Abstract
Hydrogen sulfide (H2S) has been considered as a phytotoxin for almost 300 years, having deleterious effects on plant growth and survival. However, in recent years, H2S has been added to nitric oxide (NO) and carbon monoxide (CO) as a newly categorized group of biologically active gases termed as gasotransmitters, due to its capacity to control a range of physiological responses. It is recognized that for H2S to have an effect on plants cells it has to be present in a high enough concentration. From the environment or from within are two main sources of H2S in plants. Natural sources include the discharge from volcanoes, coastal marine sediments, or anoxic soils such as found in marshland, while man-made sources include waste treatment installations, agricultural industries, and geothermal power plants. Likewise, intracellular sources of H2S in plants include the production by desulfhydrase enzymes. However, although at present there is no direct evidence that H2S acts as an endogenous regulator or signal molecule in plants, the induction of l-cysteine desulfhydrase upon pathogen attack, emission of H2S from plants exposed to SO2 injury, abiotic stress tolerance in plants supplied with endogenous H2S donor, and its involvement in guard cell signaling and root organogenesis, all suggest that this is indeed the case. Furthermore, endogenous and exogenous postharvest applications of H2S have been used for improvement of shelf life and quality attributes of food products. Recently and for future research, several research groups are focusing on H2S and its role as a signal in plants.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
Hydrogen Sulfide General Description
Hydrogen sulfide (H2S) is a colorless, flammable, extremely hazardous gas with a strong odor of “ rotten egg” that can be smelt at levels of 0.02 μl L−1 and higher (Fig. 3.1) (Lloyd 2006; Beauchamp et al. 1984). H2S toxicity has been substantiated for almost 300 years due to its implication in several mass extinctions, including one at the end of the Permian period that wiped out more than three-quarters of all species on Earth (Kump et al. 2005; Erwin 1993). However, more recently, H2S has been added to nitric oxide (NO) and carbon monoxide (CO) as a newly categorized group of biologically active gases termed gasotransmitters and gasomediators, due to its capacity to control a range of physiological responses in animals (Wang 2003; Mancardi et al. 2009). Many studies have revealed that H2S in low concentrations can act as a signaling molecule in animals, and participate in various biological processes, such as smooth muscle, relaxation, brain development, blood pressure, and inflammation (Chen et al. 2011). Therefore, it is reasonable to suspect that this gas molecule has a similar role in plants and can affect a range of their physiological responses and could be used to control postharvest plant function (Zhang et al. 2010).
H2S is synthesized in mammalian tissues via endogenous enzymes and by nonenzymatic pathways (e.g., reduction of thiols and thiol-containing molecules) (Li et al. 2011). Moreover, there is evidence that H2S in plants is released from cysteine via reversible O-acetylserine (thiol)lyase (OAS-TL) reaction, catalyzed by several l- and d-cysteine-specific desulfhydrase candidates. l-Cystathionine specifically metabolized l-cysteine to produce H2S, pyruvate, and ammonium, while d-cystathionine only decomposes d-cysteine and produces H2S (Fig. 3.2) (Wirtz et al. 2004; Chen et al. 2011). Although the biochemical properties of the H2S make it difficult to study, this gas can be measured in biological systems using a method based on the formation of methylene blue from sulfide and N,N-dimethyl-p-phenylenediamine in the presence of Fe3+ and its spectrophotometric detection at 675 nm (Hancock et al. 2012). Also, other methods include the use of gas chromatography which assess the release of H2S and the use of fluorescent probes for the gas (Liu et al. 2011a; Sasakura et al. 2011). Such and other similar assays can be applied to detect H2S in plants and their environments.
Hydrogen Sulfide in Plants
H2S is often thought to be a phytotoxin, being harmful to the growth and development of plants. However, there is accumulating evidence that H2S also could act as a gaseous regulator in plants. For example, 35 years ago it was found to inhibit oxygen release from young seedling of six rice cultivars. Likewise, in some cultivars of rice, nutrient uptake was also reduced, while in others it was increased. Phosphorous uptake was also inhibited in this plant species (Joshi et al. 1975). Also, a constant fumigation of H2S (3000 parts per billion) caused lesions on leaves, defoliation, and reduced growth of Medicago, grapes, lettuce, sugars beets, and pine. Interestingly, lower levels of fumigation (100 part per billion) caused a significant increase in the growth of Medicago, lettuce, and sugar beet (Thompson and Kats 1978). More recently, a study showed that the H2S donor NaSH would alleviate the osmotic-induced decrease in chlorophyll concentration in sweet potato. Furthermore, spraying NaSH increased the activity of the antioxidant enzymes superoxide dismutase, catalase, and ascorbate peroxidase while decreasing the concentration of reactive oxygen species (ROS) such as hydrogen peroxide and lipoxygenase (Zhang et al. 2009). These results clearly suggested that H2S can have intracellular effects which impinge on cell signaling events in the plant cells.
