledge that V-ATPases bind actin filaments in osteoclasts via an interaction between F-actin and the B2 subunit [195]. This interaction is crucial for cycling of ruffled border V-ATPase between bone resorptive activity at the ruffled-border plasma membrane and “storage” in cytoplasmic vesicles. Using computer modeling of the docking sites for this interaction, Ostrov et al. [196] were able to perform virtual screening for small molecule inhibitors, the most promising hit being enoxacin, a fluoroquinolone antibiotic. Enoxacin appears to inhibit the vesicular trafficking of the osteoclast ruffled-border V-ATPase, which is dependent on its interaction with the actin cytoskeleton, thus making it a promising candidate for osteoporosis treatment [197]. A bone-targeted enoxacin–bisphosphonate conjugate has been shown to inhibit in vivo orthodontic tooth movement in rats [198]. What separates this work to identify inhibitors of protein interaction from the previous examples is the use of virtual screening, where both the site of protein interaction and the library of compounds screened are computer models. Such methods clearly are highly cost effective, and this proof-of-concept example highlights their potential. With regard specifically to therapeutic V-ATPase targeting, a broader application of virtual drug screening must await broader databases of high-resolution 3D subunit structures and their interactions, both intra-V-ATPase isocomplex interactions and their, as yet poorly defined, extra-complex quinary interactomes.
4.1.3 RNAi and Gene Therapy Approaches
In periodontal disease, inflammation induces local osteoclastogenesis and consequent bone loss [199, 200]. In activated osteoclasts, expression of V-ATPase containing the a3 subunit is upregulated. Additionally, the a3 splice-variant, TIRC7, plays a role in T cell activation [123, 201]. Jiang et al. [202] proposed that regulating a3/TIRC7 could be a means of stemming both bone loss and inflammation in periodontal disease, simultaneously. In a mouse Porphyromonas gingivalis maxillary infection model, they used local injection of adeno-associated virus (AAV) to deliver short hairpin RNA (shRNA) constructs designed for RNA interference (RNAi) targeting of both Atp6i and Tirc7 transcripts . Knockdown of a3/Tirc7 expression caused reduced osteoclast formation and maturation, resulting in a reduction of maxillary bone loss by over 85 % relative to controls, and a similar reduction in gingival inflammation in the infected mice.
In a similar approach, Feng et al. [203] demonstrated that the C1 subunit isoform predominates in the V-ATPase of the osteoclast ruffled border. As with the a3 subunit, C1 is highly upregulated during osteoclastogenesis and is required for formation of the actin-ring sealing zone of activated osteoclasts, and for the subsequent lacunar acidification required for bone resorption. Unlike the RNAi knockdown of a3 described above [202], knockdown of C1 expression using Lentivirus-mediated siRNA delivery in vitro did not affect osteoclast formation or maturation, although actin sealing-rings were also completely disrupted.
Gene therapy is commonly regarded as a gain-of-function modality, and such approaches may, in the future, have utility for relatively rare diseases of V-ATPase subunit mutations [63]. As the above proofs of concept suggest, gene-silencing approaches have broader clinical utility using, for example, localized periarticular or periodontal injections of viral RNAi vectors for treating bone-loss disease . For the present, however, safety and cost remain major issues, and systemic gene therapy for bone loss treatment or prevention, such as for osteoporosis, will likely be a long time coming. More acutely life-threatening diseases, such as metastatic cancers, however, may see the first applications of such V-ATPase targeting approaches.
4.2 Novel V-ATPase Targeting Strategies in Agriculture
It is worth pointing out that the potential for V-ATPase targeting transcends human medicine, with wide applicability as well to veterinary medicine and agriculture, all of which ultimately impact human well-being. Examples of strategies and applications follow.
4.2.1 RNAi by dsDNA Dusting
Much early and ongoing work on V-ATPases has been accomplished in insect models. In insects, V-ATPases play a particularly important role in maintaining the pH gradient of the gut, which is vital for nutrient acquisition. Consequently, insect V-ATPases have long been recognized as targets for potentially highly specific RNAi-based insecticides [204]. The corn planthopper, Peregrinus maidis , is a major pest that feeds on maize crops, and is a mold and plant virus vector. Yao et al. [205] observed significant knockdown of V-ATPase activity in P. maidis that were fed dsRNA to achieve RNAi for V-ATPase A and D subunits, with a resultant decrease in nymph survival and female fecundity.
4.2.2 RNAi by Crop Transgenics
In a small-scale screen, Baum et al. [206] fed Western corn rootworm larvae (Diabrotica virgifera) 290 different dsRNA constructs and found 14 causing significant mortality at low doses. One, a dsRNA targeting the V-ATPase A subunit, was transformed into corn, which then showed significant resistance to D. virgifera feeding damage. In these examples, simple ingestion of dsRNA was likely successful because the RNAi target is the V-ATPase of the gut epithelium. This strategy, using carefully selected and relatively short dsRNA sequences could be useful in producing transgenic crops with insecticidal qualities only against pest species, without collateral effects. Off-target effects of traditional insecticides are of growing public concern, but the utility of RNAi methods will depend on public acceptance of the transgenic crops, or enhanced methods of production of dsRNA for large-scale crop spraying, and will require the availability of complete genomic sequences for both pest target and beneficial nontarget insect species.
4.2.3 Peptide Inhibition of the V0 Rotor
The small (37-mer) polypeptide, pea albumin 1b (PA1b), is a potent M. sexta V-ATPase inhibitor that binds c subunits, possibly interfering with rotor movement, by jamming against the e subunit within the proton-translocating V0 sector, or by binding a c and an e subunit simultaneously [207, 208]. The extracellular termini and loops available on the c subunit for PA1b binding are the least conserved regions of the otherwise highly conserved c subunit polypeptide. Consequently, though PA1b has potent insecticidal properties toward M. sexta , it has little effect on yeast or mammalian V-ATPases, or even some other insect species. These observations support the notion that species selectivity is possible in strategies aimed at eliminating pests and parasites by targeting the non-conserved sequences of the V0 rotor. Furthermore, it points to the possibility that peptides may offer exquisitely engineered selectivity, where small molecules fail.
