Abstract
In 1969 pioneering work was made by Srivastava and Beutler (J Biol Chem 244:9–16, 1969) who reported that elimination of GSSG from human erythrocytes to the incubation medium is a unidirectional and energy-dependent process. Since then, a large number of biochemical/physiological studies were performed to characterize that energy-dependent unidirectional transporter. In 1992 Cole et al. made a giant leap in identifying molecular nature of that transporter to name MRP1 (ABCC1 according to the international nomenclature). Thereafter, completion of the Human Genome Project and advanced bioinformatics technology enabled researchers to gain more insight into the gene structure of a total of 48 ABC transporters in the human genome. Today, rapid growth of personalized medicine is being supported by emerging new technologies together with accumulating knowledge of pharmacogenomics. In this chapter, we show technological developments hitherto we made and will introduce a new SNP-typing method for human ABCC11 gene that is expected to provide a practical tool for clinical diagnosis of axillary osmidrosis.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- ABC transporter
- ABCC11
- Glutathione
- GS-X pump
- MRP
- Oxidative stress
- Personalized medicine
- Apocrine gland
- Axillary osmidrosis
- Earwax
Introduction
In aerobic living cells on the earth, there is a balance between oxidants and antioxidants, or reduction–oxidation (redox) homeostasis under normal physiological conditions. Reactive oxygen species, such as superoxide anion radical (.O2 −), hydroxyl radical (.OH), and hydrogen peroxide (H2O2), are continuously generated within living cells and play important roles in many cellular pathophysiological functions, including inflammation, apoptosis, mutation, and aging (Finkel 2003). Cytotoxic agents, including antitumor agents, xenobiotics, and carcinogens, induce oxidative stress. One of the important strategies in counteracting these stresses is the glutathione (GSH) system (Winyard et al. 2005). Under oxidative stress, GSH is oxidized by GSH peroxidase to glutathione disulfide (GSSG), which is eliminated by members of the GS-X pump family, commonly known as multidrug resistance protein (MRP) efflux proteins. Excess GSSG can be catalytically reduced back to GSH by NADPH-dependent GSSG reductase (Fig. 1). GSH also regulates the activities and biosynthesis of other redox-regulating enzymes such as superoxide dismutase (SOD) and DT-diaphorases (NQO1 and NQO2). Because of its abundance (1–10 mM inside cells) (Hwang et al. 1992; Smith et al. 1996), the GSH/GSSG system represents a major pathway for redox regulation in the cells.
Role of the GS-X pump transporter in the regulation of intracellular redox conditions. Under oxidative conditions, GSH is oxidized to GSSG by GSH peroxidase. GSSG can interact with cellular proteins to form glutathione-mixed sulfide (GS-S-protein). GSSG is either eliminated by the GS-X pump in an ATP-dependent manner or reduced back to GSH by GSSG reductase
GSH is a ubiquitous tripeptide thiol (L-γ-glutamyl-L-cysteinyl-glycine) that is present in virtually all eukaryotes (Meister and Anderson 1983). It is a vital intra- and extracellular cytoprotectant (Droge 2002; Reed 1990) and an effective scavenger of reactive oxygen species (ROS). It is estimated that GSH biosynthesis originated about 3.5 billion years ago. GSH is found in the vast majority of eukaryotes, whereas in eubacteria, GSH biosynthesis is limited to only two groups, i.e., cyanobacteria and purple bacteria (Fahey and Sundquist 1991). The former appeared on earth about 3.5 billion years ago and was capable of oxygenic photosynthesis. The cyanobacteria are considered to have given rise to plant chloroplasts, whereas the purple bacteria are considered to have introduced the ancestor responsible for eukaryotic mitochondria. GSH production appears to be closely associated with those prokaryotes responsible for the oxygen-producing and oxygen-utilizing pathways of eukaryotes, suggesting that the ability of GSH biosynthesis may have been acquired by eukaryotes during the endosymbiotic process that give rise to chloroplasts and mitochondria (Fahey and Sundquist 1991). In fact, GSH plays a pivotal role in the protection of living cells under conditions of oxidative stress (Chance et al. 1979; Sies 1985).
GSH also serves as a cofactor for conjugation reactions involved in the elimination of various xenobiotics such as chemical carcinogens, environmental pollutants, and antitumor agents (Sies 1988). The formation of hydrophilic glutathionyl conjugates is catalyzed by glutathione S-transferases (GSTs), a family of so-called phase II enzymes that mediate the conjugation reaction in a substrate-dependent fashion (Ishikawa 1992; Hayes et al. 2005; Townsend and Tew 2003). These GST isoenzymes are also involved in the endogenous biosynthesis of leukotrienes, prostaglandins, testosterone, and progesterone. Among their substrates, GSTs conjugate 4-hydroxynonenal, leukotriene A4, and the signaling molecules 15-deoxy-Δ12,14-prostaglandin J2 (15Δ-PGJ2) and Δ7-prostaglandin A1 (Ishikawa 1992; Ishikawa et al. 1998; Suzuki et al. 1997).
Plant and animal cells eliminate a broad range of lipophilic toxins from the cytosol following their conjugation with glutathione (GSH) (Ishikawa 1992; Ishikawa et al. 1997; Li et al. 1995; Martinoia et al. 1993; Rea et al. 2003). This transport process is mediated by GS-X pumps, namely organic anion transporters with Mg2+-ATPase activity. The term “GS-X pump” was originally proposed based on its transport activity and high affinity for glutathione S-conjugates (GS-conjugates), glutathione disulfide (GSSG), and cysteinyl leukotrienes (Ishikawa 1992). This pump was thought to shuffle these glutathionyl metabolites into the appropriate cellular compartments (Ishikawa 1992; Ishikawa et al. 1997). Accumulated data has suggested that the GS-X pump exhibits broad substrate specificity toward different types of organic anions and thereby play a physiologically important role in inflammation, oxidative stress, xenobiotic metabolism, and antitumor drug resistance.
Pioneers’ Odyssey to Discover GS-X Pump
In 1969, pioneering work was carried out by Srivastava and Beutler (1969) who reported that elimination of GSSG from human erythrocytes to the incubation medium is a unidirectional and energy-dependent process (Srivastava and Beutler 1969) (see Table 1 for historical background). GSSG transport occurred even against a concentration gradient of GSSG, and the transport was halted almost entirely when endogenous ATP was depleted by preliminary incubation of erythrocytes in a glucose-free medium for 8 h or by the presence of fluoride in the incubation medium. Their report provided the first evidence that GSSG efflux is mediated not by simple diffusion but rather by active membrane transport. Although they suggested the energy dependence of GSSG transport, the direct requirement of ATP was not elucidated. In 1980, the transport of GSSG across the plasma membrane was proven to be an ATP-dependent “primary” active process in inside-out membrane vesicles from human erythrocytes (Kondo et al. 1980). Subsequently, ATP-dependent GSH conjugate transport was demonstrated by Board (1981), Kondo et al. (1982), and Labelle et al. (1986).