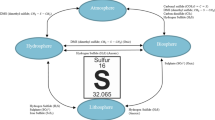
For H2S to have an effect on plants cells, it has to be present and in a high enough concentration. There are two main sources of H2S to which a plant cell may wish to respond: from the environment or from within (Fig. 3.3). The aerial parts and roots of plants may be often exposed to atmospheric H2S because it is commonly emitted from many natural and man-made sources. Natural sources of H2S include the discharge from volcanoes, coastal marine sediments, or anoxic soils such as found in marshland (Aiuppa et al. 2005; Hansen et al. 1978; Morse et al. 1987). Man-made sources of this gas are also common, and include waste treatment installations, agricultural industries, and geothermal power plants. It has also been found to be at surprisingly high concentrations in some urban environments with car catalytic converters, being suggested as a potential source (Zhang et al. 2008; Aneja et al. 2008; Bacci et al. 2000). However, the fact that plants respond to such exogenous sources of H2S does not necessarily indicate that it has a signaling role. Often, the responses of the plants are associated to toxic levels of these compounds. After all, it is because of its phytotoxic effects at high levels that H2S has become well known.
Some authors reported that to truly be a cell signaling molecule, H2S must be generated by plant cells. Many species of plant have been found to generate H2S using a light-dependent activity and includes cucumber, squash, pumpkin, soybean, and cotton, among other plants (Wilson et al. 1978). Intracellular sources of H2S would include the production by enzymatic mechanisms. As mentioned before, it appears that the enzymes responsible for H2S in plants are desulfhydrases. A plastid located cysteine desulfhydrase has been reported in Arabidopsis, while others report the presence of a similar enzyme in the mitochondria (Léon et al. 2002; Riemenschneider et al. 2005). However, activities of such enzymes are not static and have been shown to change after some circumstances, for example, pathogen challenge (Bloem et al. 2004). This would be expected if the enzymes are to perform a role in the creation of a molecule which is to act as a signal. Other enzymes have also been proposed as being able to generate H2S too, and includes d-cysteine desulfhydrase that produces pyruvate, ammonia, and H2S, and an enzyme involved in cyanide metabolism, β-cyanoalanine synthase, which converts cysteine and cyanide to β-cyanoalanine and H2S (Papenbrock et al. 2007; García et al. 2010).
Bacteria have been shown to generate H2S too. Microorganisms that are invading plants, such as pathogenic bacteria, may be able to release H2S, which will then affect the activities of the plant. Recently, Bloem et al. (2012) reported that H2S emissions were assessed in oilseed rape (Brassica napus L.) and after fungal infection with Sclerotinia sclerotiorum. It was found that infection caused a significant rise in H2S release, but they also reported that under different conditions, depending on the air concentration and the sulfur demand of the plant that H2S was sometimes taken up and not released. This may suggest that H2S is important for sulfur metabolism rather than acting solely as a signal.
Hydrogen Sulfide as Regulator of Plant Physiological Processes
Plant physiology is concerned with the fundamental processes of plants, its survival, metabolic activities, water relations, mineral nutrition, development, movement, irritability, organization, growth, and transport processes (Nilsen and Orcutt 1996). Likewise, a lot of physiological processes of plants are regulated by interactions among different plant growth molecules due to plant cells are able to sense and respond to a wide range of external and internal signals. Plant regulators are considered organic compounds, other than nutrients, which in small amounts promote, inhibit, or otherwise affect the physiological processes of plants (Moore 1979).
Although at present there is no direct evidence that H2S acts as an endogenous regulator or signal molecule in plants, the induction of l-cysteine desulfhydrase upon pathogen attack, emission of H2S from plants exposed to SO2 injury, abiotic stress tolerance in plants supplied with endogenous H2S donor, and its involvement in guard cell signaling and root organogenesis, all suggest that this is indeed the case (Hällgren and Fredriksson 1982; Bloem et al. 2004; García‐Mata and Lamattina 2010). Moreover, it is now known that H2S causes inhibition of photosynthesis at high concentrations and that it can decrease the time to germination, but also increases the resilience to drought and heavy metal toxicity (Lisjak et al. 2011; Chen et al. 2011; Oren et al. 1979; Dooley et al. 2013). Recent emerging evidence has also suggested a possible signaling role for stomatal apertures, and in promoting chloroplast biogenesis (Hancock et al. 2012; García-Mata and Lamattina 2010; Chen et al. 2011). In this sense, several research groups are now focusing on H2S and its role as a signal in plants (Table 3.1).
Mediator of Stomatal Movements
Stomata are by far the most influential components in gas exchange and their movements control transpiration. Consequently, stomata are important regulators of plant growth and development (Liu et al. 2011b). Although previous studies have shown stomata respond to a variety of environmental stresses, such as drought, cold, high CO2 concentration, and phytohormone, little is known about the signal transduction mechanisms that function in guard cells. The effects of H2S as a newly identified signal molecule on regulation of stomatal aperture have been recently emerging.
