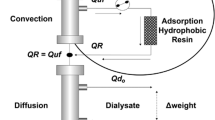
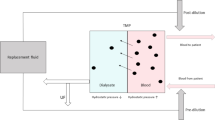
Abstract
Both observational and randomised trials comparing hemodiafiltration (HDF) with hemodialysis (HD) generally showed an improvement of the inflammatory state and oxidative stress in patients treated by HDF. Results do vary from study to study, however, not only due to differences in design and patient recruitment, but also secondary to differences in dialysis water quality, HDF mode and magnitude of the convection volume achieved. If HDF leads to a reduced (micro)inflammation in patients with chronic kidney disease, then the question arises as to whether this translates into clinically relevant measures. With respect to erythropoeitine (EPO) use, especially the earlier trials, when higher haemoglobin targets and greater use of erythropoietins were required, did suggest that HDF was associated with lower EPO requirements. These findings, however, were less clear in more recent large RCTs comparing online postdilution HDF with HD. Two prospective trials reported improved nutritional status with HDF, with objective changes in body composition as demonstrated by bioimpedance and DEXA scanning. There have been few studies which investigated whether switching from HD to HDF improved patient quality of life, and the results have been somewhat contradictory. Whether the small reduction in inflammation underlies the beneficial effect of high volume HDF on all cause and cardiovascular mortality, which is extensively discussed in Chap. 16, is an interesting, but currently unproven, option.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Inflammation
- Hemodiafiltration
- Oxidative stress
- Advanced glycosylation end products (AGEs)
- Convection
- Cytokines
Introduction
Patients with chronic kidney disease stage 5 (CKD5) have increased systemic inflammation and oxidative stress irrespective of whether they are managed conservatively or treated by dialysis. Progressive loss of residual renal function leads to the accumulation of uraemic toxins (Table 13.1). Some of these toxins, such as p-cresol and indoxyl sulfate are formed as by-product of tyrosine and phenylalanine, and tryptophan metabolism respectively, by bacteria in the gastrointestinal tract, whereas other uraemic toxins, including carbamylated albumin and other proteins accumulate due to increased production and others such as advanced glycosylation end (AGEs) products, β2 microglobulin, plasma light chains and circulating cell free DNA simply accumulate due to reduced renal clearance. However, in addition to inflammation driven directly as a consequence of the retention products of uraemia and treatments, there are additional pro-inflammatory factors (Table 13.2).
Several of these uremic toxins, including p-cresol, indoxyl sulfate, homocysteine, AGEs and β2 microglobulin have been reported to be independent risk factors for cardiovascular disease in the CKD5d patient [1–3]. After cardiovascular causes, infectious diseases are the next most common cause of death for dialysis patients with increased mortality rates being greatest for sepsis, followed in descending order by peritonitis, influenza, tuberculosis and pneumonia [4]. Patients with CKD are more susceptible to some infections, as the azotaemic state alters innate immunity, with reports of reduced monocyte Toll like receptor (TLR) 4 expression [5], reduced B lymphocyte cell populations [6], and impaired polymorphonuclear chemotaxis and phagocytosis [7] (Table 13.3). It has also been proposed that changes in the gastro-intestinal microbiota, due to the azotaemic milieu and changes in diet accompanied by increased intestinal permeability to endotoxin, results in a persistent activation of the innate immune system, with induction of regulatory mediators of the immune system which then suppress both innate and adaptive immunity [8]. Additionally, immune responses may also be impaired by poor nutritional status, malnutrition and vitamin D deficiency [9].
Inflammation leads to protein energy wasting (PEW), combining central appetite suppression, increasing risk of depression, insulin resistance with increased muscle breakdown and reduced physical activity. Inflammation leads to an increased endothelial permeability and expansion of extracellular water, which in turn leads to macrophage recruitment and activation, increasing local inflammation and the production of reactive oxygen species, AGEs and advanced oxidised protein products (AOPPs) [10, 11]. This then leads to a vicious cycle which can be difficult to break in clinical practice.