In 1984, Ishikawa and Sies reported that GSSG and GS-conjugates were released from the isolated perfused heart (Ishikawa and Sies 1984) (Fig. 2). Because the heart is continuously exposed to highly oxygenated blood from the lungs, cardiomyopathy may result from oxidative damage inflicted by hyperoxia or administration of certain drugs. Thus, GSSG efflux was thought to be an important defense mechanism against oxidative stress (Fig. 1). To characterize the GSSG efflux mechanism, we constructed a rat heart perfusion system, where electrocardiogram (ECG), heart beat rate, left ventricular presser (LVP), and oxygen consumption rate were observed to ensure that the cardiac function was normal (Fig. 2). When we correlated the GSSG efflux rate with the cellular GSSG level in rat hearts exposed to oxidative stress (t-butyl hydroperoxide), saturation kinetics was observed between these two parameters with an apparent Km value of 30 nmol × g of heart−1 and the maximal GSSG efflux rate 7.5 nmol × min−1 × g of heart−1. This finding strongly inspired us to imagine that the GSSG efflux in the heart is mediated by a certain membrane carrier (Ishikawa and Sies 1984).
Detection of GSSG efflux from isolated perfused rat hearts. Hearts from male Wistar rats of 180–250 g body weight were perfused by the method of Langendorff at 37 °C with a constant load of 80 cm of water pressure (Ishikawa and Sies 1984; Ishikawa et al. 1986a, b). Standard perfusion medium was Krebs–Henseleit bicarbonate buffer solution containing 2.5 mM CaCl2 and 5.5 mM glucose equilibrated with 95 % O2 and 5 % CO2. Effluent perfusate was collected every minute into plastic tubes maintained at 0 °C for the measurement of GSSG. GSH and GSH levels in the effluent perfusate as well as in the heart tissue were biochemically measured as described previously (Ishikawa and Sies 1984)
The heart can undergo serious pathological states associated with peroxidation of polyunsaturated fatty acids in biomembranes. Hydroxyalkenals and other diffusible reactive short-chain carbonyl compounds are considered of major importance in the cause of cytotoxicity. The hydroxyalkenals with α,β-unsaturated carbonyl structure are electrophilic and react rapidly with thiol groups of GSH, cysteine, coenzyme A, and proteins. Ishikawa et al. (1986a) reported that the glutathione S-conjugate of 4-hydroxynonenal is effectively eliminated from the heart by the action of the GS-X pump (Ishikawa et al. 1986a). Based on kinetic analysis in isolated perfused rat heart, the existence of a common export pump for both GSSG and glutathione S-conjugates is suggested.
To gain more insight into the ATP-dependent GSSG efflux from the heart, we performed in vivo nuclear magnetic resonance (NMR) measurement with an isolated perfused rat heart. Figure 3 demonstrates an in vivo NMR system where cytosolic free ATP/ADP ratio and cellular pH level were continuously monitored. The relationship of GSSG efflux rate versus cytosolic free ATP/ADP ratio shows that GSSG efflux rate is half-maximal at (ATP/ADP) free = 10 (Ishikawa et al. 1986b), suggesting that GSSG efflux is an ATP-dependent transport process. GSSG efflux from the heart is not affected by epinephrine, norepinephrine, or dibutyryl cyclic AMP; GSSG transport is independent of α- or β-adrenergic hormonal regulations (Ishikawa et al. 1986b).
Direct measurement of ATP-dependent GSSG efflux by 31P-NMR spectroscopy. 31P-NMR spectra of isolated perfused hearts were recorded with a Bruker WH 360 NMR spectrometer operated in the Fourier transform mode. The heart was placed in a 15-mm NMR sample tube and inserted into a probe which was seated in the bore of a superconducting magnet (8.5 T). NMR spectra were taken by 80 scans of a 60º broadband pulse with a 0.6 s interpulse delay. The intracellular pH was determined from the chemical shift of inorganic phosphate signal as described previously (Ishikawa et al. 1986b)
Furthermore, using plasma membrane vesicles prepared from rat hearts, Ishikawa (1989) provided direct evidence for ATP-dependent primary active transport of GSSG and GS-conjugates. The cardiac GS-X pump was shown to have high affinity for GS-conjugates with long aliphatic carbon chains (Ishikawa et al. 1986b). Leukotriene C4 (LTC4), a pro-inflammatory mediator, was found to be an endogenous substrate for the GS-X pump (Ishikawa et al. 1989, 1990).
Technological Development to Advance Transport Studies
It is important to note that Ishikawa (1989) developed the standard method to characterize ATP-dependent transport mediated by GS-X pumps, namely C-class ABC transporters. Plasma membrane fractions from many different types of cells exhibit extremely high activities of ecto-ATPases that catabolize nucleotide triphosphates (e.g., ATP, GTP, and CTP). Because of this activity, ATP in the reaction medium is rapidly degraded during transport experiments. Thus, the ATP concentration in the reaction medium must be maintained constant for a sufficient period of the transport experiment. To solve this problem, Ishikawa (1989) introduced an ATP-regenerating system by adding creatine kinase and creatine phosphate into the transport reaction medium. Accordingly, in the case of plasma membrane vesicles from rat hearts, ATP concentration was maintained almost constant for at least 20 min of the transport experiment (Ishikawa 1989).
At that time, however, the throughput of transport experiments was quite low, because whole experiments absolutely depended on manual procedures. It was urgently needed to enhance the throughput of transport experiments by creating a new automated system. Ishikawa et al. (2005) developed a semi-automated system for transport experiments. The high-speed screening system using 96-well plates (Fig. 4) enabled to analyze the substrate specificity of ABC transporters and their genetic variants (Ishikawa et al. 2005). As we will discuss later, the effect of genetic polymorphisms on the transport activity depends on the substrates tested, and therefore the functional analysis using a wide variety of substrates is of great interest. One amino acid substitution can alter interactions between the active site of an ABC transporter and substrate molecules. Therefore, it was important to quantitatively analyze and to evaluate such structure-related interactions. In this context, the high-speed screening system provided a powerful tool to quantify the impact of genetic polymorphisms on the function of ABC transporters (Ishikawa et al. 2005).