Liu et al. (2011b) reported that H2S and NO are involved in the signal transduction pathway of ethylene-induced stomatal closure, and that in Arabidopsis, H2S may represent a novel downstream indicator of NO during ethylene-induced stomatal movement. Other studies have taken advantage of the use of H2S donors. The most used donor is the compound sodium hydrosulfide (NaSH) which will dissociate rapidly to generate a very short burst of H2S. However, H2S can be relatively high if high concentrations of NaSH are used. Lisjak et al. (2011) showed a H2S-mediated stomatal opening in Arabidopsis. This was seen in plants treated with both NaSH, giving a relatively short burst of H2S, or with GYY4137 (a forerunner) giving a longer more prolonged exposure to H2S. With leaves which had not been pre-opened in the light, the effects of both NaSH and GYY4137 were larger. This work was repeated in Capsicum annuum and similar opening was induced by the treatment with both H2S donors. Other work has shown that stomatal conductance was increased by carbonyl sulfide (COS) and it was suggested that H2S mediates this effect which produced from COS hydrolysis. However, clearly further work is required (Stimler et al. 2010).
On the other hand, García-Mata and Lamattina (2010) reported a different effect of H2S on stomatal movements. They found that exogenous H2S induces stomatal closure and this effect is impaired by (1) the ATP-binding cassette (ABC) transporter inhibitor glibenclamide; (2) scavenging H2S or inhibition of the enzyme responsible for endogenous H2S synthesis partially blocks ABA-dependent stomatal closure; and (3) H2S treatment increases relative water content and protects plants against drought stress. In conclusion, their results indicate that H2S induces stomatal closure and participates in ABA-dependent signaling, possibly through the regulation of ABC transporters in guard cells. Jin et al. (2011) showed similar results, where exogenous H2S released by its donors induced stomatal closure in Arabidopsis. Also, Hou et al. (2013) suggest that the enzyme d-/l-cysteine desulfhydrase that generated H2S is involved in the regulation of ethylene-induced stomatal closure in Arabidopsis.
Regulation of Senescence
Plant senescence is considered as a highly regulated physiological process that leads to plant death (Thomas et al. 2003). Accumulating evidence shows that H2S plays various physiological roles in plants, such as senescence of cut flowers. Zhang et al. (2011) reported that H2S was found to delay flower opening and senescence in various cut flowers and branches. Cut explants of these plants were cultured in solution containing different concentrations of the H2S donor, NaHS. H2S donor treatment prolonged the vase time of cut flowers and prevented senescence in a dose-dependent manner. Also, they measured the levels of malondialdehyde (MDA) as an indicator of oxidative damage to cells and showed that it was inversely related to endogenous H2S concentration in explants. Flowers that had senesced showed higher levels of MDA and lower amounts of H2S. Furthermore, NaHS treatment increased the activities of catalase, superoxide dismutase, ascorbate peroxidase, and guaiacol peroxidase, and sustained much lower levels of H2O2 and O2 − in cut flowers. In conclusion, the study implies that H2S is involved in improving longevity of cut flowers and functions in activity of antioxidant enzymes in plants.
Moreover, H2S participation in the regulation of ripening and senescence in postharvest fruits remains unknown. A study investigated the effect of H2S on postharvest shelf life and antioxidant metabolism in strawberry fruits. Fumigation with H2S gas released from the H2S donor NaHS prolonged postharvest shelf life of strawberry fruits in a dose-dependent manner. Strawberry fruits fumigated with various concentrations of H2S sustained significantly lower rot index and higher fruit firmness, and kept lower respiration intensity and polygalacturonase activities than controls. Further investigation showed that H2S treatment maintained higher activities of catalase, guaiacol peroxidase, ascorbate peroxidase, and glutathione reductase and lower activities of lipoxygenase relative to untreated controls. H2S also reduced malondialdehyde, hydrogen peroxidase, and superoxide anion to levels below control fruits during storage. Moreover, H2S treatment maintained higher contents of reducing sugars, soluble proteins, free amino acid, and endogenous H2S in fruits. These data indicated that H2S plays an antioxidative role in prolonging postharvest shelf life and senescence of strawberry fruits (Hu et al. 2012).
Photosynthetic Response
It is well known that the increase in photosynthesis can be achieved by enhancing the activity of ribulose-1, 5-bisphosphate carboxylase (RuBISCO) (Krantev et al. 2008). Changes in RuBISCO synthesis have been primarily explained by changes in transcript abundance of its genes in response to various external and/or internal signals (Nishimura et al. 2008; Suzuki et al. 2010). In addition, the oxidation of glycolate to glyoxylate in higher plants is catalyzed by glycolate oxidase, which is located in the peroxisomes and performs an essential role in the oxidative photorespiration cycle accompanying photosynthetic CO2 assimilation (Zelitch et al. 2009). Meanwhile, photorespiration also involves a cooperative interaction among enzymes localized in chloroplasts, mitochondria, and peroxisomes, and is performed by the glycolate pathway (Yamaguchi and Nishimura 2000). A previous study showed the effect of H2S as a biologically active gas on photosynthesis, it was reported that excess sulfide (1 mM) resulted in inhibition of photosystem II (PSII) in cyanobacteria and tobacco chloroplasts (Oren et al. 1979) and that a high sulfide concentration (2 mM) depressed the growth and photosynthesis in a mangrove plant (Lin and Sternberg 1992). However, it is not clear whether a low concentration of H2S is involved in regulation of photosynthesis in plants.