Removal of Uremic Toxins by Hemodiafiltration (HDF)
HDF provides additional convective clearance compared to standard hemodialysis (HD). Small water soluble compounds such as uric acid are effectively removed by diffusion, so HDF, especially in predilution mode is less effective for urate clearance than HD. However, larger molecules such as asymmetric dimethylarginine (ADMA), with a molecular weight of just over 200 D, is more effectively cleared by postdilution HDF than HD [12]. Similarly both phosphate and β2 microglobulin clearance are increased by postdilution HDF compared to highflux HD [13]. Small peptide hormones such as leptin and FGF23 have increased clearance with on-line HDF [14, 15]. Both HDF and high flux HD have been reported to reduce circulating AGEs during a single treatment session, however postdilution HDF removes some 50 % more, and only HDF has been shown to produce a reduction in serum AGEs levels over time [16]. Similarly postdilution HDF clears more mitochondrial and cell free DNA fragments, during a treatment session, than highflux HD [17].
Studies measuring protein bound solute clearance have not demonstrated an advantage for postdilutional HDF over other dialytic modalities in removing p-cresyl or indoxyl sulfate [13, 18]. However, a recent report has suggested that predilution HDF infusing a combination of hypertonic sodium infusate, coupled with a hyponatremic dialysate increases protein bound solutes by altering protein binding, so increasing the free proportion and allowing greater clearances [19].
As such, HDF generally offers advantages over standard HD in terms of clearance of the small and middle sized water soluble azotaemic toxins. Although convective clearance would in theory be greater with predilution mode for middle sized molecules, high convective volumes also dilute the concentration gradient and reduce diffusional losses. However for most middle sized solutes, clearance is equal or greater with postdilutional mode, as the concentration entering the dialyzer is higher, and membrane adsorption is also increased. When used in conventional pre or postdilution mode, HDF does not offer any increased clearance of protein bound azotaemic toxins.
Does Hemodiafiltration Reduce the Inflammatory Effect of Hemodialysis
As blood passes out through the patient’s vascular access into the extracorporeal circuit (ECC), across the dialyzer, through the venous air detector chamber and then returns through the access, leukocytes, monocytes and platelets are activated. As the dialyzer has the greatest surface area of the extracorporeal circuit, this is the main site of activation. Complement proteins are also activated by dialyzers, with different dialyzer membrane compositions activating complement by different pathways; with polysulphone dialyzers causing classic complement pathway activation or lectin pathway activation and cellulosic dialyzers causing alternative pathway activation. Cellular activation leads to transcription of several proinflammatory cytokines, including TNF\( \alpha \), IL1β, IL-6, and IL-8, as well as chemokine receptors CXCR4 CCR7 CX3CR1, and other inflammatory mediators such as TWEAK, TRAIL and pentraxin 3. Monocyte and leukocyte activation also leads to surface blebbing and release of microparticles which trigger thrombin generation and clotting, and activation of the kinin-bradykin system. As bradykinin generation is pH dependent, then on-line priming with bicarbonate solutions increases pH and reduces bradykinin generation compared to priming with 0.9 % saline with haemodialysis [20]. HDF using ultrapure fluids has been reported to induce less monocyte and leukocyte activation and cytokine release compared to HD [21, 22].
HDF has been reported to reduce the frequency of hypotensive episodes during dialysis sessions compared to HD. Intermittent hypotensive episodes can potentially result in hypoperfusion and visceral ischemia. Although most interest has centred on reduction in cardiac blood supply and cardiac “stunning” during dialysis, other organs including the gastro-intestinal tract also suffer from ischaemia. Ischaemia, per se induces inflammatory changes. However intestinal ischaemia also leads to alteration in gut permeability, and so allows the potential for the passage of bacterial derived endotoxin into the portal circulation. As such, some of the reduction in inflammatory changes reported with on-line HDF, may be consequent on a reduction in the inflammatory response to dialysis, due to the combination of improved dialysis water quality, reduced production or increased clearance of cytokines and inflammatory mediators generated by the passage of blood through the extracorporeal circuit, and reduced gut ischemia and endotoxin translocation.
Dialysis Water Risk in Hemodiafiltration
As large volumes of dialysis water are infused directly into the patient during on-line HDF treatments, then water quality is of paramount importance, and should comply with both microbiological standards for endotoxin and bacterial contamination to ensure ultra-pure water grade (<0.1 colony forming bacterial/ml and <0.03 EU/ml) as well as chemical purity [23]. In part some of the reports of reduced inflammatory changes associated with HDF may simply reflect switching to ultrapure dialysis water.
Bacterial may form biofilm in the pipes supplying water to a dialysis unit, or contaminate bicarbonate or electrolyte mixtures. Although the current endotoxin filters will remove endotoxin and large bacterial DNA fragments [24], smaller fragments may pass through. Small fragments of bacterial DNA, up to 20 base pairs can potentially cross the current highflux dialyzers from the dialysate into the plasma water [25]. Bacterial DNA differs from human DNA in terms of methylation, and as such bacterial DNA fragments are detected and directly activate Toll like receptor 9 and provoke an inflammatory reaction.