High-speed screening for assessment of the function of ABC transporter expressed in Sf9 insect cell membranes. ABC transporter of interest was expressed in Sf9 insect cells by using recombinant virus, and plasma membrane vesicles were prepared from Sf9 cells, as described previously (Ishikawa et al. 2005). Membrane vesicles are formed by passing the membrane suspension through a 27-gauge needle. To measure the ATP-dependent transport, the standard incubation medium should contain plasma membrane vesicles (10 or 50 μg of protein), 1–200 μM radio-labeled substrate (e.g., [3H]methotrexate or [3H]dinitrophenyl glutathione), 0.25 M sucrose, 10 mM Tris/Hepes, pH 7.4, 10 mM MgCl2, 1 mM ATP, 10 mM creatine phosphate, and 100 μg/ml of creatine kinase in a final volume of 100 μl. The incubation was carried out at 37 °C. After a specified time (20 min for the standard condition), the reaction medium was mixed with 1 ml of the ice-cold stop solution (0.25 M sucrose, 10 mM Tris/Hepes, pH 7.4, and 2 mM EDTA) to terminate the transport reaction. Subsequently, aliquots (280 μl per well) of the resulting mixture were transferred to MultiScreenTM plates (Millipore). Under aspiration, each well of the plate was rinsed with the 0.25 M sucrose solution containing 10 mM Tris/Hepes, pH 7.4, four times (4 × 200 μl for each well) in an EDR384S system (BioTec, Tokyo, Japan). The radio-labeled substrate thus incorporated into the vesicles was measured by counting the radioactivity remaining on the filter of MultiScreenTM plates, where each filter was placed in 2 ml of liquid scintillation fluid (Ultima Gold, Packard BioScience). Detailed procedures are described in Ishikawa et al. (2005)
Furthermore, biological materials for transport experiments emerged as another critical issue, since the throughput of transport experiments was significantly enhanced by introducing the high-speed screening method. Preparation of plasma membrane vesicles from human tissues at a large amount was not realistic at all. A new approach was needed. Today, isolated membrane vesicles from insect cells provide a practical tool for low cost and high-throughput analysis of ABC transporters (Saito et al. 2009). Baculovirus-infected insect cells have been successfully employed to give relatively high protein expression yields; for example, Spodoptera frugiperda (Sf9) cells are widely used to obtain membranes overexpressing various ABC transporters. We usually infect Sf9 cells (1 × 106 cells/ml) with human ABC transporter-recombinant baculovirus and culture them at 26 °C with gentle shaking. The baculovirus has a 130-kb double-stranded DNA genome, packaged in a cigar-shaped (25 by 260 nm) enveloped nucleocapsid. Baculovirus enters insect cells via receptor-mediated endocytosis. The viral fusion protein gp64 is responsible for acid-induced endosomal escape. In the cytoplasm, the nucleocapsid probably induces the formation of actin filaments, which provide a possible mode of transport toward the nucleus.
The use of low ionic strength buffers during the membrane preparation steps promotes the formation of open membrane sheets and inside-out membrane vesicles. It is important to maintain high integrity of the plasma membrane vesicles used in the transport assay. In other words, the membrane vesicles have to be completely sealed. To examine the quality of plasma membrane vesicles prepared from Sf9 cells, Saito et al. (2009) used electron microscopy (TEM and SEM; Fig. 4) technologies and identified the optimal conditions required to prepare the membrane vesicles. The timing of harvesting Sf9 cells after baculovirus infection appears to be very critical. The membrane morphology of infected Sf9 cells changed greatly; in particular, numerous pores were observed in the cell membrane on day 5. Membrane vesicles prepared from those cells (day 5) are useless for transport experiments. It is also important to prepare membrane vesicles in the presence of serine/cysteine protease inhibitors. Leupeptin (10 μg/ml) inhibited the degradation of ABC transporter protein in membrane vesicles prepared from baculovirus-infected Sf9 cells during repetitive freeze–thaw cycles.
Membrane vesicles (suspended in 250 mM sucrose and 10 mM Tris/Hepes, pH 7.4) can be stored at −80 °C or in liquid nitrogen until used. For long-term (over one year) storage of membrane vesicles, however, it is recommended to substitute tolehalose for sucrose in the membrane vesicle preparations. Trehalose (α-d-glucopyranosyl α-d-glucopyranoside) is a non-reducing disaccharide composed of two glucose molecules joined by an α,α-1,1 linkage. Trehalose is a stress protectant in biological systems as it interacts with and directly protects lipid membranes and proteins from desiccation and during freezing (Saito et al. 2009).
Discovery of ABCC1 (MRP1) Gene Encoding GS-X Pump
In 1992, Cole et al. made a giant leap in identification of the molecular nature of GS-X pump (Table 1). They used a differential screening approach to clone a cDNA encoding a deduced protein of 190 kDa, which they named “MRP1”. This protein was overexpressed in a doxorubicin-resistant cell line (Cole et al. 1992). Sequence analyses of this MRP1 cDNA revealed it to be an ABC transporter, with closer sequence similarity to cystic fibrosis transmembrane conductance regulator (CFTR/ABCC7) than to P-glycoprotein-encoded MDR1 (ABCB1). Further studies demonstrated that membrane vesicles prepared from MRP1 (ABCC1)-transfected cells exhibited elevated transport activities for GSH-conjugates, such as LTC4 and S-(2,4-dinitrophenyl)-glutathione (GS-DNP) (Leier et al. 1994; Muller et al. 1994), the steroid conjugate 17-β-glucuronosyl [3H]estradiol, and bile salt conjugates 6-α-[14C]glucuronosylhypodeoxycholate and 3-α-sulfatolithocholyl [3H]taurine (Deeley and Cole 2006; Jedlitschky et al. 1996). Finally, these studies provided direct evidence that MRP1 (ABCC1) was the long-sought GS-X pump.
Thereafter, completion of the Human Genome project and advances in bioinformatics enabled researchers to identify a total of 48 ABC transporters in the human genome. It has been reported that mutations of ABC protein genes are causative in several genetic disorders in humans (Dean et al. 2001). Many of human ABC proteins are involved in membrane transport of drugs, xenobiotics, endogenous substances, or ions, thereby exhibiting a wide spectrum of biological functions. Based on the arrangement of molecular structural components, i.e., nucleotide-binding domains and topologies of transmembrane domains, the hitherto reported human ABC proteins are classified into seven subfamilies (A to G). The HUGO Human Gene Nomenclature Committee developed a standard nomenclature for the human ABC-transporter gene family.
