Abstract
The eukaryotic vacuolar-type ATPase (V-ATPase) is a multi-subunit membrane protein complex, which is evolutionarily conserved from yeast to human. It is also functionally conserved and operates as a rotary proton pumping nano-motor. In the first part of this chapter we discuss the structure and function of the yeast V-ATPase (V1VO) holoenzyme, We focus on the structural features of its subunits forming both catalytic V1 and proton conducting VO sectors. Particularly, the recently solved structure of DF-subunit complex is discussed in relation to the energy coupling and regulation of yeast V-ATPase. It is noteworthy that the structure could contribute to understanding the function and regulation of V-ATPases of eukaryotes including human, leading to the rational design of specific inhibitors for medical applications. In addition to the well characterized role as proton pump, V-ATPases have acquired alternative cellular functions during evolution. In the second part we analyze novel roles of V-ATPase in function, signaling, and vesicular trafficking of cellular receptors. Our recent studies have uncovered that V-ATPase itself functions as an evolutionarily conserved pH-sensing and signaling receptor, which forms super-complex with aldolase/cytohesin-2/Arf1,6 small GTPases in early endosomes. On the other hand, V-ATPase forms a super-complex with mTORC1/Ragulator/Rag/Rheb small GTPases in late endosome/lysosomes and is involved in amino-acids sensing and monitoring nutritional state of cells. Finally, we discuss the role of V-ATPase in the development and progression of various diseases including cancer, diabetes, and osteopetrosis among others. We also present emerging approaches and future perspectives for specific drug targeting to V-ATPase and its super-complexes.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Eukaryotic V-ATPase
- Holoenzyme
- Acidification
- pH-sensing
- Receptor signaling
- Super-complex
- Vesicular trafficking
- Human diseases
- Drug targeting
1 Introduction
Eukaryotic vacuolar-type ATPases (V-ATPases) are ATP-dependent proton pumps , which are localized in plasma membrane and the organelle membranes, and involved in various cellular processes [1–12]. This enzyme consists of a cytosolic V1 and a membrane embedded VO sectors. The subunit stoichiometry of the V1 and VO sectors are proposed to be A3:B3:C1:D1:E3:F1:G3:H1 and a1:d1:cx:c′y:c″z, respectively [1–5]. Although yeast enzyme has only two isoforms (Vph1p and Stv1p) for a subunit, human and mice have multiple subunit isoforms including: (1) two for the B, E, H and d-subunits; (2) three for the C and G-subunits; and (3) four for a-subunit (a1, a2, a3, and a4). The expression and targeting of V-ATPase with these isoforms are specific for cells and organelles [1–5].
The hydrolysis of ATP into ADP and phosphate (Pi) in the A3B3 catalytic hexamer of V1 sector drives the proton translocation by a ring of c, c′, and c″ subunits of VO sector. The coupling of both events is mediated through the rotation of a complex of DFdcc′c″. The reversible assembly/disassembly of the V1 and VO is a crucial mechanism for the regulation of V-ATPase [13–16]. Originally, this mechanism was discovered in Manduca sexta (M. sexta) and Saccharomyces cerevisiae (S. cerevisiae) in response to ceased feeding [17, 18] and glucose depletion [13, 19], respectively. However, similar mechanism should be essential for mammalian V-ATPase [20–24].
V-ATPases play a central role in the maintenance of pH-homeostasis at the cellular and organism level in mammals [1–5]. This enzyme is also involved in the endosomal pH-sensing [1, 2, 25–30] and has most recently been uncovered as a signaling receptor that modulates the activity of cytohesin-2 (CTH2) and Arf small GTPases [31]. In addition, V-ATPase is involved in sensing of amino acids and monitoring nutritional status of cells via its interaction with mTORC1/Ragulator/Rag and Rheb small GTPases [32–35]. An alternative direct role of eukaryotic ATPases in membrane fusion has been previously proposed [36–39] and the VO has been implicated in this process during exocytosis and insulin secretion in mammalian pancreatic β-cells [2, 8]. Moreover, V-ATPase with a3 and d2 isoforms is assembled in the osteoclast plasma membrane, and a direct role of the d2 isoform in the fusion of osteoclast progenitors has been described [2, 40–43]. Here, we discuss the current understanding of the structure of eukaryotic V-ATPase, focusing on the recently determined crystal structure of S. cerevisiae DF-subunit complex [44]. The finding of their interaction interface could reveal functional insights into coupling and regulation of all eukaryotic V-ATPases. In addition, we describe the emerging novel roles of V-ATPases in acidification of compartments, modulation of the function of critical cellular receptors as well as pH and nutrient sensing and signaling via its super-complexes.
In recent years, the V-ATPase has been implicated in the pathophysiology of a variety of human diseases including primary distal renal tubular acidosis accompanied by sensorial deafness [45], autosomal recessive osteopetrosis [41, 46], and autosomal recessive cutis laxa [47–49]. In addition, a role of the V-ATPase in cancer has recently emerged, since its increased expression at the plasma membrane correlates with the invasive characteristics of various malignant cells [50–53]. Based on these findings, perspectives and strategies in drug targeting to V-ATPase in human disease is discussed.
2 Structure of the Multi-subunit Eukaryotic V-ATPase
2.1 The Two Sector Composition of V-ATPase
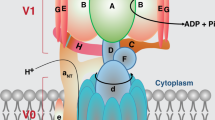
Eukaryotic V-ATPases are multi-protein complexes composed by 14 different subunits A3B3CDE3FG3HacXc′Yc″Zde. V-ATPase holoenzyme have a bipartite structure formed by a cytoplasmic V1 (A3B3CDE3FG3H) and a membrane-embedded VO (acxc′yc′′zde) sectors (Figs. 16.1 and 16.2). The stoichiometry of three VO subunits (cXc′Yc″Z) is unknown, although they are multiple. Both sectors are linked by connecting regions that are important for coupling between ATP hydrolysis in V1 and proton translocation in VO. These connecting regions consist of a central (D,F,d) and three peripheral (E,G) stalks, which are also important for reversible disassembly/assembly of V-ATPase (Fig. 16.1a). Within two V1VO sectors, however, there are functionally identifiable “stator” (A3B3EGCHae) and “rotor” (DFdcXc′Yc″Z) subcomplexes responsible for implementation of rotary mechanism of V-ATPase nano-motor [1–5, 12].
Arrangement of the existing individual atomic subunit structures in the cryo-EM-map of the S. cerevisiae V-ATPase. (a) Subunits C (1U7L; salmon), H (1HO8, brown), and the D (4RND, red) and F assembly (4RND; blue) from S. cerevisiae were fitted into the cryo-EM map. The two conformations of EG subunits, the straight (4DL0; green and cyan) and more bent (4EFA; lemon and pale cyan) are fitted to the three peripheral stalks. The crystallographic structure of two conformations of subunit D in ensemble with the stalk subunit F of S. cerevisiae (ScDF1 and ScDF2) V-ATPase are shown. (Insert) Region of the EM-map showing the interaction of modeled subunit H (S381) (yellow) through the sulfhydryl cross-linker 4-(N-maleimido)benzophenone (MBP) (stick; green) to the S. cerevisiae subunit F1-94 (E31) [44]. Cartoon representation of the structures of the individual S. cerevisiae subunits C (1U7L; salmon), F1-94 (4IX9, blue), H (1HO8, brown) and EG in two conformations, straight (4DL0; green and cyan) and bent (4EFA; lemon and pale cyan). (b) Intermolecular interactions of the subunit DF-assembly. Superimposition of the ScDF1 (pink and blue) with ScDF2 molecule (orange and grey)
Structure and composition of a novel V-ATPase/cytohesin-2/Arf1,6/aldolase signaling super-complex. The diagram shows the structure of the novel V-ATPase/cytohesin-2/aldolase/Arf1,6 super-complex localized on early endosomal (EE) membrane (see Fig. 16.3, Complex 1). It illustrates the binding site of cytohesin-2 (CTH2, in yellow) with an N-terminal tail of a2-subunit (a2N, in green) of V-ATPases [25, 29]. On the left is shown the CTH2 molecule interacting with a2N thorough Sec7-domain and with aldolase through PH-domain. Roman numbers indicate interfaces and affinities of interaction: (I) CTH2 with a2N(1–402) and (II) Sec7 domain with a2N(1–17) and III, IV) CTH2 with aldolase [28, 167]. On the right is shown the aldolase molecule interacting with a2N of V-ATPase
2.2 Catalysis and Energy Coupling in V1 Sector
V-ATPases exist in a dynamic equilibrium between fully assembled holoenzyme and reversibly disassembled V1 and VO [13–19]. Depending on the energy state of the cell, this equilibrium can be rapidly shifted [16, 54]. Recently, S. cerevisiae and M. sexta V-ATPase holoenzymes have been isolated and its three-dimensional (3D) structure was shown using high resolution cryo-electron microscopy (cryo-EM) analysis [55–57]. The structure reveals that three A and B subunits form a hexagon with the nonhomologous regions at the top of subunit A [7, 55, 56, 58, 59]. The two nucleotide-binding subunits A and B alternate around a central cavity, which narrows toward its center and opens at both ends. Subunit D inside the cavity forms different interaction with domains of the three subunits A and B [12, 55, 56, 58]. The crystallographic structure of two conformations of subunit DF complex (ScDF1 and ScDF2) forming the central stalk of S. cerevisiae V-ATPase has been solved recently [44]. Subunit D in the complex consists of a long pair of α-helices, connected by a short helix (79IGYQVQE85) and a β-hairpin region, which is flanked by two flexible loops (Fig. 16.1b). The long pair of helices is composed of the N- and the C-terminal helix, respectively, and show structural alterations in the ScDF1 and ScDF2 structures. The subunit F consists of an N-terminal domain of four β-strands (β1-β4) connected by four α-helices (α1-α4). α1 and β2 are linked via the loop 26GQITPETQEK35, which is unique in eukaryotic V-ATPases (Fig. 16.1a). Adjacent to the N-terminal domain is a flexible loop (P95-D106), followed by a C-terminal α5-helix. A perpendicular and extended conformation of the α5-helix was observed in the two crystal structures and in solution X-ray scattering experiments, respectively (Fig. 16.1a, left) [44]. The concerted interaction of the DF complex, including the P95-D106-loop with helix α5 of subunit F and bended C-terminal helix of subunit D, may activate ATP hydrolysis in the catalytic A3B3 hexamer [44, 60].
The 26GQITPETQEK35-loop of the subunit F is facing to the C-terminal serine (S381) of subunit H, revealed to be involved in cross-linking subunit F of the V1 (Fig. 16.1a, right) [60, 61]. The unique stalk of subunit H is characterized by a large, primarily α-helical N-terminal domain, which is forming a shallow groove connected by a four-residue loop to the C-terminal domain [62]. This arrangement led to the proposal, that in the process of V1 and VO dissociation the flexible C-terminal domain of subunit H moves slightly closer to the exposed 26GQITPETQEK35-loop of subunit F, where it causes conformational changes, leading to an inhibitory effect of ATPase activity in the V1 [44, 60]. 15N-[1H] heteronuclear NOE studies on the subunit F revealed a rigid core formed by β-strands, β1–β4, and α2–α4. In comparison, the N- and C-terminal helices α1 and α5 with their adjacent loops 26GQITPETQEK35 and 94IPSKDHPYD102, respectively, are more flexible in solution [60]. The N-terminal helix α1 of subunit F and the bottom segment of subunit D are in proximity to subunit d, forming the tip of the central stalk and being in direct neighborhood of the proton translocating ring of c-, c′-, c″-subunits of VO [60]. This area might be modified during the process of reversible assembly/disassembly of the V1 and VO, as shown previously. The higher flexibility of α1 in subunit F would allow to transmit the conformational change of subunit d during dissociation from the DF-heterodimer and also the movement of subunit H closer to F, through the neighboring 26GQITPETQEK35-loop [44, 60].
In addition to the central stalk, the catalytic A3B3 hexamer is connected to VO by three peripheral stalks with a different degree of twisting in the C-terminal and/or middle part (Fig. 16.1a, left) [1]. The subunit E and G, forming the peripheral stalk, are arranged in a ~150 Å long complex. The peripheral stalk is connected to the A3B3 through the globular C-terminus of subunit E, formed by α-helices and β-sheets, arranged as β1:α1:β2:β3:β4:α2 [63, 64]. This C-terminus is connected by a flexible loop region with N-termini of both subunit E and G, which are folded in a noncanonical, right-handed coiled coil. The coiled coil is disrupted by a bulge of partially unfolded secondary structure in subunit G, which provides the necessary flexibility of the peripheral stalk during detachment and assembly of the V1 from the VO (Fig. 16.1a, left) [63].
The reversible disassembly of the V1 and VO is initiated by the dissociation of subunit C [65]. As shown for its hydrated [66] and crystallized form [67] subunit C is a boot-shaped protein with an upper head domain formed from α-helices and β-strands (residues 167–262) and a globular foot domain (residues 1–55 and 320–392). Both domains are connected by an elongated helical neck domain (Fig. 16.1a, left) [67]. Location and orientation of subunit C in the enzyme enables its binding to actin [67], ADP/ATP nucleotides [68], and WNT-kinase [69]. These interactions are taking place through the foot domain allocated in proximity to the N-termini of an EG-heterodimer, as well as in neighborhood to the N-terminal region of subunit a (Fig. 16.1a, left). It was suggested that binding of ATP/ADP [68] or WNT-kinase dependent phosphorylation [69] of subunit C could alter the stability of an subunit EGC assembly by affecting its binding properties with either EG-heterodimer or with actin.