Effects of Hemodiafiltration on Inflammation and Oxidative Stress
As renal function declines, the clearance of inflammatory mediators declines, and as such HDF, by adding convective clearance, may be expected to reduce the inflammatory milieu and oxidative stress of chronic kidney disease. Hence reports of HDF reducing circulating levels of IL-6 and TNF\( \alpha \), associated with a reduction in circulating proinflammatory monocytes (CD14+CD16+ positive cells) and C creative protein [26, 27]. Similarly HDF has been reported to reduce markers of oxidative stress, such as p22phox (the subunit of NAD(P)H oxidase), PAI-1, and oxidised plasma low density lipoproteins [28]. Others have demonstrated a reduction in reactive oxygen metabolites, and an increase in total anti-oxidant activity in both whole blood and lymphocytes [29, 30] and also increased heme-oxygenase-1, a protein involved in protection against the effects of oxidative damage and inflammation compared to patients treated by standard HD [31]. However longer term studies showed that changes in anti-oxidant activity were more modest, than those reported in short term studies, with if anything a reduction in the antioxidant capacity of lymphocytes, with reduced concentrations of superoxide dismutase [32].
Inflammation is linked to endothelial dysfunction, with release of endothelial microparticles. Reports have suggested that CKD patients treated by HDF have lower circulating endothelial microparticles [33]. Although inducible monocyte nitric oxide synthase activity was shown not to be altered by HDF [31], the response to endothelial nitric oxide appears to be improved with increased brachial artery flow-mediated vasodilatation and carotid artery distensibility with HDF [34].
Clinical Effects of Hemodiafiltration
If HDF leads to a reduction in microinflammation in CKD patients, then the question arises as to whether this translates into clinically relevant measures. Comparative studies using ultrapure dialysate water comparing haemodialysis with hemodiafiltration have shown a variable effect on serum albumin, with some studies reporting an increase with HDF [35], and more recent reports not showing any differences in serum albumin over time [36, 37]. This may be due to the potentially greater losses of albumin with higher convection volume exchanges used in the more recent studies [36, 37]. Earlier studies also reported an improvement in nutritional status with HDF, as assessed by body mass index and fat mass [35]. However more importantly two studies observed that treatment with HDF led to an increase in lean body mass, measured by bioimpedance and DEXA techniques [35, 38].
Although earlier studies reported that treatment with HDF increased the response to erythropoietins, and reduced erythropoietin resistance [39, 40], this was not supported by more recent studies [41]. However both the targets for haemoglobin, and biologically available iron, have changed over time and as such lower doses of erythropoiesis stimulating agents are now used in clinical practice, which may well explain why the initial reports showed a positive effect for HDF when much higher doses were used compared to the current day. See also Chap. 12.
Inflammation has been linked to a greater prevalence of low mood and depression. Studies which have investigated whether HDF improves quality of life have produced varied results, with one study reporting that quality of life scores improved with HDF [38], whereas another failed to show any significant benefit [42] (Table 13.4).
Summary
Observational and randomised trials of HDF generally have reported that the introduction of HDF generally decreases the inflammatory milieu and increased oxidative stress of CKD. Results do vary from study to study, not only due to differences in patient recruitment, but also secondary to differences in dialysis water quality and HDF mode – predilution, mid-dilution and postdilution and mixed pre and postdilution, but more importantly the convective volume exchanged. In terms of translating these improvements in reducing microinflammation, then the earlier trials when higher haemoglobin targets and greater use of erythropoietins were required did suggest that HDF was associated with lower erthyropoietin requirements. In addition two prospective trials reported improved nutritional status with HDF with objective changes in body composition as demonstrated by bioimpedance and DEXA scanning. There have been few studies which investigated whether switching from HD to HDF improved patient quality of life, and the results have been somewhat contradictory [38, 42]. However the trials differed in terms of the mode of HDF and convective volumes delivered to be able to compare studies.
More recently randomised controlled trials have reported an overall survival benefit for HDF. Several studies have shown that the survival benefit was dependent upon the amount of convective clearance delivered [47, 48]. In addition as the survival benefit was predominantly for cardiovascular disease, then HDF by reducing microinflammation could potentially reduce vascular disease by modifying atheroma.