4.2.4 A Gain of Function V-ATPase Strategy: Genetic Engineering of Salt Resistance
While inhibitory strategies are foremost in current agricultural research targeting V-ATPases, gain of function approaches have had some traction for crop improvement. For example, it has been shown that salt stress causes upregulation of V-ATPase subunit expression in wheat, resulting in enhanced Na+ sequestration in the central vacuole. Furthermore, overexpression of wheat V-ATPase subunit genes in A. thaliana results in improved salt and osmotic stress tolerance [84, 209]. Genetic engineering of salt stress resistance in crop species has significant potential for improving worldwide food production.
5 Conclusions
5.1 V-ATPases Represent an Emerging Target of Broad Significance
We have described here the wide variety of impacts that V-ATPases have on human health and welfare. This is unique among potential pharma/agro targets and has recently been a considerable source of motivation for drug discovery. It seems likely that this interest will drive research into V-ATPase structure and function at an accelerating pace into the foreseeable future. Furthermore, new knowledge acquired for any of the diverse areas of application that we have described will enhance the development of V-ATPase targeting as a whole. The most pressing issue for targeting of V-ATPases is the matter of specificity: how to distinguish specialized V-ATPases whose inhibition would be of benefit from those V-ATPases that are vital to survival. Presently, knowledge of the structure of any V-ATPase holoenzyme at atomic resolution is lacking, as is a detailed knowledge of its interactome. Advances in these areas have been slow due to the complexity of the V-ATPase holoenzyme, but it is not unreasonable to think that a complete V-ATPase structure and a list of many more fully characterized functional interactions might be at hand within a decade. Whether V-ATPase targeting breakthroughs come in the form of novel small molecule inhibitors, engineered peptides, RNAi methods, or gene therapy, the potential for health, welfare and economic impact is great.
References
Futai M, Nakanishi-Matsui M, Okamoto H et al (2012) Rotational catalysis in proton pumping ATPases: from E. coli F-ATPase to mammalian V-ATPase. Biochim Biophys Acta 1817:1711–1721
Kane PM (2007) The long physiological reach of the yeast vacuolar H+-ATPase. J Bioenerg Biomembr 39:415–421
Toei M, Saum R, Forgac M (2010) Regulation and isoform function of the V-ATPases. Biochemistry 49:4715–4723
Forgac M (2007) Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8:917–929
Beyenbach KW, Wieczorek H (2006) The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J Exp Biol 209:577–589
Nordström T, Rotstein OD, Romanek R et al (1995) Regulation of cytoplasmic pH in osteoclasts: contribution of proton pumps and a proton-selective conductance. J Biol Chem 270:2203–2212
Schewe B, Schmälzlin E, Walz B (2008) Intracellular pH homeostasis and serotonin-induced pH changes in Calliphora salivary glands: the contribution of V-ATPase and carbonic anhydrase. J Exp Biol 211:805–815
Grinstein S, Nanda A, Lukacs G et al (1992) V-ATPases in phagocytic cells. J Exp Biol 172:179–192
Saw NMN, Kang S-YA, Parsaud L et al (2011) Vacuolar H+-ATPase subunits Voa1 and Voa2 cooperatively regulate secretory vesicle acidification, transmitter uptake, and storage. Mol Biol Cell 22:3394–3409
Manolson MF, Yu H, Chen W et al (2003) The a3 isoform of the 100-kDa V-ATPase subunit is highly but differentially expressed in large (≥10 nuclei) and small (≤5 nuclei) osteoclasts. J Biol Chem 278:49271–49278
Henriksen K, Sørensen MG, Jensen VK et al (2008) Ion transporters involved in acidification of the resorption lacuna in osteoclasts. Calcif Tissue Int 83:230–242
Brown D, Smith PJS, Breton S (1997) Role of V-ATPase-rich cells in acidification of the male reproductive tract. J Exp Biol 200:257–262
Breton S, Brown D (2013) Regulation of luminal acidification by the V-ATPase. Physiology 28:318–329
Brown D, Paunescu TG, Breton S et al (2009) Regulation of the V-ATPase in kidney epithelial cells: dual role in acid–base homeostasis and vesicle trafficking. J Exp Biol 212:1762–1772
Hinton A, Sennoune SR, Bond S et al (2009) Function of a subunit isoforms of the V-ATPase in pH homeostasis and in vitro invasion of MDA-MB231 human breast cancer cells. J Biol Chem 284:16400–16408
Xu M, Xia M, Li X-X et al (2012) Requirement of translocated lysosomal V1 H+-ATPase for activation of membrane acid sphingomyelinase and raft clustering in coronary epithelial cells. Mol Biol Cell 23:1546–1557
Strasser B, Iwaszkiewicz J, Michielin O et al (2011) The V-ATPase proteolipid cylinder promotes the lipid-mixing stage of SNARE-dependent fusion of yeast vacuoles. EMBO J 30:4126–4141
Hiesinger PR, Fayyazuddin A, Mehta SQ et al (2005) The v-ATPase V0 subunit a1 is required for a late step in synaptic vesicle exocytosis in Drosophila. Cell 121:607–620
Schumacher K (2006) Endomembrane proton pumps: connecting membrane and vesicle transport. Curr Opin Plant Biol 9:595–600
Sabota JA, Bäck N, Eipper BA et al (2009) Inhibitors of the V0 subunit of the vacuolar H+-ATPase prevent segregation of lysosomal- and secretory-pathway proteins. J Cell Sci 122:3542–3553