Chen et al. (2011) used a NaHS as a donor of H2S to understand further the roles of this gas in physiological processes of photosynthesis and grana lamella formation in S. oleracea. The results indicated that photosynthesis, RuBISCO, OAS-TL, and l-cysteine desulfhydrase activities and other photosynthetic characteristics were altered by exogenous application of a low concentration of NaHS. The number of grana lamellae stacking into functional chloroplasts was also increased markedly. Furthermore, it was demonstrated that seedlings treated with 100 μM NaHS increased the expression of RuBISCO genes but significantly decreased the gene expression of glycolate oxidase and cytochrome oxidase. They concluded that H2S acts as a signaling molecule that participates in enhancing photosynthesis and chloroplast development during S. oleracea growth.
On the other hand, highly reducing sediments are prevalent in sea grass environments. Under anoxic conditions, H2S can accumulate as an end product of anaerobic respiration at levels which may be toxic to halophytes. The photosynthetic response of Zostera marina L. (eelgrass) to manipulations in sediment sulfide concentration and light regimes was examined by Goodman et al. (1995). Sediment sulfide levels were enriched using Na2S and lowered using FeSO4. Photosynthesis vs. irradiance relationships were determined experimentally at ten light levels throughout the 21 day experiment. Photoadaptation was detected in response to the previous 4-day light history of the plants, as maximum photosynthesis decreased in response to lower daily light levels. Negative impacts of sulfide on eelgrass in this study were observed, increases in the light intensity at which gross photosynthesis equals respiration, and decreases in the initial slope of the photosynthesis-irradiance curve. The effects of eutrophication through reduced light and increased sediment sulfide were additive. Elevated sediment sulfide levels may contribute to sea grass loss in stressed areas as the potential for utilization of available light is reduced.
Pre- and Postharvest Applications of Hydrogen Sulfide
Due to the abovementioned roles that H2S plays in various physiological roles in plants, new studies are trying to apply this chemical as a novel pre- and postharvest technology applied in fresh produce to control their physiological processes. Its application in fresh produce could be through H2S donors, such as NaHS, or saturated solutions obtained by passing H2S gas into carbon dioxide-free water (Zhu et al. 2014; Li et al. 2012). However, both H2S application methods obtain similar results as it is reported by several studies. For example, Hu et al. (2014a) stated that when H2S is applied to mulberry fruits using an aqueous solution of NaHS, it could result in a decrease in the ripening rate as well as a lowering in the respiration intensity and anthocyanin content. Likewise, Zhu et al. (2014) applied H2S in kiwifruits by dipping them in gas-saturated water solutions. They found that some antioxidant-related enzymes, such as CAT, SOD, APX, and POD significantly increased after H2S treatment, delaying its senescence.
This small gas, along with others such as carbon monoxide (CO) and nitric oxide (NO), has been target of novel research projects with the purpose of developing new postharvest technologies (Abdollahi et al. 2013; Li et al. 2014; Fu et al. 2014). Some of the oldest pesticides, such as calcium polysulfide, can release H2S, especially when the pesticide solution is acidified (Smilanick and Sorenson 2001). The knowledge provided by the elucidation of role of H2S in the above mentioned physiological responses “stomatal movements” and “photosynthetic responses,” is essential to the novel technologies applied to alleviate abiotic types of stress and provide tolerance in plants (Shi et al. 2013). For example, Zhang et al. (2009) found that the application of NaHS as H2S donor can alleviate osmotic stress and prevent chlorophyll losses in seedling leaves of sweet potato (Ipomoea batatas). However, and excessive air exposure to H2S (3000 parts per billion) could result in leaves lesions, defoliation, and reduced growth of plants (Li et al. 2013). Moreover, at proper concentrations, H2S could act as a powerful preharvest technology alleviating even root tip death in pea seedlings induced by flooding of soils (Cheng et al. 2013).
On the other hand, H2S can also be successfully applied as postharvest technology to preserve fresh produce. In this context, a recent study suggested that the application of aqueous solutions of NaHS (0.5–2.5 mM) releases about 0.05–0.5 ppm of H2S gas in a sealed container, which was enough to prolong the storage of pears at 20 °C (Hu et al. 2014b). Moreover, Zhu et al. (2014) found that treatments with 45 and 90 H2S, applied as a saturated aqueous solution, could delay maturation and senescence of kiwifruits and maintain higher quality attributes. These results suggest that H2S could be applied as pre- and postharvest technology to plants and their fruits, either if they are climacteric or non-climacteric fruits.