2.3 Structure of the VO Enabling Proton Conduction
The proton conducting VO sector of V-ATPase may be formed by five or six subunits and two accessory proteins. Indeed, S. cerevisiae VO consists of six different subunits (a, c, c′, c′′, d, and e). However, the c′ subunit gene was not found in mammalian genome, and thus, the mouse and human VO are formed only by five subunits: a (a1, a2, a3, or a4), c, c′′, d (d1 or d2), and e (e1 or e2). In turn, the mammalian VO also contains the two additional accessory subunits Ac45 and M8-9 [1, 5, 70]. Structurally, the VO is composed of a ring of c- and c″-subunits (c/c″ ring) and the adjacent single copies of the a, e, Ac45, and M8-9 subunits. As suggested by the 3D map of the VO from bovine clathrin-coated vesicles V-ATPase, the accessory subunit Ac45 interacts with the c/c″ ring from the luminal side [71].
On the other hand, the N-terminal cytosolic tail of subunit a (aN) is oriented parallel to the cytoplasmic surface of the membrane in the close proximity to the N-terminus of subunit H (Fig. 16.2) [1, 55]. Recent small-angle X-ray scattering studies of the N-terminal tail a 104-363, suggested the connection between the cytoplasmic N-terminal (aN) and the transmembrane C-terminal (aC) domains of subunit a (Fig. 16.2) [72]. This arrangement makes the aN of V-ATPase accessible for cytohesin-2 (CTH2) and Arf1, Arf6 small GTPases (Fig. 16.2), which is essential for signaling and trafficking of various receptors, including EGFR/ErbB receptors (Fig. 16.3) [1, 31, 72]. Although the structure and orientation of the aN is available, the transmembrane topology of its aC remains controversial (Fig. 16.2) [1, 26]. In yeast a six [73, 74], eight [75, 76], and nine [77, 78] transmembrane helix models have been proposed. According to the model with eight helices, both N- and the C-termini of the a subunit (Vph1p) are located in the cytosol, which is supported by the results showing interaction of phosphofructokinase-1 with the C-terminal tail of the human a4- and a1-isoforms [79]. The moving membrane part of the yeast V-ATPase “rotor” is a ring composed by the c-, c′-, and c″-subunits. The c- and c′-subunits are 16 kDa proteins, proposed to contain four transmembrane helices with two loops exposed to the cytosol, while the c′′-subunit is a 23 kDa polypeptide with five putative transmembrane helices, two loops and a C-terminal tail exposed to the cytosol [80, 81]. Recently, cryo-EM observation of rotational states in S. cerevisiae V-ATPase has revealed the involvement of ten c-, c′-, and c″-subunits in the ring formation [56]. Each of these subunits is contributing two transmembrane helices to the inner ring and two helices to the outer ring. In addition, this study supported the eight transmembrane helices model of a-subunit [75, 76]. Remarkably, it was found that two of the helices are highly tilted and span the membrane where the a-subunit is in contact with the ring of ten c-, c′-, and c″-subunits, providing the new insights on the proton conducting mechanist of VO sector [56]. The subunit composition and number of transmembrane helices in the c/c″-rings of mammalian V-ATPases are open questions. Recently, the first evidence for the position of subunit e within VO has been provided [82].
Signaling and trafficking of EGFR/ErbB-receptors in endosomal/lysosomal pathway. Scheme shows signaling of epidermal growth factor (EGF) and trafficking of EGFR/ErbB-receptors in clathrin-dependent endocytosis and endosomal/lysosomal protein degradative pathway. The compartments are shown in yellow/red as follows: CCV clathrin-coated vesicles, EE early endosomes, MVB multi-vesicular bodies, LE late endosomes, RE recycling endosomes, LS lysosomes, AP autophagosomes, ER endoplasmic reticulum. Vesicular trafficking steps for the degradation branch are indicated with black arrows and for recycling branch with blue arrows. (EE and super-complex-1) Structure and composition of V-ATPase/CTH2/aldolase/Arf1,6 super-complex located in early endosomes. V-ATPase in this super-complex functions as pH-sensing and cytohesin-2/Arf1,6 signaling receptor which may regulate the trafficking and signaling EGFR/ErbB-receptors. (LE/LS and super-complex-2) Structure and composition of a novel V-ATPase/Ragulator/RagA/C/mTORC1/Rheb super-complex located in late endosomes and lysosomes. This complex is involved in sensing levels of amino acids and modulation of mTORC1-dependent downstream cellular programs and cell growth. Moreover, reversible association/dissociation of V1VO sectors of lysosomal V-ATPase is regulated by signaling of EGFR/ErbB-receptors through Akt/Erk pathway (dashed red arrows)
The cryo-EM map of yeast V-ATPase revealed that a proton conducting channel is formed by the interface between transmembrane helixes of a-subunit and the ring of c-, c′-, c″-subunits. This channel is very narrow and occurs near the middle of the membrane region [12, 83]. It is important to note that the potent V-ATPase inhibitors bafilomycin A1 (BafA1) and concanamycin A (ConA) bind to the interface between a-subunit and the ring of c-, c′-, c″-subunits inhibiting both rotary and proton-pumping mechanisms of yeast V-ATPase [84–86]. Although these compounds are very useful to analyze the role of V-ATPases in inside-acidic organelles of cultured mammalian cells [87, 88], they are unable to distinguish between V-ATPases with different isoforms, which are targeted to specific compartments. Therefore, there is an urgent need for the development of a new generation of inhibitors capable of selective inhibition of the subset of V-ATPases located at the specific compartments, such as Golgi, endosomes and lysosomes among others. Thus, an accumulating knowledge of high resolution structures of individual V-ATPase subunits as well as their interaction interfaces will be important for future developments of new generation of: (1) isoform and (2) organelle and/or (3) cell specific V-ATPase inhibitors (Figs. 16.1 and 16.2).
3 The Role of V-ATPases and Their Super-complexes in Acidification, Signaling, and Sensing
V-ATPase generates an electrochemical proton gradient, forming acidic intracellular compartments [1–5]. This enzyme is also targeted to the plasma membrane and is involved in extracellular acidification of specialized cells in kidney [89–91], epididymis [90, 92, 93], and bone [40, 74, 94–97]. It also acidifies extracellular environment in metastatic cancer cells [50–53]. The regulation of V-ATPase is achieved by the following three major processes: (1) modulation of acidification by the chemiosmotic mechanism; (2) targeting to specific organelles; and (3) regulation of the enzyme activity through reversible assembly/disassembly V1 and VO.
3.1 Chemiosmotic Process
The electrochemical proton gradient generated by V-ATPase consists of a proton gradient (ΔpH) and membrane potential (ΔΨ). Using a FRET approach , the values ΔpH = 2.2 units and ΔΨ = 27 mV were experimentally determined in intracellular organelles [98]. Acidification is predominant function of V-ATPase, which depends on its coupled function with Cl−/H+- and Na+/H+-exchangers as an important chemiosmotic mechanism regulating acidification of intracellular organelles [99–102].
3.2 Process of Subunit-Specific Targeting of V-ATPase
The S. cerevisiae V-ATPase is targeted by the two a-subunit isoforms Vph1p and Stv1p to the vacuole and Golgi/endosomes, respectively [103, 104]. Studies with chimeric proteins revealed that the targeting information is located in the cytosolic N-terminal domain of the a-subunit [104]. Similarly, in mammalian cells, localization of V-ATPase in endocytic and exocytic compartments and targeting to the plasma membrane depend on a-subunit isoforms (Figs. 16.2 and 16.3) [1–4, 94]. Of four subunit a isoforms (a1, a2, a3 and a4) [1–4], V-ATPase with a1 isoform is specifically targeted to presynaptic membranes and exocytic synaptic vesicles in mammalian neurons [105, 106]. Studies with neurosecretory PC12 cells revealed that both a1 and a2 regulate the acidification and neurotransmitter uptake and release by exocytic vesicles [107]. In contrast, V-ATPase with a1 is targeted to the endocytic pathway in brain microglial cells, and plays role in the fusion between phagosomes and lysosomes during phagocytosis, a process of microglial-mediated neuronal degeneration [108].
The a2 isoform targets V-ATPases to early endosomes of the endocytic pathway of kidney proximal tubule epithelial cells [25, 26, 30, 109, 110]. In these cells, the overexpressed recombinant a2-isoform (a2-EGFP) is also targeted V-ATPase to endosomal compartments [31]. However, in cultured osteoclast cells and B16 cells, both endogenous a2 and a1 are targeted to secretory vesicles of Golgi complex in the exocytic pathway [94, 107]. Similarly, over-expression of recombinant a2 isoform (a2-EmGFP) in neuroendocrine PC12 cells targets V-ATPase to the Golgi apparatus [94, 107].
The V-ATPase with a3 is a lysosome specific enzyme of osteoclast progenitor. During differentiation, the V-ATPase with a3 is targeted and localized to the plasma membrane of osteoclasts. The osteoclast V-ATPase is involved in bone reabsorption and its defect caused infantile malignant osteopetrosis in humans [40, 74, 94–97]. V-ATPase with a3 is also targeted to the plasma membrane of breast cancer cells and plays an important role in metastatic processes [50–53]. Phagosomes in macrophage also acquire the same enzyme from lysosomes [111], whereas it is specifically targeted to insulin secretory granules of pancreatic β-cells [2, 94].
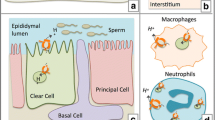
The V-ATPase with a4 is highly abundant in epididymis and kidney, where they are specifically targeted to the apical plasma membrane of epididymal clear cells and kidney collecting duct intercalated cells [89–93], indicting that this V-ATPase is involved in sperm-maturation [92, 93] and maintenance of acid-balance [90, 91], respectively. These results indicate that the a-subunit isoforms differentially target V-ATPase to the plasma membrane or intracellular compartments of the endocytic/exocytic pathways. In turn, the specific targeting and assembly of the enzyme may modulate acidification of extracellular milieu and intracellular organelles [1–4, 93, 112].
3.3 Reversible Assembly/Disassembly of V-ATPase
The regulation of V-ATPases by assembly/disassembly of the V1VO sectors was first described in response to ceased feeding in M. sexta [17, 18] and in response to glucose depletion in S. cerevisiae [13, 14, 19]. In S. cerevisiae, assembly/disassembly of the V1VO may be regulated by both a-isoform (Vph1 or Stv1) and E subunit. Evidence for the role of the E subunit in assembly was first obtained from yeast/mouse hybrid V-ATPase [113, 114]. A null mutant of yeast subunit E expressing the mouse (E1 or E2) is functional, indicating that this hybrid V-ATPase with E1 or E2 is functional as proton pump. However, assembly of the hybrid with E1 became less dependent on glucose [114]. Furthermore, a domain between residues K33 and K83 of S. cerevisiae subunit E could be replaced by the corresponding region of mouse E1 [114, 115]. Alanine scanning mutations revealed that the residue E44 of yeast subunit E is a key amino acid in regulation of subunit assembly and thus enzyme activity [115].
In S. cerevisiae, the reversible assembly and disassembly of V1VO sectors is controlled by two distinct mechanisms. While the assembly requires the cytosolic RAVE/Rab-Connectins complex (Rav-1, Rav-2, and Skp1), the disassembly process involves the cytosolic microtubular network [1, 2, 4, 5, 15, 116]. Recent study revealed, that RAVE/Rab-Connectins in yeast is an a-isoform specific complex, which is necessary for assembly of V-ATPase with Vph1p but not with Stv1p [117]. Moreover, the reversible assembly/disassembly of the yeast V1 and VO is also controlled by the direct interaction of V-ATPase with cytosolic aldolase, a central enzyme of the glycolysis (Fig. 16.2). The assembly/disassembly and interaction with aldolase is modulated by the Ras/cAMP/PKA pathway [118], suggesting that V-ATPases may act as a cytosolic glucose-sensor (Fig. 16.2) [119–121]. However, in mammalian kidney proximal tubule cells, glucose regulation on V-ATPase is modulated by the phosphatidylinositol 3-kinase (PI3K) pathway [21]. In dendritic cells, the assembly of V1VO is also regulated through the PI3K/mTOR-dependent pathway and is critical for lysosomal acidification, protein degradation, and antigen presentation (Fig. 16.3) [20, 24]. Thus, it is generally accepted that various regulatory pathways are involved in controlling assembly/disassembly of V-ATPase in eukaryotic cells and, therefore, in modulation of its function as well as acidification of intracellular organelles and extracellular milieu [22].
3.4 Novel Role of V-ATPases in Regulation of Signaling and Trafficking of Cellular Receptors
A novel role of V-ATPases in regulation of signaling, trafficking, and degradation of various cellular receptors has emerged recently. Endocytosis is an essential cellular process that is used by eukaryotic cells for the internalization of various receptors localized in the plasma membrane. As shown previously, the clathrin-dependent endocytosis pathway mediates internalization of Fz/LRP6, PRR, Notch, transferrin, megalin/cubilin, and EGFR/ErbB receptors among others (Fig. 16.3) [1, 31, 122]. Mounting evidence indicates that the V-ATPase is not only establishing the acidic pH in endocytic organelles but is also functioning as a cytohesin-2/Arf1,6 small GTPases signaling receptor (Fig. 16.2). Moreover, the V-ATPase is involved in direct interactions with critical cellular receptors, and thus, could modulate their signaling, traffic, and degradation along the endocytic pathway (Fig. 16.3). These emerging roles of V-ATPase will be discussed below.
3.5 V-ATPase and Epidermal Growth Factor Receptors (EGFR/ErbB’s)
The epidermal growth factor receptor (EGFR ) was among the first discovered growth receptors that regulate crucial cell biological processes including cell proliferation [123, 124]. The EGFR/ErbB-receptors (EGFR/ErbB’s) family comprises four members (EGFR/ErbB1, ErbB-2, ErbB-3, and ErbB-4) and are involved in the development of a variety of cancers [124–127]. Activation of EGFR/ErbB-receptors by extracellular EGF ligand promotes their hetero-dimerization with subsequent activation of TK-domains and tyrosine trans-phosphorylation of the cytoplasmic tail. However, the cytoplasmic proteins that could modulate EGF-induced activation and signaling of EGFR/ErbB-receptors were largely unknown.