Sun-Wada G-H, Toyomura T, Murata Y et al (2006) The a3 isoform of V-ATPase regulates insulin secretion from pancreatic β-cells. J Cell Sci 119:4531–4540
Hurtado-Lorenzo A, Skinner M, El Annan J et al (2006) V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol 8:124–136
Merkulova M, Bakulina A, Thaker YR et al (2010) Specific motifs of the V-ATPase a2-subunit isoform interact with catalytic and regulatory domains of ARNO. Biochim Biophys Acta 1797:1398–1409
Hofmann I, Thompson A, Sanderson CM et al (2007) The Arl4 family of small G proteins can recruit the cytohesin Arf6 exchange factors to the plasma membrane. Curr Biol 17:711–716
Hosokawa H, Dip PV, Merkulova M et al (2013) The N termini of a-subunit isoforms are involved in signaling between vacuolar H+-ATPase (V-ATPase) and cytohesin-2. J Biol Chem 288:5896–5913
Marshansky V, Rubinstein JL, Grüber G (2014) Eukaryotic V-ATPase: novel structural findings and functional insights. Biochim Biophys Acta 1837:857–879
Poëa-Guyon S, Ammar MR, Erard M et al (2013) The V-ATPase membrane domain is a sensor of granular pH that controls the exocytic machinery. J Cell Biol 203:283–298
Coonrod EM, Graham LA, Carpp LN et al (2013) Homotypic vacuole fusion in yeast requires organelle acidification and not the V-ATPase membrane domain. Dev Cell 27:462–468
Yan Y, Denef N, Schüpbach T (2009) The vacuolar proton pump (V-ATPase) is required for Notch signaling and endosomal trafficking in Drosophila. Dev Cell 17:387–402
Cruciat C-M, Ohkawara B, Acebron SP et al (2010) Requirement of prorenin receptor and vacuolar H+-ATPase-mediated acidification for Wnt signaling. Science 327:459–463
Jewell JL, Russel RC, Guan K-L (2013) Amino acid signalling upstream of mTOR. Nat Rev Mol Cell Biol 14:133–139
Peña-Llopis S, Vega-Rubin-de-Celis S, Schwartz JC et al (2011) Regulation of TFEB and V-ATPases by mTORC1. EMBO J 30:3242–3258
Laplante M, Sabatini DM (2013) Regulation of mTORC1 and its impact on gene expression at a glance. J Cell Sci 126:1713–1719
Efeyan A, Zoncu R, Sabatini DM (2012) Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med 18:524–533
Sancak Y, Sabatini DM (2009) Rag proteins regulate amino acid-induced mTORC1 signaling. Biochem Soc Trans 37:289–290
Zoncu R, Bar-Peled L, Efeyan A et al (2011) mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H+-ATPase. Science 334:678–683
Bar-Peled L, Schweitzer LD, Zoncu R et al (2012) Ragulator is a GEF for the Rag GTPases that signal amino acid levels to mTORC1. Cell 150:1196–1208
Abraham RT (2015) Making sense of amino acid sensing. Science 347:128–129
Wang S, Tsun Z-Y, Wolfson RL et al (2015) Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science 347:188–194
Xu Y, Parmar A, Roux E et al (2012) Epidermal growth factor-induced vacuolar (H+)-ATPase assembly: a role in signaling via mTORC1 activation. J Biol Chem 287:26409–26422
Muench SP, Trinick J, Harrison MA (2011) Structural divergence of the rotary ATPases. Q Rev Biophys 44:311–356
Zhang Z, Zheng Y, Mazon H et al (2008) Structure of the yeast vacuolar ATPase. J Biol Chem 283:35983–35995
Smith AN, Lovering RC, Futai M et al (2003) Revised nomenclature for mammalian vacuolar-type H+-ATPase subunit genes. Mol Cell 12:801–803
Miranda KC, Karet FE, Brown D (2010) An extended nomenclature for mammalian V-ATPase subunit genes and splice variants. PLoS One 5, e9531
Lee BS (2012) Regulation of V-ATPase expression in mammalian cells. Curr Protein Peptide Sci 13:107–116
Holliday LS (2014) Vacuolar H+-ATPase: an essential multitasking enzyme in physiology and pathophysiology. New J Sci 2014:1–21. doi:10.1155/2014/675430
Finnigan GC, Hanson-Smith V, Houser BD et al (2011) The reconstructed ancestral subunit a functions as both V-ATPase isoforms Vph1p and Stv1p in Saccharomyces cerevisiae. Mol Biol Cell 22:3176–3191
Manolson MF, Wu B, Proteau D et al (1994) STV1 gene encodes functional homologue of 95-kDa yeast vacuolar H+-ATPase subunit Vph1p. J Biol Chem 269:14064–14074
Kawasaki-Nishi S, Bowers K, Nishi T et al (2001) The amino-terminal domain of the vacuolar proton-translocating ATPase a subunit controls targeting and in vivo dissociation, and the carboxyl-terminal domain affects coupling of proton transport and ATP hydrolysis. J Biol Chem 276:47411–47420
Karet FE, Finberg KE, Nelson RD et al (1999) Mutations in the gene encoding B1 subunit of H+-ATPase cause renal tubular acidosis with sensorineural deafness. Nat Genet 21:84–90
Păunescu TG, Rodriguez S, Benz E et al (2012) Loss of the V-ATPase B1 subunit isoform expressed in non-neuronal cells of the mouse olfactory epithelium impairs olfactory function. PLoS One 7, e45395
Guillard M, Dimopoulou A, Fischer B et al (2009) Vacuolar H+-ATPase meets glycosylation in patients with cutis laxa. Biochim Biophys Acta 1792:903–914
Fischer B, Dimopoulou A, Egerer J et al (2012) Furher characterization of ATP6V0A2-related autosomal recessive cutis laxa. Hum Genet 131:1761–1773
Sobacchi C, Schulz A, Coxon FP et al (2013) Osteopetrosis: genetics, treatment and new insights into osteoclast function. Nat Rev Endocrinol 9:522–536
Stover EH, Borthwick KJ, Bavalia C et al (2002) Novel ATP6V1B1 and ATP6V0A4 mutations in autosomal recessive distal renal tubular acidosis with new evidence for hearing loss. J Med Genet 39:796–803