Teaching Points
-
CKD patients not yet on dialysis already show signs of (micro)inflammation and oxidative stress
-
Its causes are multifactorial, and result from reduced renal clearance, abnormal metabolic pathways and increased intestinal permeability for bacterial endotoxins
-
During HD, the (micro)inflammatory state is aggravated by cellular and humoral activation within the ECC
-
HDF may reduce (micro)inflammation by a reduction in IDH, and consequently, less intestinal hypoperfusion and a lower passage of bacterial endotoxins into the portal circulation
-
In addition, several cytokines and other inflammatory mediators in the MMW range are better removed by HDF than by (highflux) HD
-
Despite these findings, large recent RCTs failed to show clear differences in serum CRP and albumin levels between HD and HDF patients
Abbreviations
- CKD:
-
Chronic kidney disease
- CRP:
-
C-reactive protein
- ECC:
-
Extra- corporeal circuit
- HD:
-
Hemodialysis
- HDF:
-
Hemodiafiltration
- IDH:
-
Intra-dialytic hypotension
References
Liabeuf S, Lenglet A, Desjardins L, Neirynck N, Glorieux G, Lemke HD, et al. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 2012;82(12):1297–303.
Liabeuf S, Drueke TB, Massy ZA. Protein-bound uremic toxins: new insight from clinical studies. Toxins (Basel). 2011;3(7):911–9.
Furuya R, Kumagai H, Miyata T, Fukasawa H, Isobe S, Kinoshita N, et al. High plasma pentosidine level is accompanied with cardiovascular events in hemodialysis patients. Clin Exp Nephrol. 2012;16(3):421–6.
Wakasugi M, Kawamura K, Yamamoto S, Kazama JJ, Narita I. High mortality rate of infectious diseases in dialysis patients: a comparison with the general population in Japan. Ther Apher Dial. 2012;16(3):226–31.
Koc M, Toprak A, Arikan H, Odabasi Z, Elbir Y, Tulunay A, et al. Toll-like receptor expression in monocytes in patients with chronic kidney disease and haemodialysis: relation with inflammation. Nephrol Dial Transplant. 2011;26(3):955–63.
Pahl MV, Gollapudi S, Sepassi L, Gollapudi P, Elahimehr R, Vaziri ND. Effect of end-stage renal disease on B-lymphocyte subpopulations, IL-7, BAFF and BAFF receptor expression. Nephrol Dial Transplant. 2010;25(1):205–12.
Cohen G, Haag-Weber M, Horl WH. Immune dysfunction in uremia. Kidney Int Suppl. 1997;62:S79–82.
Anders HJ, Andersen K, Stecher B. The intestinal microbiota, a leaky gut, and abnormal immunity in kidney disease. Kidney Int. 2013;83(6):1010–6.
Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol. 2010;21(2):353–61.
Sukriti S, Tauseef M, Yazbeck P, Mehta D. Mechanisms regulating endothelial permeability. Pulm Circ. 2014;4(4):535–51.
Steyers III CM, Miller Jr FJ. Endothelial dysfunction in chronic inflammatory diseases. Int J Mol Sci. 2014;15(7):11324–49.
Zhang DL, Liu J, Liu S, Zhang Y, Liu WH. The differences of asymmetric dimethylarginine removal by different dialysis treatments. Ren Fail. 2010;32(8):935–40.
Meert N, Eloot S, Waterloos MA, Van LM, Dhondt A, Glorieux G, et al. Effective removal of protein-bound uraemic solutes by different convective strategies: a prospective trial. Nephrol Dial Transplant. 2009;24(2):562–70.
Mandolfo S, Borlandelli S, Imbasciati E. Leptin and beta2-microglobulin kinetics with three different dialysis modalities. Int J Artif Organs. 2006;29(10):949–55.
Patrier L, Dupuy AM, Granger VA, Chalabi L, Morena M, Canaud B, et al. FGF-23 removal is improved by on-line high-efficiency hemodiafiltration compared to conventional high flux hemodialysis. J Nephrol. 2013;26(2):342–9.
Lin CL, Huang CC, Yu CC, Yang HY, Chuang FR, Yang CW. Reduction of advanced glycation end product levels by on-line hemodiafiltration in long-term hemodialysis patients. Am J Kidney Dis. 2003;42(3):524–31.
Cao H, Ye H, Sun Z, Shen X, Song Z, Wu X, et al. Circulatory mitochondrial DNA is a pro-inflammatory agent in maintenance hemodialysis patients. PLoS One. 2014;9(12):e113179.