Shelf Life Prolongation of Food Products
The shelf life of fresh produce is one of the most important objectives of food industry and many research groups around the world. In that sense, the application of natural compounds as technology to preserve foods has been suggested by several authors (González-Aguilar et al. 2010; Mastromatteo et al. 2010; Juneja et al. 2012). Applications of H2S to fresh produce reduce the damage of ROS by upregulating antioxidant enzymes (Zhu et al. 2014). According to Hu et al. (2012), strawberry fruits fumigated with H2S maintained higher contents of reducing sugars, soluble proteins, free amino acids, and endogenous H2S due to the antioxidative role in the postharvest life of this fruits. The same protective effect was observed by Hu et al. (2014a) in a study where they applied NaSH as H2S donor to mulberry fruits resulting in an enhancement of antioxidant enzyme activity. Likewise, Fu et al. (2014) suggested that besides the enhancement in the antioxidant enzyme activity in fruits after treatment, H2S acts also as an antifungal agent inhibiting the growth of Saccharomyces cerevisiae, Rhizopus oryzae, Candida albicans, and several food-borne bacteria.
Hu et al. (2014b) reported similar inhibition of pathogens Aspergillus niger and Penicillium expansum in pear fruits after fumigation with H2S. These antimicrobial and antifungal properties are similar to those reported to NO, which has shown growth inhibition of Aspergillus niger, Monilinia fructicola, Penicillium italicum, and Rhizopus nigricans (Manjunatha et al. 2010). As both gases have similar chemical properties, both enhance antioxidant enzyme activity, inhibit pathogens, and also protect fresh produce against chilling injury (Luo et al. 2015; Zaharah and Singh 2011) as well as remarkable benefits to the enhancement of fresh produce when applied, both could be applied in combination to achieve a cooperative effect in the shelf life extension of food products. For example, Chang et al. (2014) observed that there was a greater effect prolonging the postharvest shelf life of strawberry fruit combining H2S and NO than that effect observed when used separately (Fig. 3.4).
Conclusions and Future Trends
So far, the role of H2S molecules has been underestimated because this was considered an undesirable phytotoxin which causes deleterious effects on plant growth. However, it has been demonstrated through diverse research projects that, in proper amounts, H2S could perform signaling functions that drive or enhance physiological responses in plant tissues. These recent breakthroughs over the truly effects of H2S are leading to the development of novel technologies to preserve or enhance diverse quality attributes in plant tissues, or as well directly over fresh produce prolonging its postharvest shelf life storage. Likewise, besides the prevention of spoilage microorganism growth, the effect of H2S as a signaling molecule triggers a series of events that induce and enhance the activity of antioxidant enzymes which may result in an added value to the plant tissue of food product treated.
-
The studies about the synergistic effects of H2S with other gases or signaling molecules are scarce and should be addressed in order to properly understand how H2S could interact with other natural molecules and its biological effect.
-
There’s a lack of studies that evaluate the effect over firmness and color and bioactive compound content changes after H2S treatments.
-
Further studies should be addressing the use of H2S molecules in emergent technologies such as MAP or active packaging to prolong shelf life of either climacteric or non-climacteric fruits and vegetables.
References
Abdollahi, R., Asghari, M., Esmaiili, M., & Abdollahi, A. (2013). Postharvest nitric oxide treatment effectively reduced decays of selva strawberry fruit. Nitric Oxide, 3, 5.459.
Aiuppa, A., Inguaggiato, S., Mcgonigle, A., O’Dwyer, M., Oppenheimer, C., Padgett, M., et al. (2005). H 2 S fluxes from Mt. Etna, Stromboli, and Vulcano (Italy) and implications for the sulfur budget at volcanoes. Geochimica et Cosmochimica Acta, 69, 1861–1871.
Aneja, V. P., Schlesinger, W. H., & Erisman, J. W. (2008). Farming pollution. Nature Geoscience, 1, 409–411.
Bacci, E., Gaggi, C., Lanzillotti, E., Ferrozzi, S., & Valli, L. (2000). Geothermal power plants at Mt. Amiata (Tuscany–Italy): Mercury and hydrogen sulphide deposition revealed by vegetation. Chemosphere, 40, 907–911.
Beauchamp, R., Bus, J. S., Popp, J. A., Boreiko, C. J., Andjelkovich, D. A., & Leber, P. (1984). A critical review of the literature on hydrogen sulfide toxicity. CRC Critical Reviews in Toxicology, 13, 25–97.
Bloem, E., Haneklaus, S., Kesselmeier, J. R., & Schnug, E. (2012). Sulfur fertilization and fungal infections affect the exchange of H2S and COS from agricultural crops. Journal of Agricultural and Food Chemistry, 60, 7588–7596.
Bloem, E., Riemenschneider, A., Volker, J., Papenbrock, J., Schmidt, A., Salac, I., et al. (2004). Sulphur supply and infection with Pyrenopeziza brassicae influence L-cysteine desulphydrase activity in Brassica napus L. Journal of Experimental Botany, 55, 2305–2312.
Chang, Z., Jingying, S., Liqin, Z., Changle, L., & Qingguo, W. (2014). Cooperative effects of hydrogen sulfide and nitric oxide on delaying softening and decay of strawberry. International Journal of Agricultural and Biological Engineering, 7, 114–122.