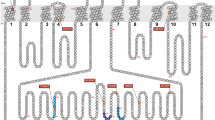
It is now known that MRPs belong to the C-class of the human ABC protein gene family, according to the HUGO Human Gene Nomenclature. This C-class contains a total of 12 genes, of which nine are MRP-related and designed MRP1 through MRP9 (ABCC1-6 and ABCC10-12, respectively) (Fig. 5a). The other three are the cystic fibrosis transmembrane conductance regulator (CFTR/ABCC7) and the sulfonylurea receptors (SUR1/ABCC8 and SUR2/ABCC9). All of the members belonging to the C-class contain two ATP-binding cassettes in the intracellular part and two core membrane-spanning domains MSD1 and MSD2. In addition, ABCC1-3, 6, and 10 contain one additional membrane-spanning domain (MSD0) (Fig. 5b). Topologically, MSD0 is not essential for the catalytic function of ABCC1 (MRP1), because deleting this domain does not compromise its LTC4 transport activity (Bakos et al. 1998). Mutations at certain Cys residues within MSD0, however, substantially reduce LTC4 transport activities (Leslie et al. 2003; Yang et al. 2002), and a photoaffinity labeling study demonstrated that MSD0 interacts with a photoreactive azido analog of LTC4 (Karwatsky et al.2005) suggesting that MSD0 may not be entirely functionless. The C-class ABC transporters, in particular MRPs, were hitherto reviewed in many excellent articles (Borst and Elferink 2002; Borst et al. 2000, 2006; Deeley and Cole 2006; Kruh and Belinsky 2003; Kruh et al. 2007). In this article, therefore, we will provide clinical aspects focusing on ABCC11 (MRP8).
a Schematic diagram representing the relationship among the multidrug resistance-associated protein (MRP) class members, cystic fibrosis transmembrane conductance regulator (CFTR), and sulfonylurea reseptors (SUR) in the human ABC protein gene C-family. b Schematic illustration of the structures of MRP class members. ABCC1, 2, 3, 6, 10 (MRP1, 2, 3, 6, and 7) have a total of 17 transmembrane regions (cylinders), clustered into three membrane-spanning domains (MSD0, MSD1, MSD2), and two intracellular ABCs. ABCC4, 5, 11, and 12 (MRP4, 5, 8, and 9) have 12 transmembrane regions, as they lack MSD0
Genetic Polymorphisms in ABCC11 (MRP8) Gene
The ABCC11 (MRP8) gene was identified by searching the human genome database. In 2001, three research groups independently cloned ABCC11 (MRP8) together with ABCC12 (MRP9) from the cDNA library of human adult liver (Yabuuchi et al. 2001; Bera et al. 2001; Tammur et al. 2001). Both ABCC11 (MRP8) and ABCC12 (MRP9) genes are located on human chromosome 16q12.1 in a tail-to-head orientation with a separation distance of about 20 kb (Fig. 6). The predicted amino acid sequences of both gene products show a high similarity to those of ABCC4 (MRP4) and ABCC5 (MRP5) (Fig. 6). However, there is no putative mouse or rat orthologous gene corresponding to human ABCC11 (Shimizu et al. 2003). This fact indicates that ABCC11 is not an orthologous gene but rather a paralogous gene generated by gene duplication in the human genome (Shimizu et al. 2003).
When transfected exogenously, the ABCC11 protein was localized in the apical membrane of Madin–Darby canine kidney strain II cells (MDCK II cells) (Bortfeld et al. 2006). The substrate specificity of ABCC11 was characterized in more detail by an in vitro transport assay with plasma membrane vesicles prepared from pig LLC-PK1 cells transfected with an ABCC11 expression vector (Chen et al. 2005). The results of this assay demonstrated that ABCC11 WT is able to transport a variety of lipophilic anions including cyclic nucleotides, glutathione conjugates such as leukotriene C4 (LTC4) and S-(2,4-dinitrophenyl)-glutathione (DNP-SG), steroid sulfates such as estrone 3-sulfate (E13S) and dehydroepiandrosterone 3-sulfate (DHEAS), glucuronides such as estradiol 17-β-D-glucuronide (E217βG), the monoanionic bile acids glycocholate and taurocholate, as well as folic acid and its analog methotrexate (Bortfeld et al. 2006; Chen et al. 2005).
Impressively, a single nucleotide polymorphism (SNP) in the ABCC11 gene was reported to determine the human earwax type (Yoshiura et al. 2006). Earwax (cerumen) is a secretory product of the ceruminous apocrine glands, which can be classified into two phenotypes in humans, wet (sticky) and dry. By using a positional cloning and linkage disequilibrium analysis of genetics of earwax type, Yoshiura et al. (2006) revealed that the nonsynonymous SNP (538G > A, Gly180Arg) in the ABCC11 gene is the determinant of human earwax type. The A/A genotype gives the dry phenotype, whereas both G/A and G/G genotypes give the wet phenotype. This is consistent with observations that earwax type is a Mendelian trait and that the wet phenotype is dominant over the dry one. Loss of ABCC11 function is considered to be a cause for the dry phenotype. ABCC11 WT may function to secrete such “wet” components of earwax.
Functional studies with ABCC11-expressing LLC-PK1 cells demonstrated that cells expressing ABCC11 SNP with allele A (Gly180Arg) show a significantly lower level of cGMP transport, as compared with those expressing ABCC11 WT with allele G (Yoshiura et al. 2006). Gly180 is located in the first transmembrane helix of ABCC11 protein, and substitution of Gly180 to Arg180 gives a positive charge to the first transmembrane helix. This electrostatic change might affect the structure of this helix and thereby N-glycosylation of ABCC11 protein is impaired (Toyoda et al. 2009).
Toyoda et al. (2009) provided evidence that proteasomal degradation of the SNP variant (Arg180) of ABCC11 is the underlying molecular mechanism (Toyoda et al. 2009). ABCC11 WT with Gly180 is an N-linked glycosylated protein, which is localized within intracellular granules and large vacuoles as well as at the luminal membrane of secretory cells in the cerumen apocrine gland (Toyoda et al. 2009). N-linked glycosylation occurs at both Asn838 and Asn844 in the extracellular loop between transmembrane domains 7 (TM7) and 8 (TM8) of the ABCC11 WT protein (Toyoda et al. 2009). The N-linked glycans are thought to be subjected to extensive modification as glycoproteins mature and move through the endoplasmic reticulum (ER) via the Golgi apparatus to their final destinations as, for example, intracellular granules and large vacuoles of secretory cells in the apocrine gland. In contrast, the SNP variant (Arg180) lacks N-linked glycosylation and readily undergoes proteasomal degradation, most probably via ubiquitination. As a consequence, no granular or vacuolar localization was detected in the cerumen apocrine glands of people homozygous for the SNP variant.