Batlle D, Haque SK (2012) Genetic causes and mechanisms of distal renal tubular acidosis. Nephrol Dial Transplant 27:3691–3704
Lee S-H, Rho J, Jeong D et al (2006) v-ATPase V0 subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med 12:1403–1409
Korvatska O, Strand NS, Berndt JD et al (2013) Altered splicing of ATP6AP2 causes X-linked parkinsonism with spasticity (XPDS). Hum Mol Genet 22:3259–3268
Ramser J, Abidi FE, Burckle CA et al (2005) A unique exonic splice enhancer mutation in a family with X-linked mental retardation and epilepsy points to a novel role of the renin receptor. Hum Mol Genet 14:1019–1027
Holliday LS (2012) Vacuolar H+-ATPase: Targeting a “housekeeping” enzyme for drug development. Curr Protein Peptide Sci 13:105–106
Kartner N, Manolson MF (2014) Novel techniques in the development of osteoporosis drug therapy: the osteoclast ruffled-border vacuolar H+-ATPase as an emerging target. Expert Opin Drug Discov 9:505–522
Kartner N, Manolson MF (2012) V-ATPase subunit interactions: the long road to therapeutic targeting. Curr Protein Peptide Sci 13:164–179
Bhargava A, Voronov I, Wang Y et al (2012) Osteopetrosis mutation R444L causes ER retention and misprocessing of vacuolar H+-ATPase a3 subunit. J Biol Chem 287:26829–26839
Gharanei S, Zatyka M, Astuti D et al (2013) Vacuolar-type H+-ATPase V1A subunit is a molecular partner of Wolfram syndrome 1 (WFS1) protein, which regulates its expression and stability. Hum Mol Genet 22:203–217
Capecci J, Forgac M (2013) Function of vacuolar ATPase (V-ATPase) a subunit isoforms in invasiveness of MCF10a and MCF10CA1a human breast cancer cells. J Biol Chem 288:3271–32741
Nishisho T, Hata K, Nakanishi M et al (2011) The a3 isoform vacuolar type H+-ATPase promotes distant metastasis in the mousse B16 melanoma cells. Mol Cancer Res 9:845–855
Sennoune SR, Luo D, Martinez-Zaguilán R (2004) Plasmalemmal vacuolar-type H+-ATPase in cancer biology. Cell Biochem Biophys 40:185–206
Sennoune SR, Bakunts K, Martínez GM et al (2004) Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol Cell Physiol 286:C1443–C1452
Pérez-Sayáns M, Somoza-Martín JM, Barros-Angueira F et al (2010) Multidrug resistance in oral squamous cell carcinoma: the role of vacuolar ATPases. Cancer Lett 295:135–143
Olsson AH, Yang BT, Hall E et al (2011) Decreased expression of genes involved in oxidative phosphorylation in human pancreatic islets from patients with type 2 diabetes. Eur J Endocrinol 165:589–595
Molina MF, Qu H-Q, Rentfro AR et al (2011) Decreased expression of ATP6V1H in type 2 diabetes: a pilot report on the diabetes risk study of Mexican Americans. Biochem Biophys Res Commun 412:728–731
Mangieri LR, Mader BJ, Thomas CE et al (2014) ATP6V0C knockdown in neuroblastoma cell alters autophagy-lysosome pathway function and metabolism of proteins that accumulate in neurodegenerative disease. PLoS One 9, e93257
Ramachandran N, Munteanu I, Wang P et al (2013) VMA21 deficiency prevents vacuolar ATPase assembly and causes autophagic vacuolar myopathy. Acta Neuropathol (Berl) 125:439–457
Price PA, June HH, Buckley JR et al (2002) SB 242784, a selective inhibitor of the osteoclastic V-H+-ATPase, inhibits arterial calcification in the rat. Circ Res 91:547–552
Rojas JD, Sennoune SR, Martinez GM et al (2004) Plasmalemmal vacuolar H+-ATPase is decreased in microvascular endothelial cells from a diabetic model. J Cell Physiol 201:190–200
Rojas JD, Sennoune SR, Maita D et al (2006) Vacuolar-type H+-ATPases at the plasma membrane regulate pH and cell migration in microvascular endothelial cells. Am J Physiol Heart Circ Physiol 291:H1147–H1157
Knight AJ, Behm CA (2012) Minireview: the role of the vacuolar ATPase in nematodes. Exp Parasitol 132:47–55
Jia C, Yu Q, Zhang B et al (2014) Role of TFP1 in vacuolar acidification, oxidative stress and filamentous development in Candida albicans. Fungal Genet Biol 71:58–67
Kang S, Shields AR, Jupatanakul N et al (2014) Supressing dengue-2 infection by chemical inhibition of Aedes aegypti host factors. PLoS Negl Trop Dis 8, e3084
Geyer M, Yu H, Mandic R et al (2002) Subunit H of the V-ATPase binds to the medium chain of adaptor protein complex 2 and connects Nef to the endocytic machinery. J Biol Chem 277:28521–28529
Müller KH, Kainov DE, El Bakkouri K et al (2011) The proton translocation domain of cellular vacuolar ATPase provides a target for the treatment of influenza A virus infections. Br J Pharmacol 164:344–357
Dhaliwal GS, Jindal V, Dhawan AK (2010) Insect pest problems and crop losses: changing trends. Indian J Ecol 37:1–7
Rahdari P, Hoseini SM (2011) Salinity stress: a review. Tech J Eng Appl Sci 1:63–66
He X, Huang X, Shen Y et al (2014) Wheat V-H+-ATPase subunit genes significantly affect salt tolerance in Arapidopsis thaliana. PLoS One 9, e86982
Parra KJ (2014) Saccharomyces cerevisiae vacuolar H+-ATPase regulation by disassembly and reassembly: One structure and multiple signals. Eukaryot Cell 13:706–714
Maxson ME, Grinstein S (2014) The vacuolar-type H+-ATPase at a glance – more than a proton pump. J Cell Sci 127:4987–4993
Lee BS, Underhill DM, Crane MK et al (1995) Transcriptional regulation of the vacuolar H+-ATPase B2 subunit gene in differentiating THP-1 cells. J Biol Chem 270:7320–7329