Krieter DH, Hackl A, Rodriguez A, Chenine L, Moragues HL, Lemke HD, et al. Protein-bound uraemic toxin removal in haemodialysis and post-dilution haemodiafiltration. Nephrol Dial Transplant. 2010;25(1):212–8.
Bohringer F, Jankowski V, Gajjala PR, Zidek W, Jankowski J. Release of uremic retention solutes from protein binding by hypertonic predilution hemodiafiltration. ASAIO J. 2015;61(1):55–60.
Coppo R, Amore A, Cirina P, Scelfo B, Giacchino F, Comune L, et al. Bradykinin and nitric oxide generation by dialysis membranes can be blunted by alkaline rinsing solutions. Kidney Int. 2000;58(2):881–8.
Todeschini M, Macconi D, Fernandez NG, Ghilardi M, Anabaya A, Binda E, et al. Effect of acetate-free biofiltration and bicarbonate hemodialysis on neutrophil activation. Am J Kidney Dis. 2002;40(4):783–93.
Kawabata K, Nakai S, Miwa M, Sugiura T, Otsuka Y, Shinzato T, et al. Changes in Mac-1 and CD14 expression on monocytes and serum soluble CD14 level during push/pull hemodiafiltration. Nephron. 2002;90(3):273–81.
Tattersall JE, Ward RA. Online haemodiafiltration: definition, dose quantification and safety revisited. Nephrol Dial Transplant. 2013;28(3):542–50.
Handelman GJ, Megdal PA, Handelman SK. Bacterial DNA in water and dialysate: detection and significance for patient outcomes. Blood Purif. 2009;27(1):81–5.
Tao X, Hoenich N, Handelman SK, Levin NW, Kotanko P, Handelman GJ. Transfer of low-molecular weight single-stranded DNA through the membrane of a high-flux dialyzer. Int J Artif Organs. 2014;37(7):529–38.
Carracedo J, Merino A, Nogueras S, Carretero D, Berdud I, Ramirez R, et al. On-line hemodiafiltration reduces the proinflammatory CD14+CD16+ monocyte-derived dendritic cells: a prospective, crossover study. J Am Soc Nephrol. 2006;17(8):2315–21.
Panichi V, Manca-Rizza G, Paoletti S, Taccola D, Consani C, Filippi C, et al. Effects on inflammatory and nutritional markers of haemodiafiltration with online regeneration of ultrafiltrate (HFR) vs online haemodiafiltration: a cross-over randomized multicentre trial. Nephrol Dial Transplant. 2006;21(3):756–62.
Calo LA, Naso A, Carraro G, Wratten ML, Pagnin E, Bertipaglia L, et al. Effect of haemodiafiltration with online regeneration of ultrafiltrate on oxidative stress in dialysis patients. Nephrol Dial Transplant. 2007;22(5):1413–9.
Filiopoulos V, Hadjiyannakos D, Metaxaki P, Sideris V, Takouli L, Anogiati A, et al. Inflammation and oxidative stress in patients on hemodiafiltration. Am J Nephrol. 2008;28(6):949–57.
Gonzalez-Diez B, Cavia M, Torres G, Abaigar P, Muniz P. Effect of a hemodiafiltration session with on-line regeneration of the ultrafiltrate on oxidative stress. Comparative study with conventional hemodialysis with polysulfone. Blood Purif. 2008;26(6):505–10.
Calo LA, Naso A, Davis PA, Pagnin E, Corradini R, Tommasi A, et al. Hemodiafiltration with online regeneration of ultrafiltrate: effect on heme-oxygenase-1 and inducible subunit of nitric oxide synthase and implication for oxidative stress and inflammation. Artif Organs. 2011;35(2):183–7.
Gonzalez-Diez B, Cavia M, Torres G, Abaigar P, Camarero V, Muniz P. The effects of 1-year treatment with a haemodiafiltration with on-line regeneration of ultrafiltrate (HFR) dialysis on biomarkers of oxidative stress in patients with chronic renal failure. Mol Biol Rep. 2012;39(1):629–34.
Ariza F, Merino A, Carracedo J, Alvarez de Lara MA, Crespo R, Ramirez R, et al. Post-dilution high convective transport improves microinflammation and endothelial dysfunction independently of the technique. Blood Purif. 2013;35(4):270–8.