Immunohistochemical studies with cerumen gland-containing tissue specimens revealed that the ABCC11 WT protein with Gly180 was expressed in the cerumen gland (Toyoda et al. 2009). The cerumen gland is one of the apocrine glands. In addition to their presence in the external auditory canal, apocrine glands can be found in the axillary region and breast, whose physical characteristics also are concerned with apocrine glands. In fact, there is a positive association among the wet earwax type, axillary osmidrosis (Yoo et al. 2006), and colostrum secretion from the breast (Miura et al. 2007).
a The genomic structures of ABCC11 (MRP8) and ABCC12 (MRP9) genes on human chromosome 16q12.1. The cytogenetic location of the ABCC11 and ABCC12 genes as well as the structures of exons and introns were analyzed by BLAST search on the human genome. The SNP 538G > A (Gly180Arg) resides on exon 4 of the ABCC11 gene
It is of great interest to note that the nonsynonymous SNP of 538 G > A (Gly180Arg) in the ABCC11 gene exhibits wide ethnic differences in the allele frequency (Fig. 7). Among Mongoloid populations in Asia, the frequency of the 538 A allele is predominantly high, whereas its allele frequency is low among Caucasians and Africans. The frequency of the 538 A allele exhibits a east–west downward geographical gradient with the highest peak in northeast Asia. It is suggested that the A allele arose in northeast Asia (Yoshiura et al. 2006), apparently reflecting the intercontinental migration of Homo sapiens (Ishikawa et al. 2013a, b).
Association Between SNP 538G > A (Gly180Arg) in ABCC11 Gene and Axillary Osmidrosis
Recently, it has been demonstrated that the ABCC11 WT allele is intimately associated with axillary osmidrosis as well as the wet type of earwax (Table 2). Several studies have already concluded that the genotypes at ABCC11 538G > A would be useful biomarkers for the diagnosis of axillary osmidrosis (Martin et al. 2010; Inoue et al. 2010; Toyoda et al. 2009; Nakano et al. 2009). Therefore, it was expected that genotyping of the ABCC11 gene would provide an accurate and practical criterion for guidance of appropriate treatment and psychological management of patients (Toyoda et al. 2009; Inoue et al. 2010; Ishikawa and Hayashizaki 2013).
Sweat produced by the axillary apocrine glands is odorless. Secretions from the apocrine glands, however, can be converted to odoriferous compounds by bacteria (Corynebacteria), which results in the formation of the unique “human axillary odor” (Shehadeh and Kligman 1963). Axillary osmidrosis patients (538G/G homozygote or G/A heterozygote) were observed to have significantly more numerous and larger-sized axillary apocrine glands as compared with those in subjects carrying the A/A homozygote. Indeed, the 538G allele in the ABCC11 gene is associated with axillary osmidrosis (Martin et al. 2010; Inoue et al. 2010; Toyoda et al. 2009; Nakano et al. 2009), and ABCC11 WT (Gly180) would be responsible for the secretion of preodoriferous compounds from the axillary apocrine gland. In primates, axillary odors may play a role in olfactory communication, although no documented behavioral or endocrine changes resulting from volatiles produced in the axillae have been reported to occur in humans. Previous studies have described the presence of androgen steroids in the axillary area. Androsterone sulfate (AS) and DHEAS were detected in an extract of axillary hairs in addition to high levels of cholesterol (Julesz 1968). It was also demonstrated, following injection of radioactive pregnenolone or progesterone, that steroid secretion was concentrated in the axillary area (Brooksbank 1970). In those studies, however, the axillary sweat collected from the skin surface contained a mixture of materials from apocrine, eccrine, and sebaceous glands, in addition to desquamating epidermal cells. In this respect, Labows et al. (1979) demonstrated that pure apocrine secretions contained at least two androgen steroids, AS and DHEAS, in addition to cholesterol. It is strongly suggested that one of the physiological functions of ABCC11 WT is the active transport of steroid metabolites, such as AS and DHEAS, into the lumen of apocrine glands.
Diagnosis of Axillary Osmidrosis by Genotyping ABCC11 538G
Today in Japan, axillary osmidrosis is recognized as a disease that is covered by the national health insurance system. Axillary osmidrosis, which is exemplified by unpleasant odors, sweating and staining of clothes, is often perceived, especially by young women, as a distressing and troublesome problem (Wu et al. 1994). Certain people display an excessive fear, aversion, or psychological hypersensitivity to unpleasant smells or odors. They tend to opt for aggressive surgical treatments and are sometimes categorized as having osmophobia. Interestingly, an association between wet-type earwax and axillary osmidrosis had already been recognized more than half a century ago (Matsunaga 1962). Hence, the wet type of earwax has frequently been used as one of the diagnostic criteria and characteristics in the clinic. This relationship, however, had only been based on the observations of those two respective phenotypes. Therefore, there has been a need for objective evidence for diagnosis of axillary osmidrosis to prevent unnecessary treatments for such patients.
The rapid growth of personalized medicine is being supported by emerging new technologies together with accumulating knowledge of pharmacogenomics. We have created a clinical method to genotype the SNP 538G > A in the human ABCC11 gene (Ishikawa and Hayashizaki 2013). The clinical method based on isothermal DNA amplification reactions enables us to detect the genetic polymorphism in about 30 min under isothermal conditions. This method requires neither DNA isolation nor PCR process for sample preparation (Toyoda et al. 2009; Ishikawa and Hayashizaki 2013). To determine the SNP 538G > A (Gly180Arg) in the ABCC11 gene, we prepared one set of primers designated TP, FP, BP, OP, and CP (Ishikawa & Hayashizaki 2013). The TPs discriminate the polymorphism 538G or 538A in the ABCC11 gene, and the CPs inhibit the background amplification from mismatch sequence pairs (Toyoda et al. 2009; Ishikawa and Hayashizaki 2013). These primers selectively recognized the SNP 538G > A of the ABCC11 gene to discriminate homozygous 538G/G, heterozygous 538G/A, and homozygous 538A/A. Genotype-specific reactions are monitored by measuring the fluorescence of SYBR®Green that is intercalated into amplified DNA products. The end-point determination can be achieved by introducing a CCD camera (Fig. 8), which enables simple and cost-effective detection. Thus, this genotyping method is expected to provide a practical tool for clinical diagnosis of axillary osmidrosis in Japan (Fig. 9).