Lee BS, Krits I, Crane-Zelkovic MK et al (1997) A novel transcription factor regulates expression of the vacuolar H+-ATPase B2 subunit through AP-2 sites during monocytic differentiation. J Biol Chem 272:174–181
Wang S-P, Krits I, Bai S et al (2002) Regulation of enhanced vacuolar H+-ATPase expression in macrophages. J Biol Chem 277:8827–8834
Izumi H, Ise T, Murakami T et al (2003) Structural and functional characterization of two human V-ATPase subunit gene promoters. Biochim Biophys Acta 1628:97–104
Gardiner-Garden M, Frommer M (1987) CpG islands in vertebrate genomes. J Mol Biol 196:261–282
Robinson PN, Böhme U, Lopez R et al (2004) Gene-ontology analysis reveals association of tissue-specific 5′CpG-island genes with development and embryogenesis. Hum Mol Genet 13:1969–1978
Illingworth RS, Bird AP (2009) CpG islands – ‘a rough guide’. FEBS Lett 583:1713–1720
Chatterjee R, Vinson C (2012) CpG methylation recruits sequence specific transcription factors essential for tissue specific gene expression. Biochim Biophys Acta 1819:763–770
Jaenisch R, Bird A (2003) Epigenetic regulation of gene expression: how the genome integrates intrinsic and environmental signals. Nat Genet 33:245–254
Vidarsson H, Westergren R, Heglind M et al (2009) The forkhead transcription factor Foxi1 is a master regulator of vacuolar H+-ATPase proton pump subunits in the inner ear, kidney and epididymis. PLoS One 4, e4471
Blomqvist SR, Vidarsson H, Fitzgerald S et al (2004) Distal renal tubular acidosis in mice that lack the forkhead transcription factor Foxi1. J Clin Invest 113
Jackson BC, Carpenter C, Nebert DW et al (2010) Update of human and mouse forkead box (FOX) gene families. Hum Genomics 4:345–352
Lacey DL, Timms E, Tan HL et al (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Song I, Kim JH, Kim K et al (2009) Regulatory mechanism of NFATc1 in RANKL-induced osteoclast activation. FEBS Lett 583:2435–2440
Beranger GE, Momier D, Rochet N et al (2006) RANKL treatment releases the negative regulation of the poly(ADP-ribose) polymerase-1 on Tcirg1 gene expression during osteoclastogenesis. J Bone Miner Res 21:1757–1769
Beranger GE, Momier D, Guigonis J-M et al (2007) Differential binding of poly(ADP-ribose) polymerase-1 and JunD/Fra2 accounts for RANKL-induced Tcirg1 gene expression during osteoclastogenesis. J Bone Miner Res 22:975–983
Takayanagi H (2007) The role of NFAT in osteoclast formation. Ann N Y Acad Sci 116:227–237
Feng H, Cheng T, Steer JH et al (2009) Myocyte enhancer factor 2 and microphthalmia-associated transcription factor cooperate with NFATc1 to transactivate the V-ATPase d2 promoter during RANKL-induced osteoclastogenesis. J Biol Chem 284:14667–14676
Jouret F, Auzanneau C, Debaix H et al (2005) Ubiquitous and kidney-specific subunits of vacuolar H+-ATPase are differentially expressed during nephrogenesis. J Am Soc Nephrol 16:3235–3246
Lee BS, Holliday LS, Krits I et al (1999) Vacuolar H+-ATPase activity and expression in mouse bone marrow cultures. J Bone Miner Res 14:2127–2136
Jeyaraj S, Dakhlallah D, Hill SR et al (2005) HuR stabilizes vacuolar H+-translocating ATPase mRNA during cellular energy depletion. J Biol Chem 280:37957–37964
Khabar KSA, Bakheet T, Williams BRG (2005) AU-rich transient response transcripts in the human genome: expressed sequence tag clustering and gene discovery approach. Genomics 85:165–175
Peng SS-Y, Chen C-YA XN et al (1998) RNA stabilization by the AU-rich element binding protein, HuR, an ELAV protein. EMBO J 17:3461–3470
Fan XC, Steitz JA (1998) Overexpression of HuR, a nuclear-cytoplasmic shuttling protein, increases the in vivo stability of ARE-containing mRNAs. EMBO J 17:3448–3460
Jeyaraj S, Dakhlallah D, Hill SR et al (2006) Expression and distribution of HuR during ATP depletion and recovery in proximal tubule cells. Am J Physiol Renal Physiol 291:F1255–F1263
López de Silanes I, Zhan M, Lal A et al (2004) Identification of a target RNA motif for RNA-binding protein HuR. Proc Natl Acad Sci U S A 101:2987–2992
Hobert O (2008) Gene regulation by transcription factors and microRNAs. Science 319:1785–1786
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Stark A, Brennecke J, Bushati N et al (2005) Animal microRNAs confer robustness to gene expression and have a significant impact on 3'UTR evolution. Cell 123:1133–1146
O'Connor DT, Zhu G, Rao F et al (2008) Heritability and genome-wide linkage in US and Australian twins identify novel genomic regions controlling chromogranin A: implications for secretion and blood pressure. Circulation 118:247–257
Wei Z, Biswas N, Courel M et al (2011) A common genetic variant in the 3′-UTR of vacuolar H+-ATPase ATP6V0A1 creates a micro-RNA motif to alter chromogranin A processing and hypertension risk. Circ Cardiovasc Genet 4:381–389
Poëa-Guyon S, Amar M, Fossier P et al (2006) Alternative splicing controls neuronal expression of V-ATPase subunit a1 and sorting to nerve terminals. J Biol Chem 281:17164–17172
Kawasaki-Nishi S, Yamaguchi A, Forgac M et al (2007) Tissue specific expression of the splice variants of the mouse vacuolar proton-translocating ATPase a4 subunit. Biochem Biophys Res Commun 364:1032–1036