Chen, J., Wu, F.-H., Wang, W.-H., Zheng, C.-J., Lin, G.-H., Dong, X.-J., et al. (2011). Hydrogen sulphide enhances photosynthesis through promoting chloroplast biogenesis, photosynthetic enzyme expression, and thiol redox modification in Spinacia oleracea seedlings. Journal of Experimental Botany, 62, 4481–4493.
Cheng, W., Zhang, L., Jiao, C., Su, M., Yang, T., Zhou, L., et al. (2013). Hydrogen sulfide alleviates hypoxia-induced root tip death in Pisum sativum. Plant Physiology and Biochemistry, 70, 278–286.
Dooley, F. D., Wyllie-Echeverria, S., Roth, M. B., & Ward, P. D. (2013). Tolerance and response of Zostera marina seedlings to hydrogen sulfide. Aquatic Botany, 105, 7–10.
Erwin, D. H. (1993). The great Paleozoic crisis; life and death in the Permian (Critical moments in paleobiology and earth history series). New York: Columbia University Press.
Fu, L.-H., Hu, K.-D., Hu, L.-Y., Li, Y.-H., Hu, L.-B., Yan, H., et al. (2014). An antifungal role of hydrogen sulfide on the postharvest pathogens Aspergillus niger and Penicillium italicum. PloS One, 9, e104206.
García, I., Castellano, J. M., Vioque, B., Solano, R., Gotor, C., & Romero, L. C. (2010). Mitochondrial β-cyanoalanine synthase is essential for root hair formation in Arabidopsis thaliana. The Plant Cell Online, 22, 3268–3279.
García-Mata, C., & Lamattina, L. (2010). Hydrogen sulphide, a novel gasotransmitter involved in guard cell signalling. New Phytologist, 188, 977–984.
González-Aguilar, G. A., Ayala-Zavala, J., Olivas, G., DE LA Rosa, L., & Álvarez-Parrilla, E. (2010). Preserving quality of fresh-cut products using safe technologies. Journal für Verbraucherschutz und Lebensmittelsicherheit, 5, 65–72.
Goodman, J. L., Moore, K. A., & Dennison, W. C. (1995). Photosynthetic responses of eelgrass (Zostera marina L.) to light and sediment sulfide in a shallow barrier island lagoon. Aquatic Botany, 50, 37–47.
Hällgren, J.-E., & Fredriksson, S.-Å. (1982). Emission of hydrogen sulfide from sulfur dioxide-fumigated pine trees. Plant Physiology, 70, 456–459.
Hancock, J. T., Lisjak, M., Teklic, T., Wilson, I. D., & Whiteman, M. (2012). Hydrogen sulphide and signalling in plants. Plant Sciences Reviews, 2011, 33.
Hansen, M. H., Ingvorsen, K., & Jøgensen, B. B. (1978). Mechanisms of hydrogen sulfide release from coastal marine sediments to the atmosphere. Limnology and Oceanography, 23, 68–76.
Hou, Z., Wang, L., Liu, J., Hou, L., & Liu, X. (2013). Hydrogen sulfide regulates ethylene-induced stomatal closure in Arabidopsis thaliana. Journal of Integrative Plant Biology, 55, 277–289.
Hu, L.-Y., Hu, S.-L., Wu, J., Li, Y.-H., Zheng, J.-L., Wei, Z.-J., et al. (2012). Hydrogen sulfide prolongs postharvest shelf life of strawberry and plays an antioxidative role in fruits. Journal of Agricultural and Food Chemistry, 60, 8684–8693.
Hu, H., Shen, W., & Li, P. (2014a). Effects of hydrogen sulphide on quality and antioxidant capacity of mulberry fruit. International Journal of Food Science & Technology, 49, 399–409.
Hu, K.-D., Wang, Q., Hu, L.-Y., Gao, S.-P., Wu, J., Li, Y.-H., et al. (2014b). Hydrogen sulfide prolongs postharvest storage of fresh-cut pears (Pyrus pyrifolia) by alleviation of oxidative damage and inhibition of fungal growth. PloS One, 9, e85524.
Jin, Z., Shen, J., Qiao, Z., Yang, G., Wang, R., & Pei, Y. (2011). Hydrogen sulfide improves drought resistance in Arabidopsis thaliana. Biochemical and Biophysical Research Communications, 414, 481–486.
Joshi, M., Ibrahim, I., & Hollis, J. (1975). Hydrogen sulfide: Effects on the physiology of rice plants and relation to straighthead disease. Phytopathology, 65, 1165–1170.
Juneja, V. K., Dwivedi, H. P., & Yan, X. (2012). Novel natural food antimicrobials*. Annual Review of Food Science and Technology, 3, 381–403.
Krantev, A., Yordanova, R., Janda, T., Szalai, G., & Popova, L. (2008). Treatment with salicylic acid decreases the effect of cadmium on photosynthesis in maize plants. Journal of Plant Physiology, 165, 920–931.