Schematic illustration for end-point detection of SmartAmp-based SNP typing with a CCD camera-linked digital processor. Before being applied to the SmartAmp reaction, each blood sample is diluted 4-fold with 40 mM NaOH and then heated at 98 °C for 3 min. During this pretreatment process, proteins and RNA are denatured and degraded under alkaline conditions. After chilling on ice, 1 μl of the pretreated sample is added directly into the reaction mixture (final volume of 25 μl). To determine the SNP 538G > A (Gly180Arg) in the ABCC11 gene, the reaction mixture contains one set of primers designated TP, FP, BP, OP, and CP (Ishikawa and Hayashizaki 2013)
Decision tree for the treatment of axillary osmidrosis. The genetic test of SNP in the ABCC11 gene is one of the clinical factors that underlies doctor’s decision. Patients carrying genotypes of 538G/G and 538G/A may be subjected to surgical operation of excising apocrine glands, whereas no surgical operation is needed for those who are carrying the genotype of 538A/A
Concluding Remarks
During the past three decades, remarkable advances were made in terms of analysis of the molecular structure and the genetics of human ABC transporters. Meanwhile, new technologies were also developed to accelerate the speed of basic research and clinical applications. The ABC transporters are a family of large proteins in membranes and are able to transport a variety of compounds including metabolites and drugs through membranes at the cost of ATP hydrolysis. Besides drug transport, the physiological function of ABC transporters includes the transport of lipids, bile salts, toxic compounds, and peptides for antigen presentation or other purposes, such as ion-channel regulation. The human genome contains 48 ABC transporter genes; at least 14 of human ABC transporter genes are reportedly associated with heritable human diseases that are rare and heavily transmitted in families. Mutations in ABC transporter genes have been reported to be associated with inherited diseases including Tangier disease T1 (ABCA1); Stargardt disease, retinitis pigmentosa, and age-related macular degeneration (ABCA4); progressive familial intrahepatic cholestasis (ABCB11); Dubin–Johnson syndrome (ABCC2); pseudoxanthoma elasticum (ABCC6); cystic fibrosis (CFTR/ABCC7); X-linked adrenoleukodystrophy (ABCD1 and ABCD2); some forms of Zellweger syndrome (ABCD3 and ABCD2), and sitosterolemia(a rare lipid metabolic disorder inherited as an autosomal recessive trait) (ABCG5 and ABCG8). Furthermore, it has recently been reported that SNPs in ABCC11 and ABCG2 genes are related to axillary osmidrosis and gout risk, respectively. Understanding the molecular mechanisms and clinical relevance of interindividual variability in drug response remains an important challenge (Ishikawa et al. 2013b). Pharmacogenomics, the study of genetic variation in the genes that influence drug effect, can provide insight into interindividual variability and a more accurate prediction of drug response than may be obtained by relying solely on a patient’s clinical information. Membrane transporters have been increasingly recognized as contributing to variability in drug exposure and response and are important to evaluate during drug development and regulatory review to understand how relevant they are to benefit and risk of drugs. Therefore, the goal of drug transporter pharmacogenomics is to understand the impact of genetic variation on the function of transporters that interact with medications.
References
Akerboom TP, Bilzer M, Sies H (1982) Competition between transport of glutathione disulfide (GSSG) and glutathione S-conjugates from perfused rat liver into bile. FEBS Lett 140:73–76
Bakos E, Evers R, Szakacs G, Tusnady GE, Welker E, Szabo K, de Haas M, van Deemter L, Borst P, Varadi A, Sarkadi B (1998) Functional multidrug resistance protein (MRP1) lacking the N-terminal transmembrane domain. J Biol Chem 273:32167–32175
Bera TK, Lee S, Salvatore G, Lee B, Pastan I (2001) MRP8, a new member of ABC transporter superfamily, identified by EST database mining and gene prediction program, is highly expressed in breast cancer. Mol Med 7:509–516
Board PG (1981) Transport of glutathione S-conjugate from human erythrocytes. FEBS Lett 124:163–165
Borst P, Elferink RO (2002) Mammalian ABC transporters in health and disease. Annu Rev Biochem 71:537–592
Borst P, Evers R, Kool M, Wijnholds J (2000) A family of drug transporters: the multidrug resistance-associated proteins. J Natl Cancer Inst 92:1295–1302
Borst P, Zelcer N, van de Wetering K, Poolman B (2006) On the putative co-transport of drugs by multidrug resistance proteins. FEBS Lett 580:1085–1093
Bortfeld M, Rius M, Konig J, Herold-Mende C, Nies AT, Keppler D (2006) Human multidrug resistance protein 8 (MRP8/ABCC11), an apical efflux pump for steroid sulfates, is an axonal protein of the CNS and peripheral nervous system. Neuroscience 137:1247–1257
Brooksbank BW (1970) Labelling of steroids in axillary sweat after administration of 3H-delta-5-pregnenolone and 14C-progesterone to a healthy man. Experientia 26:1012–1014
Chance B, Sies H, Boveris A (1979) Hydroperoxide metabolism in mammalian organs. Physiol Rev 59:527–605
Chen ZS, Guo Y, Belinsky MG, Kotova E, Kruh GD (2005) Transport of bile acids, sulfated steroids, estradiol 17-β-D-glucuronide, and leukotriene C4 by human multidrug resistance protein 8 (ABCC11). Mol Pharmacol 67:545–557
Cole SP, Bhardwaj G, Gerlach JH, Mackie JE, Grant CE, Almquist KC, Stewart AJ, Kurz EU, Duncan AM, Deeley RG (1992) Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science 258:1650–1654
Dean M, Rzhetsky A, Allikmets R (2001) The human ATP-binding cassette (ABC) transporter superfamily. Genome Res 11:1156–1166
Deeley RG, Cole SP (2006) Substrate recognition and transport by multidrug resistance protein 1 (ABCC1). FEBS Lett 580:1103–1111
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Elferink RP, Ottenhoff R, Liefting W, de Haan J, Jansen PL (1989) Hepatobiliary transport of glutathione and glutathione conjugate in rats with hereditary hyperbilirubinemia. J Clin Invest 84:476–483
Fahey RC, Sundquist AR (1991) Evolution of glutathione metabolism. Adv Enzymol Relat Areas Mol Biol 64:1–53