Bellien J, Freguin-Bouilland C, Joannides R, Hanoy M, Remy-Jouet I, Monteil C, et al. High-efficiency on-line haemodiafiltration improves conduit artery endothelial function compared with high-flux haemodialysis in end-stage renal disease patients. Nephrol Dial Transplant. 2014;29(2):414–22.
Savica V, Ciolino F, Monardo P, Mallamace A, Savica R, Santoro D, et al. Nutritional status in hemodialysis patients: options for on-line convective treatment. J Ren Nutr. 2006;16(3):237–40.
den Hoedt CH, Bots ML, Grooteman MP, van der Weerd NC, Penne EL, Mazairac AH, et al. Clinical predictors of decline in nutritional parameters over time in ESRD. Clin J Am Soc Nephrol. 2014;9(2):318–25.
den Hoedt CH, Bots ML, Grooteman MP, van der Weerd NC, Mazairac AH, Penne EL, et al. Online hemodiafiltration reduces systemic inflammation compared to low-flux hemodialysis. Kidney Int. 2014;86(2):423–32.
Beerenhout CH, Luik AJ, Jeuken-Mertens SG, Bekers O, Menheere P, Hover L, et al. Pre-dilution on-line haemofiltration vs low-flux haemodialysis: a randomized prospective study. Nephrol Dial Transplant. 2005;20(6):1155–63.
Vaslaki L, Major L, Berta K, Karatson A, Misz M, Pethoe F, et al. On-line haemodiafiltration versus haemodialysis: stable haematocrit with less erythropoietin and improvement of other relevant blood parameters. Blood Purif. 2006;24(2):163–73.
Bonforte G, Grillo P, Zerbi S, Surian M. Improvement of anemia in hemodialysis patients treated by hemodiafiltration with high-volume on-line-prepared substitution fluid. Blood Purif. 2002;20(4):357–63.
van der Weerd NC, den Hoedt CH, Blankestijn PJ, Bots ML, van den Dorpel MA, Levesque R, et al. Resistance to erythropoiesis stimulating agents in patients treated with online hemodiafiltration and ultrapure low-flux hemodialysis: results from a randomized controlled trial (CONTRAST). PLoS One. 2014;9(4):e94434.
Mazairac AH, de Wit GA, Grooteman MP, Penne EL, van der Weerd NC, den Hoedt CH, et al. Effect of hemodiafiltration on quality of life over time. Clin J Am Soc Nephrol. 2013;8(1):82–9.
Kuo HL, Chou CY, Liu YL, Yang YF, Huang CC, Lin HH. Reduction of pro-inflammatory cytokines through hemodiafiltration. Ren Fail. 2008;30(8):796–800.
Oates T, Pinney JH, Davenport A. Haemodiafiltration versus high-flux haemodialysis: effects on phosphate control and erythropoietin response. Am J Nephrol. 2011;33(1):70–5.
Penne EL, van der Weerd NC, van den Dorpel MA, Grooteman MP, Levesque R, Nube MJ, et al. Short-term effects of online hemodiafiltration on phosphate control: a result from the randomized controlled Convective Transport Study (CONTRAST). Am J Kidney Dis. 2010;55(1):77–87.
Ramirez R, Carracedo J, Merino A, Nogueras S, varez-Lara MA, Rodriguez M, et al. Microinflammation induces endothelial damage in hemodialysis patients: the role of convective transport. Kidney Int. 2007;72(1):108–13.
Grooteman MP, van den Dorpel MA, Bots ML, Penne EL, van der Weerd NC, Mazairac AH, et al. Effect of online hemodiafiltration on all-cause mortality and cardiovascular outcomes. J Am Soc Nephrol. 2012;23(6):1087–96.
Maduell F, Moreso F, Pons M, Ramos R, Mora-Macia J, Carreras J, et al. High-efficiency postdilution online hemodiafiltration reduces all-cause mortality in hemodialysis patients. J Am Soc Nephrol. 2013;24(3):487–97.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this chapter
Cite this chapter
Davenport, A. (2016). Effects of Hemodiafiltration of Inflammation and Oxidative Stress. In: Nubé, M., Grooteman, M., Blankestijn, P. (eds) Hemodiafiltration. Springer, Cham. https://doi.org/10.1007/978-3-319-23332-1_13
Download citation
DOI: https://doi.org/10.1007/978-3-319-23332-1_13
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-23331-4
Online ISBN: 978-3-319-23332-1
eBook Packages: MedicineMedicine (R0)