Smith AN, Skaug J, Choate KA et al (2000) Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat Genet 26:71–75
Smith AN, Borthwick KJ, Karet FE (2002) Molecular cloning and characterization of novel tissue-specific isoforms of the human vacuolar H+-ATPase C, G and d subunits, and their evaluation in autosomal recessive distal renal tubular acidosis. Gene 297:169–177
Sun-Wada G-H, Murata Y, Namba M et al (2003) Mouse proton pump ATPase C subunit isoforms (C2-a and C2-b) specifically expressed in kidney and lung. J Biol Chem 278:44843–44851
Heinemann T, Bulwin G-C, Randall J et al (1999) Genomic organization of the gene coding for TIRC7, a novel membrane protein essential for T cell activation. Genomics 57:398–406
Kane PM (1999) Biosynthesis and regulation of the yeast vacuolar H+-ATPase. J Bioenerg Biomembr 31:49–56
Graham LA, Flannery AR, Stevens TH (2003) Structure and assembly of the yeast V-ATPase. J Bioenerg Biomembr 35:301–312
Davis-Kaplan SR, Compton MA, Flannery AR et al (2006) PKR1 encodes an assembly factor for the yeast V-type ATPase. J Biol Chem 281:32025–32035
Li X, Su RTC, H-t H et al (1998) The molecular chaperone calnexin associates with the vacuolar H+-ATPase from oat seedlings. Plant Cell 10:119–130
Kane PM, Smardon AM (2003) Assembly and regulation of the yeast vacuolar H+-ATPase. J Bioenerg Biomembr 35:313–322
Merzendorfer H, Gräf R, Huss M et al (1997) Regulation of proton-translocating V-ATPases. J Exp Biol 200:225–235
Kane PM (2012) Targeting reversible disassembly as a mechanism of controlling V-ATPase activity. Curr Protein Peptide Sci 13:117–123
Sumner J-P, Dow JAT, Earley FGP et al (1995) Regulation of plasma membrane V-ATPase activity by dissociation of peripheral subunits. J Biol Chem 270:5649–5653
Kane PM (1995) Disassembly and reassembly of the yeast vacuolar H+-ATPase in vivo. J Biol Chem 270:17025–17032
Merkulova M, Hurtado-Lorenzo A, Hosokawa H et al (2011) Aldolase directly interacts with ARNO and modulates cell morphology and acidic vesicle distribution. Am J Physiol Cell Physiol 2011:C1442–C1455
Lu M, Ammar D, Ives H et al (2007) Physical interaction between aldolase and vacuolar H+-ATPase is essential for the assembly and activity of the proton pump. J Biol Chem 282:24495–24503
Marshansky V, Futai M (2008) The V-type H+-ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol 20:415–426
Tabke K, Albertmelcher A, Vitavska O et al (2014) Reversible disassembly of the yeast V-ATPase revisited under in vivo conditions. Biochem J 462:185–197
Su Y, Blake-Palmer KG, Sorrell S et al (2008) Human H+ATPase a4 subunit mutations causing renal tubular acidosis reveal a role for interaction with phosphofructokinase-1. Am J Physiol Renal Physiol 295:F950–F958
Hong-Hermesdorf A, Brüx A, Grüber A et al (2006) A WNK kinase binds and phosphorylates V-ATPase subunit C. FEBS Lett 580:932–939
Armbrüster A, Hohn C, Hermesdorf A et al (2005) Evidence for major structural changes in subunit C of the vacuolar ATPase due to nucleotide binding. FEBS Lett 579:1961–1967
Chan C-Y, Parra KJ (2014) Yeast phosphofructokinase-1 subunit Pfk2p is necessary for pH homeostasis and glucose-dependent vacuolar ATPase reassembly. J Biol Chem 289:19448–19457
Lu M, Sautin YY, Holliday S et al (2004) The glycolytic enzyme aldolase mediates assembly, expression, and activity of vacuolar H+-ATPase. J Biol Chem 279:8732–8739
Smardon AM, Diab HI, Tarsio M et al (2014) The RAVE complex is an isoform-specific V-ATPase assembly factor in yeast. Mol Biol Cell 25:356–367
Tuttle AM, Hoffman TL, Schilling TF (2014) Rabconnectin-3a regulates vesicle endocytosis and canonical Wnt signaling in zebrafish neural crest migration. PLoS Biol 12, e1001852
Dechant R, Binda M, Lee SS et al (2010) Cytosolic pH is a second messenger for glucose and regulates the PKA pathway through V-ATPase. EMBO J 29:2515–2526
Sautin YY, Lu M, Gaugler A et al (2005) Phosphatidylinositol 3-kinase-mediated effects of glucose on vacuolar H+-ATPase assembly, translocation, and acidification of intracellular compartments in renal epithelial cells. Mol Cell Biol 25:575–589
Oehlke O, Schlosshardt C, Feuerstein M et al (2012) Acidosis-induced V-ATPase trafficking in salivary ducts is initiated by cAMP/PKA/CREB pathway via regulation of Rab11b expression. Int J Biochem Cell Biol 44:1254–1265
Diakov TT, Kane PM (2010) Regulation of vacuolar proton-translocating ATPase activity and assembly by extracellular pH. J Biol Chem 285:23771–23778
Parra KJ, Keenan KL, Kane PM (2000) The H subunit (Vma13p) of the yeast V-ATPase inhibits the ATPase activity of cytosolic V1 complexes. J Biol Chem 275:21761–21767
Gräf R, Harvey WR, Wieczorek H (1996) Purification and properties of a cytosolic V1-ATPase. J Biol Chem 271:20908–20913
Diab H, Ohira M, Liu M et al (2009) Subunit interactions and requirements for inhibition of the yeast V1-ATPase. J Biol Chem 284:13316–13325
Jefferies KC, Forgac M (2008) Subunit H of the vacuolar (H+) ATPase inhibits ATP hydrolysis by the free V1 domain by interaction with thte rotary subunit F. J Biol Chem 283:4512–4519
Qi J, Forgac M (2007) Cellular environment is important in controlling V-ATPase dissociation and its dependency on activity. J Biol Chem 282:24743–24751