Finkel T (2003) Oxidant signals and oxidative stress. Curr Opin Cell Biol 15:247–254
Hayes JD, Flanagan JU, Jowsey IR (2005) Glutathione transferases. Annu Rev Pharmacol Toxicol 45:51–88
Hopper E, Belinsky MG, Zeng H, Tosolini A, Testa JR, Kruh GD (2001) Analysis of the structure and expression pattern of MRP7 (ABCC10), a new member of the MRP subfamily Cancer Lett 162:181–191
Huber M, Guhlmann A, Jansen PL, Keppler D (1987) Hereditary defect of hepatobiliary cysteinyl leukotriene elimination in mutant rats with defective hepatic anion excretion. Hepatology 7:224–228
Hwang C, Sinskey AJ, Lodish HF (1992) Oxidized redox state of glutathione in the endoplasmic reticulum. Science 257:1496–1502
Inoue Y, Mori T, Toyoda Y, Sakurai A, Ishikawa T, Mitani Y, Hayashizaki Y, Yoshimura Y, Kurahashi H, Sakai Y (2010) Correlation of axillary osmidrosis to a SNP in the ABCC11 gene determined by the smart amplification process (SmartAmp) method. J Plast Reconstr Aesthet Surg 63:1369–1374
Ishikawa T (1989) ATP/Mg2+-dependent cardiac transport system for glutathione S-conjugates. A study using rat heart sarcolemma vesicles. J Biol Chem 264:17343–17348
Ishikawa T (1992) The ATP-dependent glutathione S-conjugate export pump. Trends Biochem Sci 17:463–468
Ishikawa T, Hayashizaki Y (2013) Clinical SNP detection by SmartAmp method. Methods Mol Biol 1015:55–69
Ishikawa T, Sies H (1984) Cardiac transport of glutathione disulfide and S-conjugate. Studies with isolated perfused rat heart during hydroperoxide metabolism. J Biol Chem 259:3838–3843
Ishikawa T, Esterbauer H, Sies H (1986a) Role of cardiac glutathione transferase and of the glutathione S-conjugate export system in biotransformation of 4-hydroxynonenal in the heart. J Biol Chem 261:1576–1581
Ishikawa T, Zimmer M, Sies H (1986b) Energy-linked cardiac transport system for glutathione disulfide. FEBS Lett 200:128–132
Ishikawa T, Kobayashi K, Sogame Y, Hayashi K (1989) Evidence for leukotriene C4 transport mediated by an ATP-dependent glutathione S-conjugate carrier in rat heart and liver plasma membranes. FEBS Lett 259:95–98
Ishikawa T, Muller M, Klunemann C, Schaub T, Keppler D (1990) ATP-dependent primary active transport of cysteinyl leukotrienes across liver canalicular membrane. Role of the ATP-dependent transport system for glutathione S-conjugates. J Biol Chem 265:19279–19286
Ishikawa T, Li ZS, Lu YP, Rea PA (1997) The GS-X pump in plant, yeast, and animal cells: structure, function, and gene expression. Biosci Rep 17:189–207
Ishikawa T, Akimaru K, Nakanishi M, Tomokiyo K, Furuta K, Suzuki M, Noyori R (1998) Anti-cancer-prostaglandin-induced cell-cycle arrest and its modulation by an inhibitor of the ATP-dependent glutathione S-conjugate export pump (GS-X pump). Biochem J 336:569–576
Ishikawa T, Sakurai A, Kanamori Y, Nagakura M, Hirano H, Takarada Y, Yamada K, Fukushima K, Kitajima M (2005) High-speed screening of human ATP-binding cassette transporter function and genetic polymorphisms: new strategies in pharmacogenomics. Methods Enzymol 400:485–510
Ishikawa T, Toyoda Y, Yoshiura K, Niikawa N (2013a) Pharmacogenetics of human ABC transporter ABCC11: New insights into apocrine gland growth and metabolite secretion. Frontiers Genetics 3(306):2012. doi:10.3389/fgene.2012.00306.eCollection
Ishikawa T, Kim RB, König J (eds) (2013b) Pharmacogenomics of human drug transporters: clinical impacts. Wiley, Hoborken
Jansen PL, Groothuis GM, Peters WH, Meijer DF (1987) Selective hepatobiliary transport defect for organic anions and neutral steroids in mutant rats with hereditary-conjugated hyperbilirubinemia. Hepatology 7:71–76
Jedlitschky G, Leier I, Buchholz U, Barnouin K, Kurz G, Keppler D (1996) Transport of glutathione, glucuronate, and sulfate conjugates by the MRP gene-encoded conjugate export pump. Cancer Res 56:988–994
Julesz M (1968) New advances in the field of androgenic steroidogenesis of the human skin. Acta Med Acad Sci Hung 25:273–285
Karwatsky J, Leimanis M, Cai J, Gros P, Georges E (2005) The leukotriene C4 binding sites in multidrug resistance protein 1 (ABCC1) include the first membrane multiple spanning domain. Biochemistry 44:340–351
Kobayashi K, Sogame Y, Hara H, Hayashi K (1990) Mechanism of glutathione S-conjugate transport in canalicular and basolateral rat liver plasma membranes. J Biol Chem 265:7737–7741
Kondo T, Dale GL, Beutler E (1980) Glutathione transport by inside-out vesicles from human erythrocytes. Proc Natl Acad Sci USA 77:6359–6362
Kondo T, Murao M, Taniguchi N (1982) Glutathione S-conjugate transport using inside-out vesicles from human erythrocytes. Eur J Biochem 125:551–554
Kool M, de Haas M, Scheffer GL, Scheper RJ, van Eijk MJ, Juijn JA, Baas F, Borst P (1997) Analysis of expression of cMOAT (MRP2), MRP3, MRP4, and MRP5, homologues of the multidrug resistance-associated protein gene (MRP1), in human cancer cell lines. Cancer Res 57:3537–3547
Kool M, van der Linden M, de Haas M, Baas F, Borst P (1999) Expression of human MRP6, a homologue of the multidrug resistance protein gene MRP1, in tissues and cancer cells. Cancer Res 59:175–182
Kruh GD, Belinsky MG (2003) The MRP family of drug efflux pumps. Oncogene 22:7537–7552
Kruh GD, Guo Y, Hopper-Borge E, Belinsky MG, Chen ZS (2007) ABCC10, ABCC11, and ABCC12. Pflugers Arch 453:675–684
LaBelle EF, Singh SV, Srivastava SK, Awasthi YC (1986) Dinitrophenyl glutathione efflux from human erythrocytes is primary active ATP-dependent transport. Biochem J 238:443–449
Labows JN, Preti G, Hoelzle E, Leyden J, Kligman A (1979) Steroid analysis of human apocrine secretion. Steroids 34:249–258
Leier I, Jedlitschky G, Buchholz U, Cole SP, Deeley RG, Keppler D (1994) The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J Biol Chem 269:27807–27810
Leslie EM, Bowers RJ, Deeley RG, Cole SP (2003) Structural requirements for functional interaction of glutathione tripeptide analogs with the human multidrug resistance protein 1 (MRP1). J Pharmacol Exp Ther 304:643–653
Li ZS, Zhao Y, Rea PA (1995) Magnesium adenosine 5[prime]-triphosphate-energized transport of glutathione-S-conjugates by plant vacuolar membrane vesicles. Plant Physiol 107:1257–1268
Martin A, Saathoff M, Kuhn F, Max H, Terstegen L, Natsch A (2010) A functional ABCC11 allele is essential in the biochemical formation of human axillary odor. J. Invest. Dermatol. 130:529–540