Cytohesin-2 (CTH2) has been identified recently as a cytoplasmic activator of EGFR/ErbB-receptors (Fig. 16.3) [128, 129]. CTH2 enhances trans-dimerization and activation of EGFR/ErbB’s by direct binding with TK-domains of dimerized receptors and by facilitating conformational changes and trans-phosphorylation of these domains. Figure 16.3 illustrates the signaling of the epidermal growth factor (EGF) through EGFR/ErbB’s localized in the plasma membrane and early endosomes. The crucial role of CTH2 in hetero-dimerization of these receptors is also indicated. The figure also shows the V-ATPase dependent trafficking and signaling of EGFR/ErbB-receptors through the clathrin-dependent endocytosis endosomal/lysosomal protein degradation pathway. In particular, a novel important role of the assembly/disassembly of V1VO and V-ATPase-dependent late endosomal/lysosomal acidification in EGFR/ErbB-receptors function has been recently revealed [23]. It was demonstrated that EGF/EGFR-dependent signaling promotes the rapid recruiting of cytosolic V1 sectors of the V-ATPase and increases its assembly with VO on late endosomal/lysosomal compartments. This assembly in turn increases V-ATPase driven lysosomal acidification, protein degradation, and release of amino acids needed for Rheb(GTP) and mTORC1 activation (Fig. 16.3). V-ATPase is playing an indirect role in EGF-dependent activation of mTORC1 signaling pathway by modulating the assembly/disassembly of V1 and VO [32]. This is the first evidence showing the functional assembly of V-ATPase in response to the signaling of EGFR/ErbB-receptors. In this way, V-ATPase regulates mTORC1 signaling and trafficking EGFR/ErbB-receptors within the endosomal/lysosomal protein degradation pathway (Fig. 16.3) [23, 32]. Thus, V-ATPase-dependent acidification and cytohesin-2/Arf1,6 small GTPases signaling (Fig. 16.2) may play a key role in the modulation of EGFR/ErbB-receptors function, and is pivotal for the sustained signaling, recycling, or degradation (Fig. 16.3).
3.6 V-ATPase, Insulin-Like Growth Factor-1 Receptor (IGF-1R) , and Heme-Responsive Gene 1 (HRG-1) Protein
Both the growth hormone (GH) and insulin-like growth factor 1 (IGF-1) exert powerful control over lipid, protein and glucose metabolism. The function of GH/IGF-I signaling pathway is associated with longevity, and thus, aging related morbidities including diabetes and cancer [130, 131]. This pathway also plays roles in maintenance and repair of muscles [132]. Signaling by insulin-like growth factor receptor (IGF-1R) controls expression of heme-responsive gene 1 (HRG-1) that encodes a 16 kDa transmembrane protein. A recent study revealed specific targeting of this protein to early endosomes and its direct interaction with V-ATPase c-subunit [133]. Increased expression of HGR-1 enhances V-ATPase activity in the plasma membrane, lowers the extracellular pH and activates pH-dependent matrix metalloproteinases. HRG-1 also increases endosomal V-ATPase activity, which promotes trafficking of the IGF-1R, β1-integrin and glucose transporter-1 (GLUT-1) with concomitant increase of glucose uptake, cancer cell growth, migration and invasion. Thus, HRG-1 may represent a novel target for selectively disrupting V-ATPase activity and the metastatic potential of cancer cells [133, 134].
3.7 V-ATPase and Frizzled (Fz) and Low-Density Receptor-Related Protein (LRP6 ) Receptors
The Wnt/β-catenin, Wnt/PCP(planar cell polarity) and Wnt/Ca2+ signaling pathways are essential mechanisms that control embryonic tissue development, homeostasis, cell proliferation, polarity, and apoptosis [135, 136]. They are strongly linked to the development of a variety of human diseases including metastatic cancers [135, 136]. The direct role of V-ATPase in regulation of Wnt/β-catenin and Wnt/PCP signaling pathways has been uncovered recently [137, 138]. It was shown that signal transmission after association of Wnt ligands with Fz/LRP6 co-receptors requires direct interaction of LRP6 with an accessory M8-9 subunit of V-ATPase, also called V-ATPase lysosomal accessory protein-2 (ATP6AP2). This interaction takes place in early endosomes and the ATP6AP2 subunit acts as an adaptor that brings together V-ATPase and the Wnt/Fz/LRP6 receptor complex. Thus, both direct and electrochemical regulation by V-ATPases are involved in signaling of Wnt/Fz/LRP6 in endosomes involved in the protein degradation pathway.
3.8 V-ATPase and (Pro)renin Receptor (PRR )
The (pro)renin receptor (PRR) , a single transmembrane cell surface receptor, plays a central role in activating the local renin-angiotensin system. Binding of prorenin to PRR induces its conformational change, allowing conversion of angiotensinogen to angiotensin-I, which is subsequently converted to angiotensin-II by an angiotensin-converting enzyme [139, 140]. However, an angiotensin-independent function of PRR has also been recently reported, which was identified as an accessory ATP6AP2 subunit of V-ATPase [137, 138, 140, 141]. Tissue-specific conditional knockout experiments confirmed a role of PRR/ATP6AP2 in assembly of V-ATPase in murine cardiomyocytes [142]. Importantly, the level of prorenin is elevated during diabetes and over-activation of PRR is associated with development of hypertension and diabetic kidney disease [122, 143]. The role of PRR in kidney function and its association with diabetes and hypertension has been recently reviewed [122, 140, 141, 143]. Thus, future studies in this area could lead to the novel therapeutic approaches for the treatment of hypertension, diabetes, and their complications [122].
3.9 V-ATPase and Notch Receptor
The cell-to-cell signaling by the Notch receptor pathway is critical during development and tissue regeneration for controlling the balance between cell proliferation and apoptosis. Pathological loss of regulation of Notch receptor signaling is also a hallmark of different cancers [144, 145]. Activation of the Notch receptor by ligands gives rise to its cleavage by γ-secretase-mediated intra-membrane proteolysis followed by activation of specific target genes. Surprisingly, recent studies revealed that V-ATPase driven acidification may control two opposite processes in the Notch signaling in Drosophila: (1) lysosomal degradation and loss of regulation of Notch receptors; and (2) γ-secretase-mediated Notch receptor activation in early endosomes [146]. Moreover, Notch receptor signaling is also controlled by Rabconnectin-3A/B (Rbcn-3) through regulating V-ATPase both in Drosophila and mammalian cells [147, 148]. It is noteworthy that mammalian Rbcn-3 protein is a homolog of yeast Rav-1, which forms a part of the RAVE (Rav-1, Rav-2, and Skp1) complex. Interaction of Rav-1 with V-ATPase is essential for reversible assembly/disassembly of the yeast V1 and VO [15, 116, 149]. Similar to Wnt-signaling, these studies also revealed two mechanisms of Notch-signaling modulation by V-ATPase in mammalian cells: (1) through subsequent Rbcn-3/V-ATPase interaction and (2) V-ATPase-driven acidification leading to activation of γ-secretase [138, 146–148].
3.10 V-ATPase as pH-Sensor and Cytohesin-2/Arf Small GTPases Signaling Receptor
The Arf-family small GTPases belong to the Ras-superfamily that are involved in regulation of a great variety of cellular pathways [150]. These GTPases function as “molecular switches” and the transition between “on” and “off” is mediated by a GDP/GTP cycle. In particular, activation of Arf small GTPases is accomplished by the cytohesin-family of guanine nucleotide exchange factors (GEFs) . Cytohesin-family GEFs include cytohesin-1, cytohesin-2 (CTH2) (also known as ARNO), cytohesin-3 (also known as GRP1), and cytohesin-4. The generally accepted functions of cytohesin/Arf small GTPases are regulation of organelle biogenesis, modulation of vesicular trafficking, and actin cytoskeleton remodeling [151–153]. However, cytohesins have also emerged recently as central modulators of signaling and trafficking of plasma membrane receptors including: (1) EGFR/ErbB [128, 129], (2) insulin-receptor (IR) and insulin-like growth factor-1 receptor (IGF-1R) [154–158], (3) VEGF-2R [159], and (4) integrins [160, 161]. In particular, cytohesin-1 and -2 have been identified as activators of EGFR/ErbB’s that are involved in regulation of cell proliferation and oncogenesis [128, 129]. Cytohesins are also crucial downstream effectors for the IR and IGF-1R signaling cascade involved in regulation of calorie restriction pathway, longevity, and aging process [154–158, 162–164].
Previously, our laboratory focused on studies of the functional interplay between V-ATPase and cytohesin-2/Arf1,6 small GTPases in endosomal/lysosomal protein degradation pathway [30, 109, 165, 166]. The cytohesin-2 and Arf1, Arf6 are specifically targeted to early endosomes of this pathway and co-localized with V-ATPase in kidney proximal tubule cells (Figs. 16.2 and 16.3) [30, 109]. Moreover, subsequent work from our laboratory revealed that V-ATPase itself functions as a novel long-sought pH-sensor or pH-sensing receptor. In particular, V-ATPase containing a2 isoform is specifically targeted to early endosomes and directly interacts with cytohesin-2 (CTH2) in acidification-dependent manner, suggesting that the a2 is a putative pH-sensing receptor (Fig. 16.2) [25, 26]. According to this concept, V-ATPase is not only responsible for proton pumping and the generation of a pH gradient but also involved in sensing the levels of acidification and transmitting this information through membrane. Moreover, other isoforms (a1, a3, and a4) were also found to interact with CTH2 [167]. Taken together, these results suggest that pH-sensing by V-ATPases and interaction with cytohesin-2/Arf1,6 small GTPase is a general phenomenon, which may take place in other acidic organelles of both the exocytotic and the endocytic pathways. We have uncovered the molecular details of unexpected function of V-ATPases, as an evolutionarily conserved pH-sensing and cytohesin-2/Arf1,6 small GTPases signaling receptor (Fig. 16.2) [1, 2, 25–27, 31, 167].
4 Functions of V-ATPase in Normal and Disease States
In this section, we discuss roles of V-ATPases in regulating vesicular trafficking and the development of various disease states. First, our focus is the role of V-ATPases in cancer development and metastasis process. Second, we analyze its role in modulation of exocytic/secretory pathway during pathogenesis of diabetes and bone diseases. Third, we discuss the roles of V-ATPases in regulating two novel super-complexes localized in endocytic or endosomal/lysosomal protein degradation pathway (Fig. 16.3). We also discuss their roles in cancer as well as aging and age-related diseases.
4.1 V-ATPase in Cancer Development and Metastasis
Human and mouse a1 and a2 are expressed ubiquitously under physiological conditions, whereas a3 and a4 are specifically expressed and targeted to the plasma membranes of bone osteoclast and kidney intercalated cells, respectively [73, 92, 93, 168, 169]. Previous studies suggested that the a subunit targets V-ATPase to different compartments and plasma membranes as well as senses the pH-gradien t formed during acidification (Fig. 16.2) [1, 2]. V-ATPases with specific a-isoforms have been detected at the plasma membrane of the breast, ovarian, and prostate cancer cells. The role of V-ATPase dependent extracellular acidification was studied extensively in development and invasive phenotype of these metastatic tumors.
4.1.1 Breast Cancer
Early studies have demonstrated that V-ATPases are expressed in the plasma membrane of invasive cancer cells [170]. V-ATPase was found to be more prominently expressed in the highly metastatic MDA-MB231 breast cancer cells, which are more invasive and migratory than the less metastatic MCF-7 breast cancer cells. Moreover, inhibition of V-ATPase by BafA1 significantly reduces the in vitro invasion of MDA-MB231 but not MCF7 cells [50]. These results indicate that targeting of V-ATPase to the plasma membrane is correlated with metastatic phenotype of the breast cancer cells. However, the mechanism of V-ATPase function and the subunits involved in its targeting to the plasma membrane remain obscure. Further studies have focused on the role of the a-subunit isoforms in cancer. Although all four isoforms are found in MDA-MB231 but not MCF7 cell lines, the expression levels of a3 and a4 are significantly higher in metastatic MDA-MB231 than in non-metastatic MCF-7 cells. In addition, siRNA knockdown experiments further demonstrated the role of a3 and a4 isoforms in targeting V-ATPase to the plasma membrane and invasive phenotype of MDA-MB231 cells [51]. Similar role of a3 was found in invasiveness of MCF10CA1 but not MCF10a breast cancer cells [52]. Finally, the recent studies demonstrated that invasiveness of breast cancer cells could be modulated by cell-impermeable molecules targeting extracellular parts of V-ATPase: (1) a biotin-conjugated BafA1 or (2) the monoclonal antibody directed against the V5 epitope constructed on the extracellular loop of VO c-subunit [53].
Other roles of V-ATPase in breast cancer development and metastasis have been revealed recently. First, V-ATPase is required for trafficking of Rab27B small GTPase dependent pro-invasive secretory vesicles which promote an invasive growth and metastasis in estrogen receptor (ER) alpha-positive breast cancer patients. Therefore, a role of V-ATPase in invasive growth and metastasis of ER-alpha-positive breast cancer has been suggested [171]. This study demonstrated that inhibiting V-ATPase by BafA1 or silencing of a1 or d subunits might be an effective strategy for blocking Rab27B-dependent pro-invasive secretory vesicles which are involved in secretion of pro-invasive growth regulators. Second, V-ATPase driven acidosis of tumors may also control the pro-apoptotic protein Bnip3 death pathway [172]. Accordingly, it was reported that pharmacologic inhibition of V-ATPase with BafA1 could effectively activate Bnip3 pathway, promote death of breast cancer cells and significantly reduced tumor growth in MCF7 and MDA-MB-231 mouse xenografts in vivo. Importantly, the combined treatment of mice bearing the breast MDA-MB-231 xenografts with BafA1 and ERK1/2 inhibitor sorafenib has potentiated action of two inhibitors for tumor regression. These results present a novel mechanism to kill cancer cells and reverse resistance of breast hypoxic tumors. Third, breast cancer invasive cells are resistant to anoikis, a specific type of apoptosis promoted by loss of cell–matrix contact. A recent study demonstrated, that triggering of anoikis by V-ATPase inhibitor archazolid is promising therapeutic approach to reduce metastasis of breast cancer cells in mouse model in vivo [173]. Forth, very recent study has identified a novel tumor-metastasis related gene 1 (TMSG1) as a regulator of activity of V-ATPase and secreted metalloproteinase-2 in breast cancer cells [174].