Kump, L. R., Pavlov, A., & Arthur, M. A. (2005). Massive release of hydrogen sulfide to the surface ocean and atmosphere during intervals of oceanic anoxia. Geology, 33, 397–400.
Léon, S., Touraine, B., Briat, J., & Lobréaux, S. (2002). The AtNFS2 gene from Arabidopsis thaliana encodes a NifS-like plastidial cysteine desulphurase. Biochemical Journal, 366, 557–564.
Li, Z.-G., Gong, M., Xie, H., Yang, L., & Li, J. (2012). Hydrogen sulfide donor sodium hydrosulfide-induced heat tolerance in tobacco (Nicotiana tabacum L) suspension cultured cells and involvement of Ca 2+ and calmodulin. Plant Science, 185, 185–189.
Li, L., Rose, P., & Moore, P. K. (2011). Hydrogen sulfide and cell signaling. Annual Review of Pharmacology and Toxicology, 51, 169–187.
Li, Z. G., Yang, S. Z., Long, W. B., Yang, G. X., & Shen, Z. Z. (2013). Hydrogen sulphide may be a novel downstream signal molecule in nitric oxide‐induced heat tolerance of maize (Zea mays L.) seedlings. Plant, Cell & Environment, 36, 1564–1572.
Li, Q., Zhang, S., & Mao, Y. (2014). Effect of carbon monoxide on active oxygen metabolism of postharvest jujube. Journal of Food Technology Research, 1, 146–155.
Lin, G., & Sternberg, L. D. S. (1992). Effect of growth form, salinity, nutrient and sulfide on photosynthesis, carbon isotope discrimination and growth of red mangrove (Rhizophora mangle L.). Functional Plant Biology, 19, 509–517.
Lisjak, M., Teklić, T., Wilson, I. D., Wood, M., Whiteman, M., & Hancock, J. T. (2011). Hydrogen sulfide effects on stomatal apertures. Plant Signaling & Behavior, 6, 1444–1446.
Liu, J., Hou, L., Liu, G., Liu, X., & Wang, X. (2011a). Hydrogen sulfide induced by nitric oxide mediates ethylene-induced stomatal closure of Arabidopsis thaliana. Chinese Science Bulletin, 56, 3547–3553.
Liu, C., Pan, J., Li, S., Zhao, Y., Wu, L. Y., Berkman, C. E., et al. (2011b). Capture and visualization of hydrogen sulfide by a fluorescent probe. Angewandte Chemie, 123, 10511–10513.
Lloyd, D. (2006). Hydrogen sulfide: Clandestine microbial messenger? Trends in Microbiology, 14, 456–462.
Luo, Z., Li, D., Du, R., & Mou, W. (2015). Hydrogen sulfide alleviates chilling injury of banana fruit by enhanced antioxidant system and proline content. Scientia Horticulturae, 183, 144–151.
Mancardi, D., Penna, C., Merlino, A., Del Soldato, P., Wink, D. A., & Pagliaro, P. (2009). Physiological and pharmacological features of the novel gasotransmitter: Hydrogen sulfide. Biochimica et Biophysica Acta-Bioenergetics, 1787, 864–872.
Manjunatha, G., Lokesh, V., & Neelwarne, B. (2010). Nitric oxide in fruit ripening: Trends and opportunities. Biotechnology Advances, 28, 489–499.
Mastromatteo, M., Conte, A., & DEL Nobile, M. (2010). Combined use of modified atmosphere packaging and natural compounds for food preservation. Food Engineering Reviews, 2, 28–38.
Moore, T. C. (1979). Biochemistry and physiology of plant hormones. New York: Springer.
Morse, J. W., Millero, F. J., Cornwell, J. C., & Rickard, D. (1987). The chemistry of the hydrogen sulfide and iron sulfide systems in natural waters. Earth-Science Reviews, 24, 1–42.
Nilsen, E. T., & Orcutt, D. M. (1996). Physiology of plants under stress Abiotic factors. New York: John Wiley and Sons Inc.
Nishimura, K., Ogawa, T., Ashida, H., & Yokota, A. (2008). Molecular mechanisms of RuBisCO biosynthesis in higher plants. Plant Biotechnology, 25, 285–290.
Oren, A., Padan, E., & Malkin, S. (1979). Sulfide inhibition of photosystem II in cyanobacteria (blue-green algae) and tobacco chloroplasts. Biochimica et Biophysica Acta-Bioenergetics, 546, 270–279.
Papenbrock, J., Riemenschneider, A., Kamp, A., Schulz‐Vogt, H. N., & Schmidt, A. (2007). Characterization of cysteine‐degrading and H2S‐releasing enzymes of higher plants‐from the field to the test tube and back. Plant Biology, 9, 582–588.
Riemenschneider, A., Wegele, R., Schmidt, A., & Papenbrock, J. (2005). Isolation and characterization of a D-cysteine desulfhydrase protein from Arabidopsis thaliana. FEBS Journal, 272, 1291–1304.