Martinoia E, Grill E, Tommasini R, Kreuz K, Amrhein N (1993) ATP-dependent glutathione S-conjugate ‘export’ pump in the vacuolar membrane of plants. Nature 364:247–249
Matsunaga E (1962) The dimorphism in human normal cerumen. Ann Hum Genet 25:273–286
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760
Miura K, Yoshiura K, Miura S, Shimada T, Yamasaki K, Yoshida A, Nakayama D, Shibata Y, Niikawa N, Masuzaki H. (2007) A strong association between human earwax-type and apocrine colostrum secretion from the mammary gland. Hum Genet 121:631–633
Muller M, Meijer C, Zaman GJ, Borst P, Scheper RJ, Mulder NH, de Vries EG, Jansen PL (1994) Overexpression of the gene encoding the multidrug resistance-associated protein results in increased ATP-dependent glutathione S-conjugate transport. Proc Natl Acad Sci USA 91:13033–13037
Nakano M, Miwa N, Hirano A, Yoshiura K, Niikawa N (2009) A strong association of axillary osmidrosis with the wet earwax type determined by genotyping of the ABCC11 gene. BMC Genet 10:42
Paulusma CC, Bosma PJ, Zaman GJ, Bakker CT, Otter M, Scheffer GL, Scheper RJ, Borst P, Oude Elferink RP (1996) Congenital jaundice in rats with a mutation in a multidrug resistance-associated protein gene. Science 271:1126–1128
Rea PA, Sanches-fernandez R, Chen S, Peng M, Klein M, Geisler M, Martinoia E (2003) The plant ABC transporter superfamily: the functions of a few and identities of many. In: Holland IB, Cole SP, Kuchler K, Higgins CF (eds) ABC proteins from bacteria to man. Academic Press, Amsterdam, pp 335–355
Reed DJ (1990) Glutathione: toxicological implications. Annu Rev Pharmacol Toxicol 30:603–631
Saito H, Hirano H, Shin Wangsoo, Nakamura R, Osumi M, Ishikawa T (2009) Technical pitfalls and improvements in high-speed xcreening and QSAR analysis to predict durg-drug interactions of ABC transporter ABCB11 (bile salt export pump). AAPS J 11:581–589
Shehadeh NH, Kligman AM (1963) The effect of topical antibacterial agents on the bacterial flora of the axilla. J Invest Dermatol 40:61–71
Shimizu H, Taniguchi H, Hippo Y, Hayashizaki Y, Aburatani H, Ishikawa T (2003) Characterization of the mouse Abcc12 gene and its transcript encoding an ATP-binding cassette transporter, an orthologue of human ABCC12. Gene 310:17–28
Sies H (1985) Oxidative stress. Sies H, editor. Orlando: Academic Press
Sies H, Ketterer B (1988) Glutathione conjugation: mechanisms and biological significance. Academic Press, London
Smith CV, Jones DP, Guenthner TM, Lash LH, Lauterburg BH (1996) Compartmentation of glutathione: implications for the study of toxicity and disease. Toxicol Appl Pharmacol 140:1–12
Srivastava SK, Beutler E (1969) The transport of oxidized glutathione from human erythrocytes. J Biol Chem 244:9–16
Suzuki M, Mori M, Niwa T, Hirata R, Furuta K, Ishikawa T, Noyori R (1997) Chemical implications for antitumor and antiviral prostaglandins—reaction of delta(7)-prostaglandin A(1) and prostaglandin A(1) methyl-esters with thiols. J Am Chem Soc 119:2376–2385
Takikawa H, Sano N, Narita T, Uchida Y, Yamanaka M, Horie T, Mikami T, Tagaya O (1991) Biliary excretion of bile acid conjugates in a hyperbilirubinemic mutant Sprague-Dawley rat. Hepatology 14:352–360
Tammur J, Prades C, Arnould I, Rzhetsky A, Hutchinson A, Adachi M, Schuetz JD, Swoboda KJ, Ptacek LJ, Rosier M, Dean M, Allikmets R (2001) Two new genes from the human ATP-binding cassette transporter superfamily, ABCC11 and ABCC12, tandemly duplicated on chromosome 16q12. Gene 273:89–96
Townsend DM, Tew KD (2003) The role of glutathione-S-transferase in anti-cancer drug resistance. Oncogene 22:7369–7375
Toyoda Y, Sakurai A, Mitani Y, Nakashima M, Yoshiura K, Nakagawa H, Sakai Y, Ota I, Lezhava A, Hayashizaki Y, Niikawa N, Ishikawa T (2009) Earwax, osmidrosis, and breast cancer: why does one SNP (538G > A) in the human ABC transporter ABCC11 gene determine earwax type? FASEB J 23:2001–2013
Winyard PG, Moody CJ, Jacob C (2005) Oxidative activation of antioxidant defence. Trends Biochem Sci 30:453–461
Wu WH, Ma S, Lin JT, Tang YW, Fang RH, Yeh FL (1994) Surgical treatment of axillary osmidrosis: an analysis of 343 cases. Plast Reconstr Surg 94:288–294
Yabuuchi H, Shimizu H, Takayanagi S, Ishikawa T (2001) Multiple splicing variants of two new human ATP-binding cassette transporters, ABCC11 and ABCC12. Biochem Biophys Res Commun 288:933–939
Yabuuchi H, Takayanagi S, Yoshinaga K, Taniguchi N, Aburatani H, Ishikawa T (2002) ABCC13, an unusual truncated ABC transporter, is highly expressed in fetal human liver. Biochem Biophys Res Commun 299:410–417
Yang Y, Chen Q, Zhang JT (2002) Structural and functional consequences of mutating cysteine residues in the amino terminus of human multidrug resistance-associated protein 1. J Biol Chem 277:44268–44277
Yoo WM, Pae NS, Lee SJ, Roh TS, Chung S, Tark KC (2006) Endoscopy-assisted ultrasonic surgical aspiration of axillary osmidrosis: a retrospective review of 896 consecutive patients from 1998 to 2004. J Plast Reconstr Aesthet Surg 59:978–982
Yoshiura K, Kinoshita A, Ishida T, Ninokata A, Ishikawa T, Kaname T, Bannai M, Tokunaga K, Sonoda S, Komaki R, Ihara M, Saenko VA, Alipov GK, Sekine I, Komatsu K, Takahashi H, Nakashima M, Sosonkina N, Mapendano CK, Ghadami M, Nomura M, Liang DS, Miwa N, Kim DK, Garidkhuu A, Natsume N, Ohta T, Tomita H, Kaneko A, Kikuchi M, Russomando G, Hirayama K, Ishibashi M, Takahashi A, Saitou N, Murray JC, Saito S, Nakamura Y, Niikawa N (2006) A SNP in the ABCC11 gene is the determinant of human earwax type. Nat Genet 38:324–330
Acknowledgements
The authors thank Drs. Helmut Sies, Dietrich Keppler, Tsuneaki Gomi, Norio Niikawa, and Koh-ichiro Yoshiura for their valuable discussions throughout our collaborative research.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Ishikawa, T., Toyoda, Y. (2016). Human ABC Transporter ABCC11: Looking Back Pioneers’ Odyssey and Creating a New Path Toward Clinical Application. In: George, A. (eds) ABC Transporters - 40 Years on. Springer, Cham. https://doi.org/10.1007/978-3-319-23476-2_12
Download citation
DOI: https://doi.org/10.1007/978-3-319-23476-2_12
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23475-5
Online ISBN: 978-3-319-23476-2
eBook Packages: Biomedical and Life SciencesBiomedical and Life Sciences (R0)