All together, these studies suggest that V-ATPase could modulate invasiveness and breast tumor metastasis due to acidification of extracellular milieu and subsequent activation of metalloproteases. On the other hand, V-ATPase also controls apoptotic cell death and regulates vesicular trafficking and secretion of pro-invasive growth factors. Importantly, in breast cancer cells both invasiveness and metastasis could be controlled by targeting extracellular part of plasma membrane V-ATPase [175–177].
4.1.2 Ovarian Cancer
The role of V-ATPase in invasiveness and metastasis has also been recently addressed in ovarian carcinoma. Particularly, the expression, targeting, and function of a-subunit isoforms were studied in ovarian cancer tissues as well as in ovarian cancer A2780, SKOV-3, TOV-112D cell lines [178]. The a2-isoform is predominantly expressed in these cells and targeted V-ATPase to the plasma membrane. Under physiological conditions, a2-isoform is predominantly located in early endosomal compartment or Golgi and, thus, it relocates to the plasma membrane during tumorigenesis and metastasis. This study also reveals co-association of a2-isoform with cortactin, a protein involved in invasion of tumor cells. Targeting of a2 with monoclonal antibody reduces the activity of matrix MMP-2 and MMP-9 metalloproteinases and invasiveness of these ovarian cancer cells. These findings suggest that a2-isoform could be promising target for developing novel therapeutic anti-V-ATPase antibodies against ovarian carcinoma [178]. Finally, an important role of the a2-isoform derived secretory peptide in inflammation, angiogenesis, and tumorigenesis was also proposed [179–182].
4.1.3 Prostate Cancer and Tumor Angiogenesis
Angiogenesis is recognized as one of the hallmarks of cancer which enable tumor growth and metastatic dissemination [127]. Moreover, tumors are currently recognized as abnormal organs consisted of a complex mixture of the cells interacting and signaling with each other and required stable supply of nutrients and oxygen for their needs [183]. The cancer-induced neovascularization is triggered by pro-angiogenic signaling and cell-to-cell cross talk. In particular, plasma membrane V-ATPase was implicated in regulation of intracellular pH and migration of microvascular endothelial cells [184, 185]. Moreover, V-ATPase is taking part in cross talk and regulation of communication between microvascular endothelial and metastatic cells promoting acidification of extracellular space and favors protease activity [186]. A different mechanism of cross talk in metastatic prostate cancer cells involves regulation of V-ATPase by pigment epithelium-derived factor (PEDF) , a potent endogenous inhibitor of angiogenesis. Thus, PEDF was identified as novel regulator of V-ATPase and suggested the mechanism of its inhibition of prostate tumor growth.
In summary, during last decade V-ATPase emerged as a crucial enzyme for tumorigenesis and metastatic phenotype of breast, ovarian, and prostate cancers. However, during last years the pathophysiological role of V-ATPase in the development of other tumors including: melanoma [187], hepatocellular carcinoma [188], oral squamous carcinoma [189], esophageal squamous carcinoma [190], non-small-cell lung cancer [191], gastric cancer [192], colon cancer [193], and pancreatic cancer [194] were also studied.
4.2 V-ATPase in Insulin Secretion and Diabetes Mellitus
V-ATPases play also essential roles in specific organelles of differentiated cells, especially those involved in exocytosis. The secretory granules have acidic luminal pH (5.0–5.5), important for condensation and maturation of their content. It has been suggested that the inside acidic pH or proton gradient across organelle membranes is involved in fusion of the vesicles to target membranes. Interesting question is which a-subunit isoform is expressed in secretory vesicles of mammalian cells. We have focused on the role of a-isoforms in insulin secretion, since previous studies suggested that V-ATPases may be pertinent for the insulin secretion [8, 195]. Of four isoforms, the a3 was detected in almost all cells in pancreatic Langerhans islet, and localized to the membranes of insulin containing granules in β-cells. Consistent with this finding, oc/oc mutant mice, homozygous 1.6 kb deletion of the a3-locus, exhibited reduced insulin secretion into blood upon glucose administration. However, the mutant β-cell contained essentially the same amount of mature insulin as the wild-type cell. Thus, the secretion of insulin was impaired in mutant β-cells. These results suggest that the a3-isoform has a direct function in exocytosis, possibly for fusion of the secretory vesicles to plasma membranes. The human ATP6i gene encoding the a3-isoform was mapped to a chromosome 11q13, and is located about 200 kb apart from LRP5 locus, which shows strong linkage to the disease. It is highly likely that alteration of ATP6i could also contribute to Type 1 diabetes [196]. We have identified that V-ATPase with a3 is highly expressed in endocrine tissues such as adrenal, parathyroid, thyroid and pituitary gland. Thus, a3 may be commonly involved in exocytosis of endocrine tissue and play an important role in the pathogenesis of endocrine and metabolic diseases including diabetes mellitus [8].
4.3 V-ATPase in Bone Homeostasis and Diseases
Bone homeostasis is maintained through the equilibrium between bone genesis and resorption by osteoblast and osteoclast, respectively. Defects of these cells are related to bone diseases such as osteopetrosis and osteoporosis. Osteoclast generates proton flux into bone resorption lacuna to mobilize bone calcium. This acidification is carried out by V-ATPases, which are localized in the osteoclast plasma membrane. We have shown that a3 and d2 subunits are components of the osteoclast enzyme [40, 75]. Mammalian subunit d has two isoforms: ubiquitous d1 isoform and osteoclast/kidney/epididymis specific d2 isoform [40]. In contrast to the plasma membrane localization in osteoclast, the a3 isoform was found in late endosome/lysosome in NIH3T3 or RAW264.7 cells.
Murine macrophage line RAW264.7 can differentiate to multinuclear osteoclast-like cells. Upon stimulation with RANKL (Receptor Accepter Nuclear κB Ligand) from osteoblast, RAW264.7 cell differentiate into multinuclear osteoclast. During the differentiation, V-ATPase containing a3 isoform was transported to the cell periphery together with lysosome marker proteins, and finally assembled to the plasma membrane. The d2 isoform was also induced and assembled to the plasma membrane V-ATPase. Thus, secretory lysosomes should be involved in the formation of osteoclast plasma membranes. The splenic macrophage (from a3 knockout mice) lacking the a3-isoform could differentiate to multinuclear cells, which express osteoclast marker enzymes and V-ATPase with d2 and a1 or a2 isoforms [40, 197]. However, the multinuclear cell could not adsorb calcium phosphate, indicating that V-ATPase with d2/a2- or d2/a1-subunit isoforms could not perform the function of that with d2/a3-subunit isoforms containing V-ATPase.
In summary, we have studied the targeting and function of a3-isoform both in endocrine tissues and in bone osteoclasts. Remarkably, while in pancreatic β-cells a3 is targeting the V-ATPase to the membranes of insulin containing secretory granules of exocytic pathway, in bone osteoclast the V-ATPase with a3-isoform is expressed in plasma membrane and late endosomes/lysosomes of endocytic pathway. However, our results strongly suggest that V-ATPase a3 is localized into specialized secretory lysosomes in osteoclasts. These specialized lysosomes are not functioning as common endocytic lysosomes, but instead are transported to cell periphery and fused with plasma membrane, using the mechanism similar to exocytosis. In accordance with the pivotal role of V-ATPase in bone homeostasis, multiple mutations of the a3-isoform give rise to diseases of bone resorption and are associated with osteopetrosis in both mice models and humans [41, 46, 95, 198, 199].
4.4 V-ATPase and mTORC1 /Ragulator/Rag/Rheb Small GTPases Super-complex in Cancer, Diabetes, and Age-Related Diseases
The mammalian target of rapamycin (mTOR) is a large cytosolic serine-threonine kinase that controls cellular growth and metabolism. Under physiological conditions both mTORC1 and mTORC2 are involved in neonatal autophagy and survival as well as development of obesity and aging processes in adulthood. Abnormal function of mTORC1 and mTORC2 are implicated in the pathogenesis of many diseases including cancer, diabetes, age-related diseases, aging, and longevity [34, 35, 163, 200–204]. The mTOR belongs to the superfamily of phosphatidylinositol-3 kinase related-kinases (PI3KK) that forms the core of two functionally distinct complexes: mTORC1 and mTORC2. As mTORC1 primarily responds to the levels of amino acids, oxygen, energy and cellular stress stimuli, mTORC2 plays a central role in the growth factor and insulin signaling cascades (Fig. 16.3) [33–35]. mTORC2 also regulates cytoskeleton function, metabolism, and cell survival [35, 205]. V-ATPase has been identified recently as an important component of the mTORC1 regulatory super-complex and signaling pathway [32]. This super-complex, formed by V-ATPase, Ragulator, Rag, mTORC1, and Rheb, is associated with late endosomes and lysosomes of the protein degradation endocytic pathway (Fig. 16.3) [32, 206, 207]. Localization of mTORC1 on the late endosoaml/lysosomes membrane is also critical for its activation as a multifunctional serine–threonine kinase and is regulated by two types of small GTPases: (1) Rheb GTPase (Ras homolog enriched in brain); and (2) Rag GTPases. It is well recognized, that Rheb is a potent activator of mTORC1, which transmit signals of growth factors, oxygen, energy supply, and stress via the tuberous sclerosis complex (TSC) , acting as a GTPase activating protein (GAP) for Rheb small GTPase.
Although amino acids are known to modulate cell growth and homeostasis, the molecular aspects of their regulation of mTORC1 function remained elusive. However, Sabatini and coworkers have shown recently that V-ATPase is a major player in the amino acids dependent recruitment, activation and signaling of mTORC1 [32]. V-ATPase is involved in sensing the levels of intra-lysosomal amino acids through the direct interaction with the Ragulator complex, that acts as a GTPase GTP/GDP-exchange factor for Rag small GTPases [33, 208]. They have proposed that the primary function of amino acid-dependent V-ATPase/Ragulator/Rag-signaling complex is to promote recruiting mTORC1 to lysosomal membrane, and thus trigger the TSC/Rheb-driven “ignition key” for the activation of the kinase activity of lysosomal mTORC1 complex. In their scenario, V-ATPase plays a direct role in intra-lysosomal sensing of amino acids and trans-membrane signaling to mTORC1.
Recent studies demonstrated, that mTORC1 is also activated by EGF via EGFR-receptor signaling pathway (Fig. 16.3). Then, the activation of mTORC1 involves in the Akt/Erk activation, TSC complex inhibition, and Rheb(GTP) formation. In contrast to amino acid-induction studies [32], EGF signaling pathway does not accompany mTORC1 recruitment from the cytosol and its translocation to the lysosomal membrane [23, 32]. Instead, the EGF signaling promoted the rapid recruitment of V1 sectors and increased assembly of ATPase in late endosomal/lysosomal compartments. Thus, the novel role of V-ATPase in regulation of mTORC1 signaling and trafficking EGFR/ErbB-receptors within the endosomal/lysosomal protein degradation pathway is a crucial mechanism controlled by the assembly/disassembly of the V1 and VO (Fig. 16.3) [23, 32].
In summary, lysosomes and related organelles play a regulatory role in cellular protein degradation and energy production using V-ATPase/mTORC1 “sensing machinery” to monitor both lysosomal and cytosolic amino acid content as indicator of nutritional status of the cell. This physiological information is further communicated to the nucleus to activate the gene expression programs allowing lysosomes to regulate their own function [209]. This quality control process is declining over life span, contributing to cancer and aging associated diseases, and thus, V-ATPase/mTORC1/Ragulator/Rag/Rheb small GTPases super-complex is considered as an important drug target.
4.5 V-ATPase and Cytohesin-2/aldolase/Arf1,6 Small GTPases Super-complex in Cancer, Diabetes, and Age-Related Diseases
Our recent studies uncovered, that in addition to its primary role as a proton-pump, V-ATPase also functions as a pH-sensing and cytohesin-2/Arf1,6 small GTPases signaling receptor (Fig. 16.2) [1, 2, 25, 27, 31]. However, the molecular mechanism of interaction between these proteins as well as functional significance of the signaling remain unclear. Thus, we also focused on the mechanism of interaction between N-terminal cytosolic tail of a2-subunit (a2N) of V-ATPase and cytohesin-2 (CTH2) [28]. The interaction sites between these two proteins were mapped using the combination of recombinant proteins/synthetic peptides pull-down and surface plasmon resonance (SPR) experiments. Two structural elements involved in specific and high affinity association of the a2 isoform with CTH2 were identified: (1) an N-terminal binding motif formed by the first 17 amino acids of the a2N (a2N1–17 peptide) and (2) the catalytic Sec7 domain of CTH2 [28, 31]. The SPR experiments further confirmed that these structural elements are major binding sites between a2N of V-ATPase and CTH2. Furthermore, this analysis revealed a strong binding affinity between this a2N1-17 peptide and the Sec7-domain of CTH2, with a dissociation constant of K D = 3.44 × 10−7 M, similar to the binding affinity K D = 3.13 × 10−7 M between full-length a2N and CTH2 proteins (Fig. 16.2, interfaces I and II). Analysis of enzyme activity confirmed that a2N1-17 peptide is crucial for V-ATPase/CTH2 signaling and regulate the cytohesin-2 enzymatic Arf-GEF activity. Indeed, these studies revealed that a2N1-17 peptide is a potent inhibitor of the GDP/GTP-exchange activity of CTH2, that is acting through its direct interaction with the catalytic Sec7 domain. The α-helical structure of a2N1-17 and its residues F5, M10, Q14 binding with the Sec7 domain were also identified by NMR spectroscopy analysis (Fig. 16.4a, b). In silico docking studies have shown that a2N1-17 epitope of V-ATPase competes with the switch-2 region of Arf1,6 for binding to the Sec7 domain of CTH2 (Fig. 16.4c, d). Together, these experiments revealed the structural basis and molecular details of mechanism of signaling between V-ATPase and CTH2/Arf1,6 small GTPases (Fig. 16.4).