Sasakura, K., Hanaoka, K., Shibuya, N., Mikami, Y., Kimura, Y., Komatsu, T., et al. (2011). Development of a highly selective fluorescence probe for hydrogen sulfide. Journal of the American Chemical Society, 133, 18003–18005.
Shi, H., Ye, T., & Chan, Z. (2013). Exogenous application of hydrogen sulfide donor sodium hydrosulfide enhanced multiple abiotic stress tolerance in bermudagrass (Cynodon dactylon (L). Pers.). Plant Physiology and Biochemistry, 71, 226–234.
Smilanick, J., & Sorenson, D. (2001). Control of postharvest decay of citrus fruit with calcium polysulfide. Postharvest Biology and Technology, 21, 157–168.
Stimler, K., Montzka, S. A., Berry, J. A., Rudich, Y., & Yakir, D. (2010). Relationships between carbonyl sulfide (COS) and CO2 during leaf gas exchange. New Phytologist, 186, 869–878.
Suzuki, Y., Kihara-Doi, T., Kawazu, T., Miyake, C., & Makino, A. (2010). Differences in Rubisco content and its synthesis in leaves at different positions in Eucalyptus globulus seedlings. Plant, Cell & Environment, 33, 1314–1323.
Thomas, H., Ougham, H. J., Wagstaff, C., & Stead, A. D. (2003). Defining senescence and death. Journal of Experimental Botany, 54, 1127–1132.
Thompson, C. R., & Kats, G. (1978). Effects of continuous hydrogen sulfide fumigation on crop and forest plants. Environmental Science & Technology, 12, 550–553.
Wang, R. (2003). The gasotransmitter role of hydrogen sulfide. Antioxidants and Redox Signaling, 5, 493–501.
Wilson, L. G., Bressan, R. A., & Filner, P. (1978). Light-dependent emission of hydrogen sulfide from plants. Plant Physiology, 61, 184–189.
Wirtz, M., Droux, M., & Hell, R. (2004). O-acetylserine (thiol) lyase: An enigmatic enzyme of plant cysteine biosynthesis revisited in Arabidopsis thaliana. Journal of Experimental Botany, 55, 1785–1798.
Yamaguchi, K., & Nishimura, M. (2000). Reduction to below threshold levels of glycolate oxidase activities in transgenic tobacco enhances photoinhibition during irradiation. Plant and Cell Physiology, 41, 1397–1406.
Zaharah, S., & Singh, Z. (2011). Postharvest nitric oxide fumigation alleviates chilling injury, delays fruit ripening and maintains quality in cold-stored ‘Kensington Pride’ mango. Postharvest Biology and Technology, 60, 202–210.
Zelitch, I., Schultes, N. P., Peterson, R. B., Brown, P., & Brutnell, T. P. (2009). High glycolate oxidase activity is required for survival of maize in normal air. Plant Physiology, 149, 195–204.
Zhang, L., DE Schryver, P., DE Gusseme, B., DE Muynck, W., Boon, N., & Verstraete, W. (2008). Chemical and biological technologies for hydrogen sulfide emission control in sewer systems: A review. Water Research, 42, 1–12.
Zhang, H., Hu, S.-L., Zhang, Z.-J., Hu, L.-Y., Jiang, C.-X., Wei, Z.-J., et al. (2011). Hydrogen sulfide acts as a regulator of flower senescence in plants. Postharvest Biology and Technology, 60, 251–257.
Zhang, H., Tan, Z. Q., Hu, L. Y., Wang, S. H., Luo, J. P., & Jones, R. L. (2010). Hydrogen sulfide alleviates aluminum toxicity in germinating wheat seedlings. Journal of Integrative Plant Biology, 52, 556–567.
Zhang, H., Ye, Y.-K., Wang, S.-H., Luo, J.-P., Tang, J., & Ma, D. F. (2009). Hydrogen sulfide counteracts chlorophyll loss in sweetpotato seedling leaves and alleviates oxidative damage against osmotic stress. Plant Growth Regulation, 58, 243–250.
Zhu, L., Wang, W., Shi, J., Zhang, W., Shen, Y., Du, H., et al. (2014). Hydrogen sulfide extends the postharvest life and enhances antioxidant activity of kiwifruit during storage. Journal of the Science of Food and Agriculture, 94, 2699–2704.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Quirós-Sauceda, A.E., Velderrain-Rodríguez, G.R., Ovando-Martínez, M., Goñi, M.G., González-Aguilar, G.A., Ayala-Zavala, J.F. (2016). Hydrogen Sulfide. In: Siddiqui, M., Ayala Zavala, J., Hwang, CA. (eds) Postharvest Management Approaches for Maintaining Quality of Fresh Produce. Springer, Cham. https://doi.org/10.1007/978-3-319-23582-0_3
Download citation
DOI: https://doi.org/10.1007/978-3-319-23582-0_3
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23581-3
Online ISBN: 978-3-319-23582-0
eBook Packages: Chemistry and Materials ScienceChemistry and Material Science (R0)