Kawasaki-Nishi S, Nishi T, Forgac M (2001) Yeast V-ATPase complexes containing different isoforms of the 100-kDa a-subunit differ in coupling efficiency and in vivo dissociation. J Biol Chem 276:17941–17948
Trombetta ES, Ebersold M, Garrett W et al (2003) Activation of lysosomal function during dendritic cell maturation. Science 299:1400–1403
Liberman R, Bond S, Shainheit MG et al (2014) Regulated assembly of vacuolar ATPase is increased during cluster disruption-induced maturation of dendritic cells through a phosphatidyl 3-kinase/mTOR-dependent pathway. J Biol Chem 289:1355–1363
Chintagari NR, Mishra A, Su L et al (2010) Vacuolar ATPase regulates surfactant secretion in rat alveolar typeII cells by modulating lamellar body calcium. PLoS One 5, e9228
Nakamura S (2004) Glucose activates H+-ATPase in kidney epithelial cells. Am J Physiol Cell Physiol 287:C97–C105
Silva P, Gerós H (2009) Regulation by salt of vacuolar H+-ATPase and H+-pyrophosphatase activities and Na+/H+ exchange. Plant Signal Behav 4:718–726
Voss M, Vitavska O, Walz B et al (2007) Stimulus-induced phosphorylation of vacuolar H+-ATPase by protein kinase A. J Biol Chem 282:33735–33742
Finnigan GC, Cronan GE, Park HJ et al (2012) Sorting of the yeast vacuolar-type, proton-translocating ATPase enzyme complex (V-ATPase). J Biol Chem 287:19487–19500
Collaco AM, Geibel P, Lee BS et al (2013) Functional vacuolar ATPase (V-ATPase) proton pumps traffic to the enterocyte brush border membrane and require CFTR. Am J Physiol Cell Physiol 305:C981–C996
Păunescu TG, Ljubojevic M, Russo LM et al (2010) cAMP stimulates apical V-ATPase accumulation, microvillar elongation and proton extrusion in kidney collecting duct A-intercalated cells. Am J Physiol Renal Physiol 298:F643–F654
Pastor-Soler NM, Hallows KR, Smolak C et al (2008) Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am J Physiol Cell Physiol 294:C488–C494
Pech V, Pham TD, Verlander JW et al (2008) Angiotensin II activates H+-ATPase in type A intercalated cells. J Am Soc Nephrol 19:84–91
Alzamora R, Thali RF, Gong F et al (2010) PKA regulates vacuolar H+-ATPase localization and activity via direct phosphorylation of the A subunit in kidney cells. J Biol Chem 285:24676–24685
Rieg T, Rieg JD (2013) Connecting type A intercalated cell metabolic state to V-ATPase function: phosphorylation does matter! Am J Physiol Renal Physiol 305:F1105–F1106
Hallows KR, Alzamora R, Li H et al (2009) AMP-activated protein kinase inhibits alkaline pH- and PKA-induced apical vacuolar H+-ATPase accumulation in epididymal clear cells. Am J Physiol Cell Physiol 296:C672–C681
Alzamora R, Al-Bataineh M, Liu W et al (2013) AMP-activated protein kinase regulates the vacuolar H+-ATPase via direct phosphorylation of the A subunit (ATP6V1A) in the kidney. Am J Physiol Renal Physiol 305:F943–F956
Lee BS, Gluck SL, Holliday LS (1999) Interaction between vacuolar H+-ATPase and microfilaments during osteoclast activation. J Biol Chem 274:29164–29171
Chen S-H, Bubb MR, Yarmola EG et al (2004) Vacuolar H+-ATPase binding to microfilaments: regulation in response to phosphatidylinositol 3-kinase activity and detailed characterization of the actin-binding site in subunit B. J Biol Chem 279:7988–7998
Nelson N, Sacher A, Nelson H (2002) The significance of molecular slips in transport systems. Nat Rev Mol Cell Biol 3:876–881
Grabe M, Wang H, Oster G (2000) The mechanochemistry of V-ATPase proton pumps. Biophys J 78:2798–2813
Kettner C, Bertl A, Obermeyer G et al (2003) Electrophysiological analysis of the yeast V-type proton pump: variable coupling ratio and proton shunt. Biophys J 85:3730–3738
Li SC, Diakov TT, Xu T et al (2014) The signaling lipid PI(3,5)P2 stabilizes V1–Vo sector interactions and activates the V-ATPase. Mol Biol Cell 25:1251–1262
Chung J-H, Lester RL, Dickson RC (2003) Sphingolipid requirement for generation of a functional V1 component of vacuolar ATPase. J Biol Chem 278:28872–28881
van der Poel S, Wolthoorn J, van den Heuvel D et al (2011) Hyperacidification of trans-Golgi network and endo/lysosomes in melanocytes by glucosylceramide-dependent V-ATPase activity. Traffic 12:1634–1647
Yamaguchi M, Kasamo K (2001) Modulation in the activity of purified tonoplast H+-ATPase by tonoplast glycolipids prepared from cultured rice (Oryza sativa L. var. Boro) cells. Plant Cell Physiol 42:516–523
Yoshida K, Ohnishi M, Fukao Y et al (2013) Studies on vacuolar membrane microdomains isolated from Arabidopsis suspension-cultured cells: local distribution of vacuolar membrane proteins. Plant Cell Physiol 54:1571–1584
Zhang Y-Q, Gamarra S, Garcia-Effron G et al (2010) Requirement for ergosterol in V-ATPase function underlies antifungal activity of azole drugs. PLoS Pathog 6, e1000939
Sterling D, Reithmeier RAF, Casey JR (2001) A transport metabolon: functional interaction of carbonic anhydrase II and chloride/bicarbonate exchangers. J Biol Chem 276:47886–47894
Mindell JA (2012) Lysosomal acidification mechanisms. Annu Rev Physiol 74:69–86
Kasper D, Planells-Cases R, Fuhrmann JC et al (2005) Loss of the chloride channel ClC-7 leads to lysosomal storage disease and neurodegeneration. EMBO J 24:1079–1091
Graves AR, Curran PK, Smith CL et al (2008) The Cl-/H+ antiporter ClC-7 is the primary chloride permeation pathway in lysosomes. Nature 453:788–792