The molecular features of the protein–protein interaction interface and signaling between V-ATPase and cytohesin-2/Arf1,6 small GTPases. (a) NMR structure a2N(1–17) peptide derived from V-ATPase and mapping of its interaction protein–protein binding interface (formed by amino acids F5, M10, Q14, shown in red square) involved in binding with catalytic Sec7 domain of cytohesin-2. (b) In silico docking experiments of the a2N(1–17) peptide at the interface between catalytic site of the Sec7-domain of cytohesin-2 and Arf1,6 small GTPases. (c) The binding of the a2N(1–17) peptide involves the αG, αH, and αI helixes of Sec7 and switch 2 of the Arf1,6 small GTPases
Although our previous work uncovered a functional cross talk between V-ATPase, cytohesin-2, and Arf1,6 small GTPases, other downstream effectors and related cell biological events have not been unraveled. However, since V-ATPase interacts with both cytohesin-2 and aldolase, we suggested that these proteins could in turn interact with each other, forming V-ATPase/cytohesin-2/Arf1,6/aldolase super-complex. The super-complex coordinates endocytic vesicle trafficking and downstream signaling of receptors (Fig. 16.3). The direct interaction of aldolase with cytohesin-2 through its PH-domain was shown by the pull-down and SPR experiments (Fig. 16.2). This approach revealed a two-step interaction between these two proteins with K D1 = 1.1 × 10−4 M and K D2 = 2.7 × 10−6 M, clearly indicating a potential regulatory mechanism of this interaction (Fig. 16.2, interfaces III and IV). Moreover, using a cell fractionation approach, we demonstrated the association of aldolase with early endosomes and formation of V-ATPase/cytohesin-2/Arf1,6/aldolase super-complex (Figs. 16.2 and 16.3). The aldolase knockdown experiments further uncovered the functional significance of interactions within V-ATPase/cytohesin-2/Arf1,6/aldolase super-complex. It was shown that the direct interaction between aldolase and cytohesin-2 are important in: (1) gelsolin gene expression, (2) actin cytoskeletal rearrangement, and (3) redistribution of endosomal vesicles within endocytic protein-degradation pathway [167].
In summary, a novel V-ATPase/cytohesin-2/Arf1,6/aldolase super-complex identified on early endosomes may be involved in regulation of the signaling receptors and function of the protein degradation pathway (Figs. 16.2 and 16.3). Moreover, these data indicate that pH-dependent binding and signaling between V-ATPase and cytohesin-2 may modulate the interaction of the a-subunit isoforms with aldolase and/or the GE-heterodimer forming peripheral stalks and consequently modulate the reversible association/dissociation of the V1 and VO of V-ATPase (Fig. 16.2) [1, 2]. Thus, evolutionarily acquired pH-sensing and cytohesin-2/Arf1,6-signaling function of V-ATPase, and its interaction with aldolase, may be an integral part of the self-regulatory mechanism of the enzyme as a proton-pumping rotary nano-motor.
On the other hand, downstream effectors of V-ATPase/cytohesin-2/Arf1,6/aldolase super-complex have also recently emerged. Cytohesin-1/2 family Arf-GEFs are shown to play role in regulation of signaling and trafficking of EGFR/ErbB and IR/IGF-1R receptors [128, 129, 154, 156, 157]. In particular, these studies demonstrated the direct interaction of cytohesin-2 with insulin receptor and regulation of the PI3K signaling pathway. Thus, both in mice and fly cytohesin-2 is essential for signaling of IR/IGF-1R, cell growth, regulation of metabolism, and function of calorie restriction pathway (CRP) during aging process [154, 156, 157, 162, 164, 202]. In cancer cells, cytohesin-2 serves as a cytoplasmic activator of EGFR/ErbB, that modulates phosphorylation-dependent dimerization, oncogenic signaling of these receptors, and development of cancer (Fig. 16.3) [128, 129]. In conclusion, cell biological insights of V-ATPase/cytohesin-2/Arf1,6/aldolase super-complex (Figs. 16.2 and 16.3) shed light on the regulation of endocytic protein degradation pathway, trafficking and signaling EGFR/ErbB and IR/EGF-1R receptors under both physiological (longevity and aging process) and pathological (cancer and diabetes) conditions.
5 Conclusions
5.1 Drug Design and Target ing to Diverse V-ATPases and Their Super-complexes
As discussed above, extensive research in the last few years made an astounding breakthrough in understanding the structure and function of V-ATPase [1–5, 7, 9–12]. The advances include following discoveries: (1) low resolution structure of V-ATPase using cryo-EM; (2) the crystal-structure of individual subunits and their protein–protein interfaces; (3) endosomal V-ATPase/cytohesin-2/Arf1,6/aldolase super-complex; (4) lysosomal V-ATPase/Ragulator/Rag/mTORC1/Rheb super-complex, (5) novel roles of V-ATPase in trafficking and signaling of receptors, and (6) critical role of V-ATPases in the development and pathogenesis of human diseases. Thus, V-ATPase could be considered as a potential target providing powerful approaches for the development of therapeutic agents. The seven different approaches could be considered as discussed below.
First, targeting to V-ATPases in diverse compartments with a large spectrum of subunit isoforms could be productive for pharmaceutical research. The expressions of these isoforms are specific for tissues, cells, and compartments. Recent experiments demonstrated that V-ATPases with unique combinations of subunit isoforms are localized in specific cell membranes which could dictate their functions [1–5, 8]. Thus, isoform-specific subunits of V-ATPase have been suggested as attractive targets for the treatment of human diseases. Targeting a-subunit and other isoforms by small molecule inhibitors is proposed in treating lytic bone disorders [210–212]. V-ATPase a-subunit isoforms are also potential targets for the treatment of metastatic cancers. Thus, the small molecule V-ATPase inhibitors and siRNA have been studied extensively with their potential application in cancer treatment and prevention of metastasis [213–217].
Secondly, targeting the extracellular domains of a- and c-subunits with specific antibodies has been recently successfully applied for selective inhibition of plasma membrane V-ATPase and reduction of metastatic phenotype of the cancer cells [53, 178]. In addition, the first V-ATPase inhibitory peptide was also identified and its selectivity was demonstrated [82]. Thus, this approach should be considered for developing of novel anticancer pharmaceuticals including antibodies and peptides which have high specificity and low toxicity.
Third, unique mechanism of regulating V-ATPase is reversible assembly/disassembly, which could be a promising target in drug design. This approach would be even more fruitful if combined with targeting cell or tissue specific V-ATPase isoforms [13–15, 19].
Forth, V-ATPases in regulation of signaling, trafficking, and degradation of cellular receptors could be a potential target. As discussed above, endocytosis, signaling, and degradation of Fz/LRP6, PRR, and Notch receptors are regulated by the direct interaction with V-ATPase [1, 122]. Therefore, targeting to the interactions between V-ATPase and these receptors could provide a promising approach in treatment of diseases related to cellular trafficking such as cancer, diabetes, and neurodegenerative diseases.
Fifth, regulatory proteins interacting with V-ATPase could be a potential target. The V-ATPase dependent super-complexes in early endosomes (V-ATPase/cytohesin-2/Arf1,6/aldolase) and late endosomes/lysosomes (V-ATPase/Ragulator/Rag/mTORC1/Rheb) pathway have been discovered recently in our [1, 2, 25, 26, 28, 30, 31, 109, 167] and Sabatini [32–34, 208] laboratories, respectively. The specific cascade of different protein–protein interactions within these complexes could modulate pH and amino-acids sensing, targeting, assembly, and activity of V-ATPase, linked to the regulation of intravesicular acidification and trafficking. For example, mTOR-signaling pathway is critical for the pathogenesis of the cancer and age-related diseases [34, 35, 163, 200–204]. It is noteworthy that extensive studies in animal models and clinical trials have uncovered the beneficial action of rapamycin, a potent mTOR inhibitor approved by FDA for treatment of variety age-associated diseases including cancers, neurodegenerative disorders, aging, and longevity. However, due to its side effects caused by the action of rapamycin on both mTORC1 and mTORC2, there is growing necessity for pharmacological research producing more specific and efficient drugs targeting of these pathways. Therefore, small molecules specifically targeting of protein–protein interactions in V-ATPase/Ragulator/Rag/mTORC1/Rheb super-complex will provide an attractive therapeutic agents to control aberrant signaling of mTORC1 and mTORC2 complexes in cancer, diabetes, age-related diseases, aging, and longevity [34, 35, 163, 200–204].
Sixth, novel endosomal V-ATPase/cytohesin-2/Arf1,6/aldolase super-complex and especially its protein–protein interaction interfaces could be a potential therapeutic targets (Figs. 16.2, 16.3, and 16.4) [1, 2, 25, 26, 28, 31, 167]. We propose that this approach will develop novel protein–protein interaction (PPI) inhibitors including therapeutic peptides or small molecule drugs [218–220]. These compounds may be also used to regulate assembly/disassembly of V-ATPase and signaling of cytohesin-2/Arf1,6 small GTPases. Modulation of the function of EGFR/ErbB or IR/IGF-1R receptors by the similar approach may be useful for treating variety of cancers, age-related diseases, slowing aging process and extending human longevity [154–158, 162–164].
Seventh, in our recent study the V-ATPase interactome was mapped for the first time. This systematic approach, has revealed a novel interacting proteins involved in trafficking, folding, assembly, and phosphorylation of V-ATPase [221]. These cell biological processes regulate V-ATPase-dependent acidification, and thus, these pathways and proteins could serve as potential drug targets for the therapeutic regulation V-ATPase function in health and disease states.
Abbreviations
- a2N:
-
N-terminal cytosolic tail of a2-subunit V-ATPase
- Arf1:
-
ADP-ribosylation factor 1
- Arf6:
-
ADP-ribosylation factor 6
- BafA1 :
-
bafilomycin A1
- c/c″-ring:
-
Ring composed by the c- and c″-subunits
- ConA :
-
concanamycin A
- CRP:
-
Calorie restriction pathway
- cryo-EM:
-
Cryo-Electron microscopy
- CTH2:
-
Cytohesin-2
- dErbB:
-
Dimeric EGFR/ErbB-receptor
- EGF:
-
Epidermal growth factor
- EmGFP:
-
Emerald green fluorescent protein
- FKPB12:
-
FK506/rapamycin binding protein
- FRET:
-
Fluorescence resonance energy transfer
- Fz:
-
Frizzled
- GH:
-
Growth hormone
- HRG-1:
-
Heme-responsive gene 1 protein
- IGF-1R:
-
Insulin-like growth factor-1 receptor
- IR:
-
Insulin receptor
- LRP6:
-
Low-density receptor-related protein
- M. sexta :
-
Manduca sexta
- mErbB:
-
Monomeric EGFR/ErbB-receptor
- mTORC1:
-
Mammalian target of rapamycin complex 1
- mTORC2:
-
Mammalian target of rapamycin complex 2
- NMR:
-
Nuclear magnetic resonance
- NOE:
-
Nuclear Overhauser effect
- PAT1:
-
Proton coupled amino acid transporter 1
- PI3K:
-
Phosphatidylinositol 3-kinase pathway
- PKA:
-
Protein kinase A
- PPI:
-
Protein–protein interaction interface inhibitors
- RagA/C:
-
Rag A/C GTPases
- Ragulator:
-
Ragulator complex
- Ras:
-
Rat sarcoma small GTPase
- RAVE:
-
Regulator of ATPase of vacuoles and endosomes
- Rbcn-3:
-
Rabconnectin-3A/B
- Rheb GTPase:
-
Ras homolog enriched in brain
- S. cerevisiae :
-
Saccharomyces cerevisiae
- SAXS:
-
Small-angle X-ray scattering
- ScDF1 and ScDF2:
-
Two conformations of subunit DF complex
- TFEB :
-
Transcription factor EB
- TSC complex:
-
Tuberous sclerosis complex
- V-ATPase:
-
V-type ATPase
- ΔpH:
-
Proton gradient
- ΔΨ:
-
Membrane potential
References
Marshansky V, Rubinstein JL, Grüber G (2014) Eukaryotic V-ATPase: novel structural findings and functional insights. Biochim Biophys Acta 1837:857–879
Marshansky V, Futai M (2008) The V-type H + -ATPase in vesicular trafficking: targeting, regulation and function. Curr Opin Cell Biol 20:415–426
Grüber G, Marshansky V (2008) New insights into structure-function relationships between archeal ATP synthase A1Ao and vacuolar type ATPase V1Vo. BioEssays 30:1–14
Nishi T, Forgac M (2002) The vacuolar H + -ATPases—nature’s most versatile proton pumps. Nat Rev Mol Cell Biol 3:94–103
Forgac M (2007) Vacuolar ATPases: rotary proton pumps in physiology and pathophysiology. Nat Rev Mol Cell Biol 8:917–929
Hinton A, Bond S, Forgac M (2009) V-ATPase functions in normal and disease processes. Pflugers Arch 457:589–598
Zhang Z, Zheng Y, Mazon H et al (2008) Structure of the yeast vacuolar ATPase. J Biol Chem 283:35983–35995
Sun-Wada GH, Tabata H, Kawamura N et al (2007) Differential expression of a subunit isoforms of the vacuolar-type proton pump ATPase in mouse endocrine tissues. Cell Tissue Res 329:239–248
Saroussi S, Nelson N (2009) Vacuolar H + -ATPase-an enzyme for all seasons. Pflugers Arch 457:581–587
Muench SP, Huss M, Song CF et al (2009) Cryo-electron microscopy of the vacuolar ATPase motor reveals its mechanical and regulatory complexity. J Mol Biol 386:989–999
Maxson ME, Grinstein S (2014) The vacuolar-type H + -ATPase at a glance – more than a proton pump. J Cell Sci 127:4987–4993
Rawson S, Phillips C, Huss M et al (2015) Structure of the vacuolar H + -ATPase rotary motor reveals new mechanistic insights. Structure 23:461–471
Kane PM (2000) Regulation of V-ATPases by reversible disassembly. FEBS Lett 469:137–141
Kane PM (2006) The where, when, and how of organelle acidification by the yeast vacuolar H + -ATPase. Microbiol Mol Biol Rev 70:177–191
Kane PM (2012) Targeting reversible disassembly as a mechanism of controlling V-ATPase activity. Curr Protein Pept Sci 13:117–123
Parra KJ, Chan CY, Chen J (2014) Saccharomyces cerevisiae vacuolar H + -ATPase regulation by disassembly and reassembly: one structure and multiple signals. Eukaryot Cell 13:706–714
Sumner JP, Dow JA, Earley FG et al (1995) Regulation of plasma membrane V-ATPase activity by dissociation of peripheral subunits. J Biol Chem 270:5649–5653
Beyenbach KW, Wieczorek H (2006) The V-type H+ ATPase: molecular structure and function, physiological roles and regulation. J Exp Biol 209:577–589
Kane PM (1995) Disassembly and reassembly of the yeast vacuolar H + -ATPase in vivo. J Biol Chem 270:17025–17032
Trombetta ES, Ebersold M, Garrett W et al (2003) Activation of lysosomal function during dendritic cell maturation. Science 299:1400–1403
Sautin YY, Lu M, Gaugler A et al (2005) Phosphatidylinositol 3-kinase-mediated effects of glucose on vacuolar H + -ATPase assembly, translocation, and acidification of intracellular compartments in renal epithelial cells. Mol Cell Biol 25:575–589
Lafourcade C, Sobo K, Kieffer-Jaquinod S et al (2008) Regulation of the V-ATPase along the endocytic pathway occurs through reversible subunit association and membrane localization. PLoS One 3, e2758
Xu Y, Parmar A, Roux E et al (2012) Epidermal growth factor-induced vacuolar H + -ATPase assembly: a role in signaling via mTORC1 activation. J Biol Chem 287:26409–26422
Liberman R, Bond S, Shainheit MG et al (2014) Regulated assembly of the V-ATPase is increased during cluster disruption-induced maturation of dendritic cells through a phosphatidylinositol 3-kinase/mTOR-dependent pathway. J Biol Chem 289:1355–1363
Hurtado-Lorenzo A, Skinner M, El Annan J et al (2006) V-ATPase interacts with ARNO and Arf6 in early endosomes and regulates the protein degradative pathway. Nat Cell Biol 8:124–136
Marshansky V (2007) The V-ATPase a2-subunit as a putative endosomal pH-sensor. Biochem Soc Trans 35:1092–1099
Recchi C, Chavrier P (2006) V-ATPase: a potential pH sensor. Nat Cell Biol 8:107–109
Merkulova M, Bakulina A, Thaker YR et al (2010) Specific motifs of the V-ATPase a2-subunit isoform interact with catalytic and regulatory domains of ARNO. Biochim Biophys Acta 1797:1398–1409
Merkulova M, McKee M, Dip PV et al (2010) N-terminal domain of the V-ATPase a2-subunit displays integral membrane protein properties. Protein Sci 19:1850–1862
El-Annan J, Brown D, Breton S et al (2004) Differential expression and targeting of endogenous Arf1 and Arf6 small GTPases in kidney epithelial cells in situ. Am J Physiol Cell Physiol 286:C768–C778
Hosokawa H, Dip PV, Merkulova M et al (2013) The N termini of a-subunit isoforms are involved in signaling between vacuolar H + -ATPase (V-ATPase) and cytohesin-2. J Biol Chem 288:5896–5913
Zoncu R, Bar-Peled L, Efeyan A et al (2011) mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H + -ATPase. Science 334:678–683
Bar-Peled L, Sabatini DM (2012) SnapShot: mTORC1 signaling at the lysosomal surface. Cell 151:1390
Efeyan A, Zoncu R, Sabatini DM (2012) Amino acids and mTORC1: from lysosomes to disease. Trends Mol Med 18:524–533
Laplante M, Sabatini DM (2012) mTOR signaling in growth control and disease. Cell 149:274–293
Peters C, Bayer MJ, Buhler S, Andersen JS, Mann M, Mayer A (2001) Trans-complex formation by proteolipid channels in the terminal phase of membrane fusion. Nature 409:581–588
Bayer MJ, Reese C, Buhler S et al (2003) Vacuole membrane fusion: Vo functions after trans-SNARE pairing and is coupled to the Ca2 + -releasing channel. J Cell Biol 162:211–222
Baars TL, Petri S, Peters C, Mayer A (2007) Role of the V-ATPase in regulation of the vacuolar fission-fusion equilibrium. Mol Biol Cell 18:3873–3882
Strasser B, Iwaszkiewicz J, Michielin O, Mayer A (2011) The V-ATPase proteolipid cylinder promotes the lipid-mixing stage of SNARE-dependent fusion of yeast vacuoles. EMBO J 30:4126–4141
Matsumoto N, Daido S, Sun-Wada GH et al (2014) Diversity of proton pumps in osteoclasts: V-ATPase with a3 and d2 isoforms is a major form in osteoclasts. Biochim Biophys Acta 1837:744–749
Kornak U, Schulz A, Friedrich W et al (2000) Mutations in the a3 subunit of the vacuolar H(+)-ATPase cause infantile malignant osteopetrosis. Hum Mol Genet 9:2059–2063
Smith AN, Jouret F, Bord S et al (2005) Vacuolar H + -ATPase d2 subunit: molecular characterization, developmental regulation, and localization to specialized proton pumps in kidney and bone. J Am Soc Nephrol 16:245–1256
Lee SH, Rho J, Jeong D et al (2006) V-ATPase Vo subunit d2-deficient mice exhibit impaired osteoclast fusion and increased bone formation. Nat Med 12:1403–1409
Balakrishna AM, Basak S, Manimekalai MS, Grüber G (2015) Crystal structure of subunits D and F in complex gives insight into energy transmission of the eukaryotic V-ATPase from Saccharomyces cerevisiae. J Biol Chem 290:3183–3196
Smith AN, Skaug J, Choate KA et al (2000) Mutations in ATP6N1B, encoding a new kidney vacuolar proton pump 116-kD subunit, cause recessive distal renal tubular acidosis with preserved hearing. Nat Genet 26:71–75
Frattini A, Orchard PJ, Sobacchi C et al (2000) Defects in TCIRG1 subunit of the vacuolar proton pump are responsible for a subset of human autosomal recessive osteopetrosis. Nat Genet 25:343–346
Kornak U, Reynders E, Dimopoulou A et al (2008) Impaired glycosylation and cutis laxa caused by mutations in the vesicular H + -ATPase subunit ATP6V0A2. Nat Genet 40:32–34
Hucthagowder V, Morava E, Kornak U et al (2009) Loss-of-function mutations in ATP6V0A2 impair vesicular trafficking, tropoelastin secretion and cell survival. Hum Mol Genet 18:2149–2165
Morava E, Guillard M, Lefeber DJ, Wevers RA (2009) Autosomal recessive cutis laxa syndrome revisited. Eur J Hum Genet 17:1099–1110
Sennoune SR, Bakunts K, Martinez GM et al (2004) Vacuolar H + -ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity. Am J Physiol Cell Physiol 286:C1443–C1452
Hinton A, Sennoune SR, Bond S et al (2009) Function of a subunit isoforms of the V-ATPase in pH homeostasis and in vitro invasion of MDA-MB231 human breast cancer cells. J Biol Chem 284:16400–16408
Capecci J, Forgac M (2013) The function of vacuolar ATPase (V-ATPase) a subunit isoforms in invasiveness of MCF10a and MCF10CA1a human breast cancer cells. J Biol Chem 288:32731–32741
Cotter K, Capecci J, Sennoune S et al (2015) Activity of plasma membrane V-ATPases is critical for the invasion of MDA-MB231 breast cancer cells. J Biol Chem 290:3680–3692
Qi J, Forgac M (2007) Cellular environment is important in controlling V-ATPase dissociation and its dependence on activity. J Biol Chem 282:24743–24751
Benlekbir S, Bueler SA, Rubinstein JL (2012) Structure of the vacuolar-type ATPase from Saccharomyces cerevisiae at 11Å resolution. Nat Struct Mol Biol 19:1356–1362
Zhao J, Benlekbir S, Rubinstein JL (2015) Electron cryomicroscopy observation of rotational states in a eukaryotic V-ATPase. Nature 521:241–245
Muench SP, Scheres SH, Huss M et al (2014) Subunit positioning and stator filament stiffness in regulation and power transmission in the V1 motor of the Manduca sexta V-ATPase. J Mol Biol 426:286–300
Radermacher M, Ruiz T, Wieczorek H, Grüber G (2001) The structure of the V1-ATPase determined by three-dimensional electron microscopy of single particles. J Struct Biol 135:26–37
Hilario E, Gogarten JP (1998) The prokaryote-to-eukaryote transition reflected in the evolution of the V/F/A-ATPase catalytic and proteolipid subunits. J Mol Evol 46:703–715
Basak S, Lim J, Manimekalai MS, Balakrishna AM, Grüber G (2013) Crystal and NMR structures give insights into the role and dynamics of subunit F of the eukaryotic V-ATPase from Saccharomyces cerevisiae. J Biol Chem 288:11930–11939
Jefferies KC, Forgac M (2008) Subunit H of the vacuolar H + -ATPase inhibits ATP hydrolysis by the free V1 domain by interaction with the rotary subunit F. J Biol Chem 283:4512–4519
Sagermann M, Stevens TM, Matthews BW (2001) Crystal structure of the regulatory subunit H of the V-type ATPase of Saccharomyces cerevisiae. Proc Natl Acad Sci U S A 98:7134–7139
Oot RA, Huang LS, Berry EA, Wilkens S (2012) Crystal structure of the yeast vacuolar ATPase heterotrimeric EGC(head) peripheral stalk complex. Structure 20:1881–1892
Rishikesan S, Grüber G (2011) Structural elements of the C-terminal domain of subunit E (E133-222) from the Saccharomyces cerevisiae V1Vo ATPase determined by solution NMR spectroscopy. J Bioenerg Biomembr 43:447–455
Vitavska O, Merzendorfer H, Wieczorek H (2005) The V-ATPase subunit C binds to polymeric F-actin as well as to monomeric G-actin and induces cross-linking of actin filaments. J Biol Chem 280:1070–1076
Armbrüster A, Svergun DI, Coskun U et al (2004) Structural analysis of the stalk subunit Vma5p of the yeast V-ATPase in solution. FEBS Lett 570:119–125
Drory O, Frolow F, Nelson N (2004) Crystal structure of yeast V-ATPase subunit C reveals its stator function. EMBO Rep 5:1148–1152
Ambrüster A, Hohn C, Hermesdorf A et al (2005) Evidence for major structural changes in subunit C of the vacuolar ATPase due to nucleotide binding. FEBS Lett 579:1961–1967
Hong-Hermesdorf A, Brux A, Grüber A, Grüber G, Schumacher K (2006) A WNK kinase binds and phosphorylates V-ATPase subunit C. FEBS Lett 580:932–939