Venta PJ, Welty RJ, Johnson TM et al (1991) Carbonic anhydrase II deficiency syndrome in a Belgian family is caused by a point mutation at an invariant histidine residue (107 His → Tyr): complete structure of the normal human CA II gene. Am J Hum Gene 49:1082–1090
Meyers SN, McDaneld TG, Swist SL et al (2010) A deletion in bovine SLC4A2 is associated with osteopetrosis in Red Angus cattle. BMC Genomics 11:337
Kornak U, Kasper D, Bösl MR et al (2001) Loss of the ClC-7 chloride channel leads to osteopetrosis in mice and man. Cell 104:205–215
Schaller S, Henriksen K, Sveigaard C et al (2004) The chloride channel inhibitor N53736 prevents bone resorption in ovariectomized rats without changing bone formation. J Bone Miner Res 19:1144–1153
Thudium CS, Jensen VK, Karsdal MA et al (2012) Disruption of the V-ATPase functionality as a way to uncouple bone formation and resorption – a novel target for treatment of osteoporosis. Curr Protein Peptide Sci 13:141–151
Kartner N, Yao Y, Li K et al (2010) Inhibition of osteoclast bone resorption by disrupting vacuolar H+-ATPase a3–B2 subunit interaction. J Biol Chem 285:37476–37490
Toyomura T, Oka T, Yamaguchi C et al (2000) Three subunit a isoforms of mouse vacuolar H+-ATPase: preferential expression of the a3 isoform during osteoclast differentiation. J Biol Chem 275:8760–8765
Bartkiewicz M, Hernando N, Reddy SV et al (1995) Characterization of the osteoclast vacuolar H(+)-ATPase B-subunit. Gene 160:157–164
Crasto GJ, Kartner N, Yao Y et al (2013) Luteolin inhibition of V-ATPase a3–d2 interaction decreases osteoclast resorptive activity. J Cell Biochem 114:929–941
Shin D-K, Kim M-H, Lee S-H et al (2012) Inhibitory effects of luteolin on titanium particle-induced osteolysis in a mouse model. Acta Biomater 8:3524–3531
Toro EJ, Ostrov DA, Wronski TJ et al (2012) Rational identification of enoxacin as a novel V-ATPase-directed osteoclast inhibitor. Curr Protein Peptide Sci 13:180–191
Holliday LS, Lu M, Lee BS et al (2000) The amino-terminal domain of the B subunit of vacuolar H+-ATPase contains a filamentous actin binding site. J Biol Chem 275:32331–32337
Ostrov DA, Magis AT, Wronski TJ et al (2009) Identification of Enoxacin as an inhibitor of osteoclast formation and bone resorption by structure-based virtual screening. J Med Chem 52:5144–5151
Toro EJ, Zuo J, Ostrov DA et al (2012) Enoxacin directly inhibits osteoclastogenesis without inducing apoptosis. J Biol Chem 287:17894–17904
Toro EJ, Zuo J, Guiterrez A et al (2013) Bis-enoxacin inhibits bone resorption and orthodontic tooth movement. J Dent Res 92:925–931
Lin X, Han X, Kawai T et al (2011) Antibody to receptor activator of NF-κB ligand ameliorates T cell-mediated periodontal bone resorption. Infect Immun 79:911–917
Kawai T, Matsuyama T, Hosokawa Y et al (2006) B and T lymphocytes are the primary source of RANKL in the bone resorptive lesion of periodontal disease. Am J Pathol 169:987–998
Utku N, Heinemann T, Tullius SG et al (1998) Prevention of acute allograft rejection by antibody targeting of TIRC7, a novel T cell membrane protein. Immunity 9:509–518
Jiang H, Chen W, Zhu G et al (2013) RNAi-mediated silencing of Atp6i and Atp6i haploinsufficiency prevents both bone loss and inflammation in a mouse model of periodontal disease. PLoS One 8, e58599
Feng S, Deng L, Chen W et al (2009) Atp6v1c1 is an essential component of the osteoclast proton pump and in F-actin ring formation in osteoclasts. Biochem J 417:195–203
Whyard S, Singh AD, Wong S (2009) Ingested double-stranded RNAs can act as species-specific insecticides. Insect Biochem Mol Biol 39:824–832
Yao J, Rotenberg D, Afsharifar A et al (2013) Development of RNAi methods for Peregrinus maidis, the corn planthopper. PLoS One 8, e70243
Baum JA, Bogaert T, Clinton W et al (2007) Control of colepteran insect pests through RNA interference. Nat Biotechnol 25:1322–1326
Chouabe C, Eyraud V, Da Silva P et al (2011) New mode of action for a knottin protein bioinsecticide: pea albumin 1 subunit b (PA1b) is the first peptidic inhibitor of V-ATPase. J Biol Chem 286
Muench SP, Rawson S, Eyraud V et al (2014) PA1b inhibitor binding to subunits c and e of the vacuolar ATPase reveals its insecticidal mechanism. J Biol Chem 289:16399–16408
Zhang X-H, Li B, Hu Y-G et al (2014) The wheat E subunit of V-type H+-ATPase is involved in the plant response to osmotic stress. Int J Mol Sci 15:16196–16210
Sun-Wada G-H, Imai-Senga Y, Yamamoto A et al (2002) A proton pump ATPase with testis-specific E1-subunit isoform required for acrosome acidification. J Biol Chem 277:18098–18105
Acknowledgements
The authors wish to acknowledge research funding from the Canadian Institutes of Health Research (CIHR). The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Kartner, N., Manolson, M.F. (2016). The Vacuolar Proton ATPase (V-ATPase): Regulation and Therapeutic Targeting. In: Chakraborti, S., Dhalla, N. (eds) Regulation of Ca2+-ATPases,V-ATPases and F-ATPases. Advances in Biochemistry in Health and Disease, vol 14. Springer, Cham. https://doi.org/10.1007/978-3-319-24780-9_20
Download citation
DOI: https://doi.org/10.1007/978-3-319-24780-9_20
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-24778-6
Online ISBN: 978-3-319-24780-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)