Graham LA, Flannery AR, Stevens TH (2003) Structure and assembly of the yeast V-ATPase. J Bioenerg Biomembr 35:301–312
Wilkens S, Inoue T, Forgac M (2004) Three-dimensional structure of the vacuolar ATPase. Localization of subunit H by difference imaging and chemical cross-linking. J Biol Chem 279:41942–41949
Dip PV, Saw WG, Roessle M, Marshansky V, Grüber G (2012) Solution structure of subunit a, a104-363, of the Saccharomyces cerevisiae V-ATPase and the importance of its C-terminus in structure formation. J Bioenerg Biomembr 44:341–350
Smith AN, Finberg KE, Wagner CA et al (2001) Molecular cloning and characterization of Atp6n1b: a novel fourth murine vacuolar H + -ATPase a-subunit gene. J Biol Chem 276:42382–42388
Ochotny N, van Vliet A, Chan N et al (2006) Effects of human a3 and a4 mutations that result in osteopetrosis and distal renal tubular acidosis on yeast V-ATPase expression and activity. J Biol Chem 281:26102–26111
Toyomura T, Oka T, Yamaguchi C et al (2000) Three subunit a isoforms of mouse vacuolar H + -ATPase. Preferential expression of the a3 isoform during osteoclast differentiation. J Biol Chem 275:8760–8765
Toei M, Toei S, Forgac M (2011) Definition of membrane topology and identification of residues important for transport in subunit a of the vacuolar ATPase. J Biol Chem 286:35176–35186
Nishi T, Forgac M (2000) Molecular cloning and expression of three isoforms of the 100-kDa a subunit of the mouse vacuolar proton-translocating ATPase. J Biol Chem 275:6824–6830
Duarte AM, de Jong ER, Wechselberger R et al (2007) Segment TM7 from the cytoplasmic hemi-channel from VO-H + -V-ATPase includes a flexible region that has a potential role in proton translocation. Biochim Biophys Acta 1768:2263–2270
Su Y, Zhou A, Al-Lamki RS, Karet FE (2003) The a-subunit of the V-type H + -ATPase interacts with phosphofructokinase-1 in humans. J Biol Chem 278:20013–20018
Flannery AR, Graham LA, Stevens TH (2004) Topological characterization of the c, c′, and c″ subunits of the vacuolar ATPase from the yeast Saccharomyces cerevisiae. J Biol Chem 279:39856–39862
Wang Y, Cipriano DJ, Forgac M (2007) Arrangement of subunits in the proteolipid ring of the V-ATPase. J Biol Chem 282:34058–34065
Muench SP, Rawson S, Eyraud V et al (2014) PA1b inhibitor binding to subunits c and e of the vacuolar ATPase reveals its insecticidal mechanism. J Biol Chem 289:16399–16408
Junge W, Nelson N (2005) Structural biology. Nature’s rotary electromotors. Science 308:642–644
Wang Y, Inoue T, Forgac M (2005) Subunit a of the yeast V-ATPase participates in binding of bafilomycin. J Biol Chem 280:40481–40488
Bowman BJ, Bowman EJ (2002) Mutations in subunit C of the vacuolar ATPase confer resistance to bafilomycin and identify a conserved antibiotic binding site. J Biol Chem 277:3965–3972
Bowman EJ, Bowman BJ (2005) V-ATPases as drug targets. J Bioenerg Biomembr 37:431–435
Umata T, Moriyama Y, Futai M, Mekada E (1990) The cytotoxic action of diphtheria toxin and its degradation in intact Vero cells are inhibited by bafilomycin A1, a specific inhibitor of vacuolar-type H + -ATPase. J Biol Chem 265:21940–21945
Yoshimori T, Yamamoto A, Moriyama Y et al (1991) Bafilomycin A1, a specific inhibitor of vacuolar-type H + -ATPase, inhibits acidification and protein degradation in lysosomes of cultured cells. J Biol Chem 266:17707–17712
Brown D, Marshansky V (2004) Renal V-ATPase: physiology and pathophysiology. In: Futai M, Wada Y, Kaplan JH (eds) Handbook of ATPases. Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim, pp 413–442
Brown D, Breton S, Ausiello DA, Marshansky V (2009) Sensing, signaling and sorting events in kidney epithelial cell physiology. Traffic 10:275–284
Brown D, Paunescu TG, Breton S, Marshansky V (2009) Regulation of the V-ATPase in kidney epithelial cells: dual role in acid–base homeostasis and vesicle trafficking. J Exp Biol 212:1762–1772
Shum WW, Da Silva N, Brown D, Breton S (2009) Regulation of luminal acidification in the male reproductive tract via cell-cell crosstalk. J Exp Biol 21:1753–1761
Breton S, Brown D (2013) Regulation of luminal acidification by the V-ATPase. Physiology (Bethesda) 28:318–329
Sun-Wada GH, Wada Y (2013) Vacuolar-type proton pump ATPases: acidification and pathological relationships. Histol Histopathol 28:208–215
Bhargava A, Voronov I, Wang Y, Glogauer M, Kartner N, Manolson MF (2012) Osteopetrosis mutation R444L causes endoplasmic reticulum retention and misprocessing of vacuolar H + -ATPase a3 subunit. J Biol Chem 287:26829–26839
Kartner N, Manolson MF (2012) V-ATPase subunit interactions: the long road to therapeutic targeting. Curr Protein Pept Sci 13:164–179
Toro EJ, Ostrov DA, Wronski TJ, Holliday LS (2012) Rational identification of enoxacin as a novel V-ATPase-directed osteoclast inhibitor. Curr Protein Pept Sci 13:80–191
Steinberg BE, Touret N, Vargas-Caballero M, Grinstein S (2007) In situ measurement of the electrical potential across the phagosomal membrane using FRET and its contribution to the proton-motive force. Proc Natl Acad Sci U S A 104:9523–9528
Scheel O, Zdebik AA, Lourdel S, Jentsch TJ (2005) Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature 436:424–427
Jentsch TJ (2007) Chloride and the endosomal-lysosomal pathway: emerging roles of CLC chloride transporters. J Physiol 578:633–640
Stauber T, Weinert S, Jentsch TJ (2012) Cell biology and physiology of CLC chloride channels and transporters. Compr Physiol 2:1701–1744
Orlowski J, Grinstein S (2007) Emerging roles of alkali cation/proton exchangers in organellar homeostasis. Curr Opin Cell Biol 19:483–492
Manolson MF, Wu B, Proteau D et al (1994) STV1 gene encodes functional homologue of 95-kDa yeast vacuolar H + -ATPase subunit Vph1p. J Biol Chem 269:14064–14074
Kawasaki-Nishi S, Bowers K, Nishi T et al (2001) The amino-terminal domain of the vacuolar proton-translocating ATPase a subunit controls targeting and in vivo dissociation, and the carboxyl-terminal domain affects coupling of proton transport and ATP hydrolysis. J Biol Chem 276:47411–47420
Morel N, Dedieu JC, Philippe JM (2003) Specific sorting of the a1 isoform of the V-H + ATPase a subunit to nerve terminals where it associates with both synaptic vesicles and the presynaptic plasma membrane. J Cell Sci 116:4751–4762
Hiesinger PR, Fayyazuddin A, Mehta SQ et al (2005) The v-ATPase VO subunit a1 is required for a late step in synaptic vesicle exocytosis in Drosophila. Cell 121:607–620
Saw NM, Kang SY, Parsaud L et al (2011) Vacuolar H + -ATPase subunits Voa1 and Voa2 cooperatively regulate secretory vesicle acidification, transmitter uptake, and storage. Mol Biol Cell 22:3394–3409
Peri F, Nusslein-Volhard C (2008) Live imaging of neuronal degradation by microglia reveals a role for VO-ATPase a1 in phagosomal fusion in vivo. Cell 133:916–927
Maranda B, Brown D, Bourgoin S et al (2001) Intra-endosomal pH-sensitive recruitment of the Arf-nucleotide exchange factor ARNO and Arf6 from cytoplasm to proximal tubule endosomes. J Biol Chem 276:18540–18550
Marshansky V, Ausiello DA, Brown D (2002) Physiological importance of endosomal acidification: potential role in proximal tubulopathies. Curr Opin Nephrol Hypertens 11:527–537
Sun-Wada GH, Tabata H, Kawamura K et al (2009) Direct recruitment of H + -ATPase from lysosomes for phagosomal acidification. J Cell Sci 122:2504–2513
Toei M, Saum R, Forgac M (2010) Regulation and isoform function of the V-ATPases. Biochemistry 49:4715–4723
Sun-Wada GH, Imai-Senga Y, Yamamoto A, Murata Y, Hirata T, Wada Y, Futai M (2002) A proton pump ATPase with testis-specific E1-subunit isoform required for acrosome acidification. J Biol Chem 279:18098–18105
Hayashi K, Sun-Wada GH, Wada Y et al (2008) Defective assembly of a hybrid vacuolar H + -ATPase containing the mouse testis-specific E1 isoform and yeast subunits. Biochim Biophys Acta 1777:1370–1377
Okamoto-Terry H, Umeki K, Nakanishi-Matsui M, Futai M (2013) Glu-44 in the amino-terminal alpha-helix of yeast vacuolar ATPase E subunit (Vma4p) has a role for VoV1 assembly. J Biol Chem 288:36236–36243
Smardon AM, Tarsio M, Kane PM (2002) The RAVE complex is essential for stable assembly of the yeast V-ATPase. J Biol Chem 277:13831–13839
Smardon AM, Diab HI, Tarsio M et al (2014) The RAVE complex is an isoform-specific V-ATPase assembly factor in yeast. Mol Biol Cell 25:356–367
Bond S, Forgac M (2008) The Ras/cAMP/protein kinase A pathway regulates glucose-dependent assembly of the vacuolar H + -ATPase in yeast. J Biol Chem 283:36513–36521
Lu M, Holliday LS, Zhang L et al (2001) Interaction between aldolase and vacuolar H + -ATPase: evidence for direct coupling of glycolysis to the ATP-hydrolyzing proton pump. J Biol Chem 276:30407–30413
Lu M, Sautin YY, Holliday LS, Gluck SL (2004) The glycolytic enzyme aldolase mediates assembly, expression, and activity of vacuolar H + -ATPase. J Biol Chem 279:8732–8739
Lu M, Ammar D, Ives H et al (2007) Physical interaction between aldolase and vacuolar H + -ATPase is essential for the assembly and activity of the proton pump. J Biol Chem 282:24495–24503
Marshansky V (2014) Role of V-ATPase, cytohesin-2/Arf6 and aldolase in regulation of endocytosis: Implication for diabetic nephropathy. In: Nakamura S (ed) Handbook of H + -ATPases. Pan Stanford Publishing Pte. Ltd, Singapore, pp 169–199
Cohen S (1962) Isolation of a mouse submaxillary gland protein accelerating incisor eruption and eyelid opening in the new-born animal. J Biol Chem 237:1555–1562
Cohen S, Carpenter G, King L (1980) Epidermal growth factor-receptor-protein kinase interactions: Co-purification of receptor and epidermal growth factor-enhanced phosphorylation activity. J Biol Chem 255:4834–4842
Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, Carotenuto A, De Feo G, Caponigro F, Salomon DS (2006) Epidermal growth factor receptor (EGFR) signaling in cancer. Gene 366:2–16
Tomas A, Futter CE, Eden ER (2014) EGF receptor trafficking: consequences for signaling and cancer. Trends Cell Biol 24:26–34
Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144:646–674
Bill A, Schmitz A, Albertoni B et al (2010) Cytohesins are cytoplasmic ErbB receptor activators. Cell 143:201–211
Bill A, Schmitz A, Konig K et al (2013) Anti-proliferative effect of cytohesin inhibition in gefitinib-resistant lung cancer cells. PLoS One 7, e41179
Junnila RK, List EO, Berryman DE et al (2013) The GH/IGF-1 axis in ageing and longevity. Nat Rev Endocrinol 9:366–376
Berryman DE, Glad CA, List EO, Johannsson G (2013) The GH/IGF-1 axis in obesity: pathophysiology and therapeutic considerations. Nat Rev Endocrinol 9:346–356
Song YH, Song JL, Delafontaine P, Godard MP (2013) The therapeutic potential of IGF-I in skeletal muscle repair. Trends Endocrinol Metab 24:310–319
O'Callaghan KM, Ayllon V, O'Keeffe J et al (2010) Heme-binding protein HRG-1 is induced by insulin-like growth factor I and associates with the vacuolar H + -ATPase to control endosomal pH and receptor trafficking. J Biol Chem 285:381–391
Fogarty FM, O'Keeffe J, Zhadanov A, Papkovsky D, Ayllon V, O'Connor R (2014) HRG-1 enhances cancer cell invasive potential and couples glucose metabolism to cytosolic/extracellular pH gradient regulation by the vacuolar-H + -ATPase. Oncogene 33:4653–4663
Niehrs C (2012) The complex world of WNT receptor signaling. Nat Rev Mol Cell Biol 13:767–779
Niehrs C, Acebron SP (2012) Mitotic and mitogenic Wnt signaling. EMBO J 31:2705–2713
Cruciat CM, Ohkawara B, Acebron SP et al (2010) Requirement of prorenin receptor and vacuolar H + -ATPase-mediated acidification for Wnt signaling. Science 327:459–463
Niehrs C, Boutros M (2010) Trafficking, acidification, and growth factor signaling. Sci Signal 3:26
Nguyen G, Delarue F, Burckle C et al (2002) Pivotal role of the renin/prorenin receptor in angiotensin II production and cellular responses to renin. J Clin Invest 109:1417–1427
Danser AN (2009) (Pro)renin receptor and vacuolar H + -ATPase. Hypertension 54:219–221
Ichihara A, Kinouchi K (2011) Current knowledge of (pro)renin receptor as an accessory protein of vacuolar H + -ATPase. J Renin Angiotensin Aldosterone Syst 12:638–640
Kinouchi K, Ichihara A, Sano M et al (2010) The (pro)renin receptor/ATP6AP2 is essential for vacuolar H + -ATPase assembly in murine cardiomyocytes. Circ Res 107:30–34
Danser AH, Deinum J (2005) Renin, prorenin and the putative (pro)renin receptor. Hypertension 46:1069–1076
Kopan R, Ilagan MX (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137:216–233
Wang Z, Li Y, Sarkar FH (2010) Notch signaling proteins: legitimate targets for cancer therapy. Curr Protein Pept Sci 11:398–408
Vaccari T, Duchi S, Cortese K et al (2010) The vacuolar ATPase is required for physiological as well as pathological activation of the Notch receptor. Development 137:1825–1832
Yan Y, Denef N, Schupbach T (2009) The vacuolar proton pump, V-ATPase, is required for notch signaling and endosomal trafficking in Drosophila. Dev Cell 17:387–402
Sethi N, Yan Y, Quek D, Schupbach T, Kang Y (2010) Rabconnectin-3 is a functional regulator of mammalian Notch signaling. J Biol Chem 285:34757–34764
Seol JH, Shevchenko A, Deshaies RJ (2001) Skp1 forms multiple protein complexes, including RAVE, a regulator of V-ATPase assembly. Nat Cell Biol 3:384–391
Bourne HR, Sanders DA, McCormick F (1990) The GTPase superfamily: a conserved switch for diverse cell functions. Nature 348:125–132
Donaldson JG, Jackson CL (2000) Regulators and effectors of the ARF GTPases. Curr Opin Cell Biol 12:475–482
Casanova JE (2007) Regulation of Arf activation: the Sec7 family of guanine nucleotide exchange factors. Traffic 8:1476–1485
D'Souza-Schorey C, Chavrier P (2006) ARF proteins: roles in membrane traffic and beyond. Nat Rev Mol Cell Biol 7:347–358
Hafner M, Schmitz A, Grune I et al (2006) Inhibition of cytohesins by SecinH3 leads to hepatic insulin resistance. Nature 444:941–944
Hafner M, Vianini E, Albertoni B et al (2008) Displacement of protein-bound aptamers with small molecules screened by fluorescence polarization. Nat Protoc 3:579–587
Fuss B, Becker T, Zinke I, Hoch M (2006) The cytohesin Steppke is essential for insulin signalling in Drosophila. Nature 444:945–948
Jackson C (2006) Diabetes: kicking off the insulin cascade. Nature 444:833–834
Lim J, Zhou M, Veenstra TD, Morrison DK (2010) The CNK1 scaffold binds cytohesins and promotes insulin pathway signaling. Genes Dev 24:1496–1506
Mannell HK, Pircher J, Chaudhry DI et al (2012) ARNO regulates VEGF-dependent tissue responses by stabilizing endothelial VEGFR-2 surface expression. Cardiovasc Res 93:111–119
Kolanus W, Nagel W, Schiller B et al (1996) Alpha L beta 2 integrin/LFA-1 binding to ICAM-1 induced by cytohesin-1, a cytoplasmic regulatory molecule. Cell 86:233–242
Kolanus W (2007) Guanine nucleotide exchange factors of the cytohesin family and their roles in signal transduction. Immunol Rev 218:102–113
Anisimov AN, Bartke A (2013) The key role of growth hormone-insulin-IGF-1 signaling in aging and cancer. Crit Rev Oncol Hematol 87:201–223
Anisimov AN (2015) Conservative growth hormone/IGF-1 and mTOR signaling pathways as a target for aging and cancer prevention: do we really have an antiaging drug? Interdiscip Top Gerontol 40:177–188
Blagosklonny MV (2013) Selective anti-cancer agents as anti-aging drugs. Cancer Biol Ther 14:1092–1097
Marshansky V, Bourgoin S, Londono I et al (1997) Identification of ADP-ribosylation factor-6 in brush-border membrane and early endosomes of human kidney proximal tubules. Electrophoresis 18:538–547
Marshansky V, Bourgoin S, Londono I et al (1997) Receptor-mediated endocytosis in kidney proximal tubules: recent advances and hypothesis. Electrophoresis 18:2661–2676
Merkulova M, Hurtado-Lorenzo A, Hosokawa H et al (2011) Aldolase directly interacts with ARNO and modulates cell morphology and acidic vesicle distribution. Am J Physiol Cell Physiol 300(6):1442–1455
Oka T, Murata Y, Namba M et al (2001) a4, a unique kidney-specific isoform of mouse vacuolar H + -ATPase subunit a. J Biol Chem 276:40050–40054
Pietrement C, Sun-Wada GH, Da Silva N et al (2006) Distinct expression patterns of different subunit isoforms of the V-ATPase in the rat epididymis. Biol Reprod 74:185–194
Martinez-Zaguilan R, Lynch RM, Martinez GM, Gillies RJ (1993) Vacuolar-type H + -ATPases are functionally expressed in plasma membranes of human tumor cells. Am J Physiol 265:C1015–C1029
Hendrix A, Sormunen R, Westbroek W et al (2013) Vacuolar H+ ATPase expression and activity is required for Rab27B-dependent invasive growth and metastasis of breast cancer. Int J Cancer 133:843–854
Graham RM, Thompson JW, Webster KA (2014) Inhibition of the vacuolar ATPase induces Bnip3-dependent death of cancer cells and a reduction in tumor burden and metastasis. Oncotarget 5:1162–1173
Schempp CM, von Schwarzenberg K, Schreiner L et al (2014) V-ATPase inhibition regulates anoikis resistance and metastasis of cancer cells. Mol Cancer Ther 13:926–937
Mei F, You J, Liu B et al (2015) LASS2/TMSG1 inhibits growth and invasion of breast cancer cell in vitro through regulation of vacuolar ATPase activity. Tumour Biol 36:2831–2844
Sennoune SR, Luo D, Martinez-Zaguilan R (2004) Plasmalemmal vacuolar-type H + -ATPase in cancer biology. Cell Biochem Biophys 40:185–206
Sennoune SR, Martinez-Zaguilan R (2007) Plasmalemmal vacuolar H + -ATPases in angiogenesis, diabetes and cancer. J Bioenerg Biomembr 39:427–433
Sennoune SR, Martinez-Zaguilan R (2012) Vacuolar H + -ATPase signaling pathway in cancer. Curr Protein Pept Sci 13:152–163
Kulshrestha A, Katara GK, Ibrahim S et al (2015) Vacuolar ATPase ‘a2’ isoform exhibits distinct cell surface accumulation and modulates matrix metalloproteinase activity in ovarian cancer. Oncotarget 6:3797–3810
Ntrivalas E, Derks R, Gilman-Sachs A et al (2007) Novel role for the N-terminus domain of the a2 isoform of vacuolar ATPase in interleukin-1beta production. Hum Immunol 68:469–477
Ntrivalas E, Gilman-Sachs A, Kwak-Kim J, Beaman K (2007) The N-terminus domain of the a2 isoform of vacuolar ATPase can regulate interleukin-1beta production from mononuclear cells in co-culture with JEG-3 choriocarcinoma cells. Am J Reprod Immunol 57:201–209
Kwong C, Gilman-Sachs A, Beaman K (2011) Tumor-associated a2 vacuolar ATPase acts as a key mediator of cancer-related inflammation by inducing pro-tumorigenic properties in monocytes. J Immunol 186:1781–1789
Katara GK, Jaiswal MK, Kulshrestha A et al (2014) Tumor-associated vacuolar ATPase subunit promotes tumorigenic characteristics in macrophages. Oncogene 33:5649–5654
Ziyad S, Iruela-Arispe ML (2011) Molecular mechanisms of tumor angiogenesis. Genes Cancer 2:1085–1096
Rojas JD, Sennoune SR, Maiti D et al (2004) Plasmalemmal V-H + -ATPases regulate intracellular pH in human lung microvascular endothelial cells. Biochem Biophys Res Commun 320:1123–1132
Rojas JD, Sennoune SR, Maiti D et al (2006) Vacuolar-type H + -ATPases at the plasma membrane regulate pH and cell migration in microvascular endothelial cells. Am J Physiol Heart Circ Physiol 291:H1147–H1157
Sennoune SR, Arutunyan A, del Rosario C et al (2014) V-ATPase regulates communication between microvascular endothelial cells and metastatic cells. Cell Mol Biol (Noisy-le-grand) 60:19–25
Nishisho T, Hata K, Nakanishi M et al (2011) The a3 isoform vacuolar type H(+)-ATPase promotes distant metastasis in the mouse B16 melanoma cells. Mol Cancer Res 9:845–855
Xu J, Xie R, Liu X et al (2012) Expression and functional role of vacuolar H(+)-ATPase in human hepatocellular carcinoma. Carcinogenesis 33:2432–2440
Perez-Sayans M, Garcia-Garcia A, Reboiras-Lopez MD, Gandara-Vila P (2009) Role of V-ATPases in solid tumors: importance of the subunit C. Int J Oncol 34:1513–1520
Huang L, Lu Q, Han Y et al (2012) ABCG2/V-ATPase was associated with the drug resistance and tumor metastasis of esophageal squamous cancer cells. Diagn Pathol 7:180
Lu Q, Lu S, Huang L et al (2013) The expression of V-ATPase is associated with drug resistance and pathology of non-small-cell lung cancer. Diagn Pathol 8:145
Liu P, Chen H, Han L et al (2015) Expression and role of V1A subunit of V-ATPases in gastric cancer cells. Int J Clin Oncol 20(4):725–735
Lozupone F, Borghi M, Marzoli F et al (2015) TM9SF4 is a novel V-ATPase-interacting protein that modulates tumor pH alterations associated with drug resistance and invasiveness of colon cancer cells. Oncogene (PMID: 25659576)
Chung C, Mader CC, Schmitz JC et al (2011) The vacuolar-ATPase modulates matrix metalloproteinase isoforms in human pancreatic cancer. Lab Invest 91:732–743
Sun-Wada GH, Toyomura T, Murata Y et al (2006) The a3 isoform of V-ATPase regulates insulin secretion from pancreatic beta-cells. J Cell Sci 119:4531–4540
Twells RC, Mein CA, Payne F et al (2003) Linkage and association mapping of the LRP5 locus on chromosome 11q13 in type 1 diabetes. Hum Genet 113:99–105
Toyomura T, Murata Y, Yamamoto A et al (2003) From lysosomes to the plasma membrane: localization of vacuolar-type H + -ATPase with the a3 isoform during osteoclast differentiation. J Biol Chem 278:22023–22030
Scimeca JC, Franchi A, Trojani C et al (2000) The gene encoding the mouse homologue of the human osteoclast-specific 116-kDa V-ATPase subunit bears a deletion in osteosclerotic (oc/oc) mutants. Bone 26:207–213
Kartner N, Yao Y, Bhargava A, Manolson MF (2013) Topology, glycosylation and conformational changes in the membrane domain of the vacuolar H + -ATPase a subunit. J Cell Biochem 114:1474–1487
Efeyan A, Zoncu R, Chang S et al (2013) Regulation of mTORC1 by the Rag GTPases is necessary for neonatal autophagy and survival. Nature 493:679–683
Parkhitko AA, Favorova OO, Khabibullin DI et al (2014) Kinase mTOR: regulation and role in maintenance of cellular homeostasis, tumor development, and aging. Biochemistry (Mosc) 79:88–101
Blagosklonny MV (2010) Calorie restriction: decelerating mTOR-driven aging from cells to organisms (including humans). Cell Cycle 9:683–688
Blagosklonny MV (2011) Molecular damage in cancer: an argument for mTOR-driven aging. Aging (Albany NY) 3:1130–1141
Blagosklonny MV (2013) M(o)TOR of aging: mTOR as a universal molecular hypothalamus. Aging (Albany NY) 5:490–494
Lamming DW, Ye L, Katajisto P et al (2012) Rapamycin-induced insulin resistance is mediated by mTORC2 loss and uncoupled from longevity. Science 335:1638–1643
Flinn RJ, Backer JM (2010) mTORC1 signals from late endosomes: taking a TOR of the endocytic system. Cell Cycle 9:1869–1870
Flinn RJ, Yan Y, Goswami S et al (2010) The late endosome is essential for mTORC1 signaling. Mol Biol Cell 21:833–841
Bar-Peled L, Schweitzer LD, Zoncu R, Sabatini DM (2012) Ragulator is a GEF for the Rag GTPases that signal amino acid levels to mTORC1. Cell 150:1196–1208
Settembre C, Zoncu R, Medina DL et al (2012) A lysosome-to-nucleus signalling mechanism senses and regulates the lysosome via mTOR and TFEB. EMBO J 31:1095–1108
Xu J, Cheng T, Feng HT, Pavlos NJ, Zheng MH (2007) Structure and function of V-ATPases in osteoclasts: potential therapeutic targets for the treatment of osteolysis. Histol Histopathol 22:443–454
Qin A, Cheng TS, Pavlos NJ et al (2012) V-ATPases in osteoclasts: structure, function and potential inhibitors of bone resorption. Int J Biochem Cell Biol 44:1422–1435
Qin A, Cheng TS, Lin Z et al (2012) Prevention of wear particle-induced osteolysis by a novel V-ATPase inhibitor saliphenylhalamide through inhibition of osteoclast bone resorption. PLoS One 7, e34132
Lebreton S, Jaunbergs J, Roth MG et al (2008) Evaluating the potential of vacuolar ATPase inhibitors as anticancer agents and multigram synthesis of the potent salicylihalamide analog saliphenylhalamide. Bioorg Med Chem Lett 18:5879–5883
Perez-Sayans M, Somoza-Martin JM, Barros-Angueira F et al (2009) V-ATPase inhibitors and implication in cancer treatment. Cancer Treat Rev 35:707–713
You H, Jin J, Shu H, Yu B et al (2009) Small interfering RNA targeting the subunit ATP6L of proton pump V-ATPase overcomes chemoresistance of breast cancer cells. Cancer Lett 280:110–119
Niikura K (2006) Vacuolar ATPase as a drug discovery target. Drug News Perspect 19:139–144
Huss M, Wieczorek H (2009) Inhibitors of V-ATPases: old and new players. J Exp Biol 212:341–346
Pommier Y, Cherfils J (2005) Interfacial inhibition of macromolecular interactions: Nature's paradigm for drug discovery. Trends Pharmacol Sci 26:138–145
Mullard A (2012) Protein-protein interaction inhibitors get into the groove. Nat Rev Drug Discov 11:173–175
Azzarito V, Long K, Murphy NS, Wilson AJ (2013) Inhibition of alpha-helix-mediated protein-protein interactions using designed molecules. Nat Chem 5:161–173
Merkulova M, Păunescu TG, Azroyan A et al (2015) Mapping the H + -V-ATPase interactome: identification of proteins involved in trafficking, folding, assembly and phosphorylation. Sci Rep 5:14827. (doi: 10.1038/srep14827)
Acknowledgements
Original work in the authors’ laboratories is supported by NIH DK038452, BADERC DK057521-08 (Marshansky), Ministry of Education Tier 2 (MOE2011-T2-2-156; ARC 18/12), Singapore (Grüber), and Japan Science and Technology Agency (Futai).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Marshansky, V., Futai, M., Grüber, G. (2016). Eukaryotic V-ATPase and Its Super-complexes: From Structure and Function to Disease and Drug Targeting. In: Chakraborti, S., Dhalla, N. (eds) Regulation of Ca2+-ATPases,V-ATPases and F-ATPases. Advances in Biochemistry in Health and Disease, vol 14. Springer, Cham. https://doi.org/10.1007/978-3-319-24780-9_16
Download citation
DOI: https://doi.org/10.1007/978-3-319-24780-9_16
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-24778-6
Online ISBN: 978-3-319-24780-9
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)